Abstract
Phellinus igniarius, which is called Sanghuang in Chinese, is a fungal herb widely used in Traditional Chinese Medicine to treat stomachache, inflammation and tumors. Recent studies have demonstrated the antitumor, anti-diabetic, anti-inflammatory and immunity-modulating activities of P. igniarius. In the present study, we investigated that ameliorating effect of the aqueous extract of P. igniarius fruiting body (SH) on dextran sodium sulfate (DSS)-induced colitis in C57BL/6 mice. Treatment with SH (250 and 400 mg/kg) for 8 weeks effectively alleviated the pathological indicators of colitis such as bodyweight reduction, disease activity index score, shortening of colon length and abnormal colon histology. The plasma levels of lipopolysaccharide (LPS) and inflammatory factors such as interleukin-6 (IL-6), IL-1β and tumor necrosis factor (TNF)-α were all significantly reduced. Supplementation of SH (10 mg/L) also inhibited LPS-elicited IL-1β production by RAW264.7 macrophages. Real-time PCR and western blot showed that treatment with SH significantly inhibited the phosphorylation of nuclear factor kappa B inhibitor alpha (IκBα) and decreased the expression of IL-6/IL-1β-maturation genes such as apoptosis-associated speck-like protein (ASC3) and caspase-1 in the colon of DSS-induced colitis mice. These results suggest that SH is adequate for the treatment of colitis. Inhibiting the expression and release of inflammatory factors may participate in the colitis-ameliorating effect of SH.
Introduction
Inflammatory bowel disease (IBD) is a chronic remittent and relapsing inflammatory condition, which is characterized by chronic inflammation in the colon and mainly comprises ulcerative colitis (UC) and Crohn’s disease (CD)[1]. The typical symptoms of IBD are diarrhea, rectal bleeding, intermittent abdominal pain and weight loss, seriously deteriorate the quality of life and lead to increased risk of colon cancer[1]. The precise pathogenesis of IBD has not been completely elucidated, but the chronic relapsing inflammation is implicated to the exaggerated immune response to gut microbiota. This dysregulated response results in increased expression and secretion of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interferon-γ, interleukin (IL)-1β and IL-6, which ultimately damages colon tissues[2].
Lipopolysaccharide (LPS) is an important immune stimulatory factor that is derived from bacteria and induces systemic inflammation[3]. Intensive investigations have shown that LPS triggers inflammation through a series of interactions with multiple proteins including Toll-like receptor 4 (TLR4), Myeloid differentiation primary response 88 (MyD88), TNF receptor associated factor 6 (TRAF6), mitogen-activated protein kinases (MAPKs) and nuclear factor kappa B (NFκB), which ultimately results in enhanced expression of pro-inflammatory cytokines such as IL-1β and IL-6 [4, 5]. LPS also stimulates inflammatory through activation of the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome which consists of NLRP3, caspase-1, and apoptosis-associated speck-like protein (ASC3) [6].
The medicinal mushroom Phellinus igniarius (Linnearus: Fries) Quél, which is called Sanghuang in Chinese, is a basidiomycetous fungus belonging to the Hymenochaetaceae family in Basidiomycetes [7, 8]. The fruiting body of P. igniarius has been widely used in Traditional Chinese Medicine to treat stomachache, inflammation and tumors [8]. Multiple investigations have demonstrated that the extract of P. igniarius possesses antitumor, anti-diabetes and immunity-modulating effect [7–14]. Polyphenols and polysaccharides are implicated as the major active components of P. igniarius [9, 11]. Although multiple pharmacological activities of P. igniarius have been demonstrated [12], whether it is beneficial for the treatment of colitis is largely unknown.
In the present study, the effect of the aqueous extract of P. igniarius fruiting body (SH) on dextran sodium sulfate (DSS)-induced colitis was investigated in C57BL/6 mice. The therapeutic efficiency was evaluated by monitoring the colitis symptoms, histopathological damage of colon and plasma levels of inflammatory cytokines. The potential mechanisms underlying SH-elicited anti-inflammation effects were also explored.
Materials and methods
Preparation of Phellinus igniarius extract (SH)
The fruiting body of cultured P. igniarius was obtained from Xin Hong Biotech Corp., Ltd. (Hai Ning, Zhejiang province, China). The fruiting body was cut into slices and dried. Then the samples were boiled with water (w/v = 1:10) for 1 h, the filtrate was separated with filter, and the residue was further extracted under the same condition twice. The filtrates collected from three separate extractions were combined, concentrated rotary evaporation and lyophilized by freeze vacuum drying. The dried extract of P. igniarius was collected, weighed and stored at -20°C until use. One kilogram of P. igniarius fruiting body yielded 226 g of P. igniarius aqueous extract (SH). The polyphenol content in SH was 37.23% as determined by colorimetric method[15].
Animals and experiment design
All the animal experiments were performed in accordance with the National Institutes of Health regulations for the care and use of animals in research and were approved by the Medical Ethics Committee of Zhejiang Academy of Agricultural Sciences (No.ZAAS-20171005021). All efforts were taken to minimize animals suffering.
Forty male C57BL/6 mice (6-week-old, male) were purchased from Shanghai Experimental Animal Center (Shanghai, China). The mice were acclimatized under standard conditions for 1 week, fed with standard laboratory chow and kept in an animal room with 12-h light/12-h dark cycles. Animals were then randomly divided into 4 groups (n = 10 per group), which are named Normal, DSS, DSS+SH-250 and DSS+SH-400. Chronic colitis was established by supplementation of dextran sodium sulfate (DSS) (molecular weight 36–50 kDa, MP Biologicals, USA) into drinking water (1.0% w/v) for 48 days. The Normal group was not induced colitis and was administered with drinking water. The DSS group was established colitis and continued to drink DSS only. The DSS+SH-250 and DSS+SH-400 groups were established colitis and treated with SH at dosage of 250 and 400 mg/kg/day, respectively. Body weight and disease activity index were assessed every 3 days. At the end of the experiment, animals were fasted overnight and blood samples were collected for estimation of plasma parameters by kits. Animals were then euthanized by spinal dislocation after ether anesthesia, and the colon tissue was taken for histological and biochemical analysis.
Evaluation of disease activity index (DAI)
The DAI was used for evaluating the grade and extent of intestinal inflammation [16]. Stool consistency and bloody stool were monitored every 3 days for determination of DAI. Each parameter was scored as follows: diarrhea (0, normal; 2, loose stools; 4, watery diarrhea) and bloody stool (0, normal; 2, slight bleeding; 4, gross bleeding). The DAI score ranged from 0 to 8 (total score).
Histological analysis
The entire colon of each mouse was taken, and its length was immediately recorded. Then a piece of colon tissue was removed from each mouse, fixed in 4% paraformaldehyde in PBS, embedded in paraffin, and cut into 5-μm-thick sections. The sections were stained with hematoxylin and eosin (H&E) and the histology of colon tissue was examined under a light microscopy (Nikon Co., Japan).
Cell experiment
RAW264.7 cells were originated from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and obtained from the Peking Union Medical College (Beijing, China). RAW264.7 cells, were maintained in DMEM containing 10% FBS at 37 °C and 5% CO2. When grown to 70–80% confluence, cells were kept in serum-free DMEM and pre-incubated with SH (10 mg/L) for 24 h, then treated with LPS (10 ng/mL) for 4 h. The blank group was incubated with serum-free DMEM alone. After LPS treatment, the medium level of IL-1β was determined using ELISA kit (Cell signaling, Beverly, USA) according to the instruction of manufacturer.
Measurement of plasma cytokines
The plasma concentrations of lipopolysaccharide (LPS), interleukin-6 (IL-6), IL-1β and tumor necrosis factor (TNF)-α were assessed using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis MN) according to the manufacturer’s instructions. Biotin-conjugated secondary antibodies were used, followed by streptavidin-HRP (Amersham Biosciences, Arlington Heights, IL) and an OPD substrate system (Sigma). The colorimetric reaction was read in an automated ELISA microplate reader (Versamax, Molecular Devices) at 492 nm.
Real-time PCR
The colon tissues were homogenized using a tissue homogenizer. The total RNA extraction, cDNA synthesis and real-time PCR were performed as previously described [17]. Briefly, the total RNA was isolated and the cDNA was synthesized from the colon tissue, and these were processed using a universal mRNA Purification Kit (TaKaRa, Japan) and RT reagent Kit with gDNA Eraser (TaKaRa, Japan), respectively. Real-time PCR was performed using the SYBR Fast qPCR Mix (TaKaRa, Japan) via the StepOne Plus Real-time PCR system (Life Technologies, Carlsbad, CA, USA). The PCR volume was 15 μL, and it included 0.3 mL of each primer (10 nmol/L), 7.5 μL of the SYBR qPCR mix, 0.3 μL of ROX reference dye, 2.0 μL of the cDNA template, and 4.6 μL of easy dilution buffer (TaKaRa, Japan). The PCR program included 30 s at 95°C, then 40 cycles of 95°C for 5 s, and finally 62°C for 34 s. At least three independent biological replicates were performed to check the reproducibility of the data. The gene-specific primers used for quantitative PCR are listed in Table 1.
Table 1. Primers used in quantitative real-time reverse transcription-PCR.
| Primer | Sequence 5’-3’ | PCR product size(bp) |
|---|---|---|
| ASC-F | TTCTGTGACCCTGGCAATGAG | 198 |
| ASC-R | CTGGAGTCGTATGGCTTGGAG | |
| Caspase-1-F | CAGGCAAGCCAAATCTTTATCACT | 173 |
| Caspase-1-R | GTGCCATCTTCTTTGTTCTGTTCTT | |
| GAPDH- F | CAGCCTTCCTTCTTGGGTAT | 105 |
| GAPDH- R | CTGTGTTGGCATAGAGGTCTT | |
| IKKα- F | CACAGCCTCTAACTCCATCTA | 114 |
| IKKα- R | AGCGTGAAACAGGAATAAATA | |
| IKKβ- F | ACTGGAAGGCTGGGACATTAG | 107 |
| IKKβ- R | TGAAGATCGCCTGTAGCAAAG | |
| IL-1β-F | TGTGTTTTCCTCCTTGCCTCTGAT | 105 |
| IL-1β-R | TGCTGCCTAATGTCCCCTTGAAT | |
| IL-6-F | GAGGATACCACTCCCAACAGACC | 141 |
| IL-6-R | AAGTGCATCATCGTTGTTCATACA | |
| IRAK-2- F | CCGGGGTATTGGAAGATGAGC | 240 |
| IRAK-2- R | TGGGGAAGGCAGTGGTGAAAG | |
| JNK1- F | TCCTCCAAATCCATTACCTCC | 149 |
| JNK1- R | CTCCAGCACCCATACATCAAC | |
| JNK2-F | AGGACGAGTTCACGGTAGGCT | 190 |
| JNK2-R | GCCATCTTTGATAACACCACT | |
| MEKK-1- F | CTCCAAAGTCTGCAATTCTCA | 153 |
| MEKK-1- R | CTTTCAAGGAGTCAGTCGTCA | |
| MyD88-F | GTAGAGGCAGGAGAATCAGGAG | 161 |
| MyD88-R | ACAGGTAGAGAAAAGAGAGGAGAAA | |
| NLRP3-F | GTCTGGAAGAACAGGCAACAT | 144 |
| NLRP3-R | AGAACTGTCATAGGGTCAAAACG | |
| p38α-F | GTTTCAGTCCATCATTCACGC | 93 |
| p38α-R | ACATCCAACAGACCAATCACAT | |
| p65-F | GTGTCTTGGTGGTATCTGTGC | 166 |
| p65-R | GATCTGTTTCCCCTCATCTTT | |
| TAK1- F | CTGGATGATCGGGACTAAAGA | 129 |
| TAK1- R | GGATAAAGCAAGTGATGGAGC | |
| TLR-4-F | GGAACAAACAGCCTGAGACAC | 151 |
| TLR-4-R | CAAGGGATAAGAACGCTGAGAA | |
| TRAF6- F | CCAGGGGAGGTGGCTGTCATA | 209 |
| TRAF6- R | GGAAGATTGGCAACTTTGGGATG | |
| Ubc13-F | TGCTGGGGACCACTTATCTTT | 205 |
| Ubc13-R | CAACGCCCGTTATTTTCATGT | |
| UEV1A-F | TTCGGTTTTCATAGATTGTTCGTG | 111 |
| UEV1A-R | GAGTAGGCGACGGCACAGTTA | |
| ZO-1-F | AGGACACCAAAGCATGTGAG | 192 |
| ZO-1-R | GGCATTCCTGCTGGTTACA |
Western blot
Western blot analysis was performed on tissue extract as previously reported[18]. Briefly, tissue samples were lysed in lysis buffer containing 10% glycerol, 1% Triton X-100, 135 mM NaCl, 20 mM Tris (pH 8.0), 2.7 mM KCl, 1 mM MgCl2, and protease and phosphatase inhibitors (0.5 mM PMSF, 2 μM pepstatin and 2 μM okadaic acid). Aliquots of samples were subjected to SDS-PAGE followed by transfer to polyvinylidene difluoride (PVDF) membranes (Amersham Pharmacia, Uppsala, Sweden). Immunoblotting was performed with respective antibodies (1:1000). Following incubation with horseradish peroxidase-conjugated secondary antibody (SigmaAldrich, Shanghai, China), proteins were detected with ECL plus kits (Amersham, Piscataway, NJ, USA).
Statistical analyses
Data were presented as mean ± standard error (sem). Statistical analyses were performed using Student's t-test using SPSS, version 16 (SPSS Inc., Chicago, IL, USA). A P-value < 0.05 was considered statistically significant.
Results
SH attenuated the symptoms of DSS-induced chronic colitis
The clinical symptoms of colitis were estimated by DAI score and body weight reduction. The body weight of all animals increased constantly in the first 39 days. Afterward, the body weight of DSS group declined sharply and was significant lower than the Normal group. Treatment with SH (400 mg/kg) significantly prevented the reduction of body weight (Fig 1A). Similarly, the DAI score of DSS group increased after DSS-drinking for 18 days, and the DAI score reached 4 after induction for 8 weeks (Fig 1B), revealing severe diarrhea and gross anal bleeding. Treatment with SH decreased the DAI score in a dose-dependent manner. The DAI score of SH-400 group was significantly lower than that treated with DSS alone (Fig 1B). These results suggested that SH was effective to ameliorate clinical symptoms of colitis.
Fig 1. SH attenuated the symptoms of DSS-induced chronic colitis.
(A) Changes of body weight. (B) Disease activity index (DAI) score. Data are shown as means ± sem. *P<0.05, ***P<0.001.
SH improved the histopathological damage of colon
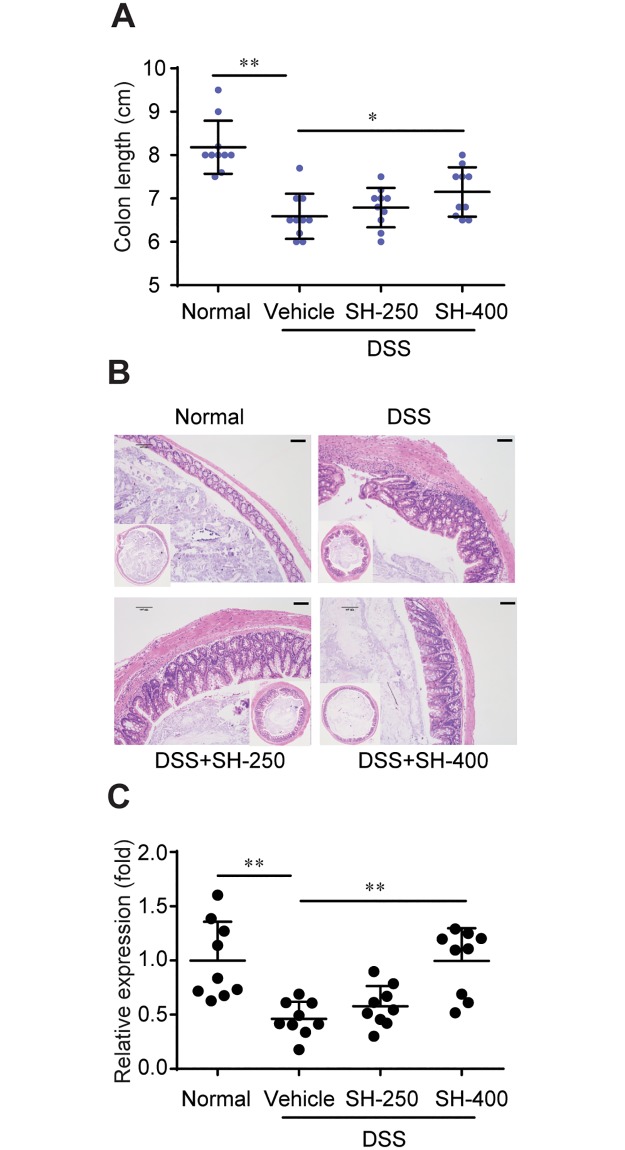
Shortening of the colon length reflects the extent of colon damage in DSS-induced colitis [19]. The average colon length was approximately 8.2 cm in healthy controls, while it was reduced to 6.6 cm in mice with DSS-induced colitis. Treatment with SH (400 mg/kg) significantly increased the colon length as compared to DSS group (Fig 2A).
Fig 2. SH improved the histopathological damage of colon.
(A) Colon length. (B) H&E staining of colon tissue sections. (C) Relative mRNA levels of tight junction protein ZO-1 in colon tissue. Data are shown as means ± sem. *P<0.05, **P<0.01.
Histological analysis also confirmed the alleviating effect of SH on colon pathology. The colon of Normal group revealed large numbers of goblet cells, preserved crypts and muscularis propria, and intact mucosal lining. Supplementation of DSS led to significant colonic damage, as evidenced by loss of epithelial barrier, decrease in the number of crypts and goblet cells, and submucosal edema (Fig 2B). Treatment with SH improved these pathological changes of colon such as abnormal crypts, loss of epithelial cells and goblet cells, and inflammatory infiltration (Fig 2B).
The intestinal tight junctions are key structures defending intestine against leaking of adverse macromolecules such as LPS. We quantified the relative expression of tight junction protein ZO-1 in colon tissue by realtime PCR. The results showed that the expression of ZO-1 in DSS group was significantly lower than normal mice, and treatment with SH (400 mg/kg) significantly increased the expression of ZO-1 (Fig 2C). This indicates that SH has protective effect on intestinal mucosal barrier.
SH reduced the levels of inflammatory cytokines in mice with DSS-induced colitis
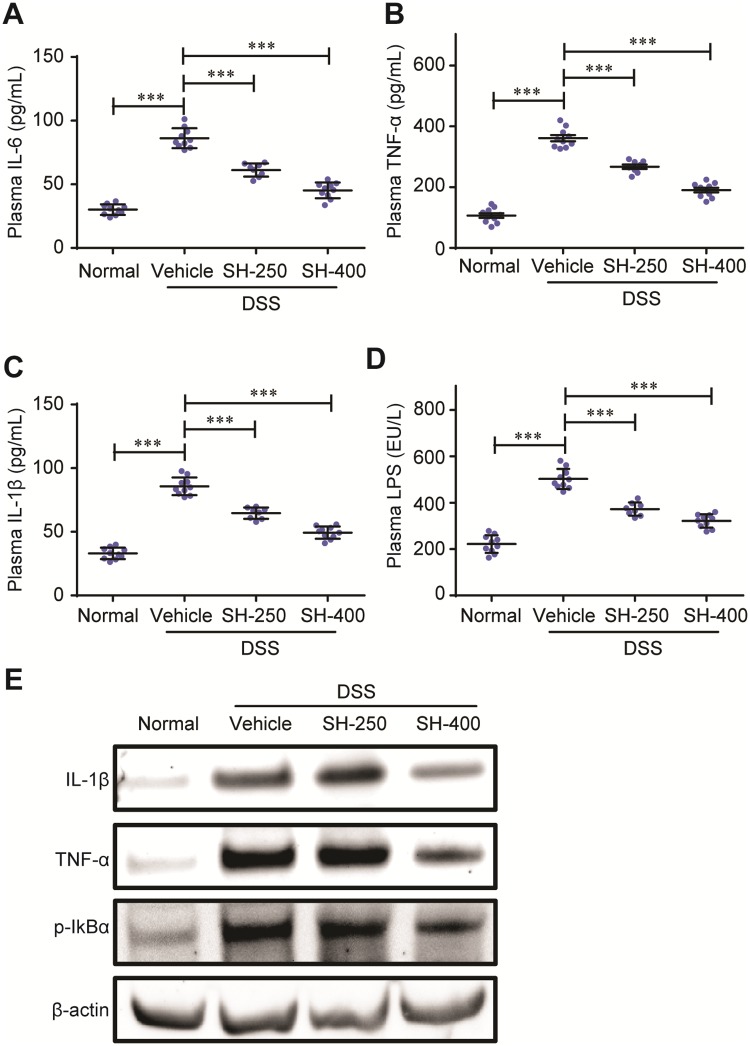
We first quantified the plasma levels of inflammatory cytokines such as IL-6, IL-1β, TNF-α, and LPS by enzyme-linked immunosorbent assay (ELISA). Supplementation with DSS remarkably increased the plasma levels of IL-6, IL-1β and TNF-α, revealing a severe inflammatory condition. DSS also increased the plasma concentration of LPS, a potent inflammation inductor that was derived from gram-negative gut bacterium (Fig 3A–3D). Treatment with SH significantly and dose-dependently decreased the plasma levels of IL-6, IL-1β and TNF-α, exhibiting potent anti-inflammatory activities. The administration of SH also significantly lowered the LPS level as compared to the vehicle control (DSS) group. We also determined the protein levels of IL-1β and TNF-α in colon tissues by western blot analysis. The results showed that treatment with SH (400 mg/kg) significantly decreased the contents of IL-1β and TNF-α in colon (Fig 3E).
Fig 3. SH reduced the levels of inflammatory cytokines.
The plasma levels of IL-6 (A), TNFα (B), IL-1β (C) and LPS (D) were determined by ELISA. (E) Western blot for colon levels of IL-1β, TNFα and phosphorylated IκBα (p-IκBα). Data are shown as means ± sem. ***P<0.001.
SH inhibited LPS-induced IL-1β production by RAW264.7 macrophages
We further investigated the direct inhibiting effects of SH on inflammatory factors production in RAW264.7 macrophages. The cells were pretreated with SH (10 mg/L) or solvent for 24 hours, then elicited by LPS for 4 hours. The production of IL-1β was quantified by ELISA kit. The results showed that treatment with SH (10 mg/L) significantly reduced the production of IL-1β elicited by LPS (Fig 4), suggesting that SH may inhibit inflammatory reaction of macrophages cells directly.
Fig 4. SH inhibited the LPS-elicited IL-1β production in RWA264.7 macrophages.
Data are shown as means ± sem. **P<0.01.
SH decreased the phosphorylation of IkBα
To explore the potential mechanism underlying SH-mediated anti-inflammatory effects in DSS-induced colon colitis, we first quantified the mRNA levels of inflammatory genes in the TLR4-TRAF6-NFκB pathway by real-time PCR, that comprised, from the upstream to the downstream, TLR-4, MyD88, TRAF6, IRAK2, Ubc13, UEV1A, TAK1, IKKα/β and p65 (a subunit of NFκB). As shown in Fig 5, the transcription of most members except Ubc13 in the TLR4-TRAF6-NFκB pathway was not largely decreased by SH. However, the mRNA levels of IL-1β and IL-6 were significantly declined after SH treatment. These results indicated that the anti-inflammatory effects of SH were not due to the inhibition of transcription of TLR4-TRAF6-NFκB pathway.
Fig 5. SH did not inhibit the transcription of TLR4-TRAF6-NFκB pathway.
Data are shown as means ± sem. *P<0.05, **P<0.01.
IkBα is a key inhibitor of NFκB, which inhibits NFκB to enter the nucleus and initiate the expression of downstream inflammation-related genes such as IL-1β, IL-6, TNFα [20]. The action of IkBα is regulated by phosphorylation. The phosphorylated IkBα tends to be ubiquitinated and degraded, thus relieving the inhibition normally imposed on NFκB. We therefore evaluated the influence of SH on the phosphorylation levels of IkBα using western blot. The results showed that SH significantly decreased the phosphorylation of IkBα (Fig 3E), suggesting that SH may exert its anti-inflammatory effect by inhibiting IkBα phosphorylation, thus suppressing the activity of NFκB and decreasing inflammatory factors production.
SH decreased the transcription of ASC3 and caspase-1
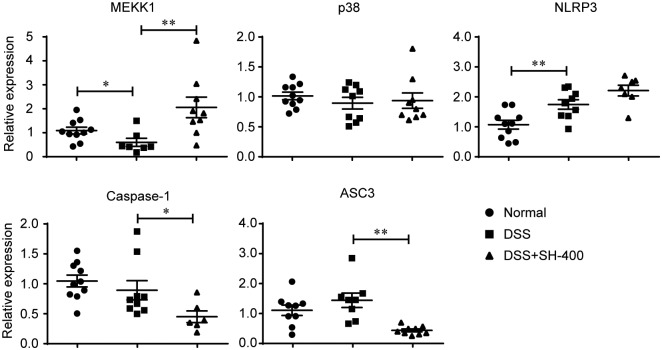
The NLRP3-ASC3-caspase-1 pathway is another inflammatory pathway which promotes the maturation and release of IL-1β and IL-6 cytokines [6]. We quantified the mRNA levels of NLRP3-ASC3-caspase-1 inflammatory pathway, which included MEKK, p38, NLRP3, ASC3 and caspase-1. The results showed that SH did not impact the transcription of p38 and NLRP3, increased the expression of MEKK1 and significantly decreased the mRNA levels of ASC3 and caspase-1 in DSS-induced colitis mice (Fig 6), suggesting that SH may inhibit release of inflammatory cytokines through downregulating ASC3-caspase-1 pathway.
Fig 6. Effects of SH on the mRNA levels of genes in NLRP3-ASC3-caspase-1 pathway.
Data are shown as means ± sem. *P<0.05, **P<0.01.
Discussion
Although multiple pharmacological activities of P. igniarius extract have been well-documented [7–14] but its effect on colitis is largely unknown. Recently, Song et al [21] reported that the ethanol and ethyl acetate extract of P. linteus exerted adequate colitis-ameliorating effect in DSS-induced colitis mice [21]. However, P. igniarius is traditionally used as decoction not by ethanol extraction. Therefore, whether the traditional usage of P. igniarius is beneficial for the treatment of colitis is remained to be elucidated.
Our results demonstrated that oral administration of aqueous extract of the fruiting body of P. igniarius (SH) is effective to ameliorate DSS-induced colitis in mice. Treatment with SH for 48 days significantly improved all clinical symptoms of DSS-induced chronic colitis including bodyweight reduction, DAI score, colon length and pathological changes such as abnormal crypts, loss of epithelial cells and goblet cells, and inflammatory infiltration. The dosage of P. igniarius aqueous extract (SH) we used in this study was similar to that used by Song et al for P. linteus ethanol and ethyl acetate extract (PBR). The effectiveness of SH on clinical symptoms of DSS-induced chronic colitis (48 days) was comparable to that of PBR on acute colitis (10 days). These results suggest that the decoction of P. igniarius is adequate to ameliorate DSS-induced colitis and thus provide a potential utility of Sanghuang for the treatment of colitis.
Colitis is a chronic remittent and relapsing inflammatory disease and multiple anti-colitis drugs exert their therapeutical effects through alleviating inflammation. Many Phellinus species have been demonstrated to possess potent anti-inflammatory activities [21–24] but whether P. igniarius (SH) possesses anti-inflammatory activity is still largely unknown. In this study, we showed that treatment with SH significantly and dose-dependently decreased the plasma levels of IL-6, IL-1β and TNF-a, suggesting that SH may ameliorate colitis through alleviating inflammation. Cell experiment showed that treatment with SH efficiently inhibited the LPS-elicited IL-1β production by RAW264.7 macrophages. These results suggested that SH could inhibit inflammation in vitro and in vivo.
Previous studies showed that some Phellinus species such as P. linteus [21, 25, 26] and P. baumii [23] exert their anti-inflammatory effects through inhibiting the expression of nuclear factor-κB (NFκB) p65 and mitogen-activated protein kinases (MAPKs) (e.g., extracellular signal-regulated protein kinase (ERK) and p38). We therefore quantified the transcription levels of all genes in LPS-elicited TLR4-TRAF6-NFκB inflammatory pathway in colon tissue. Our results showed that although the expression of IL-1β and IL-6 was significantly and dose-dependently by SH, but the mRNA levels of most genes in the TLR4-TRAF6-NFκB pathway such as TLR-4, TRAF6, IRAK2, UEV1A, TAK1, IKKα/β and p65 were not significantly decreased. We then checked the influence of SH on the phosphorylation of IkBα, a key inhibitor of NFκB which inhibits NFκB to enter the nucleus thus preventing the initiation of the expression of downstream inflammation-related genes [20]. Western blot showed that SH significantly decreased phosphorylation level of IkBα. As the phosphorylated IkBα is prone to degraded, inhibiting IkBα phosphorylation would enhance the inhibitory action of IkBα on NFκB, thus decreasing the expression of downstream inflammatory genes such as IL-1β and IL-6.
The NLRP3-ASC3-caspase-1 pathway is another important inflammatory pathway that promotes the production and release of IL-1β and IL-6 pro-inflammatory cytokines [6]. Treatment with SH did not decrease the transcription of MEKK, p38 and NLRP3, but significantly decreased the mRNA levels of ASC3 and caspase-1. As ASC3 and caspase-1 are key regulators for the maturation of IL-1β and IL-6 [6], SH may reduce the production and release of these two pro-inflammatory cytokines through inhibiting ASC3 and caspase-1. Therefore, the aqueous extract of Phellinus igniarius (SH) may suppressing the plasma levels of IL-1β and IL-6 through two pathways, (1) decreasing the phosphorylation of IkBα thus preventing NFκB to initiate the expression of downstream inflammatory genes, (2) inhibiting the expression of ASC3 and caspase-1 to reduce the maturation of IL-1β and IL-6, which ultimately ameliorates DSS-induced colitis in mice.
In conclusion, our work demonstrated that the aqueous extract of Phellinus igniarius (SH) effectively ameliorates DSS-induced colitis, which provides a potential utility of SH for the treatment of colitis. Inhibiting the expression and release of inflammatory factors may participate in the colitis-ameliorating effect of SH.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported financially by Foundation from Science and Technology Department of Zhejiang Province (2018C02003, LGN18C170005), National Natural Science Foundation of China (81673663), Modern Agro-industry Technology Research System of China (CARS-22-ZJ0105), CAMS Innovation Fund for Medical Sciences (CIFMS) 2016-I2M-3-015 and Key Laboratory of Creative Agriculture(pilot run), Ministry of Agriculture, P.R. China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stewart DC, Berrie D, Li J, Liu X, Rickerson C, Mkoji D, et al. Quantitative assessment of intestinal stiffness and associations with fibrosis in human inflammatory bowel disease. PLoS One. 2018;13(7):e0200377 10.1371/journal.pone.0200377 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dionne S, Hiscott J, D'Agata I, Duhaime A, Seidman EG. Quantitative PCR analysis of TNF-alpha and IL-1 beta mRNA levels in pediatric IBD mucosal biopsies. Dig Dis Sci. 1997;42(7):1557–66. . [DOI] [PubMed] [Google Scholar]
- 3.Sun Z, Li G, Tong T, Chen J. Micheliolide suppresses LPS-induced neuroinflammatory responses. PLoS One. 2017;12(10):e0186592 10.1371/journal.pone.0186592 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng P, Wang T, Li W, Muhammad I, Wang H, Sun X, et al. Baicalin Alleviates Lipopolysaccharide-Induced Liver Inflammation in Chicken by Suppressing TLR4-Mediated NF-kappaB Pathway. Front Pharmacol. 2017;8:547 10.3389/fphar.2017.00547 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He A, Ji R, Shao J, He C, Jin M, Xu Y. TLR4-MyD88-TRAF6-TAK1 Complex-Mediated NF-kappaB Activation Contribute to the Anti-Inflammatory Effect of V8 in LPS-Induced Human Cervical Cancer SiHa Cells. Inflammation. 2016;39(1):172–81. 10.1007/s10753-015-0236-8 . [DOI] [PubMed] [Google Scholar]
- 6.Vyleta ML, Wong J, Magun BE. Suppression of ribosomal function triggers innate immune signaling through activation of the NLRP3 inflammasome. PLoS One. 2012;7(5):e36044 10.1371/journal.pone.0036044 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suabjakyong P, Saiki R, Van Griensven LJ, Higashi K, Nishimura K, Igarashi K, et al. Polyphenol extract from Phellinus igniarius protects against acrolein toxicity in vitro and provides protection in a mouse stroke model. PLoS One. 2015;10(3):e0122733 10.1371/journal.pone.0122733 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song TY, Lin HC, Yang NC, Hu ML. Antiproliferative and antimetastatic effects of the ethanolic extract of Phellinus igniarius (Linnearus: Fries) Quelet. J Ethnopharmacol. 2008;115(1):50–6. 10.1016/j.jep.2007.09.001 . [DOI] [PubMed] [Google Scholar]
- 9.Zheng S, Deng S, Huang Y, Huang M, Zhao P, Ma X, et al. Anti-diabetic activity of a polyphenol-rich extract from Phellinus igniarius in KK-Ay mice with spontaneous type 2 diabetes mellitus. Food Funct. 2018;9(1):614–23. 10.1039/c7fo01460k . [DOI] [PubMed] [Google Scholar]
- 10.Wang FF, Liu F, Shi C, Ma W, Wang KJ, Li N. Cytotoxic Activities of Fractions of the Willow Bracket Medicinal Mushroom, Phellinus igniarius (Agaricomycetes), and the Induction of Cell Cycle Arrest and Apoptosis in MGC-803 Cells. Int J Med Mushrooms. 2017;19(6):561–70. 10.1615/IntJMedMushrooms.v19.i6.70 . [DOI] [PubMed] [Google Scholar]
- 11.Gao W, Wang W, Sun W, Wang M, Zhang N, Yu S. Antitumor and immunomodulating activities of six Phellinus igniarius polysaccharides of different origins. Exp Ther Med. 2017;14(5):4627–32. 10.3892/etm.2017.5191 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suabjakyong P, Nishimura K, Toida T, Van Griensven LJ. Structural characterization and immunomodulatory effects of polysaccharides from Phellinus linteus and Phellinus igniarius on the IL-6/IL-10 cytokine balance of the mouse macrophage cell lines (RAW 264.7). Food Funct. 2015;6(8):2834–44. 10.1039/c5fo00491h . [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Jiang SS, Wang CY, Li R, Che HL. Different immunology mechanisms of Phellinus igniarius in inhibiting growth of liver cancer and melanoma cells. Asian Pac J Cancer Prev. 2014;15(8):3659–65. . [DOI] [PubMed] [Google Scholar]
- 14.Li L, Wu G, Choi BY, Jang BG, Kim JH, Sung GH, et al. A mushroom extract Piwep from Phellinus igniarius ameliorates experimental autoimmune encephalomyelitis by inhibiting immune cell infiltration in the spinal cord. Biomed Res Int. 2014;2014:218274 10.1155/2014/218274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brat P, George S, Bellamy A, Du Chaffaut L, Scalbert A, Mennen L, et al. Daily polyphenol intake in France from fruit and vegetables. J Nutr. 2006;136(9):2368–73. 10.1093/jn/136.9.2368 . [DOI] [PubMed] [Google Scholar]
- 16.Azuma YT, Nishiyama K, Matsuo Y, Kuwamura M, Morioka A, Nakajima H, et al. PPARalpha contributes to colonic protection in mice with DSS-induced colitis. Int Immunopharmacol. 2010;10(10):1261–7. 10.1016/j.intimp.2010.07.007 . [DOI] [PubMed] [Google Scholar]
- 17.Wu C, Feng J, Wang R, Liu H, Yang H, Rodriguez PL, et al. HRS1 acts as a negative regulator of abscisic acid signaling to promote timely germination of Arabidopsis seeds. PLoS One. 2012;7(4):e35764 10.1371/journal.pone.0035764 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Guo Y, Su Y, Zhang X, Luan H, Zhang X, et al. Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the gamma1 subunit. J Cell Mol Med. 2014;18(2):293–304. 10.1111/jcmm.12187 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15(1):79–94. 10.1128/CMR.15.1.79-94.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dey G, Bharti R, Ojha PK, Pal I, Rajesh Y, Banerjee I, et al. Therapeutic implication of 'Iturin A' for targeting MD-2/TLR4 complex to overcome angiogenesis and invasion. Cell Signal. 2017;35:24–36. 10.1016/j.cellsig.2017.03.017 . [DOI] [PubMed] [Google Scholar]
- 21.Song M, Park HJ. Anti-inflammatory effect of Phellinus linteus grown on germinated brown rice on dextran sodium sulfate-induced acute colitis in mice and LPS-activated macrophages. J Ethnopharmacol. 2014;154(2):311–8. 10.1016/j.jep.2013.12.059 . [DOI] [PubMed] [Google Scholar]
- 22.Im KH, Nguyen TK, Kim JK, Choi JH, Lee TS. Evaluation of Anticholinesterase and Inflammation Inhibitory Activity of Medicinal Mushroom Phellinus pini (Basidiomycetes) Fruiting Bodies. Int J Med Mushrooms. 2016;18(11):1011–22. 10.1615/IntJMedMushrooms.v18.i11.60 . [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Lee D, Jang TS, Kang KS, Nam JW, Lee HJ, et al. Anti-Inflammatory Phenolic Metabolites from the Edible Fungus Phellinus baumii in LPS-Stimulated RAW264.7 Cells. Molecules. 2017;22(10). 10.3390/molecules22101583 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang BS, Kim JC, Bae JS, Rhee MH, Jang KH, Song JC, et al. Extracts of Phellinus gilvus and Phellinus baumii inhibit pulmonary inflammation induced by lipopolysaccharide in rats. Biotechnol Lett. 2004;26(1):31–3. . [DOI] [PubMed] [Google Scholar]
- 25.Yan GH, Choi YH. Phellinus linteus Extract Exerts Anti-asthmatic Effects by Suppressing NF-kappaB and p38 MAPK Activity in an OVA-induced Mouse Model of Asthma. Immune Netw. 2014;14(2):107–15. 10.4110/in.2014.14.2.107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CJ, Lien HM, Chang HY, Huang CL, Liu JJ, Chang YC, et al. Biological evaluation of Phellinus linteus-fermented broths as anti-inflammatory agents. J Biosci Bioeng. 2014;118(1):88–93. 10.1016/j.jbiosc.2014.01.001 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.