Abstract
This study describes the pathologic findings and most probable causes of death (CD) of 224 cetaceans stranded along the coastline of the Canary Islands (Spain) over a 7-year period, 2006–2012. Most probable CD, grouped as pathologic categories (PCs), was identified in 208/224 (92.8%) examined animals. Within natural PCs, those associated with good nutritional status represented 70/208 (33.6%), whereas, those associated with significant loss of nutritional status represented 49/208 (23.5%). Fatal intra- and interspecific traumatic interactions were 37/208 (17.8%). Vessel collisions included 24/208 (11.5%). Neonatal/perinatal pathology involved 13/208 (6.2%). Fatal interaction with fishing activities comprised 10/208 (4.8%). Within anthropogenic PCs, foreign body-associated pathology represented 5/208 (2.4%). A CD could not be determined in 16/208 (7.7%) cases. Natural PCs were dominated by infectious and parasitic disease processes. Herein, our results suggest that between 2006 and 2012, in the Canary Islands, direct human activity appeared responsible for 19% of cetaceans deaths, while natural pathologies accounted for 81%. These results, integrating novel findings and published reports, aid in delineating baseline knowledge on cetacean pathology and may be of value to rehabilitators, caregivers, diagnosticians and future conservation policies.
Introduction
The occurrence of disease in aquatic organisms will be probably one of the long-term consequences of climate change and environmental degradation [1]. Cetaceans are regarded as sentinel species to monitor marine and marine-terrestrial interface ecosystems wherein humans are strictly integrated [1]. Cetaceans are exposed to environmental stressors either anthropogenic, e.g., chemical and acoustic pollution, fisheries, maritime traffic, tourism industry, and non-anthropogenic, hereafter ‘natural,’ e.g., biotoxins, pathogens (bacteria, fungi, parasites, viruses) [2]. Several emerging and reemerging pathogens (EREPs) affecting mysticetes and odontocetes have been recognized over the last decades [1,3]. Some of these pathogens have epizootic potential, are zoonotic or display complex pathogeneses in which biotic, e.g., genetic stock, immunologic dysfunction, and abiotic, e.g., chemical pollutants, may play a major role [1,4,5].
Major cetacean EREPs include cetacean morbilliviruses, herpesviruses, poxviruses, marine Brucella species, Toxoplasma gondii, and Paracoccidioides brasiliensis (formerly Lacazia loboi) [1,3]. Furthermore, neoplasia associated with viral pathogens, such as Papillomavirus have been reported [3]. Also, of utmost public health relevance is the isolation of antibiotic resistant bacteria from free-ranging and captive cetaceans [6]. All these pathogens may have the capability to modulate environmental change, mediating decline of species, ecological proportions of predators, prey, competitors, and recyclers; and alter habitat already threatened by habitat fragmentation and climate change [7]. Additionally, novel pathogens have been identified over the last years such as polyomavirus [8], adenovirus [9] and parainfluenza [10], among others. The constantly evolving interplay between marine hosts, pathogens and environmental scenarios demands continuous health and disease monitoring.
The Canary archipelago (Spain) has the greatest cetacean biodiversity of the European coastline with 30 species described, including 7 mysticetes and 23 odontocetes [11]. These include migratory, seasonal and resident species. The first systematic, large-scale and long-term (1999–2005) pathology-based study on free-ranging cetaceans off Canarian waters suggested direct human activity was responsible for 33% of cetaceans deaths, while 62% involved natural disease processes [12]. Our multidisciplinary research team is committed to prolonged and continuous monitoring of health and disease aspects of free-ranging cetaceans off the Canaries; thus, the present study aimed to report the pathologic findings and most probable causes of deaths on cetaceans stranded along the coastline of the Canary Islands between January 2006 to December 2012.
Materials and methods
The required permission for the management of stranded cetaceans anywhere within the Canarian archipelago was issued by the environmental department of the Canary Islands’ Government. No experiments were performed on live animals because our work was based on dead stranded cetaceans. Biological and stranding epidemiology data for each individual were recorded (S1 Table). Age category was based on total body length and gonadal development, including: fetus/neonate/calf, juvenile/subadult, and adult [13]. The nutritional status (syn. body condition) was subjectively classified into good, moderate, poor, and emaciated according to anatomical parameters such as the osseous prominence of the spinous and transverse vertebral processes and ribs, the mass of the epaxial musculature, and the amount of fat deposits, taking into account the species and the age of the animal [12]. Carcasses were classified as very fresh, fresh, moderate autolysis, advanced autolysis or very advanced autolysis [14].
Necropsies followed standardized protocols [13,14]. Representative samples of skin, longissimus dorsi and rectus abdominis muscles, peritoneum, diaphragm, central nervous system, eye, pterygoid sac, tympanoperiotic complexes, tongue, oral mucosa, pharyngeal and laryngeal tonsils, esophagus, stomach, small and large intestine, liver, pancreas, trachea, lung, heart, aorta, kidney, ureter, urinary bladder, urethra, lymph nodes, spleen, testicle, penis, prepuce, ovary, uterus, vagina and vulva, were collected and fixed in 10% neutral buffered formalin. All these tissues were processed routinely, embedded in paraffin-wax and 5 μm-thick sections were stained with hematoxylin and eosin (H&E) for microscopic analysis. Special histochemical techniques (4–10 μm-thick sections) to better characterize microscopic changes on selected tissue sections included: periodic acid-Schiff, luxol fast blue, Prussian blue, Bielchowsky’s, Gram/Twort, Hall’s, Grocott- Gomori’s Methenamine Silver, Masson’s trichrome, Movat-Russel pentachrome, osmium tetroxide (post-fixation), rhodamine, Congo red, von Kossa, and Ziehl-Neelsen.
We employed a set of primary antibodies for immunohistochemical (IHC) analysis to better define the nature of some lesions in certain animals. Primary antibodies utilized were: calponin, calretinin, cluster of differentiation (CD)-3, CD79α, cytokeratin (CK)-7, CK14, CK20, glial fibrillary acid protein (GFAP), lysozyme, myeloid/histiocyte antigen (MAC)-387, neuron specific enolase (NSE), neurofilament, pancytokeratin AE1/AE3, S100 protein, and vimentin. When indicated by histopathology or observation of specific etiologic agents in sections, targeted infectious agents, i.e., cetacean morbillivirus (CeMV), Herpesvirus (HV), T. gondii, and Erysipelothrix rhusiopathiae, via IHC was undertaken. Published methodologies for IHC analyses were followed [15,16]. Detailed information on IHC methodologies are recorded in S2 Table. Negative controls consisted of serial tissue sections in which primary antibodies were substituted by non-immune homologous serum. Positive controls included appropriate cetacean, canine and/or human tissue sections, accordingly.
Fresh tissue samples (skin, muscle, lung, prescapular, pulmonary, mediastinal and mesenteric lymph nodes, liver, intestine, kidney, spleen, brain) collected routinely during necropsy, were frozen (-80°C) and selectively submitted for bacteriological analysis. These included routine culture and surface plating on routine media, e.g., Columbia blood agar and preliminary identification of isolates via API system (API 20E, API Rapid 20E, API Staph, API 20 Strep, API Coryne, API 20A). PCR targeting the 16S rRNA gene coupled with pulsed-field gel electrophoresis were performed on selected isolates [17]. Cerebrum of animal no. 63 was subjected to mycological analysis, including culture on Sabouraud agar and morphologic colony identification [18]. Tissues cultured and respective results are recorded in S3 Table. PCR analysis for detection of CeMV and HV followed published protocols and have been partially published [19,20].
For parasitological analysis, epibionts, ectoparasites and endoparasites were preserved in 70% alcohol. Identification relied on macroscopic, submacroscopic and histologic features [21]. Molecular analysis using primers 930F and 1200R for the 18S ribosomal gene were used in two Crassicauda sp. nematodes from animal no. 180 and 207 [22].
The classification herein implemented focuses on differentiating between natural and anthropogenic disease processes, hereafter ‘pathologic categories’ (PCs), according to current knowledge on cetacean pathology [12]. We considered five major natural PCs: 1) pathology associated with significant loss of nutritional status (NPSLNS; including poor and emaciated animals), 2) pathology associated with good nutritional status (NPGNS; including good and moderate animals), 3) neonatal/perinatal pathology (NPP), 4) intra- and interspecific traumatic interactions (ITI), and 5) typical mass-stranding pathology. Three major anthropogenic PCs were considered: 1) interaction with fishing activities (IFA), 2) foreign body-associated pathology (FBAP), and 3) vessel collision (VC) [12]. Furthermore, gross and microscopic lesions typically associated with the ‘stress response syndrome’ or ‘alarm reaction’ [23] and ‘capture myopathy’ [24,25] were also addressed in this classification system [12].
Material access
The material used in this study is deposited in the Marine Mammal Tissue Bank held by the Animal Histology and Pathology division of the Institute for Animal Health and Food Hygiene, Veterinary School, University of Las Palmas of Gran Canaria, Gran Canaria, Spain.
Results
Stranding epidemiology
A total of 320 cetaceans stranded between 1st January, 2006, and 31st December, 2012, including 22 species. From these, postmortem examinations were conducted on 224 (70%), representing 21 species. Odontocetes (n = 216; 17 species) were overrepresented compared to mysticetes (n = 8; four species) (S1 Table). Sex distribution of animals studied included 105 (46.9%) females, 109 (48.7%) males, and 10 (4.5%) undetermined. Age categories were: 51/224 (22.8%) neonate/calf, 59/224 (26.3%) juvenile/subadult and 113/224 (50.4%) adult. Age was unknown in one animal. Annual strandings during this 7-year period averaged 45.7; strandings occurred throughout the year with peaks between March, April and May. Necropsies were performed on 13/224 (5.8%) very fresh, 79/224 (35.3%) fresh, 61/224 (27.2%) moderate autolysis, 60/224 (26.8%) advanced autolysis and 11/224 (4.9%) very advanced autolysis carcasses. Thirty of 224 (13.4%) animals stranded alive; 194/224 (86.6%) stranded dead or were retrieved adrift. Distribution based on nutritional status included: 56 (25%) good, 60 (26.8%) moderate, 64 (28.6%) poor and 16 (7.1%) cachectic animals. The nutritional status could not be inferred in 28 (12.5%) animals due to decomposition phenomena.
The most probable CD, grouped in PC s, was identified in 208/224 (92.8%) examined individuals (Table 1). Etiologic diagnoses by pathologic categories are recorded in Table 2.
Table 1. Species studied and pathologic categories in stranded cetaceans from the Canary Islands (2006–2012).
| Pathologic categories | ND | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Natural | Anthropogenic | |||||||
| PSLNS | PGNS | NPP | IITI | FBAP | VC | IFA | |||
| Balaenoptera acutorostrata | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 3 |
| Balaenoptera borealis | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 3 |
| Balaenoptera physalus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Delphinus delphis | 9 | 9 | 0 | 3 | 0 | 0 | 1 | 2 | 24 |
| Globicephala macrorhynchus | 3 | 8 | 3 | 8* | 0 | 1 | 1 | 2 | 26 |
| Grampus griseus | 5 | 1 | 0 | 3 | 1 | 0 | 0 | 0 | 10 |
| Kogia breviceps | 3 | 3 | 1 | 4 | 0 | 4 | 0 | 1 | 16 |
| Kogia sima | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Lagenodelphis hosei | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Megaptera novaeangliae | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Mesoplodon bidens | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Mesoplodon europaeus | 0 | 0 | 0 | 1 | 1 | 3 | 0 | 2 | 7 |
| Mesoplodon mirus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Phocoena phocoena | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Physeter macrocephalus | 0 | 1 | 1 | 2 | 0 | 11* | 0 | 1 | 16 |
| Pseudorca crassidens | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| Stenella coeruleoalba | 18* | 14 | 4* | 4 | 0 | 0 | 2 | 1 | 43 |
| Stenella frontalis | 6 | 16* | 2 | 4 | 1 | 0 | 4* | 3 | 36 |
| Steno bredanensis | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 4 |
| Tursiops truncatus | 2 | 9 | 0 | 4 | 0 | 0 | 0 | 2 | 17 |
| Ziphius cavirostris | 0 | 5 | 0 | 1 | 1 | 2 | 0 | 0 | 9 |
| Total | 49 | 70 | 13 | 37 | 5 | 24 | 10 | 16 | 224 |
PSLNS: pathology associated with significant loss of nutrional status; PGNS: pathology with good nutritional status; NPP: neonatal-perinatal pathology; IITI: intra-/interspecific traumatic interactions; FBAP: foreign body-associated pathology; VC: vessel collisions; IFA: interaction with fishing activities. ND: not determined.
*Species most affected in each category.
Table 2. Pathologic categories and etiologic diagnoses for cetaceans studied that stranded in the Canary Islands (2006–2012).
| Pathologic category | Etiologic diagnosis | No |
|---|---|---|
| Natural | ||
| Pathology associated with good nutritional status | Infectious (non-parasitic) disease | 42 |
| Parasitic disease | 20 | |
| Gas embolism | 2 | |
| Encephalopathy (unknown origin) | 1 | |
| Neoplasia | 1 | |
| Idiopathic hemorrhage | 1 | |
| Uterine rupture | 1 | |
| Senile changes | 1 | |
| Intestinal torsion | 1 | |
| Pathology associated with significant loss of nutritional status | Infectious (non-parasitic) disease | 33 |
| Parasitic disease | 13 | |
| Neoplasia | 1 | |
| Senile changes | 1 | |
| Trauma | 1 | |
| Intra- and interespecific traumatic interactions | Traumata, bite scars and/or tooth-rake marks | 36 |
| Gas embolism | 1 | |
| Neonatal or perinatal pathology | Fetal distress | 6 |
| Dystocia | 2 | |
| Infectious | 2 | |
| Neonatal weakness | 1 | |
| Abortion (prematurity) | 1 | |
| Developmental anomalies | 1 | |
| Anthropogenic | ||
| Vessel collision | Keel or propeller sharp trauma with/without blunt traumata | 24 |
| Interaction with fishing activities | Blunt trauma | 6 |
| Bycatch | 2 | |
| Fishing tool sharp trauma | 2 | |
| Foreign body-associated pathology | Foreign body ingestion | 4 |
| Foreign body entanglement (presumed floating net) | 1 | |
1. Natural pathologic categories
1.1 Pathology associated with good nutritional status
NPGNS involved 70/208 (33.6%) animals, representing 13 species (Table 1). The main morphologic diagnoses for NPGNS cases are recorded in S4 Table. Etiologic diagnoses identified were: infectious (60%), parasitic (28.6%), other (gas encephalopathy, idiopathic hemorrhage, uterine rupture; 5.7%), gas embolism (2.8%), neoplasia (1.4%), and senile changes (1.4%).
CeMV infection (Fig 1a) was confirmed via IHC and/or PCR analysis in six animals: two short-finned pilot whales (Globicephala macrorhynchus; no. 17 and 172), two striped dolphins (Stenella coeruleoalba; no. 152 and 202), one Atlantic spotted dolphin (Stenella frontalis; no. 126), and one short-beaked common dolphin (Delphinus delphis; no. 36). These animals often had multicentric lymphoid depletion. Furthermore, 14% (10/70) of NPGNS animals presented varying degrees of central nervous system (CNS) inflammation; however, the etiology was not apparent ‘unknown origin meningoencephalitides’.
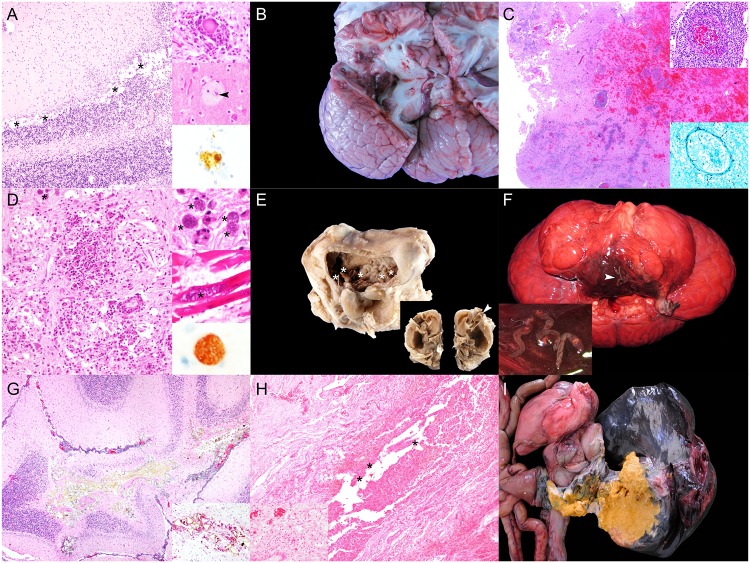
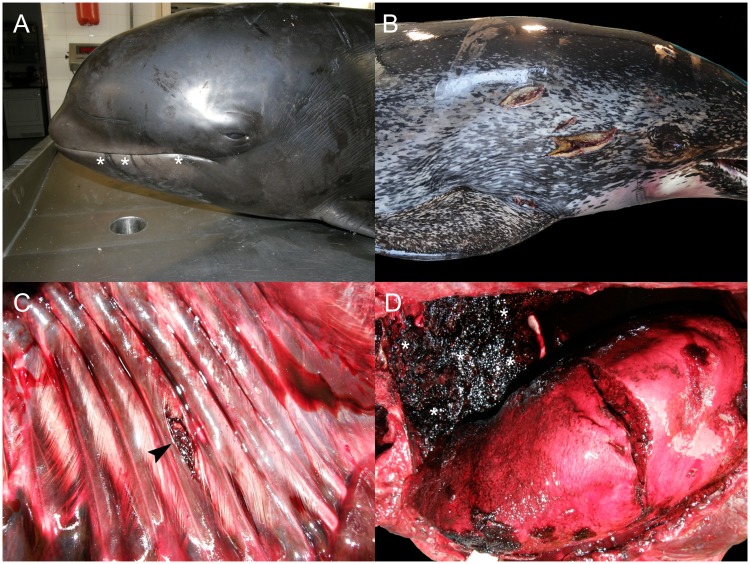
Fig 1. Panel of ‘natural pathologies associated with good nutritional status’ in cetaceans stranded in the Canary Islands (2006–2012).
A) Cetacean morbillivirus infection (animal no. 202; Stenella coeruleoalba). There is severe mononuclear inflammation at the cerebellar foliar grey and white matter interface with rarefaction at the Purkinje cell layer (asterisks). Hematoxylin and eosin (H&E). Right upper inset: focal neuronal degeneration and neuronophagia. H&E. Right middle inset: Swollen and chromatolytic cortical neuron with intranuclear eosinophilic inclusion body (arrowhead). Right lower inset: Degenerating neuron shows strong intranuclear and intracytoplasmic granular morbilliviral immunolabeling. Immunohistochemistry (IHC) for CDV. B) Aspergillus fumigatus encephalitis (animal no. 63; S. coeruleoalba). Multifocal to coalescing necrohemorrhagic encephalitis in the left cerebral hemisphere (asterisk). C) Aspergillus fumigatus encephalitis (animal no. 63; S. coeruleoalba). Severe pleocellular and necrohemorrhagic cerebral inflammation. H&E. Right upper inset: focal necrotizing vasculitis with hemorrhage and perivascular cuffing. H&E. Right lower inset: Necrotizing vasculitis with intralesional hyphae. Gomori Methenamine-Silver nitrate (GMS). D) Systemic toxoplasmosis (animal no. 46; S. frontalis). Severe lymphohistiocytic to granulomatous white matter inflammation with neuroparenchyma necrosis and rarefaction and rare T. gondii protozoal cysts (asterisk). H&E. Right upper inset: some protozoal cysts had no evident inflammation (asterisks). Right middle inset: T. gondii cysts within sarcoplasm of cardiomyocytes (asterisk). H&E. Right lower inset: cerebral protozoal cyst is strongly positive for anti-T. gondii antibody. IHC for T. gondii. E) Middle and inner ear nasitremiasis (animal no. 220; Tursiops truncatus). Lateral window (removed) of tympanic bulla (middle ear) after fixation and decalcification. There is marked proliferative otitis media with numerous intralesional adult Nasitrema sp. (asterisks). Inset: longitudinal section through the cochlea (inner ear). The cochlear nerve is swollen, dark and fragmented (arrowhead). F) Rhombencephalic nasitremiasis (animal no. 220; T. truncatus). Ventral surface of brainstem shows locally extensive necrotizing and hemorrhagic meningoencephalitis with intralesional adult Nasitrema sp. (arrowhead). Inset: detail of adult Nasitrema sp. G) Cerebellar nasitremiasis (animal no. 157; T. truncatus). Pyogranulomatous and necrotizing encephalitis with intralesional Nasitrema sp. eggs. H&E. Inset: Detail of pleocellular pyogranulomatous inflammation and Nasitrema sp. eggs. Periodic acid–Schiff (PAS). H) Inner ear nasitremiasis (animal no. 220; T. truncatus). Pyogranulomatous and necrotizing cochlear neuritis with intralesional Nasitrema sp. eggs (asterisks). H&E. Inset: endoneural lymphoplasmacytic inflammatory infiltrate in proximal cochlear nerve. H&E. I) Gastrohepatic abscess (animal no. 208; S. coeruleoalba). Severe transmural pyogranulomatous gastritis and hepatitis leading to large right hepatic lobe abscess.
Septicemia by E. rhusiopathiae was diagnosed in an Atlantic bottlenose dolphin and an Atlantic spotted dolphin (animal no.133 and 189) [17]. Septicemia by Streptococcus phocae was diagnosed in an adult female short-beaked common dolphin (animal no. 36) [26]. Fungal encephalitis by Aspergillus fumigatus (Fig 1b and 1c) was observed in a male calf striped dolphin (animal no. 63) stranded alive. PCR analyses for CeMV and HV in cerebrum were negative.
Four Atlantic spotted dolphins including three adults and one calf (animal no. 7, 46, 126 and 131) had toxoplasmosis. Most severe and extensive lesions in these cases were confined to the CNS (Fig 1d); however, there was also involvement of the heart, adrenal glands and gastrointestinal system in animal no. 46, 126 and 131 Severe arterial and renal crassicaudiasis was diagnosed in five Cuvier’s beaked whales (CBW; Ziphius cavirostris) (animal no. 92, 118, 180, 167 and 207) [22]. Severe pterygoid sinusitis by Nasitrema sp. with middle and inner ear involvement (Fig 1e) and extension to the CNS (Fig 1f and 1g) was observed in three Atlantic bottlenose dolphins (Tursiops truncatus) (animal no. 157, 220 and 224) and one short-beaked common dolphin (animal no. 195). Inflammatory changes included pyogranulomatous and necrotizing meningoencephalomyelitis, meningomyelitis and vestibulocochlear neuritis (Fig 1h) with intralesional adult and trematode ova, spongiosis, vascular necrosis and vasculitis. Animal no. 224 also presented intralesional Gram-positive bacilli; bacterial culture of cerebrum yielded Clostridium sp.
An adult, female striped dolphin (animal no. 208) presented a large hepatic abscess [analogous to case 134, NPSLNS], firmly adhered to the pyloric stomach wall (Fig 1i). The abscess ruptured and led to septic peritonitis. Severe parasitization by Brachycladium atlanticum and Pholeter gastrophilus in the biliary tract and the pyloric stomach, respectively, were associated with the ruptured abscess and adjacent inflamed areas. Severe Bolbosoma sp. intestinal parasitization was noted in an adult female sei whale (Balaenoptera borealis) (animal no. 188). Numerous acanthocephalans were embedded in the submucosa and led to complete luminal obliteration and hemorrhage. Peritoneal migrating Bolbosoma sp. were common. Similarly, severe Bolbosoma sp. parasitization was observed in a false killer whale (Pseudorca crassidens) (animal no. 192); however, hemorrhage was not a major component. Severe Crassicauda sp. parasitization affecting the cervical and thoracic subcutis, fascia, and muscle, the cervical gland and the rete mirabile was determined in an adult female pigmy sperm whale (Kogia breviceps) (animal no. 198). Additionally, Crassicauda sp. was associated with massive urethral infestation in an Atlantic spotted dolphin (animal no. 83).
Fatal gas embolism was diagnosed in two animals (animal no.147 and 163) [27,28]. A thalamic high-grade astrocytoma (glioblastoma multiforme) was diagnosed in an adult male Atlantic spotted dolphin (animal no. 50) [29]. A live-stranded adult male pigmy sperm whale (animal no. 56), evidenced severe spongiosis of the neuroparenchyma, mainly in the brainstem, thalamus and cerebral white matter. Other microscopic changes in these areas were axonal spheroids, cytotoxic and perivascular edema, astrocytosis, Alzheimer type II-like astrocytes, Gitter cells and scattered neuronal satellitosis in the cerebral cortex and thalamus. These changes were bilateral and asymmetrical. No demyelination or axonal anomalies were observed by means of luxol fast blue and Bielchowsky’s stains, respectively. Other unusual etiologic diagnoses associated with single deaths included uterine rupture (animal no. 191) and segmental intestinal torsion with mesenteric venous infarction, intestinal ischemic necrosis and luminal hemorrhage (animal no. 65).
1.2 Pathology associated with significant loss of nutritional status
NPSLNS involved 49/208 (23.5%) animals, representing 9 species (Table 1). The main morphologic diagnoses for NPSLNS cases are recorded in S5 Table. Etiologic diagnoses identified were: infectious (69.4%), parasitic (26.5%), neoplasia (2.0%), and senile changes (2.0%).
Among infectious disease processes, the CNS was commonly targeted. Viral agents identified were CeMV and HV, as determined by IHC and/or PCR analysis. Five odontocetes of three species: three striped dolphins (animal no. 43, 61 and 97), one Risso’s dolphins (Grampus griseus) (animal no. 77), and one short-finned pilot whale (animal no. 183) presented positive immunolabeling and/or a positive PCR result for CeMV. Cerebral HV infection (Fig 2a) was diagnosed in an adult male striped dolphin (animal no. 165) and confirmed by PCR [19]. Additionally, Mycoplasma sp. was isolated from the atlanto-occipital joint, which displayed severe, chronic osteoarthritis (Fig 2b). About 30.6% (15/49) of the NPSLNS were meningoencephalitides of undetermined etiology.
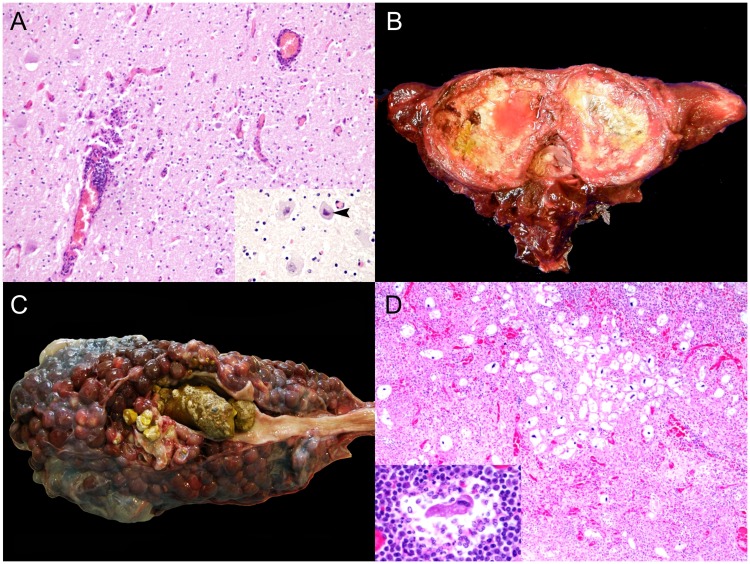
Fig 2. Panel of natural pathologies associated with loss of nutritional status in cetaceans stranded in the Canary Islands (2006–2012).
A) Cerebral herpesvirus infection (animal no. 165; S. coeruleoalba). Lymphocytic encephalitis with perivascular cuffing and gliosis. H&E. Inset: Focal eosinophilic intranuclear inclusion body (arrowhead). H&E. B) Atlanto-occipital osteoarthritis (animal no. 165; S. coeruleoalba). Marked, chronic osteoarthritis and synovitis with cartilage loss at the cranial articular facets of atlas vertebra. C) Nephrolithiasis (animal no. 127; Delphinus delphis). Severe nephrolithiasis with hydronephrosis, proximal hydroureter and chronic renicular disease with atrophy and hypertrophy. D) Ciliate protozoal lymphadenitis (animal no. 94; S. bredanensis). Pyogranulomatous and necrotizing prescapular lymphadenitis with numerous intralesional ciliate protozoan. H&E. Inset: Detail of ciliate protozoon surrounded by neutrophils and necrotic cell debris, and lymphocytes. H&E.
A juvenile female rough-toothed dolphin (Steno bredanensis) (animal no. 68) presented ulcerative glossitis, palatitis, esophagitis and gastritis, and dermatitis with scattered eosinophilic intranuclear inclusion bodies, highly suggestive of HV infection. Numerous intravascular coccobacilli, leukocytosis and disseminated intravascular coagulation were also noted. Bacteriologic cultures yielded Pseudomonas taetrolens (liver, brain); Aeromonas hydrophila/caviae (kidney, lung); P. putida (lung, brain); and A. salmonicida (brain). Wohlfahrtiimonas chitiniclastica septicemia was diagnosed in an adult male short-beaked common dolphin (animal no. 213) [30]. Pseudomonas aeruginosa was isolated from renal tissue in an adult male short-beaked common dolphin (animal no. 127) with severe nephrolithiasis (Fig 2c) and ureterolithiasis. Microscopically, there was suppurative pyelonephritis with tubular necrosis and Gram-negative bacilli, ischemic necrosis (infarcts), interstitial fibrosis and tubular proteinosis. Additionally, mild multifocal lymphocytic meningitis with occasional intravascular Gram-negative bacilli and lymphoplasmacytic cortical adrenalitis were seen.
Other interesting cases with severe and extensive lesions suggestive of systemic infectious disease albeit without confirmed etiologies involved an adult pigmy sperm whale (animal no. 6), an adult female striped dolphin (animal no. 54), and an adult male striped dolphin (animal no. 110). All these animals presented septic peritonitis. The first presented a large hepatic abscess that ruptured and led to peritonitis and hemoabdomen. An incidental hepatocellular carcinoma was also observed. The second animal had septic peritonitis due to a perforating ulcer in the keratinized stomach. The third animal presented peritonitis along with pyothorax/empyema, fibrinosuppurative pleuropneumonia and pericarditis.
Disseminated toxoplasmosis was observed in a female calf Atlantic spotted dolphin (animal no. 96). An adult male short-beaked common dolphin (animal no. 45) and a subadult male common bottlenose dolphin (animal no. 184), presented with severe and extensive pterygoid sinusitis, otitis and meningoencephalitis due to Nasitrema sp. trematodes. Nasitrema delphini [31] was identified in animal no. 184. Animal no. 94 had multisystemic ciliate protozoosis characterized by multifocal pyogranulomatous and ulcerative dermatitis and panniculitis with marked necrosis, hemorrhage and numerous ciliated protozoa; multicentric pyogranulomatous lymphadenitis with ciliated protozoa (Fig 2d); and histiocytic and neutrophilic encephalitis with necrosis and hemorrhage.
A primary uterine T cell lymphoma with disseminated metastasis was observed in an adult female Atlantic spotted dolphin (animal no. 49) [32].
1.3 Neonatal/Perinatal pathology
NPP was determined in 13/208 (6.2%), representing 7 species (Table 1). The main morphologic diagnoses for animals included in this category are shown in S6 Table. Etiologic diagnoses identified were: fetal distress (46%), dystocia (15%), infectious (15%), abortion (7.7%), congenital malformation (7.7%), and neonatal maternal-filial separation/maternal neglect; 7.7%.
All animals with presumed fetal distress (animal no. 21, 23, 29, 38, 121 and 176) had moderate to severe pulmonary edema (Fig 3a and 3b) with individualized intraalveolar, intrabronchiolar and intrabronchial squames or most often large aggregates of aspirated keratinized stratified epithelium with retained nuclei. Other common findings were diffuse pulmonary atelectasis with alternate areas of emphysema, bronchodilation, bronchiolar constriction, and intraalveolar foamy macrophages. Animal no. 23 had bronchointerstitial pneumonia with intraalveolar and intrabronchiolar meconium along with squames and alveolar histiocytosis. Animal no. 121 also presented alveolar proteinosis and hyaline membranes. Animal no. 88, with presumed dystocia, had multifocal extensive areas of congestion and hemorrhage, lineal to circumferential, typically in the subcutis of the mandibular and cranial regions, bilaterally in the thoracolumbar area, the pectoral flippers and the abdominal wall.
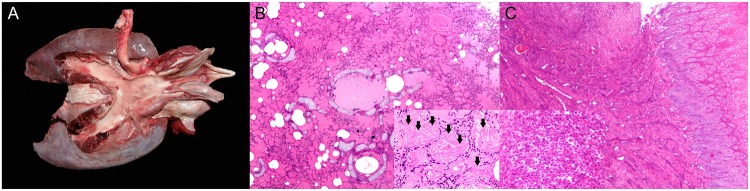
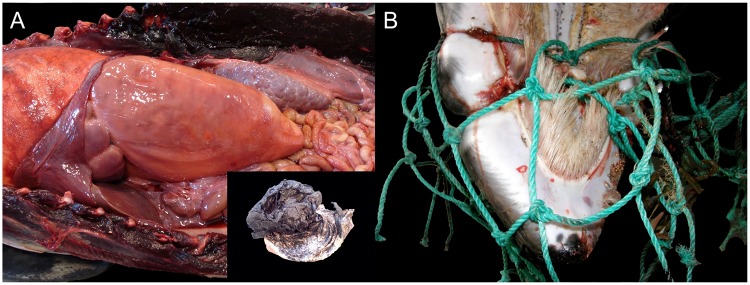
Fig 3. Panel of neonatal/perinatal pathologies in cetaceans stranded in the Canary Islands (2006–2012).
A) Pulmonary edema (animal no. 132; Balaenoptera acutorostrata). The trachea, mainstem bronchi and primary bronchi are filled with abundant frothy fluid. B) Pulmonary edema (animal no. 21; Stenella frontalis). The bronchioles and alveoli are diffusely filled by abundant proteinaceous fluid. Inset: numerous keratin squames (arrows) fill in the alveoli. H&E. C) Omphaloarteritis (animal no. 213; Kogia breviceps). The umbilical margin is infiltrated by abundant pleocellular suppurative inflammatory cells and fibrin. H&E. Inset: Numerous viable and degenerate neutrophils with macrophages, edema, fibrin and necrotic cell debris. H&E.
Several animals in this group had evidence of neonatal/perinatal infection. Animal no. 48, a presumably premature short-finned pilot whale (total body length: 130 cm-long; reference range: 140–185 cm) [33] presented findings suggestive of sepsis (multiorgan intravascular bacteria including kidney, lung, pulmonary and prescapular lymph node, spleen and brain, and hemorrhage) and fetal distress. A calf pygmy sperm whale (animal no. 213) presented with fibrinosuppurative omphaloarteritis and phlebitis (Fig 3c), neutrophilic omentitis, and pulmonary edema and hemorrhage. The etiology in these cases was unknown, but bacterial infection was suspected. No cultures were attempted. Additionally, animal no. 30, a striped dolphin calf had several congenital malformations including segmental intestinal atresia and spinal meningocele; however, autolysis limited detailed microscopic examination. There was also lack of development of multiple cranial bones and incomplete closure of fontanels.
1.4 Intra- and interspecific traumatic interactions
IITI involved 37/208 (17.8%) animals, representing 13 species (Table 1). The main morphologic and etiologic diagnoses for IITI cases are recorded in S7 Table.
Consistent trauma-associated findings in these animals were focally extensive to suffusive hemorrhage, hematomas and myonecrosis (Fig 4a), mostly in the subcutis and axial musculature, but also in internal viscera. Tooth rakes-associated erosions and lacerations were often associated with above vascular changes. Other common findings were fractures of the axial skeleton and intracavitary and visceral hemorrhage. Pulmonary fat emboli and myo-/hemoglobinuric nephrosis were occasionally observed. In some of these cases, underlying inflammatory infectious disease was observed, for instance, meningoencephalomyelitis and proliferative and sclerosing bronchitis (animal no. 123 and 206), disseminated toxoplasmosis (animal no.135), and severe arterial and renal crassicaudiasis (animal no. 211). Furthermore, two unusual interspecific traumatic events were seen. In the first case, a juvenile male false killer whale (animal no. 64) in poor body condition presented severe, focal, chronic perforating glossitis and stomatitis with an intralesional stingray spine (Fig 4b). The second case involved a male adult Risso’s dolphin with systemic gas embolism associated with complicated predatory attempts on squids [28].
Fig 4. Panel of pathologies associated with fatal intra- and interspecific interactions in cetaceans stranded in the Canary Islands (2006–2012).
A) Subcutaneous hemorrhage (animal no. 221; Mesoplodon mirus). The subcutis and suprascapular musculature is focally expanded by hemorrhage. B) Traumatic glossitis (animal no. 64; Pseudorca crassidens). Full-thickness perforating traumatic glossitis with intralesional stingray spine (asterisks).
2. Anthropogenic pathologic categories
2.1 Interaction with fishing activities
Fatal IFA involved 10/208 (4.8%) animals, representing 6 species (Table 1). The main morphologic diagnoses in IFA cases are recorded in S8 Table. Etiologic diagnoses identified were: blunt trauma (60%), fishing tool (e.g., harpoon, hook, palangre) sharp trauma (20%) and bycatch (20%).
Bycatch-associated lesions included linear and perpendicular superficial cutaneous erosions and lacerations around the rostrum and gingiva in a neonate female short-fined pilot whale (animal no. 57; Fig 5a), and lacerations in the rostrum, forehead and ventrocaudal aspect of the body along with ulcers on the buccal commissures in an adult female striped dolphin (animal no. 194). Blunt trauma with subcutaneous and cranioencephalic contusions, fractures and hemorrhage were noted in several individuals (animal no. 34, 37 and 71). Furthermore, lesions presumably linked to release from fishing nets were observed in animal no. 34 (Fig 5b). Multiple cutaneous penetrating wounds with right lung perforation and hemothorax were observed in animal no. 66 (Fig 5c and 5d). An Atlantic spotted dolphin (animal no.199) had necrotic stomatitis and mandibular osteomyelitis by perforating hook in addition to multiple cutaneous incisions of presumptive anthropogenic origin.
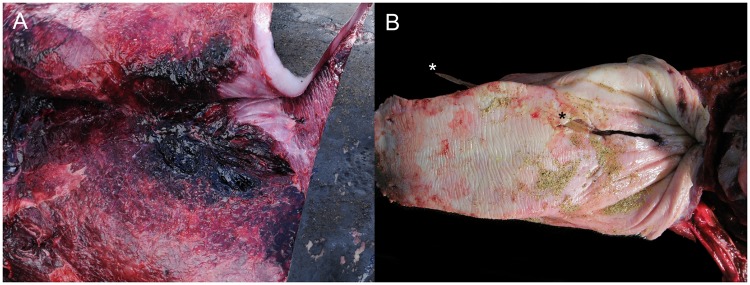
Fig 5. Panel of pathologies associated with fatal interaction with fishing activities in cetaceans stranded in the Canary Islands (2006–2012).
A) Fishing net markings (animal no. 57; Globicephala macrorhynchus). The left mouth commissure and adjacent mandibular skin has multiple, linear, parallel erosions and lacerations due to net entanglement (asterisks). B) Anthropogenic fishing-related incisive wounds (animal no. 199; S. frontalis). There are two deep incised skin cuts on the right suprascapular region. C) Thoracic wall perforation (animal no. 66; S. frontalis). There is a focal mid-diaphyseal rib fracture with muscle tearing and hemorrhage (arrowhead) on the left thoracic wall. D) Lung perforation (animal no. 66; S. frontalis). The left lung is focally perforated and lacerated and there is abundant hemothorax (asterisks) (there is some freezing artifact).
2.2 Foreign body-associated pathology
FBAP was determined in 5/208 (2.4%) animals, representing 5 species (Table 1). From these, four presented profound emaciation while one of the animals was in good nutritional status. The main morphologic diagnoses in animals included in this category are recorded in S9 Table. Foreign bodies were associated with gastric obstruction and impaction (60%), gastric perforation (20%), and chronic entanglement (20%).
In a juvenile female Gervais’ beaked whale (Mesoplodon europaeus) (animal no. 13), gastric perforation by a linear foreign body, i.e., coiled wire, led to septic fibrinosuppurative peritonitis and death. In a juvenile Cuvier’s beaked whale (animal no. 19) and an adult Risso’s dolphin (animal no. 219), a large number of plastics including packing tape, trash bags, and a rope, respectively, had lodged and severely impacted the keratinized gastric compartment. Luminal gastrointestinal hemorrhage was noted in the first animal, whereas the second had multifocal non-bleeding gastric ulcers. An adult female Atlantic spotted dolphin (animal no. 27) presented ulcerative and hemorrhagic gastritis associated with gastric impaction by 20 plastic bags mixed with abundant melena (Fig 6a). Animal no. 107, a common minke whale calf had profound cachexia associated with chronic entanglement linked to fishing net (Fig 6b).
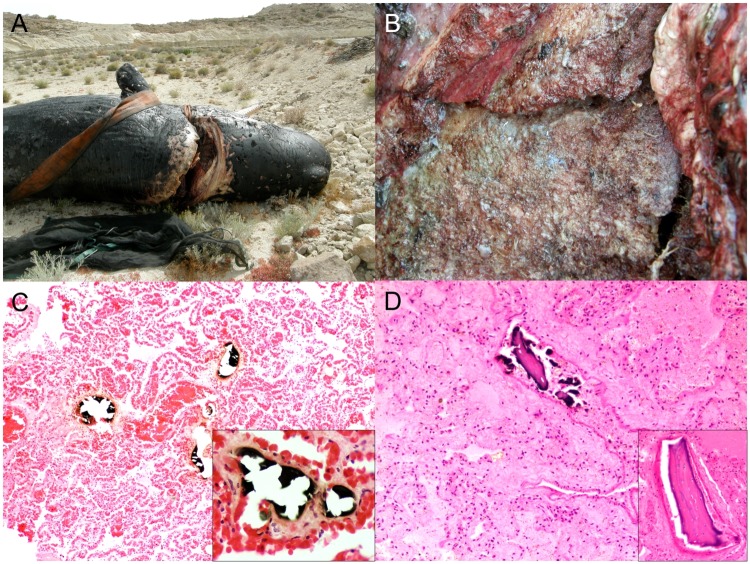
Fig 6. Panel of foreign body-associated pathologies in cetaceans stranded in the Canary Islands (2006–2012).
A) Gastric impaction (animal no. 27; Stenella frontalis). The keratinized stomach is filled with abundant plastics. Inset: detail of plastic mass mixed melena. B) Chronic entanglement (animal no. 42; Balaenoptera acutorostrata). The fishing net had lodged deeply around the maxilla leading to soft tissue constrictive necrosis, periostitis and osteomyelitis (not visible in the image) and exuberant granulation tissue.
2.3 Vessel collisions
VC involved 24/208 (11.5%) animals, representing 8 species (Table 1). The main morphologic diagnoses in this category are recorded in S10 Table. The etiologic diagnosis in these animals largely consisted of trauma, sharp or blunt, and was typically severe. Main findings included: cutaneous lacerations with muscle tearing; linear body sectioning of varying depth with exposure of viscera (thoracic, abdominal) and eventration (animal no. 15, 32, 74, 103, 104, 142, 150 and 216); single and multiple costal soft and bone tissue loss; evisceration (animal no. 3 and 200); cranioencephalic trauma (animal no. 10); and partial (animal no. 215) or complete (amputation) sectioning of the vertebral column (Fig 7a and 7b). Pulmonary and/or systemic fat embolism (Fig 7c) was observed in animal no. 10, 32, 74, 124 and 142. Animal no. 60 had multiple intravascular pulmonary osseous emboli (Fig 7d). Other findings included: hemothorax, hemoabdomen; and left pneumothorax with focal coronary arterial thrombosis (animal no. 200). Some animals had relevant coexisting diseases: animal no. 212 had multifocal pulmonary thromboembolism with intralesional Crassicauda sp. nematodes, chronic mesenteric arteritis and granulomatous nephritis with intralesional Crassicauda sp. nematodes; and a calf sperm whale (animal no. 215) had mycotic pneumonia.
Fig 7. Panel of vessel collision-associated pathologic findings in cetaceans stranded in the Canary Islands (2006–2012).
A) Cervico-occipital fracture (animal no. 142; Physeter macrocephalus). There is a single, well-demarcated, deep incisive cut through the dorsal occipital region and the atlas vertebra. B) Cervico-occipital fracture (animal no. 142; P. macrocephalus). Multiple bone fracture surfaces are stained with blue antifouling paint from the vessel cutting edges (presumably the keel). C) Fat embolism (animal no. 86; Kogia breviceps). The microvasculature of the lung parenchyma, mainly alveolar capillaries, contains multiple osmium-tetroxyde-positive (black) fat emboli. OsO₄ (postfixation technique) and H&E. Inset (animal no. 86; K. breviceps): fat embolus obliterates and expands the vascular lumen. OsO₄ (postfixation technique) and H&E. D) Pulmonary bone emboli (animal no. 60; K. breviceps). There are multiple osseous emboli in the pulmonary microvasculature. H&E. Inset: Embolic osseous fragments consists of lamellar (mature) bone with multiple presumably viable osteocytes in lacunae. H&E.
3. Active stranding pathology
A total of 35/224 (15.6%) animals stranded alive, representing 11 species (Table 1). Gross lesions suggestive of live-stranding were: superficial well-demarcated or irregular linear cutaneous erosions and lacerations of variable extent, mainly in the rostrum, the ventral aspect of the head, neck, thorax, abdomen, flanks and pectoral fins and tail fluke (Fig 8a); hemorrhage associated with myodegeneration, mainly in the rectus abdominis; multifocal pale foci in the myocardium; and systemic congestion, edema and petechiae in the brain, lungs, adrenal glands, liver, kidney and gastrointestinal tract. Classic microscopic findings of varying severity and extent were identified: mild to marked, multifocal, acute monophasic segmental skeletal (Fig 8b) and cardiac myodegeneration with contraction band necrosis, vacuolation and edema (Fig 8c); cytoplasmic eosinophilic globules within hepatocytes and ‘pink points’ (comprising acute phase proteins; Fig 8d) [34]; systemic and subendocardial and myocardial hemorrhage along with generalized congestion; acute adrenocortical degeneration and necrosis; and acute renal tubular degeneration and necrosis (Fig 8e) with or without myoglobin casts. The above findings often featured pre-existing conditions that were generally thought as the cause of stranding; CNS inflammation predominated in these cases (51.4%; 18/35).
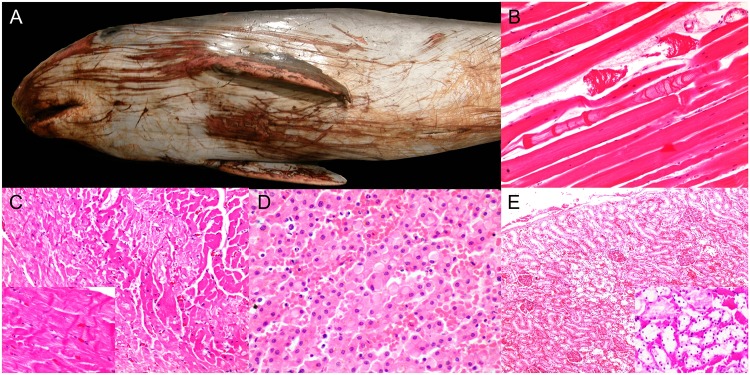
Fig 8. Panel of pathologic findings associated with active stranding in cetaceans stranded in the Canary Islands (2006–2012).
A) Cutaneous erosions, lacerations and abrasions (animal no. 56; Kogia breviceps). There are multiple, longitudinal and somewhat parallel skin erosions, lacerations and abrasions with hemorrhage linked to live-stranding in a mildly abrupt substrate. B) Myodegeneration (animal no. 9; Mesoplodon europaeus). There are multifocal acutely degenerating myofibers showing variable patterns of segmental sarcoplasmic loss and edema. H&E. C) Cardiomyocyte degeneration (animal no. 56; K. breviceps). Multifocally, cardiomyocytes are swollen, hypercontracted and hypereosinophilic or pale and vacuolated. Inset: cardiomyocyte contraction band necrosis. H&E. D) Hepatocytic globules (animal no. 24; Globicephala macrorhynchus). Hepatocytes are multifocally expanded by intracytoplasmic, lightly eosinophilic single vacuoles with occasional pink points or strands. H&E. E) Tubulonephrosis (animal no. 138; Grampus griseus). Multifocally, the renal cortex has foci of pallor and tubular disruption. H&E. Inset: Multiple proximal convoluted tubules have degenerating, necrotic and sloughed epithelial cells and there is marked congestion. H&E.
Discussion
Long-term pathology-based health monitoring of cetacean populations involves arduous, complex and demanding research and logistics. These pathologic investigations have obvious biases and limitations, e.g., the examined animals are not fully representative of the population, advanced decomposition of corpses at necropsy [35], the gain of scientific knowledge and associated outcomes exceed and justify the troubles. Furthermore, since the determination of cause of death often relies, to a certain extent, on the professional judgment of the supervising pathologist (from field necropsy to final report issuing in best case scenarios), interstudy variations in the criteria to assign causes of mortality are likely to occur [35].
Stranding epidemiology
From January 2006 to December 2012, 320 cetaceans including 22 species stranded along the coastline of the Canary Islands. Postmortem examinations were performed on 70% of the animals, including 21 species (four mysticetes and 17 odontocetes). A previous study in the area (1999–2005) recorded 19 stranded cetacean species [12]. The variety of species recorded in both studies was similar, however, the common minke whale (Balaenoptera acutorostrata), Sowerby’s beaked whale (Mesoplodon bidens), harbor porpoise (Phocoena phocoena), and false killer whale were not recorded in the previous study. By contrast, the current study did not include Blainville’s beaked whale (M. densirostris) and spinner dolphin (Stenella longirostris) [12]. A cause for such subtle species differences is not apparent; further evaluation is precluded by the small number of individuals for those species.
Compared to 1999–2005 period [12], in the present study, the sex distribution of animals studied was more homogeneous (58 females, 79 males, 1 undetermined vs 105 females, 109 males, 10 undetermined); no sex-biased stranding tendency was apparent. Regarding age, we observed that mortality by species (e.g., short-beaked common dolphin, sperm whale [Physeter macrocephalus], pygmy sperm whale, striped dolphin, and Atlantic spotted dolphin) was high in young animals, decreasing in mature ones and finally increasing in aged ones [12,36,37]. In agreement with previous observations [12], stranding events occurred most often along the eastern coasts of the oriental islands. The yearly average of strandings (n = 45.7) during 2006–2012 was slightly higher than the period 1999–2005 (n = 39) yet peak months (March, April and May) were identical to those previously reported [12]. This small difference is likely not statistically significant.
The most probable CD was identified or inferred in 98.9% (91/92) ‘fresh’ and ‘very fresh’, 96.7% (59/61) ‘moderate autolysis’ and 81.7% (59/71) ‘advanced’ ‘very advanced autolysis’ carcasses. These results attested to the value of postmortem examinations in all animals regardless their decomposition status [12]; however, cautious interpretation of findings is mandatory in these cases to avoid overinterpretations.
1. Natural pathologic categories
1.1 Pathology associated with good nutritional status
Etiologic diagnoses in NPGNS cases were: infectious (60%), parasitic (28.6%), other (e.g., gas encephalopathy, idiopathic hemorrhage, uterine rupture; 5.7%); gas embolism (2.8%), neoplasia (1.4%) and senile changes (1.4%). Our findings are somewhat similar to those previously observed in the area [12]. CeMV infection was confirmed in six animals; microscopic findings in these animals were in agreement with previous reports [38,39,40,41]. One of these animals had concomitant septicemia by S. phocae [26]. Lymphocytic meningoencephalitis in any odontocete species worldwide, particularly striped and bottlenose dolphins should prompt CeMV investigation [42]. Although IHC and PCR were not simultaneously performed with histologic examinations in a previous study in the Canary archipelago [12], retrospective IHC and PCR investigations confirmed CeMV infection in 36.8% previous ‘unknown origin meningoencephalitis’ cases [19]. Further studies to identify potential etiologic agents in cases of undetermined meningoencephalitis are warranted.
E. rhusiopathiae was isolated in an Atlantic bottlenose dolphin and an Atlantic spotted dolphin [17]. In both animals, lesions were suggestive of an acute septicemia [43,44,45,46,47]. Primary fungal meningoencephalitis by A. fumigatus was diagnosed in a striped dolphin calf. CNS infection by Aspergillus sp. has been recognized in a northern bottlenose whale (Hyperoodon ampullatus) [48], a harbor porpoise [49], three striped dolphins and a bottlenose dolphin co-infected by CeMV [38,50]. In this case, there was no evidence of systemic fungal infection and concomitant disease processes. CeMV and HV PCR analysis were negative. Four striped dolphins had toxoplasmosis agreeing with high prevalences for this species in the area [12]. The pathologic findings observed were similar to encephalitic and systemic presentations reported in different hosts and geographic locations [51].
Severe arterial and renal crassicaudiasis was observed in five CBW in this group [22]. Crassicauda magna was identified in two of them. Severe arterial lesions could result in fatalities from disseminated intravascular coagulation, multisystemic failure and severe renal damage, or major aggravation of coexisting disease [22]. Severe pterygoid sinusitis by Nasitrema sp. with middle and inner ear involvement and extension to the brain was observed in three common bottlenose dolphins and one short-beaked common dolphin. Gross and histologic findings were in agreement with previous reports [52,53,54,55,56,57,58,59]. An adult female striped dolphin had a large hepatic abscess firmly adhered to the pyloric compartment that ruptured and led to septic peritonitis. This process was associated with severe parasitism by Brachycladium atlanticum and P. gastrophilus. To the authors’ knowledge, this is a novel fatal outcome of severe digestive parasitism in cetaceans. Severe Bolbosoma sp. intestinal parasitism likely led to death in an adult female sei whale and a false killer whale. Different acanthocephalan species of genus Corynosoma and Bolbosoma parasitize the intestine of many odontocete and mysticete cetaceans [21,60,61,62,63]. All acanthocephalans are potentially pathogenic, as a result of their proboscids that penetrates the intestinal wall and may lead to peritonitis and severe disease [64]. Bolbosoma capitatum has been involved in mortalities of false killer whales due to intestinal obstruction, hemorrhagic enteritis, serositis (peritonitis) and severe anemia [65].
Severe Crassicauda sp. parasitization affecting the cervical and thoracic subcutis, fascia, and muscle, the cervical gland, and the rete mirabile was seen in an adult female pigmy sperm whale. These findings supported previous observations in which C. magna was identified in the cervico-thoracic subcutis and vascular plexuses that supply the CNS and other thoracic organs [66,67]. We believe severe Crassicauda sp. parasitization of the rete mirabile may contribute to severe vascular dysfunction. Severe urethral crassicaudiasis was diagnosed in an Atlantic spotted dolphin, recapitulating findings observed in baleen whales with urethral C. boopis infestation [68,69,70] and Crassicauda spp. in the lower urinary tract of other cetacean species, leading to partial or complete luminal obstruction [71,72].
Two animals presented lesions compatible with ‘gas embolism’ [73,74]. Two similar cases involving old Cuvier’s beaked whales with less pronounced hemorrhages were previously identified in the Canary archipelago [12]. Although gas embolism was linked to stranded ziphiids during military maneuvers and predation-related outcomes [28,73]; it is likely that gas embolism may be triggered by other non-sound-related sources, and individual factors such as age, health status and behavior, may be associated with this condition [28].
A glioblastoma multiforme was observed in an adult male Atlantic spotted dolphin [29]. CNS neoplasia is rare in cetaceans [75,76,77,78,79,80,81]. A live-stranded adult male pigmy sperm whale had CNS pathology suggestive of spongy myelinopathy with features resembling those observed in ammonia toxicity, endogenous metabolic toxicity (hepatic and renal encephalopathy), mitochondrial and idiopathic encephalopathies [82]. Nonetheless, the etiology in this case remains unknown.
Other uncommon causes of stranding and death were observed in this group. Idiopathic uterine rupture leading to hemoperitoneum and hypovolemic shock was seen in an adult female short-beaked common dolphin. In this case, the presence of a large post-gestational corpus albicans and marked dilatation of the ruptured uterine horn, suggested peri- or post-partum rupture. Uterine rupture was observed in an adult female Risso’s dolphin with an intraabdominal mummified fetus [83]. Focal intestinal torsion with strangulation and venous infarction led to fibrinous peritonitis and subsequent septic shock in an adult male Atlantic spotted dolphin, recapitulating features in previous reports [84]. Although uncertain, intestinal parasitism by Bolbosoma sp. might have been a predisposing factor in this case.
1.2 Pathology associated with significant loss of nutritional status
Etiologic diagnoses were infectious (69.4%), parasitic (26.5%), senile changes (2%) and neoplasia (2%). Most individuals (29/33, 87.9%) with evidence of infectious disease had high endo- and ectoparasitic burdens. Our results suggest an overall higher number of deaths associated with infectious diseases (39/138; 28% vs 75/224, 33.5%), and lower age-related (14/138, 10.1% vs 2/224, 0.9%) morbidity and mortality than a previous study in the area [12]. Comparison with other small or large-scale pathology-based studies is somewhat troublesome, since a standardized etiopathological classification scheme was not followed. For instance, some studies reported only bacterial infections [85], and others considered viral, bacterial, fungal and parasitic processes altogether [4]. Nonetheless, when most studies from other geographical areas are scrutinized our data show similar or higher prevalence of infectious disease [4,12,79,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109].
CeMV was detected by IHC and/or PCR in 10 NPSLNS cases representing five species. The chronic systemic and the chronic CNS-localized presentations predominated among animals in this group [42]. No characteristic eosinophilic intranuclear and intracytoplasmic inclusion bodies were observed in these animals. Herpesviral encephalitis was diagnosed in an adult male striped dolphin. Additionally, Mycoplasma sp. was isolated from chronic atlanto-occipital osteoarthritis in this animal. This case represents the first coinfection by Herpesvirus and Mycoplasma sp.in a cetacean species. Furthermore, about 30.6% (15/49) of NPSLNS cases had some degree of CNS inflammation of unknown etiology.
A juvenile female rough-toothed dolphin presented pathologic findings suggestive of upper digestive herpesviral disease along with bacterial septicemia, involving Aeromonas sp. and Pseudomonas sp. Fatal herpesviral systemic infections have been documented in several cetacean species, generally involving α-herpesvirus [110,111,112,113,114]. The individual pathogenic role of each bacteria is uncertain yet both genera have been linked to disease in cetaceans [2,106,115]. W. chitiniclastica septicemia was diagnosed in an adult male short-beaked common dolphin. This was the first description for this emerging human pathogen in a marine mammal species [30]. This agent had been linked to fatal septicemia in two human patients [116,117] and a white-tailed deer (Odocoileus virginianus) [118]. P. aeruginosa was associated with severe nephrolithiasis and ureterolithiasis with unilateral hydronephrosis and hydroureter, suppurative pyelonephritis and renal infarcts in an adult male short-beaked common dolphin. Additional histopathologic evidence (i.e., multiorgan leukocytosis, meningitis with intravascular bacteria, adrenalitis and mesenteric lymph node sinus histiocytosis with bacteria) supported bacterial septicemia; however, no bacteria were grown from liver, lung and brain tissue. P. aeruginosa has been previously linked to pyelonephritis [119] and as a primary pathogen in lower urinary tract infections [120]. Moreover, several species of Pseudomonas have been implicated in localized and systemic infections in cetaceans [2,106]. In the present case, P. aeruginosa infection could have played a role in chronic renal insufficiency and could explain additional systemic findings.
We observed other NPSLNS cases characterized by systemic infectious inflammatory disease of unknown etiology involving peritonitis in an adult male pigmy sperm whale, an adult female and a male striped dolphin. In the first, a hepatic abscess was associated with peritonitis and hemoabdomen. Hepatic changes included multiple infarcts, thrombosis and hepatocellular carcinoma. The later was likely incidental. The only other report of this neoplasm in a cetacean involved beluga whales [121]. A bacterial cause for hepatic abscesses is suspected albeit it remains unknown. In the second case, a perforating ulcer in the keratinized compartment led to septic peritonitis; however, its etiology remains unknown. Infectious or not, areas of gastric erosion and ulceration may perforate and lead to peritonitis [103,122,123,124]. The third case had fibrinosuppurative peritonitis, pyothorax, pleuropneumonia and pericarditis with intralesional bacteria. Compelling evidences support a bacterial septicemia; however, etiologic confirmation was not attained because no bacterial culture was performed in these cases.
Disseminated toxoplasmosis was determined in an Atlantic spotted dolphin calf. Gross and histologic findings were similar to those reported in many other cetacean species [51]. In this case, transplacental infection was highly suspected [125]. Risk factors for toxoplasmosis in cetaceans may include coinfection with CeMV [126], a low genetic diversity [127] and a coastal habitat [128]. Other potentially fatal parasitosis in this group involved severe Nasitrema sp. pterygoid sinusitis and otitis media and otitis interna with brain invasion [52,53,54,55,56,57,58,59]. Nasitrema delphini [31] was identified in one of the animals. We believe the severity and extent of these lesions could explain the stranding and death in both individuals. Systemic ciliated protozoosis was determined in an adult male rough-toothed dolphin, recapitulating lesions similar to previous reports [12,71,129,130,131]. Descriptions of ciliated protozoa in cetaceans include Haematophagus megapterae in humpback whales, fin whales and blue whales; Kyaroikeus cetarius, K. cetarius-like, Planilamina ovata, P. magna, Balantidium spp., and unidentified ciliates in mucosae and feces of several odontocetes and mysticetes worldwide [51]. These microorganisms are considered opportunistic, often concomitant in CeMV-infected hosts, and their pathogenicity is largely unknown. Systemic spread is not uncommon [51]. In the present case, PCR analysis for CeMV was negative. Additionally, a juvenile male short-finned pilot whale also had localized cutaneous and oral ciliate protozoal infestation. Parasitism as a cause of stranding and/or death has been widely debated [107,129,132,133]. This study corroborates the high occurrence of certain endoparasitoses and provides compelling evidence on the lethal potential for arterial and renal crassicaudiasis, pterygoid and CNS nasitremiasis, CNS and/or disseminated toxoplasmosis, hepatic brachycladiasis and urethral crassicaudiasis, particularly when vital organs, e.g., CNS, liver, kidneys, are affected.
A primary uterine T-cell lymphoma with disseminated metastases was diagnosed in an Atlantic spotted dolphin [32]. This case adds to the relatively common myeloid and lymphoid neoplasms in cetaceans [81,134,135,136,137,138,139].
1.3 Neonatal/Perinatal pathology
NPP in cetaceans may include: abortion, dystocia, early maternal-filial separation or maternal neglect, early fatal intra- and interspecific interactions, infections, loss of passive transfer immunity, prematurity, and congenital malformations, among others [12,86]. Disturbances during gestation, labor, lactation and early behavioral abnormalities may be also included in this category [12,86]. We observed evidences of fetal distress (46%), dystocia (15%), infectious (15%), abortion (7.7%), congenital malformation (7.7%) and neonatal maternal-filial separation/maternal neglect (7.7%). Somewhat similar findings were found in a previous study in the area, predominantly involving ziphiids [12]. By contrast, this apparent predisposition of ziphiids was not observed in the present work.
A major neonatal/gestational pathologic condition in common bottlenose dolphins is prematurity [140]. The main risk factors for prematurity include: premature rupture of placental membranes; intrauterine infection; uterine, cervical and placental structural abnormalities; and multiple gestation. Our results suggest that fetal distress, based on compatible gross and histologic findings (e.g., pulmonary edema, aspirated squames and/or meconium, hyaline membranes) occurs in this category, regardless the primary underlying cause. Thus, perinatal respiratory distress or asphyxia is a common pathogenic mechanism in NPP-fatalities in cetaceans [86]. We also observed a case of presumed dystocia and of presumed maternal-filial separation. Distinguishing the former from a potential non-lethal dystocic or non-dystocic birth followed by neonatal weakness, traumatic interaction and posterior maternal-filial separation may prove extremely difficult; however, distribution of congestive and hemorrhagic areas, particularly circumferential ones potentially related to uterine contraction or birth canal pressure may be suggestive. Furthermore, several animals in this study presented evidence of neonatal/perinatal infection: a presumptively premature short-finned pilot whale with sepsis and fetal distress; and a calf pigmy sperm whale with omphaloarteritis and phlebitis, and evidence of sepsis. In both cases, the etiologic agent remains unknown. Noteworthy, a presumably premature striped dolphin presented segmental intestinal atresia, a spinal meningocele and incomplete neurocranium development. Intestinal atresia bore similarity with jejuno-ileal atresia described in humans, the most common form of intestinal atresia [141]. Meningocele is the most simple ‘open neural tube’ type defect, and is characterized by cystic meningeal dilatation containing cerebrospinal fluid without neural tissue [142]. These developmental malformations have not been reported to date in cetacean species.
1.4 Intra- and interspecific traumatic interactions
Intra- and interspecific interactions among cetaceans are diverse and complex, and often aggressive in nature [143,144,145,146,147]. Several reports describe severe injuries in calves, including infanticide [145,146,148,149,150]. Predation of other cetaceans is well known for the killer whale (Orcinus orca), which has been observed attacking or harassing over 20 different species of cetaceans, including families Balaenopteridae, Balaenidae, Eschrichtiidae, Physeteridae, Ziphiidae, Monodontidae, Delphinidae and Phocoenidae [151,152,153,154,155,156,157,158,159].
We detected higher fatal intra-/interspecific interactions than a previous study in the area (17.8% vs 4.3%), involving a wider range of species (13vs 5) [12]. We found short-finned pilot whales were most prevalent, which is in agreement with previous studies in other geographic areas, often involving young adult males and linked to reproductive initiation [160]. Consistent trauma-associated pathologic findings in this category were associated with cutaneous tooth rake marking [161], external and internal hemorrhages and bone fractures [12]. Less often, pulmonary fat emboli and myo/hemoglobinuric tubulonephrosis were observed. Evidence of traumatic events and concomitant infectious inflammatory disease, largely involving the CNS or systemic disease was observed in 12/37 (32.4%) animals. Noteworthy, an unusual fatal traumatic event involved a juvenile male false killer whale in poor body condition and a stingray’s spine perforating the tongue. Stingray spine injury was considered a potential cause of stranding or beaching in killer whales [162]. Another unusual case involved a male adult Risso’s dolphin with systemic gas embolism likely associated with complicated predation on squids [28].
2. Anthropogenic pathologic categories
2.1 Interaction with fishing activities
Bycatch is the greatest anthropogenic threat to cetaceans; gillnets, trawl nets, trammel nets, purse seine nets and longlines pose the greatest risks for these species worldwide [163]. Often, small whales, dolphins and porpoises trapped in fishing nets die by asphyxia; conversely, large whales may be able to release themselves or continue entangled during long periods leading to chronic debilitating lesions and/or eventual death [164]. Very few studies have successfully evaluated and quantified real fishing impact on cetacean populations [165,166]; thus, bycatch is largely believed to be under-reported. A considerable number of publications have reviewed the gross, and to a lesser extent, the histologic findings associated with bycatch in cetaceans [105,167,168,169,170]. The greater the number of indicator criteria identified in an individual case, the greater the likelihood of an accurate diagnosis of bycatch [170].
We detected a lower prevalence of fatal IFA than a previous study in the area [12]. In these animals, we observed lesions associated with contact with fishing nets, entanglement, lacerations, as well as craneoencephalic contusions, hematomas and bone fractures, analogous to previous reports [12,168]. Other findings in this category were deep linear cutaneous wounds along with thoracic wall and lung perforation that lead to hemothorax, and necrotizing osteomyelitis due to perforating hook in the mandible of an Atlantic spotted dolphin. Additionally, we detected 4/10 (40%) animals with moderate to severe underlying inflammatory processes including CNS inflammation and verminous pneumonia. Despite the presence of algae, diatoms, dinoflagellates, foraminifera and mineral grains as a common feature in bycaught harbor porpoises due to terminal aspiration of water [171,172], we did not observe such marine elements in any of the animals of this group by histologic examination.
2.2 Foreign body-associated pathology
Marine debris are recognized as a major worldwide environmental, economic, public health and esthetic issue, posing a complex and multidimensional challenge with important consequences for the marine environment and human activity [173,174]. Between 40–80% of marine debris derive from plastics, mostly originating on land, although lost or discarded fishing nets might represent a considerable proportion, especially along the continental shelves and remote islands [175]. Excellent review articles on this issue have been published [176,177,178,179]. For marine fauna, particularly cetaceans, major impacts linked to marine debris include ingestion and entanglements [180]. Ingestion may cause obstruction of the digestive tract leading to inanition, while entanglement may result in drowning, asphyxiation, or strangulation [178]. Sublethal effects are also recognized: chronic ingestion and entanglement may compromise feeding and digestion capacity, resulting in malnutrition, disease, and reduction in reproductive performance, growing rates and longevity [181,182]. In addition, they may transfer persistent organic pollutants among others, phenomenon especially concerning for microplastics [183]. Predisposing factors for foreign body ingestion in cetaceans remain obscure, and several hypotheses have been formulated [184,185].
In this study, we found a lower prevalence of FBAP as a CD than a previous study in the area (6/138, 4.3% vs 5/224, 2.2%). [12]. The main conditions were: gastric obstruction and impaction, followed by gastric perforation and prolonged entanglement. Gastric impactions were mainly associated with abundant plastics and the single case of gastric perforation was secondary to coiled wire, leading to septic fibrinosuppurative peritonitis and death. A common minke whale calf had severe cachexia associated with prolonged entanglement linked to a presumably fishing net adrift, which had led to severe constrictive maxillary and mandibular disease. Lesions inflicted by fishing nets adrift are analogous to those of bycatch, and sometimes discerning between the two situations is not possible. In these cases, progressive constriction and tissue damage of any component of the alimentary tract and musculoskeletal system could preclude foraging/feeding behavior and locomotion, resulting in inanition and death [186,187]. Species with apparent predisposition to foreign body ingestion include: sperm whales [188,189], beaked whales [185,189,190,191,192], Franciscana (Pontoporia blainvillei)[193], and kogiids [185,194,195].
2.3 Vessel collisions
Ship collision is a major threat to cetaceans worldwide [196,197,198,199]. A wide range of vessels have been reported to have struck whales yet most severe and lethal lesions are typically inflicted by large vessels (≥80 m-length) and by those sailing at speeds greater than 14 knots [200]. Vessel collisions with cetaceans have been widely described in cetaceans in the Canary archipelago [12,201,202,203] over the last decades, aggravated by increased maritime traffic, the increased size and speed of the vessels, and the convergence of some of the main sailing routes with areas where cetaceans congregate [12].
VC-associated lesions in cetaceans are broadly divided into two categories: 1) sharp trauma, i.e., thrust, incise and cutting, often as a result of direct contact with propeller edges and rudder; and 2) blunt trauma, i.e., abrasions, lacerations, contusions and skeletal fractures [164]. This study found a higher prevalence of fatal VC than a previous study in the area (24/224, 10.7% vs 8/138, 5.8%) [12], involving eight species, mostly deep and long-lasting divers. In our study, sperm whales were the most frequently affected, agreeing with previous observations in the area [12,201]. Long periods of socialization and surface breaks after prolonged dives would be risk factors in this species [201]. In the present study, eight sperm whales were calves, two juveniles and one adult, denoting a clear predisposition of the youngest individuals [12]. This age-biased predisposition is probably related to larger times the offspring spend on the surface compared to adults, and a possible ability acquired by adults to avoid collisions [200]. Likewise, argued risk factors for deep and prolonged diving species would be long surface rests with relatively slow swimming [12,200]. Other species appear at higher risk worldwide: fin whale (Balaenoptera physalus), the northern right whales (Eubalaena glacialis), southern right whales (Eubalaena australis), humpback whales and gray whales (Eschrichtius robustus) [200].
VC-diagnostic criteria have been revised [164,204]. Briefly, these would include one or more cuts, demonstrated ante-mortem bone fractures, hematoma(s) and/or hemorrhage(s). Microscopically, typical findings of acute trauma may include edema and subcutaneous hemorrhage, and hemorrhage with myodegeneration, necrosis, and muscle contracture underlying the collision site. A recent study further characterized microscopic lesions in axial muscles in VC cases [205]. In the present study, all animals with VC diagnosis and microscopic examination of grossly injured or adjacent muscle had acute myodegenerative processes analogous to above studies [164,204,205]. The occurrence of non-fatal and fatal VC could be underestimated because some collisions only inflict blunt trauma that could go unnoticed in postmortem examinations and because some whales struck by vessels may sink and go unnoticed to scientists or official authorities [164,206]. Nonetheless, they may also have been overestimated due to incorrect interpretation of collisions with floating dead whales; determination of fatty, cartilaginous and/or bone embolism may aid to achieve a more accurate diagnosis in these cases. Our findings further attest to the importance of VC in cetaceans of the Canary archipelago [12,201,202,203].
2.4. Active stranding pathology
Regardless of whether or not the animal is seriously ill, active stranding by itself involves an anomalous and extreme situation for an organism that is not anatomically and physiologically adapted to a solid surface, or to environmental conditions different from those of the aquatic environment [207,208]. Live-stranded cetaceans are often weakened at the time of rescue, their condition deteriorates during the capture and restraint, and they may die after a period in captivity [2]. In cetaceans, acute deaths after stranding may be attributed to hyperthermia and catecholaminergic and neurogenic shock (so called ‘stress response syndrome’ or ‘alarm reaction’), and multiorgan failure, e.g., renal dysfunction. This is typically referred to and thought to be analogous to capture myopathy in terrestrial mammals, particularly in prolonged rescues and rehabilitation efforts [13,24,25,124].
Although macroscopic lesions are often not very evident, histopathologic findings in capture myopathy may include areas of necrosis in the CNS, lung, liver, intestine, pancreas and lymph nodes; acute skeletal and cardiac myodegeneration; and tubular nephrosis often with intratubular myoglobin casts [23,24,25]. Furthermore, serum creatinine phosphokinase, aspartate aminotransferase and alanine aminotransferase may be elevated [209,210,211]. Presumably, the muscle damage is a direct consequence of stranding and not the cause, reflecting stress, overexertion, trauma, and crush injury [25,209,210]. In the present study, nearly 16% of the animals were confirmed to live-strand, lower than previous observations in the area (37/138, 26.8%) [12]. All these animals had lesions of varying severity and extent, compatible with ‘active stranding pathology’ and major involvement of the integumentary, musculoskeletal, cardiovascular and urinary systems. The above lesions generally overlapped and sometimes were masked by underlying pathologies, presumably responsible (or not) for active stranding [23,24,25,212]. These were of diverse nature yet CNS inflammation was most frequent (18/35, 51.4%) [12,24]. Despite the high incidence of active stranding-associated lesions, the pathogenetic mechanisms in these species are not fully resolved. Major players would include local to generalized vasospasm (catecholamines, neurogenic), local to generalized vasodilation (shock, impeded venous flow return by body compression), direct traumatic injury of muscle and viscera, and reperfusion damage. The latter particularly deserves further research in cetaceans.
Conclusion
Herein, our results suggest that direct human activity was responsible for 19% of cetaceans’ deaths, while natural pathologic categories would account for 81% in the Canary archipelago between 2006 and 2012. Within natural PCs, those associated with good nutritional status represented 33.6%, whereas, those associated with significant loss of nutritional status represented 23.5%. Fatal intra- and interespecific traumatic interactions were 17.8%. Vessel collisions included 11.5%. Neonatal/perinatal pathology involved 6.2%. Fatal interaction with fishing activities comprised 4.8%. Within anthropogenic PCs, foreign body-associated pathology represented 2.4%. A CD could not be determined in 7.7% cases. Natural PCs were dominated by infectious and parasitic disease processes. These results, integrating novel findings and published reports, aid in delineating baseline knowledge on cetacean pathology and may be of value to rehabilitators, caregivers, diagnosticians and future conservation policies.
Supporting information
Sex: female (F), male (M). Age: fetus (F), neonate (N), calf (C), juvenile (Jv), subadult (Sad), adult (Ad). Stranding date (SD; mm/dd/yy). Type of stranding (TS). Stranding location, island (IS): Gran Canaria (GC), Fuerteventura (FT), Lanzarote (LZ), Tenerife (TF), La Gomera (LG), El Hierro (EH), La Palma (LP), La Graciosa (LGra). Conservation status (CS): Very fresh (VF), fresh (F), moderate autolysis (MA), advanced autolysis (AA), very advanced autolysis (VAA).
(DOCX)
CD: Cluster of differentiation; CK: cytokeratin; GFAP: glial fibrillary acid protein, NSE: neuron specific enolase, MAC387: myeloid/histiocytic antigen; CDV: canine distemper virus; HSV1: Herpes Simplex virus 1: HIER: Heat-induced epitope retrieval; ABC: Avidin-Biotin-Peroxidase complex.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This manuscript is dedicated to the memory of my beloved mother Andrea (Josué Díaz Delgado) who fought against cancer in silence and relentlessly and never ceased to provide love and wisdom for all of us until her last breath; I shall see you soon. Requiescat in pace. The authors thank all the members of the Cetacean Stranding Network and associated non-governmental organizations: SECAC and Canary Conservation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was designed and was held as part of a long-lasting collective effort for monitoring health of cetacean populations inhabiting the Canary archipelago. It was performed and defended as a PhD Thesis (JDD) that was funded by the Ministry of Education of Spain (https://www.mecd.gob.es/ministerio-mecd/en/organizacion.html) through an FPU grant (AP2010–0002). Additional funding included a Regional National Research Project CGL2012–39681 and a Technical Assistant Contract by Canary Islands Government (TEC0002955).
References
- 1.Bossart GD. Marine Mammals as Sentinel Species for Oceans and Human Health. Vet Pathol. 2011; 48 (3): 676–690. 10.1177/0300985810388525 [DOI] [PubMed] [Google Scholar]
- 2.Dierauf LA, Whitman KL, Gulland FMD.CRC Handbook of Marine Mammal Medicine; Dierauf LA, Whitman KL, Gulland FMD, editors. Portland, US: Taylor & Francis Inc; 2018. [Google Scholar]
- 3.Van Bressem MF, Raga JA, Di Guardo G, Jepson PD, Duignan PJ, Siebert U, et al. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis Aquat Organ. 2009; 86 (2): 143–157. 10.3354/dao02101 [DOI] [PubMed] [Google Scholar]
- 4.Lair S, Measures LN, Martineau D. Pathologic findings and trends in mortality in the beluga (Delphinapterus leucas) population of the St Lawrence Estuary, Quebec, Canada, from 1983 to 2012. Vet Pathol. 2016; 53 (1): 22–36. 10.1177/0300985815604726 [DOI] [PubMed] [Google Scholar]
- 5.Lebeuf M, Measures L, Noel M, Raach M, Trottier S. A twenty-one year temporal trend of persistent organic pollutants in St. Lawrence Estuary beluga, Canada. Sci Total Environ. 2014; 485: 377–386. 10.1016/j.scitotenv.2014.03.097 [DOI] [PubMed] [Google Scholar]
- 6.Stewart JR, Townsend FI, Lane SM, Dyar E, Hohn AA, Rowles TK, et al. Survey of antibiotic-resistant bacteria isolated from bottlenose dolphins Tursiops truncatus in the southeastern USA. Dis Aquat Org. 2014; 108 (2): 91–102. 10.3354/dao02705 [DOI] [PubMed] [Google Scholar]
- 7.Epstein PR, Chivian E, Frith K. Emerging diseases threaten conservation. Environ Health Perspect. 2003; 111 (10): A506 10.1289/ehp.111-a506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthony SJ, St Leger JA, Navarrete-Macias I, Nilson E, Sanchez-Leon M, Liang E, et al. Identification of a novel cetacean polyomavirus from a common dolphin (Delphinus delphis) with tracheobronchitis. PLoS One. 2013; 8 (7): e68239 10.1371/journal.pone.0068239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Beurden SJ, IJsseldijk LL, van de Bildt MWG, Begeman L, Wellehan JFX, Waltzek TB, et al. A novel cetacean adenovirus in stranded harbour porpoises from the North Sea: detection and molecular characterization. Arch Virol. 2017; 162 (7): 2035–2040. 10.1007/s00705-017-3310-8 [DOI] [PubMed] [Google Scholar]
- 10.Eberle KC, Neill JD, Venn-Watson SK, McGill JL, Sacco RE. Novel Atlantic bottlenose dolphin parainfluenza virus TtPIV-1 clusters with bovine PIV-3 genotype B strains. Virus Genes. 2015; 51 (2): 198–208. 10.1007/s11262-015-1224-7 [DOI] [PubMed] [Google Scholar]
- 11.Tejedor M, Martín V (2013) The Canary Islands are the richest and major hotspot of cetaceans in European waters, where it is possible to observe 30 cetacean species. Sociedad Española de Cetáceos. Tarifa, Spain.
- 12.Arbelo M, de los Monteros AE, Herráez P, Andrada M, Sierra E, Rodríguez F, et al. Pathology and causes of death of stranded cetaceans in the Canary Islands (1999–2005). Dis Aquat Organ. 2013; 103 (2): 87–99. 10.3354/dao02558 [DOI] [PubMed] [Google Scholar]
- 13.Geraci JR, Lounsbury VJ. Marine Mammals Ashore: A Field Guide for Strandings. Baltimore, MD, USA: National Aquarium in Baltimore; 2005. [Google Scholar]
- 14.Kuiken T, Hartmann MG. Dissection techniques and tissue sampling; 1991; Leiden, The Netherlands: ECS; pp. 1–39. [Google Scholar]
- 15.Hsu SM, Raine L, Fanger HX. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981; 29 (4): 577–580. 10.1177/29.4.6166661 [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Vara JA, Kiupel M, Baszler T, Bliven L, Brodersen B, Chelack B, et al. American association of veterinary laboratory diagnosticians subcommittee on standardization of immunohistochemistry suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J Vet Diagn Invest. 2008; 20: 393–413. [DOI] [PubMed] [Google Scholar]
- 17.Díaz-Delgado J, Arbelo M, Sierra E, Vela A, Domínguez M, Paz Y, et al. Fatal Erysipelothrix rhusiopathiae septicemia in two Atlantic dolphins (Stenella frontalis and Tursiops truncatus). Dis Aquat Organ. 2015; 116 (1): 75–81. 10.3354/dao02900 [DOI] [PubMed] [Google Scholar]
- 18.Leslie CE, Flannigan B, Milne LJ. Morphological studies on clinical isolates of Aspergillus fumigatus. J Med Vet Mycol. 1988; 26 (6): 335–341. [PubMed] [Google Scholar]
- 19.Sierra E, Sánchez S, Saliki JT, Blas-Machado U, Arbelo M, Zucca D, et al. Retrospective study of etiologic agents associated with nonsuppurative meningoencephalitis in stranded cetaceans in the Canary Islands. J Clin Microbiol. 2014; 52 (7): 2390–2397. 10.1128/JCM.02906-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sierra E, Fernández A, Zucca D, Câmara N, Felipe-Jiménez I, Suárez-Santana C, et al. Morbillivirus infection in Risso’s dolphin Grampus griseus: a phylogenetic and pathological study of cases from the Canary Islands. Dis Aquat Organ. 2018. 129 (3): 165–174. 10.3354/dao03248 [DOI] [PubMed] [Google Scholar]
- 21.Delyamure SL. Helminthofauna of marine mammals: ecology and phylogeny (Gelʹmintofauna morskikh mlekopitayushchikh v svete ikh ekologii i filogenii)1955.
- 22.Díaz-Delgado J, Fernández A, Xuriach A, Sierra E, Bernaldo de Quirós Y, Mompeo B, et al. Verminous arteritis due to Crassicauda sp. in Cuvier’s beaked whales (Ziphius cavirostris). Vet Pathol. 2016; 53 (6): 1233–1240. 10.1177/0300985816642228 [DOI] [PubMed] [Google Scholar]
- 23.Cowan DF, Curry BE. Histopathology of the alarm reaction in small odontocetes. J Comp Pathol. 2008; 139 (1): 24–33. 10.1016/j.jcpa.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 24.Herráez P, Espinosa de los Monteros A, Fernández A, Edwards JF, Sacchini S, Sierra E. Capture myopathy in live-stranded cetaceans. Vet J. 2013; 196 (2): 181–188. 10.1016/j.tvjl.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 25.Herráez P, Sierra E, Arbelo M, Jaber JR, de Los Monteros AE, Fernández A. Rhabdomyolysis and myoglobinuric nephrosis (capture myopathy) in a striped dolphin. J Wildl Dis. 2007; 43 (4): 770–774. 10.7589/0090-3558-43.4.770 [DOI] [PubMed] [Google Scholar]
- 26.Díaz-Delgado J, Sierra E, Vela AI, Arbelo M, Zucca D, Groch KR, et al. Coinfection by Streptococcus phocae and cetacean morbillivirus in a short-beaked common dolphin Delphinus delphis. Dis Aquat Organ. 2017; 124 (3): 247–252. 10.3354/dao03124 [DOI] [PubMed] [Google Scholar]
- 27.Díaz-Delgado J, Quesada-Canales O, Arbelo M, Sierra E, Edwards JF, Suárez-Bonnet A, et al. Multicentric benign epithelial inclusions in a free-ranging Risso’s dolphin (Grampus griseus). J Comp Pathol. 2016; 155 (2–3): 267–271. 10.1016/j.jcpa.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 28.Fernandez A, Sierra E, Diaz-Delgado J, Sacchini S, Sanchez-Paz Y, Suarez-Santana C, et al. Deadly acute Decompression Sickness in Risso’s dolphins. Sci Rep. 2017; 7 (1): 13621 10.1038/s41598-017-14038-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Díaz-Delgado J, Sacchini S, Suárez-Bonnet A, Sierra E, Arbelo M, Espinosa A, et al. High-grade astrocytoma (glioblastoma multiforme) in an Atlantic spotted dolphin (Stenella frontalis). J Comp Pathol. 2015; 152 (2–3): 278–282. 10.1016/j.jcpa.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 30.Díaz-Delgado J, Sierra E, Vela AI, Domínguez L, Andrada M, Arbelo M, et al. Endocarditis associated with Wohlfahrtiimonas chitiniclastica in a short-beaked common dolphin (Delphinus delphis). J Wildl Dis. 2015; 51 (1): 283–286. 10.7589/2014-03-072 [DOI] [PubMed] [Google Scholar]
- 31.Neiland KA, Rice D. W., Holden BL. Helminths of marine mammals, I. The genus Nasitrema, air sinus flukes of delphinid Cetacea. J Parasitol. 1970: 305–316. [Google Scholar]
- 32.Díaz-Delgado J, Sierra E, Arbelo M, Suárez-Bonnet A, Suárez-Santana C, Grau-Bassas E, et al. Primary uterine T-cell lymphoma with metastasis in an Atlantic spotted dolphin (Stenella frontalis), Canary Islands, Spain. J Wildl Dis. 2015; 51 (2): 538–541. 10.7589/2014-08-199 [DOI] [PubMed] [Google Scholar]
- 33.Reidenberg JS, Laitman JT. Cetacean prenatal development In: Perrin WF, Wursig B, Thewissen JGM, editors. Encyclopedia of marine mammals. 2nd ed San Diego, California: Elsevier; 2009. pp. 220. [Google Scholar]
- 34.Godinho AIA (2010) Estudio morfológico e inmunohistoquímico de glóbulos hialinos en hígado de cetáceos varados [Tesis doctoral]. Las Palmas de Gran Canaria. 307 p.
- 35.Lair S, Measures LN, Martineau D. Pathologic Findings and Trends in Mortality in the Beluga (Delphinapterus leucas) Population of the St Lawrence Estuary, Quebec, Canada, From 1983 to 2012. Vet Pathol. 2015; 53 (1): 22–36. 10.1177/0300985815604726 [DOI] [PubMed] [Google Scholar]
- 36.Ralls K, Brownell RL Jr., Ballou J. Diferencial mortality by sex and age in mammals, with specific referente to the sperm whale. Rep Int Whal Comm. 1980; 2(special issue): 223–243. [Google Scholar]
- 37.Stolen MK, Barlow J. A model life table for bottlenose dolphins (Tursiops truncatus) from the Indian River Lagoon System, Florida, USA. Mar Mam Sci. 2003; 19 (4): 630–649. [Google Scholar]
- 38.Domingo M, Visa J, Pumarola M, Marco AJ, Ferrer L, Rabanal R, et al. Pathologic and immunocytochemical studies of morbillivirus infection in striped dolphins (Stenella coeruleoalba). Vet Pathol. 1992; 29 (1): 1–10. 10.1177/030098589202900101 [DOI] [PubMed] [Google Scholar]
- 39.Kennedy S. Morbillivirus infections in aquatic mammals. J Comp Pathol. 1998; 119 (3): 201–225. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy S, Smyth JA, Cush PF, McAliskey M, McCullough SJ, Rima BK. Histopathologic and immunocytochemical studies of distemper in harbor porpoises. Vet Pathol. 1991; 28 (1): 1–7. 10.1177/030098589102800101 [DOI] [PubMed] [Google Scholar]
- 41.Duignan PJ, Geraci JR, Raga JA, Calzada N. Pathology of morbillivirus infection in striped dolphins (Stenella coeruleoalba) from Valencia and Murcia, Spain. Can J Vet Res. 1992; 56 (3): 242–248. [PMC free article] [PubMed] [Google Scholar]
- 42.Van Bressem MF, Duignan PJ, Banyard A, Barbieri M, Colegrove KM, De Guise S, et al. Cetacean morbillivirus: current knowledge and future directions. Viruses. 2014; 6 (12): 5145–5181. 10.3390/v6125145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chastel C, Masure O, Balouet G, Laban P, Lucas A. The student, the cetacean and swine-fever: a minor epidemic after dissection of a globicephale. Nouv Presse Med 1975; 4: 1803–1805. [PubMed] [Google Scholar]
- 44.Kinsel MJ, Boehm JR, Harris B, Murnane RD. Fatal Erysipelothrix rhusiopathiae septicemia in a captive Pacific white-sided dolphin (Lagenorhynchus obliquidens). J Zoo Wildl Med. 1997; 28 (4): 494–497. [PubMed] [Google Scholar]
- 45.Thurman GD, Downes SJ, Fothergill MB, Goodwin NM, Hegarty MM. Diagnosis and successful treatment of subacute erysipelas in a captive dolphin. J S Afr Vet Assoc. 1983; 54 (3): 193–200. [PubMed] [Google Scholar]
- 46.Wang Q, Chang BJ, Riley TV. Erysipelothrix rhusiopathiae. Vet Microbiol. 2010; 140 (3–4): 405–417. 10.1016/j.vetmic.2009.08.012 [DOI] [PubMed] [Google Scholar]
- 47.Melero M, Rubio-Guerri C, Crespo JL, Arbelo M, Vela AI, García-Párraga D, et al. First case of erysipelas in a free-ranging bottlenose dolphin (Tursiops truncatus) stranded in the Mediterranean Sea. Dis Aquat Org. 2011; 97 (2): 167–170. 10.3354/dao02412 [DOI] [PubMed] [Google Scholar]
- 48.Dagleish MP, Foster G, Howie FE, Reid RJ, Barley J. Fatal mycotic encephalitis caused by Aspergillus fumigatus in a northern bottlenose whale (Hyperoodon ampullatus). Vet Rec. 2008; 163 (20): 602–604. [DOI] [PubMed] [Google Scholar]
- 49.Dagleish MP, Patterson IA, Foster G, Reid RJ, Linton C, Buxton D. Intracranial granuloma caused by asporogenic Aspergillus fumigatus in a harbour porpoise (Phocoena phocoena). Vet Rec. 2006; 159 (14): 458–460. [DOI] [PubMed] [Google Scholar]
- 50.Cassle SE, Landrau-Giovannetti N, Farina LL, Leone A, Wellehan JF Jr, Stacy NI, et al. Coinfection by Cetacean morbillivirus and Aspergillus fumigatus in a juvenile bottlenose dolphin (Tursiops truncatus) in the Gulf of Mexico. J Vet Diagn Invest. 2016; 28 (6): 729–734. 10.1177/1040638716664761 [DOI] [PubMed] [Google Scholar]
- 51.Miller M, Shapiro K, Murray MJ, Haulena M, Raverty S. Protozoan parasites of marine mammals In: Gulland FMD, Dierauf LA, Whitman KL, editors. CRC Handbook of Marine Mammal Medicine. 3rd ed Boca Raton, FL: CRC Press; 2018. pp. 425–470. [Google Scholar]
- 52.Brieva LM, Oporto JA. Prevalencia e intensidad de la infección por tremátodos del género Nasitrema en el delfín chileno Cephalorhynchus eutropia (Cetacea: Delphinidae). Arch Med Vet. 1991; 23: 97–100. [Google Scholar]
- 53.Degollada E, Andre M, Arbelo M, Fernandez A. Incidence, pathology and involvement of Nasitrema species in odontocete strandings in the Canary Islands. Vet Rec. 2002; 150 (3): 81–82. [DOI] [PubMed] [Google Scholar]
- 54.Ebert MB, Valente ALS. New records of Nasitrema atenuatta and Nasitrema globicephalae (Trematoda: Brachycladiidae) Neiland, Rice and Holden, 1970 in delphinids from South Atlantic. CheckList 2013; 9 (6): 1538 [Google Scholar]
- 55.Lewis RJ, Berry K. Brain lesions in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). J Wildl Dis 1988; 24 (3): 577–581. 10.7589/0090-3558-24.3.577 [DOI] [PubMed] [Google Scholar]
- 56.Morimitsu T, Nagai T, Ide M, Ishii A, Koono M. Parasitogenic octavus neuropathy as a cause of mass stranding of Odontoceti J Parasitol 1986; 72 (3): 469–472. [PubMed] [Google Scholar]
- 57.Morimitsu T, Nagai T, Ide M, Kawano H, Naichuu A, Koono M, et al. Mass stranding of Odontoceti caused by parasitogenic eighth cranial neuropathy. J Wildl Dis. 1987; 23 (4): 586–590. [DOI] [PubMed] [Google Scholar]
- 58.O'Shea TJ, Homer BL, Greiner EC, Layton AW. Nasitrema sp.-associated encephalitis in a striped dolphin (Stenella coeruleoalba) stranded in the Gulf of Mexico. J Wildl Dis. 1991; 27 (4): 706–709. 10.7589/0090-3558-27.4.706 [DOI] [PubMed] [Google Scholar]
- 59.Oliveira JB, Morales JA, González-Barrientos RC, Hernández-Gamboa J, Hernández-Mora G. Parasites of cetaceans stranded on the Pacific coast of Costa Rica. Vet Parasitol. 2011; 182 (2–4): 319–328. 10.1016/j.vetpar.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 60.Balbuena JA, Raga JA. Intestinal helminth communities of the long-finned pilot whale (Globicephala melas) off the Faroe Islands. Parasitology. 1993; 106 (Pt 3): 327–333. [DOI] [PubMed] [Google Scholar]
- 61.Cowan DF. Helminth parasites of the pilot whale Globicephala melaena (Traill 1809). J Parasitol. 1967; 53 (1): 166–167. [PubMed] [Google Scholar]
- 62.Hoberg EP, Pierre-Yves D, McBurney S. Bolbosoma capitatum and Bolbosoma sp. (Acanthocephala) from Sperm Whales (Physeter macrocephalus) Stranded on Prince Edward Island, Canada. J Helminthol Soc Wash. 1993; 60 (2): 205–210. [Google Scholar]
- 63.Gibson DI, Harris EA, Bray RA, Jepson PD, Kuiken T, Baker JR, et al. A survey of the helminth parasites of cetaceans stranded on the coast of England and Wales during the period 1990–1994. J Zool 1998; 244 (4): 563–574. [Google Scholar]
- 64.Félix JR. Reported incidences of parasitic infections in marine mammals from 1892 to 1978. Zea Books; 2013. [Google Scholar]
- 65.Kikuchi S, Nakajima M. Bolbosoma capitatum (Acanthocephala) from false killer whales, Pseudorca crassidens, and its pathogenicity. Jpn J Parasitol. 1993; 42: 398–408. [Google Scholar]
- 66.Bateman TFK, Mclellan W, Piscitelli M, Harms C, Barco S, Thayer V, et al. Crassicauda infections in Kogiid whales. Integrative and Comparative Biology. Cary, NC Oxford Univ. Press INC; 2014 pp. E241-E241.
- 67.Jabbar A, Beveridge I, Bryant MS. Morphological and molecular observations on the status of Crassicauda magna, a parasite of the subcutaneous tissues of the pygmy sperm whale, with a re-evaluation of the systematic relationships of the genus Crassicauda. Parasitol Res. 2014; 114 (3): 835–841. 10.1007/s00436-014-4245-6 [DOI] [PubMed] [Google Scholar]
- 68.Lambertsen RH. Disease of the common fin whale (Balaenoptera physalus): Crassicaudiosis of the urinary system. J Mammal. 1986; 353–366. [Google Scholar]
- 69.Lambertsen RH. Disease biomarkers in large whale populations of the North Atlantic and other oceans In: McCarthy J, Shugart L, editors. Biomarkers of environmental contamination. Chelsea, Michigan: Lewis Publishers; 1990. pp. 395–417. [Google Scholar]
- 70.Lambertsen RH. Crassicaudosis: a parasitic disease threatening the health and population recovery of large baleen whales. Rev Sci Tech. 1992; 11 (4): 1131–1141. [PubMed] [Google Scholar]
- 71.Dailey MD. Diseases of Mammalia: Cetacea In: Kinne O, editor. Diseases of Marine Animals. Hamburg, Germany: Biologische Anstalt Helgoland; 1985. pp. 805–847. [Google Scholar]
- 72.Raga JA, Balbuena JA. A new species of the genus Crassicauda Leiper et Atkinson, 1914 (Nematoda: Spiruroidea) from the penis of Globicephala melas (Traill, 1809)(Cetacea: Globicephalidae) in the western Mediterranean Sea. Ann Parasitol Hum Comp. 1990; 65 (5–6): 255–261. [Google Scholar]
- 73.Fernández A, Edwards JF, Rodriguez F, Espinosa de los Monteros A, Herraez P, Castro P, et al. "Gas and fat embolic syndrome" involving a mass stranding of beaked whales (family Ziphiidae) exposed to anthropogenic sonar signals. Vet Pathol. 2005; 42 (4): 446–457. 10.1354/vp.42-4-446 [DOI] [PubMed] [Google Scholar]
- 74.Jepson PD, Arbelo M, Deaville R, Patterson IA, Castro P, Baker JR, et al. Gas-bubble lesions in stranded cetaceans. Nature. 2003; 425 (6958): 575–576. 10.1038/425575a [DOI] [PubMed] [Google Scholar]
- 75.Baily JL, Morrison LR, Patterson IA, Underwood C, Dagleish MP. Primitive Neuroectodermal tumour in a striped dolphin (Stenella coeruleoalba) with features of ependymoma and neural tube differentiation (medulloepithelioma). J Comp Pathol. 2013; 149 (4): 514–519. 10.1016/j.jcpa.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 76.Miclard J, Mokhtari K, Jouvion G, Wyrzykowski B, Van Canneyt O, Wyers M, et al. Microcystic meningioma in a dolphin (Delphinus delphis): immunohistochemical and ultrastructural study. J Comp Pathol. 2006; 135 (4): 254–258. 10.1016/j.jcpa.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 77.Pilleri G. Brain lipoma in the Humpback whale, Megaptera novaeangliae. Pathol Vet. 1966; 3: 341–349. [DOI] [PubMed] [Google Scholar]
- 78.Pilleri G. Cerebral neurofibroma in the fin whale, Balaenoptera physalus. Pathol Vet 1968; 5 (1): 35–40. [PubMed] [Google Scholar]
- 79.Pintore MD, Mignone W, Di Guardo G, Mazzariol S, Ballardini M, Florio CL, et al. Nueropathologic findings in cetaceans stranded in Italy (2002–14). J Wildl Dis. 2018; 54 (2): 295–303. 10.7589/2017-02-035 [DOI] [PubMed] [Google Scholar]
- 80.Ridgway SH, Marino L, Lipscomb TP. Description of a poorly differentiated carcinoma within the brainstem of a white whale (Delphinapterus leucas) from magnetic resonance images and histological analysis. Anat Rec. 2002; 268 (4): 441–449. 10.1002/ar.10183 [DOI] [PubMed] [Google Scholar]
- 81.Arbelo M, Espinosa de los Monteros A, Herráez P, Suárez-Bonnet A, Andrada M, Rivero M, et al. Primary central nervous system T-cell lymphoma in a common dolphin (Delphinus delphis). J Comp Pathol. 2014; 150 (2–3): 336–340. 10.1016/j.jcpa.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 82.Vandevelde M, Higgins RJ, Oevermann A. Veterinary neuropathology: Essentials of theory and practice; Vandevelde M, Higgins RJ, Oevermann A, editors. Oxford, UK: Wiley-Blackwell; 2012. [Google Scholar]
- 83.Jepson PD, Deaville R, Patterson IA, Pocknell AM, Ross HM, Baker JR, et al. Acute and chronic gas bubble lesions in cetaceans stranded in the United Kingdom. Vet Pathol. 2005; 42 (3): 291–305. 10.1354/vp.42-3-291 [DOI] [PubMed] [Google Scholar]
- 84.Begeman L, St Leger JA, Blyde DJ, Jauniaux TP, Lair S, Lovewell G, et al. Intestinal volvulus in cetaceans. Vet Pathol. 2013; 50 (4): 590–596. 10.1177/0300985812465327 [DOI] [PubMed] [Google Scholar]
- 85.Stroud RK, Roffe TJ. Causes of death in marine mammals stranded along the Oregon coast. J Wildl Dis. 1979; 15 (1): 91–97. [DOI] [PubMed] [Google Scholar]
- 86.Groch KR, Díaz-Delgado J, Marcondes MCC, Colosio AC, Santos-Neto EB, Carvalho VL, et al. Pathology and causes of death in stranded humpback whales (Megaptera novaeangliae) from Brazil. PLOS One. 2018; 13 (5): e0194872 10.1371/journal.pone.0194872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giorda F, Ballardini M, Di Guardo G, Pintore MD, Grattarola C, Iulini B, et al. Postmortem findings in cetaceans found stranded in the Pelagos sanctuary, Italy, 2007–14. J Wildl Dis. 2017; 53 (4): 795–803. 10.7589/2016-07-150 [DOI] [PubMed] [Google Scholar]
- 88.Fenton H, Daoust PY, Forzán MJ, Vanderstichel RV, Ford JK, Spaven L, et al. Causes of mortality of harbor porpoises Phocoena phocoena along the Atlantic and Pacific coasts of Canada. Dis Aquat Organ. 2017; 122 (3): 171–183. 10.3354/dao03080 [DOI] [PubMed] [Google Scholar]
- 89.Alstrup AK, Jensen LF, Hansen MS, Kinze CC, Jensen TH. Necropsy findings of 11 white-beaked dolphins (Lagenorhynchus albirostris) stranded in Denmark during 2008–2014. Aquat Mamm. 2016; 42 (3): 292–299. [Google Scholar]
- 90.McAloose D, Rago MV, Di Martino M, Chirife A, Olson SH, Beltramino L, et al. Post-mortem findings in southern right whales Eubalaena australis at Península Valdés, Argentina, 2003–2012. Dis Aquat Organ. 2016; 119 (1): 17–36. 10.3354/dao02986 [DOI] [PubMed] [Google Scholar]
- 91.Tajima Y, Maeda K, Yamada TK. Pathological findings and probable causes of the death of Stejneger’s beaked whales (Mesoplodon stejnegeri) stranded in Japan from 1999 and 2011. J Vet Med Sci. 2015; 77 (1): 45–51. 10.1292/jvms.13-0454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Casalone C, Mazzariol S, Pautasso A, Di Guardo G, Di Nocera F, Lucifora G, et al. Cetacean strandings in Italy: an unusual mortality event along the Tyrrhenian Sea coast in 2013. Dis Aquat Organ. 2014; 109 (1): 81–86. 10.3354/dao02726 [DOI] [PubMed] [Google Scholar]
- 93.Bossart GD, Hurley W, Biedenbach G, Denny M, Borkowski R, Goricki C, et al. Pathologic findings in stranded cetaceans from northeastern florida. Florida Scientist. 2013: 36–50. [Google Scholar]
- 94.Bogomolni AL, Pugliares KR, Sharp SM, Patchett K, Harry CT, LaRocque JM, et al. Mortality trends of stranded marine mammals on Cape Cod and southeastern Massachusetts, USA, 2000 to 2006. Dis Aquat Organ. 2010; 88 (2): 143–155. 10.3354/dao02146 [DOI] [PubMed] [Google Scholar]
- 95.McFee WE, Lipscomb TP. Major pathologic findings and probable causes of mortality in bottlenose dolphins stranded in South Carolina from 1993 to 2006. J Wildl Dis. 2009; 45 (3): 575–593. 10.7589/0090-3558-45.3.575 [DOI] [PubMed] [Google Scholar]
- 96.Stockin KA, Duignan PJ, Roe WD, Meynier L, Alley M, Fettermann T. Causes of mortality in stranded common dolphin (Delphinus sp.) from New Zealand waters between 1998 and 2008. Pacific Conservation Biology. 2009; 15 (3): 217–227. [Google Scholar]
- 97.Siebert U, Tolley K, Vikingsson GA, Olafsdottir D, Lehnert K, Weiss R, et al. Pathological findings in harbour porpoises (Phocoena phocoena) from Norwegian and Icelandic waters. J Comp Pathol. 2006; 134 (2–3): 134–142. 10.1016/j.jcpa.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 98.Bossart GD, Meisner R, Varela R, Mazzoil M, McCulloch SD, Kilpatrick D, et al. Pathologic findings in stranded Atlantic bottlenose dolphins (Tursiops truncatus) from the Indian River Lagoon, Florida. Florida Scientist. 2003: 226–238. [Google Scholar]
- 99.Jauniaux T, Petitjean D, Brenez C, Borrens M, Brosens L, Haelters J, et al. Post-mortem findings and causes of death of harbour porpoises (Phocoena phocoena) stranded from 1990 to 2000 along the coastlines of Belgium and Northern France. J Comp Pathol. 2002; 126 (4): 243–253. 10.1053/jcpa.2001.0547 [DOI] [PubMed] [Google Scholar]
- 100.Siebert U, Wunschmann A, Weiss R, Frank H, Benke H, Frese K. Post-mortem findings in harbour porpoises (Phocoena phocoena) from the German North and Baltic Seas. J Comp Pathol. 2001; 124 (2–3): 102–114. 10.1053/jcpa.2000.0436 [DOI] [PubMed] [Google Scholar]
- 101.Parsons EC, Jefferson TA. Post-mortem investigations on stranded dolphins and porpoises from Hong Kong waters. J Wildl Dis. 2000; 36 (2): 342–356. 10.7589/0090-3558-36.2.342 [DOI] [PubMed] [Google Scholar]
- 102.Cornaglia E, Rebora L, Gili C, Di Guardo G. Histopathological and immunohistochemical studies on cetaceans found stranded on the coast of Italy between 1990 and 1997. J Vet Med A Physiol Pathol Clin Med. 2000; 47 (3): 129–142. [DOI] [PubMed] [Google Scholar]
- 103.Martineau D, Lagace A, Beland P, Higgins R, Armstrong D, Shugart LR. Pathology of stranded beluga whales (Delphinapterus leucas) from the St. Lawrence Estuary, Quebec, Canada. J Comp Pathol. 1988; 98 (3): 287–311. [DOI] [PubMed] [Google Scholar]
- 104.Diguardo G, Agrimi U, Morelli L, Cardeti G, Terracciano G, Kennedy S. Post-mortem investigations on cetaceans found stranded on the coasts of Italy between 1990 and 1993. Vet Rec. 1995; 136 (17): 439–442. [DOI] [PubMed] [Google Scholar]
- 105.Baker JR. Causes of mortality and parasites and incidental lesions in dolphins and whales from British waters. Vet Rec. 1992; 130 (26): 569–572. [DOI] [PubMed] [Google Scholar]
- 106.De Guise S, Lagace A, Beland P, Girard C, Higgins R. Non-neoplastic lesions in beluga whales (Delphinapterus leucas) and other marine mammals from the St Lawrence Estuary. J Comp Pathol. 1995; 112 (3): 257–271. [DOI] [PubMed] [Google Scholar]
- 107.Dailey M, Stroud R. Parasites and associated pathology observed in cetaceans stranded along the Oregon coast. J Wildl Dis 1978; 14 (4): 503–511. [DOI] [PubMed] [Google Scholar]
- 108.Jauniaux T, Brosens L, Jacquinet E, Lambrigts D, Addink M, Smeenk C, et al. Postmortem investigations on winter stranded sperm whales from the coasts of Belgium and The Netherlands. J Wildl Dis. 1998; 34 (1): 99–109. 10.7589/0090-3558-34.1.99 [DOI] [PubMed] [Google Scholar]
- 109.Sweeney JC, Ridgway SH. Common diseases of small cetaceans. J Am Vet Med Assoc. 1975; 167 (7): 533–540. [PubMed] [Google Scholar]
- 110.Arbelo M, Belliere EN, Sierra E, Sacchinni S, Esperón F, Andrada M, et al. Herpes virus infection associated with interstitial nephritis in a beaked whale (Mesoplodon densirostris). BMC Vet Res. 2012; 8: 243 10.1186/1746-6148-8-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arbelo M, Sierra E, Esperón F, Watanabe TT, Belliere EN, Espinosa de los Monteros A, et al. Herpesvirus infection with severe lymphoid necrosis affecting a beaked whale stranded in the Canary Islands. Dis Aquat Organ. 2010; 89 (3): 261–264. 10.3354/dao02208 [DOI] [PubMed] [Google Scholar]
- 112.Belliere EN, Esperón F, Arbelo M, Munoz MJ, Fernández A, Sánchez-Vizcaíno JM. Presence of herpesvirus in striped dolphins stranded during the cetacean morbillivirus epizootic along the Mediterranean Spanish coast in 2007. Arch Virol. 2010; 155 (8): 1307–1311. 10.1007/s00705-010-0697-x [DOI] [PubMed] [Google Scholar]
- 113.Blanchard TW, Santiago NT, Lipscomb TP, Garber RL, McFee WE, Knowles S. Two novel alphaherpesviruses associated with fatal disseminated infections in Atlantic bottlenose dolphins. J Wildl Dis. 2001; 37 (2): 297–305. 10.7589/0090-3558-37.2.297 [DOI] [PubMed] [Google Scholar]
- 114.Esperón F, Fernández A, Sánchez-Vizcaíno JM. Herpes simplex-like infection in a bottlenose dolphin stranded in the Canary Islands. Dis Aquat Organ. 2008; 81 (1): 73–76. 10.3354/dao01915 [DOI] [PubMed] [Google Scholar]
- 115.Cusick PK, Bullock BC. Ulcerative dermatitis and pneumonia associated with Aeromonas hydrophila infection in the bottle-nosed dolphin. J Am Vet Med Assoc. 1973; 163 (6): 578–579. [PubMed] [Google Scholar]
- 116.Almuzara MN, Palombarani S, Tuduri A, Figueroa S, Gianecini A, Sabater L, et al. First Case of Fulminant Sepsis Due to Wohlfahrtiimonas chitiniclastica. J Clin Microbiol. 2011; 49 (6): 2333–2335. 10.1128/JCM.00001-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rebaudet S, Genot S, Renvoise A, Fournier PE, Stein A. Wohlfahrtiimonas chitiniclastica Bacteremia in Homeless Woman. Emerg Infect Dis. 2009; 15 (6): 985–987. 10.3201/eid1506.080232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thaiwong T, Kettler NM, Lim A, Dirkse H, Kiupel M. First Report of Emerging Zoonotic Pathogen Wohlfahrtiimonas chitiniclastica in the United States. J Clin Microbiol. 2014; 52 (6): 2245–2247. 10.1128/JCM.00382-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giamarellos-Bourboulis EJ, Adamis T, Laoutaris G, Sabracos L, Koussoulas V, Mouktaroudi M, et al. Immunomodulatory clarithromycin treatment of experimental sepsis and acute pyelonephritis caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2004; 48 (1): 93–99. 10.1128/AAC.48.1.93-99.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health. 2009; 2 (3): 101–111. 10.1016/j.jiph.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 121.De Guise S, Lagace A, Beland P. Tumors in St. Lawrence beluga whales (Delphinapterus leucas). Vet Pathol. 1994; 31 (4): 444–449. 10.1177/030098589403100406 [DOI] [PubMed] [Google Scholar]
- 122.Fletcher D, Gulland FMD, Haulena M, Lowenstine LJ, Dailey M. Nematode-associated gastrointestinal perforations in stranded California sea lions (Zalophus californianus); 1998; San Diego, CA: pp. 59. [Google Scholar]
- 123.Ridgway SH. Medical care of marine mammals J Am Vet Med Assoc. 1965; 71: 237–242. [PubMed] [Google Scholar]
- 124.Simpson JG, Cornell LH. Diseases associated with stranding and captivity Pathobiology of Marine Mammal Diseases. Boca Raton, FL, USA: CRC Press; 1983. pp. 29–64. [Google Scholar]
- 125.Resendes AR, Almeria S, Dubey JP, Obon E, Juan-Salles C, Degollada E, et al. Disseminated toxoplasmosis in a Mediterranean pregnant Risso’s dolphin (Grampus griseus) with transplacental fetal infection. J Parasitol. 2002; 88 (5): 1029–1032. 10.1645/0022-3395(2002)088[1029:DTIAMP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 126.Di Guardo G, Di Francesco CE, Eleni C, Cocumelli C, Scholl F, Casalone C, et al. Morbillivirus infection in cetaceans stranded along the Italian coastline: pathological, immunohistochemical and biomolecular findings. Res Vet Sci. 2013; 94 (1): 132–137. 10.1016/j.rvsc.2012.07.030 [DOI] [PubMed] [Google Scholar]
- 127.Roe WD, Howe L, Baker EJ, Burrows L, and Hunter SA. An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector’s dolphins (Cephalorhynchus hectori) Vet Parasitol. 2013; 192: 67–74. 10.1016/j.vetpar.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 128.Bowater RO, Norton J, Johnson S, Hill B, O’Donoghue P, Prior H. Toxoplasmosis in Indo-Pacific humpbacked dolphins (Sousa chinensis) from Queensland. Aust Vet J. 2003; 81 (10): 627–632. [DOI] [PubMed] [Google Scholar]
- 129.Howard EB, Britt JO, Matsumoto GK. Parasitic diseases In: Howard EB, editor. Pathobiology of Marine Mammals Diseases Boca Raton, Florida: CRC Press; 1983. pp. 119–232. [Google Scholar]
- 130.Woodard JC, Zam SG, Caldwell DK, Caldwell MC. Some parasitic diseases of dolphins. Pathol Vet. 1969; 6 (3): 257–272. [DOI] [PubMed] [Google Scholar]
- 131.Choi YK, Kang MS, Sohn HR, Kim DY. Disseminated ciliated protozoan infection in a Pacific dolphin (Tursiops gilli). Vet Rec 2003; 153 (23): 714–715. [PubMed] [Google Scholar]
- 132.Dailey MD. Parasitic Diseases In: Dierauf LA, Gulland FMD, editors. CRC Handbook of Marine Mammal Medicine. Second Edition ed Boca Raton, FL: CRC Press; 2001. [Google Scholar]
- 133.Raga JA, Balbuena JA, Aznar J, Fernández M. The impact of parasites on marine mammals: a review. Parasitologia 1997; 39 (4): 293–296. [PubMed] [Google Scholar]
- 134.Bossart GD, Ewing R, Herron AJ, Cray C, Mase B, Decker SJ, et al. Immunoblastic malignant lymphoma in dolphins: histologic, ultrastructural, and immunohistochemical features. J Vet Diagn Invest. 1997; 9 (4): 454–458. 10.1177/104063879700900427 [DOI] [PubMed] [Google Scholar]
- 135.De Guise S, Lagace A, Beland P. Tumors in cetaceans: updated review with special reference to the St Lawrence belugas; 1992; Hong Kong. pp. 115–117.
- 136.Howard EG, Britt FO Jr., Simpson JG. Neoplasms in marine mammals In: Howard EG, editor. Pathobiology of marine mammal diseases. Boca Raton, FL: CRC Press; 1983. pp. 95–162. [Google Scholar]
- 137.Jaber JR, Pérez J, Carballo M, Arbelo M, Espinosa de los Monteros A, Herráez P, et al. Hepatosplenic large cell immunoblastic lymphoma in a bottlenose dolphin (Tursiops truncatus) with high levels of polychlorinated biphenyl congeners. J Comp Pathol. 2005; 132 (2–3): 242–247. 10.1016/j.jcpa.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 138.Stolk A. Tumors in whales III. Proc kon Ned Akad Wet. 1962; C65: 250–268. [PubMed] [Google Scholar]
- 139.Yonezawa M, Nakamine H, Tanaka T, Miyaji T. Hodgkin’s disease in a killer whale (Orcinus orca). J Comp Pathol. 1989; 100 (2): 203–207. [DOI] [PubMed] [Google Scholar]
- 140.Joseph BE, Duffield DA, Robeck TR (2000) Summary data on reprodution of bottlenose dolphins in controlled environments. In: D.A. D, T.R. R, editors. Report from the Bottlenose Dolphin Breeding Workshop. Silver Spring, MD,: American Zoological Asoociation Marine Mammal Taxon Advisory Group. pp. 43–56.
- 141.Adams SD, Stanton MP. Malrotation and intestinal atresias. Early Hum Dev. 2014; 90 (12): 921–925. 10.1016/j.earlhumdev.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 142.Cantile C, Youseff S. Nervous system In: Maxie MG, editor. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th ed St. Louis: Elsevier; 2016. pp. 250–406. [Google Scholar]
- 143.Gerson HB, Hickie JP. Head scarring on male narwhals (Monodon monoceros): Evidence for aggressive tusk use. Can J Zool. 1985; 63: 2083–2087. [Google Scholar]
- 144.Campagna C. Aggressive Behavior, Intraspecific In: Perrin WF, Wursig B, Thewissen JGM, editors. Encyclopedia of Marine Mammals. 2 ed California, US: Academic Press; 2009. pp. 18–24. [Google Scholar]
- 145.Kaplan JD, Lentell BJ, Lange W. Possible evidence for infanticide among bottlenose dolphins (Tursiops truncatus) off St. Augustine, Florida. Mar Mam Sci. 2009; 25 (4): 970–975. [Google Scholar]
- 146.Nery MF, Simao SM. Sexual coercion and aggression towards a newborn calf of marine tucuxi dolphins (Sotalia guianensis). Mar Mam Sci. 2009; 25: 450–454. [Google Scholar]
- 147.Parsons KM, Durban JW, Claridge DE. Male-male aggression renders bottlenose dolphin (Tursiops truncatus) unconscious. Aquat Mamm. 2003; 29: 360–362 [Google Scholar]
- 148.Dunn DG, Barco SG, Pabst DA, McLellan WA. Evidence for infanticide in bottlenose dolphins of the western North Atlantic. J Wildl Dis. 2002; 38 (3): 505–510. 10.7589/0090-3558-38.3.505 [DOI] [PubMed] [Google Scholar]
- 149.Patterson IA, Reid RJ, Wilson B, Grellier K, Ross HM, Thompson PM. Evidence for infanticide in bottlenose dolphins: an explanation for violent interactions with harbour porpoises? Proc Biol Sci. 1998; 265 (1402): 1167–1170. 10.1098/rspb.1998.0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Towers JR, Halle MJ, Symonds HK, Sutton GJ, Morton AB, Spong P, et al. Infanticide in a mammal-eating killer whale population. Sci Rep. 2018; 8 (1): 4366 10.1038/s41598-018-22714-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baldridge. Killer whales attack and eat a gray whale. J Mammal. 1972; 53 (4): 898. [Google Scholar]
- 152.George JC, Philo LM, Hazard K, Withrow D, Carroll GM, Suydam R. Frequency of killer-whale (Orcinus orca) attacks and ship collisions based on scarring on bowhead whales (Balaena mysticetus) of the Bering-Chukchi-Beaufort Seas stock. Arctic. 1994; 47 (3): 247–255. [Google Scholar]
- 153.Hancock D. Killer whales kill and eat a minke whale. J Mammal. 1965; 46: 341–342. [Google Scholar]
- 154.Jefferson TA, Stacey PJ, Baird RW. A review of killer whale interactions with other marine mammals—Predation to Coexistence. Mammal Rev. 1991; 21 (4): 151–180. [Google Scholar]
- 155.Jonsgård Å, Lyshoel PB. A contribution to the knowledge of the biology of the killer whale (Orcinus orca). ICES; 1969: 1–12. [Google Scholar]
- 156.Madsen PT, de Soto NA, Tyack PL, Johnson M. Beaked whales. Curr Biol. 2014; 24 (16): R728–R730. 10.1016/j.cub.2014.06.041 [DOI] [PubMed] [Google Scholar]
- 157.Pitman RL, Ballance LT, Mesnick SI, Chivers SJ. Killer whale predation on sperm whales: Observations and implications. Mar Mam Sci. 2001; 17 (3): 494–507. [Google Scholar]
- 158.Whitehead H, Glass C. Orcas (killer whales) attack humpback whales. J Mammal. 1985; 66 (1): 183–185. [Google Scholar]
- 159.Wellard R, Lightbody K, Fouda L, Blewitt M, Riggs D, Erbe C. Killer Whale (Orcinus orca) Predation on Beaked Whales (Mesoplodon spp.) in the Bremer Sub-Basin, Western Australia. PLoS One. 2016; 11 (12): e0166670 10.1371/journal.pone.0166670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oremland MS, Allen BM, Clapham PJ, Moore MJ, Potter C, Mead JG. Mandibular fractures in short-finned pilot whales, Globicephala macrorhynchus. Mar Mammal Sci. 2010; 26 (1): 1–16. [Google Scholar]
- 161.Greenwood AG, Harrison RJ, Whitting HW. Functional and pathological aspects of the skin of marine mammals; Harrison RJ, editor. London: Academic Press; 1974. [Google Scholar]
- 162.Duignan PJ, Hunter JE, Visser IN, Jones GW, Nutman A. Stingray spines: A potential cause of killer whale mortality in New Zealand. Aquat Mamm. 2000; 26 (2): 143–147. [Google Scholar]
- 163.Reeves RR, Mcclellan K, Werner TB. Marine mammal bycatch in gillnet and other entangling net fisheries. Endanger Species Res. 2013; 20: 71–97. [Google Scholar]
- 164.Moore MJ, der Hoop J, Barco SG, Costidis AM, Gulland FM, Jepson PD, et al. Criteria and case definitions for serious injury and death of pinnipeds and cetaceans caused by anthropogenic trauma. Dis Aquat Organ. 2013; 103 (3): 229–264. 10.3354/dao02566 [DOI] [PubMed] [Google Scholar]
- 165.Barlow J, Baird RW, Heyning JE, Wynne K, Manville AM, Lowry DH, et al. A review of cetacean and pinniped mortality in coastal fisheries along the west coast of the USA and Canada and the east coast of the Russian Federation. Rep Int Whal Commn. 1994. Special 15: 405–425. [Google Scholar]
- 166.Young NM, Ludicello S (2007) Worldwide Bycatch of Cetaceans. 276 p.
- 167.Cox TM, Read AJ, Barco S, Evans J, Gannon DP, Koopman HN, et al. Documenting the by-catch of harbour porpoises, Phocoena phocoena, in coastal gillnet fisheries from stranded carcasses Fish Bull. 1998; 96: 727–734. [Google Scholar]
- 168.Kuiken T, Simpson VR, Allchin CR, Bennett PM, Codd GA, Harris EA, et al. Mass mortality of common dolphins (Delphinus delphis) in south west England due to incidental capture in fishing gear. Vet Rec. 1994; 134 (4): 81–89. [DOI] [PubMed] [Google Scholar]
- 169.de Quirós YB, Hartwick M, Rotstein DS, Garner MM, Bogomolni A, Greer W, et al. Discrimination between bycatch and other causes of cetacean and pinniped stranding. Dis Aquat Organ. 2018; 127 (2): 83–95. 10.3354/dao03189 [DOI] [PubMed] [Google Scholar]
- 170.García-Hartmann M, Couperus AS, Addink MJ. The diagnosis of by-catch: preliminary results of research in the Netherlands. In: Kuiken T, editor; 1994. 2 March 1994; Montpellier, France: pp. 16–23. [Google Scholar]
- 171.Heje LB, Nars HC. Microscopical examination of bronchial fluid from harbour porpoises (Phocoena phocoena L.) for the presence of marine flora and fauna and mineral grains as a possible method to diagnose by-catch. In: Kuiken T, editor; 1994; Montpellier, France: pp. 4–9. [Google Scholar]
- 172.Rubini S, Frisoni P, Russotto C, Pedriali N, Mignone W, Grattarola C, et al. The diatoms test in veterinary medicine: A pilot study on cetaceans and sea turtles. Forensic science international. 2018; 290: e19–e23. 10.1016/j.forsciint.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 173.Panel-GEF (2012) Impacts of Marine Debris on Biodiversity: Current Status and Potential Solutions. In: Panel SotCoBDatSaTA, editor. Montreal: Technical Series pp. 61.
- 174.UNEP. Marine Litter: A Global Challenge. United Nations Environment Program 2009; Nairobi.
- 175.Ivar do Sul JA, Costa MF. Marine debris review for Latin America and the Wider Caribbean Region: From the 1970s until now, and where do we go from here? Mar Pollut Bull. 2007; 54 (8): 1087–1104. 10.1016/j.marpolbul.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 176.Baulch S, Perry C. Evaluating the impacts of marine debris on cetaceans. Mar Pollut Bull. 2014; 80 (1–2): 210–221. 10.1016/j.marpolbul.2013.12.050 [DOI] [PubMed] [Google Scholar]
- 177.Laist DW. Overview of the Biological Effects of Lost and Discarded Plastic Debris in the Marine-Environment. Mar Pollut Bull. 1987; 18 (6B): 319–326. [Google Scholar]
- 178.Laist DW. Impacts of marine debris: entanglement of marine life in marine debris, including a comprehensive list of species with entanglement In: Coe JM, Rogers DB, editors. Marine Debris-Sources, Impacts and Solutions. New York: Springer-Verlag; 1997. pp. 99–139. [Google Scholar]
- 179.Simmonds MP. Cetaceans and marine debris: the great unknown. J Mar Biol. 2012; Article ID 684279. [Google Scholar]
- 180.Gregory MR. Environmental implications of plastic debris in marine settings-entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos Trans R Soc Lond B Biol Sci. 2009; 364 (1526): 2013–2025. 10.1098/rstb.2008.0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Katsanevakis S. Marine debris, a growing problem: sources, distribution, composition and impacts In: Hofer TN, editor. Science, Science Publishers Inc; 2008. pp. 53–100. [Google Scholar]
- 182.Macfayden G, Huntinton T, Cappell R (2009) Abandoned, Lost or Otherwise Discarded Fishing Gear. In: Paper FFaAT, editor. UNEP Regional Seas Reports and Studies Rome: UNEP/FAO. pp. 115.
- 183.Teuten EL, Saquing JM, Knappe DR, Barlaz MA, Jonsson S, Bjorn A, et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc Lond B Biol Sci. 2009; 364 (1526): 2027–2045. 10.1098/rstb.2008.0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Di Beneditto AP, Ramos RM. Marine debris ingestion by coastal dolphins: What drives differences between sympatric species? Mar Pollut Bull. 2014; 83 (1): 298–301. 10.1016/j.marpolbul.2014.03.057 [DOI] [PubMed] [Google Scholar]
- 185.MacLeod CD. Understanding the impacts of human activities on beaked whales: from individuals to species, and from local to global. In: S. J. Dolman, C. D. MacLeod, P. G. H. Evans, editors; 2007 April San Sebastián, Spain.
- 186.Knowlton AR, Kraus SD. Mortality and serious injury of northern right whales (Eubalaena glacialis) in the western North Atlantic Ocean. J Cetacean Res Manage. 2001: 193–208. [Google Scholar]
- 187.Moore MJ, Bogomolni AL, Bowman R, Hamilton PK. Fatally entangled whales can die extremely slowly. Ocean’06 MTS/IEEE, Boston, MA: 2006. [Google Scholar]
- 188.Jacobsen JK, Massey L, Gulland F. Fatal ingestion of floating net debris by two sperm whales (Physeter macrocephalus). Mar Pollut Bull. 2010; 60 (5): 765–767. 10.1016/j.marpolbul.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 189.Walker WA, Coe JM, editors (1989) Survey of marine debris ingestion by odontocete cetaceans. Honolulu, Hawaii, USA: United States Department of Commerce; 747–774 p. [Google Scholar]
- 190.Poncelet E, van Canneyt O., Boubert J.-J. Considerable amount of plastic debris in the stomach of a Cuvier’s beaked whale (Ziphius cavirostris) washed ashore on the French Atlantic coast. European Research on Cetaceans. 2000; 14: 44–47. [Google Scholar]
- 191.Santos MB, Martin V, Arbelo M, Fernández A, Pierce GJ. Insights into the diet of beaked whales from the atypical mass stranding in the Canary Islands in September 2002. J Mar Biol Assoc UK. 2007; 87 (1): 243–251. [Google Scholar]
- 192.Santos MB, Pierce GJ, Herman J, Lopez A, Guerra A, Mente E, et al. Feeding ecology of Cuvier’s beaked whale (Ziphius cavirostris): a review with new information on the diet of this species. J Mar Biol Assoc UK. 2001; 81 (4): 687–694. [Google Scholar]
- 193.Denuncio P, Bastida R, Dassis M, Giardino G, Gerpe M, Rodríguez D. Plastic ingestion in Franciscana dolphins, Pontoporia blainvillei (Gervais and d’Orbigny, 1844), from Argentina. Mar Pollut Bull. 2011; 62 (8): 1836–1841. 10.1016/j.marpolbul.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 194.Faris F, Hart K (1994) Seas of debris: a summary of the third international conference on marine debris. Miami, Florida. [Google Scholar]
- 195.Tarpley RJ, Marwitz S. Plastic debris ingestion by cetaceans along the Texas coast: two case reports. Aquat Mamm. 1993; 19: 93–98. [Google Scholar]
- 196.Panigada S, Pesante G, Zanardelli M, Capoulade F, Gannier A, Weinrich MT. Mediterranean fin whales at risk from fatal ship strikes. Mar Pollut Bull. 2006; 52: 1287–1298. 10.1016/j.marpolbul.2006.03.014 [DOI] [PubMed] [Google Scholar]
- 197.Van der Hoop JM, Moore MJ, Barco SG, Cole TVN, Daoust PY, Henry AG, et al. Assessment of management to mitigate anthropogenic effects on large whales. Conserv Biol. 2013; 27 (1): 121–133. 10.1111/j.1523-1739.2012.01934.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Van Waerebeek K, Leaper R (2007) Report from the IWC vessel strike data standardization group. Anchorage: International Whaling Commission, Scientific Committee Document SC/59/BC12. 6 p.
- 199.Van Waerebeek K, Leaper R (2008) Second report of the IWC Vessel Strike Data Standardisation Working Group. Santiago, Chile: International Whaling Commission, Scientific Committee document SC/60/BC5 8p.
- 200.Laist DW, Knowlton AR, Mead JG, Collet AS, Podesta M. Collisions between ships and whales. Mar Mam Sci 2001; 17: 35–75. [Google Scholar]
- 201.André M, Terada M, Watanabe Y (1997) Sperm whale (Physeter macrocephalus) behavioral response after the playback of artificial sound. Report of the International Whaling Commission. 499–504 p.
- 202.Carrillo M, Fabián R. Increasing numbers of ship strikes in the Canary Islands: proposal for immediate action to reduce risk of vessel-whale collisions. J Cetacean Res Manage. 2010; 11: 131–138. [Google Scholar]
- 203.Carrillo M, Tejedor M (2006) Marine traffic and the conservation of sperm whale Physeter macrocephalus populations in Canary Islands. Cetacean stranded canarian net 1980–2004. Proceedings of the 20th Conference of the ECS. Gdynia, Polland.
- 204.Campbell-Malone R, Barco SG, Daoust PY, Knowlton AR, McLellan WA, Rotstein DS, et al. Gross and histologic evidence of sharp and blunt trauma in North Atlantic right whales (Eubalaena glacialis) killed by vessels. J Zoo Wildl Med. 2008; 39 (1): 37–55. 10.1638/2006-0057.1 [DOI] [PubMed] [Google Scholar]
- 205.Sierra E, Fernández A, Espinosa de los Monteros A, Arbelo M, Díaz-Delgado J, Andrada M, et al. Histopathological muscle findings may be essential for a definitive diagnosis of suspected sharp trauma associated with ship strikes in stranded cetaceans. PLoS One. 2014; 9 (2): e88780 10.1371/journal.pone.0088780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kraus SD. Rates and Potential Causes of Mortality in North-Atlantic Right Whales (Eubalaena glacialis). Mar Mam Sci. 1990; 6 (4): 278–291. [Google Scholar]
- 207.Reynolds JE, Odell DK, Rommel SA. Biology of marine mammals; Rommel SA, editor. Washington, DC: Smithsonian Institution Press; 1999. [Google Scholar]
- 208.Simmonds MP. The meaning of cetacean strandings. Bull Inst R Sci Nat Belg Biol. 1997; 67: 29–34. [Google Scholar]
- 209.Colgrove GS. Suspected transportation-associated myopathy in a dolphin. J Am Vet Med Assoc. 1978; 173 (9): 1121–1123. [PubMed] [Google Scholar]
- 210.Gales NJ. Mass stranding of striped dolphin, Stenella coeruleoalba, at Augusta, Western Australia: notes on clinical pathology and general observations. J Wildl Dis. 1992; 28 (4): 651–655. 10.7589/0090-3558-28.4.651 [DOI] [PubMed] [Google Scholar]
- 211.Bonsembiante F, Centelleghe C, Rossi G, Giglio S, Madeo E, Gelain ME, et al. Clinico-pathological findings in a striped dolphin (Stenella coeruleoalba) affected by rhabdomyolysis and myoglobinuric nephrosis (capture myopathy). J Vet Med Sci. 2017; 79 (6): 1013–1018. 10.1292/jvms.17-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sierra E, Espinosa de Los Monteros A, Fernández A, Díaz-Delgado J, Suárez-Santana C, Arbelo M, et al. Muscle pathology in free-ranging stranded cetaceans. Vet Pathol. 2017; 54 (2): 298–311. 10.1177/0300985816660747 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sex: female (F), male (M). Age: fetus (F), neonate (N), calf (C), juvenile (Jv), subadult (Sad), adult (Ad). Stranding date (SD; mm/dd/yy). Type of stranding (TS). Stranding location, island (IS): Gran Canaria (GC), Fuerteventura (FT), Lanzarote (LZ), Tenerife (TF), La Gomera (LG), El Hierro (EH), La Palma (LP), La Graciosa (LGra). Conservation status (CS): Very fresh (VF), fresh (F), moderate autolysis (MA), advanced autolysis (AA), very advanced autolysis (VAA).
(DOCX)
CD: Cluster of differentiation; CK: cytokeratin; GFAP: glial fibrillary acid protein, NSE: neuron specific enolase, MAC387: myeloid/histiocytic antigen; CDV: canine distemper virus; HSV1: Herpes Simplex virus 1: HIER: Heat-induced epitope retrieval; ABC: Avidin-Biotin-Peroxidase complex.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.