Supplemental Digital Content is available in the text.
Keywords: Breastfeeding, Perfluoroalkyl substance, Norwegian Mother and Child Cohort Study, perfluorooctanoic acid, perfluorooctane sulfonate
Abstract
Background:
Per- and polyfluoroalkyl substances (PFASs) have been widely produced, many of them persist in the environment, and have been associated with various health effects. Previous studies have identified inverse associations between perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), and breastfeeding duration, but have been limited in investigation of other PFASs.
Methods:
We measured the associations between plasma concentrations of nine different PFASs and cessation of breastfeeding before 3 and 6 complete months using women from the Norwegian Mother and Child Cohort Study (MoBa). The study population includes 1716 primarily nulliparous women from two previous studies of MoBa participants, enrolled from 2003 to 2007. The association was measured using Cox proportional hazards model. Mixtures analyses were performed using Elastic net regularization to identify interactive effects and control for copollutant confounding.
Results:
Concentrations of PFASs in this population were lower than concentrations in the previous studies on this topic. We found associations between increasing concentrations of perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), and decreased breastfeeding cessation (increased duration). The strongest associations were seen between PFDA and PFUnDA and cessation before 3 months: (both hazard ratios = 0.73; 95% confidence intervals = 0.62, 0.86). In our population, the other PFASs appeared to be unassociated with breastfeeding cessation. The mixtures analyses identified meaningful interactions between PFUnDA:PFDA, perfluorohexane sulfonate (PFHXS):PFOA, and PFOA:PFOS.
Conclusions:
The identification of associations between previously unexamined PFASs concentrations and increased breastfeeding duration is novel and may be explained by differences in transplacental transfer rates.
What this study adds
This study adds to the body of literature on polyfluoroalkyl substances (PFASs) exposure and breastfeeding duration. Results from this study differ from those found in previous studies, as we identified null results where associations had previously been detected and unexpected positive associations between concentrations of certain PFASs and breastfeeding duration. This study will be of interest to Environmental Epidemiology readers due to the relevance of PFASs in current health research. Additionally, our mixtures analysis is a novel application in this setting and may be relevant to many other epidemiological questions.
The World Health Organization and the American Academy of Pediatrics both recommend 6 months of exclusive breastfeeding followed by complementary breastfeeding for up to 2 years.1,2 Among many other health benefits, breastfeeding is associated with decreased risk of Sudden Infant Death Syndrome and childhood leukemia for the child and decreased risk of type 2 diabetes, breast cancer, and ovarian cancer for the mother.3 Despite these recommendations, most women do not breastfeed for the suggested duration; the global rate for exclusive breastfeeding is 40% for infants under 6 months.4 There are many sociodemographic and psychosocial factors that influence breastfeeding practices, but inadequate milk supply and perception of an unsated infant are some of the most common reasons for cessation.5,6
Per- and polyfluoroalkyl substances (PFASs) are a group of more than 3000 compounds, where perfluoroalkyl carboxylic acids and perfluoroalkyl sulfonates—defined by the addition of functional groups such as a carboxylic acid or sulfonate group, respectively—are two of the most studied.7 PFASs do not occur naturally in the environment but are used in fire-fighting foams and are abundant in industrial applications and consumer products due to their oil, stain, grease, and water repellency.8 Humans are primarily exposed through consumption of ingestion of contaminated food or water, though migration from consumer products can also be a low-level exposure source.8 Many of these compounds persist both in the environment and in the human body, and detectable concentrations of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) have been observed in multiple global populations.8 PFOA and structurally similar compounds are categorized as perfluoroalkyl carboxylic acids, while PFOS and similar PFASs are classified as perfluoroalkyl sulfonates.9
Animal studies have shown that PFOA and PFOS act as endocrine disruptors, reduce immune function, cause developmental problems in offspring exposed prenatally, and exhibit hepatotoxicity.10,11 The human health effects resulting from PFASs exposure are still being discovered, but research has suggested a link between maternal PFASs concentrations and decreased breastfeeding duration. The three studies to examine this association utilized Danish, American, and Faroe Island cohorts and focused primarily on PFOA and PFOS exposure, though perfluorohexane sulfonate (PFHxS), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA) were also examined.12–14 In all three studies, there were consistent inverse associations between PFOA and PFOS exposure and duration of breastfeeding. However, two of the studies were missing information on previous breastfeeding duration, an important confounder.15 Previous breastfeeding is both a major elimination route for PFASs and a predictor of breastfeeding practices in the current pregnancy.15–18
In the present study, we examined the association between prenatal PFASs exposure and duration of breastfeeding in the Norwegian Mother and Child cohort study (MoBA), a study that contains detailed information on previous breastfeeding duration. Compared with the other studies addressing this question, the current study population is larger and contains information on exposures to other PFASs beyond PFOA and PFOS. Additionally, among the PFASs measured, we sought to detect the most important contributors to breastfeeding duration and identify interactions using mixtures analyses.
Methods
Study Population
MoBa is a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health. Participants were recruited from across Norway from 1999 to 2008. Pregnant women who scheduled a routine ultrasound visit between 17 and 20 weeks of pregnancy were invited to participate in the study through postal invitation in gestational week 15. The women consented to participation in 41% of the pregnancies. The cohort now includes 114,500 children, 95,200 mothers, and 75,200 fathers.19 Data used in this study were based on version 10 of the quality-ensured data files released for research in 2017.
The establishment and data collection in MoBa has obtained a license from the Norwegian Data Inspectorate and approval from The Regional Committee for Medical Research Ethics. References for approvals were obtained for MoBa, The Norwegian Data Inspectorate (01/4325), and The Regional Committee for Medical Research Ethics (S-97045, S-95113). The current study was approved by The Regional Committee for Medical Research Ethics (REK 2017/427). Participant data were linked with data from the Medical Birth Registry of Norway (MBRN). This dataset was produced by combining two prior studies using MoBa participants; these studies examined the relationship between PFASs plasma concentrations and subfecundity and preeclampsia.20,21 Information on inclusion criteria can be found in the source studies.20,21 The preeclampsia study contributed 883 women, 879 of whom were nulliparous. The subfecundity study contributed 833 women, 406 nulliparas and 427 multiparas. Women were included in this analysis if they had a plasma PFAS measurement and gave birth to a live singleton and were excluded if they were missing information on breastfeeding duration or never initiated breastfeeding (initiation addressed in sensitivity analysis).
Exposure Assessment
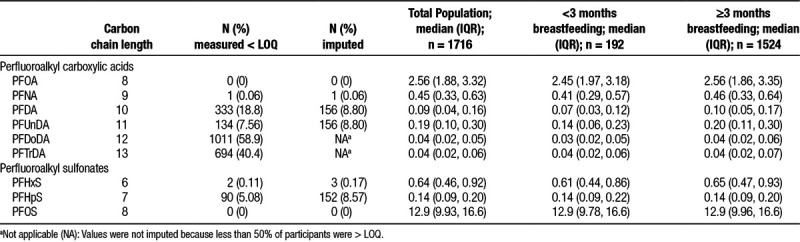
Blood samples were collected from participants at the time of the routine ultrasound examination and shipped at ambient temperature to the MoBa biorepository in Oslo.22 Samples were collected from women between approximately 17 and 20 weeks gestation. The selected PFASs are chemically stable, and significant changes in concentration due to shipment were not expected.23,24 The plasma was stored at −80°C before analysis for each of the studies. Concentrations of the compounds were determined using high-performance liquid chromatography/tandem mass spectrometry at the Norwegian Institute of Public Health. Additional details on the analytic process have been previously published.25 While 13 PFASs were measured, only 7 met our a priori threshold of >50% values above the limit of quantification (LOQ): PFOA, PFNA, PFDA, perfluoroundecanoic acid (PFUnDA), PFHxS, perfluoroheptane sulfonate (PFHpS), and PFOS. The LOQ for all compounds was 0.05 ng/mL. For this analysis, we imputed missing data using the Markov chain Monte Carlo method. We specified minimums and maximums for each PFAS based on LOQ and performed 10 imputations using proc MI in SAS 9.4 (SAS Institute Inc., Cary, NC 2015-2016). Data were considered missing if no machine reported value was available. Imputation was not performed for measured values below the LOQ. Perfluorodecanoic acid (PFDoDA) and perfluorotridecanoic acid (PFTrDA) were included in the analysis as dichotomous variables (≤ LOQ or > LOQ) because they had a high percentage of measured values (83% and 67%, respectively) but quantitation <50%. Measurements at concentrations < LOQ are considered less reliable. The following chemicals were analyzed but not included in analysis due to <50% quantitated: perfluoroheptanoic acid, perfluorobutanoate, perfluorotetradecanoic acid, and perfluorooctanesulfonamide.
Covariates
Covariates were identified through a literature review, and the adjustment set for this analysis was determined using a Directed Acyclic Graph (Figure 1). The following covariates were included in the model a priori: maternal age (continuous), previous breastfeeding duration (continuous), prepregnancy body mass index (BMI) (underweight, normal, overweight, obese), smoking during pregnancy (any versus none), and parity (multiparous versus nulliparous). A 4-level nominal variable was included to account for participants’ status in source studies (subfecundity case, subfecundity control, preeclampsia case, preeclampsia control). The women who participated in both studies (n = 17) were categorized based on their status from the subfecundity study.
Figure 1.
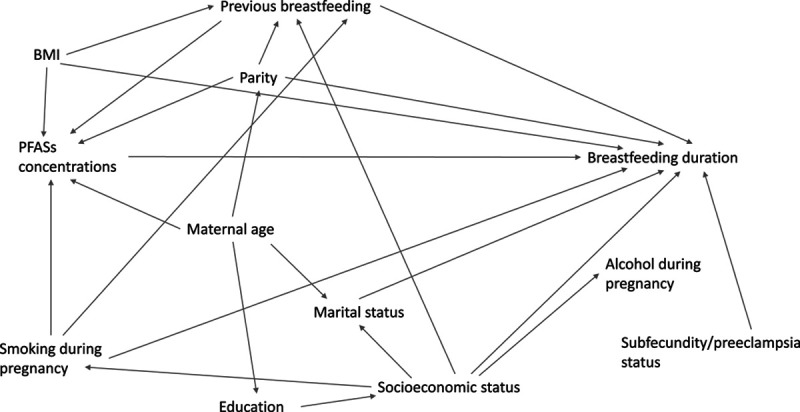
DAG for MoBa population.
Information on previous breastfeeding duration, BMI, and smoking during pregnancy was collected from self-reported surveys administered during early pregnancy (median: 18 weeks). A variable for total previous breastfeeding duration was calculated by summing the number of months women reported breastfeeding following their previous pregnancies. Prepregnancy BMI was calculated using women’s self-reported height and prepregnancy weight. Smoking during pregnancy was determined by participants’ response to the question, “Do you smoke now (after you became pregnant)?” Information on parity and maternal age at delivery was obtained from the MBRN. Covariates with missing information were imputed using fully conditional specification methods and the discriminant function.26 We included the following variables in our imputation model to inform the imputation of missing covariates: BMI, maternal age, gestational weight gain, education, alcohol consumption during pregnancy, marital status, parity, ever smoking, and current smoking. We performed 10 imputations and examined distributions of imputed variables pre- and postimputation to ensure realistic imputed values.
Maternal education and income were considered as covariates but not included in the analysis due to the homogeneity of the population. Though PFASs levels can vary during pregnancy, gestational age at sample collection was not included as samples were taken within the range of a few gestational weeks.
Breastfeeding Duration
Information on breastfeeding duration was completed by participants in a self-reported questionnaire 6 months after delivery.27 Women were asked to provide information on the frequency and duration of breastfeeding. One questionnaire item asked about infant feeding in the first week of life, one item asked about the kinds of liquids that the infant received at months 0, 1, 2, 3, 4, 5, and 6, and one item asked in which month the mother started giving complementary food to the child. Breastfeeding can be categorized as full breastfeeding (the infant receives no supplementary milk and/or infant food) or partial breastfeeding. When no other information is given, breastfeeding refers to both full and partial breastfeeding (any breastfeeding). After excluding women with missing breastfeeding data, information was available on the month of breastfeeding cessation for all women in the sample. The two outcomes of interest were cessation of breastfeeding before the end of 3 complete months and cessation of breastfeeding before the end of 6 complete months. Women were required to breastfeed in some capacity for the entire month to be categorized as completing the month. This analysis focuses on cessation of any breastfeeding rather than cessation of full breastfeeding.
Statistical Analyses
Demographic characteristics were examined within the study population and by breastfeeding duration. Distributions of PFASs concentrations were described overall and by breastfeeding duration. All analyses were performed with imputed exposure and covariate data.
To address our primary research question, we used Cox proportional hazards models to measure the association between PFASs plasma concentrations and breastfeeding cessation. PFASs concentrations were natural log transformed for analysis and were modeled continuously as well as in quartiles, with the lowest quartile serving as the referent group. The proportional hazards assumption was assessed through inclusion of time-dependent variables in the model, use of Schoenfeld residuals and goodness of fit tests, and observation of ln–ln survival curves.28 If a woman reported that she did not complete a full month of breastfeeding, her survival time was set to the midpoint of that month. Women were censored if they continued breastfeeding beyond the time of the outcome of interest. All analyses up to this point were performed in SAS.
Mixtures Analysis
Dimension reduction tools were used to identify PFASs and pairwise interactions most strongly associated with breastfeeding cessation at 3 months. Because of the correlation between PFASs, the parameter estimates produced from multiple linear models may be unstable. Thus, we utilized Elastic net regularization (ENET) for the Cox proportional hazards model, which searches for the best model with linear combinations of L1 and L2 penalties. Least absolute shrinkage and selection operator (LASSO) and Ridge regression are special cases of ENET.29 Ridge regression shrinks correlated parameters’ estimates toward the null, while LASSO selects one parameter over another, producing a sparser final model. All mixtures analyses were performed using R Statistical Software (R Foundation for Statistical Computing, Vienna, Austria).
These analyses only included women with complete covariate information (n = 1,662) and used one dataset with an average of the imputed exposure measures rather than collating results from the 10 imputations. All covariates were forced into each model. R package glmnet was used for the elastic net, and the cross-validation function (cv.glmnet) was used to test the alpha set, where alpha represents the balance between the LASSO (α = 1) and ridge (α = 0) penalties.30,31 To ensure model stability, a bootstrap procedure with 1000 iterations was conducted. We explored both a main effects only model and a model with both main effects and pairwise interactions.
Final selected models were identified based on inclusion in >70% of bootstrapped dataset model estimates. The selected models were then refit using the Cox proportional hazards models. We also performed Model Selection (MS) using R package My.stepwise for comparison.32
Sensitivity Analyses
We performed logistic regression modeling the odds of cessation of breastfeeding before 3 or 6 completed months to ensure that our results were consistent across modeling technique. We excluded noninitiators from our primary analysis, as factors unrelated to biology (including dislike of breastfeeding, other children to care for, no intention to breastfeed) may be the best explanation for noninitiation.5,33 However, a sensitivity analysis looking at odds of breastfeeding initiation was performed. Last, although a covariate was included to account sampling mechanism in the source studies, we ran models using only controls to identify the relationship between PFASs concentrations and breastfeeding duration independent of preeclampsia or subfecundity status.
Results
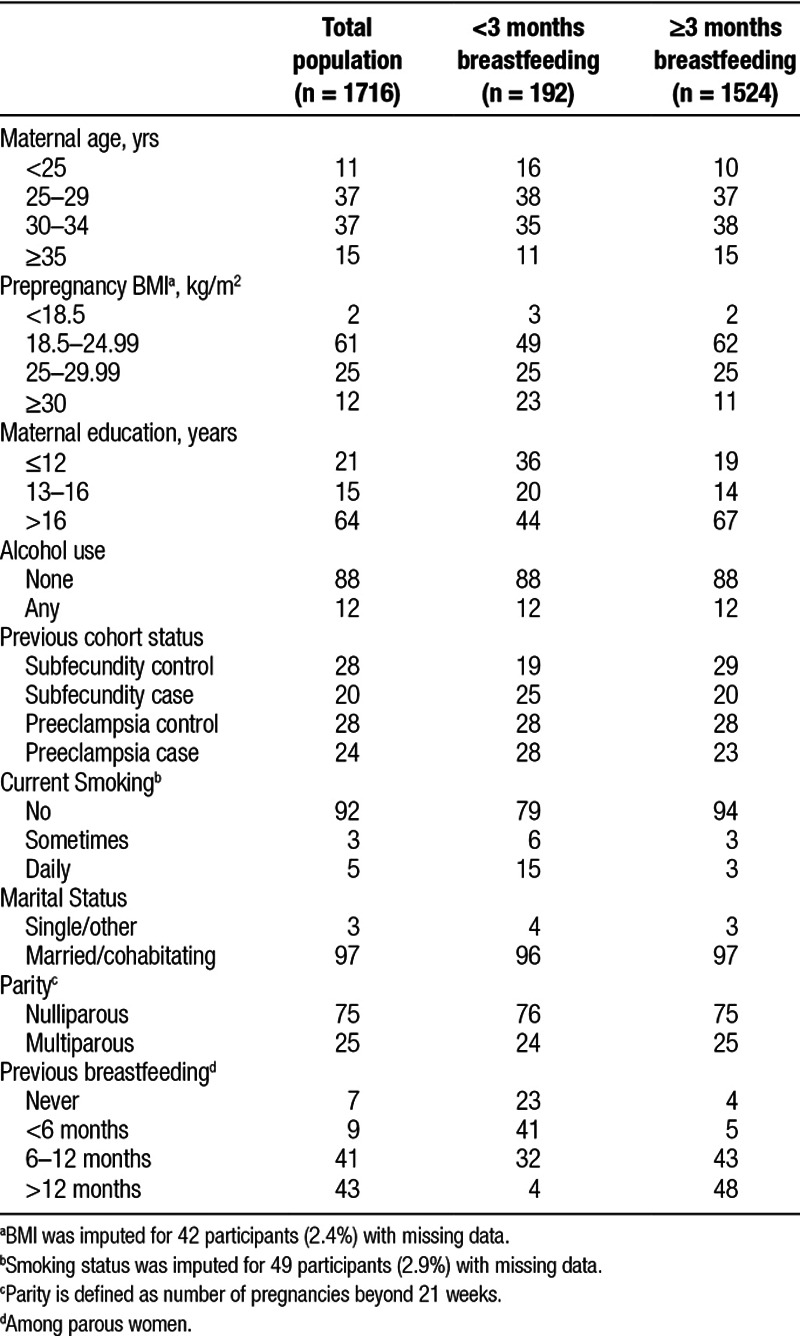
Our study included 1716 women who were primarily of normal BMI, highly educated, nonsmokers, married, and nulliparous (Table 1). Women with missing breastfeeding information (n = 183) were excluded from these analyses. In our population, approximately 11% (n = 192) of women ceased all breastfeeding before the end of 3 complete months and 21% (n = 362) of women did not reach 6 full months of breastfeeding, consistent with general breastfeeding trends in Norway.34 Duration of breastfeeding did not differ by alcohol consumption during pregnancy, parity, or marital status. Compared with women who breastfed beyond 3 months, women who breastfed for a shorter duration were more likely to be overweight or obese, less educated, younger, and current smokers. Women with shorter breastfeeding duration were more likely to have preeclampsia or exhibit characteristics of subfecundity. Among parous women, a history of prior breastfeeding was associated with longer duration of breastfeeding following the current pregnancy. On average, parous women who stopped breastfeeding before 3 months had previously breastfed for approximately 4 total months, whereas parous women who continued beyond 3 months had previously breastfed for approximately 14 total months. Missingness was low and we imputed values for prepregnancy BMI (2.4%) and smoking during pregnancy (2.9%) only.
Table 1.
Characteristics of the study population, overall, and by duration of breastfeeding: %
Both PFOS and PFOA were quantifiable in 100% of samples, while an additional 5 PFASs were measured above LOQ in >50% of samples (Table 2). Approximately 24% (n = 419) of PFDoDA and approximately 27% (n = 455) of PFTrDA samples were > LOQ; the rest of the samples were either missing or detected < LOQ. PFOS had the highest median concentration (12.9 ng/mL) followed by PFOA (2.56 ng/mL). Concentrations of most PFASs were lower among women with shorter breastfeeding durations.
Table 2.
Distribution of per- and polyfluoroalkyl substances (ng/mL) overall and by duration of breastfeeding in the study population
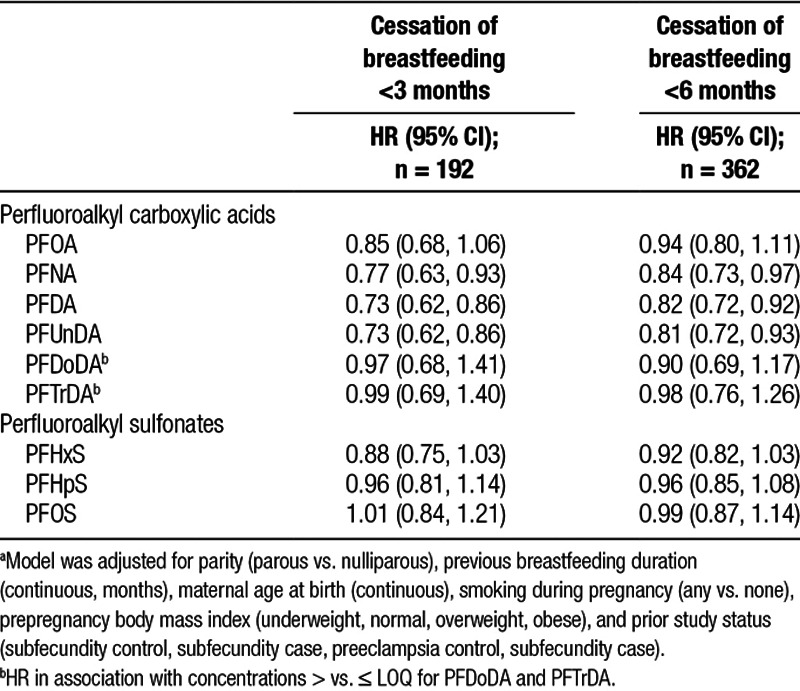
The proportional hazards assumption was met for all variables included in the model. Results from Cox proportional hazards models predominantly showed no association of PFASs plasma concentrations with breastfeeding cessation (Table 3). However, elevated concentrations of PFNA, PFDA, and PFUnDA were associated with less cessation (increased duration) of breastfeeding at both 3 and 6 months. At 3 months, the hazard of stopping breastfeeding was approximately 30% lower per interquartile range (IQR) increase in plasma concentrations for both PFDA and PFUnDA: hazard ratio (HR) = 0.73 (95% confidence interval [CI] = 0.62, 0.86). Per IQR difference for PFNA, the hazard ratio was 0.77 (95% CI = 0.63, 0.93). Analyses conducted using logistic regression with an outcome of cessation/no cessation at 3 and 6 months yielded similar results (Supplemental Material, Table 1; http://links.lww.com/EE/A20). Because breastfeeding cessation was not a rare outcome, results from the logistic regression may be an overestimate of the true effect.
Table 3.
Adjusteda HRs and 95% CIs for cessation of breastfeeding at 3 and 6 months in association with an interquartile range difference in plasma for per- and polyfluoroalkyl substances (n = 1,716)
Quartile analysis showed a similar trend of generally no association with the exceptions of PFDA and PFUnDA (Figure 2). Compared with women in the lowest quartile of PFDA plasma concentrations, women in the highest quartile had a hazard ratio of 0.44 (95% CI = 0.28, 0.71) for cessation of breastfeeding before 3 months, indicating a protective effect of higher concentrations on breastfeeding duration. The highest quartile of PFDA had an associated hazard for stopping breastfeeding before 6 months of 0.62 (95% CI = 0.45, 0.86). When compared with the referent group, the third quartile of PFUnDA had the strongest association with cessation of breastfeeding before 3 months (HR = 0.57; 95% CI = 0.38, 0.88).
Figure 2.
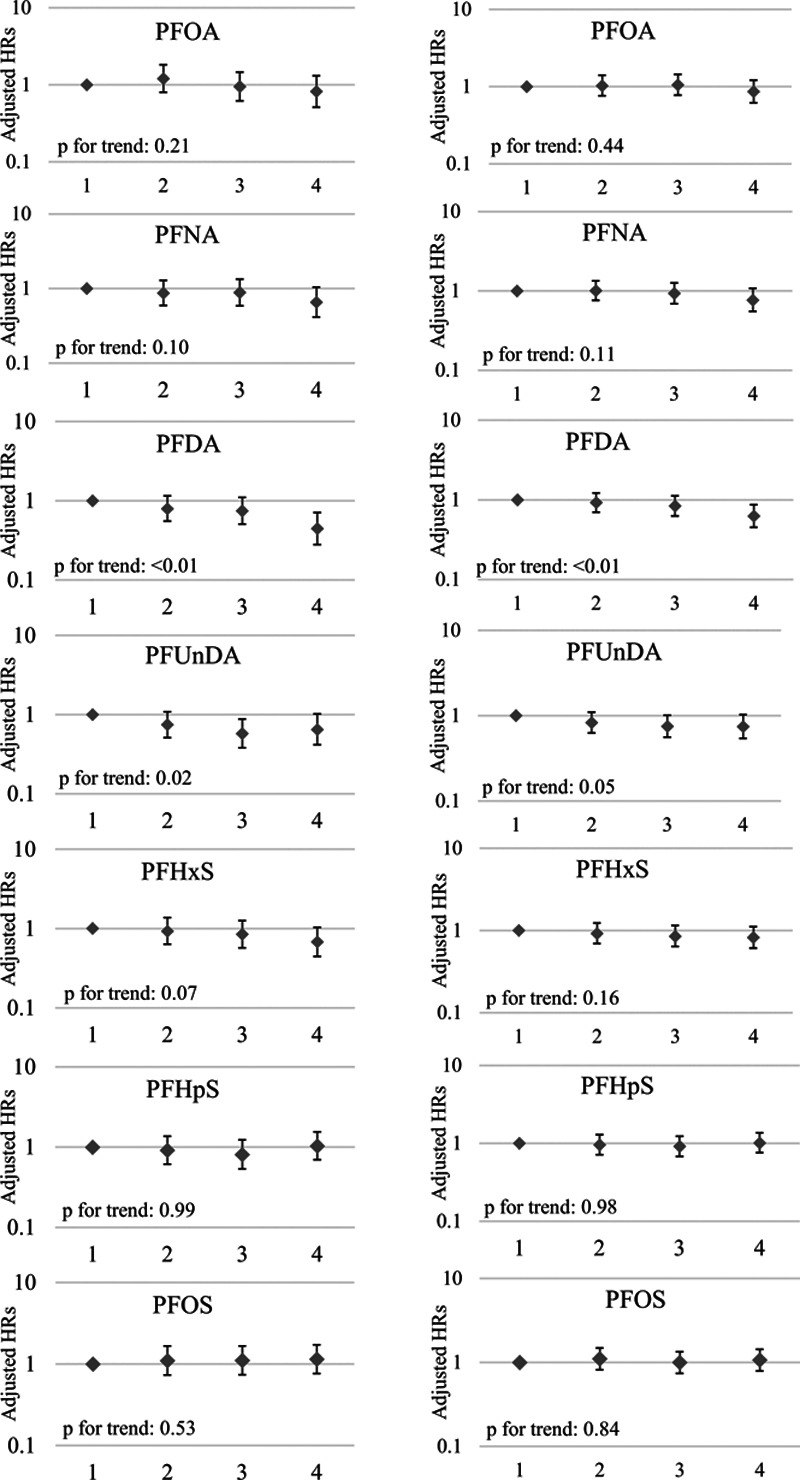
Adjusted hazard ratios (95% CIs) for cessation of breastfeeding at (a) 3 months and (b) 6 months in association with quartiles of plasma concentrations for per- and polyfluoroalkyl substances. (a) Model was adjusted for parity (parous vs. nulliparous), previous breastfeeding duration (continuous, months), maternal age at birth (continuous), smoking during pregnancy (any vs. none), prepregnancy BMI (underweight, normal, overweight, obese), and prior study status (subfecundity control, subfecundity case, preeclampsia control, subfecundity case). (b) Median concentrations of quartile 4 (n = 429) of PFASs and n cessation at 3 months: PFOA (3.97 ng/mL, n = 38); PFNA (0.79 ng/mL, n = 36); PFDA (0.22 ng/mL, n = 26); PFUnDA (0.39 ng/mL, n = 33); PFHxS (1.23 ng/mL, n = 39); PFHpS (0.27 ng/mL, n = 53); PFOS (19.84 ng/mL, n = 49).
When stratified by parity, the inverse associations between PFNA, PFDA, and PFUnDA and breastfeeding cessation remained only in nulliparous women (Supplemental Material, Table 2; http://links.lww.com/EE/A20). The effect estimates in multiparous women tended to be greater than 1, though confidence intervals were wide.
Generally, adjusted odds ratios (ORs) for breastfeeding initiation all approximated 1, indicating that initiation of breastfeeding was unassociated with PFASs concentrations (Supplemental Material, Table 3; http://links.lww.com/EE/A20). There was some suggestion that PFHpS levels are associated with decreased breastfeeding initiation. Lastly, we conducted a sensitivity analysis that included only the controls from the two source studies. The effect estimates were comparable to the results seen in the total population (Supplemental Material, Table 4; http://links.lww.com/EE/A20).
In this study, many of the PFASs were correlated, necessitating dimension reduction techniques to investigate the associations with multiple compounds simultaneously (Supplemental Material, Table 5; http://links.lww.com/EE/A20). The main effects model included more shrinkage of parameters (α = 0.1), while the main effects and interaction model tended toward sparseness (α = 0.9). As selected by both ENET and MS methods, the main effects only model identified PFHxS, PFDA, and PFOS as important contributors to the outcome (Table 4). The HR of PFOS exposure as generated by MS methods was 1.65 (95% CI = 1.06, 2.57), and the HR generated by our ENET procedure was comparable at 2.00 (95% CI = 1.19, 3.35), suggesting that in our mixtures analyses, higher PFOS levels were associated with increased hazard of cessation.
Table 4.
Adjusteda hazard ratios and 95% confidence intervals for cessation of breastfeeding at 3 months in association with plasma per- and polyfluoroalkyl substances using multi-pollutant models with dimension reduction technique: (1) Elastic Net Regularization and (2) Model Selection
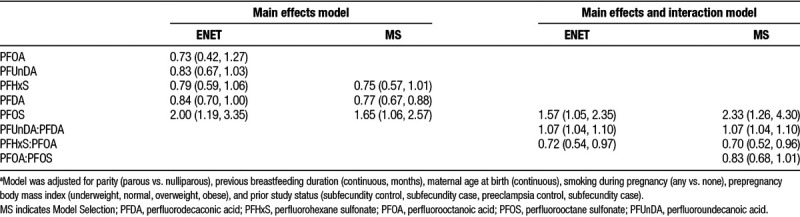
Using both selection methods, the effect estimates of PFHxS and PFDA showed an inverse association with outcome, where higher PFASs concentrations were associated with less cessation or more breastfeeding. ENET models additionally selected PFUnDA as being associated with breastfeeding duration. In the analysis involving main effects and all possible pairwise interactions, PFOS remained important in both ENET and MS. Both model reduction techniques identified PFUnDA:PFDA and PFHxS:PFOA interactions as associated with breastfeeding duration. MS techniques further selected PFOA:PFOS as a meaningful driver of outcome. PFOS and PFUnDA:PFDA were inversely associated with breastfeeding duration, suggesting a hazardous exposure, while PFHxS:PFOA was positively associated with breastfeeding duration, suggesting protective exposure effects.
Discussion
In this study estimating the association between maternal PFASs plasma concentrations and breastfeeding duration in the MoBa cohort, we found protective associations with PFNA, PFDA, and PFUnDA. Higher concentrations of these compounds were associated with a reduced hazard of breastfeeding cessation at both 3 and 6 months, indicating longer breastfeeding durations. There did not appear to be any association between breastfeeding cessation at either 3 or 6 months and plasma concentrations of other PFASs. However, when we examined multiple PFASs simultaneously, our results suggest that higher PFOS concentrations were associated with shorter durations of breastfeeding when accounting for the effects of other PFASs. Though previous studies did not perform mixtures analyses, this result agrees with their primary analyses showing that higher PFOS concentrations are associated with shorter breastfeeding duration. PFDA and PFUnDA are independently associated with longer breastfeeding durations and appear to interact with each other such that their combined effect was associated with shorter breastfeeding duration. No known toxicological studies have investigated possible interaction effects between PFASs. Along with PFDA and PFUnDA, PFNA appeared to be associated with increased breastfeeding duration in traditional regression models. The mixtures analyses, however, did not identify PFNA as a meaningful exposure, suggesting that the effects of PFNA may have been driven by its high correlation with PFDA. Results from the mixtures analyses should be interpreted cautiously, however, due to the newness of this method and the fact that ENET chooses important exposures statistically, rather than through known biological mechanisms.
All three prior studies found an association between elevated PFOA concentrations and decreased breastfeeding duration (increased cessation), and Fei et al12 and Timmermann et al14 additionally observed associations between elevated PFOS concentrations and decreased breastfeeding duration.13 Only Timmermann et al14 ran multivariate models with PFASs beyond PFOA and PFOS. Using regression, they found null results for PFHxS but results indicating that PFNA and PFDA were associated with shorter durations of breastfeeding. To our knowledge, no studies have examined the effects of PFUnDA.
Differences between our findings and previous research may be explained by a variety of reasons. Notably, PFASs concentrations in this cohort were lower than those in the other studies. In Fei et al,12 the median PFOA level for cases was 5.9 ng/mL (IQR = 4.7, 7.5). In the Romano et al13 study, the median PFOA concentration was 5.5 ng/mL (IQR = 3.8, 7.7), while the median in the present analysis was 2.6 ng/mL (IQR = 1.9, 3.3). In the Timmermann et al14 study, PFOA concentrations were comparable to those measured in our study (median: 2.4 ng/mL [IQR = 1.5, 3.6]), but PFOS concentrations were higher (median: 19.5 ng/mL [IQR = 18.7, 28.2] vs. median in our study: 12.9 ng/mL [IQR = 9.9, 16.6]). PFOS levels were higher in the Fei et al12 and Romano et al13 studies (median for cases in Fei et al12: 37.0 ng/mL [IQR = 28.0, 46.9] and median in Romano et al13: 13.9 ng/mL [IQR = 9.6, 18.2]). Concentrations of PFNA, PFDA, and PFHxS in the Timmermann et al14 study population were higher than those measured in our population as well (PFNA: 0.62 vs. 0.49; PFDA: 0.28 vs. 0.10; PFHxS: 1.45 vs. 0.67). This may suggest that the compounds only exhibit deleterious physiological effects at a certain threshold, below which effects are not seen.
A second explanation for the observed differences could be that both Timmermann et al14 and Fei et al12 did not have information on prior breastfeeding duration and were only able to control for parity. To determine the importance of data on previous breastfeeding in addition to parity, we ran adjusted models with and without breastfeeding information. Exclusion of information on prior breastfeeding inflated effect estimates approximately 18% for PFOA (HRs = 0.85 vs. 1.00). A similar analysis performed by Romano et al13 yielded concordant results, in which the absence of breastfeeding information biased estimates. Inclusion of parity alone does not fully account for this confounding.
The finding that higher concentrations of PFNA, PFDA, and PFUnDA were associated with protective effects was unexpected. No known toxicological research examining PFNA, PFDA, or PFUnDA concentrations on mammary gland development exists. Our results may be attributed to a residual confounding, a chance finding, or a yet unknown biological mechanism. Approximately 27% of participants had values < LOQ for PFDA and approximately 16% had values < LOQ for PFUnDA. The uncertainty associated with these low concentrations may partially explain our unexpected findings. However, similar findings were observed with PFNA, and very few participants had levels < LOQ. Unmeasured confounding is unlikely to explain how our results differ from previous studies as we considered all of their included covariates as possible confounders.
There is evidence that PFASs accumulate in fetal tissue with different efficiencies, and differential transfer of compounds from maternal plasma to fetal tissue may explain our findings.35–37 A study of Danish women found that of five PFASs analyzed (PFOS, PFOA, PFNA, PFDA, and PFUnDA), PFDA and PFUnDA had the highest concentrations in both placental and fetal tissues relative to maternal concentrations.36 Additionally, a study of Spanish women found that the more hydrophobic compounds (the sulfonates) were retained in maternal tissue more readily than the carboxylates.35 Given varying transplacental transfer rates, maternal levels of PFASs may not appropriately represent fetal exposure. Fetal concentrations may also be relevant for breastfeeding cessation; no known human research has examined the effect of infant PFASs concentrations on ability to sustain breastfeeding, but some toxicological research suggests that PFOA may impact the offspring’s ability to nurse, attenuating feeding stimulation in dams that is key for optimal lactation.38 If PFDA and PFUnDA are transferred to the fetus at higher rates than other PFASs, fetal exposure to those compounds would be higher than to the other PFASs, relative to the measured maternal concentrations. If PFASs in the placenta or fetus are responsible for early breastfeeding cessation, e.g., through interfering with the infant’s ability to properly breastfeed, then a protective effect of higher maternal plasma concentrations would be observed.
Rodent studies examining breast development have found numerous potential interference points for PFOA or PFOS. Multiple studies have found that female mice exposed to PFOA during gestation had visible delays in epithelial differentiation and alterations in functional mammary gland differentiation.38,39 Another study exposed female mice to PFOA at serum concentrations similar to those experienced by humans and found that prenatal exposure to PFOA causes significant mammary developmental delays in offspring.40 Thus, toxicological evidence suggests that higher maternal plasma concentrations of PFASs would lead to attenuated breastfeeding duration due to decreased milk supply.
There are many strengths to this study. First, it includes PFASs that have not previously been investigated with regard to breastfeeding duration. Secondly, our population contains a high percentage of nulliparous women. Nulliparas are the preferred study group for assessment of this question because results are not confounded by parity or prior breastfeeding. Even control of these variables cannot preclude the possibility of residual confounding. In addition, this is the largest study of this question to date. Furthermore, none of the previous studies on this topic analyzed potential mixture effects of PFASs. Because the compounds were correlated, the individual concentrations of compounds may not accurately represent the true exposures.41,42 Using ENET, we identified the most important PFASs contributing to breastfeeding duration after adjusting for correlation between compounds and identified interaction effects. However, results from the ENET analysis should be interpreted cautiously due to the limitations of the method.
There are also potential sources of imprecision in our study. If exposure in the newborn is relevant for duration of breastfeeding, then our measurements in maternal plasma are imperfect for capturing the exposure of interest. Additionally, women in this study were not asked to provide reasons for cessation of breastfeeding; thus, it is difficult to separate the effects of physiological limitations from other factors. However, the cohort is homogenous and Norway’s generous maternity leave policies likely limit the chance of cessation for work-related reasons.43
Our sample is unique in that it is a subset of women combined from two previous studies using the MoBa cohort. Though the Whitworth et al21 study found no association between PFASs and preeclampsia, another study noted a link between elevated PFOS concentrations and preeclampsia, though concentrations in that population were notably higher.44 In our study, we observed that breastfeeding duration was shorter among women with preeclampsia and other studies have noted similar findings.45 Similarly, subfecundity has been associated with both PFAS levels and was association with decreased breastfeeding duration in our study.46 There is evidence that status in the source studies may confound results in our study, but we included this variable as a covariate in our models. Additionally, we performed a controls-only sensitivity analysis which yielded similar results to our main analysis, reducing the concerns of bias due to our study design.
In the two source studies, both cases and controls were selected randomly from the eligible populations, limiting the possibility of selection bias. However, only 41% of invited women participated in MoBa, allowing for the possibility of differential selection into the cohort. Furthermore, not all women in MoBa provided biological samples. It is possible that women who accepted the invitation and subsequently provided samples were more health conscious and more likely to be involved in the healthcare system. These women may also choose to breastfeed longer. Though exposure to contaminated water would likely not be differential by health behavior, exposure to PFASs from food, often found in fast food packaging and pizza boxes, may be. In this case, the MoBa cohort contains women who are less likely to have high PFASs concentrations and more likely to breastfeed longer. Our results may then underestimate the true hazard of PFASs exposure in association with breastfeeding.
In this Norwegian cohort with relatively low PFASs exposures, we observed surprising inverse associations of PFNA, PFDA, and PFUnDA and breastfeeding cessation. We observed positive associations with PFOS and breastfeeding cessation, but only when accounting for other exposures. Last, we observed no association with PFOA though this has been noted in other studies.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
Supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS). This work was also supported by NIEHS training grant T32ES007018. The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/National Institute of Neurological Disorders and Stroke (grant no. 1 UO1 NS 047537-01 and grant no. 2 UO1 NS 047537-06A1).
Access to Data: No data can be shared due to data protection regulations. Researchers can apply for access to data through the Norwegian Institute of Public Health (https://www.fhi.no/en/more/access-to-data/).
Acknowledgments
We are grateful to all the participating families in Norway who take part in this on-going cohort study. We thank Dr. Sue Fenton for subject matter expertise.
Supplementary Material
Footnotes
Published online 21 August 2018
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.World; Health Organization. Infant and Young Children Nutrition. Global Strategy for Infant and Young Child Feeding 2002 [cited A55/A15 January 10, 2018] Available at: http://www.who.int/mediacentre/factsheets/fs342/en/. Accessed 10 November 2017.
- 2.Eidelman AI. Breastfeeding and the use of human milk: an analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeed Med. 2012;7(5):323–324. doi: 10.1089/bfm.2012.0067. [DOI] [PubMed] [Google Scholar]
- 3.Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. 2007 Apr Report No.: 1530-4396 (Print) 1530-4396 Contract No.: 153. [PMC free article] [PubMed] [Google Scholar]
- 4.UNICEF. Infant and Young child Feeding Global Database 2017 [January 12, 2018] Available at: https://data.unicef.org/topic/nutrition/infant-and-young-child-feeding/. Accessed 14 November 2017.
- 5.Ahluwalia IB, Morrow B, Hsia J. Why do women stop breastfeeding? Findings from the Pregnancy Risk Assessment and Monitoring System. Pediatrics. 2005;116(6):1408–1412. doi: 10.1542/peds.2005-0013. [DOI] [PubMed] [Google Scholar]
- 6.Wambach K, Campbell SH, Gill SL, Dodgson JE, Abiona TC, Heinig MJ. Clinical lactation practice: 20 years of evidence. J Human Lact. 2005;21(3):245–258. doi: 10.1177/0890334405279001. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, DeWitt JC, Higgins CP, Cousins IT. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? 2017;51(5):2508–2518. doi: 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- 8.Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;212(3):239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 9.US EPA. Risk Management for Per- and Polyfluoroalkyl Substances (PFASs) Under TSCA 2017 [January 10, 2018] Available at: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-and-polyfluoroalkyl-substances-pfass. Accessed 14 November 2017.
- 10.White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 2011;127(1–2):16–26. doi: 10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45(19):7954–7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- 12.Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scand J Work Environ Health. 2010;36(5):413–421. doi: 10.5271/sjweh.2908. [DOI] [PubMed] [Google Scholar]
- 13.Romano ME, Xu Y, Calafat AM, et al. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res. 2016;149:239–246. doi: 10.1016/j.envres.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timmermann CAG, Budtz-Jorgensen E, Petersen MS, et al. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reprod Toxicol. 2017;68:164–170. doi: 10.1016/j.reprotox.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brantsaeter AL, Whitworth KW, Ydersbond TA, et al. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int. 2013;54:74–84. doi: 10.1016/j.envint.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen C, Haug LS, Stigum H, Froshaug M, Broadwell SL, Becher G. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in Norwegian breast-milk during twelve months of lactation. Environ Sci Technol. 2010;44(24):9550–9556. doi: 10.1021/es1021922. [DOI] [PubMed] [Google Scholar]
- 17.Hackman NM, Schaefer EW, Beiler JS, Rose CM, Paul IM. Breastfeeding outcome comparison by parity. Breastfeed Med. 2015;10(3):156–162. doi: 10.1089/bfm.2014.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mondal D, Weldon RH, Armstrong BG, et al. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect. 2014;122(2):187–192. doi: 10.1289/ehp.1306613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnus P, Birke C, Vejrup K, et al. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016;45(2):382–388. doi: 10.1093/ije/dyw029. [DOI] [PubMed] [Google Scholar]
- 20.Starling AP, Engel SM, Richardson DB, et al. Perfluoroalkyl substances during pregnancy and validated preeclampsia among nulliparous women in the Norwegian Mother and Child Cohort Study. Am J Epidemiol. 2014;179(7):824–833. doi: 10.1093/aje/kwt432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitworth KW, Haug LS, Baird DD, et al. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology. 2012;23(2):257–263. doi: 10.1097/EDE.0b013e31823b5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paltiel L, Haugan A, Skjerden T, et al. The biobank of the Norwegian Mother and Child Cohort Study - present status. Nor J Epidemiol. 2014;24(1–2):29–35. [Google Scholar]
- 23.Fromel T, Knepper TP. Biodegradation of fluorinated alkyl substances. Rev Environ Contam Toxicol. 2010;208:161–177. doi: 10.1007/978-1-4419-6880-7_3. [DOI] [PubMed] [Google Scholar]
- 24.Kato K, Wong LY, Basden BJ, Calafat AM. Effect of temperature and duration of storage on the stability of polyfluoroalkyl chemicals in human serum. Chemosphere. 2013;91(2):115–117. doi: 10.1016/j.chemosphere.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A. 2009;1216(3):385–393. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- 26.Lee KJ, Carlin JC. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol. 2009;171(5):624–632. doi: 10.1093/aje/kwp425. [DOI] [PubMed] [Google Scholar]
- 27.The Norwegian Mother and Child Cohort Study. The Norwegian Mother and Child Cohort Study. 2005. Questionnaire 4 - when your child is around 6 months old. pp. 1–16. [Google Scholar]
- 28.Kleinbaum DG, Klein M. Evaluating the proportional hazards assumption. In: Gail M, Krickeberg K, Samet JM, Tsiatis A, Wong W, editors. In: Survival Analysis: A Self-Learning Text. 3rd ed. New York, NY: Springer; 2012. [Google Scholar]
- 29.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol. 2005;67:301–320. [Google Scholar]
- 30.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 31.Simon N, Friedman J, Hastie T, Tibshirani R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J Stat Softw. 2011;39(5):1–13. doi: 10.18637/jss.v039.i05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International; -Harvard Statistical Consulting Company. My.stepwise: Stepwise. International-Harvard Statistical Consulting Company; 2017. [Google Scholar]
- 33.Ogbuanu CA, Probst J, Laditka SB, Liu J, Baek J, Glover S. Reasons why women do not initiate breastfeeding: a southeastern state study. Womens Health Issues. 2009;19(4):268–278. doi: 10.1016/j.whi.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Australian Government Department of Health. An International Comparison Study into the Implementation of the WHO Code and Other Breastfeeding Initiatives. The University of Sydney: 2011.
- 35.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res. 2015;142:471–478. doi: 10.1016/j.envres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Mamsen LS, Jonsson BAG, Lindh CH, et al. Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci Total Environ. 2017;596–597:97–105. doi: 10.1016/j.scitotenv.2017.04.058. [DOI] [PubMed] [Google Scholar]
- 37.Cariou R, Veyrand B, Yamada A, et al. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ Int. 2015;84:71–81. doi: 10.1016/j.envint.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 38.White SS, Calafat AM, Kuklenyik Z, et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96(1):133–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 39.White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ Health Perspect. 2011;119(8):1070–1076. doi: 10.1289/ehp.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E, Fenton SE. The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod Toxicol. 2015;54:26–36. doi: 10.1016/j.reprotox.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindh CH, Rylander L, Toft G, et al. Blood serum concentrations of perfluorinated compounds in men from Greenlandic Inuit and European populations. Chemosphere. 2012;88(11):1269–1275. doi: 10.1016/j.chemosphere.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 42.Starling AP, Engel SM, Whitworth KW, et al. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ Int. 2014;62:104–112. doi: 10.1016/j.envint.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norwegian Labour and Welfare Administration. Parental Benefit 2018 [updated January 25, 2018–February 3, 2018] Available at: https://www.nav.no/en/Home/Benefits+and+services/Relatert+informasjon/parental-benefit#chapter-1. Accessed 20 November 2017.
- 44.Savitz DA, Stein CR, Bartell SM, et al. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology. 2012;23(3):386–392. doi: 10.1097/EDE.0b013e31824cb93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cordero L, Valentine CJ, Samuels P, Giannone PJ, Nankervis CA. Breastfeeding in women with severe preeclampsia. Breastfeed Med. 2012;7(6):457–463. doi: 10.1089/bfm.2012.0019. [DOI] [PubMed] [Google Scholar]
- 46.Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod. 2009;24(5):1200–1205. doi: 10.1093/humrep/den490. [DOI] [PubMed] [Google Scholar]


