Abstract
Anthocyanins are plant secondary metabolites that, despite their chemical instability, have found many applications as natural food colorants. They are also known for their beneficial health effects because of their antioxidant and anticancer properties. More stable versions of these molecules, particularly at neutral pH conditions, are required to study the anthocyanin pharmacokinetic properties and obtain effective therapeutic results. In the present report, a cost-effective technique was developed to prepare the deuterated anthocyanin using recombinant Escherichia coli as a production host and deuterated glycerol and D2O in the culture media. This approach resulted in the formation of endogenous deuterated uridine 5′-diphosphate-glucose that was further incorporated by the recombinant anthocyanin pathway, resulting in the formation of deuterated cyanidin 3-O-glucoside (C3G). The deuterium exchange of O–D and C–D were studied by liquid chromatography (LC)–mass spectrometry and NMR analysis. The labeled C3G, purified by high-performance LC showed a stable nature at pH 7.0 as compared to nondeuterated C3G.
Introduction
Anthocyanins are a group of plant natural products and dietary polyphenols that are responsible, to a large extent, for the red color in fruits, vegetables, flowers, and red wine.1 Among these natural products, cyanidin 3-O-glucoside (C3G) is one of the most prevalent anthocyanins in nature. Because of the increasing preference for natural food colorants, anthocyanins in general and C3G in particular have received considerable attention in recent years as candidates for replacing artificial food colorants and cosmetic additives.2−5 In addition, C3G has gained attention for its potential therapeutic role related to its neuroprotective effects, including protection against amyloid-β-peptide-induced toxicity,6 antioxidant and cognitive promotion activity,7 anti-ischemic, anticancer, and anti-inflammatory properties.8,9
Because of their promising therapeutic potential, various pharmacokinetic studies have focused on analyzing the signaling mechanisms of anthocyanins such as C3G in vital organs.10−12 Unfortunately, the disappearance of C3G in the blood stream is very fast owing to its rapid biotransformation and its chemical instability at normal pH.10 As a result, there is considerable interest in using more stable anthocyanins, such as deuterated anthocyanin derivatives. The biological effect of deuterium (2H), with twice the mass of a hydrogen atom, has been extensively studied. Deuterium–carbon bonds are generally about six to 10 times more stable than the corresponding hydrogen–carbon bonds. As a result, they demonstrate slower rates of bond cleavage. The presence of deuterium also results in a kinetic isotope effect,13 which has been used to slow the metabolism of drugs when they reach vital organs and get metabolized through C–H bond breaking. One or more well-placed deuterium can actually have a significant effect on how long a drug will circulate in the bloodstream, by slowing down its clearance. Deuterated compounds also can provide critical insights into the pharmacokinetic properties of molecules.14
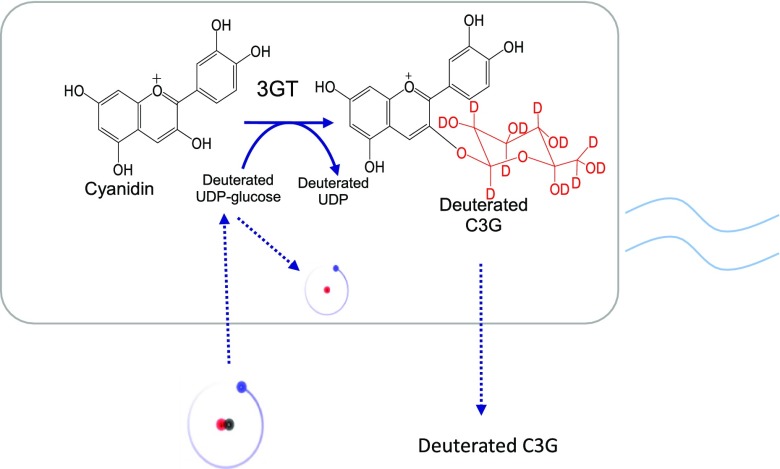
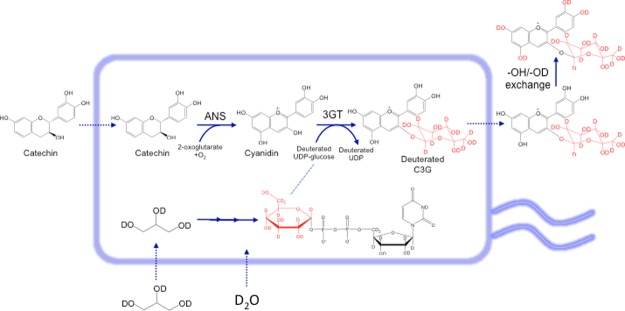
As current methods of anthocyanin production rely on extraction from plant sources, they are not amenable to modifications that can allow the facile production of non-natural anthocyanin derivatives, such as deuterated anthocyanins. However, in the recent past, microbial production of recombinant anthocyanins has emerged as an attractive alternative and more flexible production method, especially using well-characterized microbial hosts such as Escherichia coli.15 In our laboratory, we have reconstituted the metabolic pathways leading to the biosynthesis of various anthocyanins, including C3G, from flavan-3-ol precursors as well as glucose.16,17 We have shown that recombinant E. coli expressing anthocyanidin synthase (ANS) are able to convert the cheap and abundant precursor (+)-catechin into the unstable compound cyanidin, which can be significantly stabilized by glycosylation with uridine 5′-diphosphate (UDP)-glucose: 3-O-glycosyltransferase (3GT) to form C3G (Figure 1). In this work, we describe the biosynthesis of deuterated C3G through recombinant E. coli as a production host by supplementing deuterated glycerol and deuterated water into the culture media (Figure 1). This is the first time the synthesis of a deuterated anthocyanin molecule has been reported, opening the possibility of synthesizing not only other deuterated anthocyanin molecules but also, more generally, deuterated polyphenols.
Figure 1.
Schematic illustration of the biosynthesis of deuterated C3G by an engineered E. coli strain expressing ANS and flavonoid 3-glucosyltransferase (3GT) in this study. Deuterated glycerol and D2O were used to support cell growth and to supply deuterated UDP-glucose, which provided the deuterated glucosyl group in the deuterated C3G. Regular catechin was employed as the substrate for the formation of the tricyclic structure in the deuterated C3G. The −OH group in the tricyclic structure can be exchanged with the deuterated hydroxyl (−OD) of D2O in the medium.
Results and Discussion
Comparison of Glucose and Glycerol as Carbon Sources in C3G Fermentation
Glucose is the most widely utilized carbon source in industrial E. coli fermentations,18 but the excess glucose supplementation under aerobic conditions causes the formation of acidic byproducts by E. coli, the most common of which is acetate.19,20 Alternatively, glycerol can serve as an excellent carbon source despite the fact that it is a more energy-poor carbon source, and is a better source to produce regular and deuterated C3G and has increased importance in industrial biotechnology.21,22E. coli can utilize glucose and glycerol as carbon sources to produce destination compounds. Previously, our lab reported the production of flavan-3-ols using recombinant E. coli cocultures with different ratios of glycerol and glucose as a carbon source.23 In that work, increasing the glycerol percentage in the fermentation medium resulted in a shift in the production landscape toward higher titers appearing at later induction points. However, the effects of glycerol on C3G production by E. coli have not been tested so far, to the best of our knowledge. Therefore, in the present study, to select a better carbon source for C3G production, we decided to compare the C3G production by recombinant E. coli in the presence of both glucose and glycerol.
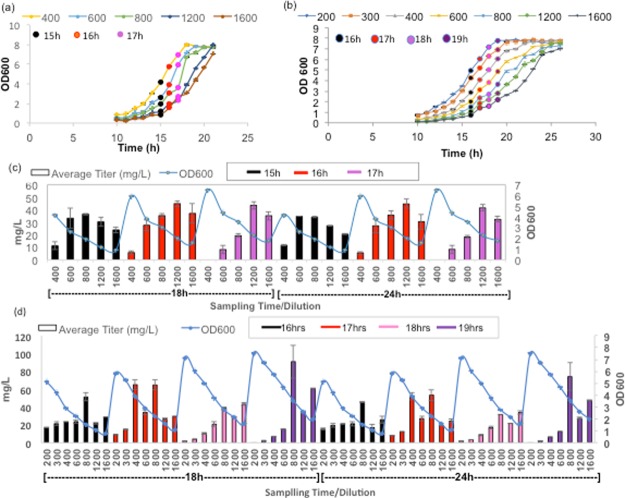
The growth of the C3G-producing strain was slower on glycerol-supplemented media than glucose (Figure S1). The recombinant strain reached the midlog phase (OD600 ≈ 3.7) in 22 h after the start of the fermentation when grown in glycerol-supplemented media (Figure S1c), whereas it reached the same growth stage faster (17 h, OD600 ≈ 3.5) in glucose-supplemented media (Figure S1b). This difference may be due to the incorporation of glycerol into the central metabolism as dihydroxyacetone phosphate that can participate in both gluconeogenic and glycolytic processes.24 Growth on glycerol resulted in a decrease of specific growth rate and low level of acetate production.25 The induction time points with isopropyl β-d-1-thiogalactopyranoside (IPTG) were also optimized for different cell dilutions separately in each glucose- and glycerol-supplemented Aspergillus minimal medium (AMM) [3.5 g/L KH2PO4, 5.0 g/L K2HPO4, 3.5 g/L (NH4)2HPO4, 100 mL 10× 3-(N-morpholino)propanesulfonic acid) (MOPS) mix (83.72 g/L MOPS, 7.17 g/L tricine, 28 mg/L FeSO4·7H2O, 29.2 g/L NaCl, 5.1 g/L NH4Cl, 1.1 g/L MgCl2, 0.48 g/L K2SO4, and 0.2 mL micronutrient stock), 1 mL 1 M MgSO4, 0.1 mL 1 M CaCl2, and 1 mL 0.5 g/L thiamine HCl. Micronutrient stock contains 0.18 g/L (NH4)6Mo7O24, 1.24 g/L H3BO3, 0.12 g/L CuSO4, 0.8 g/L MnCl2, and 0.14 g/L ZnSO4, supplemented with 20% (w/v) glucose or glycerol with ampicillin] (Figure 2c,d). The objective of optimization was to achieve the maximum C3G titer by optimizing induction time points and sampling conditions. In glucose-supplemented media, the cells were induced at 15, 16, and 17 h after subculturing and sampling was done 18 and 24 h after induction. Induction at 16 h after subculturing resulted in maximum C3G production followed by 17 and 15 h time points. Overall, the cells with dilutions of 1200 and 1600 showed better results than lower dilutions. The highest titer achieved was 44.98 mg/L, at OD600 ≈ 2.8 with induction at 16 h after inoculation, and sampled 24 h following induction (Figure 2c).
Figure 2.
Growth curve of E. coli (At3GT + PhANS) at different dilutions in AMM supplemented with glucose (a) and glycerol (b) verifying different induction time points. Dynamics of production of C3G at different induction time points with sampling at 18 and 24 h in glucose-supplemented media (c) and glycerol-supplemented media (d). The values are average of three replicates. Error bars represent standard deviation.
In glycerol-supplemented media, the cultures were induced at later induction time points, that is, 17, 18, and 19 h at different cell dilutions (Figure 2d). The induction at 19 h after subculturing showed enhanced production of C3G at 800 and 1600 dilutions. Sampling at 18 h after induction proved to be better than 24 h sampling. The highest titer of C3G was obtained with glycerol as the sole carbon source and was 90.95 mg/L at OD600 ≈ 3.7 by inducing the recombinant C3G pathway 19 h after inoculation, and sampled 18 h after induction (Figure 2d).
The better C3G production in low-cost glycerol-supplemented media than glucose-supplemented media allowed us to continue with the production of deuterated anthocyanins by using deuterated glycerol as the carbon source in the medium.
Deuterium-Labeled C3G Production
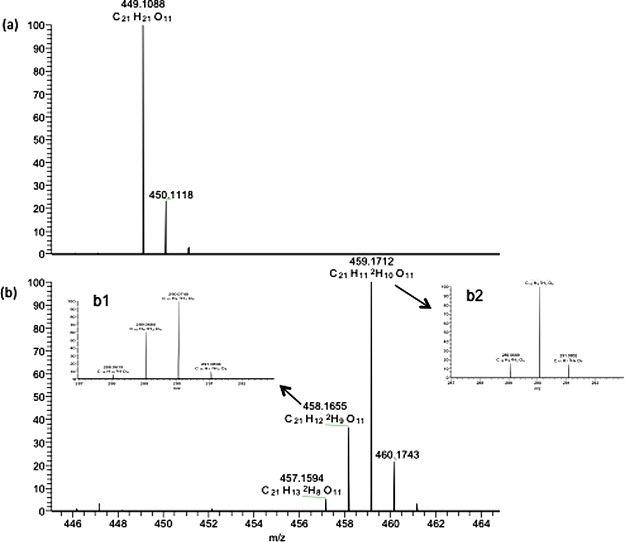
The deuterium-labeled C3G production was carried out in AMM where normal water and normal glycerol were replaced by deuterated water (D2O) and deuterated glycerol-d8. Liquid chromatography–mass spectrometry (LC–MS) analysis showed the different probabilities of deuterium incorporation to the C3G compound in the culture supernatant (Figure 3b), whereas no deuteration was found in C3G produced in normal medium (Figure 3a). The highest probability of deuterium incorporation to C3G was of 10 atoms of deuterium (mass 459) followed by nine atoms of deuterium (mass 458) (Figure 3b). The MS/MS spectrum of the selected peak clearly confirmed most of the deuterium incorporation in the glucose residue of C3G, which is consistent with the preliminary design that deuterium in deuterated glycerol will be incorporated into UDP-glucose and delivered into the glucose residue in deuterated C3G, as regular catechin was used as the precursor for the formation of the tricyclic structure of C3G. Thus, the deuterated hydrogens in deuterated C3G are mainly located in the glycosyl group. The deuterium was also incorporated into the tricyclic ring, which was possibly ascribed to the deuterium–hydrogen exchange in the presence of D2O.
Figure 3.
Fourier transform MS ESI positive-ion mode chromatogram of anthocyanidin glucoside; (a) nondeuterated sample; (b) deuterated sample; (b1,b2) MSMS ESI of the selected peak after losing glucoside residue shows different possibilities of deuteration in the aromatic ring.
For structural determination of deuterated C3G, NMR analysis was performed. Deuterated C3G was purified from the crude sample using high-performance LC (HPLC) (Figure S2) and subject to NMR analysis. Treating regular anthocyanins with 1% DCl in 500 μL MeOD-d4 for 1 h, the subsequent 1H NMR spectrum was exactly matched with the commercial C3G anthocyanin standard (Figure S3). This control experiment demonstrated that hydrogen–deuterium exchange on the carbon atoms from glucose residue of anthocyanins did not occur under the fermentation conditions. Characterization of the purified deuterated anthocyanins and regular anthocyanins by NMR spectroscopy is presented in Figure 4. Peaks at 5.0–7.5 ppm (denoted with a bracket) in both spectra correspond to aromatic protons on anthocyanins and are exactly matched, indicating that no hydrogen–deuterium exchange occurred on the aromatic ring. Peaks at 1.8–4.0 ppm (denoted in dashed boxes) in panel (a) correspond to protons of carbon atoms from glucose residue, such as peak at 3.8 ppm that corresponds to glucose-anomeric hydrogen, but did not show up in panel (b), demonstrating that hydrogen–deuterium exchange happened on glucose residue. This technique is very promising to produce labeled anthocyanins having a much stronger C–D bond, the content of which can be measured in the tissues during pharmacokinetic studies using heteronuclear NMR.
Figure 4.
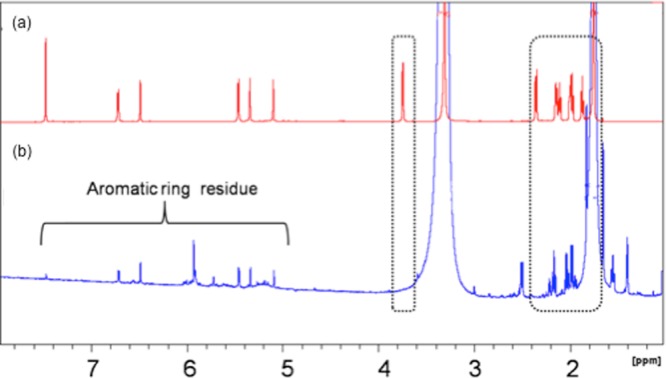
1H NMR comparison between commercial anthocyanins (a) and deuterated anthocyanins (b). Peaks at 5.0–7.5 ppm (denoted with a bracket) in both spectra correspond to aromatic protons on anthocyanins and are exactly matched. Peaks at 1.8–4.0 ppm (denoted in dashed boxes) in panel A correspond to protons from glucose residue but did not show up in panel B, demonstrating that deuterated exchange happened on glucose residue.
Stability Analysis of Deuterated C3G
At normal pH, anthocyanins are highly unstable molecules that are susceptible to degradation in response to different environmental factors such as oxygen, temperature, and light.26 Anthocyanins are more stable at acidic pH (1–3). In aqueous solutions at pH > 4, anthocyanins adopt the form of carbinol and chalcones that undergo degradation easily and produce phenolic acids.27 The degradation of anthocyanins at normal pH is an important factor that can influence their therapeutic properties, given that the pH of the human blood is around 7.3 and at 7.3.10 More generally, it plays a critical role in determining the application of these molecules in the food industry as natural food colorants. Therefore, it was necessary to test the stability of the produced deuterated C3G at normal pH.
The pH stability test of deuterated and nondeuterated C3G was performed at pH 7.0 and showed a more stable nature for deuterated C3G as compared to normal C3G at room temperature (Figure 5). The sample analysis by HPLC at regular intervals showed more decrease in residual percentage of regular C3G (from 100 at 0 h to 21.2% at 72 h) as compared to deuterated C3G (from 100 at 0 h to 71% at 72 h).
Figure 5.
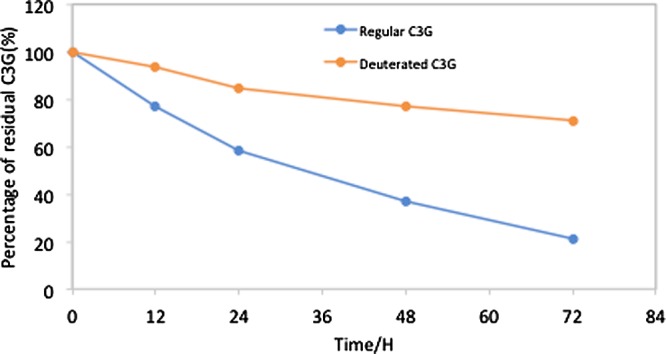
Stability comparison between regular C3G (blue line) and deuterated C3G (red line) at different intervals of time (from 0 to 72 h) at pH 7.4, demonstrating early fall for regular C3G as compared to deuterated C3G.
The improved stability of deuterated C3G will result in longer residence times of this molecule in the human body, possibly resulting in increased absorption, distribution, metabolism, and excretion in clinical trials. We envision that deuterated C3G can be used to better study the fate of anthocyanins in human bodies and to investigate their interaction with human cells.
Conclusions
In the present study, recombinant E. coli was used to convert deuterated glycerol and D2O to deuterated UDP-glucose that was incorporated into the deuterated C3G by the last step in the biosynthetic pathway, catalyzed by the glycosyltransferase 3GT. The biosynthesis process presented in this work is benign and cost-effective. Therefore, recombinant E. coli strains can provide a very convenient and effective approach to synthesizing deuterated C3G, which only requires simple deuterated glycerol and D2O in the fermentation medium. Such deuterated anthocyanins can be used in the future in pharmacokinetic studies in animal models. Moreover, this technique can also be applied for the production of other deuterated anthocyanins using engineered E. coli incorporated with other anthocyanins producing genes.
Materials and Methods
Strains and Chemicals
E. coli strains and plasmids used in this study were constructed previously in our lab,16 and are available through Addgene (plasmids #86901–86917). The culture media and other chemicals, viz., LC–MS grade methanol, water, formic acid, MeOD-d4 (99.9%), deuterated glycerol-d8 (99%), and deuterium chloride (99 atom % D), were purchased from Sigma-Aldrich.
Bacterial Culture Conditions and Growth Curve
All anthocyanin production experiments were performed using a recombinant E. coli BL21 Star (DE3) carrying plasmid pETM6-At3GT-m-PhANS where a two-step anthocyanin pathway has been introduced in monocistronic configuration.16 The working culture was maintained on Luria–Bertani agar plates supplemented with 80 μg/mL ampicillin. All growth as well as production experiments were performed in 1 mL AMM as described previously in polypropylene deep 48-well plates (5 mL, VWR) covered with breathable rayon film (VWR). Casamino acids were excluded as they are a potential carbon source.17,28 For carrying out experiments in deuterated media, buffer salts and mineral salts were pre-equilibrated with 99.9% D2O and then flash-evaporated/lyophilized to dryness to remove exchangeable hydrogen atoms. The exchanged salts were then dissolved in 99.9% D2O and filter-sterilized. Deuterated glycerol-d8 (20 g/L) was used as a carbon source.
For obtaining a bacterial growth curve, glycerol stocks of recombinant BL21 Star (DE3) strains carrying anthocyanin pathway plasmids were grown on solid media and after overnight growth, a single colony was inoculated into a single well of a polypropylene deep 48-well plate containing 1 mL of liquid media. The plate was then incubated at 37 °C with shaking at 225 rpm. Optical cell density was measured on a BioTek Synergy 4 Microplate Reader.
Optimization of Induction Time Points
For induction time point optimization in glucose-supplemented media after 17 h of overnight growth, 400-, 600-, 800-, 1200-, and 1600-fold dilutions were made, whereas for glycerol-supplemented media after 22 h of overnight growth, 200-, 300-, 400-, 600-, 800-, 1200-, and 1600-fold dilutions were made. All cultures were incubated at 225 rpm and 30 °C. Substrate (1 g/L (+)-catechin final concentration) stock dissolved in dimethylformamide/ethanol (8:2, v/v) and inducer (1 mM IPTG final concentration) was added sequentially by multichannel pipette at different time points after subculturing. These time points were 15, 16,, 17 h for glucose (Figure 2a) and 16, 17, 18, and 19 h for glycerol-supplemented media (Figure 2b). Cultures were sampled for anthocyanin quantification at 18 and 24 h postinduction. All production experiments were performed in biological triplicate, and reported production values represent mean and standard error of the mean as quantified by HPLC.
HPLC Analysis and Purification
The 200 μL of cell culture was extracted by addition of an equal volume of acidified methanol (1% HCl, v/v), followed by brief vortexing and centrifugation at 21 000g for 10 min. C3G stock solutions of known concentrations were prepared by dissolving analytical standards (purchased from Extrasynthese) in dimethyl sulfoxide and were stored at −20 °C. Standard curves for quantifying the anthocyanidin glucoside production were prepared by dilution of C3G into methanol at appropriate concentrations. The samples or standards (10 μL) were injected for analysis on an Agilent 200 series HPLC equipped with a ZORBAX SB-C18 StableBond analytical column (150 mm × 4.6 mm, 5 μm) and a diode array detector. Acetonitrile (solvent A) and water (solvent B), both containing 0.1% formic acid, were used as mobile phases. A flow rate of 1 mL/min was used with the following gradients: 10–40% A (0–10 min) and 10–40% A (10–15 min). C3G was quantified at a retention time of 4.8 min against the standard curves by peak area integration at 520 nm.
Anthocyanins were purified from substrate catechin as well as other components of media using HPLC with 60 μL injection volume. The separation was conducted using mobile phases A and B as defined above, at a flow rate of 1 mL/min with the following gradients: 1.0–15% A (0–45 min), 15–60% A (45–50 min), 60–1.0% (50–52 min), and 1% A (52–55 min).
LC–MS Analysis
LC analysis was performed by injecting 5 μL of the sample to the Agilent 1200 HPLC system with column Agilent ZORBAX Eclipse XDB-C18 4.6 × 150 mm 5 μm. Water and acetonitrile containing 0.2% formic acid were used as solvents A and B, respectively, at a flow rate of 250 μL/min, using the following gradients: 5–10% B (0–1 min), 10–30% B (1–10 min), 30–90% B (10–30 min), 90–5% B (34.9–35 min), and 5% B (35–40 min). MS analysis was done using LTQ Orbitrap XL from Agilent Technologies (Santa Clara, CA, USA), electrospray ionization (ESI), positive-ion mode, resolution 30 000, and mass accuracy 3 ppm.
NMR Analysis
All samples were dissolved in 0.5 mL of MeOD-d4 (contains 1% DCl). 1H spectra were performed at 298 K on a Bruker 800 MHz instrument recorded for 32 scans with TopSpin 2.1.6 software.
Stability Test of Deuterated Anthocyanins
The purified deuterated C3G or regular C3G (Alkemist Labs, USA) was dissolved in phosphate buffered saline (pH 7.4) with an initial concentration of ∼20 mg/L and was left at room temperature in the dark for 72 h. Samples were taken periodically and the residual C3G was measured by HPLC.
Acknowledgments
The authors are grateful to the SERB Indo-US Science and Technology Forum for their financial support. This research is also supported by the NSF CBET award number 1604547 and by the Global Research Laboratory Program (NRF 2016K1A1A2912829) through the National Research Foundation of Korea (NRF).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01134.
Structure of anthocyanidin glucosides (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wu X.; Beecher G. R.; Holden J. M.; Haytowitz D. B.; Gebhardt S. E.; Prior R. L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- Tanaka Y.; Ohmiya A. Seeing Is Believing: Engineering Anthocyanin and Carotenoid Biosynthetic Pathways. Curr. Opin. Biotechnol. 2008, 19, 190–197. 10.1016/j.copbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Ananga A.; Georgiev V.; Ochieng J.; Phills B.; Tsolov V.. Production of Anthocyanins in Grape Cell Cultures: A Potential Source of Raw Material for Pharmaceutical, Food, and Cosmetic Industries. In The Mediterranean Genetic Code—Grapevine and Olive; Poljuha D., Sladonja B., Eds.; InTech, 2013; pp 247–287. [Google Scholar]
- Aberoumand A. A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J. Dairy Food Sci. 2011, 6, 71–78. [Google Scholar]
- Bhan N.; Xu P.; Koffas M. A. G. Pathway and Protein Engineering Approaches to Produce Novel and Commodity Small Molecules. Curr. Opin. Biotechnol. 2013, 24, 1137–1143. 10.1016/j.copbio.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Tarozzi A.; Morroni F.; Merlicco A.; Bolondi C.; Teti G.; Falconi M.; Cantelli-Forti G.; Hrelia P. Neuroprotective Effects of Cyanidin 3-O-Glucopyranoside on Amyloid Beta (25-35) Oligomer-Induced Toxicity. Neurosci. Lett. 2010, 473, 72–76. 10.1016/j.neulet.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Shih P.-H.; Chan Y.-C.; Liao J.-W.; Wang M.-F.; Yen G.-C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J. Nutr. Biochem. 2010, 21, 598–605. 10.1016/j.jnutbio.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Kang T. H.; Hur J. Y.; Kim H. B.; Ryu J. H.; Kim S. Y. Neuroprotective effects of the cyanidin-3-O-β-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci. Lett. 2006, 391, 122–126. 10.1016/j.neulet.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Di Giacomo C.; Acquaviva R.; Santangelo R.; Sorrenti V.; Vanella L.; Li Volti G.; D’Orazio N.; Vanella A.; Galvano F. Effect of Treatment with Cyanidin-3-O-β-D-Glucoside on Rat Ischemic/Reperfusion Brain Damage. Evidence-Based Complementary Altern. Med. 2012, 2012, 1–10. 10.1155/2012/285750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasaro S.; Ziberna L.; Gasperotti M.; Tramer F.; Vrhovšek U.; Mattivi F.; Passamonti S. Determination of Cyanidin 3-Glucoside in Rat Brain, Liver and Kidneys by UPLC/MS-MS and Its Application to a Short-Term Pharmacokinetic Study. Sci. Rep. 2016, 6, 22815. 10.1038/srep22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T.; Sonntag S.; Strass G.; Bitsch I.; Bitsch R.; Netzel M. Urinary Pharmacokinetics of Cyanidin Glycosides in Healthy Young Men Following Consumption of Elderberry Juice. Int. J. Clin. Pharmacol. Res. 2005, 25, 47–56. [PubMed] [Google Scholar]
- Jeon S.; Han S.; Lee J.; Hong T.; Yim D.-S. The Safety and Pharmacokinetics of Cyanidin-3-Glucoside after 2-Week Administration of Black Bean Seed Coat Extract in Healthy Subjects. Korean J. Physiol. Pharmacol. 2012, 16, 249–253. 10.4196/kjpp.2012.16.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P. Kinetic Deuterium Isotope Effects in Cytochrome P450 Reactions. Methods Enzymol. 2017, 596, 217–238. 10.1016/bs.mie.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner D. J.; Baker A.; Dunstall T. G. Pharmacological Uses and Perspectives of Heavy Water and Deuterated Compounds. Can. J. Physiol. Pharmacol. 1999, 77, 79–88. 10.1139/y99-005. [DOI] [PubMed] [Google Scholar]
- Pandey R. P.; Parajuli P.; Koffas M. A. G.; Sohng J. K. Microbial Production of Natural and Non-Natural Flavonoids: Pathway Engineering, Directed Evolution and Systems/Synthetic Biology. Biotechnol. Adv. 2016, 34, 634–662. 10.1016/j.biotechadv.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Cress B. F.; Leitz Q. D.; Kim D. C.; Amore T. D.; Suzuki J. Y.; Linhardt R. J.; Koffas M. A. G. CRISPRi-Mediated Metabolic Engineering of E. Coli for O-Methylated Anthocyanin Production. Microb. Cell Factories 2017, 16, 10. 10.1186/s12934-016-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S.; Jones J. A.; Lachance D. M.; Bhan N.; Khalidi O.; Venkataraman S.; Wang Z.; Koffas M. A. G. Improvement of Catechin Production in Escherichia Coli through Combinatorial Metabolic Engineering. Metab. Eng. 2015, 28, 43–53. 10.1016/j.ymben.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Gosset G. Improvement of Escherichia Coli Production Strains by Modification of the Phosphoenolpyruvate:Sugar Phosphotransferase System. Microb. Cell Factories 2005, 4, 14. 10.1186/1475-2859-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luli G. W.; Strohl W. R. Comparison of Growth, Acetate Production, and Acetate Inhibition of Escherichia Coli Strains in Batch and Fed-Batch Fermentations. Appl. Environ. Microbiol. 1990, 56, 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Zhu J.; Bennett G. N.; San K.-Y. Succinate Production from Different Carbon Sources under Anaerobic Conditions by Metabolic Engineered Escherichia Coli Strains. Metab. Eng. 2011, 13, 328–335. 10.1016/j.ymben.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Bisen P. S.; Sanodiya B. S.; Thakur G. S.; Baghel R. K.; Prasad G. B. K. S. Biodiesel Production with Special Emphasis on Lipase-Catalyzed Transesterification. Biotechnol. Lett. 2010, 32, 1019–1030. 10.1007/s10529-010-0275-z. [DOI] [PubMed] [Google Scholar]
- Yazdani S. S.; Gonzalez R. Anaerobic Fermentation of Glycerol: A Path to Economic Viability for the Biofuels Industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. 10.1016/j.copbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Jones J. A.; Vernacchio V. R.; Sinkoe A. L.; Collins S. M.; Ibrahim M. H. A.; Lachance D. M.; Hahn J.; Koffas M. A. G. Experimental and Computational Optimization of an Escherichia Coli Co-Culture for the Efficient Production of Flavonoids. Metab. Eng. 2016, 35, 55–63. 10.1016/j.ymben.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Martínez-Gómez K.; Flores N.; Castañeda H. M.; Martínez-Batallar G.; Hernández-Chávez G.; Ramírez O. T.; Gosset G.; Encarnación S.; Bolivar F. New Insights into Escherichia Coli Metabolism: Carbon Scavenging, Acetate Metabolism and Carbon Recycling Responses during Growth on Glycerol. Microb. Cell Factories 2012, 11, 46. 10.1186/1475-2859-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L.; Shimizu K. Global Metabolic Regulation Analysis for Escherichia Coli K12 Based on Protein Expression by 2-Dimensional Electrophoresis and Enzyme Activity Measurement. Appl. Microbiol. Biotechnol. 2003, 61, 163–178. 10.1007/s00253-002-1202-6. [DOI] [PubMed] [Google Scholar]
- Fernandes I.; Faria A.; Calhau C.; de Freitas V.; Mateus N. Bioavailability of Anthocyanins and Derivatives. J. Funct. Foods 2014, 7, 54–66. 10.1016/j.jff.2013.05.010. [DOI] [Google Scholar]
- Fang J. Bioavailability of Anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. 10.3109/03602532.2014.978080. [DOI] [PubMed] [Google Scholar]
- Jones J. A.; Vernacchio V. R.; Collins S. M.; Shirke A. N.; Xiu Y.; Englaender J. A.; Cress B. F.; McCutcheon C. C.; Linhardt R. J.; Gross R. A.; Koffas M. A. G. Complete Biosynthesis of Anthocyanins Using E. coli Polycultures. mBio 2017, 8, e00621. 10.1128/mbio.00621-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.