Abstract
Objective
In renal arteries, inhibitors of G protein βγ subunits (Gβγ) reduce Kv7 activity and inhibit Kv7-dependent receptor-mediated vasorelaxations. However, the mechanisms underlying receptor mediated relaxation are artery-specific. Consequently, the aim of this study was to ascertain the role of Gβγ in Kv7-dependent vasorelaxations of the rat vasculature.
Approach
Isometric tension recording was performed in isolated rat renal, mesenteric and cerebral arteries (RA, MA and CA respectively) to study isoproterenol and calcitonin gene related peptide (CGRP) relaxations. Kv7.4 was knocked down via morpholino transfection, whilst inhibition of Gβγ was investigated with gallein and M119K. Proximity ligation assay (PLA) was performed on isolated myocytes to study the association between Kv7.4 and G protein β subunits or signalling intermediaries.
Results
Isoproterenol or CGRP-induced relaxations were attenuated by Kv7.4 knockdown in all arteries studied. Inhibition of Gβγ with gallein or M119K had no effect on isoproterenol-mediated relaxations in MA but had a marked effect on CGRP-induced responses in MA and CA and isoproterenol responses in RA. Isoproterenol increased association with Kv7.4 and Rap1a in MA which were not sensitive to gallein, whereas in RA isoproterenol increased Kv7.4-AKAP associations in a gallein-sensitive manner.
Conclusions
The Gβγ-Kv7 relationship differs between vessels and is an essential requirement for AKAP, but not Rap, mediated regulation of the channel.
Keywords: G protein βγ subunits, Kv7 channels, vascular biology
Subject Codes: Vascular Biology, Ion Channels, Cell Signalling, Basic Science Research
Introduction
The Kv7 family of potassium channels are key regulators of vascular responsiveness and contribute to receptor mediated vasorelaxations (1, 2). Of the 5 subunits identified, rodent and human arteries express predominantly Kv7.1, Kv7.4 and Kv7.5, and blockade of these channels leads to membrane depolarisation and increased vascular contractility (2). In addition, Kv7 channels are involved in numerous diverse receptor mediated relaxations to agents such as calcitonin gene related peptide ((CGRP) in the cerebral artery (3)), adenosine (coronary artery (4)) and natriuretic peptides (renal artery and aorta (5)), as well as participating in forskolin induced relaxations of cerebral (6) and coronary (7) arteries. Relaxations to the β-adrenoceptor agonist isoproterenol are Kv7 dependent in the renal and mesenteric arteries (8, 9), but the post-receptor signals that link to Kv7 channels differs between vessels. Thus in renal arteries it is PKA (Protein Kinase A) dependent, with AKAP (A-Kinase Anchoring Protein) localisation with Kv7.4 increasing, whereas in mesenteric arteries it is EPAC (Exchange Protein Activated by cAMP) dependent, and association of Kv7.4 with Rap1a and Rap2 G proteins increases (9).The Kv7.4 channel has been most implicated as having a key role in the vasculature as this subunit is downregulated in many arteries from hypertensive animals (10, 11) that exhibit impaired responses to receptor agonists, whilst Kv7.4 siRNA directed knockdown compromises vessel reactivity (3, 8).
The seminal discovery that Kv7.4 has an obligatory reliance upon G protein βγ subunits (Gβγ) independent of Gα and the key role of Kv7.4 in vascular smooth muscle suggests that Gβγ subunit regulation may have a major role in vascular reactivity (12). In renal artery myocytes the isoproterenol mediated increase in activity of Kv7 channels was abrogated by different inhibitors of Gβγ-effector interactions (12). This data suggests that Kv7 channels require constitutive interaction with Gβγ to respond to some intracellular signals, or that generation of some vasorelaxant signals are dependent upon Gβγ activity. The present study used a combination of gene-silencing, whole artery myography and protein-protein association techniques to determine if receptor mediated relaxations in other areas of the rat vasculature involved Gβγ activity akin to the renal artery. The data reveals a complex role for Gβγ which differs both between vascular bed and stimulus.
Materials and Methods
The authors declare that all supporting data are available within the article (and its online supplementary files).
Animals
Male Wistar rats (175-225g) were culled by cervical dislocation in accordance with the UK Animals (Scientific Procedures) Act (1986). Renal, mesenteric and cerebral arteries (RA, MA and CA respectively) were dissected of adherent fat and connective tissue and stored on ice in a physiological saline solution (PSS) containing (in mmol/L); 4.5 KCl, 120 NaCl, 1.2 MgSO4.7H2O, 1.2 NaH2PO4.2H2O, 25 NaHCO3 5 D-Glucose and 1.25 CaCl2.
Cell isolation
Dissected renal, mesenteric and cerebral arteries were used for isolation of individual myocytes. Vessels were bathed for 10 minutes in a nominally Ca2+ free solution (in mmol/L: 6 KCl, 120 NaCl, 1.2 MgCl2, 12 D-glucose and 10 HEPES, pH 7.4 with NaOH). Vessels were then incubated at 37°C for 17 (MA and CA) or 23 (RA) mins in Ca2+ free solution containing in mg/ml: 1.5 collagenase, 0.75 thermolysin, 1 trypsin inhibitor and 1 bovine serum albumin. Vessels were then washed in Ca2+ free solution for 10 mins and then triturated to liberate myocytes. The cell solution was plated on 13mm coverslips in a 24 well plate and supplemented with an equivalent volume of 2.5mmol/L Ca2+ solution to allow the cells to adhere.
Gene Knock-Down
To study the specific role of Kv7.4 in vascular relaxations, knockdown of Kv7.4 in MA, RA and CA was performed by transfection with morpholino nucleotides as described in vessels previously (13). Kv7.4 morpholino nucleotides (14) and mismatched control nucleotides (5µmol/L, Genetools) were mixed with Lipofectamine 2000 (Life Technologies) in Opti-MEM and left at room temperature for 2 hours. RA, MA and CA were transfected with this mix in Dulbeccos modified eagle’s medium F-12 with 1% penicillin/streptomycin at 37°C for 48 hours.
Western Blot
Lysates were run on polyacrylamide gels (4–12% Bis–Tris, Thermo-Fisher), and then transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore). The membrane was probed with primary antibodies diluted in blocking buffer (5% milk in PBS-Tween 20) overnight at 4°C. Primary antibodies used were: i) rabbit anti-Kv7.4 (1:200; sc-50417; Santa Cruz); ii) mouse anti-β-actin (1:5000; A1978; Sigma Aldrich). Anti-rabbit and anti-mouse secondary antibodies conjugated to IRDye® 800RD and IRDye® 680CW, respectively (dil.1:10,000; Li-Cor Biosciences) were used as appropriate. Protein bands were visualised using the Odyssey Infrared Imaging System (Li-Cor Biosciences, Cambridge, UK). Protein band intensities were measured using Image Studio software (Li-Cor Biosciences, Cambridge, UK), and normalized to β-actin. Data was analysed by Mann-Whitney test where ‘n’ represents the number of animals used.
Immunofluorescence
Freshly isolated myocytes from cerebral arteries from vessels transfected with mismatched or Kv7.4 morpholino were prepared as above. Cells were fixed with 3% PFA on ice for 20 mins and stored in PBS at 4°C. For immunofluorescence, cells were treated with 0.1mol/L glycine for 5 min and incubated in blocking solution (PBS containing 0.1% Triton X-100 and 1% bovine serum albumin) for 1h at RT. Cells were then incubated overnight at 4°C with the following primary antibodies: (i) rabbit anti-Kv7.4 antibody (dilution 1:100, AbCam, Cambridge, UK); (ii) mouse anti α-smooth muscle actin (dilution 1:500, Sigma, Dorset, UK). Samples were then washed with PBS and incubated for 1h with donkey anti-rabbit and donkey anti-mouse secondary antibodies conjugated to Alexa Fluor 568 or Alexa Fluor 488, respectively (dilution 1:100, Thermo-Fisher, Paisley, UK). All antibodies were diluted in blocking solution. Subsequently, samples were washed with PBS and mounted using Vectashield Antifade Medium containing DAPI for nuclei counterstaining (Vector Laboratories, Peterborough, UK). Coverslips were analysed using a Zeiss LSM 510 Meta argon/krypton laser scanning confocal microscope (Carl Zeiss, Jena, Germany). Corrected total cell fluorescence (CTCF) was calculated using ImageJ software as elsewhere described (15). The number of cells analysed is indicated by ‘n’, whereas ‘N’ represents the number of animals used. Statistical analysis were performed by Student’s t-test.
Myography
RA, MA and CA were mounted in myographs (Danish Myograph Technologies) for isometric tension recording. Chambers were filled with PSS, aerated with 95% O2/ 5% CO2 at 37°C. After normalisation to 90% of vessel diameter at 100mg Hg, vessels were constricted with 1µmol/L methoxamine (RA) and 300nmol/L U46619 (MA and CA). Relaxation dose responses to isoproterenol or CGRP were performed in the presence and absence of Gβγ inhibitors (M119K and Gallein (16)), Kv7.4 knockdown or cell signalling inhibitors (see Reagents). Detailed information on level of vessel contraction in experiments, % maximal relaxation levels, and IC50s can be found in Tables I-VI in the supplement. Statistical analyses used a repeated measures two-way ANOVA with Bonferroni post-hoc analysis. For all myography experiments, vessels from one animal were used in a 4 chamber myograph allowing for different experiments to be conducted simultaneously, but always including a same day control. The n number provided represents both the number of experiments performed and the number of animals used.
Proximity Ligation Assay
Freshly isolated cells wells were used as described above. 1mL of solution containing 2.5mmol/L CaCl2 was added to each well and cells were placed in an incubator (37°C, 5% CO2) for 30 minutes to equilibrate. Cells then underwent treatment: 50µmol/L gallein or DMSO control (30mins), 10µmol/L isoproterenol (90s) or 1nmol/L CGRP (90s) or H2O control in PSS. Coverslips were immediately fixed with 3% PFA on ice for 20 mins and stored in PBS at 4°C. For the proximity ligation assay, cells were permeablised with 0.01% Triton X for 5 mins. The Duolink in situ PLA detection kit (Sigma-Aldrich, UK) was used to detect single molecule interactions for Kv7.4 (mouse monoclonal (N43/6, RRID: AB_2131828, UC Davis/NIH NeuroMab Facility) and (i) G protein β subunit (rabbit polyclonal (sc-378, Santa Cruz)), (ii) AKAP 150 (goat polyclonal (sc-6446, Santa Cruz Biotechnology)) or (iii) Rap1a (mouse monoclonal (NBP2-22527, Novus Biologicals)). Antibody combinations for PLA have been validated previously (9). Experiments were performed as per manufacturer’s instructions; primary antibodies were incubated at 1:200 overnight at 4°C. Red fluorescent oligonucleotides produced as the end product of the procedure were visualised using a Zeiss Confocal LSM 510. Images were analysed using Image J software using the particle detector tool. The number of puncta per cell was calculated as the average of two mid sections in each cell. The number of cells analysed is indicated by ‘n’, whereas ‘N’ represents the number of animals used. Statistical analyses used one-way ANOVA multiple comparisons test with Bonferroni post-hoc analysis.
Reagents
Linopirdine, CGRP, Gallein and 8-pCPT-2Me-cAMP-AM were purchased from Tocris, UK. ESI-09 was obtained from BioLog, Germany. M119K were provided by the National Cancer Institute Drug Development Programme. All other reagents were from Sigma-Aldrich UK.
Statistics
All statistical analyses were performed using GraphPad Prism 7 (CA, USA). Different statistical tests were performed as appropriate for the data set being analysed and are defined in the relevant methods and results sections. Briefly, a Bonferroni post-hoc test was used following a repeated measures two-way ANOVA to analyse concentration effect curves, or following a one-way ANOVA to analyse PLA data. Alternatively a Mann-Whitney test was used to analyse western blot data and Students t-test for IF data. For all data significance of p<0.05 is denoted (*), p<0.01 is denoted (**) and p<0.005 is denoted (***).
Results
Kv7.4 morpholino directed knockdown in the rat vasculature
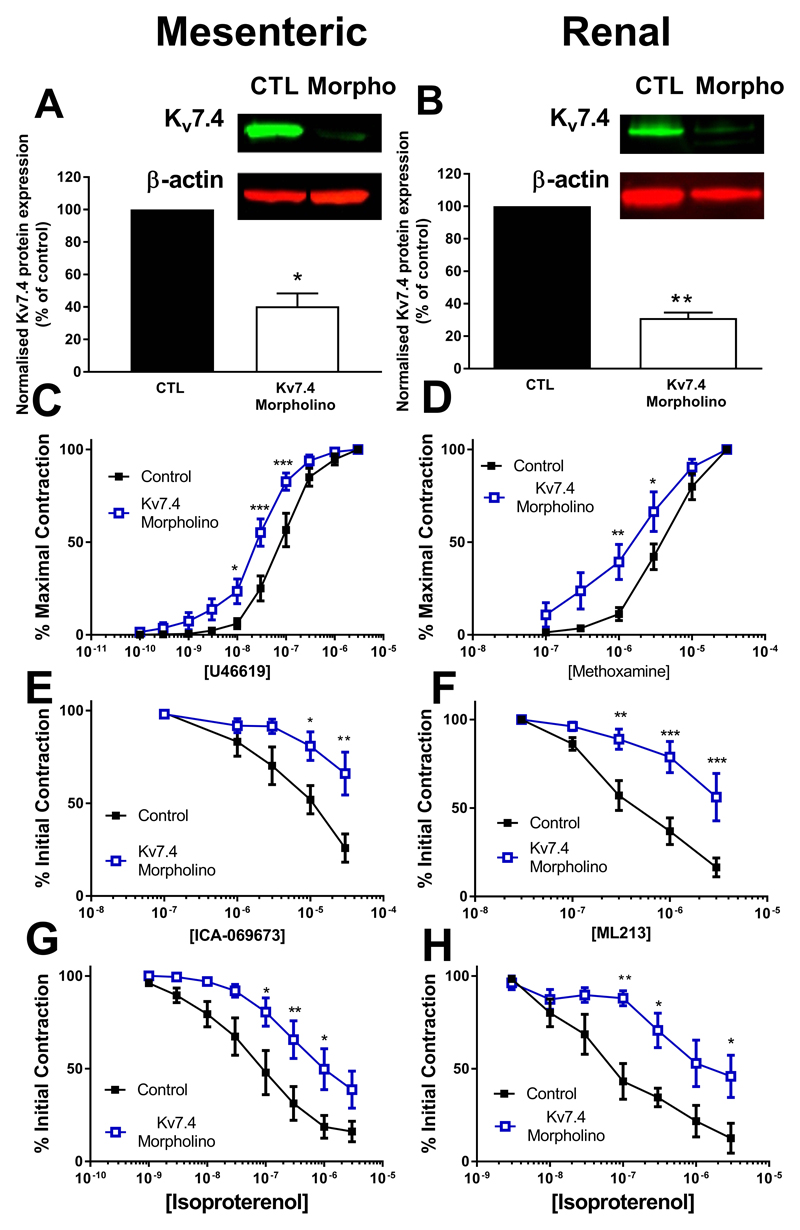
To confirm a role for Kv7.4 in vasodilator responses we used vessels where Kv7.4 was knocked down by morpholino transfection. Protein knockdown of Kv7.4 was confirmed by western blot in RA and MA (Figure 1A and 1B). Kv7.4 knockdown produced a significant leftward shift in the dose response to methoxamine (RA) and U46619 (MA) (Figure 1C and 1D). Functional impairment of channel activity was confirmed by attenuated responses to two Kv7 channel activators, ML213 and ICA-069673 (Figure 1E and 1F). We next looked at the effect of Kv7.4 knockdown on isoproterenol evoked relaxations in both RA and MA, which have previously been shown to involve Kv7 channels (5,8), and these were impaired in Kv7.4 morpholino transfected vessels (Figure 1G and 1H).
Figure 1.
Morpholino knockdown of Kv7.4 in renal and mesenteric arteries. Kv7.4 protein expression in Kv7.4 morpholino transfected mesenteric artery (A, n=5) and renal artery (B, n=4) as normalised to β-actin and expressed as a % of control transfected vessels. Representative blots depicting bands for Kv7.4 and β-actin are displayed in inserts. Concentration dependent contractile responses to U46619 in the mesenteric artery (100pmol/L - 3µmol/L, n=16, C) and to methoxamine in the renal artery (100nmol/L - 30µmol/L, n=10, D) in control (open squares) or Kv7.4 morpholino (closed squares) transfected vessels. Concentration effect relaxations to the Kv7 activator ICA-069673 in the mesenteric artery (100nmol/L - 30µmol/L, n=5, E) or ML213 in the renal artery (30nmol/L - 3µmol/L, n=5, F) in control (open squares) or Kv7.4 morpholino (closed squares) transfected vessels. Concentration effect curve in renal arteries (n=5, G) and mesenteric arteries (n=8, H) to isoproterenol in control (closed squares) and Kv7.4 morpholino (open squares) transfected vessels. Data was analysed by Mann-Whitney test for Western Blots or by Bonferroni post-hoc test following a repeated measures two-way ANOVA for functional studies, where p<0.05 is denoted (*), p<0.01 is denoted (**) and p<0.005 is denoted (***)
Basal associations between Kv7.4 and Gβ in RA and MA
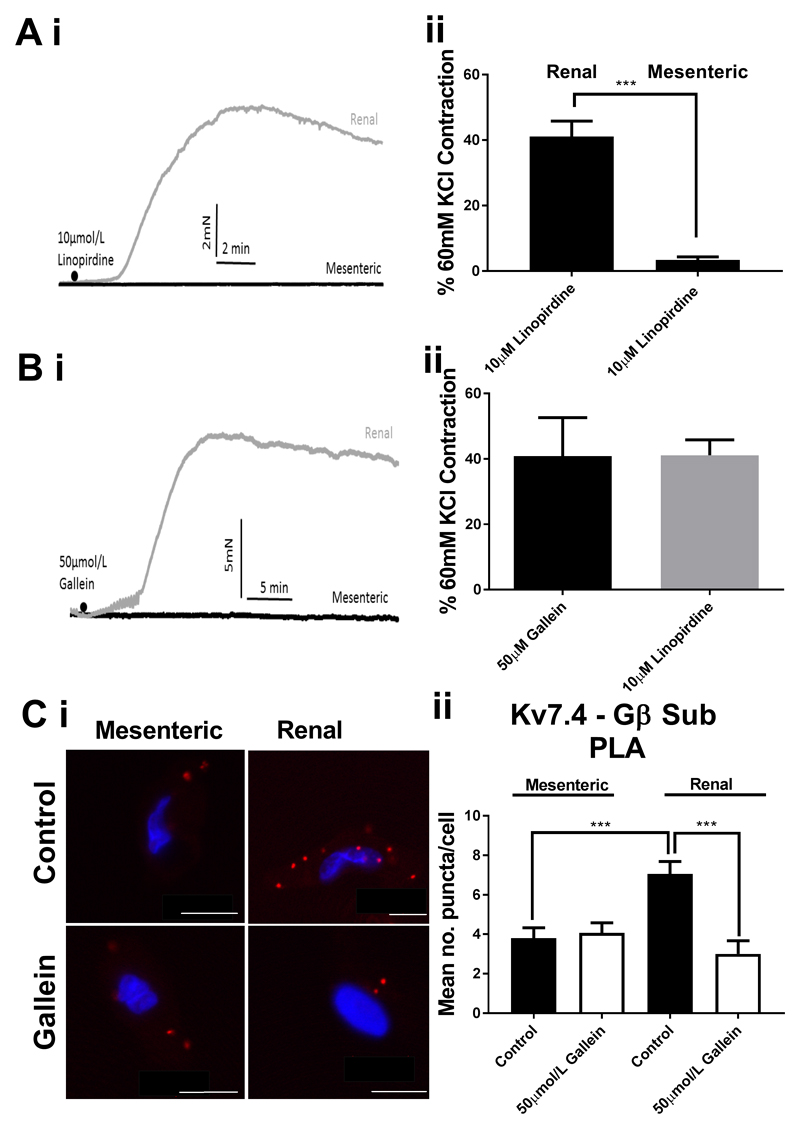
In the RA, inhibition of either Kv7 channels or Gβγ subunits produces contraction in the basal resting state (5, 8, and 12). Therefore, we considered whether there were differences in the role Kv7 channels or Gβγ subunits in the basal resting state of RA and MA. In non-stimulated vessels, inhibition of Kv7 channels with 10µmol/L linopirdine did not contract MA but produced a robust contraction of RA (Fig. 2A). Similarly, inhibition of the Gβγ interactions with 50µmol/L gallein did not produce any effect in MA but contracted RA considerably (Fig. 2B). Proximity ligation assay showed a lower level of Kv7.4 - Gβ puncta in MA compared with RA in unstimulated cells, whilst in RA 50µmol/L gallein treatment decreased Kv7.4 - Gβ puncta to similar levels seen in MA, whereas gallein treatment had no effect in MA (Fig. 2C)
Figure 2.
Basal relationship between Kv7 and Gβγ in renal and mesenteric arteries. Representative traces (i) and mean data (ii) of contractions in response to Kv7 channel inhibition (A, 10µmol/L Linopirdine) or Gbg inhibition (B, 50µmol/L gallein) in mesenteric (n=16-21) and renal (n= 8-13) arteries. Representative confocal images (Ci) of proximity ligation assay between Kv7.4 and Gβ in MA (left) and RA (right) in control (top) or after treatment with 50µmol/L gallein. Scale bars represent 10µm. (Cii) Analysis of mean number of puncta/cell (C ii, n=15-30, N=3-4). Data was analysed by unpaired t-test for (A) and (B) and by one-way ANOVA for (C) where p<0.005 is denoted (***)
Effect of Gβγ inhibition on renal and mesenteric artery responses
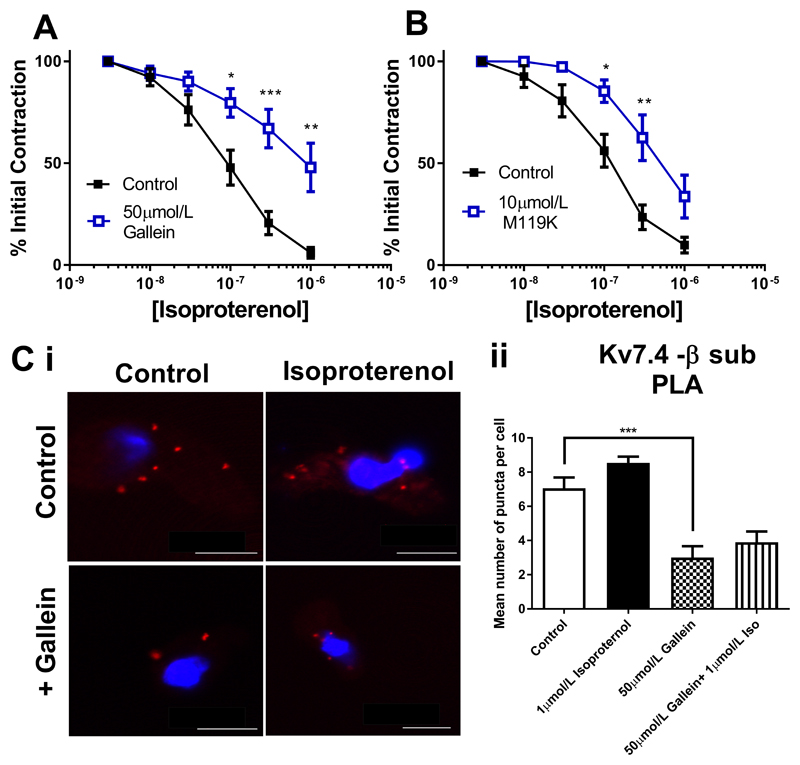
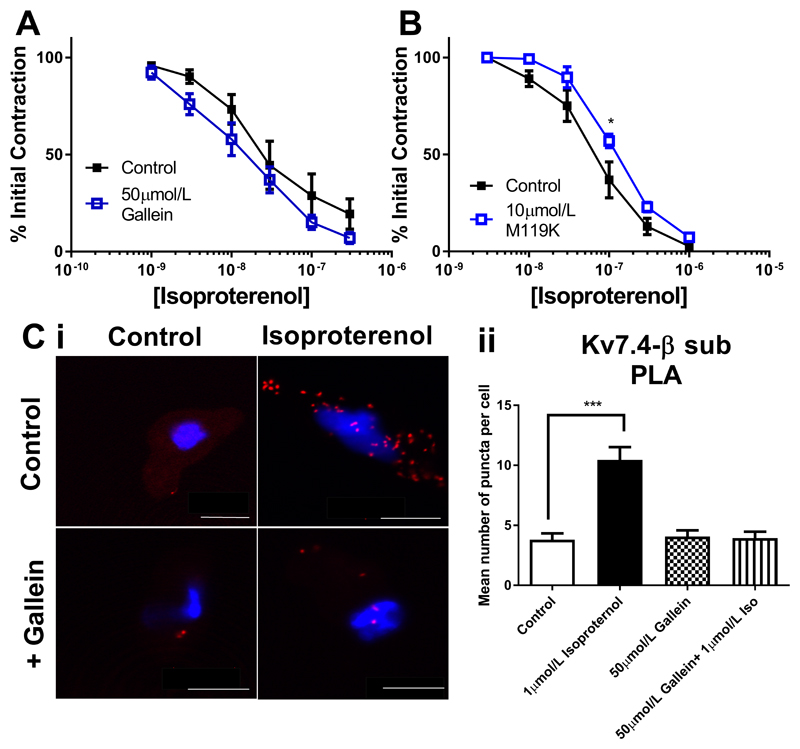
We next considered the role of Gβγ subunits in isoproterenol dependent relaxations in RA and MA. In RA isoproterenol responses were impaired after Gβγ inhibition by either 50µmol/L gallein or 10µmol/L M119K (Fig. 3A and 3B). As shown in Fig 2C, gallein treatment of RA myocytes decreased the basal level of Kv7.4-Gβ puncta significantly (data shown again here for clarity), but no increase in puncta levels is seen with subsequent isoproterenol treatment (Fig. 3C). However, in contrast to RA, isoproterenol relaxations in the MA were not sensitive to 50µmol/L gallein (Fig. 4A) although a slight attenuation was produced by 10µmol/L M119K (Fig. 4B). The number of Kv7.4-Gβ associations at rest in MA was less than in the RA (as shown in Fig 2C, data shown again here for clarity), but increased with isoproterenol treatment in a gallein-sensitive manner (Fig. 4C). These data reveal a differential reliance on Gβγ for basal and isoproterenol driven tone in MA compared to RA.
Figure 3.
Kv7 and Gβγ dependence of isoproterenol relaxations of the rat renal artery. Concentration effect curve in renal arteries to isoproterenol (3nmol/l-3µmol/L) DMSO control (closed squares) or in the presence of 50µmol/L gallein (A, n=4) or 10µmol/L M119K (B, n=9). (C i) Proximity ligation assay between Kv7.4 and Gβ representative images in control (top left), with isoproterenol alone (top right), gallein (bottom left) and isoproterenol with gallein (bottom right). Scale bars represent 10µm. (C ii) Mean data of no. puncta/cell in renal artery myocytes in control or in the presence of 50µmol/L gallein before and after stimulation with 1µmol/L isoproterenol (n=10-20, N=3). Myography data was analysed by Bonferroni post-hoc test following a repeated measures two-way ANOVA. PLA data was analysed by one-way ANOVA. p<0.05 is denoted (*), p<0.01 is denoted (**) and p<0.005 is denoted (***)
Figure 4.
Kv7 and Gβγ dependence of isoproterenol relaxations of the rat mesenteric artery. Concentration effect curve in mesenteric arteries to isoproterenol (1nmol/l-1µmol/L) (in DMSO control (closed squares) or in the presence of 50µmol/L gallein (A, n=10) or 10µmol/L M119K (B, n=7) (all open squares). (C i) Proximity ligation assay between Kv7.4 and Gβ representative images in control (top left), with isoproterenol alone (top right), gallein (bottom left) and isoproterenol with gallein (bottom right). Scale bars represent 10µm. (C ii) Mean data of no. puncta/cell in mesenteric artery myocytes in control or in the presence of 50µmol/L gallein before and after stimulation with 1µmol/L isoproterenol (n=15-30, N=3-4). Myography data was analysed by Bonferroni post-hoc test following a repeated measures two-way ANOVA. PLA data was analysed by one-way ANOVA. p<0.05 is denoted (*) and p<0.005 is denoted (***)
Examining differing Gβγ dependencies of isoproterenol effects in MA and RA
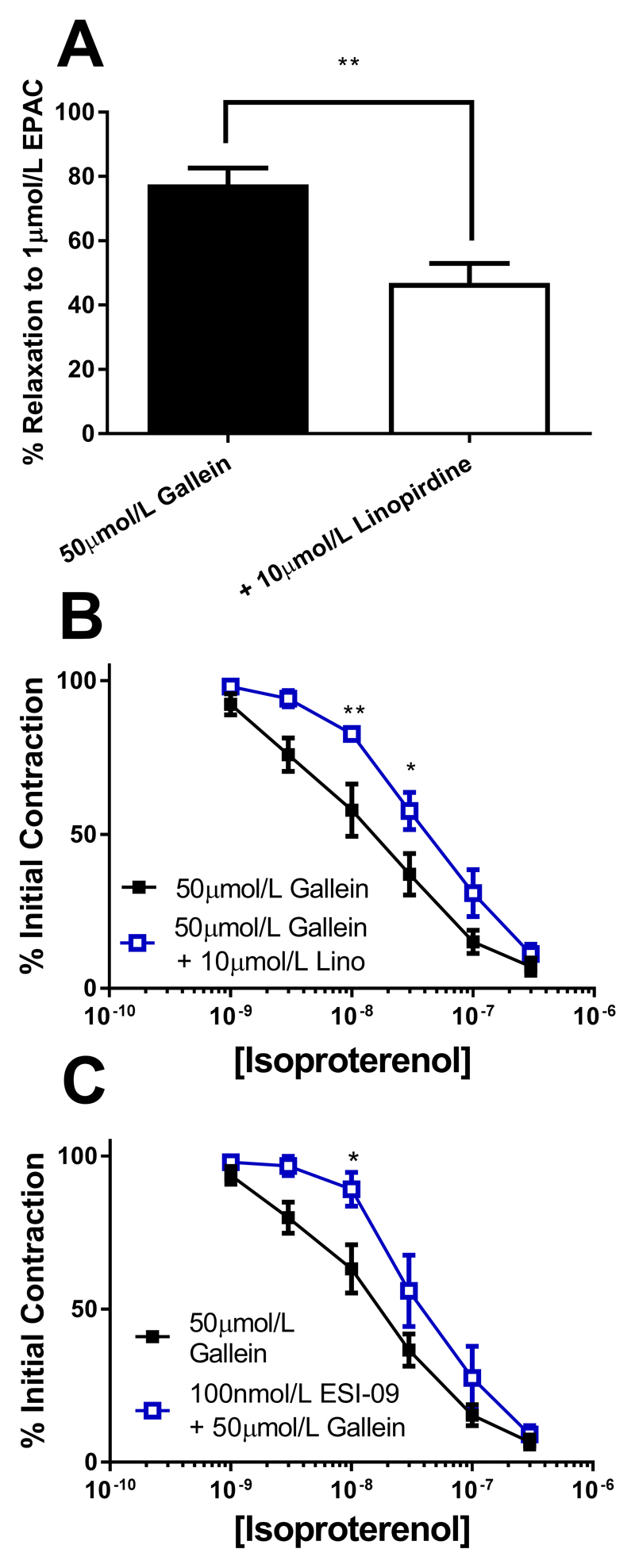
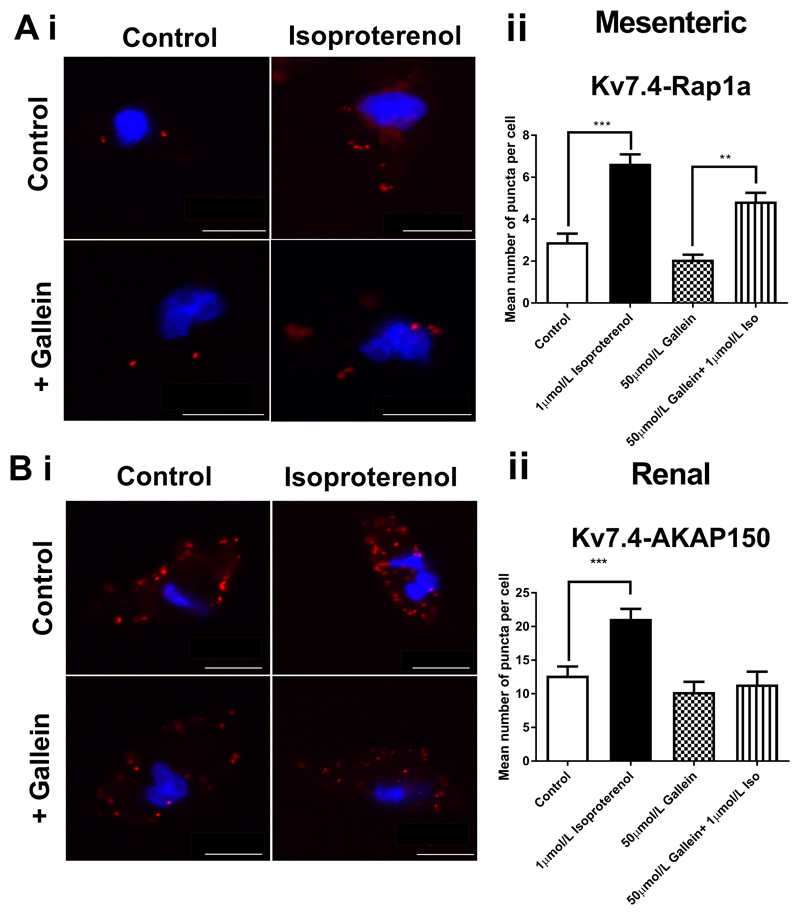
Subsequently, we investigated if the strong dependence on Gβγ for isoproterenol relaxations in RA compared to MA was due to the different intracellular mediators responsible for mediating the relaxation in the different beds. In RA this is predominantly a PKA dependent relaxation, whereas in MA this relaxation is EPAC dependent (9). Relaxations to the EPAC analogue 8-pCPT-2Me-cAMP-AM are inhibited by Kv7 channel blockers in the rat mesenteric artery (9). In the presence of gallein the 8-pCPT-2Me-cAMP-AM (1µmol/L) relaxation in MA still displayed linopirdine sensitivity (Fig. 5A). Similarly, isoproterenol relaxations in the presence of gallein were still sensitive to both Kv7 and EPAC inhibition (10µmol/L linopirdine and 100nmol/L ESI-09, respectively) (Figs. 5B and 5C). Previous work showed that the EPAC dependent isoproterenol relaxation of MA enhanced the association of Kv7.4 with Rap1a (9), a small G protein downstream of EPAC in PLA experiments and this was still apparent in the presence of gallein (Fig. 6A). Conversely, the increase in the Kv7.4-AKAP association which occurs in RA with isoproterenol treatment (9), did not occur in the presence of gallein (Fig. 6B).
Figure 5.
Role of Gβγ in EPAC dependent responses. (A) Mean relaxant effect of 1µmol/L 8-pCPT-2Me-cAMP-AM (EPAC) in mesenteric arteries in the presence of 50µmol/L alone (black), or in combination with 10µmol/L linopirdine (white) (n=6-8). Concentration effect curve in mesenteric arteries to isoproterenol (1nmol/l-300nmol/L) in the presence of 50µmol/L Gallein alone (closed squares, n=10), or in combination with (B) 10µmol/L linopirdine (n=8, open squares) or (C) 100nmol/L ESI-09 (n=5, open squares). Myography data was analysed by Bonferroni post-hoc test following a repeated measures two-way ANOVA. p<0.05 is denoted (*) and p<0.01 is denoted (**)
Figure 6.
Gβγ dependence of intracellular signalling mechanisms. (Ai) Proximity Ligation Assay between Kv7.4 and Rap1a in mesenteric artery myocytes representative images in control (top left), with isoproterenol alone (top right), gallein (bottom left) and isoproterenol with gallein (bottom right) and mean number of puncta/cell (Aii, n=15-34, N=3-4). Scale bars represent 10µm. (Bi) Proximity Ligation Assay between Kv7.4 and AKAP150 in renal artery myocytes representative images in control (top left), with isoproterenol alone (top right), gallein (bottom left) and isoproterenol with gallein (bottom right) and mean number of puncta/cell (Bii, n=10-25, N=2-3). Scale bars represent 10µm. PLA data was analysed by one-way ANOVA with Bonferroni post hoc analysis where p<0.01 is denoted (**) and p<0.005 is denoted (***)
Investigating the effect of Gβγ inhibition on CGRP relaxations of the MA and CA
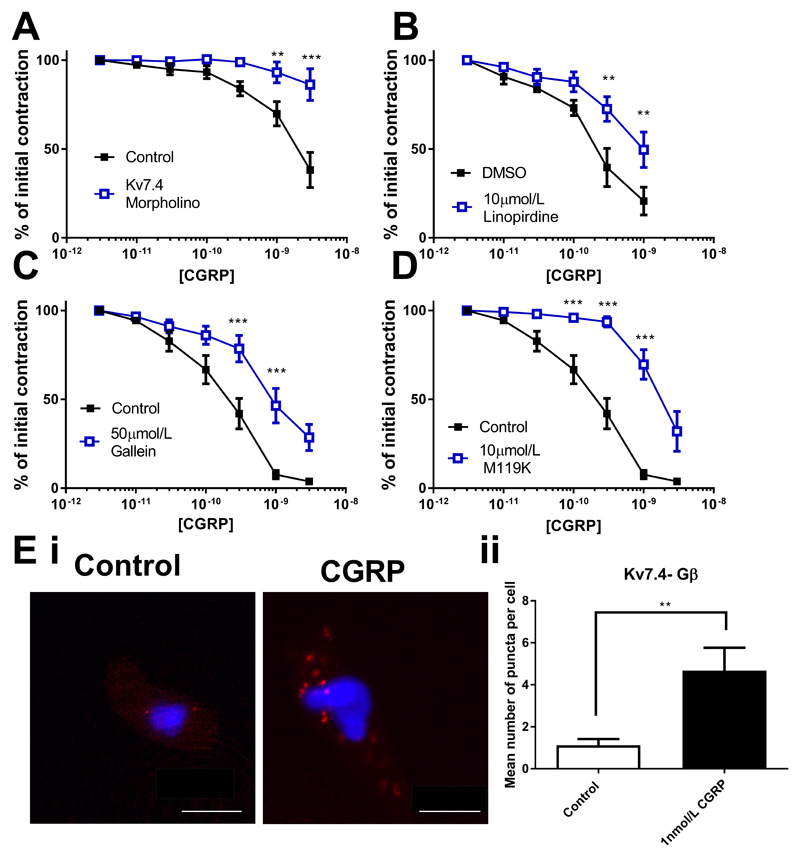
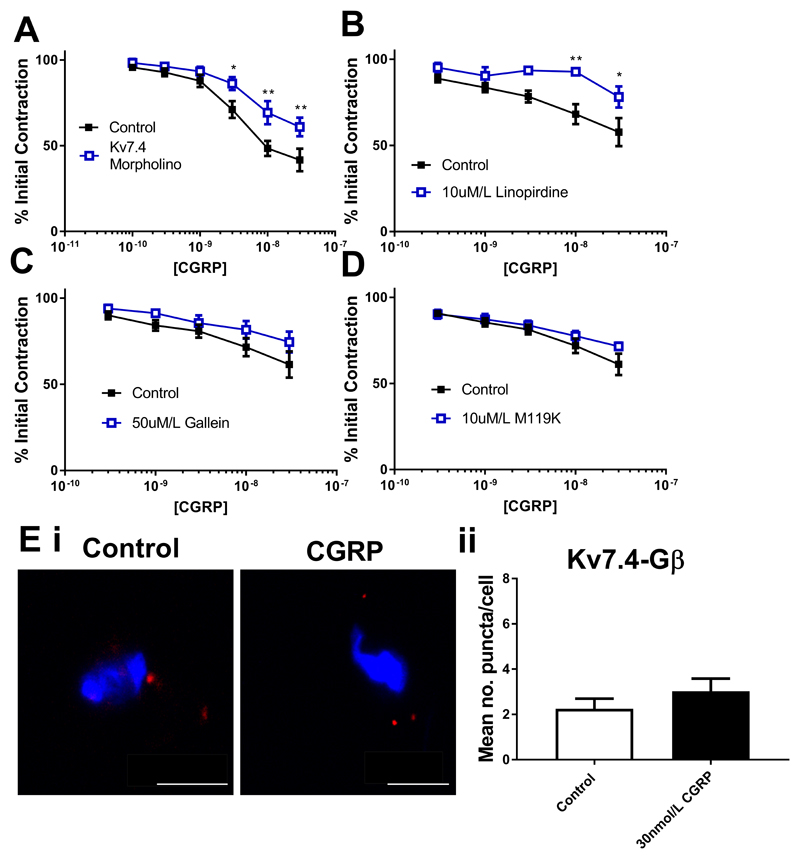
We next considered whether the role of the Gβγ-Kv7.4 relationship was something peculiar to isoproterenol, or whether other vasorelaxants working via Kv7 channels display a similar dependence. CGRP is an effective relaxant of mesenteric arteries (17) that works through a Gs-linked receptor. CGRP relaxations of the MA were markedly attenuated in vessels where Kv7.4 was knocked down by morpholino (Fig. 7A), and by 10µmol/L linopirdine (Fig. 7B). Relaxations were also sensitive to 50µmol/L gallein (Fig. 7C) and 10µmol/L M119K (Fig. 7D). However, neither inhibition of PKA (1µmol/L PKI and 1µmol/L KT5720) nor EPAC (300nmol/L ESI-09) had any effect on relaxations (Supplementary Fig. I). Proximity ligation assay showed an increase in the number of associations between Kv7.4 and the Gβ subunit in MA myocytes treated with 1nmol/L CGRP, (Fig. 7E). CGRP-dependent relaxation of CA are impaired by Kv7 blockers (3 and current study) and these relaxations were attenuated in CA where Kv7.4 was knocked down by morpholino (Fig. 8A). Protein knockdown of Kv7.4 was confirmed by immunofluorescence on isolated myocytes (western blotting was not possible in CA due to the limited amount of protein that can be obtained per experiment), whilst functional knockdown was confirmed by impaired relaxations to ML213 (Supplementary Fig. II). Kv7.4 knockdown CA vessels displayed increased contractility to U46619 when compared to control vessels (Supplementary Fig. II). Relaxations to CGRP in CA were also attenuated by 10µmol/L linopirdine (Fig. 8B). In contrast to the MA, relaxations in the CA were not sensitive to either 50µmol/L gallein (Fig. 8C) or 10µmol/L M119K (Fig. 8D). Incubation with linopirdine (0.04 ± 0.09mN) or gallein (0.04 ± 0.1mN) did not produce a significant change in basal tone in CA. Proximity ligation assay between Kv7.4 and the Gβ subunit showed no change in the number of puncta in CA myocytes upon treatment with 30nmol/L CGRP, (Fig. 8E)
Figure 7.
Kv7 and Gβγ dependence of CGRP relaxations of the rat mesenteric artery. Concentration effect curve in mesenteric arteries to CGRP (1pmol/l-10nmol/L) (A) in control (closed squares) and Kv7.4 morpholino (open squares) transfected vessels (n=6) or (B-D) DMSO control (closed squares) or in the presence of 10µmol/L linopirdine (B, n=8), 50µmol/L gallein (C, n=7) or 10µmol/L M119K (D, n=8) (all open squares). Proximity ligation assay between Kv7.4 and Gβ representative images (Ei) in control (left) and after stimulation with 1nmol/L CGRP (right) and mean number of puncta/cell in mesenteric artery myocytes (Eii, n=12-13, N=3). Scale bars represent 10µm. Myography data was analysed by Bonferroni post-hoc test following a repeated measures two-way ANOVA. PLA data was analysed by unpaired t test. p<0.01 is denoted (**) and p<0.005 is denoted (***)
Figure 8.
Kv7 and Gβγ dependence of CGRP relaxations of the rat cerebral artery. Concentration effect curve in cerebral arteries to CGRP (100pmol/l-30nmol/L) (A) in control (closed squares) and Kv7.4 morpholino (open squares) transfected vessels (n=7) or (B-D) DMSO control (closed squares) or in the presence of 10µmol/L linopirdine (B, n=5), 50µmol/L gallein (C, n=6) or 10µmol/L M119K (D, n=7) (all open squares). Proximity ligation assay between Kv7.4 and Gβ representative images (Ei) in control (left) and after stimulation with 30nmol/L CGRP (right) and mean number of puncta/cell in mesenteric artery myocytes (Eii, n=19-22, N=4). Scale bars represent 10µm. Myography data was analysed by Bonferroni post-hoc test following a repeated measures two-way ANOVA. PLA data was analysed by unpaired t test. p<0.05 is denoted (*) and p<0.01 is denoted (**)
Discussion
This study reveals that the role of the Kv7- Gβγ relationship in mediating relaxations is both receptor- and artery specific. We show vessel dependent differences in the Kv7.4-Gβγ relationship affects the basal vascular reactivity and that Gβγ signalling is a functional component of some vasorelaxant pathways. These findings demonstrate the sheer complexity involved in vascular control and response mechanisms, and begin to uncover the subtle distinctions that can exist over different vascular beds in both signalling and ion channel mechanisms.
The role of Gβγ in vascular responsiveness
Gβγ are well documented as essential components of the heterotrimeric G protein complex, with increasingly recognised diverse roles in cellular signalling such as regulation of adenylate cyclase, MAP kinases, phospholipase C and PI3 kinases (18, 19), but the role these subunits may play in vascular responsiveness remains ill defined. However, the identification that Gβγ are required for basal native vascular Kv7 channel activity in the renal artery (12), which is strongly implicated in arterial regulation, provides a new mechanism for vascular control. Indeed, inhibition of Gβγ with gallein contracts the RA but not MA or CA, mirroring the effects of Kv7 channel inhibition in these vessels. It must be noted that Gβγ can also regulate the activity of other channels such as CaV or KIR (18–21), and these interactions might play a role in vascular control mechanisms, an interesting avenue for future study. However, here we present compelling evidence that there are vascular bed differences in the activity of Kv7 channels and their associations with regulators which contribute to a complex myriad of vascular control mechanisms.
Previous work established a role for the Gβγ-Kv7 complex in basal vascular reactivity in the renal artery, showing an association between Kv7.4 and Gβ in the basal state which decreased with gallein treatment (12). Functionally this was associated with the generation of contractions of similar amplitude to those produced by Kv7 channel blockers (12). Interestingly in these studies examining different parts of the vasculature in more detail, i.e. the mesenteric and cerebral arteries, no such basal association was seen between Kv7.4 and Gβ (Figs 1 and 8) and no such basal reactivity was seen in response to either Gβγ or Kv7 blockade to that seen in renal artery (7,8, 12 and current study). This shows that there is distinct variation in the contribution of both Kv7 channels and Gβγ to basal regulatory control in different vascular beds.
Role of Gβγ in receptor mediated relaxations
Various receptor dependent relaxations in renal, mesenteric and cerebral arteries have previously implicated involvement of Kv7 channels as shown through pharmacological blockade and siRNA knockdown of Kv7.4 in the renal artery (8, 9). To extend some of these previous findings we utilised morpholino knockdown of Kv7.4, in all three vessels to study receptor dependent relaxations. It must be noted for all of our findings that previous studies in various arteries have demonstrated isoproterenol or CGRP effects to involve KATP, BKCa or other Kv channels (9, 22–29). That several potassium channels have been implicated in these relaxations is now a recurring theme in vascular physiology research. Whilst some of this may be due to species and/or vessel dependent differences, future studies must aim to integrate these many findings to establish how these individual mechanisms work in concert.
Knockdown of Kv7.4 channels resulted in marked attenuation of isoproterenol responses concomitant with decreased total Kv7.4 in all vessels. Further experiments showed that inhibition of Gβγ by gallein or the more potent M119K (16) prohibited isoproterenol relaxations in RA but had little effect on isoproterenol responses in the MA. Previous studies demonstrated that isoproterenol relaxations of the RA were PKA and AKAP dependent, whereas in the MA they were EPAC dependent (9). Therefore we considered this as a possibility for the difference in the Gβγ dependence of these relaxations. Consequently, we tested the effect of Gβγ inhibition on EPAC mediated relaxations by using the analogue 8-pCPT-2Me-cAMP-AM. These relaxations involved Kv7 channels, but were not attenuated by Gβγ inhibition. This is consistent with the EPAC dependent isoproterenol response in MA also not being gallein sensitive. However, Kv7 channel blockade still prevented EPAC and isoproterenol relaxation in MA in the presence of gallein suggesting that Gβγ were not required for EPAC to increase Kv7 activity. Indeed, PLA studies show that the increase in Kv7.4-Rap1a association with isoproterenol treatment was not affected with Gβγ inhibition. Conversely, gallein treatment prevented the isoproterenol induced increase in AKAP-Kv7.4 association in the RA. This suggests that here the presence of Gβ is required for AKAP to associate with the channel complex but not for Rap proteins.
We considered that the Kv7-Gβγ relationship might be peculiar to isoproterenol dependent effects, and so investigated CGRP as another vasorelaxant mechanism which has been previously been shown to be Kv7 dependent in the cerebral artery (3). CGRP relaxations in both MA and CA were attenuated by Kv7 blockade with linopirdine and by morpholino knockdown of Kv7.4. We also corroborated previous findings that established a role for Gβγ subunits in CGRP responses of the MA (17). PLA experiments show that CGRP treatment increased Gβ-Kv7.4 puncta consistent with a greater association of Kv7.4 and Gβγ whilst both Gβγ inhibitors attenuated the relaxant response to CGRP considerably. Remarkably, in the cerebral vasculature no such relationship was seen, with no association detected between Gβ-Kv7.4 in either control or CGRP treated myocytes, whilst Gβγ inhibitors had no effect on relaxations.
Raised cyclic AMP in response to CGRP is a reasonably consistent finding in most vessels studied, and therefore relaxations will occur via PKA or EPAC (27, 30, 31). Whilst a role for PKA has been shown by some (30), and our findings are in agreement with previous work which demonstrated PKA had no role in CGRP relaxations of the MA (17). This would rule out a traditional cAMP dependent relaxation pathway in the MA, and the high dependence on Gβγ activity would suggest that this is the main mechanism involved although the exact signals are currently unclear. The source of the Gβγ which is recruited to the Kv7.4 is unclear, but may suggest a pool of ‘free’ subunits close to the channel – a previously controversial view, but there is now a critical mass of evidence reporting the existence of such pools (32–35). Alternatively, the source of Gβγ may occur from translocation directly from receptor stimulation depending on the subcellular localisation. Future studies will investigate the role of other Gβγ effectors (e.g. enzyme cascades, other ion channels) in this process.
Of course all experiments must be interpreted with the relevant caveats; it is possible that signalling intermediaries other than those described here may be involved in these processes, and the role of Kv7 channels and how they are regulated in the endothelium is unclear but may play a role here. In addition the use of freshly isolated myocytes for PLA experiments, although in agreement with our functional data, may not represent the true picture in intact arteries as alterations may take place in the isolation process. There are many unknowns about how Gβγ function in vascular smooth muscle, although they clearly have important roles as they have been implicated in proliferation and differentiation processes (36, 37). So an added caveat is that Gβγ are involved in many different cellular processes and therefore their inhibition may produce manifold effects. There is the possibility that inhibition of these subunits prevents proper functioning of the receptor complexes. However, in the MA isoproterenol relaxations are not affected suggesting this receptor is still capable of functional signalling, whilst vasoconstrictor stimuli in both vessels are not affected. We propose that one mechanism by which Gβγ are involved in vascular responsiveness is by regulating the activity and ability of Kv7.4 to respond to some intracellular signals. Future studies will aim to investigate the physiological significance of these findings in vivo on blood flow and pressure.
These findings highlight the startling level of heterogeneity of receptor signalling across vascular beds and demonstrates the perils to extrapolate out findings from one vessel type to many. Here we show two vasorelaxants in a total of three vessels which all involve Kv7 channels to some extent, but the signalling intermediaries are different in every situation. This study reveals a complex vascular bed specific regulation of Kv7 channels by Gβγ subunits, and the underlying requirement of this regulation for some, but not all, receptor mediated effects on these channels. Overall this study demonstrates the importance of the Kv7.4- Gβγ relationship in vascular control, and gives an intriguing insight into novel vasorelaxant mechanisms by Gβγ.
Supplementary Material
Highlights.
G protein βγ subunits are important in receptor mediated vasodilations in a vascular bed/vasorelaxant specific manner and this is due in part to the relationship of vascular Kv and Gβγ
G protein βγ subunit regulation of vascular Kv7 channels is important in the transmission of further regulatory stimuli and is an essential requirement for AKAP mediated regulation of the channel. However, EPAC dependent signals do not require the Kv7-Gβγ relationship, whilst Gβγ can also be involved in mediating receptor dependent relaxations
There are many complex, differences in the coupling of receptor signalling and to Kv7 channels. This suggests highly specialised regional control of these processes throughout the vasculature.
Acknowledgements
The authors would like to acknowledge the Biological Research Facility for animal services, and also the Image Resource Facility for assistance with confocal imaging, both at St George’s University of London
Sources of funding
JBS was funded by a British Heart Foundation grant (PG/15/97/31862) awarded to IAG and JBS. VB was funded by a Medical Research Council grant (MR/K019074/1) awarded to IAG.
Abbreviations
- AKAP
A-Kinase Anchoring Protein
- CA
Cerebral Artery
- CGRP
Calcitonin Gene Related Peptide
- EPAC
Exchange Protein directly Activated by cyclic AMP
- Gβγ
G protein βγ subunits
- GPCR
G-protein Coupled Receptor
- MA
Mesenteric Artery
- PLA
Proximity Ligation Assay
- PKA
Protein Kinase A
- RA
Renal Artery
Footnotes
Disclosures
None
References
- 1.Haick JM, Byron KL. Novel treatment strategies for smooth muscle disorders: Targeting Kv7 potassium channels. Pharmacol Ther. 2016;165:14–25. doi: 10.1016/j.pharmthera.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Stott JB, Jepps TA, Greenwood IA. Kv7 potassium channels: a new therapeutic target in smooth muscle disorders. Drug Discov Today. 2014;19:413–424. doi: 10.1016/j.drudis.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, Greenwood IA. Contribution of Kv7.4/Kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol. 2014;34:887–893. doi: 10.1161/ATVBAHA.114.303405. [DOI] [PubMed] [Google Scholar]
- 4.Khanamiri S, Soltysinska E, Jepps TA, Bentzen BH, Chadha PS, Schmitt N, Greenwood IA, Olesen SP. Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertension. 2013;62:1090–1097. doi: 10.1161/HYPERTENSIONAHA.113.01244. [DOI] [PubMed] [Google Scholar]
- 5.Stott JB, Barrese V, Jepps TA, Leighton EV, Greenwood IA. Contribution of Kv7 channels to natriuretic peptide mediated vasodilation in normal and hypertensive rats. Hypertension. 2015;65:676–682. doi: 10.1161/HYPERTENSIONAHA.114.04373. [DOI] [PubMed] [Google Scholar]
- 6.Morales-Cano D, Moreno L, Barreira B, Pandolfi R, Chamorro V, Jimenez R, Villamor E, Duarte J, Perez-Vizcaino F, Cogolludo A. Kv7 channels critically determine coronary artery reactivity: left-right differences and down-regulation by hyperglycemia. Cardiovasc Res. 2015;106:98–108. doi: 10.1093/cvr/cvv020. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Yang Y, Tanner MA, Li M, Hill MA. Heterogeneity in Kv7 channel function in the cerebral and coronary circulation. Microcirculation. 2015;22:109–121. doi: 10.1111/micc.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Cole WC, Moffatt JD, Greenwood IA. Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired β-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension. 2012;59:877–884. doi: 10.1161/HYPERTENSIONAHA.111.187427. [DOI] [PubMed] [Google Scholar]
- 9.Stott JB, Barrese V, Greenwood IA. Kv7 channel activation underpins EPAC-dependent relaxations of rat arteries. Arterioscler Thromb Vasc Biol. 2016;36:2404–24115. doi: 10.1161/ATVBAHA.116.308517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, Hansen RS, Greenwood IA. Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation. 2011;124:602–611. doi: 10.1161/CIRCULATIONAHA.111.032136. [DOI] [PubMed] [Google Scholar]
- 11.Carr G, Barrese V, Stott JB, Povstyan OV, Jepps TA, Figueiredo HB, Zheng D, Jamshidi Y, Greenwood IA. MicroRNA-153 targeting of KCNQ4 contributes to vascular dysfunction in hypertension. 2016;112:581–589. doi: 10.1093/cvr/cvw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stott JB, Povstyan OV, Carr G, Barrese V, Greenwood IA. G-protein βγ subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity. Proc Natl Acad Sci USA. 2015;112:6497–6502. doi: 10.1073/pnas.1418605112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jepps TA, Carr C, Lundegaard PR, Olesen SP, Greenwood IA. Fundamental role for the KCNE4 ancillary subunit in Kv7.4 regulation of arterial tone. J Physiol. 2015;593:5325–5340. doi: 10.1113/JP271286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 15.Burgess A, Vigneron S, Brioudes E, Labbe JC, Lorca T, Castro A. Loss of human Greatwell results in G2 arrest and multiple mitotic defects due to deregulation of the cycli B-Cdc/PP2A balance. Proc Natl Acad Sci USA. 2010;107:12464–12569. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gbetagamma-subunit signalling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 17.Meens MJ, Mattheij NJ, van Loenen PB, Spijkers LJ, Lemkens P, Nelissen J, Compeer MG, Alewijnse AE, De Mey JG. G-protein βγ subunits in vasorelaxing and anti-endothelinergic effects of calcitonin gene-related peptide. Br J Pharmacol. 2012;166:297–308. doi: 10.1111/j.1476-5381.2011.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smrcka AV. G protein βγ subunits: central mediators of G protein-coupled receptor signalling. Cell Mol Life Sci. 2008;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbé JC, Miller GJ, Hébert TE. The expanding roles of Gβγ subunits in G protein-coupled receptor signalling and drug action. Pharmacol Rev. 2013;65:545–577. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- 20.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of the GTP-binding proteins activate the muscarinic K+ channel in the heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 21.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 22.Randall MD, McCulloch AI. The involvement of ATP-sensitive potassium channels in beta-adrenoceptor-mediated vasorelaxation in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1995;115:607–612. doi: 10.1111/j.1476-5381.1995.tb14975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Kwok KH. Effects of putative K+ channel blockers on beta-adrenoceptor-mediated vasorelaxation of the rat mesenteric artery. J Cardiovasc Pharmacol. 1997;29:515–519. doi: 10.1097/00005344-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 24.White R, Bottrill FE, Siau D, Hiley CR. Protein kinase A-dependent and –independent effects of isoproterenol in rat isolated mesenteric artery: interactions with levcromakalim. J Pharmacol Exp Ther. 2001;298:917–924. [PubMed] [Google Scholar]
- 25.Beleznai TZ, Yarova PL, Yuiii KH, Dora KA. Smooth muscle Ca2+-activated and voltage-gated K+ channels modulate conducted dilatation in rat isolated small mesenteric arteries. Microcirculation. 2001;18:487–500. doi: 10.1111/j.1549-8719.2011.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 27.Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994;475:9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong KW, Yoo SE, Yu SS, Lee JY, Rhim BY. Pharmacological coupling and functional role for CGRP receptors in the vasodilation of rat pial arteriole. Am J Physiol. 1996;270:H317–H323. doi: 10.1152/ajpheart.1996.270.1.H317. [DOI] [PubMed] [Google Scholar]
- 29.Reslerova M, Loutzenhiser R. Renal microvascular actions of calcitonin gene-related peptide. Am J Physiol Renal Physiol. 1998;274:F1078–F1085. doi: 10.1152/ajprenal.1998.274.6.F1078. [DOI] [PubMed] [Google Scholar]
- 30.Gangula PR, Lanlua P, Bukoski RD, Wimalawansa SJ, Yallampalli C. Mesenteric arterial relaxation to calcitonin gene-related peptide is increased during pregnancy and by sex steroid hormones. Biol Reprod. 2004;71:1739–1745. doi: 10.1095/biolreprod.104.031369. [DOI] [PubMed] [Google Scholar]
- 31.Bol M, Leybaert L, Vanheel B. Influence of methanandamide and CGRP on potassium currents in smooth muscle cells of small mesenteric arteries. Pflugers Arch. 2012:669–677. doi: 10.1007/s00424-012-1083-1. [DOI] [PubMed] [Google Scholar]
- 32.Bokoch GM. The presence of free G protein beta/gamma subunits in human neutrophils results in suppression of adenylate cyclase activity. J Biol Chem. 1987;262:589–594. [PubMed] [Google Scholar]
- 33.Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG. Differential distribution of alpha subunits and beta gamma subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–1040. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin HC, Duncan JA, Kozasa T, Gilman AG. Sequestration of the G protein beta gamma subunit complex inhibits receptor-mediated endocytosis. Proc Natl Acad Sci. 1998;95:5057–5060. doi: 10.1073/pnas.95.9.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajith Karunarathne WK, O’Neill PR, Martinez-Espinosa PL, Kalyanaraman V, Gautman N. All G protein βγ subunits are capable of translocation on receptor activation. Biochem Biophys Res Commun. 2012;421:605–611. doi: 10.1016/j.bbrc.2012.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iaccarino G, Smithwick LA, Lefkowitz RJ, Koch WJ. Targeting Gbeta gamma signalling in arterial vascular smooth muscle proliferation: a novel strategy to limit restenosis. Proc Natl Acad Sci. 1999;96:3945–3950. doi: 10.1073/pnas.96.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reusch JP, Schaefer M, Plum C, Schultz G, Paul M. Gbeta gamma mediate differentiation of vascular smooth muscle cells. J Biol Chem. 2001;276:19540–19547. doi: 10.1074/jbc.M101963200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.