Abstract
Exposure of P. aeruginosa to the aminoglycoside (AG) paromomycin (PAR) induced expression of the PA3720-armR locus and the mexAB-oprM multidrug efflux operon that AmgR controls, although PAR induction of mexAB-oprM was independent of armR. Multiple AGs promoted mexAB-oprM expression and this was lost in the absence of the amgRS locus encoding an aminoglycoside-activated envelope stress-responsive 2-component system (TCS). Purified AmgR bound to the mexAB-oprM promoter region consistent with this response regulator directly regulating expression of the efflux operon. The thiol-active reagent, diamide, which, like AGs, promotes protein aggregation and cytoplasmic membrane damage also promoted AmgRS-dependent mexAB-oprM expression, a clear indication that the MexAB-OprM efflux system is recruited in response to membrane perturbation and/or circumstances that lead to this. Despite the AG and diamide induction of mexAB-oprM, however, MexAB-OprM does not appear to contribute to resistance to these agents.
Introduction
Pseudomonas aeruginosa is a common nosocomial human pathogen [1, 2] often associated with pulmonary infections in patients with cystic fibrosis [3]. The organism has an impressive intrinsic antimicrobial resistome [4] and readily develops resistance during antimicrobial therapy via mutation and horizontal gene transfer [5–7]. Major contributors to intrinsic and acquired antimicrobial resistance in P. aeruginosa are a number of broadly-specific multidrug efflux systems of the RND family [8]. One of these, MexAB-OprM, which contributes to both intrinsic [9] and acquired (i.e., mutational) [6] resistance, exhibits one of the broadest substrate profiles of the RND pumps in P. aeruginosa, accommodating a wide range of clinically-relevant [10–12] and experimental [13] antimicrobials as well as biocides [14] and a variety of non-clinical agents (e.g., organic solvents [15], dyes [16, 17], detergents [17], herbicides [18] and acylhomoserine lactones (AHLs) associated with quorum-sensing (QS) [19]. Clinically, this efflux system is most noted for its contribution to acquired fluoroquinolone and β-lactam resistance [6].
Expression of mexAB-oprM is controlled by several regulators including two repressors, MexR [20] and NalD [21], that each act at one of two promoters occurring distal (PI, MexR) [22] and proximal (PII, NalD) [21] to the efflux locus. Mutations in each of these genes are associated with efflux gene hyperexpression and multidrug resistance in both lab [20] [23] [24, 25] and clinical [26] [25, 27, 28] isolates. MexR binding to its promoter is modulated by its redox status (in response to oxidative stress) [29, 30] and by the ArmR anti-repressor, whose binding to MexR abrogates promoter binding by the repressor [31]. armR occurs as part of the PA3720-armR operon that is regulated by the product of the divergently-transcribed nalC repressor gene [32], with nalC mutants showing elevated PA3720-armR expression and, so, elevated mexAB-oprM expression and multidrug resistance [32] as a result of ArmR modulation of MexR's repressor activity [31]. The function of PA3720, a protein with no homology to any characterised protein, remains unknown. nalC lab and clinical isolates expressing mexAB-oprM and showing a multidrug-resistant phenotype have been reported [32] [33, 34]. Recently, a homologue of the E. coli CpxR envelope stress response regulator has been identified in P. aeruginosa and shown to bind to the PI promoter of mexAB-oprM, where it positively influences efflux gene expression [35]. Interestingly, mexAB-oprM expression and the multidrug resistance of MexR- nalB mutants were shown to be partially dependent on CpxR [35]. Finally, the BrlR regulator of antimicrobial tolerance of P. aeruginosa biofilms [36] has also been shown to bind to the mexAB-oprM promoter region where it positively influences expression of the efflux operon, which thus contributes to biofilm antimicrobial tolerance [37].
Despite the identification of a number of mexAB-oprM regulators, the naturally-occurring signals to which they respond and the intended substrates and, so, function of the efflux system remain largely unknown. Chlorinated phenols, including the pesticide pentachlorophenol (PCP) that is an uncoupler of oxidative phosphorylation [38], have been shown to induce expression of the PA3720-armR and mexAB-oprM operons, in part owing to their ability to bind and, so, modulate the repressor activity of NalC [39, 40]. Still, some PCP induction of mexAB-oprM is seen in mutants lacking the ArmR anti-repressor but dependent on MexR, an indication that this agent operates though multiple channels in driving expression of this efflux operon. In any case, it seems unlikely that PCP is the intended natural substrate/inducer but may mimic naturally-occurring plant-derived phenolic compounds that could be. NalD has been shown to bind novobiocin, a known MexAB-OprM substrate, with novobiocin binding abrogating NalD interaction with its DNA target, thereby enhancing PII promoter activity [41]. Still, it is far from clear that this gyrase-targeting [42, 43] antimicrobial is an intended inducer and substrate for this system. The identification of a CpxR homologue as a regulator of mexAB-oprM would suggest that the efflux system responds to envelope stress. Still, an envelope stress-response two-component system (TCS) that regulates genes reminiscent of E. coli CpxR targets, AmgRS, has already been described in P. aeruginosa, despite AmgRS being a homologue of the E. coli osmotic stress-responsive TCS, OmpR-EnvZ [44]. As such, P. aeruginosa CpxR may respond to signals other than those related to envelope perturbation. Finally, a recent report documents a transient, early log phase induction of mexAB-oprM in response to Ca2+ [45], although the regulator mediating this and the Ca2+-generated signal(s) to which the system is responding have not been identified.
The current study was undertaken to follow up a preliminary observation that exposure of P. aeruginosa to the aminoglycoside (AG), PAR, induced the PA3720-armR operon, which suggested that this antimicrobial might also upregulate mexAB-oprM expression. We confirm that it and, in fact, several AGs, do indeed induce mexAB-oprM expression. Surprisingly, however, this was independent of the ArmR anti-repressor and instead dependent on the aforementioned AmgRS envelope stress-responsive TCS. These results suggest that the generation of certain product(s) of envelope stress and/or cellular conditions that promote envelope perturbation require MexAB-OprM function and, so, promote it’s production.
Materials and methods
Bacterial strains and plasmids
Bacterial strains and plasmids used in this study are listed in Table 1. Plasmid pEX18Tc and its derivatives were maintained in E. coli with 5 (in L-broth) or 10 (on L-agar) μg/ml tetracycline. Plasmid pET23a and its derivatives were maintained in E. coli with ampicillin (100 μg/ml).
Table 1. Bacterial strains and plasmids.
| Strain | Relevant genotypea | Source |
|---|---|---|
| E. coli | ||
| DH5α | φ80ΔlacZΔM15 endA1 recA1 hsdR17(rK-mK+) supE44 thi-1 gyrA96 relA1 F- Δ(lacZYA-argF) U169 | [46] |
| S17-1 | thi pro hsdR recA Tra+ | [47] |
| E. coli Bl21 DE3 (pLysE) | F-ompTrB- mB-; DE3 is a lambda derivative carrying lacI and a T7 RNA polymerase gene under placUV5 control | [48] |
| P. aeruginosa | ||
| K767 | PAO1 wild-type P. aeruginosa | [49] |
| K1491 | K767ΔmexR | [50] |
| K3415 | K767ΔarmR | [39] |
| K1523 | K767ΔmexB | [51] |
| K1525 | K767ΔmexXY | [52] |
| K1542 | K767ΔmexXY ΔmexB | [53] |
| K3793 | K767ΔnalD | This study |
| K3794 | K767ΔmexR ΔnalD | This study |
| K3519 | K767ΔamgR | [54] |
| K3583 | K767ΔamgS | [54] |
| K3260 | K767 derivative carrying the amgSV121G mutation |
[54] |
| K3249 | K767 derivative carrying the amgSR182C mutation |
[54] |
| Plasmids | ||
| pET23a | Expression plasmid; ApR | Novagen |
| pMJF34 | pET23a::amgR | This study |
aTcR, tetracycline resistance;
ApR, ampicillin resistance.
DNA methods
Standard protocols were used for restriction endonuclease digestions, ligations, transformations and agarose gel electrophoresis, as previously described [55]. Plasmid DNA was extracted from E. coli using the Fermentas GeneJET Plasmid Miniprep Kit or the Qiagen Plasmid Midi Kit according to protocols provided by the manufacturers. Chromosomal DNA was extracted from P. aeruginosa using the Qiagen DNeasy Blood & Tissue Kit according to a protocol provided by the manufacturer. PCR products and restriction endonuclease digest products requiring purification were purified using the Promega Wizard SV Gel and PCR Clean-Up System according to a protocol provided by the manufacturer. Plasmid DNA was introduced into CaCl2-competent E. coli cells, which were prepared as previously described [55]. Oligonucleotide synthesis was performed by Integrated DNA Technologies (Coralville, Iowa), and nucleotide sequencing was performed by ACGT Corporation (Toronto, Canada).
Susceptibility testing
The antimicrobial susceptibility of various P. aeruginosa strains was assessed using the 2-fold microtiter broth dilution method as described previously [56]. The minimum inhibitory concentration (MIC) was recorded as the lowest concentration of antibiotic inhibiting visible growth after 18 hours of incubation at 37°C.
Quantitative RT-PCR
P. aeruginosa cells grown overnight in L-broth at 37°C were subcultured (1:49) in the same medium and incubated at 37°C until cultures reached an optical density at 600 nm (OD600) of 0.6–0.8. Total RNA was isolated, purified and reverse transcribed into cDNA as described previously [57]. Where specified, the antimicrobials paromomycin (PAR; 256 μg/ml), neomycin (NEO; 64 μg/ml), gentamicin (GEN; 2 μg/ml), tobramycin (TOB; 1 μg/ml), kanamycin (KAN; 128 μg/ml), chloramphenicol (CAM; 32 μg/ml), tetracycline (TET; 16 μg/ml), erythromycin (ERY; 512 μg/ml), azithromycin (AZI; 512 μg/ml) and diamide (DIA; 4 mM) were added at their respective MICs 30 minutes prior to harvesting cells. In some experiments, P. aeruginosa was pretreated with CAM (128 μg/mL) for 15 min prior to the addition of neomycin at its MIC. The primers used in quantitative real-time PCR (qPCR) were designed to amplify gene fragments with lengths of 138 bp (mexA; Forward: CAGCAGCTCTACCAGATCG; Reverse: CGTACTGCTGCTTGCTCA), 123 bp (armR; Forward: CAACAAACCGTCCCGCAC; Reverse: GTAGAGGTCCCAGGCATTGC) and 188 bp (PA3720; Forward: GATGCCTTTCCCTTGGTCCA; Reverse: TCCTTGAGCCACAACACCAG). The rpoD reference gene was amplified as described previously [58]. The amplification efficiencies of the qRT-PCR primer sets were 102.8% (correlation co-efficient, r2 = 0.997) for mexA, 100.3% for armR (r2 = 0.997) and 101.2% for PA3720 (r2 = 0.994). The qRT-PCR reaction mixtures, amplification parameters, and melt curve analyses were performed as previously described [39, 59]. The expression levels of mexA, PA3720 and armR were normalized to that of the reference gene rpoD using the ΔΔC(t) method provided by the CFX-manager software version 1.6 (Bio-Rad) and are reported as fold change relative to that in the wild-type P. aeruginosa PAO1 strain K767, unless otherwise specified. A minimum of three biological replicates each performed in triplicate were carried out for all samples. In all instances, no-template controls were carried out to ensure the absence of DNA contamination.
Expression and purification of AmgR
To facilitate the purification of AmgR, a C-terminal polyhistidine tag was engineered onto AmgR by cloning the amgR gene into plasmid pET23a. The amgR gene was amplified by PCR using primers AmgR-His-For (5’-GATCCATATGTCGAACCCTGCCGCCCT-3’; NdeI site underlined) and AmgR-His-Rev (5’-GATCCTCGAGGGCCTTGCGCGCGTTGCCGTC-3’; XhoI site underlined) in a 50 μl PCR mixture that contained 1 μg P. aeruginosa PAO1 strain K767 chromosomal DNA, 0.6 μM of each primer, 0.2 mM of each dNTP, 1 x Phusion GC buffer and 1 U of Phusion polymerase (New England Biolabs). The mixture was heated for 30 sec at 98°C, followed by 30 cycles of 30 sec at 98°C, 30 sec at 65.0°C, 30 sec at 72°C, concluding with 7 min at 72°C. The PCR product was gel-purified, digested with NdeI and XhoI and cloned into NdeI-XhoI-restricted pET23a to yield pET23a::amgR (pMJF34) encoding His-tagged AmgR. Following nucleotide sequencing of the cloned gene to confirm the absence of PCR-generated mutations, plasmid pMJF34 was introduced into E. coli Bl21(DE3) harbouring the pLysE plasmid via transformation. AmgR-His was then expressed and purified from 50 ml of culture as previously described [39], with the exception that IPTG-induced expression of the amgR gene on pMJF34 was carried out for 90 min.
Electrophoretic mobility shift assay
The binding of purified AmgR to a DNA fragment encompassing the intergenic region upstream of mexAB-oprM was assessed using the electrophoretic mobility shift assay (EMSA) as described previously [39]. The intergenic region was amplified on a 315-bp fragment using primers K9 and K10 [22] in a 50-μl reaction mixture containing 1 μg of purified P. aeruginosa PAO1 strain K767 chromosomal DNA, 0.6 μM of each primer, 0.2 mM of each dNTP, 1 x Phusion HF buffer, 5% (vol/vol) dimethylsulphoxide and 1 U of Phusion polymerase (New England Biolabs). The mixture was heated at 98°C for 30 sec, followed by 30 cycles of 30 sec at 98°C, 30 sec at 65.0°C, 15 sec at 72°C, concluding with 7 min at 72°C. The DNA fragment was gel purified and quantified using a NanodropTM 2000 Spectrophotometer (Thermo Fisher Scientific). To assess the specificity of any binding observed, excess sheared salmon sperm DNA (100 μM) was added to the reaction mixtures prior to the addition of AmgR.
Membrane depolarization assay
A previously described fluorometric assay [60] involving the membrane potential-sensitive dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3)], was employed to measure the degree of cytoplasmic membrane (CM) depolarization promoted by diamide treatment of P. aeruginosa. Briefly, early log phase (OD600 = 0.3–0.5) L-broth subcultures of P. aeruginosa were exposed (or not) to diamide (4 mM final concentration). Samples (5 mL) were taken immediately and then hourly over 3 h and incubated with DiBAC4(3) (Invitrogen, Burlington, Ontario, Canada; 10 μg/ml final concentration) in the dark for 5 min at 37°C. Bacteria were then pelleted and resuspended in phosphate-buffered saline [61] to a final OD600 of 0.1. The membrane depolarization-dependent fluorescence emitted by cells was measured using a Varian (now Agilent, Mississauga, Ontario, Canada) Cary Eclipse fluorescent spectrophotometer with excitation and emission wavelengths of 490 and 518, respectively.
Results
Aminoglycoside-induced expression of mexAB-oprM
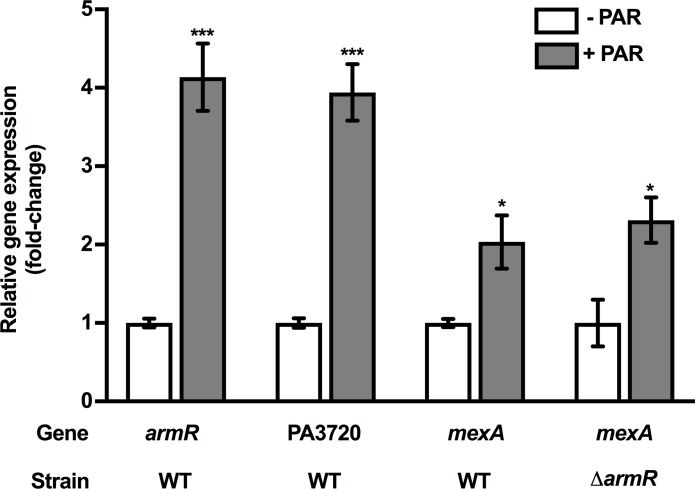
DNA microarray analysis of wild-type (WT) P. aeruginosa PAO1 strain K767 treated with the AG, PAR, revealed an increase in expression of the PA3720-armR two-gene operon (K. Poole, unpublished) whose products regulate expression of the mexAB-oprM multidrug efflux operon [31, 32]. To validate the results of the transcriptome analysis, log-phase K767 cells were similarly treated with the MIC of PAR, and expression of the PA3720 and armR genes were assessed using qRT-PCR. In agreement with the microarray data, treatment with PAR led to an increase (~4-fold) in expression of the PA3720 and armR genes (Fig 1). ArmR is an anti-repressor for the mexAB-oprM repressor [31], MexR, and its expression in so-called nalC mutants is responsible for the elevated mexAB-oprM expression and multidrug resistance of these mutants [32]. It was, therefore, hypothesized that the PAR-promoted increase in armR expression and, ultimately, ArmR production, would also drive derepression of the mexAB-oprM operon. Indeed, PAR provided for a ~2-fold increase in mexA gene expression (as a measure of mexAB-oprM expression) relative to the untreated K767 strain (Fig 1). Surprisingly, however, mexAB-oprM expression was still PAR-inducible in a mutant lacking armR (Fig 1), an indication that PAR induction of mexAB-oprM expression was not mediated by ArmR.
Fig 1. PAR induction of PA3720-armR and mexAB-oprM expression.
Expression of PA3720, armR and mexA was assessed in log-phase WT P. aeruginosa strain K767 following a 30-minute exposure to the MIC of the AG, paromomycin (PAR; 256 μg/ml) using qRT-PCR. mexA expression was also assessed in a ΔarmR derivative of K767, K3415, following exposure (30 min) to the MIC of PAR (256 μg/ml) using qRT-PCR. In all cases, expression was normalized to rpoD and is reported relative to the untreated WT P. aeruginosa strain K767 (fold-change). Values shown are means ± standard errors of the means (SEMs) from at least three independent determinations, each performed in triplicate. t-test: *, P < 0.05; ***, P < 0.001.
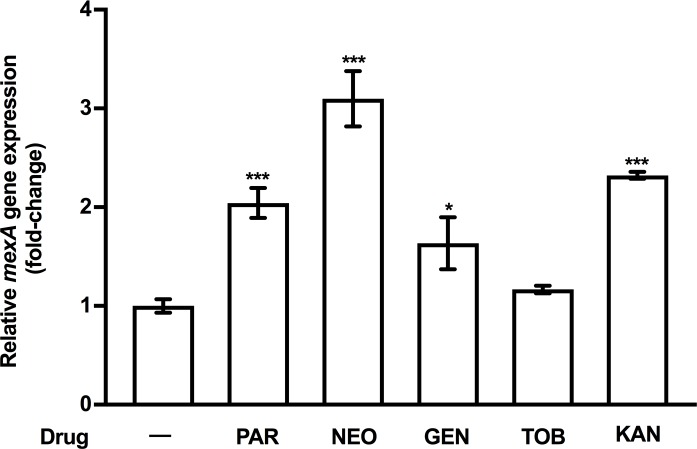
To assess whether PAR induction of this efflux system was specific to this AG or a more general property of this antimicrobial class, additional AGs were assessed for an ability to induce mexAB-oprM expression. Like PAR, the AGs KAN and NEO induced mexAB-oprM expression at their MICs, with NEO showing the best induction amongst all of the AGs tested (Fig 2). In contrast, GEN provided for a minimal increase in mexAB-oprM expression and TOB failed to induce this efflux operon, also at their MICs (Fig 2). Despite the induction of mexAB-oprM, however, this efflux system does not appear to contribute to AG resistance in P. aeruginosa–loss of MexAB-OprM in both WT strain K767 and a mutant derivative lacking the MexXY efflux system linked to intrinsic AG resistance did not impact AG susceptibility (Table 2).
Fig 2. AG induction of mexAB-oprM expression.
mexA expression was assessed in log-phase WT P. aeruginosa strain K767 following a 30-minute exposure to the MIC of the AGs, paromomycin (PAR; 256 μg/ml), neomycin (NEO; 64 μg/ml), gentamicin (GEN; 2 μg/ml), tobramycin (TOB; 1 μg/ml), and kanamycin (KAN; 128 μg/ml) using qRT-PCR. Expression was normalized to rpoD and is reported relative to the untreated K767 (fold change). Values are means ± SEMs from at least three independent determinations, each performed in triplicate. t-test: *, P < 0.05; ***, P < 0.001.
Table 2. Impact of mexAB-oprM loss on AG susceptibility.
| Strain | Genotype | MIC (μg/ml) for:a | |||
|---|---|---|---|---|---|
| GEN | PAR | NEO | KAN | ||
| K767 | WTb | 4 | 256 | 64 | 128 |
| K1523 | ΔmexB | 4 | 256 | 64 | 128 |
| K1525 | ΔmexXY | 2 | 32 | 32 | 64 |
| K1542 | ΔmexXY ΔmexB | 2 | 32 | 32 | 64 |
aGEN, gentamicin; PAR, paromomycin; NEO, neomycin; KAN, kanamycin.
bWT, wild type
AG induction of the mexAB-oprM operon is independent of the MexR and NalD repressors
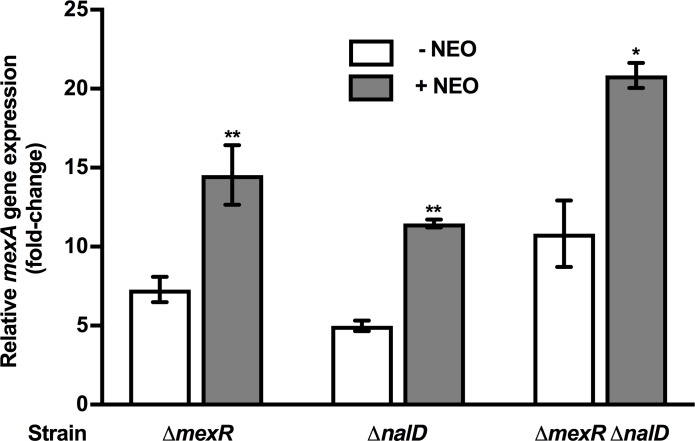
The mexAB-oprM operon is regulated by 2 direct repressors, MexR [22] and NalD [21], one or both of which might mediate the observed AG inducibility of this efflux operon. To assess this, the impact of a mexR or nalD deletion on AG induction of mexAB-oprM expression was assessed. If AGs somehow obviate MexR or NalD repressor activity in promoting increased mexAB-oprM expression, AG induction of mexAB-oprM expression should be lost in either a mexR or nalD knockout strain. Individual deletions of the mexR and nalD genes in the WT K767 strain led to the expected increase in mexAB-oprM expression (Fig 3). Still, exposure of these mutants to the AG, NEO, still produced an increase in mexAB-oprM expression (Fig 3). Similar results were obtained with a mutant lacking both repressor genes, where loss of both mexR and nalD provided for an increase in mexAB-oprM expression that was, nonetheless, elevated further upon exposure to NEO (Fig 3). Thus, AG induction of this efflux operon is independent of the MexR and NalD repressors.
Fig 3. MexR- and NalD-independent AG induction of mexAB-oprM expression.
mexA expression was assessed in P. aeruginosa strains K1491 (ΔmexR), K3793 (ΔnalD) and K3794 (ΔmexR ΔnalD) following a 30-minute exposure to the MIC of neomycin (NEO; 64 μg/ml for all strains) using qRT-PCR. Expression was normalized to rpoD and is reported relative to the untreated K767 strain (fold change). Values are means ± SEMs (error bars) from at least three independent determinations, each performed in triplicate. t-test: *, P < 0.05; **, P < 0.01.
AmgRS mediates AG induction of mexAB-oprM expression
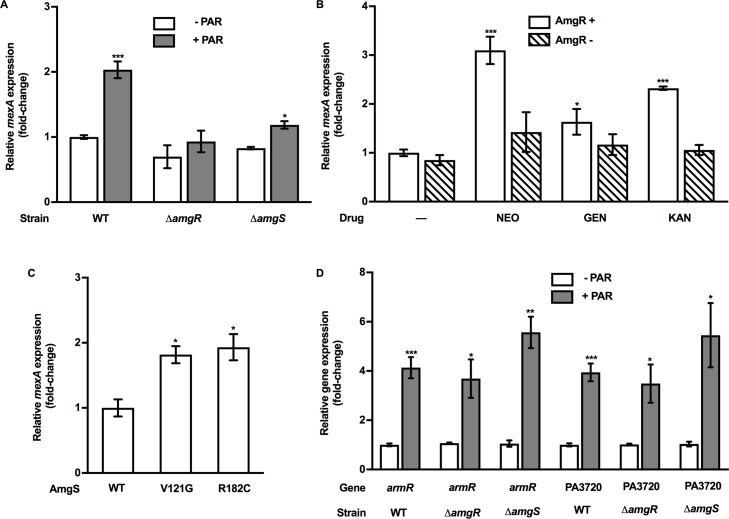
AmgRS, an AG-responsive TCS in P. aeruginosa that functions as part of an envelope stress response to AG-induced CM-damaging aberrant polypeptides, was recently shown to play a role in AG-induced expression of the AG resistance-promoting mexXY multidrug efflux operon [44, 54, 62]. This TCS might, thus, mediate the AG induction of mexAB-oprM. To assess this, the impact of AmgRS loss on PAR induction of mexAB-oprM expression was determined. As seen in Fig 4A, mutants lacking amgR or amgS were deficient for PAR-inducible expression of mexAB-oprM, an indication that AG induction of this efflux system was dependent on the AmgRS TCS. Consistent with this, mexAB-oprM expression promoted by the other AGs was similarly lost in the amgR deletion strain (Fig 4B). As expected, activation of AmgRS as a result of amgS gain-of-function mutations (V121G and R182C) also promoted an increase in mexAB-oprM expression, independent of AG exposure (Fig 4C). This did not, however, parallel an increase in resistance to representative MexAB-OprM substrate antimicrobials (e.g. carbenicillin, chloramphenicol, nalidixic acid; Table 3). As well, despite its link to mexAB-oprM and a common inducibility by PAR, the PA3720-armR operon retained its PAR inducibility in the amgR deletion strain (Fig 4D) an indication that these operons can be independently regulated.
Fig 4. AmgRS-dependent AG-inducible mexAB-oprM expression.
(A) mexA expression was assessed in log-phase cultures of WT P. aeruginosa strain K767 and its ΔamgR (strain K3159) and ΔamgS (strain K3583) derivatives following exposure to the MIC of PAR (K767, 256 μg/ml; K3159, 16 μg/ml; K3583, 16 μg/ml) for 30 min using qRT-PCR. (B) mexA expression was assessed in log-phase cultures of P. aeruginosa strains K767 (WT; AmgR+) and K3159 (ΔamgR; AmgR-) exposed to the MIC of the indicated antimicrobials (-, no antimicrobial; NEO, neomycin; GEN, gentamicin; KAN; kanamycin) using qRT-PCR. Antimicrobials were used at: NEO, 64 (K767) and 4 (K3159) μg/ml; GEN, 2 (K767) and 0.5 (K3159) μg/ml; KAN, 128 (K767) and 16 (K3159) μg/ml. (C) mexA expression was assessed in strains harbouring WT (K767) and mutant (K3288, AmgSV121G; K3249, AmgSR182C) amgS genes using qRT-PCR. (D) armR and PA3720 expression was assessed in log-phase cultures of P. aeruginosa strains K767 (WT), K3159 (ΔamgR), and K3583 (ΔamgS) exposed to the MIC of PAR for 30 min using qRT-PCR. Expression in all cases was normalized to rpoD and is reported relative to the untreated K767 strain (fold change). Values are means ± SEMs from at least three independent determinations, each performed in triplicate. t-test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Table 3. Impact of amgS gain-of-function mutations on antibiotic susceptibility.
| Strain | AmgSa | MIC (μg/ml) for:b | |||
|---|---|---|---|---|---|
| CAR | CAM | NAL | NOV | ||
| K767 | WT | 64 | 64 | 128 | 1024 |
| K3260 | V121G | 64 | 32 | 128 | 2048 |
| K3249 | R182C | 64 | 64 | 128 | 1024 |
aThe amino acid change in AmgS in the indicated mutants is highlighted. WT, wild type.
bCAR, carbenicillin; CAM, chloramphenicol; NAL, nalidixic acid; NOV, novobiocin.
AmgR binds to the mexAB-oprM promoter region
The regulation of mexAB-oprM by AmgRS is most simply explained by AmgR directly controlling expression of the efflux operon from a promoter upstream of the efflux genes. To assess this, binding of AmgR to the mexAB-oprM promoter region was assessed using an EMSA. As seen in Fig 5A, purified AmgR bound to a DNA fragment that encompassed the intergenic region upstream of mexAB-oprM. Moreover, binding was retained in the presence of excess competitor DNA (Fig 5B), an indication that binding was specific.
Fig 5. AmgR binds to the mexAB-oprM promoter region.
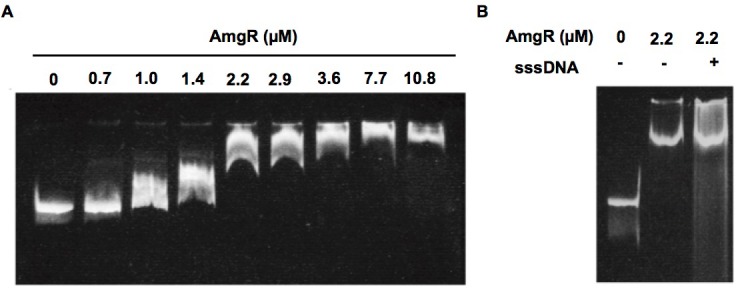
(A) Electromobility shift assay in which 40 ng of a 351-bp DNA fragment encompassing the intergenic region upstream of mexAB-oprM was incubated with increasing concentrations of purified AmgR as indicated. (B) Electromobility shift assay in which the above-mentioned 351-bp DNA fragment was incubated with the indicated concentration of AmgR in the absence (-) and presence (+) of 200 ng of sheared salmon sperm DNA (sssDNA).
Role of cytoplasmic membrane perturbation in AG-induced mexAB-oprM expression
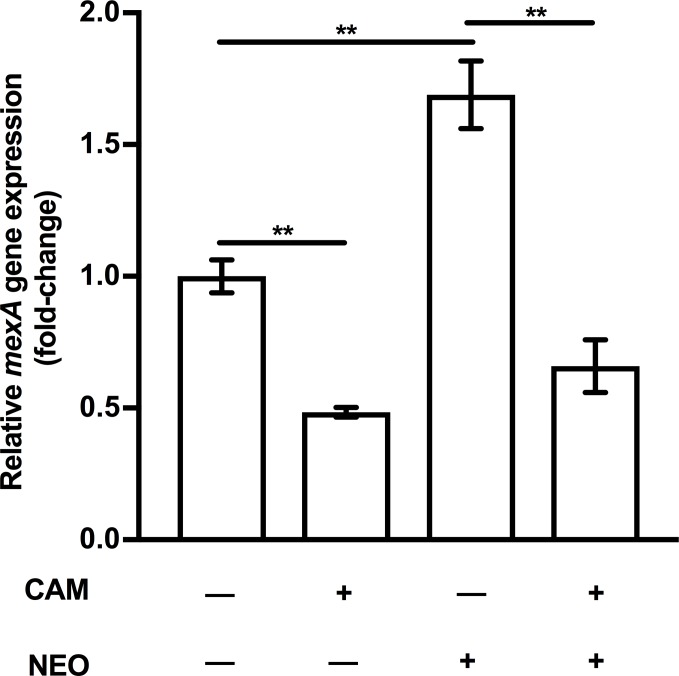
It has been demonstrated that AmgRS responds to and protects cells from envelope stress caused by AG-generated mistranslated/misfolded proteins that damage the cytoplasmic membrane (CM) [44, 54, 62]. Previous reports demonstrated that treatment of WT P. aeruginosa with CAM, a protein translation inhibitor, prior to the addition of an AG, blocked AG-promoted CM damage, consistent with is blockage of mistranslated protein synthesis [62]. To assess if AG-promoted CM damage is responsible for AmgRS-dependent induction of mexAB-oprM expression by AGs, WT P. aeruginosa K767 cells were pre-treated with CAM prior to the addition of the AG, NEO, and mexA expression was measured. CAM pre-treatment of cells prevented NEO induction of mexAB-oprM expression (Fig 6), consistent with it blocking AG-generated mistranslated/misfolded proteins and subsequent CM damage. Still, it was also observed that CAM alone negatively influenced mexAB-oprM expression (Fig 6). As such, it is unclear whether AG-induced mexAB-oprM expression follows from AG generation of membrane-damaging mistranslation products.
Fig 6. Impact of chloramphenicol on AG induction of mexAB-oprM expression.
mexA expression was assessed in log phase cells of P. aeruginosa strain K767 exposed to NEO (at MIC; 64 μg/ml) for 30 min without (-) and with (+) a 15-min pre-treatment with chloramphenicol (CAM; 128 μg/ml) using qRT-PCR. In a control experiment, K767 was also exposed to 128 μg/ml CAM in the absence of NEO, to assess its impact on mexA expression on its own. Expression was normalized to rpoD and is reported relative to the untreated K767 strain (fold change). Values are means ± SEMs from at least three independent determinations, each performed in triplicate. t-test: **, P < 0.01.
mexAB-oprM is inducible by additional ribosome-perturbing agents
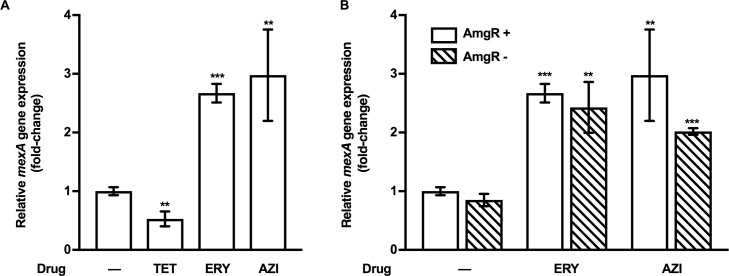
The observation that AG induction of mexAB-oprM is AmgRS-dependent and that CAM, another ribosome-targeting agent, fails to induce the efflux system, is consistent with AG-generated membrane-damaging mistranslation products being key to AG-promoted mexAB-oprM expression. As such, only mistranslation-promoting AGs are likely to induce this efflux system. To assess this, a number of additional agents that target the ribosome but do not promote mistranslation (TET, ERY, AZI) [63] were assessed for an ability to induce expression of the mexAB-oprM operon. As with CAM, treatment of WT P. aeruginosa K767 with the MIC of TET yielded a decrease in mexAB-oprM expression (2-fold; Fig 7A). In contrast, treatment of K767 with ERY or AZI increased mexAB-oprM gene expression (2.5-fold; Fig 7A). Still, this was independent of AmgR–induction of mexAB-oprM expression by ERY and AZI was retained in an amgR null mutant (Fig 7B; see K3519). Thus, ribosome perturbation alone is insufficient for AmgRS-promoted mexAB-oprM expression. While macrolide-induced mexAB-oprM expression is clearly mediated by a different regulatory pathway, it is unclear whether it follows from ribosome perturbation or some other impact of these agents on P. aeruginosa.
Fig 7. AmgRS-independent macrolide induction of mexAB-oprM expression.
(A) mexA expression was assessed in log-phase cultures of WT P. aeruginosa strain K767 exposed to the MIC of tetracycline (TET; 16 μg/ml), erythromycin (ERY; 512 μg/ml) and azithromycin (AZI; 512 μg/ml) for 30 min using qRT-PCR. Expression was normalized to rpoD and is reported to that of the untreated (-) strain K767. (B) mexA expression was assessed in log-phase cultures of P. aeruginosa strains K767 (AmgR+) and K3159 (AmgR-) exposed to the MIC of ERY or AZI (512 μg/ml for both drugs and both strains) for 30 min using qRT-PCR. In all cases, expression was normalized to rpoD and is reported relative to that of the untreated strain K767 (fold change). Values are means ± SEMs from at least three independent determinations, each performed in triplicate. t-test: **, P < 0.01; ***, P < 0.001.
Diamide-promoted mexAB-oprM expression
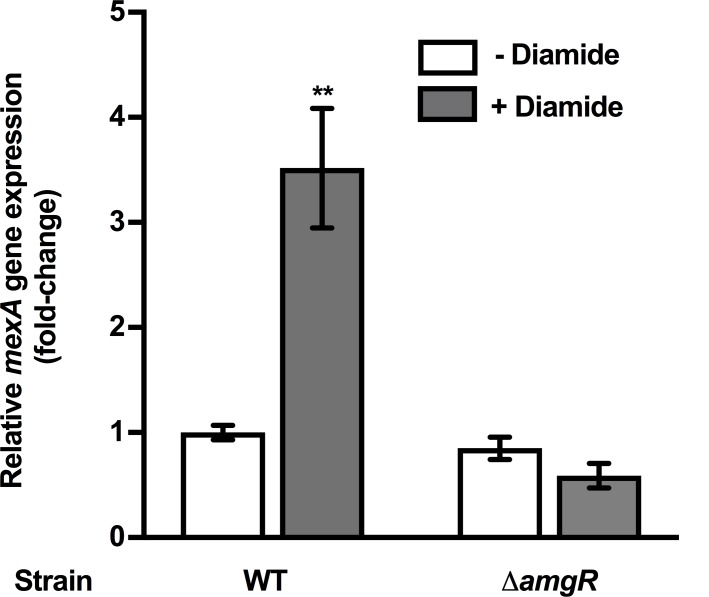
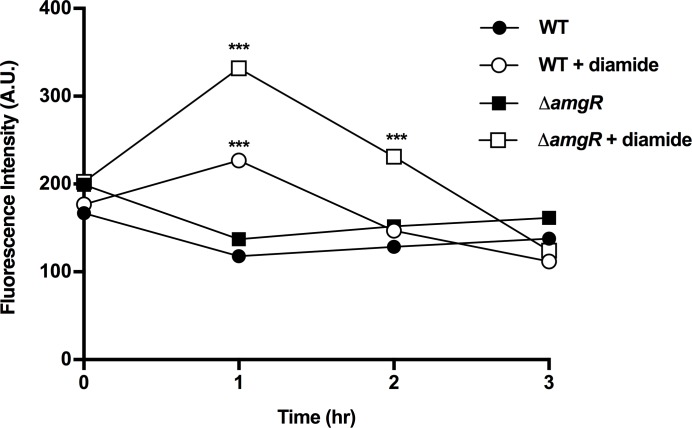
In addition to their contribution to CM damage, AGs [e.g. streptomycin (STR)] have also been shown to cause protein aggregation in E. coli, also as a result of AG-induced mistranslation [64]. Like AGs, the thiol-specific oxidant, diamide (DIA) has also been shown to promote protein misfolding and aggregation [65–67] although an impact on membranes has never been assessed. To assess whether protein aggregation might be playing a role in AmgRS-driven mexAB-oprM expression the impact of diamide on expression of the efflux operon was assessed. Treatment of WT P. aeruginosa strain K767 with the MIC of diamide induced mexAB-oprM expression (~3.5-fold; Fig 8) and this was dependent on AmgR (Fig 8). Interestingly diamide was also shown to be modestly CM perturbing, promoting a transient increase in membrane depolarization (at 1 h post-diamide addition; Fig 9) and, as with AGs [60, 62], this was enhanced in a mutant lacking amgR (Fig 9). Thus, AmgRS appears to protect P. aeruginosa from CM perturbation promoted by diamide.
Fig 8. AmgRS-dependent diamide induction of mexAB-oprM expression.
mexA expression was assessed in log-phase cultures of P. aeruginosa strains K767 (AmgR+) and K3159 (AmgR-) following a 30-min exposure to the MIC of diamide (4 mM for both strains) using qRT-PCR. Expression was normalized to rpoD and is reported to that of the untreated strain K767 (fold change). Values are means ± SEMs from at least three independent determinations, each performed in triplicate. t-test: **, P < 0.01.
Fig 9. Diamide-promoted cytoplasmic membrane depolarization.
Cytoplasmic membrane depolarization, as assessed by DiBAC4(3) fluorescence (arbitrary units; A.U.), was measured over time following exposure of WT strain P. aeruginosa K767 (circles) and its AmgR- derivative, K3159 (squares), to 4 mM diamide (filled symbols) at T = 0 hr. Unexposed cells are represented by unfilled symbols. The data are means ± SEMs of three independent experiments. Note that the symbols are larger than the error bars, which are thus not visible in the figure. t-test: ***, P < 0.001.
Discussion
AmgR represents yet another direct regulator of the mexAB-oprM multidrug efflux operon, highlighting the complexity of the regulation of this locus and the apparent diversity of signals and growth conditions to which it responds. Given the apparent responsiveness of the AmgRS TCS to envelope stress resultant from cytoplasmic membrane perturbation by AG-generated mistranslation products, it is likely that the observed AmgRS-dependent induction of mexAB-oprM by AGs reflects an envelope stress-inducibility of this efflux locus and, so, a need for MexAB-OprM under certain conditions of envelope stress. Consistent with this, another agent shown here to be membrane perturbing, diamide, also showed AmgRS-dependent induction of mexAB-oprM expression and this TCS appeared to protect P. aeruginosa somewhat from diamide-mediated membrane damage. While CM perturbation is not a heretofore reported property of diamide, it has been shown that a mutant of Xanthamonas campestris pv. campestris lacking the RpoE envelope stress response sigma is more sensitive to diamide than WT X. campestris [68], further support for this agent being membrane-damaging. It is interesting to note, too, that the AG induction of mexAB-oprM was variable and reflected the AG responsiveness of AmgRS [54] with PAR and NEO being the better inducers/activators while, for example, TOB failed to induce/activate (at 1X MIC) and, indeed, perturb membranes [62]. Still, despite the induction of this multidrug efflux locus by AGs and diamide, MexAB-OprM does not appear to play any role in resistance to these agents. It is also possible that AG induction of mexAB-oprM serves primarily to increase OprM levels, this outer membrane protein functioning with the proteins products of the similarly AmgRS-regulated and AG-inducible mexXY efflux operon, with MexXY-OprM a known contributor to AG resistance. Still, oprM appears to possess its own promoter [69], such that its expression can be driven independently of mexAB, to ensure, for example, adequate levels of OprM to partner with MexXY without the need for unnecessary and wasteful induction of the mexAB genes.
While attempts were made to show that AG-promoted mexAB-oprM expression was dependent on protein translation (as evidence for potentially membrane-damaging mistranslation products being key to AG induction of the efflux locus) the results were inconclusive since classical translation inhibitors (CAM and TET) themselves had a negative impact on mexAB-oprM expression, rendering the observed CAM prevention of AG-promoted mexAB-oprM expression not readily interpretable. Possibly, TET and CAM inhibition of translation interferes with endogenous mistranslation that might provide basal levels of membrane-damaging aberrant polypeptides that activate AmgRS and provide for some mexAB-oprM expression. Still, loss of amgR did not adversely affect endogenous mexAB-oprM expression, arguing against this. Alternatively, since CAM is known to induce expression of a 2nd RND type multidrug efflux operon, mexEF-oprN [70], and mutational upregulation of this efflux operon has been shown to parallel a possibly compensatory decrease in mexAB-oprM expression [71, 72], the CAM-driven decrease in mexAB-oprM expression may be secondary to its upregulation of mexEF-oprN. TET (and CAM) also induces the mexXY RND efflux operon [73] although there is as yet no evidence that this parallels a decrease in mexAB-oprM expression, and other mexXY inducers (AGs and macrolides) [73, 74] were shown here to promote mexAB-oprM expression. This may, however, simply reflect these ribosome-targeting agents impacting the P. aeruginosa in ways that require MexAB-OprM and, so, induce mexAB-oprM expression, masking the negative impact their possible induction of a 2nd efflux operon might otherwise have on it.
Adding to the complexity of mexAB-oprM regulation is the observed induction of this efflux locus by the macrolide antibiotics ERY and AZI. That such induction is independent of AmgRS, consistent with this TCS responding to membrane perturbation and macrolides not known to provide for membrane damage, speaks to a possibly additional mexAB-oprM regulator mediating this, though at this point one cannot rule out the involvement of one of the other regulators identified to date. Interestingly, and in contrast to results presented here, it has been reported that AZI suppresses mexAB-oprM expression in P. aeruginosa [75], which we can only attribute to differences in growth medium and/or the WT strain used in the two studies. The observed AG induction of the PA3720-armR operon that encodes the MexR antirepressor is also interesting, given that it does not provide for AG induction of mexAB-oprM expression and, so, suggests that this locus may also influence expression of additional loci.
It is becoming clear that the mexAB-oprM multidrug efflux locus is inducible by a number of antimicrobial agents although, with few exceptions, the actual inducing signals and intended efflux substrates are unknown. Thus, while clearly stress-responsive, the actual function of MexAB-OprM remains to be elucidated.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by an operating grant from the Canadian Institutes of Health Research to KP (MOP - 102740; http://www.cihr-irsc.gc.ca/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wieland K, Chhatwal P, Vonberg RP. Nosocomial outbreaks caused by Acinetobacter baumannii and Pseudomonas aeruginosa: Results of a systematic review. Amer J Infect Control. 2018. February 2 pil: S0196-6553(17)31335-4. 10.1016/j.ajic.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 2.Adam HJ, Baxter MR, Davidson RJ, Rubinstein E, Fanella S, Karlowsky JA, et al. Comparison of pathogens and their antimicrobial resistance patterns in paediatric, adult and elderly patients in Canadian hospitals. J Antimicrob Chemother. 2013;68 Suppl 1:i31–37. [DOI] [PubMed] [Google Scholar]
- 3.Hauser AR, Jain M, Bar-Meir M, McColley SA. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin Microbiol Rev. 2011;24:29–70. 10.1128/CMR.00036-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivares J, Bernardini A, Garcia-Leon G, Corona F, M BS, Martinez JL. The intrinsic resistome of bacterial pathogens. Front Microbiol. 2013;4:103 10.3389/fmicb.2013.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breidenstein EB, de la Fuente-Nunez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011;19:419–426. 10.1016/j.tim.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 6.Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol. 2011;2:65 10.3389/fmicb.2011.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Causape C, Cabot G, Del Barrio-Tofino E, Oliver A. The versatile mutational resistome of Pseudomonas aeruginosa. Front Microbiol. 2018;9:685 10.3389/fmicb.2018.00685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poole K. Pseudomonas efflux pumps In: Yu EW, Zhang Q, Brown MH, editors. Microbial efflux pumps. Norfolk, UK: Caister Academic Press; 2013. pp. 175–206. [Google Scholar]
- 9.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44:3322–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorth P, McLean K, Ratjen A, Secor PR, Bautista GE, Ravishankar S, et al. Evolved aztreonam resistance is multifactorial and can produce hypervirulence in Pseudomonas aeruginosa. mBio. 2017;8. pil: e00517-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalhoub H, Pletzer D, Weingart H, Braun Y, Tunney MM, Elborn JS, et al. Mechanisms of intrinsic resistance and acquired susceptibility of Pseudomonas aeruginosa isolated from cystic fibrosis patients to temocillin, a revived antibiotic. Sci Rep. 2017;7:40208 10.1038/srep40208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caughlan RE, Jones AK, Delucia AM, Woods AL, Xie L, Ma B, et al. Mechanisms decreasing in vitro susceptibility to the LpxC inhibitor CHIR-090 in the gram-negative pathogen Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012;56:17–27. 10.1128/AAC.05417-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuanchuen R, Beinlich K, Hoang TT, Becher A, Karkhoff-Schweizer RR, Schweizer HP. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob Agents Chemother. 2001;45:428–32. 10.1128/AAC.45.2.428-432.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li XZ, Zhang L, Poole K. Role of the multidrug efflux systems of Pseudomonas aeruginosa in organic solvent tolerance. J Bacteriol. 1998;180:2987–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srikumar R, Poole K. Demonstration of ethidium bromide efflux by multiresistant pumps of Pseudomonas aeruginosa. Clin Microbiol Infect. 1999;5:5S58–5S59. [Google Scholar]
- 17.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother. 1998;42:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ketelboeter LM, Bardy SL. Characterization of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione resistance in pyomelanogenic Pseudomonas aeruginosa DKN343. PLoS One. 2017;12:e0178084 10.1371/journal.pone.0178084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans K, Passador L, Srikumar R, Tsang E, Nezezon J, Poole K. Influence of the MexAB-OprM multidrug efflux system on quorum-sensing in Pseudmonas aeruginosa. J Bacteriol. 1998;180:5443–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srikumar R, Paul CJ, Poole K. Influence of mutations in the mexR repressor gene on expression of the MexA-MexB-OprM multidrug efflux system of Pseudomonas aeruginosa. J Bacteriol. 2000;182:1410–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita Y, Cao L, Gould G, Avison MB, Poole K. nalD encodes a second repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa. J Bacteriol. 2006;188:8649–8654. 10.1128/JB.01342-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans K, Adewoye L, Poole K. MexR repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa: identification of MexR binding sites in the mexA-mexR intergenic region. J Bacteriol. 2001;183:807–812. 10.1128/JB.183.3.807-812.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito K, Yoneyama H, Nakae T. nalB-type mutations causing the overexpression of the MexAB-OprM efflux pump are located in the mexR gene of the Pseudomonas aeruginosa chromosome. FEMS Microbiol Lett. 1999;179:67–72. [DOI] [PubMed] [Google Scholar]
- 24.Vestergaard M, Paulander W, Marvig RL, Clasen J, Jochumsen N, Molin S, et al. Antibiotic combination therapy can select for broad-spectrum multidrug resistance in Pseudomonas aeruginosa. Int J Antimicrob Agents. 2016;47:48–55. 10.1016/j.ijantimicag.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 25.Sobel ML, Hocquet D, Cao L, Plesiat P, Poole K. Mutations in PA3574 (nalD) lead to increased MexAB-OprM expression and multidrug resistance in lab and clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:1782–1786. 10.1128/AAC.49.5.1782-1786.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jalal S, Ciofu O, Hoiby N, Gotoh N, Wretlind B. Molecular mechanisms of fluoroquinolone resistance in Pseudomonas aeruginosa isolates from cystic fibrosis. Antimicrob Agents Chemother. 2000;44:710–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziha-Zarifi I, Llanes C, Koehler T, Pechere JC, Plesiat P. In vivo emergence of multidrug-resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob Agents Chemother. 1999;43:287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choudhury D, Ghose A, Dhar Chanda D, Das Talukdar A, Dutta Choudhury M, Paul D, et al. Premature termination of MexR Leads to overexpression of MexAB-OprM efflux pump in Pseudomonas aeruginosa in a tertiary referral hospital in India. PLoS One. 2016;11:e0149156 10.1371/journal.pone.0149156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Hu J, Chen PR, Lan L, Li Z, Hicks LM, et al. The Pseudomonas aeruginosa multidrug efflux regulator MexR uses an oxidation-sensing mechanism. Proc Natl Acad Sci USA. 2008;105:13586–13591. 10.1073/pnas.0803391105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Yi C, Zhang J, Zhang W, Ge Z, Yang CG, et al. Structural insight into the oxidation-sensing mechanism of the antibiotic resistance of regulator MexR. EMBO Rep. 2010;11:685–690. 10.1038/embor.2010.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daigle DM, Cao L, Fraud S, Wilke MS, Pacey A, Klinoski R, et al. Protein modulator of multidrug efflux gene expression in Pseudomonas aeruginosa. J Bacteriol. 2007;189:5441–5451. 10.1128/JB.00543-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao L, Srikumar R, Poole K. MexAB-OprM hyperexpression in NalC type multidrug resistant Pseudomonas aeruginosa: identification and characterization of the nalC gene encoding a repressor of PA3720-PA3719. Mol Microbiol. 2004;53:1423–1436. 10.1111/j.1365-2958.2004.04210.x [DOI] [PubMed] [Google Scholar]
- 33.Llanes C, Hocquet D, Vogne C, Benali-Baitich D, Neuwirth C, Plesiat P. Clinical strains of Pseudomonas aeruginosa overproducing MexAB-OprM and MexXY efflux pumps simultaneously. Antimicrob Agents Chemother. 2004;48:1797–1802. 10.1128/AAC.48.5.1797-1802.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braz VS, Furlan JP, Fernandes AF, Stehling EG. Mutations in NalC induce MexAB-OprM overexpression resulting in high level of aztreonam resistance in environmental isolates of Pseudomonas aeruginosa. FEMS Microbiol Lett. 2016;363. pil: fnw166. [DOI] [PubMed] [Google Scholar]
- 35.Tian ZX, Yi XX, Cho A, O'Gara F, Wang YP. CpxR activates MexAB-OprM efflux pump expression and enhances antibiotic resistance in both laboratory and clinical nalB-type Isolates of Pseudomonas aeruginosa. PLoS Pathogens. 2016;12:e1005932 10.1371/journal.ppat.1005932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao J, Sauer K. The MerR-like transcriptional regulator BrlR contributes to Pseudomonas aeruginosa biofilm tolerance. J Bacteriol. 2012;194:4823–4836. 10.1128/JB.00765-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao J, Schurr MJ, Sauer K. The MerR-like regulator BrlR confers biofilm tolerance by activating multidrug efflux pumps in Pseudomonas aeruginosa biofilms. J Bacteriol. 2013;195:3352–3363. 10.1128/JB.00318-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinbach EC, Garbus J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature. 1969;221:1016–1018. [DOI] [PubMed] [Google Scholar]
- 39.Starr LM, Fruci M, Poole K. Pentachlorophenoln induction of the Pseudomonas aeruginosa mexAB-oprM efflux operon: involvement of repressors NalC and MexR and the antirepressor ArmR. PLoS ONE. 2012;7:e32684 10.1371/journal.pone.0032684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh S, Cremers CM, Jakob U, Love NG. Chlorinated phenols control the expression of the multi-drug resistance efflux pump MexAB-OprM in Pseudomonas aeruginosa by activating NalC. Mol Microbiol. 2011;79:1547–1556. 10.1111/j.1365-2958.2011.07544.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, Wang D, Zhou W, Sang H, Liu X, Ge Z, et al. Novobiocin binding to NalD induces the expression of the MexAB-OprM pump in Pseudomonas aeruginosa. Mol Microbiol. 2016;100:749–758. 10.1111/mmi.13346 [DOI] [PubMed] [Google Scholar]
- 42.Sugino A, Higgins NP, Brown PO, Peebles CL, Cozzarelli NR. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978;75:4838–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamour V, Hoermann L, Jeltsch JM, Oudet P, Moras D. An open conformation of the Thermus thermophilus gyrase B ATP-binding domain. J Biol Chem. 2002;277:18947–18953. 10.1074/jbc.M111740200 [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Hinz A, Bauerle E, Angermeyer A, Juhaszova K, Kaneko Y, et al. Targeting a bacterial stress response to enhance antibiotic action. Proc Natl Acad Sci USA. 2009;106:14570–14575. 10.1073/pnas.0903619106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khanam S, Guragain M, Lenaburg DL, Kubat R, Patrauchan MA. Calcium induces tobramycin resistance in Pseudomonas aeruginosa by regulating RND efflux pumps. Cell Calcium. 2017;61:32–43. 10.1016/j.ceca.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 46.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, et al. Short protocols in molecular biology, 2nd ed New York: John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 47.Simon R, Priefer U, Puehler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology. 1983;1:784–791. [Google Scholar]
- 48.Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. [DOI] [PubMed] [Google Scholar]
- 49.Masuda N, Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1847–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adewoye L, Sutherland A, Srikumar R, Poole K. The MexR repressor of the mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: characterization of mutations compromising activity. J Bacteriol. 2002;184:4308–4312. 10.1128/JB.184.15.4308-4312.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirakata Y, Srikumar R, Poole K, Gotoh N, Suematsu T, Kohno S, et al. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J Exp Med. 2002;196:109–118. 10.1084/jem.20020005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sobel ML, McKay GA, Poole K. Contribution of the MexXY multidrug transporter to aminoglycoside resistance in Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2003;47:3202–3207. 10.1128/AAC.47.10.3202-3207.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraud S, Campigotto AJ, Chen Z, Poole K. The MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa: involvement in chlorhexidine resistance and induction by membrane damaging agents dependent upon the AlgU stress-response sigma factor. Antimicrob Agents Chemother. 2008;52:4478–4482. 10.1128/AAC.01072-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lau CH, Fraud S, Jones M, Peterson SN, Poole K. Mutational activation of the AmgRS two-component system in aminoglycoside-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57:2243–2251. 10.1128/AAC.00170-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sambrook J, Russell DW. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 56.Jo JT, Brinkman FS, Hancock RE. Aminoglycoside efflux in Pseudomonas aeruginosa: involvement of novel outer membrane proteins. Antimicrob Agents Chemother. 2003;47:1101–1111. 10.1128/AAC.47.3.1101-1111.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Purssell A, Poole K. Functional characterization of the NfxB repressor of the mexCD-oprJ multidrug efflux operon of Pseudomonas aeruginosa. Microbiology. 2013;159:2058–2073. 10.1099/mic.0.069286-0 [DOI] [PubMed] [Google Scholar]
- 58.Lau CHF, Fraud S, Jones M, Peterson SN, Poole K. Reduced expression of the rplU-rpmA ribosomal protein operon in mexXY-expressing pan-aminmoglycoside-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012;56:5171–5179. 10.1128/AAC.00846-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Starr LM, Fruci M, Poole K. Pentachlorophenol induction of the Pseudomonas aeruginosa mexAB-oprM efflux operon: involvement of repressors NalC and MexR and the antirepressor ArmR. PLoS ONE. 2012;7:e32684 10.1371/journal.pone.0032684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krahn T, Gilmour C, Tilak J, Fraud S, Kerr N, Lau CH, et al. Determinants of intrinsic aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012;56:5591–5602. 10.1128/AAC.01446-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nehme D, Li XZ, Elliot R, Poole K. Assembly of the MexAB-OprM multidrug efflux system of Pseudomonas aeruginosa: identification and characterization of mutations in mexA compromising MexA multimerization and interaction with MexB. J Bacteriol. 2004;186:2973–2983. 10.1128/JB.186.10.2973-2983.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau CH, Krahn T, Gilmour C, Mullen E, Poole K. AmgRS-mediated envelope stress-inducible expression of the mexXY multidrug efflux operon of Pseudomonas aeruginosa. MicrobiologyOpen. 2015;4:121–135. 10.1002/mbo3.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson DN. On the specificity of antibiotics targeting the large ribosomal subunit. Annals NY Acad Sci. 2011;1241:1–16. [DOI] [PubMed] [Google Scholar]
- 64.Ling J, Cho C, Guo LT, Aerni HR, Rinehart J, Soll D. Protein aggregation caused by aminoglycoside action is prevented by a hydrogen peroxide scavenger. Mol Cell. 2012;48:713–722. 10.1016/j.molcel.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engman J, von Wachenfeldt C. Regulated protein aggregation: a mechanism to control the activity of the ClpXP adaptor protein YjbH. Mol Microbiol. 2015;95:51–63. 10.1111/mmi.12842 [DOI] [PubMed] [Google Scholar]
- 66.Runde S, Moliere N, Heinz A, Maisonneuve E, Janczikowski A, Elsholz AK, et al. The role of thiol oxidative stress response in heat-induced protein aggregate formation during thermotolerance in Bacillus subtilis. Mol Microbiol. 2014;91:1036–1052. 10.1111/mmi.12521 [DOI] [PubMed] [Google Scholar]
- 67.Muller A, Hoffmann JH, Meyer HE, Narberhaus F, Jakob U, Leichert LI. Nonnative disulfide bond formation activates the σ32-dependent heat shock response in Escherichia coli. J Bacteriol. 2013;195:2807–2816. 10.1128/JB.00127-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bordes P, Lavatine L, Phok K, Barriot R, Boulanger A, Castanie-Cornet MP, et al. Insights into the extracytoplasmic stress response of Xanthomonas campestris pv. campestris: role and regulation of σE-dependent activity. J Bacteriol. 2011;193:246–264. 10.1128/JB.00884-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Q, Li XZ, Srikumar R, Poole K. Contribution of outer membrane efflux protein OprM to antibiotic resistance in Pseudomonas aeruginosa independent of MexAB. Antimicrob Agents Chemother. 1998;42:1682–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fetar H, Gilmour C, Klinoski R, Daigle DM, Dean CR, Poole K. mexEF-oprN multidrug efflux operon of Pseudomonas aeruginosa: regulation by the MexT activator in response to nitrosative stress and chloramphenicol. Antimicrob Agents Chemother. 2011;55:508–514. 10.1128/AAC.00830-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li XZ, Barre N, Poole K. Influence of the MexA-MexB-oprM multidrug efflux system on expression of the MexC-MexD-OprJ and MexE-MexF-OprN multidrug efflux systems in Pseudomonas aeruginosa. J Antimicrob Chemother. 2000;46:885–893. [DOI] [PubMed] [Google Scholar]
- 72.Maseda H, Sawada I, Saito K, Uchiyama H, Nakae T, Nomura N. Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2004;48:1320–1328. 10.1128/AAC.48.4.1320-1328.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morita Y, Sobel ML, Poole K. Antibiotic inducibility of the MexXY multidrug efflux system of Pseudomonas aeruginosa: involvement of the antibiotic-inducible PA5471 gene product. J Bacteriol. 2006;188:1847–1855. 10.1128/JB.188.5.1847-1855.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeannot K, Sobel ML, El Garch F, Poole K, Plesiat P. Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug-ribosome interaction. J Bacteriol. 2005;187:5341–5346. 10.1128/JB.187.15.5341-5346.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sugimura M, Maseda H, Hanaki H, Nakae T. Macrolide antibiotic-mediated down regulation of MexAB-OprM efflux pump expression in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52:4141–4144. 10.1128/AAC.00511-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.