Abstract
Four ammonium-based ionic liquids were synthesized for the selective extraction and degradation of lignin from coffee husk. The extracted lignin samples were characterized by Fourier transform infrared, gel permeation chromatography, gas chromatography–mass spectrometry, UV–vis, 1H and 13C NMR, heteronuclear single-quantum coherence-NMR, thermogravimetric analysis, X-ray diffraction, and field emission scanning electron microscopy analyses. The analyzed results confirmed that these ionic liquids are able to effectively extract and decompose the lignin to smaller molecules from the biomass. Experimental results show that a significantly high yield, 71.2% of the original lignin, has been achieved. This processing method is an efficient, economical, and environmentally friendly green route for producing high-added-value lignin from wasted coffee husk.
1. Introduction
A huge amount of coffee husk (CH), as waste materials, is produced after the refining of coffee beans. The management of such unwanted waste materials remains a challenging task. In general practice, those waste materials are either burned and released to the atmosphere or dumped in field. However, both ways create a serious threat to the environment. Therefore, developing a green method to extract lignin from the enormously available coffee husk not only helps us to overcome this environmental problem but also enhances the added value of those waste materials.
Basically, lignin is an amorphous polymer with highly cross-linking network.1 It is known to possess various functional groups, reactive sites, and high content of guaiacyl, syringyl, and p-hydroxyphenyl chemical units.2 Various applications of lignin are well recognized, such as an additive to composite materials,3 antioxidants,4 adsorbents,5 sorption of heavy-metal ions,6 anticancer agents,7 and dyes. The wide applications of lignin emanate from its substantial amount of functional groups and reactive sites presented in the molecule irrespective of its plant sources.2 Despite its direct use in various emerging fields of science and engineering, depolymerization of lignin to lower-molecular-weight phenolic compounds and oligomers has been recognized as a highly potential method to fulfill our future demands of valuable chemicals.8 Therefore, extracting lignin and simultaneously converting into high-added-value compounds from the nonedible and enormously available biomass, such as coffee husk, is economically and environmentally attractive for practical applications.
Several methods, such as alkali treatment,9 acid treatment,10 organic solvents treatment,11 and use of sub- or supercritical technology,12 have been proposed to extract lignin from lignocellulosic biomass. In the development of lignin extraction process from lignocellulosic biomass, the major concerns are to find the source of lignin, which possesses negligible economical values, to find a suitable solvent to dissolve and decompose biomass, and to design a process with low energy consumption and less complexity. The process of lignin recovery from biomass generally includes pretreatment, dissolution, extraction, and finally depolymerization. Although various conventional solvents are found to be highly promising in recovering major valuable components and reducing the energy requirement, green credential of the process remains doubtful.13 For example, Zhang et al.14 reported that the extraction of lignin by using mineral acid is highly advantageous in the expectations of reducing energy consumption and gaining high yield of valuable compounds. However, due to the hazardous nature of the mineral acid, the process is harmful to the environment, as well as the recycling and reusing of acid is not economical.
Finding a suitable green solvent for the pretreatment and dissolution of biomass is essentially needed. Recently, ionic liquids (ILs), as a new class of green solvents, have been found to be suitable for the dissolution of biomass.15 ILs are mainly composed of cations (generally organic) and anions (organic or inorganic). They are known to possess very interesting physiochemical properties over conventional solvents, such as high solvation ability for a range of molecules, comparatively less flammable nature, high ionic conductivity, and wide electrochemical window.16 Especially, due to their nonvolatile nature and high chemical and thermal stabilities, they are referred to as green media.17 Thus, in comparison to the conventional volatile organic solvents and the hazardous mineral acids or alkaline solutions, the process using ILs is considered as a green route for the biomass utilization.
The extraction of lignin from biomass in imidazolium-based IL has been studied by many researchers.18−20 For instance, Lan et al.18 investigated the fractionation process of the sugarcane bagasse in 1-butyl-3-methylimidazolium chloride and reported that 10.51 wt % of lignin can be extracted. They concluded that the ionic liquid disrupts the lignin cellulose or hemicellulose structural network and fractionates the biomass into its constituent components of lignin, cellulose, and hemicellulose. The extraction of lignin from wood sources (Pinus radiata and Eucalyptus globulus) by using a series of imidazolium-based ILs accompanying with microwave treatment was also reported.19 Those ILs included 1-ethyl-3-methylimidazolium acetate, 1-butyl-3-methylimidazolium acetate, 1-allyl-3-methylimidazolium chloride, 1-ethyl-3-methylimidazolium chloride, and 1-butyl-3-methylimidazolium chloride. All of the investigated ionic liquids are found to dissolve lignocellulosic biomass with an aid of microwave irradiation, and lignin was precipitated by using methanol as an antisolvent. Some other studies by using imidazolium-based ILs are also available in the literature.20 Those studies focused on optimizing the processing parameters to increase the overall yield of lignin and its valuable components.
In this work, we attempt to develop a green method to extract waste coffee husk and simultaneously depolymerize the extracted lignin into high-added-value compounds. The main objective of this work is to evaluate several potential ammonium-based ILs for the extraction and depolymerization of lignin from coffee husk. The selected ionic liquids are diisopropylethylammonium acetate ([DIPEA][Ac]), diisopropylethylammonium propanoate ([DIPEA][P]), diisopropylethylammonium octanoate ([DIPEA][O]), and diisopropylethylammonium benzoate ([DIPEA][B]). In our previous work,21 we have successfully demonstrated the suitability of the ammonium-based ionic liquids in the selective degradation of the alkali lignin (AL). The extracted lignin is characterized by means of spectroscopic and chromatographic approaches, including Fourier transform infrared (FT-IR) spectroscopy, gel permeation chromatography (GPC), ultraviolet–visible spectroscopy (UV–vis), 1H and 13C nuclear magnetic resonance (NMR) spectroscopy, heteronuclear single-quantum coherence (HSQC)-NMR spectroscopy, and thermogravimetric analysis (TGA). Material characterization techniques, such as X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), and transmission electron microscopy (TEM), are also used to characterize untreated and treated coffee husk biomass. Further, systematic studies are made to explore the effect of increasing hydrophobicity, the effect of aromatic anion over aliphatic anion, and the effects of operational parameters (temperature T, and reaction time t) on the performance of extraction and depolymerization of lignin from coffee husk.
2. Results and Discussion
In this study, we aim to develop a green route to produce valuable chemicals using waste coffee husk as a feedstock. For this purpose, we performed a detailed study to extract the lignin content of the coffee husk with three ionic liquids containing ammonium ion as cation and aliphatic anions with varying alkyl chain as anions ([DIPEA][Ac], [DIPEA][O], and [DIPEA][P]) at 80, 100, and 120 °C, respectively. The extracted lignin samples are characterized by using a series of spectroscopic and chromatographic techniques. The favorable reaction conditions of temperature and treatment time for dissolution of coffee husk in ammonium-based ionic liquid are found as 120 °C and 4 h, respectively. Further, to compare the efficiency of the ionic liquids containing ammonium ion as cation and aliphatic anion as anion to that of the ionic liquid containing common ammonium ion as cation and aromatic ion as anion, a study was performed in the presence of [DIPEA][B] only at favorable reaction conditions (120 °C and 4 h). The obtained results are discussed in the subsequent sections.
2.1. Fourier Transform Infrared (FT-IR) Spectroscopy
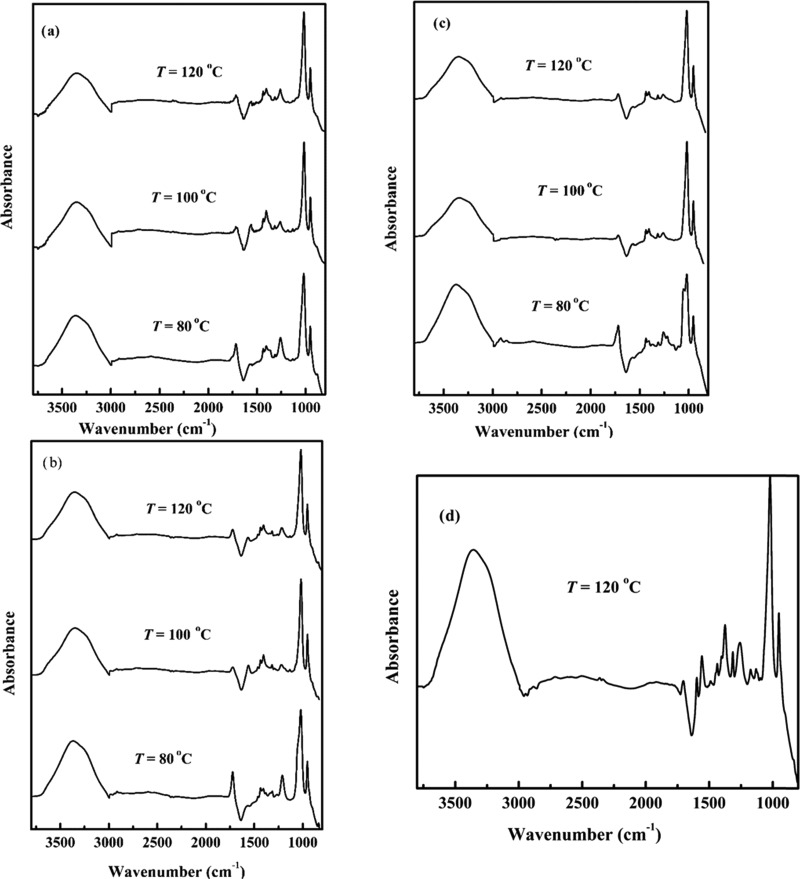
For the structural elucidation of lignin extracted from coffee husk by the ionic liquids at different extraction temperatures, the extracted solutions were analyzed by FT-IR spectroscopy. Figure 1 presents the FT-IR spectra for lignin extracted by four ionic liquids: [DIPEA][Ac], [DIPEA][B], [DIPEA][O], and [DIPEA][P] at 80, 100, and 120 °C, and all of the patterns of the spectra appear to be almost identical.
Figure 1.
FT-IR spectra of lignin extracted from coffee husk by (a) [DIPEA][Ac], (b) [DIPEA][P], (c) [DIPEA][O], and (d) [DIPEA][B].
Typical lignin peaks, such as O–H stretching (3341–3374 cm–1),22 C–H stretching in methylene groups (2914–2978 cm–1),23 aromatic skeletal vibrations (1552–1574 cm–1), and aromatic C–H in-plane deformation (1274–1174 cm–1),24 are visible in all extracted lignin spectra. The wavenumbers of the major peaks are presented in Table 1.
Table 1. Possible Functional Groups and the Corresponding Wavenumber (cm–1) of Ionic Liquid-Extracted Lignin (ILL) at Various Extraction Temperatures (T) after 4 h Treatment.
| assigned
functional groups with their wavenumber
(cm–1) |
||||||
|---|---|---|---|---|---|---|
| ILs used | T (°C) | OH | methylene C–H | benzene skeleton | aromatic C–H | C–O |
| [DIPEA][Ac] | 80 | 3363 | 2978 | 1555 | 1264 | 1013 |
| 100 | 3341 | 2920 | 1552 | 1254 | 1013 | |
| 120 | 3343 | 2924 | 1564 | 1264 | 1013 | |
| [DIPEA][B] | 120 | 3357 | 2914 | 1557 | 1250 | 1023 |
| [DIPEA][O] | 80 | 3374 | 2917 | 1552 | 1257 | 1013 |
| 100 | 3343 | 2924 | 1578 | 1255 | 1015 | |
| 120 | 3343 | 2917 | 1567 | 1272 | 1013 | |
| [DIPEA][P] | 80 | 3374 | 2937 | 1567 | 1222 | 1013 |
| 100 | 3353 | 2935 | 1568 | 1213 | 1013 | |
| 120 | 3363 | 2917 | 1574 | 1220 | 1013 | |
The explained absorption moieties were also detected in the extracted lignin from another woody biomass.25 It is also reported that the spectral peak at 1600–1400 cm–1 is the vibration band of the aromatic ring,26 which is also evident from the peak assigned in the current study. Besides the spectral peaks explained in the above sections, an absorption band around 1330 cm–1 was also found, which belongs to the condensed syringyl and guaiacyl units.27
2.2. UV Spectroscopic Analysis
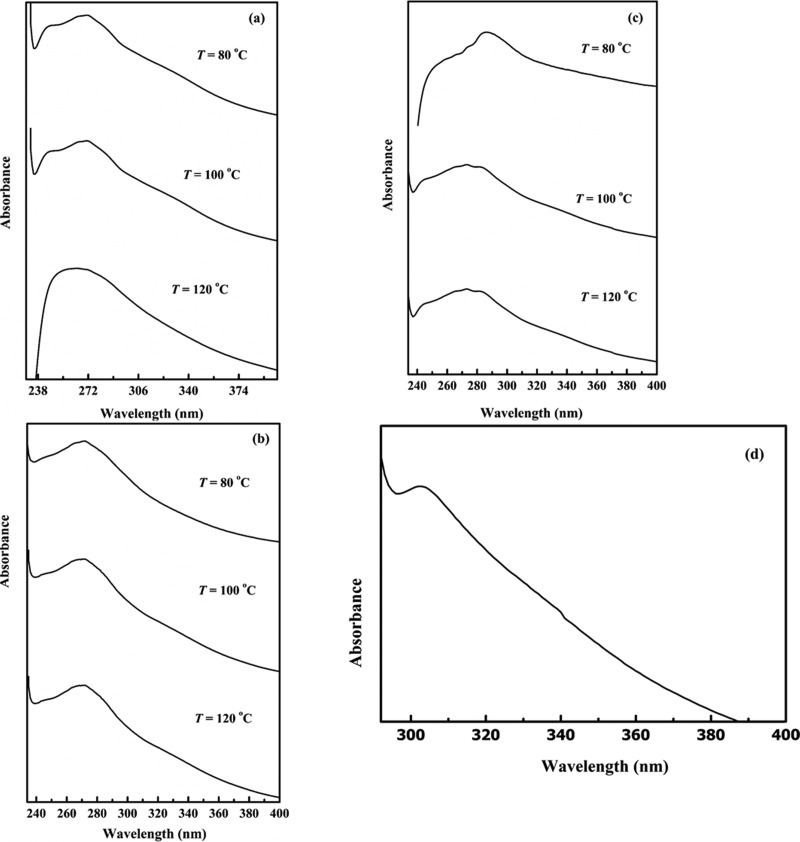
The samples of lignin extracted by different ionic liquids were also characterized by UV–vis spectroscopy technique. Figure 2a–d shows that lignin extracted by the ILs had different local maximum intensities of wavelength. For [DIPEA][Ac]-extracted lignin, the maxima of the peak shifted from 271 to 262 nm as the extraction temperature changed from 80 to 120 °C, indicating that the extracted compounds are getting smaller molecular weight with increasing temperature. The local maximum intensity of lignin extracted by [DIPEA][B] at 120 °C occurs at 303 nm, but the maximum intensities of lignin samples extracted by [DIPEA][Ac], [DIPEA][O], and [DIPEA][P] in this study are found to be at lower wavelengths in the same extraction conditions. It means that the lignin extracted by the ILs derived from aliphatic anions have lower molecular weight than that extracted by the ILs derived from aromatic anion under the same experimental conditions.
Figure 2.
UV spectra of coffee husk lignin extracted by (a) [DIPEA][Ac], (b) [DIPEA][P], (c) [DIPEA][O], and (d) [DIPEA][B].
It is worth mentioning that the UV analysis provides strong evidence for the existence of phenolic aromatic compounds in the sample solutions.28 The positions of the peaks on the UV spectra determine qualitatively the nature of the compounds. Wu et al.25 reported that lignin could absorb in different ranges, depending on whether the phenolic groups are condensed or noncondensed to aromatic ring, which may vary with the source and techniques used.
In the same manner, compounds having higher molecular weight show adsorption peak at longer wavelengths than those having lower molecular weight. In the current study, the absorption peaks of lignin extracted are found in the range of 245–303 nm for the samples taken from the experimental runs at 80–120 °C for 4 h. On the basis of the experimental results, the relative order of molecular weights of lignin extracted from coffee husk with different ionic liquids can be found. Under the same experimental conditions, the order of the averaged molecular weights of the lignin extracted follows the sequence [DIPEA][Ac] < [DIPEA][P] < [DIPEA][O] < [DIPEA][B], according to the position of peaks of the lignin samples by wavelength.
Liu et al.29 reported that lignin shows a maximum adsorption peak near 280 nm, which is good agreement with the results obtained from the ionic liquid-extracted lignin in the present study. The presence of the phenolic aromatic ring is also noted during the functional group analysis by FT-IR spectra.
2.3. Molar Mass Distribution of the Ionic Liquids-Extracted Lignin
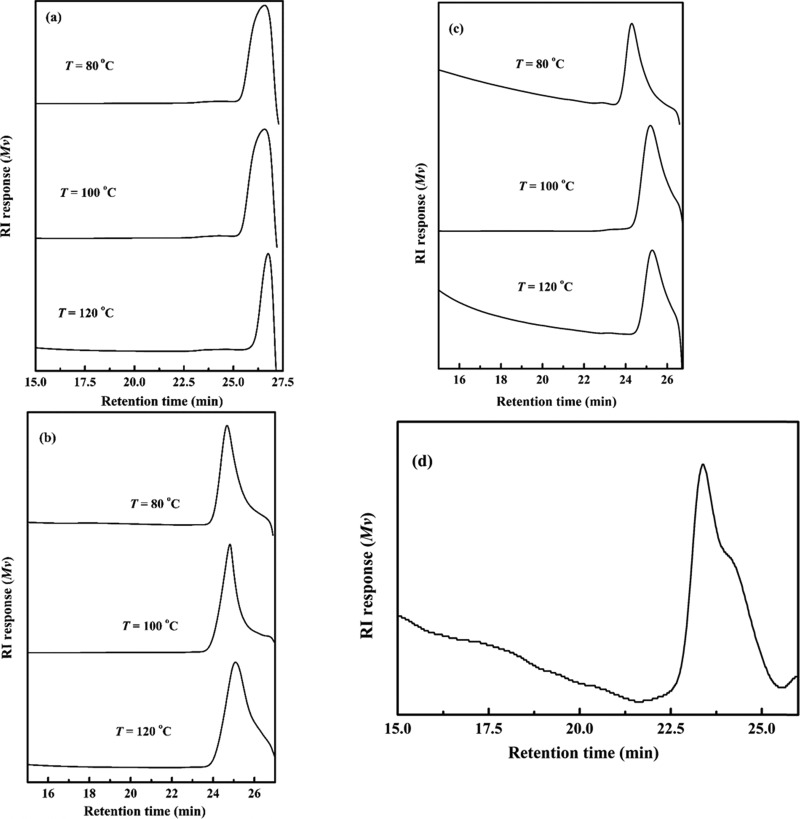
The molecular weight distributions of the lignin extracted from coffee husk by the ionic liquids are presented in Figure 3a–d. The average molecular weights (Mw and Mn) and polydispersity indices (PDIs) of [DIPEA][Ac], [DIPEA][B], [DIPEA][O], and [DIPEA][P] IL-extracted lignin were determined by gel permeation chromatography (GPC) analysis, and the results are depicted in Table 1. A higher value of average molecular weights is observed from the extracted lignin at lower temperatures. At higher temperatures, the presence of the lower average molecular weight indicates that in addition to the fractionation of the coffee husk to respective components of biomass (lignin, cellulose, and hemicellulose), the extracted lignin was degraded to its monomers and oligomers.
Figure 3.
GPC curves of coffee husk lignin extracted by (a) [DIPEA][Ac], (b) [DIPEA][P], (c) [DIPEA][O], and (d) [DIPEA][B].
For further confirmation of the degradation of lignin to lower-molecular-weight phenolic compounds, [DIPEA][Ac]-extracted solution at 120 °C for 4 h reaction was taken and the methanol-soluble portion (i.e., the major compounds obtained from the depolymerization of lignin) was analyzed with gas chromatography–mass spectrometry (GC–MS). The results of GC–MS analysis confirm that value-added chemicals were produced by treating the coffee husk with the ILs. The compounds in the product are identified from their mass spectra, which are benzeneacetic acid, 4-[(trimethylsilyl)oxy]-trimethylsilyl ester, N-benzyl-N-ethyl-p-isopropylbenzamide, and dimethyl sulfoxide (DMSO). This result is consistent with the results from GPC and UV–visible analyses. Chinnappan and Kim22 have also found similar fragmentation of the lignin product after extracting lignin from poplar wood by ionic liquids. The polydispersity indices (PDIs) of each ionic liquid-extracted lignin at 80, 100, and 120 °C were calculated. The values are in the range of 1.00–1.20 (as given in Table 2).
Table 2. Weight-Average (Mw) and Number-Average (Mn) Molecular Weights and Polydispersity Indices (PDI, Mw/Mn) of Lignin Extracted from Coffee Husk by Various Ionic Liquids.
| ionic
liquids used at various experimental
temperatures |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [DIPEA][Ac] |
[DIPEA][B] |
[DIPEA][O] |
[DIPEA][P] |
|||||||||
| T (°C) | Mw | Mn | PDI | Mw | Mn | PDI | Mw | Mn | PDI | Mw | Mn | PDI |
| 80 | 2200 | 2111 | 1.04 | ND | ND | ND | 3068 | 2852 | 1.08 | 2527 | 2403 | 1.05 |
| 100 | 1641 | 1638 | 1.00 | ND | ND | ND | 2753 | 2362 | 1.16 | 2521 | 2389 | 1.06 |
| 120 | 1582 | 1581 | 1.00 | 5796 | 5093 | 1.14 | 2735 | 2273 | 1.20 | 2238 | 2124 | 1.05 |
In comparison to the aromatic anion-derived ionic liquids, the ionic liquids with shorter chains of the aliphatic anion produce lower molecular weight of lignin extracted. It indicates that the aliphatic anions have stronger interaction with the biomass than the aromatic anions, having the same cation source. In this work, the acids used as the anion source for the synthesis of the ionic liquids are acetic acid, propanoic acid, octanoic acid, and benzoic acid. Besides the extraction of cellulose, hemicellulose, and lignin from coffee husk, the aliphatic acids-derived ionic liquids facilitate the degradation of the extracted lignin. The retention time of the molecular weight distribution of the [DIPEA][B]-extracted lignin appears to be shorter than that of the lignin extracted by the other ionic liquids. This reveals that the lignin extracted with [DIPEA][B] has comparatively less interactions with respective ionic liquid and thus possesses a lower extent of degradation, which leads to lignin with higher average molecular weight. As the chain length of the anion of aliphatic groups increases, the average molecular weight of the lignin extracted also increases. This result is in good agreement with the investigation by UV–visible spectroscopy. This further confirms that the ionic liquids with shorter-carbon chains give the lower molecular weight of lignin extracted.
2.4. Thermogravimetric Analysis (TGA)
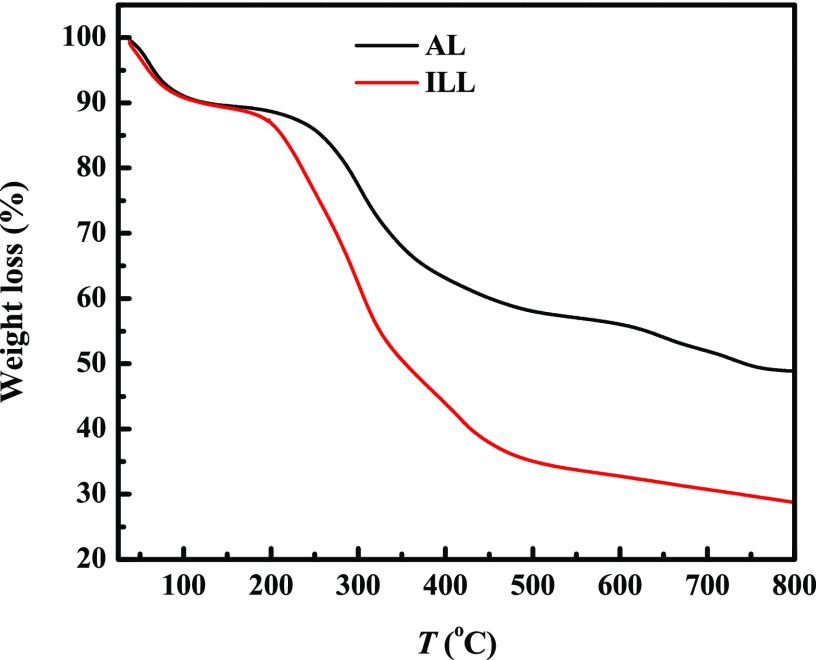
TGA under nitrogen environment is one of the crucial techniques for the study of thermal stability and decomposition of lignocellulosic biomass.30 To determine the thermal stability of lignin extracted from coffee husk by the ionic liquids, TGA study was conducted. Structurally, lignin is composed of phenylpropanoids connected with various ether bonds and carbon–carbon bonds mostly by β-O-4, with these chemical bonds leading to a wide range of degradation temperature from 100 to 800 °C. [DIPEA][Ac]-extracted coffee husk and commercial alkali lignin were taken and subjected to TGA analysis for comparison. After TGA runs, 28% of ionic liquid-extracted lignin and 48% of alkali lignin still remained unvolatized at 800 °C due to the formation of highly condensed aromatic structures, which have the ability to form char, as presented in Figure 4.
Figure 4.
Thermal decomposition profiles of alkali lignin (AL) and extracted lignin using [DIPEA][Ac] (ILL) at 120 °C after 4 h treatment.
Degradation of the lignin samples can be divided into three stages. The range of temperature and the weight loss percent in each stage are presented in Table 3 for both types of lignin. In stage I, the evaporation of absorbed moisture occurred. Decomposition of carbohydrates presented in the lignin to volatile gases, such as CO, H2, CO2, and CH4, is expected to occur at phase II.31
Table 3. Values of the Percent Weight Loss at Various Temperature Ranges for Different Sources of Lignin.
| types of lignin |
||||
|---|---|---|---|---|
| ionic liquid-extracted lignin |
alkali lignin |
|||
| degradation stages | range of T (°C) | % wt loss | range of T (°C) | % wt loss |
| phase I | 38–100 | 10 | 38–110 | 10 |
| phase II | 190–340 | 35 | 220–390 | 24 |
| phase III | above 340 | 24 | above 390 | 15 |
The thermogravimetric analysis of wood species was conducted by Poletto et al.,30 who reported that lignin degradation occurs between 250 and 500 °C. The last phase covers a wide range of temperature, in which cracking of hydroxyl groups and volatile compounds derived from lignin are supposed to be removed.32 It is also reported that the source of lignin determines its thermal property. The lignin extracted from coffee husk has lower thermal stability (with char yield of 28%) than alkali lignin (with char yield of 48%).
2.5. X-ray Diffraction (XRD)
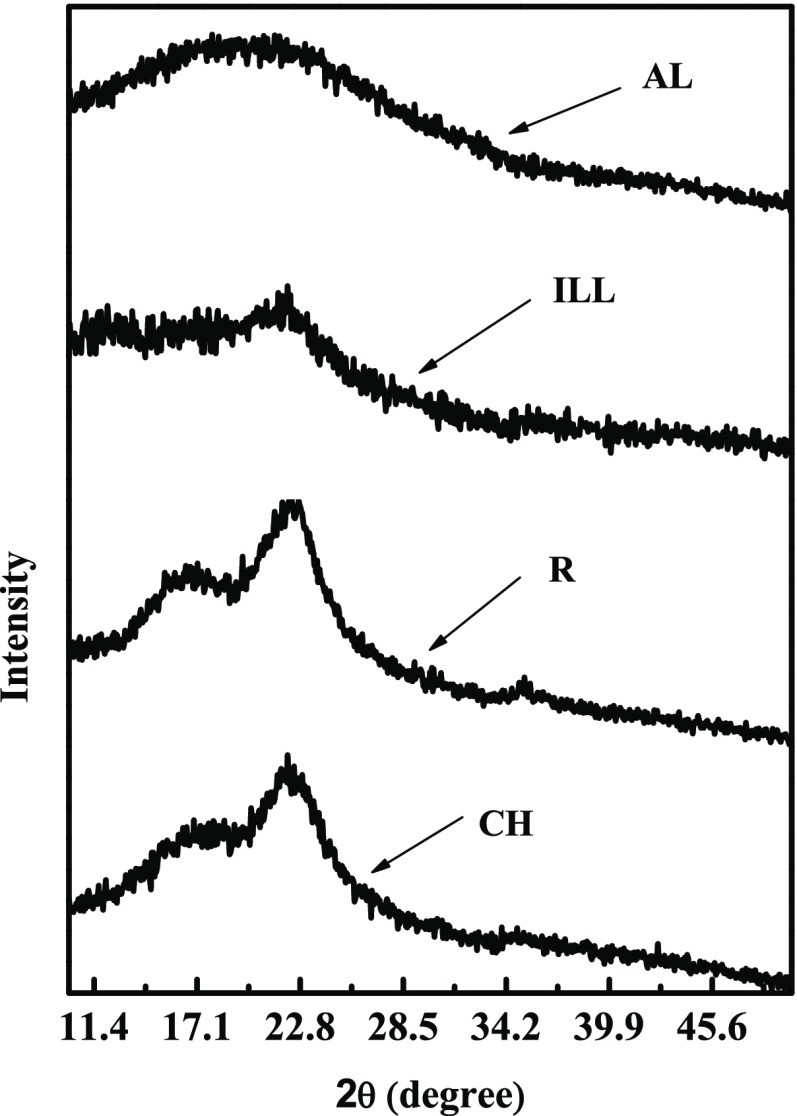
Evidences related to crystallinity or amorphousness of materials are obtained by XRD analysis.33,34 The XRD patterns of the coffee husk, the ionic liquid ([DIPEA][Ac])-extracted lignin, the residue (R) (cellulose and hemicellulose), and the alkali lignin are presented in Figure 5. The coffee husk has diffraction angles at 2θ = 16.7, 22.1, and 35.2°. After ionic liquid treatment at 120 °C for 4 h, the position of diffraction angles are retained and the broadness decreases (strength of the signal increases) in the residue isolated. However, the diffraction angles at 2θ = 16.7 and 35.2° were diminished after separating the cellulose and hemicellulose, which is an evidence for the absence carbohydrates in the lignin detached (confirmation for the effective extraction of lignin by the ionic liquid from the biomass). The increased strength of the signal of the residue further confirms the crystallinity of the material (property of cellulose).
Figure 5.
XRD patterns of coffee husk (CH), residue (R), ionic liquid-extracted lignin (ILL), and alkali lignin (AL).
The ionic liquid-extracted lignin (ILL) has a diffraction angle at 2θ = 22.1°, while the commercial alkali lignin (AL) at 2θ = 19.6°; this variation could be due to the source and type of the lignin. The signal of the diffraction angle of ILL becomes weaker and broader than that of coffee husk, which shows more amorphousness of the polymer. Goudarzi et al.34 reported that the diffraction angle of lignin from biomass is diverse due to different sources yielding various types of lignin. Generally, the treatment of the raw coffee husk with the studied ionic liquids can directly fractionate the biomass to lignin and carbohydrate effectively.
2.6. Morphological Analysis (FESEM)
The morphologies of the untreated coffee husk, the ionic liquid-extracted lignin, and the carbohydrate-enriched materials (cellulose and hemicellulose) were analyzed by FESEM to investigate the change of morphology of the materials before and after processing. As seen from Figure S1 in the Supporting Information, the FESEM images show the differences in morphology of the original coffee husk, the ionic liquid ([DIPEA][Ac])-extracted lignin, and the carbohydrate-enriched materials separated from the biomass. The untreated coffee husk has a smooth surface with cracks. Before any chemical modification, the surface of the coffee husk has wax and other components of biomass like pectin, lignin, and hemicellulose, which cover the cellulose within and providing protection against external environmental stresses.
After treatment with ionic liquids at 80, 100, and 120 °C, the interaction of the biomass with the ionic liquid and the exposure of bundles of cellulose fiber facilitate the extraction of lignin. From these images, it can be observed that the lignin fraction morphology is composed of a smaller irregular shape, as well as exhibits a porous surface and distorted structure. The morphology of carbohydrate-enriched materials has crystalline properties. It appears that carbohydrate-enriched materials show interconnected porous structure. Li et al.35 studied the two-step treatment with ionic liquids and alkali for enhancing enzymatic hydrolysis of Eucalyptus: chemical and anatomical changes. They found that the morphology of the cell walls faced disruptions such as disorder and distortion, which are observed when pretreated with [Bmim][OAc] and [Emim][OAc]. Another study was also conducted with an efficient process for pretreatment of corn stalk in functional ionic liquids.36 They found the compact ordered and rigid fibril structure from untreated corn stalk, and the structure became disordered after pretreatment of the lignocellulose.37 According to their finding, the loose structure after pretreatment with ionic liquids is probably due to the removal of lignin and decrease of cellulose crystallinity. The current study is also in good agreement with the previous reported morphologies of biomass before and after treatment with ionic liquids.
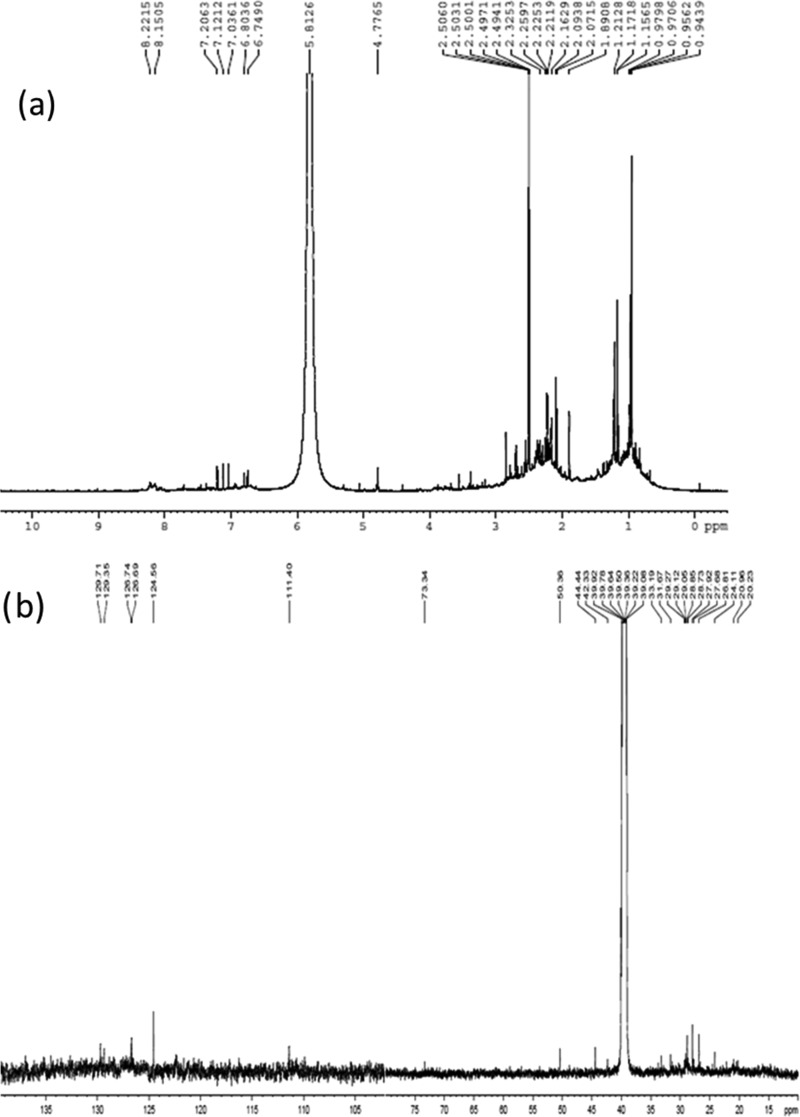
2.7. 1H and 13C NMR Spectra
For further confirmation of the chemical structure of lignin extracted from coffee husk, 1H, 13C, and two-dimensional (2D) NMR spectroscopy studies are conducted. The 1H and 13C NMR spectra are presented in Figure 6. As can be seen from Figure 6a, in the aromatic region of the spectra, the presence of G and S units is noted by the chemical shift at 6–8 ppm on proton NMR.25,38 Specifically, the integral peak located at 6.8 ppm is assigned to be aromatic protons in guaiacylpropane structure. The spectral peak that occurs at 0.95–1.2 ppm belongs to an aliphatic compound moiety.38 The spectra 2.1–2.2 ppm arise from methyl protons adjacent to the double bond. A sharp signal at 2.5 ppm relates to the protons in DMSO. The peak at 4.78 ppm comes from the Hβ of β-O-4 structures. The presence of characteristic chemical bonds such as C–C, C–O, and C=C was noted via analysis of hydrogens located at and/or near the atoms for samples under investigation.
Figure 6.
1H NMR (a) and 13C NMR (b) spectra of lignin fraction extracted by IL at 120 °C after 4 h treatment.
In addition to the proton NMR spectrum, the 13C NMR spectrum gives more information concerning the syringyl, guaiacyl, and p-hydroxyphenyl aromatic carbons, as they are located in the range of 104.5–166.6 ppm.39 The 13C NMR spectrum in Figure 6b at 111 ppm of chemical shift shows a guaiacyl unit,40 and the spectral peaks at 126.7–129.7 ppm represent condensed aromatic compounds.41 In most cases, the oxygenated carbon of aromatic compounds shows a peak around 140–154 ppm, which is currently absent in the coffee husk lignin characterized. Besides the aromatic compounds’ chemical shift noted in the 13C NMR spectrum of coffee husk lignin, the characteristic peak is observed at 73 ppm, which is Cα in β-O-4. In most chemical structures of lignin extracted from various biomasses, β-O-4 linkage is the common and the highest in occurrence.25 The aliphatic C–O on the lignin propyl side chain is noted at 24–26.8 and 29.1 ppm for Cα- and Cβ-methylene groups, respectively.
To further investigate the structural features of lignin extracted from the coffee husk, the sample was subjected to 2D HSQC NMR analysis. From the results of 2D HSQC analysis, as shown in Figure S2 in the Supporting Information, an aromatic region at δC/δH of 111–130/6.0–8.0 ppm and the aliphatic region at δC/δH of 24–73/0.9–5.8 ppm of an organic compound were observed.42 Both aliphatic and aromatic moieties are observed from the HSQC NMR spectrum of the isolated lignin by [DIPEA][Ac]. In the aromatic regions, the spectrum at δC/δH 127.0/6.7 ppm is attributed to the C2–H2, and Cα/β–Hα/β of the stilbenes units are noted. In this region, δC/δH of 131/6.9 ppm spectral peak occurs, which is a characteristic signal of the p-hydroxylphenyl unit for a carbon position at δC/δH of C2,6–H2,6.43 The presence of cinnamyl alcohol end groups is also revealed around the spectral peak at δC/δH of 128/6.8 ppm. Besides the aromatic groups, methoxy group on the ring is observed at δC/δH 57/3.5.44 Zikeli et al.45 reported that in the aliphatic region of HSQC, the presence of a peak of δC/δH at 50–90/2.5–5.8 ppm is an indication for β-O-4 linkage. In the same manner, the spectral peak is observed for δC/δH at 57/3.5 ppm, which is in good agreement with the previous piloted studies.
A recent study showed that the NMR spectra (1H, 13C and 2D HSQC) approved the characteristic structural features of lignin extracted from other biomasses, which is also noted in lignin extracted from the coffee husk. Structurally, lignin is composed of three phenylpropanoids interlinked via the C–O linkage of α- and β-ether bonds.46 It is reported that β-O-4 is the dominant one and covers half of the total chemical bonds used to link each monomer, and the value varies from biomass to biomass.47 This chemical bond is also noted in the current work, and the result is in good agreement with the previous report. In their monomeric form, these compounds may include p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol. But these monomers, linked through chemical bonds, produce p-hydroxyphenyl, guaiacyl, and syringyl residues from the polymer.1 The presence of these monomeric units of lignin polymer extracted from coffee husk is also confirmed by the structural analysis conducted in this work.
2.8. Quantification of Lignin Extracted by ILs
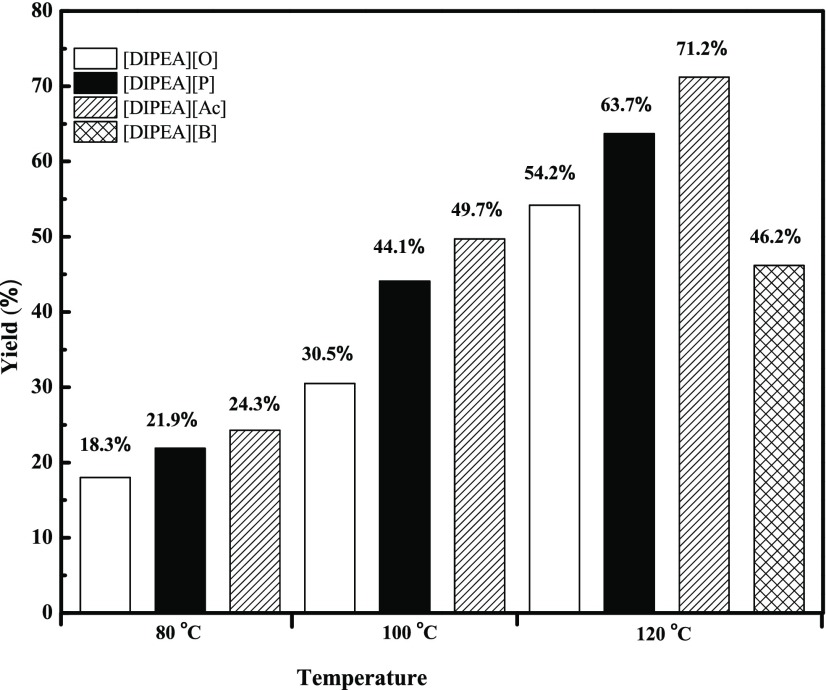
The acid-insoluble lignin content in the original coffee husk was determined using a scaled down TAPPI 222 procedure,48 and the value is found to be 28.4% on mass basis. The percentages of the original lignin extracted (yields) by various ionic liquids at different temperatures after 4 h treatment are presented in Table 4. Accordingly, the yield of the extraction and degradation processes for each IL at each temperature is shown in Figure 7. The coffee husk volatile solids and ash content (on mass basis) are also determined and found to be 92.3 and 7.7%, respectively.
Table 4. Yield of Lignin Extracted by Ionic Liquids at Various Experimental Temperatures.
| 102 yielda (%) |
||||
|---|---|---|---|---|
| T (°C) | [DIPEA][Ac] | [DIPEA][P] | [DIPEA][O] | [DIPEA][B] |
| 120 | 71.2 | 63.7 | 54.2 | 46.2 |
| 100 | 49.7 | 44.1 | 30.5 | ND |
| 80 | 24.3 | 21.9 | 18.3 | ND |
Yield = mass percentage of the extracted lignin from coffee husk/28.4%.
Figure 7.
Percentage of lignin extracted (yield) by ILs at various temperatures after 4 h treatment.
We have investigated ammonium-based ionic liquids for the dissolution of coffee husk with cleaner process and eco-friendliness. During treatment of biomass by ILs, the structure of the IL plays a great role. In the current work, we have subjected four ionic liquids to extract lignin from coffee husk and the results show a wide variation under similar condition. Since the ILs used are derived from the same cation with varying anions, the solubility seems to be strongly affected by the choice of anion, although temperature of reaction is also a determinant factor. It is reported that at lower temperatures or at room temperature, some anions such as trifluoromethanesulfonate (triflate, [OTf]−), methyl sulfate ([MeSO4]−), chloride, bromide, and acetate are assigned as inhibitors of lignin dissolution.49 But at higher extraction temperatures, the yield of the ionic liquid to isolate lignin increases.
As can be observed in Figure 7, as the length of the carbon chain of anion increases, the yield of biomass extraction decreases. The anions incorporated for the extraction of lignin in the present study are acetate, propanoate, octanoate, and benzoate. In the present work, using [DIPE][Ac] for extraction at 120 °C for 4 h, 71.2% of original lignin is extracted from coffee husk. By increasing the number of carbon of the anion by one, the yield decreases by 7.5%. Furthermore, by increasing the chain length from acetate to octanoate, the yield drops by 17%. This indicates that with the increase of the chain length or decrease of the acidity of the aliphatic anion, the dissolution of coffee husk as well as yield of the extracted lignin decreases. A similar effect of acidity has been reported in the literature,50 and it is well known that the acidity of the ionic liquid used for biomass treatment has a direct relationship with the progress of the process. Additionally, by reducing the extraction temperature from 120 to 80 °C at the same time interval for acetate-derived ionic liquids, the yield decreased by 46.9%. These show that besides the nature of anions of the ionic liquids applied, temperature also plays a vital role in the biomass extraction and degradation.
3. Conclusions
This study shows that ammonium-based ionic liquids, [DIPEA][Ac], [DIPEA][P], [DIPEA][O], and [DIPEA][B], can be used not only for dissolving the coffee husk, but also for extracting and degradating its lignin content under mild conditions. The structure of ionic liquids and temperature are found to be the crucial factors for the performance of the extraction–degradation process. Decrease of the hydrophobicity of anion and increase of extraction temperature will promote the yield of the lignin extraction. The highest yield, 71.2%, is achieved as the coffee husk is treated with [DIPEA][Ac] at 120 °C for 4 h. Moreover, the extracted lignin has been characterized by FT-IR, GPC, GC–MS, UV–vis, 1H and 13C NMR, HSQC NMR, TGA, XRD, and FESEM techniques. Value-added compounds can be obtained from the waste coffee husk by using the proposed ammonium-based ionic liquids, especially [DIPEA][Ac].
4. Materials and Methods
4.1. Materials
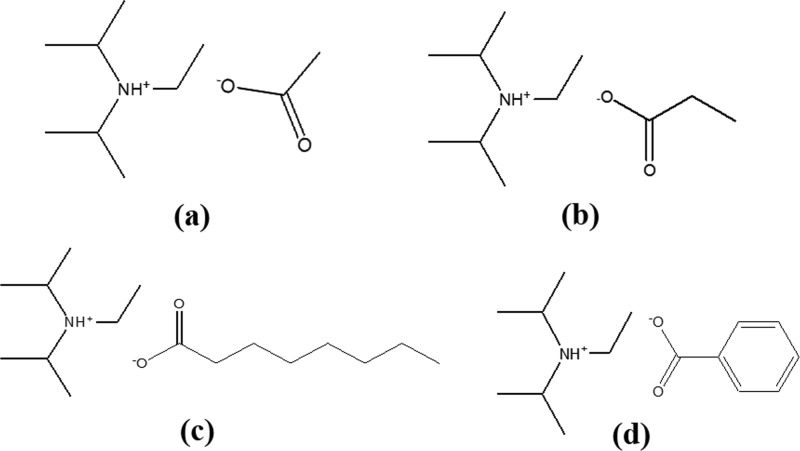
Coffee husk was collected from Mettu town, Oromia National Regional State, Ethiopia, and transported to Taipei, Taiwan. Octanoic acid, acetic acid, benzoic acid, propanoic acid, methanol, methyl sulfoxide-d6, and sodium nitrate were purchased from Sigma-Aldrich. Diisopropylethylamine and acetone were supplied by Acros Organics and sulfuric acid by Shimakyu (Japan). All of these commercial chemicals are analytical reagents and used without further purification. Double-distilled deionized water was prepared by the Nanopure-Ultrapure water system with a resistivity of 18.3 MΩ cm. The ionic liquids, [DIPEA][Ac], [DIPEA][P], [DIPEA][O], and [DIPEA][B], used in this study were synthesized in our laboratory according to the method reported in our previous paper.21 The structures of these selected ionic liquids are presented in Figure 8.
Figure 8.
Structures of the ionic liquids: (a) [DIPEA][Ac], (b) [DIPEA][P], (c) [DIPEA][O], and (d) [DIPEA][B].
4.2. Lignin Extraction from Coffee Husk
The coffee husk was ground into powder using an electric lab mill for 1 min. The husk was ground and classified with a series of brass sieves (40–80 mesh) and then dried overnight in an oven at 60 °C. In the extraction process, 0.50 g of bagasse was added to 10 g of IL in a 50 mL glass equilibrium cell and a magnetic stirrer was used to promote the mixing. The cell was connected to a thermostatic circulator (Fisher Scientific International, Inc.), which was adjusted to a desired temperature. The extraction (or reaction) time was tested from 1 to 4 h time intervals at 120 °C. The mixture in the cell became homogeneous when the extraction time is as long as 4 h and thus it was taken as the reaction time for the whole study. To investigate the temperature effect on the yield of lignin extraction from coffee husk, the experiments were conducted at 80, 100, and 120 °C, respectively, for 4 h.
4.3. Product Separation and Characterization
To separate different classes of compounds from the product mixture, the bagasse/IL solution was poured into a 250 mL beaker containing 100 mL of acetone/water (1:1 v/v). The beaker was sealed with Parafilm, and the mixture was stirred at room temperature for 1 h. The precipitated cellulose-rich materials were separated by filtration and washing through a ceramic funnel with nylon filter paper on a Buchner flask under soft vacuum. Then, acetone and water were removed from lignin/IL by a rotary concentrator under reduced pressure. Finally, the lignin was isolated from IL by dialysis using distilled water for 3 days with water exchanged at 8 h interval. The separated lignin sample was analyzed with various analytical techniques for characterization.
The samples of IL-extracted lignin at different reaction temperatures were collected, and the FT-IR spectra were recorded on a Nicolet FTIR-iS10 spectrometer in the range of 4000–650 cm–1 IR region at a spectral resolution of 8 cm–1 and 200 scans. The IR spectra were collected and analyzed by the spectroscopic software Varian 4.10. Absorption spectra for the samples of ionic liquid-extracted lignin at various extraction temperatures were measured at room temperature by using a UV–visible spectrophotometer (JASCO, V-550, Japan) equipped with a 1.0 cm quartz cell. The operating conditions were scan speed, 100 nm min–1; scan range, 200–400 nm; slit width, 2 nm; and l = 0.1 nm. The samples for UV–visible analysis were prepared by diluting the IL-extracted lignin in ethanol.
The molecular weight distribution of the extracted lignin was also measured by gel permeation chromatography (GPC). The mobile phase and sample solutions were microfiltered with a 0.2 μm membrane filter and degassed. The sample solution (2 mg mL–1) was prepared in degassed distilled water and injected into the GPC (aqueous phase, Waters 1525 HPLC pump, 2489 UV detector; aqueous phase, Waters column 3: WATO-11520 (7.8 × 300 mm), WATO-11530 (7.8 × 300 mm), WATO-11545 (7.8 × 300 mm)). Aqueous solution of 0.05 M NaNO3 was used as the mobile phase at a flow rate of 0.5 mL min–1. The calibration curve was prepared by using Pullulan as a standard.
The MS fraction of methanol-soluble products was identified by GC–MS (HP model 6890 series GC system and 5973 mass selective detector) with an Agilent, DB-5MS capillary column (J&W Scientific, Inc.; length 30 m × I.D. 0.25 mm). The temperature program was 523–573 K with a heating rate of 10 °C min–1. Helium served as the carrier gas with a flow rate of 1.0 mL min–1. The National Institute of Standards and Technology library of mass spectroscopy was used for identification of the compounds. The 1H NMR, 13C NMR, and HSQC spectra of the IL-extracted lignin in deuterated dimethyl sulfoxide (DMSO-d6), after treatment at 120 °C for 4 h, were measured by using a Bruker-Biospin 500 MHz instrument. Each NMR spectrum was recorded by using tetramethylsilane as an internal standard and with an average of 120 scans.
The thermal profiles of the extracted lignin were measured by TGA (Pyris Diamond TG-DTA). During TGA measurement, the weighted sample of about 10 mg was heated from 40 to 800 °C at a heating rate of 10 °C min–1, under a dry nitrogen atmosphere. X-ray diffraction (XRD) measurements for coffee husk, commercial alkali lignin, IL-extracted lignin, and carbohydrate residue were made using Bruker D2-phaser diffractometer with a Cu Kα radiation source (λ = 1.54056 Å), scanning between 10 and 60° (2θ) at a scan rate of 2° min–1 in steps of 0.02°. The morphologies of the coffee husk, [DIPEA][Ac]-extracted lignin, and carbohydrate residue were examined by field emission scanning electron microscopy (FESEM, JSM 6500F, JEOL) and field emission transmission electron microscopy (TEM, JEOL-2100) coupled with energy-dispersive X-ray spectroscopy, which was used to determine the elemental content.
The lignin content in the original coffee husk was determined by the TAPPI method with a scale down process. Approximately 2.5 g of dried bagasse was placed in a 50 mL vial, and 25 mL of 72% H2SO4 aqueous solution was added. The mixture was stirred at room temperature for 2 h and then transferred to a 500 mL round-bottom flask, diluted with 300 mL of deionized water, and refluxed for 4 h. The solution was filtered and dried, and acid-insoluble lignin was determined gravimetrically.
Acknowledgments
The authors are grateful for financing provided by the Ministry of Science and Technology (MOST), Taiwan, through grants MOST 105-2221-E011-144-MY3 and MOST 105-2811-E-011-012, Mettu University, and the international student scholarship from NTUST.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01447.
Field emission scanning electron microscopy images for coffee husk, [DIPEA][Ac]-extracted lignin, and carbohydrate-enriched materials (Figure S1); HSQC NMR spectrum of lignin fraction extracted by IL at 120 °C after 4 h treatment in ionic liquids (Figure S2) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Calvo-Flores F. G.; Dobado J. A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. 10.1002/cssc.201000157. [DOI] [PubMed] [Google Scholar]
- Dimmel D. In Lignin and Lignans: Advances in Chemistry; Heitner C., Dimmel D. R., Schmidt J. A., Eds.; CRC Press- Taylor & Francis Group, 2010; pp 1–10. [Google Scholar]
- Dick T. A.; Couve J.; Gimello O.; Mas A.; Robin J.-J. Chemical modification and plasma-induced grafting of pyrolitic lignin. Evaluation of the reinforcing effect on lignin/poly(l-lactide) composites. Polymer 2017, 118, 280–296. 10.1016/j.polymer.2017.04.036. [DOI] [Google Scholar]
- García A.; Spigno G.; Labidi J. Antioxidant and biocide behaviour of lignin fractions from apple tree pruning residues. Ind. Crops Prod. 2017, 104, 242–252. 10.1016/j.indcrop.2017.04.063. [DOI] [Google Scholar]
- García-Mateos F. J.; Cordero-Lanzac T.; Berenguer R.; Morallón E.; Cazorla-Amorós D.; Rodríguez-Mirasol J.; Cordero T. Lignin-derived Pt supported carbon (submicron)fiber electrocatalysts for alcohol electro-oxidation. Appl. Catal., B 2017, 211, 18–30. 10.1016/j.apcatb.2017.04.008. [DOI] [Google Scholar]
- Xu F.; Zhu T. T.; Rao Q. Q.; Shui S. W.; Li W. W.; He H. B.; Yao R. S. Fabrication of mesoporous lignin-based biosorbent from rice straw and its application for heavy-metal-ion removal. J. Environ. Sci. 2017, 53, 132–140. 10.1016/j.jes.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Saarinen N. M.; Warri A.; Airio M.; Smeds A.; Makela S. Role of dietary lignans in the reduction of breast cancer risk. Mol. Nutr. Food Res. 2007, 51, 857–866. 10.1002/mnfr.200600240. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Wan C. Biological valorization strategies for converting lignin into fuels and chemicals. Renewable Sustainable Energy Rev. 2017, 73, 610–621. 10.1016/j.rser.2017.01.166. [DOI] [Google Scholar]
- Lee R. A.; Berberi V.; Labranche J.; Lavoie J. M. Lignin extraction--reassessment of the severity factor with respect to hydroxide concentration. Bioresour. Technol. 2014, 169, 707–712. 10.1016/j.biortech.2014.07.038. [DOI] [PubMed] [Google Scholar]
- Goldmann W. M.; Ahola J.; Mikola M.; Tanskanen J. Formic acid aided hot water extraction of hemicellulose from European silver birch (Betula pendula) sawdust. Bioresour. Technol. 2017, 232, 176–182. 10.1016/j.biortech.2017.02.032. [DOI] [PubMed] [Google Scholar]
- Constant S.; Basset C.; Dumas C.; Di Renzo F.; Robitzer M.; Barakat A.; Quignard F. Reactive organosolv lignin extraction from wheat straw: Influence of Lewis acid catalysts on structural and chemical properties of lignins. Ind. Crops Prod. 2015, 65, 180–189. 10.1016/j.indcrop.2014.12.009. [DOI] [Google Scholar]
- Serna L. V. D.; Alzate C. E. O.; Alzate C. A. C. Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 113–120. 10.1016/j.biortech.2015.09.078. [DOI] [PubMed] [Google Scholar]
- Luong N. D.; Binh N. T. T.; Duong L. D.; Kim D. O.; Kim D. S.; Lee S. H.; Kim B. J.; Lee Y. S.; Nam J. D. An eco-friendly and efficient route of lignin-based copolyester synthesis. Polym. Bull. 2012, 68, 879–890. 10.1007/s00289-011-0658-x. [DOI] [Google Scholar]
- Zhang J.; Deng H.; Lin L.; Sun Y.; Pan C.; Liu S. Isolation and characterization of wheat straw lignin with a formic acid process. Bioresour. Technol. 2010, 101, 2311–2316. 10.1016/j.biortech.2009.11.037. [DOI] [PubMed] [Google Scholar]
- Sun N.; Rahman M.; Qin Y.; Maxim M. L.; Rodriguez H.; Rogers R. D. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009, 11, 646–655. 10.1039/b822702k. [DOI] [Google Scholar]
- Platzer S.; Kar M.; Leyma R.; Chib S.; Roller A.; Jirsa F.; Krachler R.; MacFarlane D. R.; Kandioller W.; Keppler B. K. Task-specific thioglycolate ionic liquids for heavy metal extraction: Synthesis, extraction efficacies and recycling properties. J. Hazard. Mater. 2017, 324, 241–249. 10.1016/j.jhazmat.2016.10.054. [DOI] [PubMed] [Google Scholar]
- Dong S. J.; Zhang B. X.; Gao Y. F.; Hu X. M. An Efficient Process for Pretreatment of Lignocelluloses in Functional Ionic Liquids. Int. J. Polym. Sci. 2015, 2015, 1–6. 10.1155/2015/978983. [DOI] [Google Scholar]
- Lan W.; Liu C. F.; Sun R. C. Fractionation of bagasse into cellulose, hemicellulose and lignin with ionic liquid treatment followed by alkaline extraction. J. Agric. Food Chem. 2011, 59, 8691–8701. 10.1021/jf201508g. [DOI] [PubMed] [Google Scholar]
- Casas A.; Oliet M.; Alonso M. V.; Rodriguez F. Dissolution of Pinus radiata and Eucalyptus globulus woods in ionic liquids under microwave radiation: lignin regeneration and characterization. Sep. Purif. Technol. 2012, 97, 115–122. 10.1016/j.seppur.2011.12.032. [DOI] [Google Scholar]
- Sidik D. A. B.; Ngadi N.; Amin N. A. S. Optimization of lignin production from empty fruit bunch via liquefaction with ionic liquid. Bioresour. Technol. 2013, 135, 690–696. 10.1016/j.biortech.2012.09.041. [DOI] [PubMed] [Google Scholar]
- Tolesa L. D.; Gupta B. S.; Lee M. J. The chemistry of ammonium-based ionic liquids in depolymerization process of lignin. J. Mol. Liq. 2017, 248, 227–234. 10.1016/j.molliq.2017.10.054. [DOI] [Google Scholar]
- Chinnappan A.; Kim H. Environmentally benign catalyst: Synthesis, characterization, and properties of pyridinium dicationic molten salts (ionic liquids) and use of application in esterification. Chem. Eng. J. 2012, 187, 283–288. 10.1016/j.cej.2012.01.101. [DOI] [Google Scholar]
- Kim J. Y.; Shin E. J.; Eom I. Y.; Won K.; Kim Y. H.; Choi D.; Choi I. G.; Choi J. W. Structural features of lignin macromolecules extracted with ionic liquid from poplar wood. Bioresour. Technol. 2011, 102, 9020–9025. 10.1016/j.biortech.2011.07.081. [DOI] [PubMed] [Google Scholar]
- Gordobil O.; Egüés I.; Labidi J. Modification of eucalyptus and spruce organosolv lignins with fatty acids to use as filler in PLA. React. Funct. Polym. 2016, 104, 45–52. 10.1016/j.reactfunctpolym.2016.05.002. [DOI] [Google Scholar]
- Wu M.; Pang J.; Zhang X.; Sun R. Enhancement of lignin biopolymer isolation from hybrid poplar by organosolv pretreatments. Int. J. Polym. Sci. 2014, 2014, 1–10. 10.1155/2014/194726. [DOI] [Google Scholar]
- Gabov K.; Gosselink R. J.; Smeds A. I.; Fardim P. Characterization of lignin extracted from birch wood by a modified hydrotropic process. J. Agric. Food. Chem. 2014, 62, 10759–10767. 10.1021/jf5037728. [DOI] [PubMed] [Google Scholar]
- Azadfar M.; Gao A. H.; Bule M. V.; Chen S. Structural characterization of lignin: a potential source of antioxidants guaiacol and 4-vinylguaiacol. Int. J. Biol. Macromol. 2015, 75, 58–66. 10.1016/j.ijbiomac.2014.12.049. [DOI] [PubMed] [Google Scholar]
- da Silva C. G.; Grelier S.; Pichavant F.; Frollini E.; Castellan A. Adding value to lignins isolated from sugarcane bagasse and Miscanthus. Ind. Crops Prod. 2013, 42, 87–95. 10.1016/j.indcrop.2012.04.040. [DOI] [Google Scholar]
- Liu Z.; Meng L.; Chen J.; Cao Y.; Wang Z.; Ren H. The utilization of soybean straw III: Isolation and characterization of lignin from soybean straw. Biomass Bioenergy 2016, 94, 12–20. 10.1016/j.biombioe.2016.07.021. [DOI] [Google Scholar]
- Poletto M.; Zattera A. J.; Forte M. M.; Santana R. M. Thermal decomposition of wood: influence of wood components and cellulose crystallite size. Bioresour. Technol. 2012, 109, 148–153. 10.1016/j.biortech.2011.11.122. [DOI] [PubMed] [Google Scholar]
- Fang Z.; Sato T.; Smith R. L. J.; Inomata H.; Arai K.; Kozinski J. A. Reaction chemistry and phase behavior of lignin in high-temperature and supercritical water. Bioresour. Technol. 2008, 99, 3424–3430. 10.1016/j.biortech.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Giudicianni P.; Cardone G.; Ragucci R. Cellulose, hemicellulose and lignin slow steam pyrolysis: Thermal decomposition of biomass components mixtures. J. Anal. Appl. Pyrolysis 2013, 100, 213–222. 10.1016/j.jaap.2012.12.026. [DOI] [Google Scholar]
- Karimi K.; Taherzadeh M. J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. 10.1016/j.biortech.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Goudarzi A.; Lin L. T.; Ko F. K. X-ray diffraction analysis of kraft lignins and lignin-derived carbon nanofibers. J. Nanotechnol. Eng. Med. 2014, 5, 021006 10.1115/1.4028300. [DOI] [Google Scholar]
- Li H.-Y.; Chen X.; Wang C.-Z.; Sun S.-N.; Sun R.-C. Evaluation of the two-step treatment with ionic liquids and alkali for enhancing enzymatic hydrolysis of Eucalyptus: chemical and anatomical changes. Biotechnol. Biofuels 2016, 9, 166. 10.1186/s13068-016-0578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.; Baker J. O.; Himmel M. E.; Parilla P. A.; Johnson D. K. Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. 10.1186/1754-6834-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J. D.; Woiciechowski A.; Zandona F. A.; Noseda M. D.; Kaur B. S.; Soccol C. R. Lignin preparation from oil palm empty fruit bunches by sequential acid/alkaline treatment-A biorefinery approach. Bioresour. Technol. 2015, 194, 172–178. 10.1016/j.biortech.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Kang S.; Xiao L.; Meng L.; Zhang X.; Sun R. Isolation and structural characterization of lignin from cotton stalk treated in an ammonia hydrothermal system. Int. J. Mol. Sci. 2012, 13, 15209–15226. 10.3390/ijms131115209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Dong S. J.; Ma H. H.; Zhang B. X.; Wang Y. F.; Hu X. M. Fractionation of corn stover into cellulose, hemicellulose and lignin using a series of ionic liquids. Ind. Crops Prod. 2015, 76, 688–696. 10.1016/j.indcrop.2015.07.037. [DOI] [Google Scholar]
- Vivas N.; Nonier M. F.; Pianet I.; Vivas N.; Fouquet É Structure of extracted lignins from oak heartwood (Quercus petraea Liebl., Q. robur L.). C. R. Chim. 2006, 9, 1221–1233. 10.1016/j.crci.2006.02.005. [DOI] [Google Scholar]
- El Hage R.; Brosse N.; Sannigrahi P.; Ragauskas A. Effects of process severity on the chemical structure of Miscanthus ethanol organosolv lignin. Polym. Degrad. Stab. 2010, 95, 997–1003. 10.1016/j.polymdegradstab.2010.03.012. [DOI] [Google Scholar]
- Sette M.; Lange H.; Crestini C. Quantitative HSQC Analyses of Lignin: A Practical Comparison. Comput. Struct. Biotechnol. J. 2013, 6, e201303016 10.5936/csbj.201303016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y. Y.; Xiao L. P.; Shi Z. J.; Sun R. C. Structural variation of bamboo lignin before and after ethanol organosolv pretreatment. Int. J. Mol. Sci. 2013, 14, 21394–21413. 10.3390/ijms141121394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J.; Tong Z.; Wang L.; Zhu J. Y.; Ingram L. Isolation and structural characterization of sugarcane bagasse lignin after dilute phosphoric acid plus steam explosion pretreatment and its effect on cellulose hydrolysis. Bioresour. Technol. 2014, 154, 274–281. 10.1016/j.biortech.2013.12.072. [DOI] [PubMed] [Google Scholar]
- Zikeli F.; Ters T.; Fackler K.; Srebotnik E.; Li J. Wheat straw lignin fractionation and characterization as lignin-carbohydrate complexes. Ind. Crops Prod. 2016, 85, 309–317. 10.1016/j.indcrop.2016.03.012. [DOI] [Google Scholar]
- Jia S.; Cox B. J.; Guo X.; Zhang Z. C.; Ekerdt J. G. Cleaving the beta--O--4 bonds of lignin model compounds in an acidic ionic liquid, 1-H-3-methylimidazolium chloride: an optional strategy for the degradation of lignin. ChemSusChem 2010, 3, 1078–1084. 10.1002/cssc.201000112. [DOI] [PubMed] [Google Scholar]
- Mahmood N.; Yuan Z.; Schmidt J.; Xu C. Depolymerization of lignins and their applications for the preparation of polyols and rigid polyurethane foams: A review. Renewable Sustainable Energy Rev. 2016, 60, 317–329. 10.1016/j.rser.2016.01.037. [DOI] [Google Scholar]
- Frei M. Lignin: characterization of a multifaceted crop component. Sci. World J. 2013, 2013, 436517 10.1155/2013/436517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffar S. H.; Fan M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenergy 2013, 57, 264–279. 10.1016/j.biombioe.2013.07.015. [DOI] [Google Scholar]
- Brandt A.; Gräsvik J.; Hallett J. P.; Welton T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. 10.1039/c2gc36364j. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.