Abstract
Introduction
Alkaline phosphatase is implicated in intestinal lipid transport and to the development of obesity. Placental alkaline phosphatase is localised to the microvillous plasma membrane of the placental syncytiotrophoblast at the maternal-fetal interface, but its role is unclear. We investigated the relations of placental alkaline phosphatase activity and mRNA expression with maternal body composition and offspring fat mass in humans.
Methods
Term human placentas from the UK Birthright cohort (n=52) and the Southampton Women’s Survey (SWS)(n=95) were studied. In the Birthright cohort, alkaline phosphatase activity was measured in placental microvillous plasma membrane vesicles. In the SWS, alkaline phosphatase mRNA was measured using Nanostring. Alkaline phosphatase gene expression was compared to other lipid related genes.
Results
In Birthright samples placental microvillous plasma membrane alkaline phosphatase activity was positively associated with maternal triceps skinfold thickness and BMI (β=0.04 (95%CI 0.01, 0.06) and β=0.02 (0.00, 0.03) µmol/mg protein/min per SD, P=0.002 and P=0.05, respectively) adjusting for potential confounders. In SWS samples placental alkaline phosphatase mRNA expression in term placenta was positively associated with maternal triceps skinfold (β=0.24 (0.04, 0.44) SD/SD, P=0.02), had no association with neonatal %fat mass (β=0.01 (-0.20, 0.21) SD/SD, P=0.93) and was negatively correlated with %fat mass at ages 4 (β=-0.28 (-0.52, -0.04) SD/SD, P=0.02), 6-7 (β=-0.25 (-0.49, -0.02) SD/SD, P=0.03) years. When compared with placental expression of other genes, alkaline phosphatase expression was positively related to genes including the lysophosphatidylcholine transporter MFSD2A (major facilitator superfamily domain containing 2A, P<0.001) and negatively related to genes including the fatty acid transport proteins 2 and 3 (P=0.001, P<0.001).
Conclusions
Our findings suggest relationships between placental alkaline phosphatase and both maternal and childhood adiposity. The inverse relationship between placental alkaline phosphatase gene expression and childhood %fat mass suggests placental alkaline phosphatase may help to protect the fetus from the adverse effects of maternal obesity.
Introduction
Maternal obesity can affect fetal development, predisposing the fetus to develop childhood obesity and perpetuating intergenerational cycles of obesity 1. While the mechanisms are not yet clear, effects of maternal obesity on the fetus are likely to be mediated at least in part via the placenta 2. Placental function could promote offspring adiposity via increased nutrient transfer affecting body composition (such as occurs in diabetes and obesity), or indirectly via persistent developmental changes in metabolism or neuroendocrine regulation.
Maternal factors including parity, body composition and smoking are associated with changes in placental gene expression, indicating that the placental tissue responds to changes in the maternal environment 3, 4. Maternal body composition has also been associated with changes in placental function which could affect fetal development 5, 6. While evolutionary pressures on placental development are likely to favour adaptations that protect the fetus from maternal undernutrition, its ability to respond to maternal overnutrition are of increasing interest 7.
Alkaline phosphatases are membrane-bound glycoproteins found in many tissues 8. There are four main alkaline phosphatase variants, intestinal, placental, placental-like and a non-tissue specific isoform. Placental alkaline phosphatase is a specific marker of the maternal-facing microvillous plasma membrane (MVM) of the syncytiotrophoblast. Alkaline phosphatase activity in the placenta is associated with preterm delivery 9 and trophoblast alkaline phosphatase activity is altered in pre-eclampsia 10 but not by well controlled maternal diabetes mellitus 11. Evidence linking alkaline phosphatases to lipid metabolism comes from studies in mice where intestinal alkaline phosphatase is implicated in lipid transport and the development of obesity 12 and the metabolic syndrome 13. Mouse studies suggest that the protective effect of intestinal alkaline phosphatase against the metabolic syndrome results at least partly from inhibition of absorption of endotoxin that occurs with dietary fats 14. In a mouse adipocyte cell line, alkaline phosphatase has direct effects on lipid metabolism, where it is found in association with lipid droplets and inhibition of its activity reduces lipolysis 15. Mice and rats have a similar placental localisation of alkaline phosphatase to humans on the maternal-facing plasma membrane of syncytiotrophoblast 16, 17.
The regulation of human placental alkaline phosphatase is not well described, but is transcriptionally regulated by steroid hormones, PPAR-γ 18 and the short chain fatty acid butyrate 19–21. In rodents, maternal prenatal undernutrition and postnatal high fat diet regulate intestinal alkaline phosphatase levels, suggesting that its activity in specific tissues may respond to humoral stimuli 12, 22. PPAR-γ also regulates the bone isoform of alkaline phosphatase which could mediate nutritional signals 18.
Significant progress is being made in determining the mechanisms of placental fatty acid transfer with both membrane transport and metabolism thought to be important 23. Placental lipid metabolism appears to be altered in obese mothers, indicating a possible mechanism by which maternal obesity could affect fetal development 6, 24. However, much remains to be understood, including the regulation of placental lipid transfer and fetal responses to altered lipid transfer. In light of the association between intestinal alkaline phosphatase and obesity, this study examined the specific hypothesis that there was an association between maternal obesity and placental alkaline phosphatase in an initial cohort (the Birthright study); having found an association, probes for measurement of placental alkaline phosphatase mRNA were added to a larger panel of probes under evaluation in relation to childhood adiposity in a second cohort (the Southampton Women’s Survey). These cohorts represent the range of maternal adiposity in the population rather than comparing lean and obese women. To place the observations with alkaline phosphatase in a broader context of placental lipid transport and metabolism its expression in relationship to other lipid-related genes are reported.
Subjects and methods
Study populations
Placentas and offspring of participants in the Birthright study 25 and Southampton Women’s Survey (SWS) were studied 26. In the Birthright cohort we measured alkaline phosphatase activity in relation and related this to maternal adiposity, birthweight and ponderal index at birth and 9 months. In the SWS placental alkaline phosphatase gene expression was measured and related to measures of maternal, neonatal and childhood fat mass.
The Birthright study was approved by the Southampton Joint Ethics Sub-Committee (246/92) 27. Research in the Southampton Women’s Survey (SWS) was approved by the Southampton and South West Hampshire Research Ethics Committee (276/97, 307/97, 089/99, 153/99, 005/03/t, 06/Q1702/104). Written informed consent was obtained from all participating women and by parents or guardians with parental responsibility on behalf of their children. Cohort characteristics were recorded in early and late pregnancy for the Birthright cohort (including recalled pre-pregnancy maternal weight from which BMI was calculated) and pre-pregnancy in the SWS.
In the Birthright study, 604 white women were approached who were aged 16 years or older with singleton pregnancies and known menstrual dates and who attended the antenatal booking clinic at the Princess Anne Hospital, Southampton, UK before 17 weeks gestation. 44 women declined to take part or could not be visited until after 20 weeks’ gestation, 11 had ultrasound scan results suggesting that their menstrual dates were incorrect, and 7 had infants that either died in the perinatal period or had major congenital abnormalities, leaving 542 who took part. Of these, 52 women had placentas collected and MVM vesicles successfully were prepared 28. Children were studied at birth and at 9 months of age.
The SWS is a prospective cohort study that assessed the diet, body composition and lifestyles of non-pregnant women recruited through General Practices across the city between April 1998 and December 2002. Each woman was invited to take part by letter, followed by a telephone call when an interview date was arranged. 12583 women agreed to take part, 75% of all women who were contacted. Trained research nurses visited the women at home and collected information about their health, diet, and lifestyles and performed anthropometric measurements. A ‘prudent diet score’ was calculated for each woman using principal component analysis, to reflect diet quality based on responses to a food frequency questionnaire 29. Women who subsequently became pregnant were followed up at 11, 19, and 34 weeks of gestation, and their children were studied in infancy and childhood. Between 1998 and 2007, there were 3158 live singleton births. For this study of placental alkaline phosphatase gene expression, a cohort of 95 placentas was selected from 300 collected rapidly after birth to allow RNA extraction based on the availability of neonatal dual energy X-ray absorptiometry (DXA) data. Gestational age of the SWS babies was determined using an algorithm based on last menstrual period and early ultrasound data.
Placental samples
Placentas were collected within 30 minutes of delivery. Placental weight was measured after removing blood clots, cutting the umbilical cord flush with its insertion into the placenta, trimming away surrounding membranes and removing the amnion from the basal plate 30. Ten villous tissue samples were selected using a stratified random sampling method and stored at -80°C.
Offspring body composition measurement in SWS
Within 3 weeks of birth and at 4, 6-7 and 8-9 years of age, subsets of children underwent an assessment of body composition by dual-energy X-ray absorptiometry (DXA) using a Lunar DPX-L instrument (GE Corp) in infancy and a Hologic Discovery instrument (Hologic Inc) in childhood, both cross-calibrated. The total X-ray dose for the whole-body scans was approximately 10.5 microsieverts (paediatric scan mode), which is equivalent to approximately 1–2 days background radiation. All scan results were checked independently by two trained operators, and agreement was reached as to their acceptability; scans showing unacceptable movement artefact were excluded. Fat mass was derived from the whole-body scan through the use of paediatric software (Hologic Inc.).
MVM membrane alkaline phosphatase activity (Birthright cohort)
After delivery, placental MVM vesicles were prepared as reported previously 27, 28 from 52 women who delivered at term after normal pregnancies. MVM vesicle alkaline phosphatase activity was measured at pH 9.8 using p-nitrophenyl phosphate as a substrate 28.
RNA isolation, quality assessment and analysis by the nCounter system (SWS cohort)
Frozen placental tissue (50 – 100 mg) was homogenised in RLT lysis buffer (Qiagen, Hilden, Germany) by using MagNa lyser green beads (Roche, Basel, Switzerland) and the MagNA lyser system (Roche, Basel, Switzerland). RNA was isolated from placenta tissue homogenates by RNeasy mini kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. The RNA quality control was performed on a 2100 Bioanalyzer Instrument (Agilent Technologies, Santa Clara, USA) and only samples with an RNA integrity number (RIN) above 7.0 were considered for analysis. Probes for analysis of placental alkaline phosphatase gene expression were added to a custom Code Set containing 53 genes selected for their association with lipid transport, lipid metabolism and inflammation (See supplementary Table 1). Analysis was performed using the nSolver 2.0 Analysis Software (NanoString Technologies, Seattle, WA).
Immunohistochemistry (SWS cohort)
Immunohistochemistry was performed on 5 µm paraffin-embedded sections from term human placenta, without antigen retrieval. The placental alkaline phosphatase antibody (Abcam #ab133602) was applied at 1:1000 and detected with Lab Vision AEC substrate system (Thermo Scientific).
Statistics
Summary data are presented as mean and standard deviation (SD) or median and interquartile range (IQR). Relationships of MVM placental alkaline phosphatase activity and gene expression levels with maternal, fetal or neonatal variables were analysed by linear regression and reported as regression coefficients (95% CIs). In the SWS measures of maternal and offspring adiposity and alkaline phosphatase gene expression were transformed to normality using Fisher-Yates transformations 31. Given the specific hypothesis testing nature of the analyses a P-value of < 0.05 was considered statistically significant. Relationships between placental alkaline phosphatase and the expression of placental genes were performed using Pearson correlations. Data were analysed using Stata version 14.1 (StataCorp, Texas, USA).
Results
Characterisation of the participants in the Birthright and SWS cohorts
Details of the women are presented in Table 1.
Table 1. Summary statistics for Birthright and SWS cohorts.
| Birthright (n = 52) |
SWS (n = 95) |
|
|---|---|---|
| Maternal age (years)1 | 27.9 (4.3) | 28.6 (3.9) |
| Primiparous, n (%) | 20 (38%) | 44 (46%) |
| Maternal education ≥ A-levels, n (%) | 22 (42%) | 59 (63%) |
| Maternal triceps skinfold (mm)2 | 20.1 (17.8, 25.3) | 21.1 (15.7, 24.4) |
| Maternal height (m)1 | 1.62 (0.07) | 1.62 (0.06) |
| Pre-pregnant Maternal BMI (kg/m2)2 | 24.3 (21.0, 26.6) | 24.9 (23.0, 29.2) |
| Offspring gestational age at birth (weeks)1 | 39.9 (1.5) | 39.8 (1.3) |
| Male offspring, n (%) | 27 (52%) | 51 (54%) |
| Birthweight (kg)1 | 3.4 (0.5) | 3.5 (0.4) |
Mean (SD)
Median (IQR)
Immunolocalisation of alkaline phosphatase
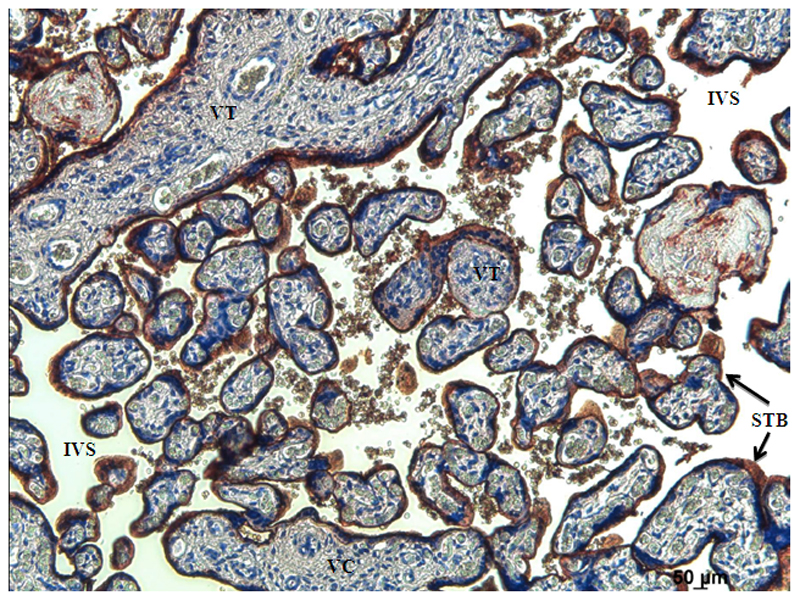
Placental alkaline phosphatase was localised to the syncytiotrophoblast of term human placenta (Figure 1).
Figure 1.
Representative image showing immunohistochemical localisation of placental alkaline phosphatase in term human placenta. Strong alkaline phosphatase staining (brown) is seen in syncytiotrophoblast the interface between the mother and the fetus. VC = villous core, IVS = intervillous space, STB = syncytiotrophoblast.
Relationships with MVM vesicle alkaline phosphatase activity (Birthright cohort)
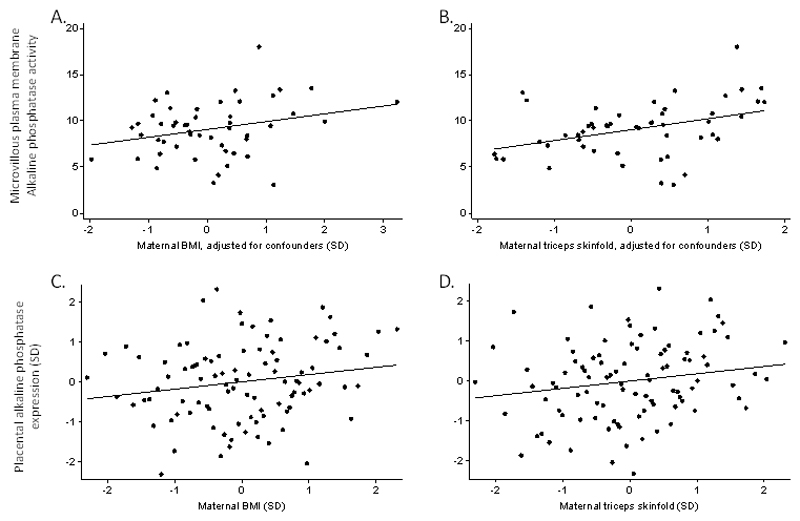
The mean (SD) placental MVM alkaline phosphatase activity was 9.14 (2.89) µmol/mg protein/min. The mean (SD) alkaline phosphatase enrichment was 23.3 (4.9) fold. Alkaline phosphatase activity was positively associated with increasing maternal body mass index and with maternal triceps skinfold thickness (adjusted for gestational age, parity, height and smoking) (Figure 2) but not with birth weight (P = 0.11) or offspring ponderal index at birth (P = 0.49) or 9 months of age (P = 0.39). Alkaline phosphatase activity (n = 52) was unrelated to fetal sex (P = 0.48) or gestational age at birth (P = 0.89). Alkaline phosphatase activity was unrelated to maternal age (P = 0.97), parity (P = 0.72), height (P = 0.22) and smoking (P = 0.36).
Figure 2.
Maternal BMI and triceps skinfold as predictors of alkaline phosphatase activity or gene expression. A, Maternal BMI was related to microvillous plasma membrane alkaline phosphatase activity (B (95% CI) 0.10 (0.00, 0.20), p= 0.05) B, Maternal triceps skinfold was related to microvillous plasma membrane alkaline phosphatase activity (B (95% CI), 0.15 (0.05, 0.24), P > 0.002). C, The relationship between maternal BMI and placental alkaline phosphatase gene expression B (95% CI) 0.18 (-0.02, 0.38) P = 0.08. D, Maternal triceps skinfold was related to placental alkaline phosphatase gene expression (B (95% CI) 0.18 (-0.02, 0.38), P = 0.02).
Maternal obesity and placental alkaline phosphatase gene expression (SWS cohort)
Placental alkaline phosphatase mRNA expression at term was positively associated with maternal triceps skinfold thickness (P = 0.02), and there was a non-significant positive association with maternal pre-pregnancy BMI (P = 0.08, Figure 2). Alkaline phosphatase expression did not differ by sex (P = 0.54), and there were no associations between placental alkaline phosphatase gene expression and maternal prudent diet score (P = 0.36), placental weight (P = 0.22) or birthweight (P = 0.82). Placental alkaline phosphatase gene expression was not associated with maternal weight gain during pregnancy (defined as inadequate, adequate, excessive according to recognised criteria 32, P = 0.17.)
Placental alkaline phosphatase gene expression was negatively associated with offspring fat mass at ages 4 and 6-7 years but not at 8-9 years (Adjusted for sex, gestational age at birth, age at DXA, maternal smoking in pregnancy, parity and maternal pre-pregnancy BMI, Table 2). In these samples maternal BMI was not associated with offspring fat mass at 4 years of age (r = 0.16, P = 0.27) but was at 6 years (r = 0.32, P = 0.03) and 8-9 years of age (r = 0.31, P = 0.04).
Table 2. Placental alkaline phosphatase gene expression as a predictor of placental weight and offspring birth weight and adiposity.
| Placental alk. phos. mRNA expression (SD) | |||
|---|---|---|---|
| B (95% CI) | P | n | |
| Placental weight (kg) | -0.01 (-0.03, 0.01) | 0.22 | 93 |
| Birth weight (kg) | 0.01 (-0.08, 0.10) | 0.83 | 94 |
| Infant fat mass (SD) | 0.02 (-0.18, 0.22) | 0.85 | 94 |
| Infant % fat mass (SD) | 0.01 (-0.20, 0.21) | 0.93 | 94 |
| 4 year fat mass (SD) | -0.28 (-0.56, 0.00) | 0.05 | 48 |
| 4 year % fat (SD) | -0.28 (-0.52, -0.04) | 0.02 | 48 |
| 6-7 year fat mass (SD) | -0.25 (-0.51, 0.00) | 0.05 | 46 |
| 6-7 year % fat (SD) | -0.25 (-0.49, -0.02) | 0.03 | 46 |
| 8-9 year fat mass (SD) | -0.16 (-0.43, 0.11) | 0.23 | 46 |
| 8-9 year % fat (SD) | -0.15 (-0.41, 0.11) | 0.24 | 46 |
Adjusted for sex, gestational age at birth, age at DXA, maternal smoking in pregnancy, parity and maternal pre-pregnancy BMI. Where the unit is SD data were transformed to Z scores.
In the SWS cohort there were no significant interactions between maternal pre-pregnancy BMI and alkaline phosphatase expression on offspring adiposity indicating that the relationship between childhood adiposity and placental alkaline phosphatase expression did not differ according to levels of maternal BMI.
The 95 SWS pregnancies used in this study were compared to the SWS cohort as a whole and there were no differences in maternal age, maternal height, pre-pregnancy maternal triceps, or birthweight. However, maternal BMI was higher in the women whose placentas were used in this study (median (IQR), 24.9 (23.0, 29.2), n = 95) compared to the overall cohort (median (IQR), 24.1 (21.7, 27.5), n = 12319, P = 0.0005).
Relationship between the expression of alkaline phosphatase and lipid-related genes (SWS Cohort)
Of the 53 genes studied, placental alkaline phosphatase mRNA expression was negatively associated with 8 genes including FATP-2 and 3, positively associated with 20 genes including the Na+ dependent lysophosphatidylcholine transporter MFSD2A (Table 3). There was no significant association with 18 genes and expression of 6 genes was below the level of detection (Supplementary table 1). The fatty acid transporters FATP-2 and 3 were negatively associated with alkaline phosphatase gene expression while positive associations were seen with genes involved extracellular matrix as well as the lysophosphatidylcholine transporter MFSD2A. Other genes whose placental expression was related to maternal pre-pregnancy BMI were FATP3 (r = -0.17, P = 0.10), FADS1, (r = -0.29, P = 0.005), TGFB1 (r = 0.24, P = 0.02) and TIMP2 (r = 0.22, P = 0.04). Other genes whose placental expression was related to maternal pre-pregnancy triceps skinfold were FATP3 (r = -0.20, P = 0.05), ELOV1 (r = -0.26, P = 0.01) and TGFB1 (r = 0.30, P = 0.003).
Table 3. Genes whose expression was significantly associated with placental alkaline phosphatase expression (n = 95).
| Gene | Function | R | P |
|---|---|---|---|
| FATP3 | Fatty acid transport | -0.34 | 0.001 |
| FADS1 | Fatty acid desaturase | -0.39 | <0.001 |
| FATP2 | Fatty acid transport | -0.39 | <0.001 |
| TSPO | Mitochondrial cholesterol transport | -0.38 | <0.001 |
| IL6 | Interleukin 6 | -0.33 | 0.001 |
| G0S2 | G0/G1 Switch 2 | -0.23 | 0.02 |
| ACAT2 | Cytosolic acetoacetyl-CoA thiolase | -0.24 | 0.02 |
| VCAN | Chondroitin sulphate proteoglycan | -0.20 | 0.05 |
| PPAR gamma | Transcription factor | 0.21 | 0.04 |
| FN1 | Fibronectin-1 | 0.23 | 0.02 |
| FAT/CD36 | Fatty acid transport | 0.24 | 0.02 |
| MMP2 | Matrix metallopeptidase 2 | 0.25 | 0.01 |
| ELOV1 | Fatty acid elongase | 0.28 | 0.006 |
| TIMP3 | Metallopeptidase inhibitor | 0.37 | <0.001 |
| MMP15 | Matrix metallopeptidase 15 | 0.38 | <0.001 |
| LEP | Leptin | 0.41 | <0.001 |
| PNPLA2 | Triglyceride hydrolysis | 0.43 | <0.001 |
| CGI58/ABHD5 | α/β hydrolase domain containing 5 | 0.45 | <0.001 |
| LTPB1 | Latent TGF beta binding protein 1 | 0.47 | <0.001 |
| STARD3 | Lipid trafficking endosomal | 0.51 | <0.001 |
| PLIN3 | Perilipin 3, lipid droplet associated | 0.52 | <0.001 |
| ANGPTL4 | Angiopoietin-like protein 4 | 0.53 | <0.001 |
| PLIN2 | Perilipin 2, lipid droplet associated | 0.53 | <0.001 |
| SRB1 | Scavenger receptor class B member 1 (HDL) | 0.53 | <0.001 |
| TGFB1 | Transforming growth factor beta 1 | 0.54 | <0.001 |
| SREBP1 | Sterol regulatory element-binding transcription factor 1 | 0.60 | <0.001 |
| TIMP2 | Metallopeptidase inhibitor 2 | 0.72 | <0.001 |
| MFSD2A | Na+-dep lysophosphatidylcholine symporter 1 | 0.79 | <0.001 |
Discussion
This study found that measures of placental alkaline phosphatase were positively associated with markers of maternal adiposity and negatively associated with childhood fat mass. These are interesting observations as maternal obesity and factors which are positively associated with it, might be expected to be linked to higher rates of childhood adiposity.
The association between maternal adiposity and both placental alkaline phosphatase activity and gene expression provides further evidence for relationships between maternal body composition and the placenta 4, 5, but the mechanism underlying this association is not immediately clear. Maternal obesity could alter plasma non esterified fatty acids (NEFA) profiles regulating alkaline phosphatase expression via PPARs or histone deacetylases (HDACs)18. Corticosteroids and oestrogen could also regulate alkaline phosphatase expression and differences in the levels of these hormones, their binding proteins or their placental receptors as a result of maternal obesity could underlie this observation 21, 33. Alternatively, it may not be the maternal obesity itself, but an obesogenic diet or lifestyle that alters regulation of placental alkaline phosphatase. Consistent with this notion, perinatal undernutrition in mice has persistent effects on intestinal alkaline phosphatase 22. Placenta-like alkaline phosphatase is regulated by the short chain fatty acid butyrate produced by intestinal bacteria 20. This type of regulation could provide a link between an obesogenic diet, likely to be lower in dietary fibre, and alkaline phosphatase activity. However, there was no association of alkaline phosphatase with the quality of the woman’s diet as assessed by the prudent diet score 29.
Maternal obesity is associated with fat mass and BMI in the offspring 34. It was, therefore, interesting that while maternal obesity was positively associated with both higher MVM alkaline phosphatase activity and placental alkaline phosphatase gene expression, placental alkaline phosphatase gene expression was negatively associated with childhood obesity. Mice deficient in intestinal alkaline phosphatase have an enhanced rate of intestinal lipid uptake and develop obesity over time 12. Moreover, mouse studies also show that the protective effect of intestinal alkaline phosphatase in regard to the metabolic syndrome 14 may be inhibited by phenylalanine, a metabolite of the sweetener aspartamine 13. If placental alkaline phosphatase also acts as a negative regulator of lipid transfer in the placenta, this may provide a mechanism for the observation that higher alkaline phosphatase expression was negatively associated with childhood obesity. As alkaline phosphatase was not associated with fat mass at birth, any effect of subsequent childhood obesity must be indirect. It is possible that fetal sensing of lipid availability in utero influences the regulation of postnatal adiposity gain. The relationship between placental alkaline phosphatase expression and childhood fat mass is intriguing as it suggests a link between placenta and the development of obesity in childhood which could serve as part of a placental biomarker panel identifying those at risk of developing obesity or as a target for prenatal intervention. Alkaline phosphatase may affect placental function in ways unrelated to fat transport, for instance through dephosphorylation of insulin-like growth factor binding protein 1 and regulation of placental exposure to insulin-like growth factors 33.
The positive association between expression of placental alkaline phosphatase and the lysophosphatidylcholine (LPC) transporter MFSD2A may also provide a link through which placental function may influence later development. MFSD2A is important for docosahexaenoic acid (DHA) transport and has been implicated in placental DHA transfer in pregnancy 35. Low cord blood DHA levels are associated neonatal insulin resistance and impaired neurodevelopment and these may influence subsequent developmental patterns 36.
Alkaline phosphatase is predominantly localised to the MVM of the placental syncytiotrophoblast, at the interface between the mother and placenta 28. While this suggests it is well placed to respond to maternal influences and mediate effects on placental function, its biological role remains unclear. This study demonstrated strong associations between the expression of placental alkaline phosphatase and other lipid-related genes, including leptin, which was positively related to placental alkaline phosphatase expression 15. However, we need to consider that placental alkaline phosphatase may not act in isolation but may participate a system of co-regulated genes. For instance, maternal obesity is linked to the differential composition of lipid droplets 6 and the expression of two lipid droplet associated genes (perilipins) were also positively related to alkaline phosphatase activity. Such relationships could be coordinated via transcription factors such as PPAR-γ whose gene expression was positively correlated with placental alkaline phosphatase. To adequately address these questions, the functional role of alkaline phosphatase needs to be more clearly elucidated along with the factors determining its regulation.
A feature of the Birthright and SWS cohorts is that they are both broadly representative of the overall population, rather than just comparing lean and obese individuals. The value of this is that our findings are likely to be applicable across normal populations.
Limitations of this study include the fact that we do not have equivalent measures of placental alkaline phosphatase in both cohorts and that we do not have childhood adiposity data in the Birthright cohort. That said, we did find associations between maternal obesity and placental alkaline phosphatase in two separate cohorts, which indicates that these observations are robust and worthy of further attention. It would also be interesting to measure the activity of this enzyme in MVM preparations from poorly controlled diabetic pregnancy, in which the babies tend to have a relatively high-fat mass 37. The use of a selected gene set meant that it was not possible to do meaningful pathways analysis.
The concept that the expression of specific genes or pathways within the placenta may act to protect the fetus and the offspring from the intergenerational effects of maternal obesity is an interesting one. If such pathways could be clearly identified, they would be prime targets for intervention studies. The observations described here are of particular interest as rather than protecting the fetus from the effects of maternal undernutrition they suggest factors that may protect the fetus from maternal over nutrition 2.
In conclusion, this study suggests that maternal obesity is related to placental alkaline phosphatase and that the expression of this gene in the placenta was inversely associated with offspring obesity. Further research is needed to clearly define the biological role of placental alkaline phosphatase and determine whether there could be a mechanistic underpinning to this association. Attention should also focus on whether the relationships with alkaline phosphatase relate to clusters of co-regulated genes whose placental expression may influence the subsequent development of obesity.
Supplementary Material
Funding
The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement n°289346. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (as an NIHR Senior Investigator (NF-SI-0515-10042) and through the NIHR Southampton Biomedical Research Centre) and the European Union's Seventh Framework Programme (FP7/2007-2013), project ODIN under grant agreement numbers 613977.
Footnotes
Conflict of interest statement.
KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. The other authors declare no conflict of interest.
References
- 1.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis RM, Desoye G. Placental Lipid and Fatty Acid Transfer in Maternal Overnutrition. Ann Nutr Metab. 2017;70(3):228–231. doi: 10.1159/000463397. [DOI] [PubMed] [Google Scholar]
- 3.Day PE, Ntani G, Crozier SR, Mahon PA, Inskip HM, Cooper C, et al. Maternal Factors Are Associated with the Expression of Placental Genes Involved in Amino Acid Metabolism and Transport. PLoS One. 2015;10(12):e0143653. doi: 10.1371/journal.pone.0143653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Tierney PF, Lewis RM, McWeeney SK, Hanson MA, Inskip HM, Morgan TK, et al. Immune Response Gene Profiles in the Term Placenta Depend Upon Maternal Muscle Mass. Reproductive Sciences. 2012;19(10):1041–1056. doi: 10.1177/1933719112440051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis RM, Greenwood SL, Cleal JK, Crozier SR, Verrall L, Inskip HM, et al. Maternal muscle mass may influence system A activity in human placenta. Placenta. 2010;31(5):418–422. doi: 10.1016/j.placenta.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Hirschmugl B, Desoye G, Catalano P, Klymiuk I, Scharnagl H, Payr S, et al. Maternal obesity modulates intracellular lipid turnover in the human term placenta. Int J Obes (Lond) 2017;41(2):317–323. doi: 10.1038/ijo.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis RM, Cleal JK, Hanson MA. Review: Placenta, evolution and lifelong health. Placenta. 2012;33:S28–S32. doi: 10.1016/j.placenta.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem. 2014;29(3):269–78. doi: 10.1007/s12291-013-0408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashiri A, Katz O, Maor E, Sheiner E, Pack I, Mazor M. Positive placental staining for alkaline phosphatase corresponding with extreme elevation of serum alkaline phosphatase during pregnancy. Arch Gynecol Obstet. 2007;275(3):211–4. doi: 10.1007/s00404-006-0212-5. [DOI] [PubMed] [Google Scholar]
- 10.Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: expression of Flt-1 and endoglin. PLoS One. 2013;8(2):e56754. doi: 10.1371/journal.pone.0056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desoye G, Hofmann HH, Weiss PA. Insulin binding to trophoblast plasma membranes and placental glycogen content in well-controlled gestational diabetic women treated with diet or insulin, in well-controlled overt diabetic patients and in healthy control subjects. Diabetologia. 1992;35(1):45–55. doi: 10.1007/BF00400851. [DOI] [PubMed] [Google Scholar]
- 12.Narisawa S, Huang L, Iwasaki A, Hasegawa H, Alpers DH, Millan JL. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol Cell Biol. 2003;23(21):7525–30. doi: 10.1128/MCB.23.21.7525-7530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gul SS, Hamilton AR, Munoz AR, Phupitakphol T, Liu W, Hyoju SK, et al. Inhibition of the gut enzyme intestinal alkaline phosphatase may explain how aspartame promotes glucose intolerance and obesity in mice. Appl Physiol Nutr Metab. 2017;42(1):77–83. doi: 10.1139/apnm-2016-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaliannan K, Hamarneh SR, Economopoulos KP, Nasrin Alam S, Moaven O, Patel P, et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2013;110(17):7003–8. doi: 10.1073/pnas.1220180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Mosqueira C, Velez-delValle C, Kuri-Harcuch W. Tissue alkaline phosphatase is involved in lipid metabolism and gene expression and secretion of adipokines in adipocytes. Biochim Biophys Acta. 2015;1850(12):2485–96. doi: 10.1016/j.bbagen.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Kusinski LC, Jones CJ, Baker PN, Sibley CP, Glazier JD. Isolation of plasma membrane vesicles from mouse placenta at term and measurement of system A and system beta amino acid transporter activity. Placenta. 2010;31(1):53–9. doi: 10.1016/j.placenta.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glazier JD, Jones CJ, Sibley CP. Preparation of plasma membrane vesicles from the rat placenta at term and measurement of Na+ uptake. Placenta. 1990;11(5):451–63. doi: 10.1016/s0143-4004(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 18.Lencel P, Delplace S, Hardouin P, Magne D. TNF-alpha stimulates alkaline phosphatase and mineralization through PPARgamma inhibition in human osteoblasts. Bone. 2011;48(2):242–9. doi: 10.1016/j.bone.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Albert JL, Sundstrom SA, Lyttle CR. Estrogen regulation of placental alkaline phosphatase gene expression in a human endometrial adenocarcinoma cell line. Cancer Res. 1990;50(11):3306–10. [PubMed] [Google Scholar]
- 20.Deng G, Liu G, Hu L, Gum JR, Jr, Kim YS. Transcriptional regulation of the human placental-like alkaline phosphatase gene and mechanisms involved in its induction by sodium butyrate. Cancer Res. 1992;52(12):3378–83. [PubMed] [Google Scholar]
- 21.Chou JY, Takahashi S. Control of placental alkaline phosphatase gene expression in HeLa cells: induction of synthesis by prednisolone and sodium butyrate. Biochemistry. 1987;26(12):3596–602. doi: 10.1021/bi00386a052. [DOI] [PubMed] [Google Scholar]
- 22.Lalles JP, Orozco-Solis R, Bolanos-Jimenez F, de Coppet P, Le Drean G, Segain JP. Perinatal undernutrition alters intestinal alkaline phosphatase and its main transcription factors KLF4 and Cdx1 in adult offspring fed a high-fat diet. J Nutr Biochem. 2012;23(11):1490–7. doi: 10.1016/j.jnutbio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG. The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res. 2017;58(2):443–454. doi: 10.1194/jlr.P072355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabuig-Navarro V, Haghiac M, Minium J, Glazebrook P, Ranasinghe GC, Hoppel C, et al. Effect of Maternal Obesity on Placental Lipid Metabolism. Endocrinology. 2017;158(8):2543–2555. doi: 10.1210/en.2017-00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001;16(9):1694–703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 26.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C, et al. Cohort profile: The Southampton Women's Survey. Int J Epidemiol. 2006;35(1):42–8. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godfrey KM, Matthews N, Glazier J, Jackson A, Wilman C, Sibley CP. Neutral amino acid uptake by the microvillous plasma membrane of the human placenta is inversely related to fetal size at birth in normal pregnancy. J Clin Endocrinol Metab. 1998;83(9):3320–6. doi: 10.1210/jcem.83.9.5132. [DOI] [PubMed] [Google Scholar]
- 28.Glazier JD, Jones CJ, Sibley CP. Purification and Na+ uptake by human placental microvillus membrane vesicles prepared by three different methods. Biochim Biophys Acta. 1988;945(2):127–34. doi: 10.1016/0005-2736(88)90475-0. [DOI] [PubMed] [Google Scholar]
- 29.Crozier SR, Inskip HM, Barker ME, Lawrence WT, Cooper C, Robinson SM, et al. Development of a 20-item food frequency questionnaire to assess a 'prudent' dietary pattern among young women in Southampton. Eur J Clin Nutr. 2010;64(1):99–104. doi: 10.1038/ejcn.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leary SD, Godfrey KM, Greenaway LJ, Davill VA, Fall CH. Contribution of the umbilical cord and membranes to untrimmed placental weight. Placenta. 2003;24(2–3):276–8. doi: 10.1053/plac.2002.0888. [DOI] [PubMed] [Google Scholar]
- 31.Armitage P, Berry G. Stat Meth Med Res. Third Edition edn. Blackwell Science Ltd.; Oxford, United Kingdom: 2002. [Google Scholar]
- 32.Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): 2009. [PubMed] [Google Scholar]
- 33.Solomon AL, Siddals KW, Baker PN, Gibson JM, Aplin JD, Westwood M. Placental alkaline phosphatase de-phosphorylates insulin-like growth factor (IGF)-binding protein-1. Placenta. 2014;35(7):520–2. doi: 10.1016/j.placenta.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM, et al. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr. 2015;101(2):368–75. doi: 10.3945/ajcn.114.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prieto-Sanchez MT, Ruiz-Palacios M, Blanco-Carnero JE, Pagan A, Hellmuth C, Uhl O, et al. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin Nutr. 2017;36(2):513–521. doi: 10.1016/j.clnu.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Madore C, Nadjar A, Delpech JC, Sere A, Aubert A, Portal C, et al. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav Immun. 2014;41:22–31. doi: 10.1016/j.bbi.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 37.de Santis MS, Taricco E, Radaelli T, Spada E, Rigano S, Ferrazzi E, et al. Growth of fetal lean mass and fetal fat mass in gestational diabetes. Ultrasound Obstet Gynecol. 2010;36(3):328–37. doi: 10.1002/uog.7575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.