Abstract
For prostate cancer detection on prostate multiparametric MRI (mpMRI), the Prostate Imaging-Reporting and Data System version 2 (PI-RADSv2) and computer-aided diagnosis (CAD) systems aim to widely improve standardization across radiologists and centers. Our goal was to evaluate CAD assistance in prostate cancer detection compared with conventional mpMRI interpretation in a diverse dataset acquired from five institutions tested by nine readers of varying experience levels, in total representing 14 globally spread institutions.
Index lesion sensitivities of mpMRI-alone were 79% (whole prostate (WP)), 84% (peripheral zone (PZ)), 71% (transition zone (TZ)), similar to CAD at 76% (WP, p=0.39), 77% (PZ, p=0.07), 79% (TZ, p=0.15). Greatest CAD benefit was in TZ for moderately-experienced readers at PI-RADSv2 <3 (84% vs mpMRI-alone 67%, p=0.055). Detection agreement was unchanged but CAD-assisted read times improved (4.6 vs 3.4 minutes, p<0.001). At PI-RADSv2 ≥ 3, CAD improved patient-level specificity (72%) compared to mpMRI-alone (45%, p<0.001).
PI-RADSv2 and CAD-assisted mpMRI interpretations have similar sensitivities across multiple sites and readers while CAD has potential to improve specificity and moderately-experienced radiologists’ detection of more difficult tumors in the center of the gland. The multi-institutional evidence provided is essential to future prostate MRI and CAD development.
Keywords: computer-aided diagnosis, prostate cancer, multiparametric MRI, PI-RADSv2, tumor detection
INTRODUCTION
Men with suspected or known prostate cancer are increasingly evaluated with prostate multiparametric MRI (mpMRI) because it aids in the detection of clinically significant disease [1–4]. However, mpMRI has been criticized because of variability in quality of exams and inconsistent interpretations across clinical centers and physicians. Interpretation can be affected by many factors including relative visibility of tumors, tumor location, and inter-observer variation. To address some of these issues the Prostate Imaging-Reporting and Data System version 2 (PI-RADSv2) was introduced in 2015 as a set of guidelines outlining standard acquisition parameters and a categorization system for cancer detection [5]. PI-RADSv2 has been widely adopted and can achieve cancer detection rates up to 80-90%; however, it is associated with a steep learning curve and exhibits a high degree of inter-reader variability, likely reflecting inherent ambiguities in the classification scheme. Moreover, many centers report a mpMRI miss rate up to 16-30% [6–11]. A large scale, prospective, multicenter trial of PI-RADSv2 has not yet been performed.
Machine learning is a highly touted method of improving feature recognition in images. Computer-aided diagnosis (CAD) systems have shown promise in the identification of prostate cancer on mpMRI in several single institution studies [12–17]. For instance, we previously developed a CAD system based on in-house images with readers from outside our institution and showed excellent results [18]. However, for a CAD system to be truly useful it must be trained with a much more diverse set of data, crossing vendors and institutions, and interpreted by multiple readers with varying experience to validate its performance.
The purpose of this study was to test a new prostate CAD on a highly heterogenous, “real-world” data set from 5 institutions against mpMRI interpretations with PI-RADSv2 using a diverse set of readers, varying in location and experience.
RESULTS
Patient and lesion characteristics
Patient and lesion characteristics are given in Table 1, stratified by institution. The final study population consisted of 144 case patients and 72 control patients. In case patients, there were a total of 285 pathologically-proven tumors, of which 10/285 were found spanning both peripheral (PZ) and transition (TZ) zones, 187/285 were PZ lesions, and 88/285 were in the TZ. Institution 1 had the highest proportion of Gleason score 3+3, at 57% (27/47), whereas Institutions 2, 3, and 5 most commonly reported Gleason 7.
Table 1. Patient and tumor demographics by providing institution.
| Institution 1 | Institution 2 | Institution 3 | Institution 4 | Institution 5 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Cases | Cases | Controls | p | ||
| Patient-based | N | 32 | 24 | 36 | 24 | 50 | 24 | 10 | 16 | 144 | 72 | |
| Age | 65.6 (51-76) | 61.3 (49-78) | 61.8 (51-71) | 59.9 (49-72) | 61.9 (47-79) | 62.8 (50-77) | 58.5 (42-68) | 63.1 (54-76) | 62.6 (42-79) | 61.3 (49-78) | 0.21 | |
| PSA | 8.4 (3.3-23) | 10.9 (3.5-24) | 9.3 (3.4-26.1) | 6.6 (0.3-11.5) | 6.7 (1.2-27.3) | 6.9 (1.3-24) | 11 (3.7-31.9) | 7.5 (3.5-17.8) | 8.1 (1.2-31.9) | 8.2 (0.3-24) | 0.5 | |
| Mean # lesions/ patient | 1.47 | 2.06 | 1.98 | 3.3 | 2 | 1.98 | ||||||
| WP | PZ | TZ | WP | PZ | TZ | WP | PZ | TZ | WP | PZ | TZ | WP | PZ | TZ | WP | PZ | TZ | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesion-based by Gleason score | Total | 47 | 35 | 14 | 74 | 54 | 22 | 99 | 65 | 38 | 33 | 19 | 16 | 32 | 24 | 8 | 285 | 197* | 98* | |
| 3+3 | 27 | 19 | 8 | 18 | 9 | 9 | 10 | 6 | 4 | 17 | 8 | 9 | 0 | 0 | 0 | 72 | 42 | 30 | ||
| 3+4 | 9 | 6 | 3 | 42 | 34 | 8 | 64 | 42 | 23 | 11 | 6 | 5 | 19 | 17 | 2 | 145 | 105 | 41 | ||
| 4+3 | 5 | 5 | 1 | 5 | 5 | 2 | 14 | 7 | 8 | 3 | 3 | 1 | 10 | 5 | 5 | 37 | 25 | 17 | ||
| 4+4 | 3 | 3 | 0 | 5 | 4 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 9 | 1 | ||
| >4+4 | 3 | 2 | 2 | 4 | 2 | 2 | 9 | 8 | 3 | 2 | 2 | 1 | 3 | 2 | 1 | 21 | 16 | 9 |
Lesion-based data is given for whole prostate (WP) and by zone (peripheral (PZ), transition (TZ)). *10 lesions located in both PZ and TZ.
Patient based baseline mpMRI and CAD performance
The areas under the receiver operating characteristic curves (AUCs) of mpMRI alone (mpMRI) and CAD-assisted mpMRI interpretation (CAD) for the detection of cancer at the patient-level were 81.9% and and 83.1%, respectively (p=0.58).
Patient-level sensitivity and specificity of mpMRI and CAD at each PI-RADSv2 category threshold is given in Table 2. Considering all detected lesions (PI-RADSv2 category ≥1), moderately experienced readers achieved comparable sensitivity with mpMRI vs CAD at 93.3% vs 92.8% (p=0.864) while highly experienced readers achieved mpMRI sensitivity 96.7% vs CAD at 90.7% (p=0.007). Overall specificity was similar between mpMRI and CAD, at 35% for both (p=0.927).
Table 2. Patient-level sensitivity and specificity of mpMRI and CAD at each PI-RADSv2 category threshold.
| Overall | Moderately experienced | Highly experienced | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PI-RADSv2 Threshold |
MRI | CAD | p | MRI | CAD | p | MRI | CAD | p | |
| 1 | Sensitivity | 95.6% (92.9-97.7%) | 91.4% (86.5-94.4%) | 0.05 | 93.3% (88.7-97.1%) | 92.8% (87.3-97.2%) | 0.86 | 96.7% (94.5-98.5%) | 90.7% (85.3-95.3%) | 0.007 |
| Specificity | 35% (27.6-42.6%) | 34.5% (23.2-46.4%) | 0.93 | 44.9% (34.2-55.6%) | 23.8% (11.9-36.8%) | 0.003 | 30.1% (21.2-39.1%) | 39.9% (27.3-53.1%) | 0.10 | |
| 2 | Sensitivity | 95.6% (92.9-97.7%) | 85.4% (79.8-91%) | <0.001 | 93.3% (88.7-97.1%) | 84.8% (77.4-91%) | 0.005 | 96.7% (94.5-98.5%) | 85.8% (79.7-91.6%) | <0.001 |
| Specificity | 35.9% (28.6-43.3%) | 52.1% (42.6-62%) | <0.001 | 44.9% (34.2-55.6%) | 46.3% (33.6-59.2%) | 0.85 | 31.4% (23-39.8%) | 55% (45.1-65.6%) | <0.001 | |
| 3 | Sensitivity | 93.9% (90.8-96.4%) | 81.5% (75-87.6%) | <0.001 | 92.7% (88.1-96.6%) | 79.4% (71.4-86.9%) | <0.001 | 94.4% (91.2-97.1%) | 82.5% (75.9-89%) | <0.001 |
| Specificity | 44.8% (37.7-51.9%) | 71.5% (63.2-79.6%) | <0.001 | 48.9% (38.1-59.6%) | 71.1% (58.9-82.6%) | 0.001 | 42.8% (35-50.9%) | 71.7% (62.6-80.3%) | <0.001 | |
| 4 | Sensitivity | 88.1% (83.6-92.3%) | 76.5% (69.6-82.8%) | <0.001 | 85.4% (79.5-90.8%) | 74.1% (65.8-82%) | 0.001 | 89.5% (84.4-93.9%) | 77.7% (70.5-84.4%) | <0.001 |
| Specificity | 61.9% (53.5-69.7%) | 85.2% (78.2-91.1%) | <0.001 | 58% (45.3-70.3%) | 81.5% (70-91.4%) | <0.001 | 63.8% (55.1-72.5%) | 87% (81-92.7%) | <0.001 | |
| 5 | Sensitivity | 47.7% (40.1-55.6%) | 43.4% (35.7-51.3%) | 0.03 | 49.8% (39.8-60%) | 44.9% (35.2-54.6%) | 0.16 | 46.7% (39-54.8%) | 42.7% (35-50.8%) | 0.07 |
| Specificity | 92.7% (89.9-95.5%) | 96.5% (93.6-98.8%) | 0.04 | 88.4% (82-94.7%) | 96% (89.4-100%) | 0.09 | 94.9% (91.4-97.9%) | 96.8% (93.8-99.3%) | 0.36 | |
For each PI-RADv2 category threshold, sensitivity and specificity are given across all readers and stratified by experience, with 95% confidence intervals given in parentheses. p<0.05 was used for significance.
When considering those lesions classified as suspicious (PI-RADSv2 category ≥3), the specificity of CAD was higher than that for mpMRI (71.5% vs 44.8% for all readers, p<0.001), while the sensitivities were comparable across reader experience levels. On mpMRI alone, highly experienced readers achieved sensitivity of 94.4% vs 92.7% for moderately experienced readers; for CAD, highly experienced readers achieved sensitivity of 82.5% vs 79.4% for moderately experienced readers.
Lesion based PI-RADSv2 performance
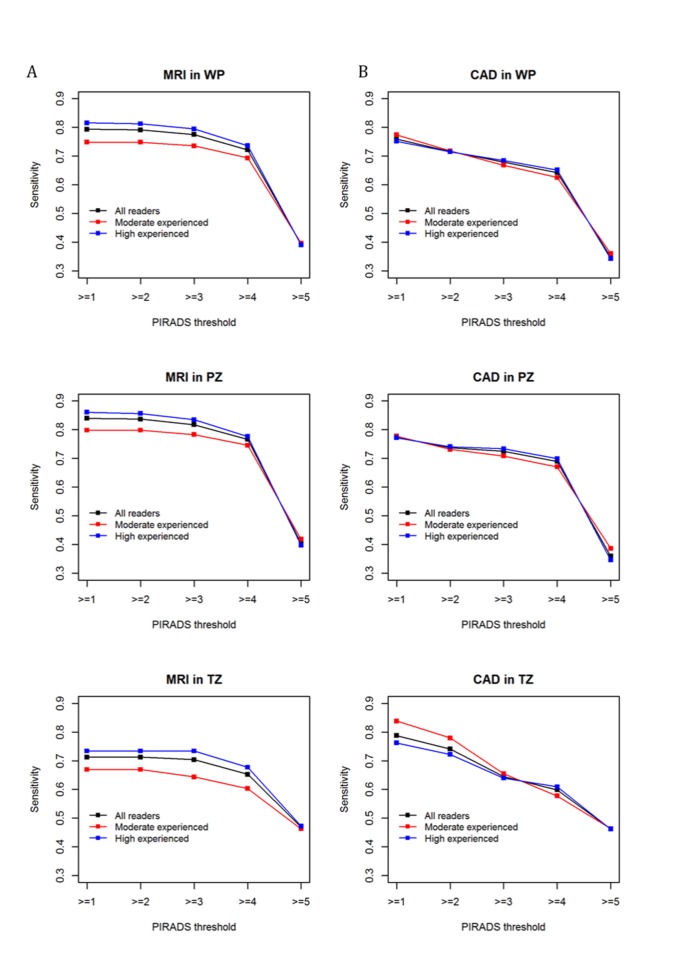
MRI-only index lesion sensitivity stratified by reader experience at each PI-RADSv2 category threshold is given for WP, PZ, and TZ in Figure 1A. At PI-RADSv2 ≥ 1, mpMRI sensitivity for all readers was 79% (75% for moderately experienced readers and 82% for highly experienced readers). For PI-RADSv2 ≥ 3, it was similar at 78% for all readers, 74% for moderately experienced readers, and 79% for highly experienced readers.
Figure 1.
Index lesion sensitivity in WP, PZ, TZ for MRI-only (A) and CAD-assisted (B) reads. Sensitivities are plotted for all readers as well as by experience level at each PI-RADSv2 category threshold. PI-RADSv2 category ≥1 threshold used for all lesions detected on MRI and CAD, while PI-RADSv2 category ≥3 threshold used to represent all lesions considered cumulatively suspicious on MRI and CAD. WP = whole prostate, PZ = peripheral zone, TZ = transition zone.
In the PZ, mpMRI sensitivity at PI-RADSv2 ≥ 1 for all readers was 84%, and at PI-RADSv2 ≥ 3 sensitivity was 82%. Stratified by experience, moderately experienced readers achieved 80% sensitivity at PI-RADSv2 ≥ 1 and 78% at PI-RADSv2 ≥ 3 in the PZ, while highly experienced readers achieved 86% at PI-RADSv2 ≥ 1 and 83% at PI-RADSv2 ≥ 3.
In the TZ, mpMRI sensitivity at PI-RADSv2 ≥ 1 was 71% across all readers and 70% across all readers at PI-RADSv2 ≥ 3. Stratification by experience revealed that while highly experienced readers achieved sensitivities of 73% at both PI-RADSv2 thresholds, moderately experienced readers had 67% sensitivity at PI-RADSv2 ≥ 1, decreased to 64% at PI-RADSv2 ≥ 3.
Lesion based CAD-assisted performance
CAD-assisted index lesion sensitivity stratified by reader experience is given for WP, PZ, and TZ in Figure 1B. CAD performance followed a similar trend to mpMRI-only PI-RADSv2 performance for readers of all experience, except achieving a higher detection sensitivity in the TZ at lower PI-RADSv2 categorization for moderately experienced readers.
In WP, CAD-assisted reader sensitivity at PI-RADSv2 ≥ 1 was 75% across all 9 readers, decreasing to 67.9% at PI-RADSv2 ≥ 3. Stratification by experience revealed the same trend, with moderately experienced readers achieving 77.4% sensitivity at PI-RADSv2 ≥ 1 but 66.8% at PI-RADSv2 ≥ 3 and highly experienced readers achieving sensitivities of 75.2% vs 68.5% for PI-RADSv2 ≥ 1 vs ≥ 3, respectively.
In zone-based analysis, PZ CAD performance was similar to the WP. Moderately experienced readers and highly experienced readers achieved similar sensitivities at PI-RADSv2 ≥ 1, at 77.7% and 77.2%, respectively. At PI-RADSv2 ≥ 3, highly experienced readers achieved a slightly higher sensitivity than moderately experienced readers, at 73.4% vs 70.8%, respectively.
The use of CAD in the TZ demonstrated better performance for baseline detection at PI-RADSv2 ≥1, at 83.5% for moderately experienced readers and 76.2% for highly experienced readers. Performance at PI-RADSv2 ≥ 3 showed a similar trend to WP and PZ, with sensitivity 65.5% for moderately experienced readers and 64% for highly experienced readers.
Lesion based comparison
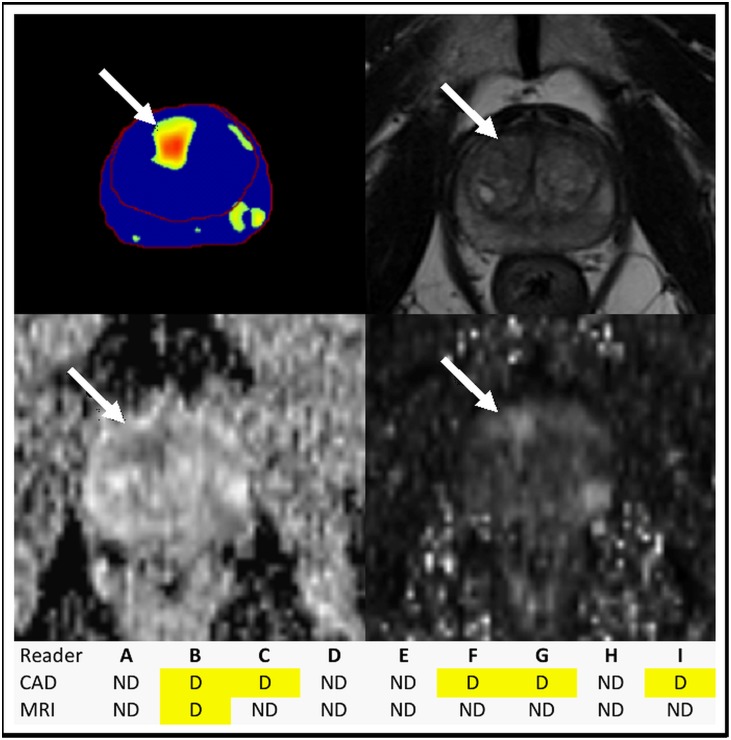
Averaged across all readers at PI-RADSv2 ≥ 1, CAD showed similar index lesion sensitivities to mpMRI-alone in WP (p=0.39), PZ (p=0.07) and TZ (p=0.15). Notably for moderately experienced readers the CAD showed a trend toward improved sensitivity in the TZ compared to mpMRI-alone for PI-RADSv2 ≥1 (83.8% CAD vs 66.9% MRI, p=0.055). Figure 2 shows an example of how CAD can benefit the detection of a TZ lesion. Similar results were observed for clinically significant cancer detection as shown in Supplementary Figure 1. In the WP, CAD lesion detection at PI-RADSv2 ≥ 1 was comparable to mpMRI-alone within experience levels (moderate: p=0.63, high: p=0.09); in the PZ, CAD detection was comparable to mpMRI-alone for moderately experienced readers (p=0.646) but not for highly experienced readers (p=0.02).
Figure 2. Benefit of CAD in TZ tumor identification.
CAD (top left) picked up a tumor (arrows) in the right apical anterior TZ, identified by more readers on MRI (T2W top right, ADC map bottom left, b-1500 bottom right) with CAD assistance. ND = not detected, D = detected; the tumor was found by 5 readers with CAD assistance versus 1 reader with mpMRI alone. Radical prostatectomy histopathology mapping revealed Gleason 4+5 prostatic adenocarcinoma within this lesion.
Investigation of image quality in the data set is reported in Supplementary Materials. Across all institutions’ images, 24% (35/144) of case patients and 24% (17/72) of control patients were classified as low quality based qualitative criteria of motion and rectal gas. CAD sensitivity at PI-RADSv2 ≥ 1 did not vary between high quality and low quality images at 54.6% vs 51.5%. However, fewer false positives were seen in higher quality images compared to lower quality images, resulting in CAD-assisted positive predictive value (PPV) difference of 9.4% at PI-RADSv2 ≥ 1 (57.6% for high quality vs 48.2% for low quality) and 7.5% at PI-RADSv2 ≥ 3 (76.8% for high quality vs 69.7% for low quality).
Reader agreement
Reader agreement for lesion detection is given in Table 3 , stratified by reader experience levels. Overall agreement was not different with CAD assistance (mpMRI-alone index of specific agreement (ISA) 90% vs CAD-assisted 92%, p=0.401), and this pattern was consistent across comparisons between readers of each experience level.
Table 3. Inter-reader agreement of lesion detection.
| Reader experience level pairing | MRI | CAD | p |
|---|---|---|---|
| Overall | 92% (86.9-95.8%) | 89.8% (83.7-94.9%) | 0.401 |
| High-High | 92.2% (86.4-96.3%) | 88.7% (81.7-94.7%) | 0.251 |
| Moderate-Moderate | 91.7% (84-97.3%) | 91.9% (84.5-97.4%) | 0.963 |
| High-Moderate | 92% (86.8-95.6%) | 90.5% (84.4-95.2%) | 0.563 |
Inter-reader agreement, measured with index of specific agreement (ISA), is given across all reads and between readers of each experience level, with 95% confidence intervals given in parentheses. A p-value <0.05 was used for significance.
Image interpretation times
For all readers, the average time to interpret mpMRI alone and with CAD assistance was 4.6 minutes and 3.7 minutes, respectively (p<0.001). Both moderately experienced readers and highly experienced readers had reduced read out times with CAD assistance compared to mpMRI alone (moderate: mpMRI 6.3 minutes vs CAD assisted 4.4 minutes; high: mpMRI 3.5 minutes vs CAD assisted 2.7 minutes, p<0.001).
DISCUSSION
Using moderately and highly experienced readers we observed that standardized PI-RADSv2 categorization and our CAD system, optimized for quantitative parameters that can be extracted from images obtained across different manufacturers and institutions, showed similar baseline detection rates. The CAD system additionally demonstrated improved specificity in conjunction with PI-RADSv2 categorization as well as slightly improved radiologist efficiency. Our findings suggest that standardization and interpretive assistance strategies such as PI-RADSv2 and CAD systems help readers detect cancer with reasonable accuracy, and CAD has potential to improve detection performance in the TZ. This is a robust result based on multi institutional data and a multi-reader study with non-overlapping affiliations.
PI-RADSv2 was released to promote global standardization of mpMRI and to diminish variation in acquisition, interpretation, and reporting [5]. As it is a system based on mostly expert consensus, there have been numerous post hoc studies validating PI-RADSv2. A recent meta-analysis published by Zhang et al. found a pooled sensitivity of 85% (range 78-91%) across 13 studies individually utilizing imaging data within their own institutions compared to radical prostatectomy specimens [19]. This is in agreement with other studies conducted at a single center but with multiple readers [6, 20]. In our study, we found an index lesion sensitivity for PI-RADSv2 ≥ 3 of 78%. PI-RADSv2’s intended aim is to broadly standardize interpretation; our findings largely support this aim.
A known weakness of mpMRI is the TZ where sensitivity is particularly low. The TZ is difficult because of its complex, variable architecture where features of cancer overlap with prostatic hyperplasia [21, 22]. Interestingly, for lesions scored PI-RADSv2 ≥1, the greatest CAD benefit was seen in the TZ where it helped moderately experienced readers to achieve 83.8% sensitivity with CAD versus 66.9% with mpMRI. Thus far, CAD has shown promise in PZ tumor detection but poor diagnostic value in the TZ [17, 23, 24]. Our CAD system utilized separate TZ segmentation and was precisely trained on TZ tumor outlines which may account for the unexpectedly good results which held up even at a multi-institutional level. The numerous additional true positive lesions identified with lower PI-RADS scores suggest that perhaps CAD can provide a special utility in identifying these difficult-to-see TZ tumors, especially for less experienced readers. Additionally, the classification of these tumors to PI-RADS 1 and 2 supports growing evidence that current PI-RADSv2 TZ criteria does not fully account for the spectrum of lesions encountered [25, 26].
Reader experience influenced the value of CAD compared to mpMRI alone at threshold PI-RADSv2 category ≥ 1 versus category ≥ 3. A threshold set at PI-RADSv2 category ≥ 1 represents all subjectively high probability spots indicated by the CAD alone without radiologist discretion in assigning a final suspicion score. Here, CAD in isolation demonstrated comparable performance to mpMRI alone. However, when CAD and the radiologist were considered a diagnostic team, fewer true positive lesions were identified as cumulatively suspicious i.e. PI-RADSv2 category ≥ 3 while per-patient specificity improved, especially for more experienced readers. One possible explanation for this decrease in sensitivity is variable trust in the CAD among readers. Trust has been identified as a major factor in reducing the effectiveness of CAD in radiology [27]. Distrust of CAD is more common in radiologists who are independently confident in lesion identification, and leads to under-reliance and subsequent misclassification of true positive lesions [28, 29]. Alternatively, over-reliance on CAD occurs when readers are uncertain and welcome the assistance of the CAD [30]. The prior first-reader CAD study we conducted also saw greater benefit at a PI-RADSv2 ≥ 1 threshold [18]. While neither study was designed for this purpose, the consistent pattern supports a complex CAD-user relationship which should inform future studies.
It should be noted that the cases used in this study were very diverse in terms of institution-specific acquisition, MRI manufacturer, and patient population. Single-institution studies designed for parallel training and testing of prostate CAD have reported AUCs ranging 76-95% on 3-Tesla images with biopsy or prostatectomy validation [12, 17, 31]. However, a reasonable concern in CAD development is whether a system can be used beyond the population it is trained to recognize patterns on. In our study, we observed AUC of 83% in validation of our CAD that was naïve to the variety of machine specifications and institutional protocols representative of the testing population. This demonstrates that a system trained on quantitative parameters extracted from standardized images can provide meaningful interpretive assistance in a diverse, real-world clinical application.
One potential explanation for the varying mpMRI-CAD discrepancies was image quality. It is widely acknowledged that prostate mpMRI is technically challenging due to motion artifact and rectal gas causing susceptibility artifacts on diffusion weighted imaging. Previously, Caglic et al. demonstrated a 17.5% reduction in positive predictive value where there was greater rectal gas distention. A significant negative correlation between rectal distension and DWI or T2W image quality has been noted [32]. In our population, 24% of cases had poor quality images based on evaluation of rectal distension and inter-slice prostate motion, and CAD pick-ups were more likely to be false positives in these patients resulting in a 7-10% positive predictive value reduction compared to image quality judged to be satisfactory. These issues are not helped by the wide variability in technique that has been observed in surveys of practices performing mpMRI. Esses et al. conducted a survey that revealed a highly variable level of adherence to PI-RADSv2 technical standards across imaging facilities, suggesting that image quality may often be compromised [33]. This problem can only be overcome by better training and education. However, to expect a CAD system to perform equally well on non-standard image acquisitions is unrealistic.
Our study has several limitations. First, a multi-institutional data set inherently suffers from incomplete standardization across institutions in imaging and in histopathology. This includes controls, who had negative imaging validated by 12 or 24-core biopsy. However, all institutions providing images and data were large centers with a genitourinary focus in both their radiology and pathology departments, and the natural variation seen among institutions aligned with the goal of this study to mimic real world clinical variability. Additionally, while the first-reader design most clearly elucidates contribution of CAD to final detection performance, its biggest limitation is that it does not capture additional reader pick-ups on the mpMRI. Studies in other organ systems have found that strict CAD-based decision thresholds, such as a focused probability map, may lead to less of an effort in identifying other abnormalities [34]. Alternatives include a second-reader study design in which CAD output is only available as an adjunct after the reader has viewed the images, or a CAD providing less specific prompting by drawing attention to general suspicious regions of the prostate rather than fully delineating a lesion. Another limitation of this study is that the institutions in this study were likely to be more experienced in mpMRI than an average center. Indeed, there were no inexperienced readers in the study. The readers were, in general, motivated, academic faculty which is unlikely to represent the novice general reader for which the benefits of CAD may be more striking. However, this is a fundamental limitation of clinical trials that are often first reported in academic settings. Finally, the training population utilized for our CAD system was relatively limited in an effort to prevent overlap with the study population. It has previously been shown that CAD sensitivity can be increased with a larger fraction of difficult cases included in the training database [35]. A focus on further diversifying the training population might improve the results of CAD validation.
In conclusion, when using PI-RADSv2 and a CAD system based on heterogeneous imaging acquisitions, readers with different experience levels were able to detect index lesions with comparable sensitivity to non-assisted interpretations. The addition of CAD improved reader specificity and provided a time efficiency advantage. In order to be robust, CAD systems must be based on diverse data sets and be tested by multiple readers with varying experience and diversity of location.
MATERIALS AND METHODS
This Health Insurance Portability and Accountability Act-compliant retrospective evaluation of prospectively acquired multi-institutional data was approved by our local ethics committee. Inclusion of outside institution anonymized data was approved in accordance with the National Institutes of Health’s Office of Human Subjects Resources protocol (Protocol #11617). Local ethics approvals to share data were obtained as needed.
Study design and statistical powering
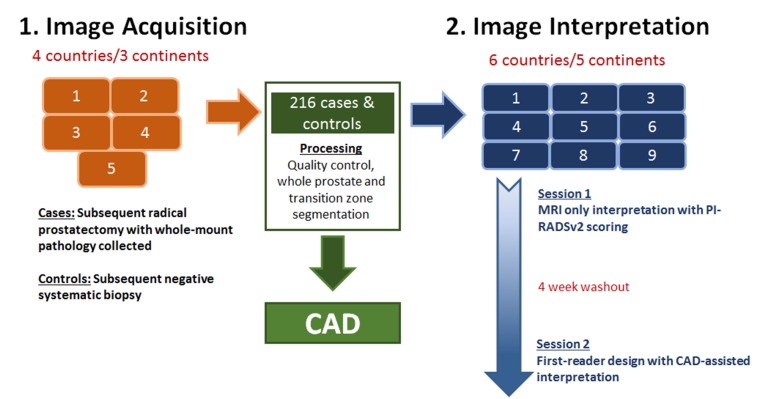
A flow diagram illustrating overall study design is given in Figure 3. Our goal was to test PI-RADSv2 interpretation and CAD-assisted interpretation on a large scale, utilizing 5 institutions for image acquisition and 9 different institutions for image interpretation thus ensuring no local bias associated with interpreting images from one’s own institution. Our primary hypothesis was that CAD-assisted mpMRI would have a higher sensitivity for cancer detection than mpMRI alone. To limit the number of cases each reader would have to interpret, a hybrid design was used to test this hypothesis. Randomization stratified by patient disease status was carried out such that one sixth of randomly selected patients were evaluated by all readers and the remaining patients were assigned at random to each pairwise combination of readers. With this design the average number of interpretations was 76 (range 75-78) with a 2:1 ratio of cancer vs control patients for each reader. The primary endpoint was the difference in average reader-specific sensitivity between CAD and mpMRI. Based on prior results, sensitivity for index lesions was set at 76% for mpMRI, and an improvement in sensitivity of 10% was targeted for CAD [18]. The standard deviation of the endpoint was estimated using these two sensitivity values. The study has 91% power to detect a 10% difference in sensitivity using the Z test at the two-sided 5% significance level.
Figure 3. Study design.
The large multi-institutional framework is shown starting with image acquisition and ending with image interpretation across multiple institutions and readers.
Patient population
Five institutions were recruited to submit de-identified data of consecutive patients who underwent prostate mpMRI at 3-Tesla without endorectal coil that met specified standard sequences as described below. Case patients were consecutive men with lesions detected on mpMRI, subsequent positive biopsy and radical prostatectomy, and whole-mount pathology with lesion mapping available. Control patients were those with negative mpMRI and subsequent negative standard 12-core systematic biopsy or 24-core transperineal template biopsy. Patients who received any prior treatment, with imaging artifact arising from hip prostheses, or with incomplete mpMRI scans were excluded. A total of 144 case patients and 72 control patients were included. Patient characteristics are given in Table 1.
MRI technique
All prostate mpMRI scans were acquired on 3T scanners without the use of an endorectal coil. Magnet brands and models, as well as scanning parameters, varied, but all protocols included axial, sagittal, and coronal T2 spin echo sequences without fat suppression, diffusion-weighted imaging (DWI) images acquired with at least 2 b-values to allow for calculation of apparent diffusion coefficient (ADC) maps and a high value b-1500 DWI, and unprocessed dynamic contrast enhanced (DCE) images compliant with PI-RADSv2 standards.
Supplementary Tables 1-5 contain sequences, coil information, and MRI acquisition parameters utilized in this study. For subsequent CAD processing, a high b-value image of b=1500 mm/sec2 was needed, and so in cases where this was not available, the high b value image was calculated utilizing the mono-exponential model [36].
Image de-identification
To comply with the Office of Human Subjects Resources guidelines for utilization of external data, all images had to be completely de-identified at their respective original institutions prior to collection to ensure patient confidentiality. This de-identification was performed using standard scripts removing patient information as well as clearing DICOM tags other than those reflecting scanner parameters. Upon our collection of the data, an additional de-identification script was used to immediately process the images for a second time to guarantee patient privacy.
Radiologist profile
Nine radiologists, from 9 different institutions, participated in the study. Six were highly experienced (>2000 prostate mpMRI cases) and three were moderately experienced (500-1000 cases). All had experience with PI-RADSv2 at their home institutions prior to this study, but none had interpreted studies from the institutions providing the images.
Computer aided diagnosis software
The CAD system was closely based on a Random Forest classifier system developed and validated for in-house images acquired at 3T with endorectal coil [37]. The system was re-designed for optimal processing of non-endorectal coil images. T2W, ADC, b-1500 DWI, and segmentations of the whole prostate and transition zone were inputs for both training and study data. DCE data was not incorporated into the CAD system. Commercial software was used for automated segmentation on axial T2W images (iCAD, Nashua, New Hampshire), and each automated segmentation was further refined by a prostate mpMRI-focused research fellow with experience in segmenting >200 axial T2W prostate scans. The T2W segmentation was also used on ADC and b-1500 DWI images, as minimal motion was assumed between the consecutively obtained sequences. The classifier was trained based on specific tumor segmentations in a training population correlating with pathologic data from whole mount pathology, given in Supplementary Table 6. The training and study data sets had no patient or institutional overlap.
Image interpretation
For each sequential interpretation session, readers were provided their respective assigned patients as full sets of de-identified DICOM images and instructed to view them on their personal workstations utilizing RadiAnt DICOM Viewer [38]. Readers were unaware of clinical and pathologic outcomes.
For Session 1, the sequences provided for each patient consisted of T2W, DWI (ADC, b-1500 DWI), and DCE. Through a Microsoft Access-designed read-out form, each reader was provided with patient pseudo-identifiers in a randomized order. Within this programmed form, readers recorded up to 4 detected lesions per mpMRI, assigned a PI-RADSv2 category from 1-5 to each lesion, recorded the location of each lesion in a standardized fashion that included the zone, side of the prostate, and an annotated screenshot of the lesion [5]. Additionally, the form was built with a timer that recorded duration of each interpretation session. All data were recorded in a linked Microsoft Access database [39].
A 4-week washout period followed the conclusion of Session 1. During the washout, a training packet was sent to each reader with 3 examples to familiarize them with interpreting the CAD results. For Session 2, readers were instructed to view the CAD output first and identify up to 4 suspicious areas which were markedly red or orange on a probability map as shown in Supplementary Figure 2. Following CAD output interpretation, readers evaluated the full corresponding mpMRI next to the annotated CAD output. Readers accepted the finding on CAD if mpMRI features were consistent with PI-RADSv2 category ≥ 3; otherwise the finding on CAD was rejected (PI-RADSv2 category ≤ 2) [5]. Each patient was assigned a new pseudo-identifier and the patient list was randomized from Session 1 to ensure that studies were interpreted in a different order. Readers input data on a similar Microsoft Access form that recorded time and linked the data to the database [39]. For both sessions, PI-RADSv2 ≥ 1 represents all recorded lesions, and PI-RADSv2 ≥ 3 represents those lesions classified as appearing more suspicious according to PI-RADSv2 guidelines [5].
Histopathologic validation
Each providing institution was instructed to supply mapped histopathology of the radical prostatectomy specimen spanning from apex to base. Lesion-specific locations and Gleason scores were determined by a genitourinary pathologist from each institution. Pathologists were unaware of mpMRI results. MRI-histopathology correlation was performed by a prostate mpMRI-focused research fellow using visible prostate landmarks and lesion morphology.
Statistical analysis
For patient-based analysis, the maximum PI-RADSv2 score assigned by a given reader was used to calculate the sensitivity and specificity at each PI-RADSv2 threshold and to construct a receiver operating characteristic (ROC) curve. For lesion-based analysis, reader sensitivity for index lesions was calculated. The index lesion was defined as the tumor with highest Gleason score and largest volume as designated on histopathology. Specificity was not estimated because negative regions were not specified. Because true positive lesions detected by CAD but rejected by readers would be assigned PI-RADSv2 categories 1 or 2, the comparison in true positives between CAD and mpMRI alone was focused on PI-RADSv2 ≥ 1 and PI-RADSv2 ≥ 3. Reader statistics were averaged across all readers and by experience level. Bootstrap resampling stratified by disease status was used to calculate the 95% confidence intervals for sensitivity, specificity, and area under the ROC curve (AUC). The confidence limits were obtained from the 2.5th and 97.5th percentiles of the 2,000 bootstrap samples. The Wald test using the bootstrap standard error was utilized to test the differences in the estimated sensitivity, specificity, and AUC between mpMRI and CAD. All tests were two-sided and p-value <0.05 was considered statistically significant.
Inter-observer agreement on lesion detection in the same location was assessed by the index of specific agreement (ISA), defined as the conditional probability that an independent reader detects a lesion in the same location as a randomly selected reader [40, 41]. Inference for ISA was made based on the bootstrap resampling procedure and Wald-test as described above.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
The National Institutes of Health (NIH) Medical Research Scholars Program is a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, The American Association for Dental Research, the Colgate-Palmolive Company, Genentech and alumni of student research programs and other individual supporters via contributions to the Foundation for the National Institutes of Health.
Abbreviations
- mpMRI
Multiparametric magnetic resonance imaging
- PI-RADSv2
Prostate Imaging-Reporting and Data System version 2
- CAD
Computer-aided diagnosis
- WP
Whole prostate
- PZ
Peripheral zone
- TZ
Transition zone
- PPV
Positive predictive value
- ISA
Index of specific agreement
- T2W
T2-weighted MRI
- DWI
Diffusion-weighted imaging
- ADC
Apparent diffusion coefficient
- DCE
Dynamic contrast-enhanced MRI
- ROC
Receiver operating characteristic
- AUC
Area under the ROC curve
| Trial design | Data acquisition (imaging) | Data processing (imaging) | Monitoring | Data acquisition (surgery and histopathology) | Data processing (Histopathology) | Data correlation | Statistics | Manuscript preparation | Manuscript editing | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sonia Gaur | X | X | X | X | X | X | X | X | X | |
| Nathan Lay | X | X | X | X | X | X | ||||
| Stephanie Harmon | X | X | X | X | X | X | X | X | X | |
| Sreya Doddakashi | X | X | X | X | X | |||||
| Sherif Mehralivand | X | X | X | X | X | |||||
| Burak Argun | X | X | X | X | X | |||||
| Tristan Barrett | X | X | X | X | X | |||||
| Sandra Bednarova | X | X | X | X | X | |||||
| Rossanno Girometti | X | X | X | X | X | |||||
| Ercan Karaarslan | X | X | X | X | X | |||||
| Ali Riza Kural | X | X | X | X | X | |||||
| Aytekin Oto | X | X | X | X | X | |||||
| Andrei S. Purysko | X | X | X | X | X | |||||
| Tatjana Antic | X | X | X | X | ||||||
| Cristina Magi-Galluzzi | X | X | X | X | ||||||
| Yesim Saglican | X | X | X | X | ||||||
| Stefano Sioletic | X | X | X | X | ||||||
| Anne Y. Warren | X | X | X | X | ||||||
| Leonardo Bittencourt | X | X | X | X | ||||||
| Jurgen J. Fütterer | X | X | X | X | ||||||
| Rajan T. Gupta | X | X | X | X | ||||||
| Ismail Kabakus | X | X | X | X | ||||||
| Yan Mee Law | X | X | X | X | ||||||
| Daniel Margolis | X | X | X | X | ||||||
| Haytham Shebel | X | X | X | X | ||||||
| Antonio C. Westphalen | X | X | X | X | ||||||
| Bradford J. Wood | X | X | X | |||||||
| Peter A. Pinto | X | X | X | |||||||
| Joanna H. Shih | X | X | X | X | X | X | ||||
| Peter L. Choyke | X | X | X | X | X | X | X | X | X | |
| Ronald M. Summers | X | X | X | X | X | X | ||||
| Baris Turkbey | X | X | X | X | X | X | X | X | X |
Author contributions
See following table.
CONFLICTS OF INTEREST
Dr. Ronald M. Summers: Patent royalties from iCAD & ScanMed, Research support from Ping An & NVidia, Software licenses to Philips & Ping An.
Dr. Bradford J. Wood reports grants from NIH Intramural Research Program , during the conduct of the study; grants and non-financial support from CRADA with Philips, grants and non-financial support from CRADA with Celsion Corp, grants and non-financial support from CRADA with Siemens, grants and non-financial support from CRADA with Biocompatibles BTG, grants and non-financial support from CRADA with NVIDIA, grants and non-financial support from CRADA with Profound Medical, grants and non-financial support from CRADA with XAct Robotics, outside the submitted work; In addition, Dr. Wood has a patent Fusion Patents with royalties paid to NIH and to Dr. Wood by Philips, but this is unrelated to this work.
FUNDING
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This research was supported in part by the Intramural Research Program of the National Institutes of Health.
Sherif Mehralivand’s postdoctoral fellowship is funded by a research grant of the Dr. Mildred Scheel Foundation (Bonn, Germany).
REFERENCES
- 1.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM, Merino MJ, Simon RM, Choyke PL, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–7. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rais-Bahrami S, Siddiqui MM, Turkbey B, Stamatakis L, Logan J, Hoang AN, Walton-Diaz A, Vourganti S, Truong H, Kruecker J, Merino MJ, Wood BJ, Choyke PL, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013;190:1721–7. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, Briganti A, Budaus L, Hellawell G, Hindley RG, Roobol MJ, Eggener S, Ghei M, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018;378:1767–1777. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehralivand S, Shih JH, Rais-Bahrami S, Oto A, Bednarova S, Nix JW, Thomas JV, Gordetsky JB, Gaur S, Harmon SA, Siddiqui MM, Merino MJ, Parnes HL, et al. A Magnetic Resonance Imaging-Based Prediction Model for Prostate Biopsy Risk Stratification. JAMA Oncol. 2018;4:678–685. doi: 10.1001/jamaoncol.2017.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, Margolis D, Schnall MD, Shtern F, Tempany CM, Thoeny HC, Verma S. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greer MD, Brown AM, Shih JH, Summers RM, Marko J, Law YM, Sankineni S, George AK, Merino MJ, Pinto PA, Choyke PL, Turkbey B. Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: A multireader study. J Magn Reson Imaging. 2016;45:579–85. doi: 10.1002/jmri.25372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao C, Gao G, Fang D, Li F, Yang X, Wang H, He Q, Wang X. The efficiency of multiparametric magnetic resonance imaging (mpMRI) using PI-RADS Version 2 in the diagnosis of clinically significant prostate cancer. Clin Imaging. 2016;40:885–8. doi: 10.1016/j.clinimag.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Muller BG, Shih JH, Sankineni S, Marko J, Rais-Bahrami S, George AK, de la Rosette JJ, Merino MJ, Wood BJ, Pinto P, Choyke PL, Turkbey B. Prostate Cancer: Interobserver Agreement and Accuracy with the Revised Prostate Imaging Reporting and Data System at Multiparametric MR Imaging. Radiology. 2015;277:741–50. doi: 10.1148/radiol.2015142818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenkrantz AB, Ayoola A, Hoffman D, Khasgiwala A, Prabhu V, Smereka P, Somberg M, Taneja SS. The Learning Curve in Prostate MRI Interpretation: Self-Directed Learning Versus Continual Reader Feedback. AJR Am J Roentgenol. 2016;208:W1–W9. doi: 10.2214/AJR.16.16876. [DOI] [PubMed] [Google Scholar]
- 10.Borofsky S, George AK, Gaur S, Bernardo M, Greer MD, Mertan FV, Taffel M, Moreno V, Merino MJ, Wood BJ, Pinto PA, Choyke PL, Turkbey B. What Are We Missing? False-Negative Cancers at Multiparametric MR Imaging of the Prostate. Radiology. 2018;286:186–95. doi: 10.1148/radiol.2017152877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le JD, Tan N, Shkolyar E, Lu DY, Kwan L, Marks LS, Huang J, Margolis DJ, Raman SS, Reiter RE. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol. 2015;67:569–76. doi: 10.1016/j.eururo.2014.08.079. [DOI] [PubMed] [Google Scholar]
- 12.Lemaitre G, Marti R, Rastgoo M, Meriaudeau F. Computer-aided detection for prostate cancer detection based on multi-parametric magnetic resonance imaging. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:3138–41. doi: 10.1109/EMBC.2017.8037522. [DOI] [PubMed] [Google Scholar]
- 13.Hambrock T, Vos PC, Hulsbergen-van de Kaa CA, Barentsz JO, Huisman HJ. Prostate cancer: computer-aided diagnosis with multiparametric 3-T MR imaging--effect on observer performance. Radiology. 2013;266:521–30. doi: 10.1148/radiol.12111634. [DOI] [PubMed] [Google Scholar]
- 14.Metzger GJ, Kalavagunta C, Spilseth B, Bolan PJ, Li X, Hutter D, Nam JW, Johnson AD, Henriksen JC, Moench L, Konety B, Warlick CA, Schmechel SC, et al. Detection of Prostate Cancer: Quantitative Multiparametric MR Imaging Models Developed Using Registered Correlative Histopathology. Radiology. 2016;279:805–16. doi: 10.1148/radiol.2015151089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litjens GJ, Barentsz JO, Karssemeijer N, Huisman HJ. Clinical evaluation of a computer-aided diagnosis system for determining cancer aggressiveness in prostate MRI. Eur Radiol. 2015;25:3187–99. doi: 10.1007/s00330-015-3743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng Y, Jiang Y, Antic T, Giger ML, Eggener SE, Oto A. Validation of quantitative analysis of multiparametric prostate MR images for prostate cancer detection and aggressiveness assessment: a cross-imager study. Radiology. 2014;271:461–71. doi: 10.1148/radiol.14131320. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Tian Z, Zhang Z, Fei B. Computer-aided Detection of Prostate Cancer with MRI: Technology and Applications. Acad Radiol. 2016;23:1024–46. doi: 10.1016/j.acra.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer MD, Lay N, Shih JH, Barrett T, Bittencourt LK, Borofsky S, Kabakus I, Law YM, Marko J, Shebel H, Mertan FV, Merino MJ, Wood BJ, et al. Computer-aided diagnosis prior to conventional interp retation of prostate mpMRI: an international multi-reader study. Eur Radiol. 2018 Apr 12 doi: 10.1007/s00330-018-5374-6. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Tang M, Chen S, Lei X, Zhang X, Huan Y. A meta-analysis of use of Prostate Imaging Reporting and Data System Version 2 (PI-RADS V2) with multiparametric MR imaging for the detection of prostate cancer. Eur Radiol. 2017;27:5204–14. doi: 10.1007/s00330-017-4843-7. [DOI] [PubMed] [Google Scholar]
- 20.Purysko AS, Bittencourt LK, Bullen JA, Mostardeiro TR, Herts BR, Klein EA. Accuracy and Interobserver Agreement for Prostate Imaging Reporting and Data System, Version 2, for the Characterization of Lesions Identified on Multiparametric MRI of the Prostate. AJR Am J Roentgenol. 2017;209:1–7. doi: 10.2214/AJR.16.17289. [DOI] [PubMed] [Google Scholar]
- 21.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15:340–5. [PubMed] [Google Scholar]
- 22.Greene DR, Wheeler TM, Egawa S, Dunn JK, Scardino PT. A comparison of the morphological features of cancer arising in the transition zone and in the peripheral zone of the prostate. J Urol. 1991;146:1069–76. doi: 10.1016/s0022-5347(17)38003-5. [DOI] [PubMed] [Google Scholar]
- 23.Dinh AH, Melodelima C, Souchon R, Moldovan PC, Bratan F, Pagnoux G, Mege-Lechevallier F, Ruffion A, Crouzet S, Colombel M, Rouviere O. Characterization of Prostate Cancer with Gleason Score of at Least 7 by Using Quantitative Multiparametric MR Imaging: Validation of a Computer-aided Diagnosis System in Patients Referred for Prostate Biopsy. Radiology. 2018;287:525–533. doi: 10.1148/radiol.2017171265. [DOI] [PubMed] [Google Scholar]
- 24.Niaf E, Lartizien C, Bratan F, Roche L, Rabilloud M, Mege-Lechevallier F, Rouviere O. Prostate focal peripheral zone lesions: characterization at multiparametric MR imaging--influence of a computer-aided diagnosis system. Radiology. 2014;271:761–9. doi: 10.1148/radiol.14130448. [DOI] [PubMed] [Google Scholar]
- 25.Rosenkrantz AB, Oto A, Turkbey B, Westphalen AC. Prostate Imaging Reporting and Data System (PI-RADS), Version 2: A Critical Look. AJR Am J Roentgenol. 2016;206:1179–83. doi: 10.2214/AJR.15.15765. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann R, Logan C, O'Callaghan M, Gormly K, Chan K, Foreman D. Does the Prostate Imaging-Reporting and Data System (PI-RADS) version 2 improve accuracy in reporting anterior lesions on multiparametric magnetic resonance imaging (mpMRI)? Int Urol Nephrol. 2018;50:13–9. doi: 10.1007/s11255-017-1753-1. [DOI] [PubMed] [Google Scholar]
- 27.Jorritsma W, Cnossen F, van Ooijen PM. Improving the radiologist-CAD interaction: designing for appropriate trust. Clin Radiol. 2015;70:115–22. doi: 10.1016/j.crad.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa RM, Schmidt RA, Linver MN, Edwards AV, Papaioannou J, Stull MA. Clinically missed cancer: how effectively can radiologists use computer-aided detection? AJR Am J Roentgenol. 2012;198:708–16. doi: 10.2214/AJR.11.6423. [DOI] [PubMed] [Google Scholar]
- 29.Halligan S, Altman DG, Mallett S, Taylor SA, Burling D, Roddie M, Honeyfield L, McQuillan J, Amin H, Dehmeshki J. Computed tomographic colonography: assessment of radiologist performance with and without computer-aided detection. Gastroenterology. 2006;131:1690–9. doi: 10.1053/j.gastro.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 30.Drew T, Cunningham C, Wolfe JM. When and why might a computer-aided detection (CAD) system interfere with visual search? An eye-tracking study. Acad Radiol. 2012;19:1260–7. doi: 10.1016/j.acra.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Burtt K, Turkbey B, Choyke P, Summers RM. Computer aided-diagnosis of prostate cancer on multiparametric MRI: a technical review of current research. Biomed Res Int. 2014;2014:789561. doi: 10.1155/2014/789561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caglic I, Hansen NL, Slough RA, Patterson AJ, Barrett T. Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur J Radiol. 2017;90:174–80. doi: 10.1016/j.ejrad.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Esses SJ, Taneja SS, Rosenkrantz AB. Imaging Facilities' Adherence to PI-RADS v2 Minimum Technical Standards for the Performance of Prostate MRI. Acad Radiol. 2017;25:188–195. doi: 10.1016/j.acra.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Berbaum KS, Caldwell RT, Schartz KM, Thompson BH, Franken EA., Jr Does computer-aided diagnosis for lung tumors change satisfaction of search in chest radiography? Acad Radiol. 2007;14:1069–76. doi: 10.1016/j.acra.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng B, Wang X, Lederman D, Tan J, Gur D. Computer-aided detection; the effect of training databases on detection of subtle breast masses. Acad Radiol. 2010;17:1401–8. doi: 10.1016/j.acra.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant KB, Agarwal HK, Shih JH, Bernardo M, Pang Y, Daar D, Merino MJ, Wood BJ, Pinto PA, Choyke PL, Turkbey B. Comparison of calculated and acquired high b value diffusion-weighted imaging in prostate cancer. Abdom Imaging. 2015;40:578–86. doi: 10.1007/s00261-014-0246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lay N, Tsehay Y, Greer MD, Turkbey B, Kwak JT, Choyke PL, Pinto P, Wood BJ, Summers RM. Detection of prostate cancer in multiparametric MRI using random forest with instance weighting. J Med Imaging (Bellingham) 2017;4:024506. doi: 10.1117/1.JMI.4.2.024506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medixant RadiAnt DICOM Viewer
- 39.Microsoft Microsoft Access Professional Plus 2010 2010 [Google Scholar]
- 40.Fleiss JL. 2nd edition. John Wiley & Sons Ltd; 1981. Statistical Methods for Rates and Proportions. [Google Scholar]
- 41.Shih JH, Greer MD, Turkbey B. The problems with the kappa statistic as a metric of inter-observer agreement on lesion detection using a third-reader approach when locations are not pre-specified. Acad Radiol. 2018 Mar 16 doi: 10.1016/j.acra.2018.01.030. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.