Abstract
Saponins are potential wide-spectrum antitumor drugs, and copper(I) catalyzed azide–alkyne 1,3-dipolar cycloaddition is a suitable approach to synthesizing saponin-like compounds by regioselective glycosylation of the C2/C3 hydroxyl and C28 carboxylic groups of triterpene aglycones maslinic acid (MA) and oleanolic acid (OA). Biological studies on the T-84 human colon carcinoma cell line support the role of the hydroxyl groups at C2/C3, the influence of the aglycone, and the bulky nature of the substituents in C28. OA bearing a α-d-mannose moiety at C28 (compound 18) focused our interest because the estimated inhibitory concentration 50 was similar to that reported for ginsenoside Rh2 against colon cancer cells and it inhibits the G1–S phase transition affecting the cell viability and apoptosis. Considering that triterpenoids from natural sources have been identified as inhibitors of nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) signaling, docking studies were conducted to evaluate whether NF-κB may be a potential target. Results are consistent with the biological study and predict a similar binding mode of MA and compound 18 to the p52 subunit from NF-κB but not for OA. The fact that the binding site is shared by the NF-κB inhibitor 6,6-dimethyl-2-(phenylimino)-6,7-dihydrobenzo[d][1,3]oxathiol-4(5H)-one supports the result and points to NF-κB as a potential target of both MA and compound 18.
Introduction
Cancer is the second cause of death and morbidity in developed countries. According to the World Health Organization, about 8.8 million people worldwide died from cancer in 2015, which represents nearly one in six of all global death and an estimated economic cost of $1.16 trillion.1 Distribution of cancer is associated with socioeconomic development, the countries with a high human development index (HDI) being responsible for 41% of the cases, whereas low-HDI countries for only less than 6%. Despite this fact, the transition of low-income countries to higher development will have profound effects on the scale and profile of the cancer.2 Among the different cancers, colorectal cancer shows a strong correlation with lifestyle, and it can be considered as a marker of developmental transition and adoption of a western lifestyle.2,3 This is one of the three most common cancers expected in United States and is rising rapidly in low-income and middle-income countries.3,4
Saponins are secondary metabolites that are gaining increasing attention as potential wide-spectrum antitumor drugs because apart from provoking membrane permeabilization when administrated at high concentrations, at low concentrations, they show cytostatic, proapoptotic, and antimetastatic effects on tumor cells.5,6 From a chemical point of view, saponins consist of a nonpolar polycyclic part, referred to as aglycone or sapogenin, attached to one or more sugar side chains (i.e., glycone). On the basis of the chemical character of the aglycone and the number of directly bound sugar chains, saponins are divided into steroidal and triterpenoid saponins and categorized into monodesmosides (one chain), bisdesmosides (two chains), or trisdesmosides (three chains). Naturally occurring triterpenoid saponins mainly contain aglycones with 30 carbon atoms, the most commonly core structures being pentacyclic oleananes and tetracyclic dammaranes, and their antitumor activity has been proven.6,7 The degree of structural diversity and the fact that minor structural changes in the molecules may lead to a major difference in activity make the prediction of the biological activity from the structure–activity relationship a real challenge. Additionally, the isolation of saponins from plant material is difficult and time-consuming and the synthesis on industrial scale is far from trivial.8 In this context, β-hederin (oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-arabinopyranoside) has been a source of inspiration to synthesize different families of new active compounds.9,10
Interestingly, pentacyclic triterpenes present in the skin of the fruit of Olea europaea have been proposed to have a positive effect on colon tumors and, among them, maslinic acid (2α,3β-dihydroxyolean-12-ene-28-oic) (MA) and oleanolic acid (3β-hydroxyolean-12-ene-28-oic) (OA) stand out.11−15 The bioactivity of these triterpenes is not limited to antitumor activity but includes cardioprotective, anti-inflammatory, antioxidative, antileukemic, antithrombotic, antidiabetic, antihypertensive, antihyperlipidemic, immunomodulating, antiviral, antibacterial, and antiprotozoal activities.16−18 The low toxicity and the broad bioactivity spectrum of MA and OA make them attractive compounds for chemical modifications to improve the potency, selectivity, and/or pharmacokinetic parameters.18 Thus, OA and, to a lesser extent, MA have been modified in three “active portions”: the hydroxyl groups at C2 and/or C3, the C12–C13 double bond, and the C28 carboxylic group, many of the resulting molecules showing improved antitumor activity.19−25 However, the poor water solubility of triterpenoids is a key factor that limits their clinical application as therapeutic agents and PEGylation and glycosylation have been demonstrated to be successful strategies that improve water solubility and preserve the biological activity.26−28
Pentacyclic triterpenes and triterponoid saponins probably act at multiple levels although the target molecules are still being identified. Cumulative experimental and epidemiologic lines of evidence suggest a link between inflammation and cancer, and recent studies have demonstrated the relevance of chronic inflammation in colorectal cancer development.29 Of particular importance is the function of pro- and anti-inflammatory cytokines. Among them, the tumor necrosis factor activates nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB), inducing the expression of various genes that stimulate tumor-associated inflammation, including cyclooxygenase-2 (COX-2).29 In this context, the fact that triterpenoids from natural sources such as ginsenosides, glycyrrhizin, betulin, lupeol, and avicins have been identified as inhibitors of NF-κB signaling may be one of the keys to understand their effect at cellular level.30 This observation is especially relevant when taking into account that aberrant NF-κB regulation has been observed in many cancers, including colon, lung, breast, and prostate, that are the main contributors to the statistics of cancer worldwide, and the inhibition of NF-κB has been identified as a promising option to improve anticancer therapy.31−33
Herein, we describe the glycosylation of the triterpenes from olive fruit MA and OA by means of click chemistry to yield a short library of synthetic mono- and bidesmosides and their evaluation against the T-84 cell line (human colorectal carcinoma derived from lung metastasis) and docking with NF-κB.
Results and Discussion
Chemistry
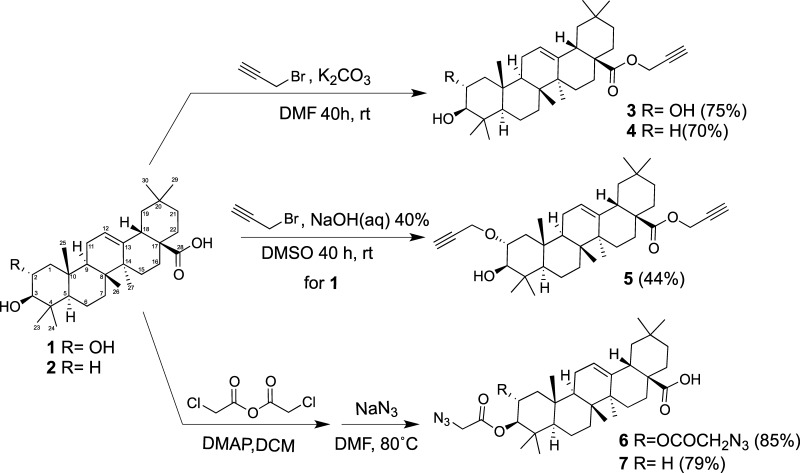
The cytostatic, proapoptotic, and antimetastatic effects of saponins on tumor cells make them potential wide-spectrum anticancer drugs.5,6 However, the degree of structural diversity and the fact that minor structural changes in the molecules may lead to major differences in the activity are obstacles for the systematic study of saponins. Because the pentacyclic triterpenes MA and OA have been proposed to have a positive effect on colon tumors and click chemistry is a very powerful tool in drug discovery, we envisaged the glycosylation of MA and OA via the copper(I)-catalyzed azide–alkyne 1,3-dipolar cycloaddition (CuAAC) as a feasible approach to synthesizing synthetic oleanane-type triterpenoids that may be biologically active.11−15,34,35 The rationale behind this strategy is that besides the general benefits of click chemistry the 1,2,3-triazole formed in CuAAC is more than a passive linker because it can form π–π interactions with aromatic rings and hydrogen bonds through the N(2) and N(3) nitrogen atoms, contributing to the interaction with the target molecule.34,35
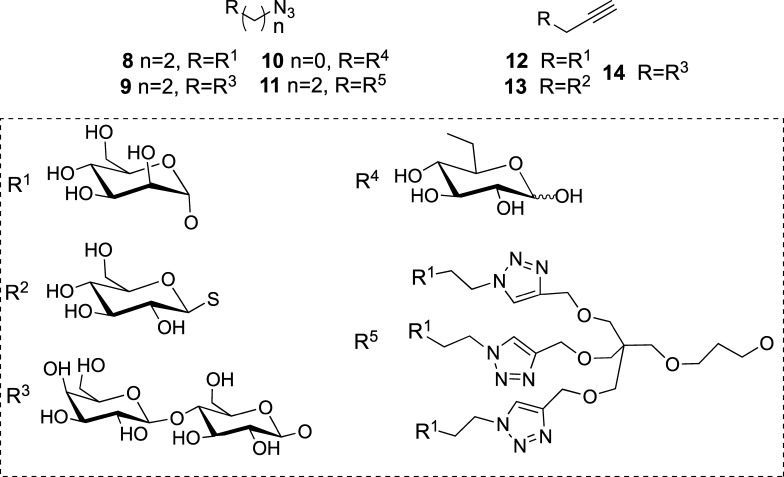
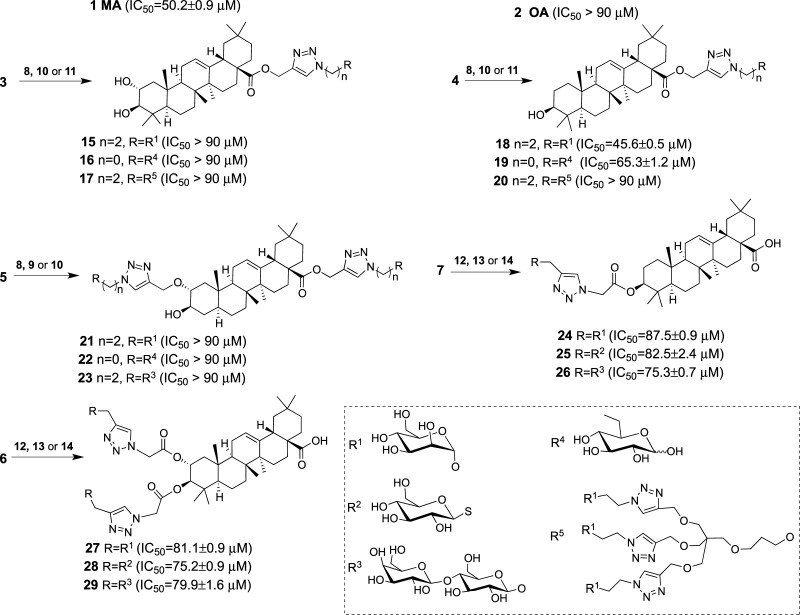
Our efforts were focused on the derivatization of the C2/C3 hydroxyl groups and the C28 carboxylic group, which according to the literature are “active portions” of the molecule.19 β-d-Lactose (Lac), α-d-mannose (Man), and β-d-glucose (Glc) were linked to aglycones MA and OA to obtain both monodesmoside- and bidesmoside-like saponins. Commercial MA (1) and OA (2) were chemoselectively propargylated in dimethylformamide (DMF)/K2CO3 at room temperature for 40 h to yield the corresponding esters 3 and 4 (Scheme 1). When the propargylation of MA was carried out in dimethyl sulfoxide (DMSO)/NaOH, bispropargylated compound 5 was obtained, as expected from the reported low reactivity of the OH group at C3.19 Alternatively, an azide group was introduced at these positions by acylation with chloroacetic anhydride, followed by nucleophilic displacement of the chlorine atom by azide to yield compounds 6 and 7 (Scheme 1). The counterparts clickable azide (8–11) and alkyne (12–14) sugars derived from monosaccharides Man and Glc, disaccharide Lac, and trivalent mannosylated dendron (Scheme 2) were synthesized following methodologies previously reported.36,37 Click assembly of complementary clickable triterperne (3–7) and sugar (8–14) partners was carried out in DMF using copper complex (EtO)3P·CuI as a soluble catalyst and under microwave irradiation (800 W, 80 °C, 15 min) to improve the yield and reduce the reaction time.20,38 Saponin-like compounds 15–29 were thus obtained with good yield (Scheme 3), and their structure was confirmed by NMR (Figures S4–S20). This short library comprises monodesmoside- and bidesmoside-like saponins with subtle differences derived from the aglycone (i.e., MA vs OA) as the pairs 15–18, 16–19, or 17–20 as well as others more divergent consequence of the increasing complexity of the glycone (monosaccharide, disaccharide, or trivalent dendron), its number (monodesmoside vs bisdesmoside), and its position (ring A vs C28) (Scheme 3).
Scheme 1. Introduction of Click Functions in MA (1) and OA (2).
Scheme 2. Structure of Clickable Azide (8–11) and Alkyne (12–14) Carbohydrates.
Scheme 3. Structure of the Synthetic Oleanane-type Triterpenoids (15–29) Obtained by the Reaction of MA (1) and OA (2) with the Clickable Carbohydrates (8–14).
In brackets are given the antiproliferative activities on the human colon carcinoma T-84 cell line expressed as the inhibitory concentration 50 (IC50) values resulting from the mean ± standard deviation of at least five independent measurements in the micromolar range.
Biological Assays
Saponins commonly found in herbs and in formulations traditionally used in Chinese medicine have been reported to exhibit promising anticancer potential.39 Among them, the triterpenoid saponins ginsenoside Rh2 and Rg3 and saikosaponin A show in vitro antiproliferative activity against colon cancer cells with the inhibitory concentration 50 (IC50) ranging from 100 to 20 μM.39 This fact led us to assay compounds 1 (MA), 2 (OA), and 15–29 on the T-84 cell line, a human colon carcinoma cell line derived from a lung metastasis of colorectal carcinoma. Their antiproliferative activity was evaluated by colorimetric quantification with sulforhodamine B.40,41 Results are included in Scheme 3. According to IC50, compounds can be grouped into three categories: (i) those with an attractive IC50, including MA and compound 18 with values of 50.2 ± 0.9 and 45.06 ± 0.6 μM, respectively, (ii) those with IC50 within the interval 60–90 μM, OA being at the border with IC50 89.4 ± 3.4 μM, and (iii) those with IC50 > 90 μM and considered as inactive. This different behavior of MA and OA has been reported in the literature for cancer cell lines EMT-6 (breast) and SW480 (colon), and this has been explained by the additional α-oriented hydroxyl group at C2 in MA (Scheme 3).22 The analysis of the synthetic oleanane-type triterpenoids revels that the modification of both hydroxyl groups of MA yielded compounds 27–29 with low antiproliferative activity, which is in the same order as that of compounds 24–26 obtained from the poorly active OA aglycone. These results support the role of the hydroxyl group at C2 of MA and suggest that the modification of the hydroxyl group at C3 confers some antiproliferative activity to those compounds derived from OA (compounds 24–26) and partially compensates the effect of the substitution at C2 of MA (compounds 27–29).
The C28 carboxyl group has been identified as one of the “active portions” of the molecule, and amide derivatization at C28 of β-hederin, a triterpenoid saponin whose aglycone is OA, results in highly cytotoxic compounds.9,19 Hence, the modification of C28 was envisaged as a synthetic approach to converting MA and OA into more toxic synthetic oleanane-type triterpenoids. Compounds 15–20 were synthesized, and their biological activity was found to be dependent on both the aglycone and the bulky nature of the substituent (Scheme 3). Thus, compounds 15–16 derived from MA are inactive, whereas their counterparts 18–19 from OA are active, with IC50 of 45.6 and 65.3 μM, respectively. The steric effect of the substituents is revealed by compounds 18–20 whose biological activity ranges from 45.6 μM for compound 18, with a single Man residue linked by the anomeric position, to the inactive compound 20, bearing a trivalent dendron. In addition, compound 19, with a Glc residue linked by C6 to test a different orientation of the glycopyranoside ring, is less active than compound 18. These results suggest that the modification of C28 disturbs the interaction of MA with its target, whereas it confers antiproliferative activity to OA by promoting either the interaction with different targets or an alternative interaction with the same target as MA.
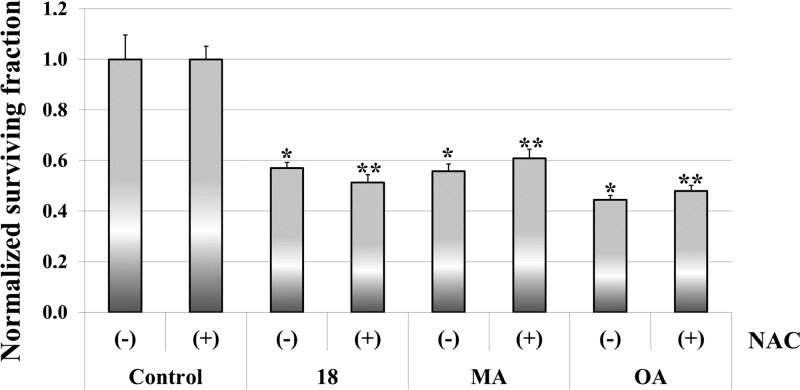
Compound 18 is more active than MA despite being derived from OA, in agreement with other authors who reported similar results for the PEGylated OA and MA.26,27 This fact attracted our interest, and further biological studies were carried out. Thus, to evaluate the effect of oxidative stress on the biological effect of compound 18, the survival rate of T-84 cells in the presence or absence of the antioxidant N-acetyl-l-cysteine (NAC) was determined by the sulforhodamine B colorimetric assay.42 NAC is an antioxidant frequently used as a tool for investigating the role of the reactive oxygen species (ROS) in biological processes.43,44 As shown in Figure 1, NAC does not confer protection to T-84 carcinoma cells lines treated either with compound 18, MA, or OA, discarding the oxidative stress as the main reason responsible for the biological action.
Figure 1.
Effect of NAC on the survival of the human colon carcinoma T-84 cell line. Cells were treated with either compound 18, OA, or MA alone (−) or in combination with 2 mM NAC (+). Values are normalized by their control counterparts. Symbols * and ** denote a significant difference with control (−) or control (+) groups, respectively, p < 0.05.
This result was expected because according to bibliography MA exerts a dual activity acting as a copper chelator and a scavenger of free radicals, whereas in vitro studies with several breast cell lines have shown the protective effect of OA on MCF10A cells (nontumoral cell line) by reducing the ROS levels in the basal state.45−48 In contrast to our results, it has been reported that NAC reverses the inhibitory effect of OA in hepatocellular carcinoma cells, but this discrepancy can be rationalized when considering that the authors of the study concluded that OA triggered a new autophagic cell death pathway in human hepatocellular carcinoma cells.49
Because the cell cycle machinery is one of the most important chemotherapeutic targets, we evaluated the cell cycle distribution of T-84 cells by flow cytometry after 24 h of induction with three different concentrations (0.5, 1, or 2 times the corresponding IC50 value) of compounds 18, MA, or OA.50,51 Results are depicted in Table 1, and the representative flow cytograms are included in the Supporting Information as Figure S1.
Table 1. Effect of Compound 18, MA, and OA on the Cell Cycle of T-84 Cells after 24 h of Induction with 0.5, 1, or 2 Times the Corresponding IC50 Valuea.
| treatment | times IC50 | sub-G1 (%) | G0–G1 (%) | S (%) | G2–M (%) |
|---|---|---|---|---|---|
| control | 2.2 ± 0.7 | 46.1 ± 1.4 | 19.9 ± 2.0 | 31.8 ± 3.0 | |
| compound 18 | 0.5× | 2.2 ± 0.5 | 46.1 ± 2.5 | 20.7 ± 5.0 | 30.9 ± 2.3 |
| 1.0× | 3.8 ± 0.4 | 67.6 ± 1.6* | 5.6 ± 1.9* | 23.0 ± 0.4* | |
| 2.0× | 5.1 ± 0.2* | 67.2 ± 1.3* | 4.4 ± 1.2* | 23.3 ± 0.7* | |
| MA | 0.5× | 2.3 ± 0.1 | 61.8 ± 3.2* | 13.2 ± 1.0* | 22.6 ± 2.1* |
| 1.0× | 4.3 ± 0.7* | 73.6 ± 2.2* | 7.1 ± 1.9* | 15.0 ± 0.9* | |
| 2.0× | 12.6 ± 1.2* | 55.4 ± 1.3* | 6.3 ± 1.9* | 25.6 ± 2.3* | |
| OA | 0.5× | 3.1 ± 1.0 | 68.7 ± 2.9* | 11.1 ± 2.0* | 17.1 ± 0.5* |
| 1.0× | 19.0 ± 0.7* | 54.5 ± 0.4* | 3.1 ± 0.6* | 23.4 ± 0.9* | |
| 2.0× | 45.8 ± 0.9* | 40.9 ± 5.2 | 8.1 ± 4.4* | 5.2 ± 1.6* |
Data are mean ± standard error of the mean of three independent determinations. Symbol * denotes a significant difference with the control group, p < 0.05.
The percentages of control cells (i.e., untreated) after 24 h were 2.2% in sub-G1, 46.1% in G0–G1, 19.9% in S, and 31.8% in G2–M. In general, the three compounds assayed provoked an effect on the cell cycle distribution in a dose-dependent manner to yield an increase in the sub-G1 phase and a decrease in S and G2–M phases, although each compound showed particular features. When cells were treated with compound 18 at a concentration of 0.5 × IC50, the cell cycle distribution remained as that of the control cells and concentrations of 1 × IC50 and 2 × IC50 provoked a similar effect, yielding an increase in G0–G1 (67%) and a decrease in S and G2–M phases (around 4–5 and 23%, respectively), without important changes in the sub-G1 population. MA led to appreciable changes in the cell cycle even at 0.5 × IC50, with a dose-dependent G0–G1 accumulation (61.8 and 73.6%, respectively) and a reduction in the percentage of cells in the two other phases. The treatment with MA at 2 × IC50 yielded an increase of sub-G1 (12.6%) and G0–G1 (55.4%) and a reduction in S and G2–M phases, in agreement with the data reported for HT-29 and Caco-2 colorectal cells.12−14,52,53 Analogous to MA, OA induction also provoked changes in the cell cycle at 0.5 × IC50, but unlike MA, the main feature of the cell cycle was the marked dose-dependent increment of sub-G1, from 3.1% (0.5×) to 45.8% (2×) accompanied by an initial increment in G0–G1 at 0.5× that lowered to reach at 2× the values of the control cell and an overall reduction of S and G2–M phases.
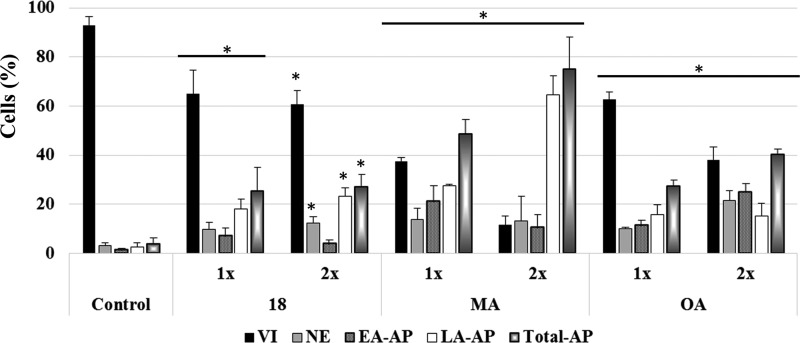
These results show that MA, OA, and compound 18 are able to inhibit G1–S phase transition. However, although the three compounds increased the sub-G1 fraction at 24 h, the values induced by OA are especially significant and in agreement with the reported induction of apoptosis and inhibition of cell proliferation.54 Interestingly, whereas OA induces a large extent of apoptosis at a concentration 2 × IC50, the cell-cycle profile of the cells treated with compound 18 remains almost invariant. To confirm whether the fraction of sub-G1 cells was due to apoptosis promotion, the viability of cells induced for 48 h with different concentrations of 18, MA, and OA was evaluated by flow cytometry with annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (Figure 2).
Figure 2.
Effects of compound 18, MA, and OA on the viability of T-84 cells induced with 0.5, 1, or 2 times the IC50 value for 48 h. Percentages of viable cells (VI), necrotic cells (NE), early apoptotic cells (EA-AP), late apoptotic cells (LA-AP), and total apoptotic cells (total-AP) are shown. The experiment was repeated independently three times yielding similar results. Symbol * denotes p < 0.05 vs control cells (i.e., untreated).
The treatment with compound 18 reduced the cell viability from 92.9% for the control group to 64.9 and 60.63% for the cultures treated with 1 and 2 times the IC50, respectively. This reduction of the viability was associated with an increase in the fraction of cells in necrosis, early apoptosis, and late apoptosis, the total apoptosis being increased from 3.8% for the control group to 25.2 and 27.1% for the cultures treated with 1 and 2 times the IC50, respectively, revealing that both cell viability and apoptosis remain almost unaffected when the dose of compound 18 is doubled. However, cultures treated with MA or OA experienced a dose-dependent reduction of cell viability, concomitant with an increment of total apoptosis that was more accentuated with MA. MA induced 48.6 and 75% of total apoptosis at 1× and 2×, respectively, whereas OA induced 27.3 and 40.2%, respectively. These results are in agreement with the studies that demonstrate that MA and OA, either alone or in combination with MA, inhibit cell proliferation, causing apoptotic death in HT-29 cells and that OA does not lead to apoptosis in other cell lines, such as the colon carcinoma HCT-15, despite the success of 3-O-acetyloleanolic acid on HCT-116.11,12,54−58
Docking Studies
Recent studies have demonstrated the importance of chronic inflammation in colorectal cancer development. Although the molecular mechanisms are not fully understood, NF-κB and cyclooxygenase-2 (COX-2) have been identified as potential therapeutic targets.29−33,59 Triterpenoids from natural sources such as ginsenosides, glycyrrhizin, betulin, lupeol, and avicins have been identified as inhibitors of NF-κB signaling, and this may be one of the keys to understand their effect at cellular level.30 Additionally, MA has been reported to suppress COX-2 expression at concentrations that also lower the activity of NF-κB, suggesting that the effect on NF-κB may lead to the downregulation of different genes, including COX-2.60,61 In this context, we hypothesized that the biological effect of MA and compound 18 might be a consequence of the interaction with NF-κB and envisaged docking as the approach to put to test the hypothesis.
The docking approach is not exempt of difficulty because, despite the fact that the output results are ranked by a scoring function, the identification of the correct solution may be far from trivial, and for this reason, it is customary to assess the goodness of the scoring function by redocking ligands that have been cocrystallized with the target.62 However, to the best of our knowledge, no structure of NF-κB bound to molecules similar to MA has been reported and an additional difficulty is the fact that NF-κB is a family of homo- and heterodimeric proteins resulting from the association of five different subunits (p50/p105 (NF-κB1), p52/p100 (NF-κB2), RelA (p65), c-Rel (v-Rel being the viral oncogenic version), and RelB), the two main NF-κB dimers being p50:RelA and p52:RelB heterodimers.63 Hence, as a preliminary step, the binding of MA and OA to the entire surface of p50:RelA (protein data bank (PDB) code 1vkx) and p52:v-Rel (PDB code 3do7) was computed. Calculations did not reach any solution for p50:RelA, and the scoring function of the docking of MA and OA on p52:v-Rel revealed two significant solutions for MA and one for OA (Table 2).
Table 2. Results of the Best Poses of the Docking of MA, OA, Compound 18, and NF-κB Inhibitor 6,6-Dimethyl-2-(phenylimino)-6,7-dihydrobenzo[d][1,3]oxathiol-4(5H)-one (BOT) with p52:v-Rel and a Comparison with Experimental IC50 for the Human Colon Carcinoma T-84 Cell Line.
| compound | ranka | fullfitnessb | binding site | ΔGc | estimated Kd (μM) | experimental IC50 (μM) |
|---|---|---|---|---|---|---|
| MA | 0 0 | –4218.29 | p52 | –8.825026 | 0.34 | 50.2 |
| 1 2 | –4202.93 | v-Rel | –6.383734 | 20.81 | ||
| OA | 0 0 | –4225.85 | v-Rel | –6.554255 | 15.61 | 89.4 |
| BOT | 0 0 | –4286.02 | p52 | –7.619894 | 2.58 | |
| 18 | 0 0 | –4156.36 | p52 | –9.658060 | 0.08 | 45.6 |
Rank resulting from the scoring function.
Total energy of the system including solvation.
Computed ΔG of the interaction.
The graphical analysis of the solutions revealed that MA is predicted to bind both p52 and v-Rel, the former being the best solution, whereas OA shares the binding mode of MA with subunit v-Rel (Figure S2). The fact that only the experimental antiproliferative activity of MA was significant led us to hypothesize that binding to v-Rel was not biologically relevant. This hypothesis was put to test by docking 6,6-dimethyl-2-(phenylimino)-6,7-dihydrobenzo[d][1,3]oxathiol-4(5H)-one (BOT), a newly discovered NF-κB inhibitor with antiproliferative effect.64 The solution with the highest score predicted the binding of BOT to p52 in the same site as MA, supporting the hypothesis that the binding to p52 is biologically relevant (Figure S3).
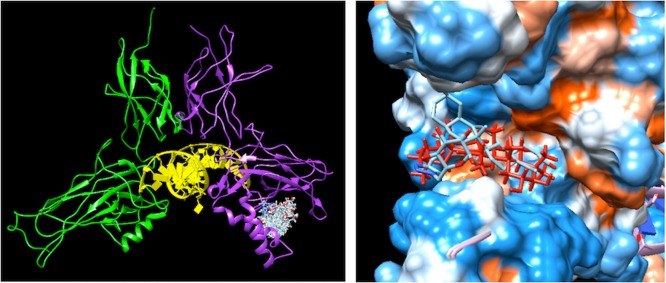
The docking of compound 18 to the entire surface of p52:v-Rel also predicted the binding to p52, at the same site as MA (Figure 3).
Figure 3.
Left: poses resulting from the docking of compound 18 on p52:v-Rel (PDB accession code 3do7). Subunit v-Rel is shown in green, subunit p52 in purple, and DNA in yellow. Right: alignment of the top score solution of MA (red) and compound 18 (cyan). The protein is shown as a surface colored according to the hydrophobicity on the Kyte–Doolittle scale, ranging from dodger blue, for the most hydrophilic, to white and orange red, for the most hydrophobic.
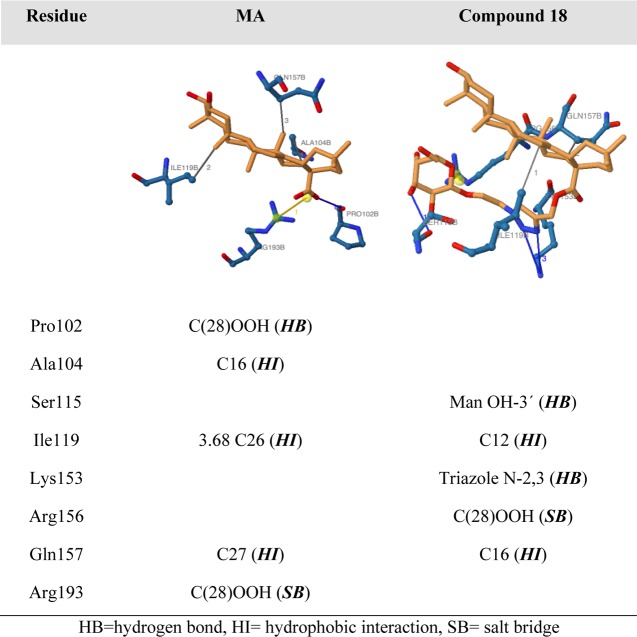
The analysis of the interactions (Table 3) reveals that MA and compound 18 share the hydrophobic interactions of the triterpenic aglycone with Lys119 and Gln157 and the salt bridge between the carboxyl group at C28 and an Arg residue. Compound 18 also forms three additional hydrogen bonds, one between the hydroxyl groups in position 3 of the mannose ring and two between nitrogen atoms of the triazole ring and Lys153, suggesting that not only the carbohydrate but also the triazole linker plays a role in the interaction.
Table 3. Analysis of the Interactions between p52 and MA and Compound 18a.
HB = hydrogen bond, HI = hydrophobic interaction, SB = salt bridge.
The Kd of the interaction was estimated from the computed values of ΔG (Table 2). Although these estimated values may differ considerably from the experimental dissociation constants, they make the evaluation of the relative affinity possible. The docking results of MA predict a lower affinity for v-Rel than for p52, and this low affinity is shared by OA, being consistent with the poor antiproliferative activity of OA. Interestingly, the Kd values predicted for MA and compound 18 are 0.34 and 0.08 μM, respectively, suggesting that compound 18 is more related to MA than to OA, as supported by the cytometric study.
Conclusions
CuAAC is a suitable approach for the regioselective modification of the C2/C3 hydroxyl and C28 carboxylic groups of the triterpene aglycones MA and OA to yield a short library of saponin-like compounds. The cytotoxicity assays reveal different behaviors of OA and MA and suggest that the modification of the hydroxyl group at C3 of these compounds confers some antiproliferative activity to the compounds derived from OA. The cytotoxicity of the compounds resulting from the click modification of the C28 carboxyl group is dependent on the aglycone and on the bulky nature of the substituent, those derived from OA that link a monosaccharide (Man or Glc) being active. In particular, OA bearing a Man moiety at C28 (compound 18) is the most cytotoxic on the human colon carcinoma T-84 cells, and it may have potential pharmacological interest, with IC50 in the range of that reported for ginsenoside Rh2. Biological assays show that although compound 18 is derived from the poorly active OA aglycone, it shares with MA the effect on the cell cycle, inhibiting DNA replication and G1–S phase transition, and unlike OA, it does not promote apoptosis to a large extent. Docking experiments suggest that the biological activity may be related to the binding to NF-κB. Both MA and compound 18 share with BOT the binding mode to the p52 subunit of NF-κB, and the predicted affinities are in agreement with the experimental IC50 values.
Experimental Section
General Chemistry Methods
All reagents and solvents were used as obtained from commercial sources unless otherwise indicated. All microwave reactions were performed in a Milestone Star Microwave Labstation at 500 W. Thin-layer chromatography (TLC) was performed on Merck Silica Gel 60 F254 aluminum sheets, and TLC plates were stained with ceric sulfate (1% w/v) and ammonium sulfate (2.5% w/v) in 10% (v/v) aqueous sulfuric acid or ethanolic sulfuric acid (10% v/v). 1H and 13C NMR spectra were recorded at room temperature on a Varian Direct Drive (400 or 500 MHz) spectrometer. Chemical shifts are given in ppm and referenced to the signal of the residual protonated solvent (1H: δ = 2.50 for DMSO-d6, 13C: δ = 39.10 for DMSO-d6). Electrospray ionization (ESI) mass spectra were recorded with a Waters LCT Premier XE spectrometer. Melting points were measured with a Gallenkamp melting point apparatus and are uncorrected. Optical rotations were recorded on a PerkinElmer 341 polarimeter at room temperature. IR spectra were recorded with a PerkinElmer spectrum two Fourier transform infrared attenuated total reflection spectrometer.
Synthetic Procedures
General Procedure for the Synthesis of Mono Alkynyl Derivatives of MA and OA (3 and 4)
To a solution of MA or OA (96%, 1 mmol) in DMF (20 mL) was added propargyl bromide (80 wt % toluene, 9.3 mmol) and potassium carbonate (2.5 mmol). The obtained suspension was stirred at room temperature for 40 h, and the solvent was removed under vacuum. The residue was dissolved in CH2Cl2 (30 mL) and washed successively with H2O, 5% HCl solution, and H2O. The organic layer was dried with anhydrous Na2SO4 and filtered, and the solvent was removed under reduced pressure. The crude was purified by column chromatography.
Compound 3
Column chromatography (CH2Cl2/MeOH, 20:1) yields 3 as a white solid (327 mg, 75%). The spectroscopic data match those reported in the literature.20
Compound 4
Column chromatography (CH2Cl2/MeOH, 40:1) yields 4 as a white solid; (350 mg, 70%). The spectroscopic data match those reported in the literature.21
Synthesis of Dialkynyl Derivative of MA (5)
To a solution of MA (96%, 825 mg, 1.68 mmol) in DMSO (8 mL) was added propargyl bromide (80 wt % toluene, 37.2 mmol) and NaOH (40% solution, 8 mL). The obtained suspension was stirred at room temperature for 40 h. The resulting mixture was diluted with Et2O/toluene 1:1 (50 mL) and washed with H2O (3 × 25 mL). The organic layer was dried with anhydrous Na2SO4 and filtered, and the solvent was removed under reduced pressure. The crude was purified by column chromatography (Et2O/hexane, 1:2), yielding 5 as a white solid (350 mg, 70%). The spectroscopic data match those reported in the literature.20
General Procedure for the Synthesis of Azide Derivatives of MA and OA (6 and 7)
A solution of MA or OA (96%, 1 mmol), chloroacetic anhydride (1.70 g, 10 mmol), and 4-(dimethylamino)pyridine (196 mg, 1.6 mmol) in CH2Cl2 (20 mL) was stirred at room temperature for 16 h. The resulting mixture was diluted with CH2Cl2 (20 mL) and washed with H2O (25 mL). The organic layer was dried with anhydrous Na2SO4, and the solvent was removed under vacuum to yield a colorless syrup. The crude and NaN3 (520 mg, 8 mmol) were dissolved in DMF (20 mL) and heated at 80 °C overnight. The solvent was removed under reduced pressure and purified by column chromatography.
Compound 6
Column chromatography (CH2Cl2/MeOH, 40:1) yields 6 as a white solid (543 mg, 85%); mp 169–170 °C; [α]D +14.4 (c 1, Cl3CH); IR (neat): υ̅ = 2944, 2105, 1740, 1691, 1461, 1364, 1281, 1182 cm–1; 1H NMR (500 MHz, DMSO-d6) δ 12.0 (brs, 1H), 5.16 (brs, 1H), 5.07 (ddd, J = 11.1, 10.4, and 4.5 Hz, 1H), 4.81 (d, J = 10.4 Hz, 1H), 4.18 (s,1H), 4.05 (d, J = 17.3 Hz, 1H), 4.01 (d, J = 17.3 Hz, 1H), 2.75 (dd, J = 13.8, 3.8 Hz, 1H), 2.02–0.70 (m, 20H), 1.14 (s, 3H), 1.02 (s, 3H), 0.89 (bs, 12H), 0.73 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 178.54, 168.42, 168.23, 143.95, 121.09, 80.81, 70.88, 53.47, 49.49, 49.47, 46.46, 45.65, 45.42, 42.83, 41.42, 40.76, 39.15, 38.89, 37.81, 33.31, 32.82, 32.07, 31.96, 30.40, 27.92, 27.19, 25.62, 23.35, 22.96, 22.57, 17.74, 17.34, 16.75, 16.04; high-resolution mass spectrometry (HRMS) (ESI+): m/z calcd for C34H50N6O6 [M]+: 639.3870; found: 639.3849.
Compound 7
Column chromatography (CH2Cl2) yields 7 as a white solid (425 mg, 79%); mp 216–217 °C; [α]D +59.2 (c 1, Cl3CH); IR (neat): υ̅ = 2941, 2103, 1741, 1690, 1463, 1357, 1281, 1195 cm–1; 1H NMR (500 MHz, DMSO-d6) δ 12.0 (brs, 1H), 5.16 (t, J = 3.3 Hz, 1H), 4.52 (dd, J = 11.5 and 4.4 Hz, 1H), 4.14 (d, J = 16.9 Hz, 1H), 4.09 (d, J = 17.2 Hz, 1H), 2.74 (dd, J = 13.8 and 4.0 Hz, 1H), 1.97–1.87 (m, 1H), 1.82 (dd, J = 8.8 and 3.1 Hz, 2H), 1.71–1.20 (m, 22H), 1.11 (s, 3H), 1.10–0.96 (m, 3H), 0.90 (s, 3H), 0.87 (bs, 6H), 0.85 (s, 3H), 0.83 (s, 3H), 0.73 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 178.54, 168.39, 143.85, 121.37, 81.77, 54.40, 49.69, 46.79, 45.66, 45.44, 41.34, 40.79, 38.85, 37.44, 37.36, 36.46, 33.30, 32.81, 32.19, 32.07, 30.38, 27.69, 27.20, 25.56, 23.36, 23.12, 22.87, 22.60, 17.76, 16.79, 16.58, 15.16, 15.03; HRMS (ESI–): m/z calcd for C32H48N3O4 [M – H]−: 538.3645; found: 538.3621.
General Procedure for Microwave-Assisted Click Reaction of MA and OA with Sugars
Alkyne (3, 4, or 5) or azide (6 or 7) derivate of MA or OA (0.07–0.19 mmol) was reacted with complementary clickable azide (8–10) and alkyne sugar (12–14) derivatives (1.2 equiv per clickable function) in DMF (5 mL) in the presence of (EtO)3P·CuI as a soluble catalyst (0.1 equiv per clickable function) under microwave irradiation (800 W, 80 °C) for 15 min. The solvent was removed under reduced pressure.
Compound 15
This was prepared according to the general procedure from 3 (80 mg, 0.157 mmol) and 8 (47 mg, 0.188 mmol) and purified by column chromatography (CH2Cl2/MeOH, 4:1) to yield a white solid (152 mg, 96%); mp 139–141 °C; [α]D +38.4 (c 0.25, py); IR (neat): υ̅ = 3376, 2943, 1714, 1458, 1364, 1229, 1137, 1093, 1050 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 7.96 (s, 1H), 5.12 (brs, 1H), 5.04 (d, J = 12.9 Hz, 1H), 5.01 (d, J = 12.9 Hz, 1H), 4.60 (d, J = 1.1 Hz, 1H), 4.56–4.43 (m, 2H), 3.90 (m, 1H), 3.82–3.66 (m, 1H), 3.56 (dd, J = 11.7 and 1.9 Hz, 1H), 3.52 (s, 1H), 3.42–3.33 (m, 5H), 3.08 (m, 1H), 2.71 (d, J = 9.4 Hz, 2H), 1.99–0.68 (several m, 20H), 1.03 (s, 3H), 0.86 (s, 3H), 0.84 (s, 3H), 0.82 (bs, 6H), 0.66 (s, 3H), 0.42 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 176.91, 143.64, 142.35, 125.41, 122.26, 108.96, 100.03, 82.50, 79.89, 76.94, 74.15, 70.98, 70.30, 69.67, 67.51, 66.90, 65.25, 63.50, 61.28, 57.34, 55.48, 55.09, 49.70, 47.37, 47.08, 46.43, 45.77, 41.63, 41.31, 39.21, 37.93, 33.49, 33.12, 32.62, 32.30, 30.69, 29.12, 27.38, 25.93, 23.69, 23.36, 22.85, 18.36, 17.46, 16.76, 16.64; HRMS (ESI+): m/z calcd for C41H66N3O10 [M + H]+: 760.4689; found: 760.4722.
Compound 16
This was prepared according to the general procedure from 3 (100 mg, 0.196 mmol) and 10 (48 mg, 0.235 mmol) and purified by column chromatography (CH2Cl2/MeOH, 3:1) to yield a white solid (119 mg, 85%); mp 173–174 °C; [α]D (c 0.25, py) +67.6; IR (neat): υ̅ = 3365, 2944, 2103, 1725, 1461, 1365, 1260, 1232, 1159, 1050 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) (Glc β-anomer 55%, Glc α-anomer 45%) δ 7.90 (s, 0.45H), 7.86 (s, 0.55H), 5.13 (d, J = 3.5 Hz, 1H), 5.03 (m, 2H), 4.86 (d, J = 3.6 Hz, 0.55H), 4.62 and 4.35 (2m, 2H), 4.24 (d, J = 7.8 Hz, 0.45H), 3.95–3.87 (m, 0.55H), 3.54–2.87 (several m, 3.45H), 1.93–0.67 (several m, 22H), 1.02 (s, 3H), 0.87 (s, 3H), 0.84 (s, 3H), 0.82 (s, 6H), 0.66 (s, 3H), 0.41 (s, 1.65H), 0.38 (s, 1.35H); 13C NMR (151 MHz, DMSO-d6) δ 177.04, 143.74, 142.39, 142.30, 125.94, 125.90, 122.37, 97.08, 92.68, 82.59, 76.31, 74.80, 74.47, 72.87, 72.29, 71.79, 71.52, 70.14, 67.61, 57.50, 55.17, 51.40, 47.45, 47.14, 46.53, 45.84, 41.72, 41.41, 38.02, 33.56, 33.19, 32.70, 32.39, 30.76, 29.20, 27.45, 26.01, 23.77, 23.43, 22.94, 18.44, 17.54, 16.88, 16.70; HRMS (ESI+): m/z calcd for C39H62N3O9 [M + H]+: 716.4486; found: 716.4484.
Compound 17
This was prepared according to the general procedure from 3 (54 mg, 0.105 mmol) and 11 (106 mg, 0.096 mmol) and purified by column chromatography (acetonitrile/H2O, 4:1) to yield a white solid foam (114 mg, 74%); mp 121–123 °C; [α]D +26.0 (c 0.25, py); IR (neat): υ̅ = 3333, 2924, 1737, 1365, 1217, 1091, 1052 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 7.93 (s, 4H), 5.11 (brs, 1H), 5.03 (d, J = 12.8 Hz, 1H), 4.99 (d, J = 12.8 Hz, 1H), 4.59 (s, 3H), 4.56–4.41 (m, 8H), 4.40 (s, 6H), 3.80–3.70 (m, 6H), 3.61–3.50 (m, 6H), 3.45–3.22 (m, 22H), 3.16–3.03 (m, 4H), 2.71 (d, J = 9.3 Hz, 2H), 1.94–1.82 (m, 2H), 1.72 (m, 4H), 1.90-0.54 (several m, 20H), 1.01 (s, 3H), 0.86 (s, 3H), 0.81 (s, 3H), 0.80 (s, 3H), 0.79 (s, 3H), 0.65 (s, 3H), 0.39 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 177.35, 144.82, 143.93, 142.62, 125.73, 124.77, 122.51, 100.26, 82.80, 74.23, 71.18, 71.00, 70.54, 69.96, 69.71, 69.27, 69.09, 67.87, 67.10, 65.58, 64.61, 61.48, 57.60, 55.32, 50.18, 50.04, 47.61, 47.25, 46.73, 46.02, 45.57, 41.88, 41.58, 38.17, 33.74, 33.34, 32.84, 32.57, 30.91, 29.34, 27.64, 26.16, 23.92, 23.60, 23.11, 18.61, 17.68, 17.00, 16.84; HRMS (ESI+): m/z calcd for C75H122N12O2 [M + H]+: 1622.9814; found: 1622.9813.
Compound 18
This was prepared according to the general procedure from 4 (90 mg, 0.183 mmol) and 8 (38 mg, 0.153 mmol) and purified by column chromatography (CH2Cl2/MeOH, 10:1) to yield a white solid (105 mg, 92%); mp 213–214 °C; [α]D +51.6 (c 0.25, py); IR (neat): υ̅ = 3382, 2942, 1714, 1462, 1387, 1251, 1161, 1135, 1092, 1048, 1027 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 8.00 (s, 1H), 5.13 (brs, 1H), 5.06 (d, J = 12.7 Hz, 1H), 5.02 (d, J = 12.6 Hz, 1H), 4.78 (m, 1H), 4.60 (s, 1H), 4.52 (m, 2H), 3.91 (m, 1H), 3.76 (m, 1H), 3.51 (s, 1H), 3.40 (dd, J = 10.8 and 5.4 Hz, 1H), 3.35 (m, 2H), 3.11 (m, 1H), 2.96 (m, 1H), 2.74 (brd, J = 10.1 Hz, 1H), 1.90–0.64 (m, 22H), 1.04 (s, 3H), 0.86 (s, 3H), 0.84 (bs, 6H), 0.81 (s, 3H), 0.65 (s, 3H), 0.47 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 176.86, 143.60, 142.35, 125.42, 122.40, 100.12, 77.27, 74.29, 71.05, 70.36, 66.98, 65.28, 61.37, 57.40, 55.22, 49.71, 47.45, 46.46, 45.81, 41.64, 41.37, 39.23, 38.76, 38.52, 36.95, 33.53, 33.16, 32.76, 32.34, 30.74, 28.63, 27.48, 27.20, 25.94, 23.75, 23.32, 22.91, 18.37, 16.81, 16.43, 15.52; HRMS (ESI+): m/z calcd for C41H66N3O9 [M + H]+: 744.4799; found: 744.4794.
Compound 19
This was prepared according to the general procedure from 4 (100 mg, 0.202 mmol) and 10 (50 mg, 0.243 mmol) and purified by column chromatography (CH2Cl2/MeOH, 5:1) to yield a white solid (118 mg, 84%); mp 170–171 °C; [α]D +72.4 (c 0.25, py); IR (neat): υ̅ = 3387, 2942, 1726, 1463, 1388, 1361, 1253, 1159, 1048 cm–1; 1H NMR (600 MHz, DMSO-d6, D2O exchange) (Glc β anomer 55%, Glc α anomer 45%) δ 7.91 (s, 0.45H), 7.87 (s, 0.55H), 5.13 (d, J = 3.6 Hz, 1H), 5.08–4.99 (m, 2H), 4.86 (d, J = 3.6 Hz, 0.55H), 4.69–4.56 (m, 1H), 4.34 (m, 1H), 4.24 (d, J = 7.8 Hz, 0.45H), 3.95–3.89 (m, 0.55H), 3.52–3.47 (m, 0.45H), 3.45 (t, J = 9.2 Hz, 0.55H), 3.21–3.14 (m, 0.45H), 3.09 (dd, J = 9.6 and 3.6 Hz, 0.55H), 3.00–2.94 (m, 1H), 2.90 (m, 1H), 2.72 (m, 1H), 1.95–0.64 (m, 22H), 1.01 (s, 3H), 0.85 (s, 3H), 0.83 (bs, 6H), 0.62 (s, 3H), 0.80 (s, 3H), 0.45 (s, 1.65H), 0.42 (s, 1.35H); 13C NMR (151 MHz, DMSO-d6) δ 177.25, 143.77, 142.46, 126.05, 122.61, 97.15, 92.77, 77.63, 76.39, 74.89, 74.56, 72.98, 72.37, 71.85, 71.60, 70.22, 57.59, 55.39, 51.50, 47.62, 46.68, 45.97, 41.78, 41.54, 38.89, 38.68, 37.08, 33.67, 33.30, 32.91, 32.50, 30.86, 28.74, 27.61, 27.21, 26.09, 23.87, 23.46, 23.07, 18.52, 16.98, 16.93, 16.54, 15.66, 15.62; HRMS (ESI+): m/z calcd for C39H62N3O8 [M + H]+: 700.4537; found: 700.4523
Compound 20
This was prepared according to the general procedure from 4 (38 mg, 0.076 mmol) and 11 (77 mg, 0.069 mmol) and purified by column chromatography (SiO2, acetonitrile/H2O, 5:1) to yield a white solid foam (88 mg, 80%); mp 102–104 °C; [α]D +16.2 (c 0.25, py); IR (neat): υ̅ = 3331, 2922, 1735, 1363, 1215, 1089, 1050 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 7.92 (s, 3H), 7.90 (s, 1H), 5.09 (s, 1H), 5.02 (d, J = 12.9 Hz, 1H), 4.97 (d, J = 12.7 Hz, 1H), 4.59 (bs, 3H), 4.56–4.41 (m, 8H), 4.39 (s, 6H), 3.90 (m, 3H), 3.78–3.70 (m, 6H), 3.58–3.51 (m, 6H), 3.45–3.24 (m, 22H), 3.21 (s, 3H), 3.07 (m, 3H), 2.96 (m, 1H), 2.67 (m, 2H), 2.63 (m, 1H), 2.36 (m, 1H), 1.90–0.54 (several m, 20H), 0.99 (s, 3H), 0.81 (s, 3H), 0.79 (s, 3H), 0.78 (s, 3H), 0.74 (s, 3H), 0.60 (s, 3H), 0.37 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 177.73, 145.05, 144.03, 142.84, 125.94, 125.00, 122.81, 100.35, 78.05, 74.24, 71.30, 71.14, 70.69, 70.15, 69.39, 69.21, 67.20, 65.74, 64.71, 61.58, 57.73, 55.56, 50.39, 50.25, 47.81, 46.96, 45.72, 41.99, 41.76, 40.19, 40.15, 40.07, 39.98, 39.94, 39.77, 39.73, 39.52, 39.31, 39.10, 38.96, 38.89, 37.26, 33.87, 33.47, 32.75, 31.04, 28.90, 26.26, 24.06, 23.27, 18.71, 17.14, 16.70, 15.81; HRMS (ESI+): m/z calcd for C75H122N12O26 [M + 2H]2+: 803.4296; found: 803.4852.
Compound 21
This was prepared according to the general procedure from 5 (50 mg, 0.091 mmol) and 8 (50 mg, 0.200 mmol) and purified by column chromatography (CH2Cl2/MeOH, 4:1) to yield a white solid (80 mg, 84%); mp 132–134 °C; [α]D +24.4 (c 0.25, py); IR (neat): υ̅ = 3356, 2942, 2104, 1722, 1455, 1363, 1260, 1230, 1136, 1092, 1054 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 7.97 (s, 1H), 7.96 (s, 1H), 5.12 (brs, 1H), 5.05 (d, J = 12.7 Hz, 1H), 5.01 (d, J = 12.8 Hz, 1H), 4.62–4.43 (m, 8H), 3.75 (m, 2H), 3.68–3.50 (m, 4H), 3.46–3.29 (m, 8H), 3.06 (m, 2H), 2.87 (d, J = 9.4 Hz, 1H), 2.71 (d, J = 10.1 Hz, 1H), 1.89–0.60 (several m, 20H), 1.01 (s, 3H), 0.88 (s, 3H), 0.83 (s, 3H), 0.82 (bs, 6H), 0.69 (s, 3H), 0.40 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 177.41, 145.65, 143.91, 142.69, 125.76, 124.78, 122.52, 100.21, 81.39, 77.18, 74.25, 74.24, 71.20, 71.15, 70.54, 67.11, 66.39, 65.57, 65.52, 62.83, 61.49, 57.55, 55.11, 50.08, 50.03, 47.60, 46.75, 46.02, 44.20, 41.90, 41.58, 38.14, 33.74, 33.38, 32.82, 32.81, 32.58, 30.94, 29.29, 27.64, 26.16, 23.94, 23.63, 23.10, 18.59, 17.77, 16.98, 16.73; HRMS (ESI+): m/z calcd for C52H83N6O16 [M + H]+: 1047.5850; found: 1047.5865.
Compound 22
This was prepared according to the general procedure from 5 (70 mg, 0.127 mmol) and 10 (58 mg, 0.281 mmol) and purified by column chromatography (acetonitrile/H2O, 7:1) to yield a white solid (99 mg, 81%); mp 174–175 °C; [α]D +24.8 (c 0.25, py); IR (neat): υ̅ = 3356, 2943, 1730, 1645, 1462, 1365, 1231, 1151, 1051 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) (Glc β anomer 55%, Glc α anomer 45%) δ 7.92, 7.89, 7.85 (3s, 2H), 5.12 (brs, 1H), 5.08–4.97 (m, 2H), 4.87 (d, J = 3.6 Hz, 1H), 4.69–4.51 (m, 4H), 4.42–4.28 (m, 2H), 4.25 (2d, J = 7.8 Hz, 1H), 3.91 (m, 1H), 3.56–2.83 (several m, 7H), 2.70 (d, J = 11.8 Hz, 1H), 1.96–0.65 (several m, 20H), 1.00 (s, 3H), 0.87 (s, 3H), 0.82 (s, 3H), 0.80 (brs, 6H), 0.67 (brs, 3H), 0.40, 0.37 (2s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 177.27, 145.26, 145.15, 143.62, 142.38, 142.30, 125.94, 125.09, 122.26, 96.84, 92.51, 92.47, 81.21, 76.96, 76.81, 76.10, 74.62, 74.37, 74.30, 72.78, 72.05, 71.62, 71.51, 71.34, 71.28, 70.11, 69.95, 62.48, 57.34, 54.84, 51.31, 47.33, 46.50, 45.73, 43.91, 41.61, 41.32, 37.85, 33.44, 33.08, 32.54, 32.30, 30.64, 28.99, 27.36, 25.89, 23.64, 23.35, 22.82, 18.33, 17.46, 16.71, 16.42; HRMS (ESI+): m/z calcd for C48H76N6O14 [M + 2H]2+: 480.2709; found: 480.2724.
Compound 23
This was prepared according to the general procedure from 5 (60 mg, 0.109 mmol) and 9 (98 mg, 0.239 mmol) and purified by column chromatography (acetonitrile/H2O, 5:1) to yield a white solid foam (109 mg, 73%); mp 186–188 °C; [α]D −6.4 (c 0.25, py); IR (neat): υ̅ = 3358, 2942, 1720, 1660, 1376, 1065 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 8.04 (brs, 2H), 5.13 (brs, 1H), 5.06–4.99 (m, 2H), 4.55 (m, 6H), 4.26 (t, J = 7.2 Hz, 2H), 4.17 (brs, 2H), 4.05 (m, 2H), 3.92–3.83 (m, 2H), 3.73 (d, J = 11.4 Hz, 2H), 3.61–3.21 (m, 21H), 3.01 (m, 2H), 2.87 (d, J = 9.0 Hz, 1H), 2.71 (d, J = 12.5 Hz, 1H), 1.95–0.80 (m, 20H), 1.01 (s, 3H), 0.90 (s, 3H), 0.83 (s, 3H), 0.81 (bs, 6H), 0.68 (s, 3H), 0.41 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 177.46, 143.97, 142.63, 126.30, 125.34, 122.54, 106.95, 104.23, 104.19, 103.22, 103.15, 81.48, 80.76, 80.67, 77.24, 76.04, 75.46, 75.24, 73.57, 73.46, 71.80, 71.17, 68.77, 68.73, 68.28, 68.21, 62.86, 61.14, 61.07, 60.87, 57.66, 55.16, 50.46, 47.65, 46.80, 46.07, 44.26, 41.95, 41.64, 40.11, 40.02, 39.94, 39.86, 39.77, 39.69, 39.60, 39.52, 39.35, 39.18, 39.02, 38.18, 33.80, 33.42, 32.88, 32.60, 30.98, 29.34, 27.71, 26.24, 24.02, 23.69, 23.16, 18.66, 17.84, 17.02, 16.80; HRMS (ESI+): m/z calcd for C64H104N6O26 [M + 2H]2+: 686.3500; found: 686.3500.
Compound 24
This was prepared according to the general procedure from 7 (100 mg, 0.185 mmol) and 12 (45 mg, 0.204 mmol) and purified by column chromatography (CH2Cl2/MeOH, 5:1) to yield a white solid (130 mg, 93%); mp 258–260 °C; [α]D +66.4 (c 0.25, py); IR (neat): υ̅ = 3375, 2943, 1743, 1693, 1463, 1366, 1270, 1214, 1134, 1055 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 8.06 (s, 1H), 5.36 (d, J = 17.7 Hz, 1H), 5.27 (d, J = 17.4 Hz, 1H), 5.11 (s, 1H), 4.71 (s, 1H), 4.65 (d, J = 12.4 Hz, 1H), 4.50 (d, J = 12.4 Hz, 1H), 4.39 (s, 1H), 3.66–3.35 (m, 7H), 2.65 (m, 1H), 1.95–0.70 (several m, 22H), 1.04 (s, 3H), 0.82 (s, 9H), 0.73 (s, 3H), 0.65 (s, 3H), 0.62 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 167.60, 144.55, 144.31, 126.58, 122.29, 99.51, 83.28, 77.79, 74.56, 74.34, 71.36, 71.30, 70.75, 70.64, 67.42, 67.26, 61.74, 61.56, 59.51, 55.37, 54.03, 51.37, 47.68, 42.06, 38.08, 37.18, 33.98, 33.58, 32.98, 31.08, 28.40, 27.94, 26.35, 24.06, 23.66, 18.46, 17.55, 16.97, 15.71; HRMS (ESI+): m/z calcd for C41H64N3O10 [M + H]+: 758.4592; found: 758.459.
Compound 25
This was prepared according to the general procedure from 7 (69 mg, 0.127 mmol) and 13 (36 mg, 0.152 mmol) after column chromatography (CH2Cl2/MeOH, 5:1) as a white solid (79 mg, 80%); mp 245 °C (dec); [α]D +3.6 (c 0.25, py); IR (neat): υ̅ = 3363, 2943, 1742, 1692, 1463, 1366, 1271, 1216, 1034 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 7.97 (s, 1H), 5.32 (d, J = 17.6 Hz, 1H), 5.23 (d, J = 17.6 Hz, 1H), 5.10 (s, 1H), 4.40 (m, 1H), 4.23 (d, J = 9.7 Hz, 1H), 3.82 (d, J = 14.3 Hz, 1H), 3.68 (d, J = 10.7 Hz, 1H), 3.42 (dd, J = 11.9 and 6.3 Hz, 1H), 3.14 (m, 2H), 3.05 (m, 2H), 2.67 (m, 1H), 1.90–0.88 (several m, 22H), 1.03 (s, 3H), 0.82 (brs, 9H), 0.73 (s, 3H), 0.65 (s, 3H), 0.64 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 179.67, 167.51, 145.09, 144.47, 125.67, 122.21, 84.64, 83.04, 81.26, 78.37, 73.47, 70.57, 61.82, 55.31, 51.29, 47.64, 46.40, 46.23, 41.99, 41.49, 38.17, 38.03, 37.13, 33.96, 33.54, 32.91, 32.80, 31.02, 28.39, 27.88, 26.31, 24.01, 23.60, 23.47, 23.25, 18.44, 17.47, 17.07, 15.66; HRMS (ESI+): m/z calcd for C41H64N3O9S [M + H]+: 774.4363; found: 774.4363.
Compound 26
This was prepared according to the general procedure from 7 (76 mg, 0.140 mmol) and 14 (59 mg, 0.154 mmol) and purified by column chromatography (CH2Cl2/MeOH, 4:1) to yield a white solid foam (120 mg, 93%); mp 261–262 °C; [α]D +8.4 (c 0.25, py); IR (neat): υ̅ = 3374, 2943, 1743, 1692, 1462, 1366, 1048 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 8.06 (s, 1H), 5.36 (d, 17.6 Hz, 1H), 5.27 (d, 17.6 Hz, 1H), 5.11 (s, 1H), 4.83 (d, J = 12.3 Hz, 1H), 4.65 (d, J = 12.4 Hz, 1H), 4.45–4.39 (m, 1H), 4.34–4.28 (m, 1H), 4.19 (d, J = 6.4 Hz, 1H), 3.76–3.29 (several multiplets, 12H), 3.08–2.99 (m, 1H), 2.72–2.59 (m, 1H), 1.86 (m, 1H), 1.77 (m, 2H), 1.65–0.85 (several m, 22H), 1.04 (s, 3H), 0.83 (s, 3H), 0.82 (bs, 6H), 0.75 (s, 3H), 0.66 (s, 3H), 0.65 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 179.93, 167.70, 144.54, 126.67, 122.28, 104.21, 102.29, 83.27, 80.70, 77.88, 76.10, 75.51, 75.41, 73.60, 71.25, 68.86, 62.10, 61.24, 60.96, 56.06, 55.32, 51.33, 47.67, 46.46, 46.35, 42.07, 41.58, 38.21, 38.12, 37.19, 33.99, 33.57, 32.97, 32.86, 31.08, 28.44, 27.92, 26.33, 24.06, 23.72, 23.65, 23.31, 18.48, 17.56, 17.08, 15.71; HRMS (ESI+): m/z calcd for C47H74N3O15 [M + H]+: 920.5127; found: 920.5120.
Compound 27
This was prepared according to the general procedure from 6 (100 mg, 0.156 mmol) and 12 (75 mg, 0.345 mmol) and purified by column chromatography (acetonitrile/H2O, 5:1) to yield a white solid (140 mg, 84%); mp 139–141 °C; [α]D +26.8 (c 0.25, py); IR (neat): υ̅ = 3359, 2943, 1751, 1694, 1215, 1132, 1058 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 8.09 (s, 1H), 8.05 (s, 1H), 5.42–5.24 (m, 5H), 5.09 (brs, 1H), 4.95 (brs, 1H), 4.73 (d, J = 4.5 Hz, 1H), 4.65 (d, J = 12.3 Hz, 2H), 4.50 (d, J = 12.0 Hz, 2H), 3.65–3.35 (m, 12H), 2.74–2.61 (brs, 1H), 1.90–0.88 (several m, 20H), 1.02 (s, 3H), 0.90 (s, 3H), 0.79 (s, 3H), 0.78 (s, 3H), 0.76 (s, 3H), 0.68 (s, 3H), 0.63 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 180.29, 167.68, 144.63, 126.90, 126.83, 122.14, 99.90, 99.81, 82.40, 74.36, 72.57, 71.50, 70.91, 67.54, 61.85, 59.96, 59.84, 54.98, 51.36, 47.75, 46.53, 44.42, 43.82, 42.28, 41.71, 38.71, 34.28, 33.82, 33.02, 31.26, 28.87, 28.14, 26.69, 24.26, 23.93, 23.50, 18.62, 17.92, 17.61, 16.82; HRMS (ESI–): m/z calcd for C52H77N6O18 [M – H]−: 1073.5294; found: 1073.5245.
Compound 28
This was prepared according to the general procedure from 6 (67 mg, 0.105 mmol) and 13 (54 mg, 0.231 mmol) and purified by column chromatography (acetonitrile/H2O, 5:1) to yield a white solid foam (94 mg, 81%); mp 146–148 °C; [α]D −14.4 (c 0.25, py); IR (neat): υ̅ = 3351, 2944, 1748, 1695, 1462, 1353, 1267, 1214, 1039 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 8.03 (s, 1H), 8.00 (s, 1H), 5.43 (d, J = 17.7 Hz, 1H), 5.32 (d, J = 17.5 Hz, 1H), 5.25 (s, 2H), 5.11 (brs, 1H), 4.97 (s, 1H), 4.69 (d, J = 10.2 Hz, 1H), 4.25 (2d, J = 10.0 Hz, 2H), 3.85 (d, J = 14.0 Hz, 2H), 3.67 (m, 2H), 3.41 (m, 2H), 3.22–3.00 (m, 8H), 2.66 (brs, 1H), 1.94–0.90 (several m, 20H), 1.07 (s, 3H), 0.98 (s, 3H), 0.92 (s, 3H), 0.82 (brs, 6H), 0.80 (s, 3H), 0.72 (s, 3H), 0.65 (bs, 3H); 13C NMR (126 MHz, DMSO-d6) δ 167.65, 167.52, 145.24, 144.67, 125.79, 125.74, 121.87, 84.78, 84.63, 82.03, 81.25, 78.37, 73.48, 70.57, 61.81, 54.32, 51.05, 47.25, 43.39, 42.09, 38.45, 33.96, 33.52, 32.63, 31.05, 28.56, 27.85, 26.30, 24.01, 23.65, 23.56, 23.48, 18.37, 17.85, 17.47, 17.10, 16.63; HRMS (ESI+): m/z calcd for C52H78N6O16S2 [M + H]+: 1107.4960; found: 1107.4960.
Compound 29
This was prepared according to the general procedure from 6 (81 mg, 0.127 mmol) and 14 (106 mg, 0.279 mmol) and purified by column chromatography (acetonitrile/H2O, 5:1) to yield a white solid (152 mg, 86%); mp 230–231 °C; [α]D +4.0 (c 0.25, py); IR (neat): υ̅ = 3363, 2942, 1749, 1692, 1373, 1213, 1057 cm–1; 1H NMR (500 MHz, DMSO-d6, D2O exchange) δ 8.13 (s, 1H), 8.08 (s, 1H), 5.46 (d, J = 17.6 Hz, 1H), 5.36 (d, J = 18.0 Hz, 1H), 5.27 (m, 2H), 5.10 (s, 1H), 4.98 (m, 1H), 4.84 (m, 2H), 4.66 (dd, J = 21.6 and 10.3 Hz, 4H), 4.34 (2d, J = 7.9 Hz, 2H), 4.19 (d, J = 4.5 Hz, 2H), 3.77 (d, J = 11.4 Hz, 2H), 3.60–3.31 (several m, 20H), 3.06 (brs, 2H), 2.67 (m, 1H), 1.95–0.95 (several m, 20H), 1.04 (s, 3H), 0.92 (s, 3H), 0.82 (brs, 6H), 0.80 (s, 3H), 0.73 (s, 3H), 0.65 (brs, 3H); 13C NMR (126 MHz, DMSO-d6) δ 167.36, 167.18, 144.45, 144.21, 126.36, 126.25, 121.43, 103.80, 101.99, 101.89, 81.81, 80.25, 75.70, 75.11, 75.00, 73.20, 71.93, 70.86, 68.48, 61.79, 61.69, 60.85, 60.55, 54.03, 50.71, 46.95, 41.75, 38.11, 33.21, 30.71, 28.22, 27.53, 25.96, 23.69, 23.31, 17.45, 17.15, 16.28; HRMS (ESI–): m/z calcd for C52H77N6O18 [M – H]−: 1397.6350; found: 1397.6377.
Bioassays
Cell Lines and Culture
Human colorectal carcinoma line T-84 was supplied by the Department of Cell Cultures of the Granada University Scientific Instrumentation Centre. This line was cultured at 37 °C in 5% CO2 and 90% humidity with Dulbecco’s modified Eagle’s medium, supplemented with 10% heat-inactivated fetal bovine serum, 10 mL/L penicillin–streptomycin 100×, and 2 mM l-glutamine. Culture media and respective supplements were supplied by Sigma-Aldrich (Madrid, Spain).
In Vitro Antiproliferative Assay
To calculate the IC50 values of the compounds, T-84 cells were seeded in 96-well plates, and after 24 h, they were induced with increasing concentrations of one of the compounds from 15 to 29 for 3 days. Subsequently, the cells were fixed with 10% cold trichloroacetic acid (4 °C) and stained with 0.4% sulforhodamine B in 1% HOAc. The colorant was solubilized with 10 mM Tris-base, pH 10.5, and optical density values were determined by colorimetry at 492 nm (Multiskan EX, Thermo Electron Corporation). IC50 values were calculated from the semilogarithmic dose–response curve by linear interpolation. For the study of the induction of oxidative stress, cells were treated with increasing concentrations of compounds 18, MA, and OA in the presence or absence of 2 mM NAC for 3 days. Then, the cultures were processed for colorimetric quantification by the sulforhodamine B assay, as described above.
Cell Cycle Analysis
T-84 cells were seeded in six-well plates, and after 24 h, they were induced with 0.5, 1, or 2 times the IC50 value of 18, MA, or OA for 24 h. Then, cultures were washed with phosphate-buffered saline (PBS), fixed with 70% cold ethanol, and incubated with a DNA extraction solution (0.2 M Na2HPO4, 0.1 M citric acid, pH 7.8) for 15 min at 37 °C. Cells were then centrifuged, washed with PBS, and resuspended in 250 μL of a solution of propidium iodide (40 μg/mL) and RNAse (100 μg/mL) for 30 min at 37 °C in the dark. Finally, samples were analyzed in a FACSCalibur (BD Biosciences). Results were analyzed using FlowJo software (v 7.6.5, Tree Star, Inc.).
Apoptosis Assays with Annexin V
Cell viability was determined by flow cytometry using the annexin V–FITC kit (Pharmingen, San Diego, CA). T-84 cells were seeded in six-well plates, and after 24 h, they were induced with 1 or 2 times the IC50 values of 18, MA, and OA for 48 h. Cells were then detached with PBS–ethylenediaminetetraacetate, washed twice with cold PBS, and collected by centrifugation at 500 g for 10 min. Cells were stained following the manufacturer’s protocol, and then samples were analyzed in a FACSCalibur (BD Biosciences). Results were analyzed using FlowJo software (v 7.6.5, Tree Star, Inc.).
Statistical Analysis
SPSS 24 for Windows (SPSS, Chicago, IL) was used for the statistical analysis. Results were compared with the Student’s test, one- or two-way analyses of variance. p < 0.05 was considered significant. Data were graphically represented using Microsoft Excel 2010 software (Microsoft Corporation).
Docking
Coordinate Preparation
Ghemical 2.95 was used to generate three-dimensional coordinates of MA, OA, BOT, and compound 18 and for geometrical minimization by molecular mechanics with the tripos 5.2 force field until the gradient energy was lower than 0.001 kJ/mol.65 The X-ray coordinates of the NF-κB1 p50:p65 and NF-κB2 p52:v-Rel complexed with DNA were extracted from the protein data bank (PDB codes 1vkx and 3do7, respectively), and they were prepared for docking with Dock Prep, a tool implemented in Chimera, that deletes water molecules and ions, repairs truncated side chains, adds hydrogens, and assigns partial charges.66
Docking
Docking was carried out at Swiss-Dock sever in accurate mode and without defining the region of interest (blind docking).67 Results were clustered and ranked by their fullfitness scores, a parameter that accounts for the total energy of the system calculated with the CHARMM22 molecular mechanics force field and that includes the solvation free energy.62,68 Analysis of the results was carried out with the help of UCSF Chimera, Poseview, and protein–ligand interaction profiles.66,69,70Kd values were estimated from the ΔG computed during the docking by applying the expression ΔG = RT ln Kd, where R is the ideal gas constant (0.0019872 kcal/(mol K)) and T is the temperature (298 K).
Acknowledgments
This work was supported by a grant from Ramón Areces Foundation (Madrid, Spain) and by grant CTQ2014-55474-C2-1-R from the Spanish Ministerio de Economia y Competitividad (MINECO) co-financed by FEDER funds. This paper is related to the Ph.D. thesis of N.M.-S. and E.R.-B.
Glossary
Abbreviations
- BOT
6,6-dimethyl-2-(phenylimino)-6,7-dihydrobenzo[d][1,3]oxathiol-4(5H)-one
- COX-2
cyclooxygenase-2
- CuAAC
copper(I)-catalyzed azide–alkyne 1,3-dipolar cycloaddition
- HDI
human development index
- MA
maslinic acid
- NAC
N-acetyl-l-cysteine
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cell
- OA
oleanolic acid
- ROS
reactive oxygen species
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01034.
Additional figures illustrating cytograms; binding modes of MA, OA, and BOT; and NMR spectra (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- International Agency for Research on Cancer . World Cancer Report 2014; Stewart B. W., Wild C. P., Eds; World Health Organization, 2014.
- Fidler M. M.; Soerjomataram S.; Bray F. A global view on cancer incidence and national levels of the development index. Int. J. Cancer 2016, 139, 2436–2446. 10.1002/ijc.30382. [DOI] [PubMed] [Google Scholar]
- Arnold M.; Sierra M. S.; Laversanne M.; Soerjomataram I.; Jemal A.; Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- Siegel R. L.; Miller K. D.; Hemal A. Cancer statistics, 2016. CA-Cancer J. Clin. 2016, 66, 7–30. 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Thakur M.; Melzig M. F.; Fuchs H.; Weng A. Chemistry and pharmacology of saponins: special focus on cytotoxic properties. Bot.: Targets Ther. 2011, 1, 19–29. 10.2147/btat.s17261. [DOI] [Google Scholar]
- Aboutalebi R.; Monfared A. Saponin Terpenoids; A brief review of mechanisms of actions and anti-cancerous effects. Am. Chem. Sci. J. 2016, 12, 1–8. 10.9734/ACSJ/2016/20638. [DOI] [Google Scholar]
- Dinda B.; Debnath S.; Mohanta B. C.; Harigaya Y. Naturally occurring triterpenoid saponins. Chem. Biodiversity 2010, 7, 2327–2580. 10.1002/cbdv.200800070. [DOI] [PubMed] [Google Scholar]
- Koczurkiewicz P.; Czyż J.; Podolak I.; Wójcik K.; Galanty A.; Janeczko Z.; Michalik M. Multidirectional effects of triterpene saponins on cancer cells - mini-review of in vitro studies. Acta Biochim. Pol. 2015, 62, 383–393. 10.18388/abp.2015_1089. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Lu W.-X.; Yan M.-C.; Yu Y.; Ikejima T.; Cheng M.-S. Synthesis and tumor cytotoxicity of novel Amide derivatives of β-hederin. Molecules 2010, 15, 7871–7883. 10.3390/molecules15117871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Wang Z.; Su S.; Xing Y.; Li Y.; Li M.; Liu J.; Yang S. Synthesis and cytotoxicity of oleanolic acid trisaccharide saponins. Carbohydr. Res. 2017, 442, 9–16. 10.1016/j.carres.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Juan M. E.; Planas J. M.. Effects of Pentacyclic Triterpenes from Olives on Colon Cancer. In Bioactive Foods and Extracts Cancer Treatment and Prevention; Watson R. R., Preedy V. R., Eds.; CRC Press, 2010; pp 403–413. [Google Scholar]
- Reyes-Zurita F. J.; Rufino-Palorames E. E.; Lupiáñez J. A.; Cascante M. Maslinic acid, a natural triterpene from Olea europaea induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett. 2009, 273, 44–54. 10.1016/j.canlet.2008.07.033. [DOI] [PubMed] [Google Scholar]
- Reyes-Zurita F. J.; Rufino-Palorames E. E.; García-Salguero L.; Peragón J.; Medina P. P.; Parra A.; Cascante M.; Lupiáñez J. A. Maslinic acid, a natural triterpene, induces a death receptor-mediated apoptotic mechanism in Caco-2 p53-deficient colon adenocarcinoma cells. PLoS One 2016, 11, e0146178 10.1371/journal.pone.0146178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Zurita F. J.; Pachón-Peña G.; Lizárraga D.; Rufino-Palorames E. E.; Cascante M.; Lupiáñez J. A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNKp53-dependent mechanism. BMC Cancer 2011, 25, 154. 10.1186/1471-2407-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiram N. B.; Indranie C.; Malisetty S. V.; Jagan P.; Steele V. E.; Rao C. V. Chemoprevention of colon carcinogenesis by oleanolic acid and its analog in male F344 rats and modulation of COX-2 and apoptosis in human colon HT-29 cancer cells. Pharm. Res. 2008, 9, 2151–2157. 10.1007/s11095-008-9582-7. [DOI] [PubMed] [Google Scholar]
- Sánchez-Quesada C.; López-Biedma A.; Warleta F.; Campos M.; Beltrán G.; Gaforio J. J. Bioactive properties of he main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. J. Agric. Food Chem. 2013, 61, 12173–12182. 10.1021/jf403154e. [DOI] [PubMed] [Google Scholar]
- Rufino-Palomares E. E.; Pérez-Jiménez A.; Reyes-Zurita F. J.; García-Salguero L.; Mokhtari K.; Herrera-Merchán A.; Medina P. P.; Peragón J.; Lupiañez J. A. Anti-cancer and anti-angiogenic properties of various natural pentacyclic tri-terpenoids and some of their chemical derivatives. Curr. Org. Chem. 2015, 19, 919–947. 10.2174/1385272819666150119225952. [DOI] [Google Scholar]
- Paszel-Jaworska A.; Romaniuk A.; Rybczynska M. Molecular mechanisms of biological activity of oleanolic acid - A source of inspiration for a new drugs design. Mini-Rev. Org. Chem. 2014, 11, 330–342. 10.2174/1570193X1103140915111839. [DOI] [Google Scholar]
- Sporn M. B.; Liby K. T.; Yore M. M.; Fu L.; Lopchuk J. M.; Gribble G. W. New synthetic triterpenoids, potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J. Nat. Prod. 2011, 74, 537–545. 10.1021/np100826q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaïb K.; Delemasure S.; Dutartre P.; Jannet H. B. Microwave-assisted synthesis, anti- inflammatory and anti-proliferative activities of new maslinic acid derivatives bearing 1,5- and 1,4-disubstituted triazoles. J. Enzyme Inhib. Med. Chem. 2016, 31, 130–147. 10.1080/14756366.2016.1193733. [DOI] [PubMed] [Google Scholar]
- Wei G.; Luan W.; Wang S.; Cui S.; Li F.; Liu Y.; Liu Y.; Cheng M. A library of 1,2,3-triazole-substituted oleanolic acid derivatives as anticancer agents: design, synthesis, and biological evaluation. Org. Biomol. Chem. 2015, 13, 1507–1514. 10.1039/C4OB01605J. [DOI] [PubMed] [Google Scholar]
- Chouaïb K.; Romdhane A.; Delemasureb S.; Dutartre P.; Elie N.; Touboul D.; Jannet H. B.; Hamzaa M. A. Regiospecific synthesis, anti-inflammatory and anticancer evaluation of novel 3,5-disubstituted isoxazoles from the natural maslinic and oleanolic acids. Ind. Crops Prod. 2016, 85, 287–299. 10.1016/j.indcrop.2016.03.024. [DOI] [Google Scholar]
- Cheng K.-G.; Su C.-H.; Yang L.-D.; Liu J.; Chen Z.-F. Synthesis of oleanolic acid dimers linked at C-28 and evaluation of anti-tumor activity. Eur. J. Med. Chem. 2015, 89, 480–489. 10.1016/j.ejmech.2014.10.066. [DOI] [PubMed] [Google Scholar]
- Bednarczyk-Cwynar B.; Zaprutko L.; Ruszkowski P.; Hladon B. Anticancer effect of A-ring or/and C-ring modified oleanolic acid derivatives on KB, MCF-7 and HeLa cell lines. Org. Biomol. Chem. 2012, 10, 2201–2205. 10.1039/c2ob06923g. [DOI] [PubMed] [Google Scholar]
- Mallavadhani U. V.; Vanga N. R.; Jeengar M. K.; Naidu V. G. M. Synthesis of novel ring-A fused hybrids of oleanolic acid with capabilities to arrest cell cycle and induce apoptosis in breast cancer cells. Eur. J. Med. Chem. 2014, 74, 398–404. 10.1016/j.ejmech.2013.12.040. [DOI] [PubMed] [Google Scholar]
- Medina-O’Donnell M.; Rivas F.; Reyes-Zurita F. J.; Martinez A.; Galisteo-Gonzales F.; Lupiañez J. A.; Parra A. Synthesis and in vitro antiproliferative evaluation of PEGylated triterpenes. Fitoterapia 2017, 120, 25–40. 10.1016/j.fitote.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Medina-O’Donnell M.; Rivas F.; Reyes-Zurita F. J.; Martinez A.; Martin-Fonseca S.; Garcia-Granados A.; Ferrer-Martin R. M.; Lupiañez J. A.; Parra A. Semi-synthesis and antiproliferative evaluation of PEGylated pentacyclic triterpenes. Eur. J. Med. Chem. 2016, 118, 64–78. 10.1016/j.ejmech.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Xu J.; Nie X.; Hong Y.; Jiang Y.; Wu G.; Yin X.; Wang C.; Wang X. Synthesis of water soluble glycosides of pentacyclic dihydroxytriterpene carboxylic acids as inhibitors of α-glucosidase. Carbohydr. Res. 2016, 424, 42–53. 10.1016/j.carres.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Wang K.; Karin M. Tumor-elicited inflammation and colorectal cancer. Adv. Cancer Res. 2015, 128, 173–196. 10.1016/bs.acr.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Jain H.; Dhingra N.; Narsinghani T.; Sharma R. Insights into the mechanism of natural terpenoids as NF-κB inhibitors: an overview on their anticancer potential. Exp. Oncol. 2016, 38, 158–168. [PubMed] [Google Scholar]
- Van Waes C. Nuclear factor-kB in development, prevention, and therapy of cancer. Clin. Cancer Res. 2007, 13, 1076–1082. 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- Baud V.; Karin M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discovery 2009, 8, 33–40. 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.; Cohen J.; Arun P.; Chen Z.; Van Waes C. NF-κB in carcinoma therapy and prevention. Expert Opin. Ther. Targets 2008, 12, 1109–1122. 10.1517/14728222.12.9.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. C.; Sharpless K. B. The growing impact of click chemistry on drug discovery. Drug Discovery Today 2003, 8, 1128–1137. 10.1016/S1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- Hou J.; Liu X.; Shen J.; Zhao G.; Wang P. G. The impact of click chemistry in medicinal chemistry. Expert Opin. Drug Discovery 2012, 7, 489–501. 10.1517/17460441.2012.682725. [DOI] [PubMed] [Google Scholar]
- Ortega-Muñoz M.; Lopez-Jaramillo J.; Hernandez-Mateo F.; Santoyo-Gonzalez F. Synthesis of glyco-silicas by Cu(I)-catalyzed “click-chemistry” and their applications in affinity chromatography. Adv. Synth. Catal. 2006, 348, 2410–2420. 10.1002/adsc.200600254. [DOI] [Google Scholar]
- Wu S. H.; Yao C.-H.; Hsiech C.-J.; Liu Y.-W.; Chao Y.-S.; Song J.-S.; Lee J.-C. Development and application of a fluorescent glucose uptake assay for the high-throughput screening of non-glycoside SGLT2 inhibitor. Eur. J. Pharm. Sci. 2015, 74, 40–44. 10.1016/j.ejps.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Pérez-Balderas F.; Ortega-Muñoz M.; Morales-Sanfrutos J.; Hernández-Mateo F.; Calvo-Flores F. G.; Calvo-Asin J. A.; Isac-García J.; Santoyo-González F. Multivalent neoglycoconjugates by regiospecific cycloaddition of alkynes and azides using organic- soluble copper catalysts. Org. Lett. 2003, 5, 1951–1954. 10.1021/ol034534r. [DOI] [PubMed] [Google Scholar]
- Xu X.-H.; Li T.; Fong C. M. V.; Chen X.; Chen X.-J.; Wang Y.-T.; Huang M.-Q.; Lu J.-J. Saponins from Chinese medicines as anticancer agents. Molecules 2016, 21, 1326. 10.3390/molecules21101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Serrano F.; Mut-Salud N.; Cruz-Bustos T.; Gomez-Samblas M.; Carrasco E.; Garrido J. M.; López-Jaramillo F. J.; Santoyo-Gonzalez F.; Osuna A. Functionalized immunostimulating complexes with protein A via lipid vinyl sulfones to deliver cancer drugs to trastuzumab-resistant HER2-overexpressing breast cancer cells. Int. J. Nanomed. 2016, 11, 4777–4785. 10.2147/IJN.S112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia J. J.; Tapia R.; Mahdjour S.; Rodriguez-Serrano F.; Mut-Salud N.; Chahboun R.; Alvarez-Manzaneda E. Antiproliferative activity of natural taiwaniaquinoids and related compounds. J. Nat. Prod. 2017, 80, 308–318. 10.1021/acs.jnatprod.6b00700. [DOI] [PubMed] [Google Scholar]
- Carrasco E.; Álvarez P. J.; Melguizo C.; Prados J.; Álvarez-Manzaneda E.; Chahboun R.; Messouri I.; Vázquez-Vázquez M. I.; Aranega A.; Rodríguez-Serrano F. Novel merosesquiterpene exerts a potent antitumor activity against breast cancer cells in vitro and in vivo. Eur. J. Med. Chem. 2014, 79, 1–12. 10.1016/j.ejmech.2014.03.071. [DOI] [PubMed] [Google Scholar]
- Zafarullah M.; Li W. Q.; Sylvester J.; Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. 2003, 60, 6–20. 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mut-Salud N.; Álvarez P. J.; Garrido J. M.; Carrasco E.; Aránega A.; Rodríguez-Serrano F. Antioxidant intake and antitumor therapy: toward nutritional recommendations for optimal results. Oxid. Med. Cell. Longevity 2016, 6719534 10.1155/2016/6719534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montilla M. P.; Agil A.; Navarro M. C.; Jiménez M. I.; García-Granados A.; Parra A.; Cabo M. M. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med. 2003, 69, 472–474. 10.1055/s-2003-39698. [DOI] [PubMed] [Google Scholar]
- Sánchez-Quesada C.; López-Biedma A.; Warleta F.; Campos M.; Beltrán G.; Gaforio J. J. Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. J. Agric. Food Chem. 2013, 61, 12173–12182. 10.1021/jf403154e. [DOI] [PubMed] [Google Scholar]
- Allouche Y.; Beltrán G.; Gaforio J. J.; Uceda M.; Mesa M. D. Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem. Toxicol. 2010, 48, 2885–2890. 10.1016/j.fct.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Sánchez-Quesada C.; López-Biedma A.; Gaforio J. J. Oleanolic acid, a compound present in grapes and olives, protects against genotoxicity in human mammary epithelial cells. Molecules 2015, 20, 13670–13688. 10.3390/molecules200813670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.; Song Q.; Hu D.; Zhuang X.; Yu S.; Teng D. Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway. Korean J. Physiol. Pharmacol. 2016, 20, 237–243. 10.4196/kjpp.2016.20.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owa T.; Yoshino H.; Yoshimatsu K.; Nagasu T. Cell cycle regulation in the G1 phase: a promising target for the development of new chemotherapeutic anticancer agents. Curr. Med. Chem. 2001, 8, 1487–1503. 10.2174/0929867013371996. [DOI] [PubMed] [Google Scholar]
- Bertoli C.; Skotheim J. M.; de Bruin R. A. M. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes F. J.; Centelles J. J.; Lupiáñez J. A.; Cascante M. (2α,3β)-2,3-Dihydroxyolean-12-en-28-oic acid, a new natural triterpene from Olea europaea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett. 2006, 580, 6302–6310. 10.1016/j.febslet.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Rufino-Palomares E. E.; Reyes-Zurita F. J.; García-Salguero L.; Mokhtari K.; Medina P. P.; Lupiáñez J. A.; Peragón J. Maslinic acid, a triterpenic anti-tumoural agent, interferes with cytoskeleton protein expression in HT29 human colon-cancer cells. J. Proteomics 2013, 83, 15–25. 10.1016/j.jprot.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Li L.; Wei L.; Shen A.; Chu J.; Lin J.; Peng J. Oleanolic acid modulates multiple intracellular targets to inhibit colorectal cancer growth. Int. J. Oncol. 2015, 47, 2247–2254. 10.3892/ijo.2015.3198. [DOI] [PubMed] [Google Scholar]
- Juan M. E.; Planas J. M.; Ruiz-Gutierrez V.; Daniel H.; Wenzel U. Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells. Br. J. Nutr. 2008, 100, 36–43. 10.1017/S0007114508882979. [DOI] [PubMed] [Google Scholar]
- Žiberna L.; Šamec D.; Mocan A.; Nabavi S. F.; Bishayee A.; Farooqi A. A.; Sureda A.; Nabavi S. M. Oleanolic acid alters multiple cell signaling pathways: implication in cancer prevention and therapy. Int. J. Mol. Sci. 2017, 18, 643. 10.3390/ijms18030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Guo W.-J.; Yang Q.-Y. Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World J. Gastroenterol. 2002, 8, 493–495. 10.3748/wjg.v8.i3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo K. H.; Park J.-H.; Cui E. J.; Kim K. I.; Kim J. Y.; Kim J.; Hong S. G.; Baek N. I.; Chung I. S. 3-O-acetyloleanolic acid induces apoptosis in human colon carcinoma HCT-116 cells. Phytother. Res. 2012, 26, 1541–1546. 10.1002/ptr.4616. [DOI] [PubMed] [Google Scholar]
- Harris R. E.; Beebe J.; Alshafie G. A. Reduction in cancer risk by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. J. Exp. Pharmacol. 2012, 4, 91–96. 10.2147/JEP.S23826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsum Y. W.; Yew W. T.; Hong P. L. V.; Soo K. K.; Hoon L. S.; Chieng Y. C.; Mooi L. Y. Cancer chemopreventive activity of maslinic acid: suppression of COX-2 expression and inhibition of NF-κB and AP-1 activation in Raji cells. Planta Med. 2011, 77, 152–157. 10.1055/s-0030-1250203. [DOI] [PubMed] [Google Scholar]
- Li C.; Yang Z.; Zhai C.; Qiu W.; Li D.; Yi Z.; Wang L.; Tang J.; Qian M.; Luo J.; Liu M. Maslinic acid potentiates the anti-tumor activity of tumor necrosis factor a by inhibiting NF-κB signaling pathway. Mol. Cancer 2010, 73. 10.1186/1476-4598-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosdidier A.; Zoete V.; Michielin O. EADock: Docking of small molecules into protein active sites with a multiobjective evolutionary optimization. Proteins 2007, 67, 1010–1025. 10.1002/prot.21367. [DOI] [PubMed] [Google Scholar]
- Huxford T.; Ghosh G. A structural guide to proteins of the NF-κB signaling module. Cold Spring Harbor Perspect. Biol. 2009, 1, a000075 10.1101/cshperspect.a000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswararao E.; Anh H. L. T.; Sharma V. K.; Lee K.-C.; Sharma N.; Kim Y.; Jung S.-H. Study on anti-proliferative effect of benzoxathiole derivatives through inactivation of NF-κB in human cancer cells. Bioorg. Med. Chem. Lett. 2012, 22, 4523–4527. 10.1016/j.bmcl.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Hassinen T.; Perakyla M. New energy terms for reduced protein models implemented in an off-lattice force field. J. Comput. Chem. 2001, 22, 1229–1242. 10.1002/jcc.1080. [DOI] [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Couch G. S.; Greenblatt D. M.; Meng E. C.; Gerrin T. E. UCSF Chimera -a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Grosdidier A.; Zoete V.; Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoete V.; Grosdidier A.; Cuendet M.; Michielin O. Use of the FACTS solvation model for protein-ligand docking calculations. Application to EADock. J. Mol. Recognit. 2010, 23, 457–461. 10.1002/jmr.1012. [DOI] [PubMed] [Google Scholar]
- Stierand K.; Rarey M. From modeling to medicinal chemistry: Automatic generation of two- dimensional complex diagrams. ChemMedChem 2007, 2, 853–860. 10.1002/cmdc.200700010. [DOI] [PubMed] [Google Scholar]
- Salentin S.; Schreiber S.; Haupt V. J.; Adasme M. F.; Schroeder M. PLIP: fully automated protein-ligand interaction profiles. Nucleic Acids Res. 2015, 43, W443–W447. 10.1093/nar/gkv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.