Abstract
Necroptosis is a subtype of regulated necrosis that occurs when caspases are inhibited or fail to activate. Stimulus of cell death receptors results in a signaling cascade that triggers caspase independent, immunogenic cell death. The core pathway relies on receptor interacting protein kinase (RIPK) 1 and 3, which interact through their receptor homotypic interacting motif (RHIM) domains, and form amyloid-like structures termed the necrosome. RIPK3 recruits and phosphorylates mixed lineage kinase domain-like pseudokinase (MLKL), the terminal mediator in the necroptotic pathway. MLKL polymerizes to form a second amyloid-like structure that causes cell membrane disruption resulting in cell death. Although the core necroptosis pathway has been elucidated, the details of MLKL membrane translocation and membrane disruption remain an open area of research.
Keywords: Necroptosis, MLKL, Thioredoxin, Amyloid-like polymer
Introduction
Necroptosis is one of the regulated cell death pathways1–4. Phenotypically necroptosis resembles necrosis, but results from a specific caspase independent, kinase dependent signaling cascade, culminating in plasma membrane rupture and immunogenic cell death5. The inflammatory response generated from expulsion of cellular contents and cytokines stimulates both innate and adaptive immune responses. Necroptotic cell death has been described in TNF mediated systemic inflammatory response syndrome (SIRS), ischemic reperfusion injury, neurodegeneration, and pathogen infection, particularly viral disease6–9. The relevance of necroptosis in viral disease is perhaps the most compelling10–16. Yet, it is increasingly clear that programmed cell death in infectious and non-infectious disease states is unlikely to be unilateral. Recent evidence suggests that multiple parallel cell death pathways may be occurring concurrently15,17–19. Beyond the pathophysiologic questions associated with necroptosis, the specific mechanism of how MLKL causes membrane permeabilization and cell death remains unresolved. Illumination of the cryptic aspects of the terminal necroptotic pathway will offer insights into how necroptosis can be medicinally manipulated.
Necroptosis signaling pathway
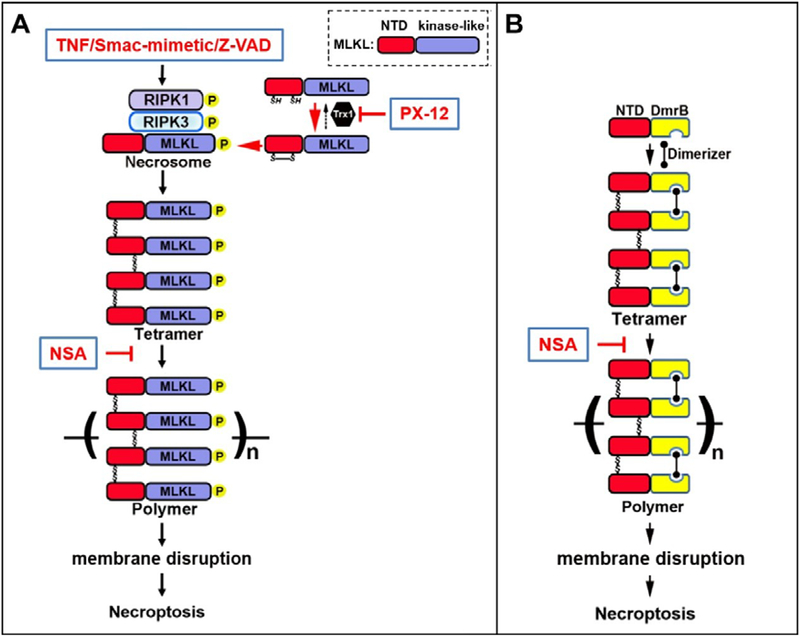
Cell extrinsic signals of necroptosis include ligands of the death receptor family (TNF, FAS or TRAIL), viral double stranded DNA or lipopolysaccharide stimulation of TLR 3 and 4, respectively, inflammatory mediators like interferon and viral stimulus of DNA-dependent activator of interferon regulatory factor18,20. The best studied is TNF mediated necroptosis. The death receptors of the TNF family contain a cytoplasmic death domain that induces regulated cell death. Ligand binding to the death receptor TNFR1 induces receptor trimerization. This conformational change enhances binding with the death domain (DD) adaptor protein RIPK1. RIPK1 binding leads to either cell survival through inflammatory cytokine signaling, or regulated cell death depending on the context. When ubiquitination of RIPK1 is disrupted through loss of the E3 ligases21, cell death signaling proceeds. Subsequently, three cell death outcomes are possible: RIPK1-dependent or independent apoptosis, or necroptosis. Of the cell death pathways, apoptosis predominates when caspase 3 and 8 are active, and necroptosis progresses when caspase activity is inhibited, but recent evidence suggests that caspase inhibition is not imperative for necroptosis and both cell death pathways can progress concurrently dependent on cellular context22. The fate decision is dictated by TAK1 phosphorylation of Ser231 in the intermediate domain of RIPK123. Sustained phosphorylation of the intermediate domain of RIPK1 stimulates interaction with RIPK3 and necroptotic cell death, as long as RIPK3 intracellular level is sufficient5,24. In the canonical necroptosis pathway RIPK1 binds to RIPK3, a serine/threonine kinase, through their C-terminal Receptor Homotypic Interacting Motif (RHIM) domains, which results in RIPK3 phosphorylation. Initially, RIPK1 kinase activity was believed responsible for RIPK3 phosphorylation but other kinases may be involved. RIPK3 activation via phosphorylation is imperative to necroptosis and seeds synthesis of amyloid-like polymers termed the necrosome25 (Figure 1A).
Figure 1.
(A) Overview of the TNF-induced necroptosis pathway. Upon treatment with TNF, Smac-mimetic and a pan-caspase inhibitor Z-VAD-FMK, receptor-interacting protein kinase 1 and 3 (RIPK1, RIPK3) hetero-oligomerize into amyloid-like fibers which recruit mixed lineage kinase-like protein MLKL to form the necrosome. MLKL contains an N-terminal domain (NTD) and a kinase-like domain. After being phosphorylated by RIPK3 in the kinase-like domain, MLKL undergoes conformational changes and forms disulfide bond-dependent tetramers. MLKL tetramers subsequently translocate to membrane fractions and further polymerize to form unique amyloid-like fibers. How MLKL polymers disrupt membrane integrity is still not clear. Importantly, disulfide bond formation of MLKL is tightly controlled by the cytosolic oxidoreductase thioredoxin 1 (Trx1). Inhibition of Trx1 with compound PX-12 in some cells leads to spontaneous activation of MLKL polymerization and subsequent necroptosis. Compound necrosulfonamide (NSA) specifically conjugates cysteine 86 of human MLKL and cysteine 32 of Trx1 to block MLKL tetramer polymerization and necroptosis. (B) Overview of necroptosis activation by induced-dimerization of MLKL-NTD. In a cell line where NTD of MLKL is fused to a dimerization domain called DmrB, addition of compound Dimerizer leads to disulfide bond-dependent tetramer formation of the fusion protein. The tetramers further polymerize to form amyloid-like fibers, leading to membrane disruption and necroptosis. Similar to the full-length MLKL, compound NSA also blocks the polymerization step of NTD-DmrB. This cell line bypasses the requirement of the upstream activators, such as TNF, TNFR, RIPK1, and RIPK3 for necroptosis activation, providing a powerful tool to study steps downstream of MLKL oligomerization.
The terminal mediator: MLKL
RIPK3 recruits mixed lineage kinase domain-like pseudokinase (MLKL), the obligatory effector of necroptosis, to the necrosome and phosphorylates human MLKL at T357 and S35826,27. Upon phosphorylation of MLKL by RIPK3, MLKL undergoes a conformational change that triggers MLKL tetramer formation which further polymerize to form unique amyloid-like fibers required for cell death28–34 (Figure 1A). The structural stability of polymeric MLKL relies on intermolecular disulfide bonds. Under steady-state conditions, the cytoplasmic thiol oxidoreductase thioredoxin-1 (Trx1) system maintains cellular redox balance by catalyzing disulfide exchange reactions, to limit disulfide bond formation between target proteins, such as monomeric MLKL. The small molecule necroptosis inhibitor necrosulfonamide (NSA) enhances this interaction by cross-linking Trx1 cysteine-32 to cysteine-86 of human MLKL, which blocks MLKL polymerization and membrane permeabilization.35 When cellular homeostatic mechanisms such as the Trx1 system are overwhelmed or inhibited, necroptosis ensues (Figure 1A).
MLKL’s preference for the plasma membrane
MLKL contains two domains including an N-terminal domain (NTD) and a C-terminal kinase-like domain. It has been shown that the recombinant NTD is sufficient to disrupt liposomes in vitro. Furthermore, induced-dimerization of a NTD fusion protein is sufficient to induce necroptosis in cells18,26,29,31–33 (Figure 1B). Multiple mechanistic models have been proposed to explain MLKL induced membrane disruption, but there is currently no definitive consensus. MLKL’s binding affinity for negatively charged phospholipids has been explored in multiple in vitro studies26,32–34. Using hydrophobic membrane lipid strips and lipid binding assays, MLKL’s binding predilection included multiple phosphoinositide species and the inner mitochondrial membrane specific cardiolipin32,33 Phosphoinositides are acidic phospholipids that interact with proteins and regulate integral membrane proteins36. Phosphoinositide species are specific to each cellular compartment membrane. PIP2, specific to the plasma membrane, attracts proteins associated with membrane budding and fusion and regulates ion channels36 MLKL’s phosphoinositide binding repertoire included phosphatidylinositol 4-phosphate (PI(4)P, trans Golgi), phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2, plasma membrane), phosphatidylinositol (3,4,5)-triphosphate (PIP3, plasma membrane), phosphatidylinositol (3)-phosphate (PI(3)P, late endosome and MVB), phosphatidylinositol (3,4)-biphosphate (PI(3,4)P2, early endosome), and phosphatidylinositol (3,5)-biphosphate (PI(3,5)P2, late endosome)26,33. Recombinant full length and N-terminal MLKL were capable of inducing leakage of PIP2 containing liposomes, and this occurred more efficiently than liposomes mimicking the mitochondrial membrane32,33. MLKL’s diverse organelle membrane binding was validated through cell fractionation experiments and immunostaining26.
MLKL binding to plasma membrane PIP2 is widely accepted as causal to cell death in necroptosis18,34, but the importance of MLKL’s affinity for the other phosphoinositides and cardiolipin remains unclear. Initially, the mitochondria was believed to play a role in necroptosis37, however, multiple studies have downplayed its significance38–40. It is significant to note that the latter studies were conducted in murine cells and the difference may reflect cell-type specificity or species divergence. NTD of mouse MLKL was shown to be sufficient to induce necroptosis in L929 cells, but not in mouse dermal fibroblasts30. Species differences among the MLKL NTD clearly exist, as human and chicken NTD are unable to kill mouse dermal fibroblasts despite orthologue sequence and structural similarities30,34.
Although the NTD of MLKL was capable of disrupting liposomes, it was not able to disrupt nanodiscs, which have a more rigid membrane surface structure due to the presence of a scaffolding protein26,32,36. This supports insertion of the NTD, at least partially, into the lipid membranes, and this insertion may be stabilized by the pseudokinase region30,34,41–43. Whether this insertion causes direct cell membrane disruption in vivo or invokes a secondary response remains contentious. Evidence also exist to supports MLKL’s membrane permealization through formation of cation channels or association with existing ion channels31,34,44,45, however, conformation and structural imaging has not yet identified the plasma membrane permeating structure.
MLKL: gain-of-function polymer
Evolutionarily conserved, prion-like signaling proteins or protein complexes such as mitochondrial antiviral signaling protein (MAVS) and the apoptosis associated spec-like protein (ASC), differ from other prion-like proteins in that they are gain-of-function polymers and their conformation is key to their function46,47. The identification of an MLKL amyloid-like fiber distinct from the RIPK1-RIPK3 amyloid-like signaling complex, makes it the second prion-like structure identified in the necroptosis signaling pathway25,28. Based on biochemical evidence, generation of the polymeric form of MLKL is a required step in the necroptotic pathway, suggesting that it too is a gain-of-function polymer. Similarities between the amyloid-like MLKL polymers and the MAVS and ASC signalosomes set them apart from other typical prion domains.
For example, their monomeric forms are not enriched in glutamine and asparagine and the monomers undergo regulated prion conversion47, 48. Furthermore, different from most other fibrils that contain mainly β-sheets, MAVS and ASC polymers are comprised of mostly helical structures, and the N-terminal domain of MLKL that forms polymers also contains mainly helical structures28. Interestingly, MLKL oligomerization and membrane translocation requires the chaperone HSP90 and its co-chaperone Cdc3748,49. Although the heat shock proteins are widely associated with prion templating, chaperones do not appear to be required for MAVS or ASC polymer assembly.
The exact function of the MLKL polymer is still unclear. The homology between the NTD of MLKL and the N-terminal HeLo-like domain (HELL) domain of the fungal protein HELLP identified by Daskalov et al. may offer a clue to MLKLs polymeric function.50 The HELL domain functions analogously to the HET-S protein from the ascomycete fungus Podospora anserine, which permeabilizes plasma membranes through an N-WHUPLQDO α-helical globular, pore-forming domain (HeLo)50–52. The HET-S/s C-terminal prion domain is required for amyloid propagation. In prion form, the C-terminal of HET-s adopts a β solenoid fold which transconforms the HET-S prion domain during cell fusion. This leads to refolding of the HET-S HeLo domain exposing a helix capable of permeating the membrane and propagating peripheral cellular migration leading to cell death via pore formation.50 HET-S does not form a prion53,54. The HeLo domain of Het-s, which differs from the HET-S HeLo domain by 13 residues, is non-toxic52. This raises the question: Which molecular species causes membrane disruption? Is the MLKL polymer a toxic amyloid-like fibril or could it serve an alternative purpose as a seed for a toxic MLKL monomer or oligomer? Alternatively, MLKL not directly involved in structure dysfunction, could it serve as an intracellular signalosome or locally transmissible prion-like protein within an extracellular vesicle pathway55?
Could PolyQ proteins and MLKL share a similar mechanism of cytotoxicity?
Another potentially applicable model for MLKL induced cell damage and dysfunction are the polyglutamine (PolyQ) fibrils associated with multiple degenerative diseases (huntingtin in Huntington’s disease, α-synuclein in Parkinson’s disease β-amyloid in Alzheimer’s disease). The polyQ stretches shared by these aggregate-prone proteins are causal to misfolding and correlated to severity of disease56, 57. Amyloidogenic protein misfolding leads to nucleation-dependent generation of polymorphic oligomers and fibrils. Oligomeric aggregates, composed of a small numbers of molecules and large numbers of non-filamentous assemblies, are believed to be the toxic species58,59. Exposure to oligomers disrupts giant unilamellar vesicle membranes through shifts in bilayer rigidity and adhesion force, inducing leakage of intravesicular contents59. Yet, the fibrils may also contribute to membrane damage as in the case of type 2 diabetes mellitus58,60–62. Human islet amyloid polypeptide (hIAPP) fibril growth at the extravesicular membrane of large unilamellar vesicles causes breakdown of cell barrier function. In vitro, the rate of leakage is accelerated by the presence of preformed seeds suggesting that fibril elongation rather than intermediate oligomers are the cause of the membrane damage. Fibrils can extract lipid at the liposome contact point of distortion62. In vivo, this is followed by intracellular calcium dysregulation and oxidative stress61,63.
Although MLKL lacks polyQ tracts, the polymeric expansion may represent a common mechanism for membrane breakdown in that both cellular damage scenarios require intimate association negatively charged membrane lipids and positively charged residues or areas of high positive charge density on the protein polymers. The possibility that MLKL polymers and polyQ polymers might use a common mechanism to disrupt membrane integrity is very intriguing given that there is aacumulating evidence linking necroptosis to neurodegenerative diseases9. Future experiments could be devised to test if the in vitro assembled MLKL polymers and polyQ polymers could disrupt the membranes of purified organelles, which may represent the cellular context better than liposomes.
Conclusion
Research in the field of necroptosis and other regulated cell death pathways has grown exponentially in the past decade. Despite the vast accumulation of data, the intricacies and inter-relatedness of necroptosis and other regulated cell death pathways hinders therapeutic application to the treatment of human disease. The identification of a polymeric MLKL fiber is an intriguing addition to the pool of prion-like protein effectors in inflammatory signaling, but it does not answer the key mechanistic question of how plasma membrane disruption occurs. Rather, it encourages further exploration of the complexities of the cell death pathways.
Acknowledgements
This work is supported by the Cellular and Molecular Biology Training Grant 2T32GM008203–26A1 to A.J. and the NIH R01 grant (RGM120502A) and the Welch Foundation grant (I1827) to Z.W. Z.W. is the Virginia Murchison Linthicum Scholar in Medical Research, and a Cancer Prevention and Research Institute of Texas (CPRIT) Scholar (Grant R1222).
Footnotes
Conflict of Interest: No conflicts declared.
References
- 1.Yang WS & Stockwell BR Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol 26, 165–176 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong T, Liao D, Liu X & Lei X Using Small Molecules to Dissect Non-apoptotic Programmed Cell Death: Necroptosis, Ferroptosis, and Pyroptosis. Chembiochem 16, 2557–2561 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Chan FK, Luz NF & Moriwaki K Programmed necrosis in the cross talk of cell death and inflammation. Annu Rev Immunol 33, 79–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res 26, 1007–1020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Duprez L, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35, 908–918 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Adameova A, et al. Evidence of necroptosis in hearts subjected to various forms of ischemic insults. Can J Physiol Pharmacol, 1–7 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Orozco S & Oberst A RIPK3 in cell death and inflammation: the good, the bad, and the ugly. Immunol Rev 277, 102–112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan J, Dawson TM & Dawson VL Cell Death Mechanisms of Neurodegeneration. Adv Neurobiol 15, 403–425 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Berger AK, et al. Viral RNA at Two Stages of Reovirus Infection Is Required for the Induction of Necroptosis. J Virol 91(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bian P, et al. MLKL Mediated Necroptosis Accelerates JEV-Induced Neuroinflammation in Mice. Front Microbiol 8, 303 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brault M & Oberst A Controlled detonation: evolution of necroptosis in pathogen defense. Immunol Cell Biol 95, 131–136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daley-Bauer LP, et al. Mouse cytomegalovirus M36 and M45 death suppressors cooperate to prevent inflammation resulting from antiviral programmed cell death pathways. Proc Natl Acad Sci U S A 114, E2786–E2795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels BP, et al. RIPK3 Restricts Viral Pathogenesis via Cell Death-Independent Neuroinflammation. Cell 169, 301–313 e311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galluzzi L, Kepp O, Chan FK & Kroemer G Necroptosis: Mechanisms and Relevance to Disease. Annu Rev Pathol 12, 103–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaiha GD, et al. Dysfunctional HIV-specific CD8+ T cell proliferation is associated with increased caspase-8 activity and mediated by necroptosis. Immunity 41, 1001–1012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mocarski ES, Kaiser WJ, Livingston-Rosanoff D, Upton JW & Daley-Bauer LP True grit: programmed necrosis in antiviral host defense, inflammation, and immunogenicity. J Immunol 192, 2019–2026 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrie EJ, Hildebrand JM & Murphy JM Insane in the membrane: a structural perspective of MLKL function in necroptosis. Immunol Cell Biol 95, 152–159 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Chien H & Dix RD Evidence for multiple cell death pathways during development of experimental cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression: apoptosis, necroptosis, and pyroptosis. J Virol 86, 10961–10978 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1, 489–495 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Vasilikos L, Spilgies LM, Knop J & Wong WW Regulating the balance between necroptosis, apoptosis and inflammation by inhibitors of apoptosis proteins. Immunol Cell Biol 95, 160–165 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Nogusa S, et al. RIPK3 Activates Parallel Pathways of MLKL-Driven Necroptosis and FADD-Mediated Apoptosis to Protect against Influenza A Virus. Cell Host Microbe 20, 13–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng J, et al. Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun 8, 359 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Li J, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 54, 133–146 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Liu S, et al. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc Natl Acad Sci U S A (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 24, 105–121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildebrand JM, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci U S A 111, 15072–15077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Z, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16, 55–65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su L, et al. A plug release mechanism for membrane permeation by MLKL. Structure 22, 1489–1500 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dondelinger Y, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep 7, 971–981 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Tanzer MC, et al. Evolutionary divergence of the necroptosis effector MLKL. Cell Death Differ 23, 1185–1197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynoso E, et al. Thioredoxin-1 actively maintains the pseudokinase MLKL in a reduced state to suppress disulfide bond-dependent MLKL polymer formation and necroptosis. J Biol Chem (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falkenburger BH, Jensen JB, Dickson EJ, Suh BC & Hille B Phosphoinositides: lipid regulators of membrane proteins. J Physiol 588, 3179–3185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Jiang H, Chen S, Du F & Wang X The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148, 228–243 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Murphy JM, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Remijsen Q, et al. Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis. Cell Death Dis 5, e1004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tait SW, et al. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep 5, 878–885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanzer MC, et al. Necroptosis signalling is tuned by phosphorylation of MLKL residues outside the pseudokinase domain activation loop. Biochem J 471, 255–265 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Quarato G, et al. Sequential Engagement of Distinct MLKL Phosphatidylinositol-Binding Sites Executes Necroptosis. Mol Cell 61, 589–601 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang D, et al. The MLKL Channel in Necroptosis Is an Octamer Formed by Tetramers in a Dyadic Process. Mol Cell Biol 37(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia B, et al. MLKL forms cation channels. Cell Res 26, 517–528 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Chen X, Gueydan C & Han J Plasma membrane changes during programmed cell deaths. Cell Res (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S, et al. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife 2, e00785 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai X, Xu H & Chen ZJ Prion-Like Polymerization in Immunity and Inflammation. Cold Spring Harb Perspect Biol 9(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao XM, et al. Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis 7, e2089 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobsen AV, et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis 7, e2051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daskalov A, et al. Identification of a novel cell death-inducing domain reveals that fungal amyloid-controlled programmed cell death is related to necroptosis. Proc Natl Acad Sci U S A 113, 2720–2725 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai X, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156, 1207–1222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saupe SJ & Daskalov A The [Het-s] prion, an amyloid fold as a cell death activation trigger. PLoS Pathog 8, e1002687 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loquet A & Saupe SJ Diversity of Amyloid Motifs in NLR Signaling in Fungi. Biomolecules 7(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenwald J, et al. The mechanism of prion inhibition by HET-S. Mol Cell 38, 889–899 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eisenberg D & Jucker M The amyloid state of proteins in human diseases. Cell 148, 1188–1203 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zoghbi HY & Orr HT Glutamine repeats and neurodegeneration. Annu Rev Neurosci 23, 217–247 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Scarafone N, et al. Amyloid-like fibril formation by polyQ proteins: a critical balance between the polyQ length and the constraints imposed by the host protein. PLoS One 7, e31253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Relini A, Marano N & Gliozzi A Misfolding of amyloidogenic proteins and their interactions with membranes. Biomolecules 4, 20–55 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho CS, Khadka NK, She F, Cai J & Pan J Polyglutamine aggregates impair lipid membrane integrity and enhance lipid membrane rigidity. Biochim Biophys Acta 1858, 661–670 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Relini A, Marano N & Gliozzi A Probing the interplay between amyloidogenic proteins and membranes using lipid monolayers and bilayers. Adv Colloid Interface Sci 207, 81–92 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Engel MF, et al. Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc Natl Acad Sci U S A 105, 6033–6038 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milanesi L, et al. Direct three-dimensional visualization of membrane disruption by amyloid fibrils. Proc Natl Acad Sci U S A 109, 20455–20460 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pieri L, Madiona K, Bousset L & Melki R Fibrillar alpha-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys J 102, 2894–2905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]