Abstract
In the context of valorization of lignin produced from the pulp and paper industries, biodegradable UV-protection films were prepared using lignin and cellulose nanocrystals (CNCs). Initially, CNC films were optimized for improving their transparency by studying the effect of various sodium hydroxide (NaOH) concentrations. Maximum (%) transmittance of CNC film was obtained for NaOH addition between 3 and 4 wt %. The optimized CNC suspensions were used for incorporating alkaline lignin (AL) and softwood kraft lignin (SKL) in various concentrations (1–10 wt %). Morphological characterization showed homogeneity of the lignin distribution in CNC/lignin films. Complete UV blocking was achieved at 10 wt % lignin (AL or SKL) in CNC films. Cross-polarized optical microscopy and scanning electron microscopic images of films showed some degrees of global alignment of CNC rods upon addition of NaOH, which remained unaffected by lignin addition. Lignin modification through acetylation reduced the lignin color and improved visible light transmission of films without significantly affecting the UV-absorption properties. Presence of lignin also enhanced the thermal and contact angle stability of the films. This work shows for the first time that CNC aqueous suspensions with and without containing lignin could be tuned through the addition of NaOH to produce transparent and homogenous films, providing a simple and green approach in engineering CNC/lignin UV-protection films.
Introduction
Biomass-derived materials are promising alternatives to the petroleum-based polymers due to their sustainability and biodegradability. The major structural components of trees and various plants, cellulose, lignin, and hemicellulose, are the most abundant naturally available biomass-based materials. Cellulose nanocrystals (CNCs) are the crystals derived from acid hydrolysis of native cellulose with a diameter between 4–25 and 100–1000 nm in length, is rapidly emerging as one of the most fascinating nanomaterials for multifunctional application, mainly due to their inherent advantages, including nanoscale dimension, high specific strength and stiffness, high surface area, ease of processing, and cost effectiveness.1−3 Moreover, CNC possess interesting optical properties by virtue of their chiral nematic self-assembly. Controlling this behavior would facilitate their use in applications, such as security papers, mirrorless lasing, and polarizing films.4−6 Lignin is the second most abundant renewable carbon source after cellulose, it is an amorphous, polyphenolic branched structure formed from enzyme-assisted dehydrogenative polymerization of the phenyl propanoid units.7 Five million metric tons of lignin is produced worldwide predominantly as a noncommercialized waste product per year.8 In pulp and paper industries, lignin is a main component of black liquor produced from the kraft process. Currently, the primary usage of this lignin-based waste product is to produce energy. Only 2% of all industrial lignins and 100 000 tons of kraft lignins available are valorized per year.9
Lignin is a natural UV blocker due to its functional groups, such as phenolic units, ketones, and other chromophores.7,10,11 Aromatic structure of lignin can increase thermal and oxidation stabilities of polymers in blends, and the free radical scavenging ability of its phenolic groups gives lignin an excellent antioxidant property.12−17 Currently, organic and inorganic UV blockers are widely used for the UV-protection applications.18,19 Organic absorbers are often toxic and degrade upon exposure to sunlight.20−22 Inorganic UV-blocking agents, such as ZnO and TiO2, are mostly used in the form of nanoparticles.23,24 However, their higher loading is required for the complete UV blocking, which often leads to agglomeration, reducing the transparency of the resultant polymer nanocomposite films.18,25 Some of the coatings developed for the UV-blocking applications using inorganic particles, such as titanium dioxides and cerium oxide doped with iron, silica, alumina, or organic liquids, showed higher absorption in a visible region, which induced the opaque nature to these coatings.26 CNC can form transparent films or coatings, and lignin is a naturally available and biodegradable UV blocker. CNC and lignin can be synergistically used to obtain the films with high transparency and UV-absorption properties.
A common challenge in engineering new nanocellulose–lignin-based materials is incompatibility between hydrophilic cellulose and hydrophobic lignin. In addition, at normal pH and in the absence of chemical additives, both CNC and lignin have a negative charge and therefore there is an electrostatic repulsion between them. It was noticed that nonderivatized cellulose/lignin films could be formed using green solvents, such as ionic liquids, but this approach is not economical due to high cost of solvent.27 Other approaches using some hazardous organic solvents, such as dimethyl sulfoxide/water and dioxane/water, did not produce homogenous films.28 To compatibilize CNC and lignin, several attempts were carried out through covalent bond formation.13,29 Sadeghifar et al.13 have produced flexible cellulose/lignin UV-blocking films containing low amounts of covalently bonded lignin. In this procedure, azide-modified cellulose microcrystals dissolved in dimethylacetamide/lithium chloride were reacted with propargylated softwood kraft lignin (SKL) to produce 0.5, 1, and 2 wt % lignin containing materials. Cellulose films were then prepared by regeneration in acetone. Cellulose film containing 2% lignin showed around 100% absorption of UV-B (280–320 nm) and majority of UV-A (320–400 nm). The obtained films were uniform, but the procedure required use of organic solvents and long preparation times. Another approach reported in literature for covalent bond formation between cellulose nanocrystal and lignin was using Fenton’s reagent (H2O2 and FeSO4) as an initiator.29 This approach increased covalent and noncovalent bonds between both polymers and contributed to the increase in the water resistance of coated films on quartz slides and in water retention in self-supported films as a hydrogel film. Natural UV-absorbent coatings prepared from CNC and dehydrogenation polymer or organosolv lignin by simple evaporation of the colloidal blended mixture have also been reported.28 These materials were in the form of thin films and are transparent on glass or quartz slides with variable antireflective or UV-absorbent properties, depending on the process used. In these materials, lignin and CNC are interacted through noncovalent and electrostatic forces.28,30 However, homogeneity and hydrophobicity of these materials were not reported. Water-soluble polymers, like poly(vinyl alcohol), have been used for the preparation of lignin-based transparent films,31 but the resultant nanocomposite films were not fully biodegradable.
The present study shows for the first time that homogenous and transparent films of CNC and lignin can be processed without the use of organic solvents. The simple and novel approach shown in this study for CNC/lignin nanocomposite film preparation uses aqueous alkaline solvent containing sodium hydroxide (NaOH). CNC and alkaline lignin (AL) or softwood kraft lignin (SKL) is compatibilized in this aqueous alkaline suspension. The CNC/AL and CNC/SKL films were casted using aqueous alkaline mixture containing optimal NaOH concentration. The resultant homogenous, transparent films were characterized for their structural, optical, thermal, and hydrophobic properties.
Results and Discussion
Structural and Morphological Properties
CNC and CNC/lignin films were prepared by casting the aqueous colloidal suspension followed by drying it at room temperature. As shown in Figure 1a, CNC film prepared at neutral pH showed iridescent colors. This iridescence is caused by the chiral nematic ordering of CNC upon drying.32−35 Films prepared by addition of 2 wt % AL and SKL in aqueous CNC suspension did not affect the chiral nematic assembly of the CNC and retained iridescence, as shown in Figure 1c,e, respectively. AL is a water-soluble form of lignin and produced homogenous films (Figure 1c), whereas nonhomogenous films were obtained for SKL due to its low solubility in aqueous CNC suspension (Figure 1e).
Figure 1.
CNC-based films from aqueous suspensions at different pHs, CNC films at (a) pH 7, (b) pH 11.5; CNC/AL (2 wt %) films at (c) pH 7, (d) pH 11.5; CNC/SKL (2 wt %) films at (e) pH 7, (f) pH 11.5.
It was observed that in alkaline conditions (pH 11.5), the chiral nematic ordering of CNC disappeared completely and produced highly transparent films (Figure 1b). Although the effects of electrolytes and pH on the aqueous phase behavior of the CNC have been studied for the various inorganic salts, their effect on film transparency has not been focussed. Most studies involved the effect of small electrolyte concentrations on ζ potential, particle size, pitch length, alignment and phase formation in aqueous CNC suspensions.33,36−38 Alkaline condition used in preparing CNC/lignin films in this study showed the effect of NaOH on the film transparency. This, to the authors knowledge, is the first attempt to show the effect of NaOH addition on the CNC film transparency. The addition of sodium counter ions hinders chiral interaction between the CNC rods leading to transparent films (detailed explanation can be found in the following section). It also helped in forming uniform films reducing the shrinkage during drying. As shown in Figure 2, CNC film with 4 wt % NaOH addition formed uniform film throughout the Petri dish, whereas pure CNC film shrank upon drying and produced an iridescent film of uneven thickness. Shrinkage in the pure CNC film is caused by the hydrogen bonding between hydroxyl groups on the surface of CNC. Addition of NaOH introduced the sodium and hydronium counter ions, which reduced the hydrogen bonding between the hydroxyl groups.
Figure 2.
Dried CNC films with 4 wt % NaOH (left), CNC film without NaOH addition (right).
The lignin addition to the alkaline aqueous CNC suspensions produced transparent and homogeneous films of CNC/AL and CNC/SKL, as shown in Figure 1d,f, respectively. Besides eliminating iridescence, addition of NaOH also helped in uniformly dispersing SKL in suspension producing the homogenous CNC/SKL films. Such uniformity resulted due to alkaline conditions, where pKa of aromatic hydroxyl lignin group is achieved. Deprotonation of this hydroxyl group produced the phenolate anion, thus stabilizing the lignin molecules in aqueous suspensions through electrostatic repulsions. Uniform dispersion of lignin in aqueous suspension is also retained in the dried films, thereby producing highly homogenous CNC/lignin films similar to those of lignin-grafted CNC films.29
The root-mean-square (RMS) roughness of the CNC/lignin films was also measured using atomic force microscopy (AFM). The average RMS roughness values were 7.3 ± 1.3, 13.3 ± 2.1, and 12.9 ± 1.4 nm for CNC, CNC/AL (10 wt %), and CNC/SKL (10 wt %), respectively. Topographical AFM images of the 10 wt % CNC/AL and 10 wt % CNC/SKL films (Figure 3) showed homogeneity of lignin dispersion in the CNC matrix.
Figure 3.
Topographical images of CNC/lignin films using alkaline aqueous mixtures (a) CNC/AL (10 wt %), (b) CNC/SKL (10 wt %).
Structural features of CNC rod self-assembly can be observed from the scanning electron microscopy (SEM) images taken across slanted cross-sections of the films. As shown in Figure 4a,b, the chiral nematically arranged rods across the film thickness are clearly visible. The different locally nematic layers of CNC are oriented in various directions, giving rise to overall helical chiral nematic self-assembly. However, in the case of the films with 4 wt % NaOH addition, these layers of CNC with chiral nematic orientations were not observed. The SEM images of this sample in Figure 4c,d, show few rods orienting out of plane, whereas most CNC rods are in the plane. This shows that there is some level of global alignment of CNC rods in the film upon addition of the NaOH. This is also supported by the cross-polarized optical microscopy images discussed in the following section. Similar morphology of CNC rods was observed for the 10 wt % CNC/SKL films (Figure 4e,f), in this case uniform distribution of lignin particles could be seen across the film thickness. Uniform and homogenous lignin dispersion was also observed in case of 10 wt % CNC/AL and 10 wt % CNC/acetylated-SKL (Ac-SKL) films (Figure S1, Supporting Information). Acetylation of SKL did not affect its morphology and dispersibility in CNC matrix.
Figure 4.
Scanning electron microscopy images of the CNC film (a, b), CNC (4 wt % NaOH) (c, d), and CNC/SKL (10 wt %) (e, f).
The thickness of the films increased linearly with the increase in lignin concentration (Figure 5), CNC/AL, CNC/SKL, and CNC/Ac-SKL films had similar thickness at certain lignin concentration. The average thickness of CNC film was 46.3 μm, which has increased upto 67 μm upon 10 wt % lignin addition.
Figure 5.
Thickness of the composite films with various lignin concentrations.
Optical Properties
Optimization of Film Transparency
To enhance the scope of CNC/lignin films in applications requiring transparent UV-protection properties, it is desirable to maximize the visible light transmission and precisely control the UV light blocking. Since addition of NaOH influences the film transparency, its concentration in suspension needs to be optimized to get the maximum transparency in the film. For this purpose, 1.64 wt % aqueous CNC suspensions with various NaOH additions ranging from 0.5 to 15 wt % were prepared. These suspensions were characterized for the ζ potential and average CNC particle size using Malvern Zetasizer, and the corresponding films were characterized for the UV–vis transmission.
As shown in Figure 6a, addition of NaOH influenced the size and the ζ potential of the CNC particles in suspension. It was clearly observed that ζ potential and average particle size of CNC slightly increased till 7 wt % NaOH addition; however, drastic changes in these parameters were observed at higher concentrations. A small decrease in the average particle size at 0.5 wt % NaOH addition was observed, which may have arisen from compression of the electrical double layer of CNC. Such behavior has been described for CNC rods upon addition of trace electrolytes by Araki and Kuga37 and Dong et al.39 Small concentration of sodium ions can cause shrinkage of effective particle size through shielding of the surface charge on CNC. This exposes its twisted morphology thereby manifesting the formation of chiral nematic ordering. Iridescence of the films (Figure 7a,b) and their characteristic transmission spectra in Figure 6b show that such morphology of CNC has retained until 1 wt % NaOH addition. At 1.5 wt % NaOH concentration, increase in double layer would obscure the chiral morphology by making the effective rods straight and smooth, which results in loss of iridescence, as shown in Figure 7c. At concentrations more than 4 wt %, the negative charge on the CNC surface is completely screened by increase in double layer thereby reducing the absolute ζ potential. When the absolute value of ζ potential is less than 30 mV, the van der Waals force dominates the electrostatic repulsion and thus charged particles tend to aggregate causing drastic increase in particle size.40 Some studies have also shown that higher concentration of NaOH could also lead to desulfation reducing the stability of the CNC rods in suspension.41,42
Figure 6.
(a) Average particle size and ζ potential measurements of 1.64 wt % aqueous alkaline CNC suspension with various NaOH additions. (b) Transmission spectra for the CNC films with various NaOH additions.
Figure 7.
CNC films at various NaOH concentrations (a) 0.5 wt %, (b) 1 wt %, (c) 1.5 wt %, (d) 2 wt %, (e) 3 wt %, (f) 4 wt %, (g) 7 wt %, (h) 10 wt %, and (i) 15 wt %.
Figure 6b shows the transmission spectra of the films prepared from suspensions with various NaOH concentrations. At lower concentrations of 0.5 and 1 wt % NaOH, dip in transmittance is observed with minimum transmittance at around 470 nm. This transmission behavior is caused by characteristic reflection of light from the iridescent CNC films.33,43,44 In CNC films with 1.5 wt % NaOH, destruction of chiral nematic structure has led to increase in transparency. As shown in Figure 7c–f, films within NaOH concentration range of 1.5–4 wt % looked completely transparent; however, their transmittance has increased within this range (Figure 6b). This may have resulted from the increase in alignment of more nematic domains with NaOH addition. Increase in global alignment of CNC reduces the variation in refractive index across the film thickness. This in turn will increase the visible light transmittance by lowering the dispersive losses in the film.45 The increased CNC particle size at higher NaOH concentrations caused more scattering of incident light reducing the transparency of resulting films. After 4 wt %, the film transmittance was reduced (Figure 6b) in accordance with the increased CNC particle size (Figure 6a) resulting in hazy films (Figure 7g–i). On the basis of the maximum optical transmittance and ability to completely solubilize the largest concentration of SKL used in this study, 4 wt % NaOH concentration was chosen to prepare CNC/lignin films.
UV-Blocking Properties of Film
Lignin has the ability to absorb the UV light due to its phenolic structure. UV-blocking properties of CNC/AL and CNC/SKL films were characterized through its UV transmission spectra after incorporating the lignin at various concentrations. The UV–vis transmittance of the films was measured in the wavelength range of 200–800 nm, as shown in Figure 8. Transmission spectra include all of the normal incident light on the films exposed perpendicular to the light source excluding the absorbed and scattered light. All of the films were prepared using the optimized (4 wt %) of the NaOH concentration.
Figure 8.
UV–vis transmission spectra of CNC and CNC/lignin films with various lignin concentrations.
The CNC films showed a higher transmittance in the UV and visible regions, maximum transmittance for this film was upto 90%. The homogenous lignin containing films exhibited high transmittance in the visible spectrum and high absorption in the UV spectrum. As shown in Figure 8, CNC/SKL films with 1 wt % lignin contents completely blocked UV-C (200–280 nm) spectrum, whereas CNC/AL (1 wt %) partially blocked UV-C. Increasing lignin concentration to 4 wt % completely blocked the UV-C and partially blocked the UV-B (280–320 nm) for both the films, whereas at 10 wt % lignin concentration total UV protection was obtained in the both films blocking UV-C, UV-B, and UV-A (320–400 nm) spectra. At this lignin concentration, visible light transmittance for CNC/AL films was slightly higher than CNC/SKL. The UV-protection behavior and visible light transparency are similar to the covalently bonded microcrystalline cellulose/SKL films prepared and reported by Sadeghifar et al.13 However, the transparency of these films was lower compared to that of the CNC/organosolv lignin films, which have a lower thickness, reported by Hambardzumyan et al.28
Sun protection factor (SPF) was also estimated using the method used by Dutra et al.46 SPFs of the 1 wt % CNC/AL and CNC/SKL films were 10.6 and 13.4, respectively, which indicates UV-B protection equal to 100 – (100/SPF), which is close to 91 and 93%, respectively. With 4 wt % lignin, UV protection has increased to 97.3 and 97.6% for CNC/AL and CNC/SKL films corresponding to their SPFs of 36.5 and 41.1.
Since UV-protection stability of films will determine their service life, the transmission spectra of films before and after exposure to UV light were compared for their UV-blocking performance for 2 h. As shown in Figure 9, the transmission spectra for CNC and CNC/lignin films did not change after UV exposure showing UV stability of the films.
Figure 9.
UV–vis transmittance of CNC and CNC/lignin films before and after UV (254 nm) exposure for 2 h (UVT refers to UV treated).
Birefringent Properties of Films
As discussed previously, the transparency of the CNC film depends on the self-assembly of CNC rods. The CNC rods in chiral nematic films are helically oriented across the thickness of the film, which exhibits various colors owing to circular polarization of the incident light,47 whereas for films with fully nematic arrangement of rods, a linear polarization of incident light is achieved.45Figure 10 shows the transmitted cross-polarized optical microscopy images of CNC and CNC/lignin films. These images were taken at two different orientations of the films, which are 45° apart from each other. The transmitted light images of CNC films at these two positions showed clear contrast. The overall image appeared dark at 0° position with respect to either polarizer or analyzer, whereas strong birefringence occurred when turned to 45° position. This indicated that there is some degrees of alignment of CNC rods within the film, which can be attributed to the nematic arrangements induced in transition regime concentration. The transition regime concentration is where the CNC rods go from fully isotropic to fully nematic arrangement. As shown in study by Bertsch et al.,48 this transition concentration starts at 4–5 wt % without addition of electrolyte and was reduced to 1–2 wt % upon addition of electrolytes. CNC and NaOH concentrations used in this study were 1.64 wt % and 16.7 mM NaOH, respectively, which would be in an isotropic regime. Although isotropic to chiral nematic ordering of CNC rods in this suspension was hindered due to charge screening, the local nematic ordering of rods would retain in these films. Nematic ordering of such CNC domains gives rise to some degrees of overall CNC alignment in the dried films. Interestingly, lignin incorporation did not hinder the NaOH-induced self-assembly of CNC. The structural details regarding the CNC self-assembly have been shown previously in Figure 4. The polarizing property of the films adds to the functionality of these films making them potential biobased film material for the applications, such as sunglasses, contact lenses, windows, etc., where visible light polarization along with UV-blocking properties are desired.
Figure 10.
Cross-polarized reflected images of the CNC and CNC/lignin films (column A: CNC alignment parallel to polarizer, column B: CNC alignment 45° to the polarizer).
Lignin Modification To Enhance the Visible Light Transmission
To improve the scope of optical applications of CNC/lignin films, it is desirable to reduce the lignin color and improve the visible light transmission. Several methods were discussed in literature related to lignin color reduction; however, most of them degrade the lignin aromatic structure reducing its UV-absorption properties.49−53 Lignin in its natural form mostly occurs as acetylated lignin in hardwoods.53 However, it is transformed into the dark brown-colored material during its isolation processes. In spite of many efforts, the isolation of totally unaltered native lignin is still a challenge.54,55 Acetylation of lignin replaces the phenolic hydroxyl groups in lignin with the acetyl groups, which reduces the lignin color by preventing the formation of quinone and quinone methide chromophores.53 AL and SKL acetylation was carried in presence of acetic anhydride and pyridine. Degree of acetylation of the lignin samples was qualitatively determined from their Fourier transform infrared (FTIR) spectra performed before and after acetylation, as shown in Figure 11. For Ac-SKL samples (Figure 11a), a stretching vibrational band of the hydroxyl groups between 3200 and 3400 cm–1 is eliminated showing complete acetylation of the hydroxyl groups, whereas for Ac-AL (Figure 11b) there is significant decrease in the peak intensity showing the partial acetylation of the hydroxyl groups. Also, the C=O stretching vibrational absorptions for phenolic and aliphatic acetyls could be observed in Ac-SKL and Ac-AL samples at 1760 and 1740 cm–1, respectively.
Figure 11.
FTIR spectra comparison of (a) SKL and Ac-SKL, (b) AL and Ac-AL.
Figure 12 shows the change in color obtained after the acetylation of SKL, the dark brown color of SKL has significantly reduced to the light brown color. 0.1 wt % solutions of AL, Ac-AL, SKL, and Ac-SKL were prepared using 1:1 (v/v) dioxane/water solutions. Figure 13 shows the transmission spectra of the 0.1 wt % solutions of these lignin samples. It was observed that due to lignin color reduction, the visible light transmission at 550 nm is enhanced by 65 and 160% for AL and SKL lignin samples, respectively. The UV-blocking properties of the lignin are only slightly affected due to the acetylation, the Ac-AL and Ac-SKL can still block the UV light upto 375 nm compared to 400 nm obtained for AL and SKL. The extinction coefficient values for the lignin samples listed in Table 1 also show slight reduction after the acetylation. The UV-absorption properties of lignin are mostly retained due to the retention of its aromatic structure after acetylation. Figure 14a,b shows the effect of lignin acetylation on the transparency and transmission spectra of CNC/lignin films, respectively. The visible light transmittance of 10 wt % CNC/Ac-SKL film at 550 nm is enhanced by 67% compared to 10 wt % CNC/SKL film, without significantly affecting the UV-blocking properties.
Figure 12.
Lignin samples before and after acetylation.
Figure 13.
Transmission spectra of 0.1 wt % lignin solution in dioxane/water.
Table 1. Extinction Coefficients of the Lignin Samples.
| sample | extinction coefficient (wt %–1 cm–1)@255 nm |
|---|---|
| AL | 205.3 |
| Ac-AL | 196.6 |
| SKL | 223.8 |
| Ac-SKL | 216.1 |
Figure 14.
(a) CNC/SKL (10 wt %) and CNC/Ac-SKL (10 wt %) films. (b) Transmission spectra of comparison for CNC/SKL (10 wt %) and CNC/Ac-SKL (10 wt %).
Surface Hydrophobicity
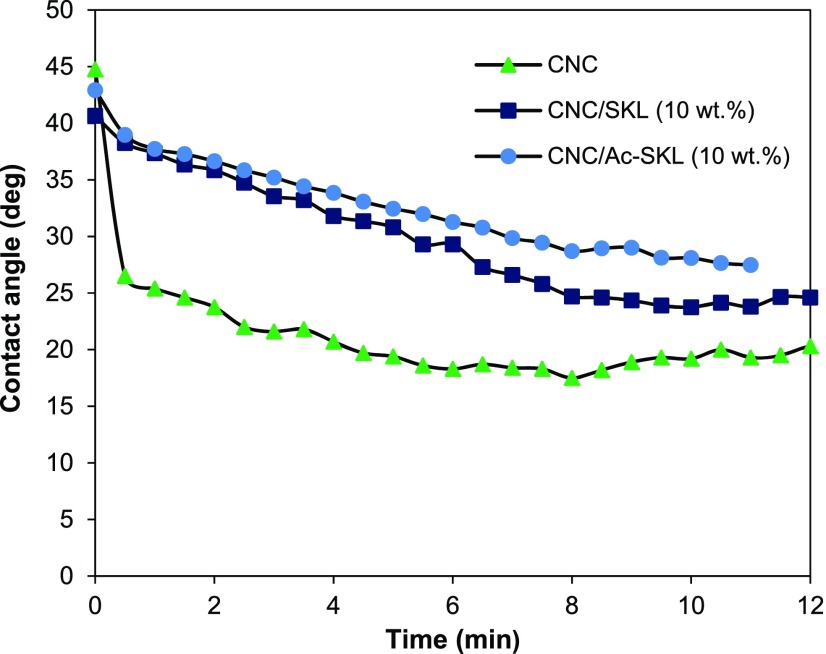
Owing to its aromatic structure and the fewer hydroxyl groups compared to CNC, lignin is generally considered relatively more hydrophobic than CNC. However, no appreciable change in hydrophobicity was observed upon addition of 10 wt % lignin to CNC, as static contact angle did not increase significantly for the CNC/lignin films. Average static contact angles measured for 10 wt % CNC/SKL, 10 wt % CNC/Ac-SKL, and neat CNC samples were in the range of 43 ± 2° (Figure 15). However, it was observed that for lignin containing CNC films, the contact angle was more stable than that of neat CNC films. Within first 100 s, the contact angle of 10 wt % lignin containing films, i.e., CNC/Ac-SKL and CNC/SKL reduced to 38°, whereas for CNC films contact angle rapidly reduced to 25°. In case of CNC films, hydrophillic hydroxyl groups are easily available for the water molecules. The water droplet spreads easily on the surface thereby rapidly reducing the contact angle. For CNC/lignin samples, penetration into the films was reduced by the uniformly dispersed hydrophobic lignin network surrounding the cellulose nanocrystals in the matrix. Unexpectedly, similar initial contact angle of CNC and lignin containing CNC films may be due to low concentration of lignin present on the surface of the film.
Figure 15.
Contact angle measurements for CNC and CNC/lignin samples as a function of time.
Thermal Stability of the Films
Thermal decompositions of CNC and CNC/lignin films were studied using thermogravimetric analysis (TGA) in an inert nitrogen medium. Decomposition behavior in terms of weight percent versus temperature and derivative weight percent versus temperature (differential thermogravimetric (DTG) plot) are shown in shown Figure 16a,b, respectively. DTG plot shows large peaks between temperature range 300–330 °C, which largely corresponds to pyrolysis of cellulose chains resulting from depolymerization, dehydration, and decomposition of glycosyl units.56,57 Similar thermal behavior was obtained for the NaOH-treated sulfonated CNC.58 Smaller peaks between 180 and 190 °C for all of the samples correspond to sulfate group decomposition, whereas peaks around 230 °C observed in cases of CNC/SKL and CNC/Ac-SKL would be due to presence of impurities induced in SKL during kraft processing.
Figure 16.
Thermogravimetric analyses for CNC and CNC/lignin films. (a) Weight percent vs temperature. (b) Temperature derivative weight percent vs temperature.
Thermal stability of the CNC films is enhanced upon incorporation of the SL. Figure 16a showed more weight loss at any given temperature for the CNC films compared to lignin containing films. Peak temperature (T1) from DTG plot corresponding to CNC pyrolysis has shifted to higher temperature upon lignin addition. T1 for neat CNC films is 307 °C, which has increased to 312 °C for 10 wt % CNC/SKL and 327 °C for 10 wt % CNC/Ac-SKL. Presence of the phenolic OH groups within the lignin structure and the aromatic char originating from the lignin at elevated temperature are responsible for the thermal stability characteristics of the lignin incorporated in polymer matrix.59−62 Acetylation of lignin further enhances the lignin thermal stability due to replacement of hydroxyl groups with more stable acetyl groups in lignin.63 The increased thermal stability of the nanocomposite films makes lignin-based films a promising candidate for applications in severe environmental conditions.
Conclusions
A simple approach to prepare the CNC and lignin-based transparent and homogenous UV-protection films is developed. The results demonstrated for the first time that CNC aqueous suspensions with and without containing lignin could be tuned through the addition of NaOH to produce transparent and homogenous films. CNC films were optimized for transparency using various NaOH additions. It was observed that the addition of NaOH in the range of 3–4 wt % resulted in the CNC films with maximum transparency. Moreover, NaOH addition enhanced the homogeneity of the films by uniformly dispersing lignin in the films. CNC/AL and CNC/SKL with 10 wt % lignin concentration provided complete UV blocking. The UV-protection behavior of these films was stable under UV irradiation. Acetylation of lignin reduced the lignin color with only slight reduction in their extinction coefficient. Incorporation of 10 wt % Ac-SKL into CNC films increased visible light transmittance at 550 nm by 67% without significantly affecting the UV-blocking property compared to 10 wt % CNC/SKL films. Presence of lignin also provided the thermal and contact angle stabilities. SKL addition (10 wt %) increased the maximum weight loss temperature (T1) of CNC by 5 °C, whereas 10 wt % Ac-SKL, being more stable, increased T1 by 20 °C. Due to the relatively more hydrophobic nature of lignin compared to CNC, contact angle of CNC/lignin films remained stable over time compared to CNC films; however, initial contact angle was unaffected by the presence of lignin. There is scope for the further optimization in terms of lignin color reduction and increasing the CNC alignment. Progress in these areas can make CNC/lignin nanocomposites a biodegradable, low-cost alternative as a coating/film material with UV blocking and optical polarization functionality for sunglasses, automobile windshields, home windows, contact lenses, and UV-sensitive polymers.
Experimental Section
Materials
CNC, purchased from the University of Maine Process Development Center, was manufactured at U.S. Forest Service’s Cellulose Nanomaterials Pilot Plant at the Forest Products Laboratory, Madison, WI. The sodium form of CNC were obtained as an aqueous gel (11.5–12.5 wt %), with crystal dimension specifications in the range of 5–20 nm for width and 150–200 nm for length. Some of the literature values are in agreement with these reported dimensions.42,64,65 Reid et al. have reported aspect ratio of this CNC to be ∼19 with an average length of 134 ± 52 nm and width 7 ± 2 nm.64 SKL was provided by our industry partner, and this softwood kraft lignin contains 95 wt % lignin with impurities mainly as residual sugars, sulfur, and ash. NaOH pellets, anhydrous pyridine, acetic anhydride were purchased from TCI America. AL, purchased from the Sigma-Aldrich, is water-soluble lignin with low sulfonate content with an average molecular weight of ∼10 000.
Methods
Acetylation of Lignin
The purpose of lignin acetylation was to replace lignin hydroxyl groups with acetyl groups to reduce the dark lignin color, which is partly caused by chromophores arising from phenolic hydroxyl groups, such as quinone and quinonemethides. SKL (1 g) was dissolved in a mixture of pyridine (10 mL) and acetic anhydride (10 mL) and stirred at room temperature for 72 h.66,67 The reaction mixture was then added dropwise into a 500 mL of ice water to precipitate the acetylated lignin. Subsequently, followed by filtration and repeated washing with ice water and ethanol.
Film Preparation
CNC films were prepared by mixing 1 g of CNC in 60 mL of deionized (DI) water with or without addition of NaOH, whereas CNC/lignin films were prepared by suspending 1 g of CNC in 30 mL of DI water, which was mixed for 30 min using magnetic stirrer. Separately, required quantity of lignin was mixed in a 30 mL of DI water with an addition of 240 mg of 5 M aqueous NaOH solution. The aqueous alkaline lignin solution was mixed for 30 min to completely dissolve all of the lignin without having any large visible aggregates. CNC suspension and lignin solution were then mixed together for another 30 min to get the uniform mixture of the CNC/lignin. This mixture was then casted on polystyrene Petri dishes and dried at room temperature in a fume hood for 48 h to obtain the uniform, transparent CNC/lignin films. CNC/acetylated-SKL (Ac-SKL) films were prepared using the similar procedure. However, in this case, Ac-SKL was solubilized in 30 mL of dioxane and CNC is suspended in 30 mL of DI water, before mixing these mixtures together. NaOH solution (240 mg, 5 M) was added to this mixture. These films were dried on a Teflon mold in fume hood for 48 h. NaOH and lignin concentration wherever mentioned throughout this study are wt % based on the total weight of 1 g of CNC instead of mixture weight. This was used for convenience as each film is prepared from 1 g of CNC.
Characterizations
UV–Vis Spectroscopy
Transmission spectra of the aqueous lignin solutions and CNC/lignin films were carried out using a Thermo Scientific GENESYS 10S UV–vis spectrometer in the wavelength range of 200–800 nm. Transmittance at the wavelength of 550 nm was used as a measure of film transparency. Sun protection factor was calculated using absorbance within UV-B range (290–320 nm). Extinction coefficient (ελ) of the lignin and acetylated lignin was calculated using Beer–Lambert equation as follows
where ελ (wt %–1 cm–1) is an extinction coefficient measured using absorbance Aλ, which is a maximum peak absorbance at wavelength (λ = 255 nm). d is the path length for the incident light, which is 1 cm in this case, and C (wt %) is the concentration of lignin solution.
Fourier Transform Infrared Spectroscopy
Fourier transform infrared (FTIR) spectra of the lignin samples were measured on a Nicolet 6700 FTIR spectrometer by Thermo Scientific. A total of 64 cumulative scans in absorption mode was taken, with a resolution of 1 cm–1 in the frequency range 4000–600 cm–1.
Atomic Force Microscopy (AFM)
AFM images of CNC/lignin films were performed under ambient conditions on a commercial AFM (Pacific Nanotechnology Nano-R AFM, Pacific Nanotechnology, Santa Clara, CA) in noncontact mode using aluminum AFM tips with resonant frequencies in the range of 150–210 kHz and force constants in the range of 4.5–14 N m–1 (MikroMasch, Wilsonville, OR). Images are collected at a scan rate of 0.5 or 1 Hz depending on the image size with resolution of 256 × 256 data points. The RMS roughness of films was obtained from 10 μm × 10 μm AFM scans.
Scanning Electron Microscopy (SEM)
SEM images of CNC and CNC/lignin films were taken using a JEOL (Tokyo, Japan) 7000-F field-emission scanning electron microscope. Films were sputter-coated with gold before taking images.
Optical Microscopy
Cross-polarized images of CNC and CNC/lignin films were taken using a Nikon (Melville, NY) Eclipse 80i microscope with an LU Plan Fluor 4×/0.13NA Nikon objective lens and a Nikon DS-Ri2 microscope camera. Each film was placed between the cross-polarizers, and images were taken in direction oriented at 0 and 45° to the polarization axis.
ζ Potential and Particle Size Measurement
The dynamic light scattering (Zetasizer Nano ZS, Malvern Instrument) was used to determine the ζ potential and CNC particle size in aqueous suspension as a function of NaOH concentration. Dilute CNC suspensions of 1.64 wt % with various NaOH concentrations (0–15 wt % of the CNC weight) were prepared by magnetically stirring the suspension mixture for 2 h. All measurements were carried out at 25 °C with at least three experiments for each sample.
Contact Angle Measurements
Static contact angle of the films was measured on a Ramé-Hart model 200 automated goniometer, using DROPimage standard software provided by Ramé-Hart. Measured contact angle was an average of three readings obtained for the water droplets, the measurement error is ±2°.
Thickness Tester
The thickness of the films was measured using the thickness tester from Testing Machine Inc. Reported thickness values were an average of at least 15 measurements along the film diameter.
Thermal Analysis
Thermogravimetric analysis (TGA) was performed on a TGA Q500 (TA instruments) under nitrogen at a rate of 10 °C min–1 from room temperature to 800 °C, with 20 min isothermal step at 120 °C to ensure the removal of residual moisture.
Acknowledgments
The authors gratefully acknowledge the financial support provided by Alabama Center for Paper and Bioresource Engineering. The authors would also like to acknowledge the assistance provided in Zetasizer Nano ZS characterizations by Dr. Allan David lab at the Department of Chemical Engineering, Auburn University and the usage of electron microscopy facility in Materials Engineering Department, Auburn University.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01345.
Scanning electron microscopy images of the 10 wt % CNC/AL and 10 wt % CNC/Ac-SKL film cross-sections (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mondal S. Preparation, properties and applications of nanocellulosic materials. Carbohydr. Polym. 2017, 163, 301–316. 10.1016/j.carbpol.2016.12.050. [DOI] [PubMed] [Google Scholar]
- Chirayil C. J.; Mathew L.; Thomas S. Review of recent research in nanocellulose preparation from different lignocellulosic fibers. Rev. Adv. Mater. Sci. 2014, 37, 20–28. [Google Scholar]
- Giri J.; Adhikari R. A brief review on extraction of nanocellulose and its application. Bibechana 2012, 9, 81–87. 10.3126/bibechana.v9i0.7179. [DOI] [Google Scholar]
- Revol J.-F.; Godbout D. L.; Gray D. G.. Solidified Liquid Crystals of Cellulose with Optically Variable Properties. US5,629,055A, 1997.
- Finkelmann H.; Kim S. T.; Munoz A.; Palffy-Muhoray P.; Taheri B. Tunable mirrorless lasing in cholesteric liquid crystalline elastomers. Adv. Mater. 2001, 13, 1069–1072. . [DOI] [Google Scholar]
- Palffy-Muhoray P.; Cao W.; Moreira M.; Taheri B.; Munoz A. Photonics and lasing in liquid crystal materials. Philos. Trans. R. Soc., A 2006, 364, 2747–2761. 10.1098/rsta.2006.1851. [DOI] [PubMed] [Google Scholar]
- Lin S. Y.; Dence C. W.. Methods in Lignin Chemistry; Springer-Verlag: Berlin, New York, 1992. [Google Scholar]
- Vishtal A. G.; Kraslawski A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar]
- Gosselink R.; De Jong E.; Guran B.; Abächerli A. Co-ordination network for lignin—standardisation, production and applications adapted to market requirements (EUROLIGNIN). Ind. Crops Prod. 2004, 20, 121–129. 10.1016/j.indcrop.2004.04.015. [DOI] [Google Scholar]
- Falkehag S. I.; Marton J.; Adler E.. Chromophores in Kraft Lignin; ACS Publications, 1966. [Google Scholar]
- Barsberg S.; Elder T.; Felby C. Lignin–Quinone Interactions: Implications for Optical Properties of Lignin. Chem. Mater. 2003, 15, 649–655. 10.1021/cm021162s. [DOI] [Google Scholar]
- Liu X.; Wang J.; Li S.; Zhuang X.; Xu Y.; Wang C.; Chu F. Preparation and properties of UV-absorbent lignin graft copolymer films from lignocellulosic butanol residue. Ind. Crops Prod. 2014, 52, 633–641. 10.1016/j.indcrop.2013.11.036. [DOI] [Google Scholar]
- Sadeghifar H.; Venditti R.; Jur J.; Gorga R. E.; Pawlak J. J. Cellulose-Lignin Biodegradable and Flexible UV Protection Film. ACS Sustainable Chem. Eng. 2017, 5, 625–631. 10.1021/acssuschemeng.6b02003. [DOI] [Google Scholar]
- Domenek S.; Louaifi A.; Guinault A.; Baumberger S. Potential of lignins as antioxidant additive in active biodegradable packaging materials. J. Polym. Environ. 2013, 21, 692–701. 10.1007/s10924-013-0570-6. [DOI] [Google Scholar]
- Pouteau C.; Dole P.; Cathala B.; Averous L.; Boquillon N. Antioxidant properties of lignin in polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. 10.1016/S0141-3910(03)00057-0. [DOI] [Google Scholar]
- Sadeghifar H.; Argyropoulos D. S. Correlations of the antioxidant properties of softwood kraft lignin fractions with the thermal stability of its blends with polyethylene. ACS Sustainable Chem. Eng. 2015, 3, 349–356. 10.1021/sc500756n. [DOI] [Google Scholar]
- Espinoza Acosta J. L.; Torres Chávez P. I.; Ramírez-Wong B.; Bello-Pérez L. A.; Vega Ríos A.; Carvajal Millán E.; Plascencia Jatomea M.; Ledesma Osuna A. I. Mechanical, thermal, and antioxidant properties of composite films prepared from durum wheat starch and lignin. Starch/Stärke 2015, 67, 502–511. 10.1002/star.201500009. [DOI] [Google Scholar]
- Silvestre C.; Cimmino S.. Ecosustainable Polymer Nanomaterials for Food Packaging: Innovative Solutions, Characterization Needs, Safety and Environmental Issues; CRC Press, 2013. [Google Scholar]
- Calvo M. E.; Castro Smirnov J. R.; Míguez H. Novel approaches to flexible visible transparent hybrid films for ultraviolet protection. J. Polym. Sci., Part B: Polym. Phys. 2012, 50, 945–956. 10.1002/polb.23087. [DOI] [Google Scholar]
- Vallejo J. J.; Mesa M.; Gallardo C. Evaluation of the avobenzone photostability in solvents used in cosmetic formulations. Vitae 2011, 18, 63–71. [Google Scholar]
- Butt S.; Christensen T. Toxicity and phototoxicity of chemical sun filters. Radiat. Prot. Dosim. 2000, 91, 283–286. 10.1093/oxfordjournals.rpd.a033219. [DOI] [Google Scholar]
- Díaz-Cruz M. S.; Llorca M.; Barceló D.; Barceló D. Organic UV filters and their photodegradates, metabolites and disinfection by-products in the aquatic environment. TrAC, Trends Anal. Chem. 2008, 27, 873–887. 10.1016/j.trac.2008.08.012. [DOI] [Google Scholar]
- Teo C. H.; Rahman F. Degradation and protection of polyaniline from exposure to ultraviolet radiation. Appl. Phys. A: Mater. Sci. Process. 2010, 99, 311–316. 10.1007/s00339-009-5533-3. [DOI] [Google Scholar]
- Tu Y.; Zhou L.; Jin Y. Z.; Gao C.; Ye Z. Z.; Yang Y. F.; Wang Q. L. Transparent and flexible thin films of ZnO-polystyrene nanocomposite for UV-shielding applications. J. Mater. Chem. 2010, 20, 1594–1599. 10.1039/b914156a. [DOI] [Google Scholar]
- Xiong H.-M.; Zhao X.; Chen J.-S. New polymer–inorganic nanocomposites: PEO–ZnO and PEO–ZnO–LiClO4 films. J. Phys. Chem. B 2001, 105, 10169–10174. 10.1021/jp0103169. [DOI] [Google Scholar]
- Aloui F.; Ahajji A.; Irmouli Y.; George B.; Charrier B.; Merlin A. Inorganic UV absorbers for the photostabilisation of wood-clearcoating systems: Comparison with organic UV absorbers. Appl. Surf. Sci. 2007, 253, 3737–3745. 10.1016/j.apsusc.2006.08.029. [DOI] [Google Scholar]
- Wu R.-L.; Wang X.-L.; Li F.; Li H.-Z.; Wang Y.-Z. Green composite films prepared from cellulose, starch and lignin in room-temperature ionic liquid. Bioresour. Technol. 2009, 100, 2569–2574. 10.1016/j.biortech.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Hambardzumyan A.; Foulon L.; Chabbert B.; Aguié-Béghin V. Natural organic UV-absorbent coatings based on cellulose and lignin: designed effects on spectroscopic properties. Biomacromolecules 2012, 13, 4081–4088. 10.1021/bm301373b. [DOI] [PubMed] [Google Scholar]
- Hambardzumyan A.; Foulon L.; Bercu N.; Pernes M.; Maigret J.-E.; Molinari M.; Chabbert B.; Aguié-Beghin V. Organosolv lignin as natural grafting additive to improve the water resistance of films using cellulose nanocrystals. Chem. Eng. J. 2015, 264, 780–788. 10.1016/j.cej.2014.12.004. [DOI] [Google Scholar]
- Hambardzumyan A.; Molinari M.; Dumelie N.; Foulon L.; Habrant A.; Chabbert B.; Aguié-Béghin V. Structure and optical properties of plant cell wall bio-inspired materials: Cellulose–lignin multilayer nanocomposites. C. R. Biol. 2011, 334, 839–850. 10.1016/j.crvi.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Xiong F.; Wu Y.; Li G.; Han Y.; Chu F. Transparent nanocomposite films of lignin nanospheres and poly(vinyl alcohol) for UV-absorbing. Ind. Eng. Chem. Res. 2018, 57, 1207–1212. 10.1021/acs.iecr.7b04108. [DOI] [Google Scholar]
- Abitbol T.; Cranston E. D.. Chiral Nematic Self-assembly of Cellulose Nanocrystals in Suspensions and Solid Films. Handbook of Green Materials, Part 3: Self- and Direct-Assembling of Bionanomaterials; World Scientific Publishing: Singapore, 2014; Chapter 4, Vol. 5, pp 37–56. [Google Scholar]
- Pan J.; Hamad W.; Straus S. K. Parameters affecting the chiral nematic phase of nanocrystalline cellulose films. Macromolecules 2010, 43, 3851–3858. 10.1021/ma902383k. [DOI] [Google Scholar]
- Picard G.; Simon D.; Kadiri Y.; LeBreux J.; Ghozayel F. Cellulose nanocrystal iridescence: a new model. Langmuir 2012, 28, 14799–14807. 10.1021/la302982s. [DOI] [PubMed] [Google Scholar]
- Liu D.; Wang S.; Ma Z.; Tian D.; Gu M.; Lin F. Structure–color mechanism of iridescent cellulose nanocrystal films. RSC Adv. 2014, 4, 39322–39331. 10.1039/C4RA06268J. [DOI] [Google Scholar]
- Phan-Xuan T.; Thuresson A.; Skepö M.; Labrador A.; Bordes R.; Matic A. Aggregation behavior of aqueous cellulose nanocrystals: the effect of inorganic salts. Cellulose 2016, 23, 3653–3663. 10.1007/s10570-016-1080-1. [DOI] [Google Scholar]
- Araki J.; Kuga S. Effect of trace electrolyte on liquid crystal type of cellulose microcrystals. Langmuir 2001, 17, 4493–4496. 10.1021/la0102455. [DOI] [Google Scholar]
- Prathapan R.; Thapa R.; Garnier G.; Tabor R. F. Modulating the zeta potential of cellulose nanocrystals using salts and surfactants. Colloids Surf., A 2016, 509, 11–18. 10.1016/j.colsurfa.2016.08.075. [DOI] [Google Scholar]
- Dong X. M.; Kimura T.; Revol J.-F.; Gray D. G. Effects of ionic strength on the isotropic–chiral nematic phase transition of suspensions of cellulose crystallites. Langmuir 1996, 12, 2076–2082. 10.1021/la950133b. [DOI] [Google Scholar]
- Zhong L.; Fu S.; Peng X.; Zhan H.; Sun R. Colloidal stability of negatively charged cellulose nanocrystalline in aqueous systems. Carbohydr. Polym. 2012, 90, 644–649. 10.1016/j.carbpol.2012.05.091. [DOI] [PubMed] [Google Scholar]
- Hasani M.; Cranston E. D.; Westman G.; Gray D. G. Cationic surface functionalization of cellulose nanocrystals. Soft Matter 2008, 4, 2238–2244. 10.1039/B806789A. [DOI] [Google Scholar]
- Abitbol T.; Kloser E.; Gray D. G. Estimation of the surface sulfur content of cellulose nanocrystals prepared by sulfuric acid hydrolysis. Cellulose 2013, 20, 785–794. 10.1007/s10570-013-9871-0. [DOI] [Google Scholar]
- Revol J.-F.; Godbout L.; Gray D. Solid self-assembled films of cellulose with chiral nematic order and optically variable properties. J. Pulp Pap. Sci. 1998, 24, 146–149. [Google Scholar]
- Beck S.; Bouchard J.; Berry R. Controlling the reflection wavelength of iridescent solid films of nanocrystalline cellulose. Biomacromolecules 2011, 12, 167–172. 10.1021/bm1010905. [DOI] [PubMed] [Google Scholar]
- Chowdhury R. A.; Peng S. X.; Youngblood J. Improved order parameter (alignment) determination in cellulose nanocrystal (CNC) films by a simple optical birefringence method. Cellulose 2017, 24, 1957–1970. 10.1007/s10570-017-1250-9. [DOI] [Google Scholar]
- Dutra E. A.; da Costa Oliveira D. A. G.; Kedor-Hackmann E. R. M.; Santoro M. I. R. M. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev. Bras. Cienc. Farm. 2004, 40, 381–385. 10.1590/S1516-93322004000300014. [DOI] [Google Scholar]
- Lagerwall J. P.; Schütz C.; Salajkova M.; Noh J.; Park J. H.; Scalia G.; Bergström L. Cellulose nanocrystal-based materials: from liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80 10.1038/am.2013.69. [DOI] [Google Scholar]
- Bertsch P.; Isabettini S.; Fischer P. Ion-Induced Hydrogel Formation and Nematic Ordering of Nanocrystalline Cellulose Suspensions. Biomacromolecules 2017, 18, 4060–4066. 10.1021/acs.biomac.7b01119. [DOI] [PubMed] [Google Scholar]
- Lin S. Y.Process for Reduction of Lignin Color. US4,184,845A, 1980.
- Zhang H.; Bai Y.; Zhou W.; Chen F. Color reduction of sulfonated eucalyptus kraft lignin. Int. J. Biol. Macromol. 2017, 201–208. 10.1016/j.ijbiomac.2017.01.031. [DOI] [PubMed] [Google Scholar]
- Dilling P.; Sarjeant P. T.. Reduction of Lignin Color. US4,454,066A, 1984.
- Wang J.; Deng Y.; Qian Y.; Qiu X.; Ren Y.; Yang D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. 10.1039/C5GC02180D. [DOI] [Google Scholar]
- Qian Y.; Deng Y.; Li H.; Qiu X. Reaction-free lignin whitening via a self-assembly of acetylated lignin. Ind. Eng. Chem. Res. 2014, 53, 10024–10028. 10.1021/ie5010338. [DOI] [Google Scholar]
- Wu S.; Argyropoulos D. An Improved Method for lsolating Lignin in High Yield and Purity. J. Pulp Pap. Sci. 2003, 29, 235–240. [Google Scholar]
- Ikeda T.; Holtman K.; Kadla J. F.; Chang H.-m.; Jameel H. Studies on the effect of ball milling on lignin structure using a modified DFRC method. J. Agric. Food Chem. 2002, 50, 129–135. 10.1021/jf010870f. [DOI] [PubMed] [Google Scholar]
- Roman M.; Winter W. T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. 10.1021/bm034519+. [DOI] [PubMed] [Google Scholar]
- Martínez-Sanz M.; Lopez-Rubio A.; Lagaron J. M. Optimization of the nanofabrication by acid hydrolysis of bacterial cellulose nanowhiskers. Carbohydr. Polym. 2011, 85, 228–236. 10.1016/j.carbpol.2011.02.021. [DOI] [Google Scholar]
- Wang N.; Ding E.; Cheng R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493. 10.1016/j.polymer.2007.03.062. [DOI] [Google Scholar]
- Gerlock J.; Kucherov A.; Smith C. Determination of active HALS in automotive paint systems II: HALS distribution in weathered clearcoat/basecoat paint systems. Polym. Degrad. Stab. 2001, 73, 201–210. 10.1016/S0141-3910(01)00002-7. [DOI] [Google Scholar]
- Glasser W. G.Lignin: Historical, Biological, and Materials Perspectives; American Chemical Society: Washington, DC, 2000; Vol. 742, pp 216–238. [Google Scholar]
- González Sánchez C.; Alvarez L. Micromechanics of lignin/polypropylene composites suitable for industrial applications. Macromol. Mater. Eng. 1999, 272, 65–70. . [DOI] [Google Scholar]
- De Chirico A.; Armanini M.; Chini P.; Cioccolo G.; Provasoli F.; Audisio G. Flame retardants for polypropylene based on lignin. Polym. Degrad. Stab. 2003, 79, 139–145. 10.1016/S0141-3910(02)00266-5. [DOI] [Google Scholar]
- Jakab E.; Faix O.; Till F. Thermal decomposition of milled wood lignins studied by thermogravimetry/mass spectrometry. J. Anal. Appl. Pyrolysis 1997, 40–41, 171–186. 10.1016/S0165-2370(97)00046-6. [DOI] [Google Scholar]
- Reid M. S.; Villalobos M.; Cranston E. D. Benchmarking cellulose nanocrystals: from the laboratory to industrial production. Langmuir 2017, 33, 1583–1598. 10.1021/acs.langmuir.6b03765. [DOI] [PubMed] [Google Scholar]
- Heggset E. B.; Chinga-Carrasco G.; Syverud K. Temperature stability of nanocellulose dispersions. Carbohydr. Polym. 2017, 157, 114–121. 10.1016/j.carbpol.2016.09.077. [DOI] [PubMed] [Google Scholar]
- Cachet N.; Camy S.; Benjelloun-Mlayah B.; Condoret J.-S.; Delmas M. Esterification of organosolv lignin under supercritical conditions. Ind. Crops Prod. 2014, 58, 287–297. 10.1016/j.indcrop.2014.03.039. [DOI] [Google Scholar]
- Gilarranz M. A.; Rodríguez F.; Oliet M.; García J.; Alonso V. Phenolic OH group estimation by FTIR and UV spectroscopy. Application to organosolv lignins. J. Wood Chem. Technol. 2001, 21, 387–395. 10.1081/WCT-100108333. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.