Abstract
Within 35 years (2049) the global population will reach an estimated nine billion people. This presents a massive challenge to agriculture: how do we feed all of these people with nutritious food in a sustainable way?
Presently the yields of most major crops are stagnating while the demand for food, both grain and animal protein, is growing. To meet the challenge of improving yields requires a constant commitment to generating a steady supply of improved cultivars and lines for all major crops. Conventional breeding cannot keep pace with what is required; to meet the targets biotechnology and the production of genetically-modified (GM) crops is filling the gap. However, there are still concerns as to the safety of GM crops for human consumption and the environment. In this review I explore the need for GM crops, the way they are produced, and their impact and safety.
The future is very promising for GM technologies to meet the future global needs for food feed and fiber in a sustainable and responsible way. GM crops are only one part of the solution. To meet the targeted yields, nutritional quality, and sustainable production, we need all of the tools at our disposal including conventional and organic food production systems.
Introduction
In August of 2013 anti-GMO (Genetically-Modified Organisms) activists destroyed the Philippine Department of Agriculture’s field trials of Golden Rice, a rice variety genetically-modified to deliver high levels of β-carotene in the seed (See Figure 1). Within the scientific community there was a rapid and unprecedented condemnation of this action, led by a widely signed petition and a strongly worded letter to science in support of GMOs by a cadre of highly respected and prominent scientists.1 This outrage and widespread condemnation by the scientific community had not been forthcoming following the many similar destructive acts perpetrated on research fields involving GMOs. However, the primary reason for such a vigorous response was that “Golden Rice” (http://www.goldenrice.org/) was a community supported effort to meet a critical humanitarian need. β-carotene is a precursor of vitamin A, an essential component of rhodopsin the fundamental light absorbing pigment in the human eye. A chronic deficiency of vitamin A in the diet leads to blindness and a compromised immune system. It is an all too common affliction in the world’s poverty stricken and malnourished, claiming the sight of half a million children a year and the lives of almost half of them. According to a recent study2 vitamin A supplements reduce the mortality rate in children aged six months to five years by 24% and deliver a large reduction in poor vision and blindness. Golden Rice was envisioned as a non-commercial venture to deliver a cheap and effective (easy to distribute and deliver) dietary source of vitamin A for areas of the world where rice is the staple and often the main source of nutrition. Golden Rice, developed by the research teams of Ingo Potrykus and Peter Beyer, has taken 25 years to reach the point where field trials can be undertaken. Nearly all scientific and regulatory hurdles have been successfully navigated. This effort took an unmatched partnership between public and private sectors to fund and required private concerns to agree to release the intellectual property rights free of charge for the many patented components involved in the gene constructs.
Figure 1.
Golden Rice (far right, yellow color) was envisioned as a non-commercial venture to deliver a cheap and effective (easy to distribute and deliver) dietary source of vitamin A for areas of the world where rice is the staple. Source: Wikipedia
Why was the reaction to the Golden Rice incident limited to the scientific community? The answer to that question is a complex one, but at its root is a lack of understanding of both Genetically-Modified Organisms (GMOs) as they pertain to crops and the food supply and the depth of the problem that agriculture faces over the next two decades and beyond. I have deliberately focused on agriculture and plants in the preceding sentence because GMOs have been fully accepted in the medical arena. Recombinant proteins are widely used to develop effective treatments of a variety of diseases and ailments and there has been no effort to ban them or to vilify the practice of producing them. The prime example of this is the use of genetically-modified bacteria to produce human insulin3 widely used in the treatment of diabetes. Biopharmaceuticals, the products obtained from the use of GMOs, were well established in the 1980s4 and have since been fully accepted. Their benefits and risks are well understood. GM crops have not experienced this widespread acceptance and remain controversial for many people and advocacy groups.5
The Need for GMOs
Before I discuss GM crops, how they are produced, what GM crops are currently grown and will be available in the future, I think it is important to understand why there is such a commitment to developing them.
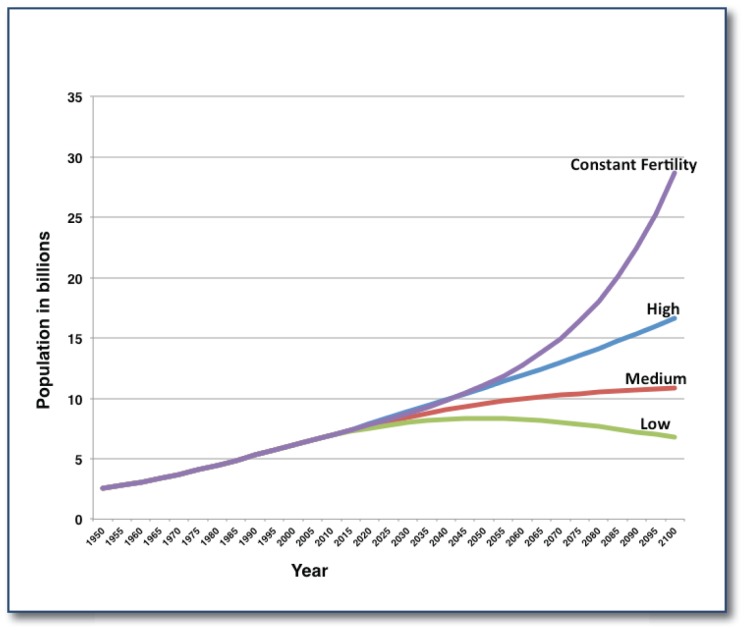
At the time of writing, the global human population is approximately 7.15 billion according to the U.S. Census Bureau population clock (http://www.census.gov/popclock/). The United Nations predicts, depending upon which growth model is used, that by 2030 (only 16 years from now) the global human population will be between 8.9 (high) to 7.9 (low) billion, and by 2050 somewhere between 10.9 and 8.3 billion (See Figure 2). The majority of the population growth will occur in what are now designated as developing countries (http://www.landcommodities.com/farmland-supply-and-investment-fundamentals/). The U.N. Food and Agricultural Organization (FAO) reported that in 2012 a total of 868 million people were suffering from hunger and malnutrition, just over two-thirds of which (563 million) live in Asia and the Pacific and a quarter (234 million) in Sub-Saharan Africa.6 Although these figures have declined from the 1,000 million people level recorded in 1990, there is still a long way to go. Consider that the death toll from hunger and malnutrition, a curable condition, is greater than “for AIDS, malaria and TB combined” (http://www.wfp.org/stories/what-need-know-about-hunger-2012). This is a complex issue with many socioeconomic and political ramifications. A major factor that drives any realistic solution is the need to match the rate of increase in global demand with rate of increase in yields of staple crops (primarily grains), feed, and livestock (including fish). Tilman et. al7 concluded that to provide sufficient food to cope with the increase in the global population, agricultural production would have to double by 2050. Even the more conservative FAO estimates that agricultural production must increase by at least 60% globally (77% in the developing economies) in the same time frame.6 In practical terms, if we focus on just the major global crops: maize, wheat, rice, and soybean (66% of calories in the “global” diet) this would require an annual increase in yield of 2.4%.8 On a global level the current rates of increase for these four crops are 1.6% for maize, 0.9% for wheat, 1% for rice, and 1.3% for soybean which is significantly less that what is required.8 On a regional level there are areas of the world that will double agricultural yields by 2050, primarily in regions where population growth is somewhat stable. There are large portions of the world that will not be able to come close to such a goal even for one of the four major crops. Historical analyses of yield data from over 13,500 political units across the globe reveal areas where current yield gains have stagnated or declined under the current agricultural production systems.9, 10 To reverse these trends and to achieve the necessary yield gains on a yearly basis is a daunting task. It will require both an ongoing improvement in the genetics of our major crops (and livestock) and how we manage our cropping and animal production systems. These improvements will have to be tailored to regional and local needs and environments as well as ensure that the agricultural systems we put in place are sustainable. Such a task will require all of the tools we have at our disposal and the development of new ones.
Figure 2.
Predictions of future global population growth.
Source: Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat, World Population Prospects: The 2012 Revision, http://esa.un.org/unpd/wpp/index.htm
The problems we face are compounded by several complicating factors foremost of which is the finite amount of arable land that we have available for agriculture. The FAO baseline scenario predicts that by 2050 there will be approximately 0.18 hectares of arable land available for food production for each person on the planet, down from the current 0.242 hectare value.11 The consensus of opinion, formulated from this FAO study is that the global yield increases required to meet future demands must be obtained from the same area of land that is currently under cultivation. Without the ability to farm more acreage yield increases must come from genetic improvement or greater agricultural inputs (fertilizer, water, and pest/weed management). The baseline scenario does not take into account additional demands on the land for biofuel feedstock production or possible alterations in land use driven by urbanization, desertification, salinization, and soil degradation. Also the increase in demand for animal protein in the diet alters land use from crops to pasture as prosperity comes to developing economies. All of these factors have the potential to further reduce the amount of arable land available for food production. Putative global climate change scenarios and water resource problems further complicate the task of maintaining arable lands for agriculture. These factors also add specific challenges to crop improvement through genetics and improved cropping systems as they directly affect crop yields rather than simply limit farmable acreage.12
To meet the challenge of improving crop yields each season requires a constant commitment to generating a conveyor belt of improved cultivars and lines for all of the major crops. Such a commitment has been in place since organized breeding programs were established in the 18th century even though genetic principles were not yet understood (pre-Mendel). Conventional breeding programs were able to sustain and advance yields for the staple grain crops to keep up with the demand for food in the developed countries of Europe and North America. In the late 1950s and early 1960s large areas of Asia were facing widespread famine. Fortuitously, in the 1940s a very insightful plant breeder, Norman Borlaug, had seen the danger of such a catastrophe occurring in Mexico and had initiated a breeding program for high-yielding and disease resistant wheats. His success in doing so, coupled with the development of new mechanized agricultural technologies and cropping systems, resulted in the aversion of famine in Mexico and allowed the country to becoming a net exporter of wheat by the early 1960s.13 Borlaug, through his tireless advocacy and the foresight of Asian governments, was able to translate his success with wheat to the development of a high-yielding disease resistant rice cultivar, IR8, which was quickly adopted. Mass famine was averted and Borlaug was credited with saving a billion lives by his breeding and advocacy efforts. He was awarded the Nobel Peace Prize in 1970. It is clear that we are currently facing a similar crisis to the one that Borlaug saw coming in the 1940s. It is also clear that conventional breeding, as practiced in the 20th century, will not be the entire solution this time around.
Conventional breeding relies on the introduction of new traits/genes into existing cultivars or commercial lines by sexual crosses i.e. crossing of one parental line with a second parental line that is expressing the desired trait (disease resistance, drought tolerance etc). Such a cross results in progeny that have inherited a complete set of genes from both parents so that although they have inherited the desired trait they have inherited a multitude of others, some of which may not be desirable and may reduce yield (a phenomenon called yield drag). To reduce yield drag breeders select progeny that best express the desired trait and cross it back to one of the parent plants in order to dilute out the negative traits inherited in the first cross (backcross). Through several iterations of this backcrossing scheme breeders eventually end up with a high yielding line that carries the desired trait. To achieve this requires many generations and several years (10 to 15years for wheat depending on the starting material) before lines can be tested in an agronomic setting or, as in the case for corn, used as a parental line in the production of commercial hybrids. Conventional breeding is also limited to what genetic variation is available in the gene pool of the crop or in a close relative that is sexually compatible. The search for genetic variation (gene variants) that can impact yield and productivity becomes more and more difficult and the incremental increases in yield become smaller and smaller with time. Yield is a complex phenotype and is the sum of the activity of a multitude of genes and rarely lends itself to rapid yield gains. Norman Borlaug’s lines dramatically altered crop yields not only by increasing the number of seeds per plants but also by adapting the plants to mechanized and high density cropping systems. Modern conventional breeding programs use varieties that are well adapted to modern production agriculture and thus yield gains based solely on plant performance are not as dramatic as those witnessed in the “Green Revolution” (http://en.wikipedia.org/wiki/Green_Revolution).
Modern breeding programs are also focused on nutritional and compositional qualities of the final product, whether it be grain, bean, fruit, or vegetative outputs. Variation can be increased using mutagenesis (chemical or radiation) but this is not selective and introduces many genetic changes into the crop and extends the breeding timeline. Currently our efforts fall short of the desired target yield increase rates (2.4% per year) if food production is to keep pace with the growing population. Modern breeding efforts are starting to be driven by molecular and genomics driven technologies, such as marker assisted breeding and genotyping-by-sequencing. These promise to dramatically reduce breeding timelines and fuel the rapid discovery of here-to-with untapped genetic variation. Thus although there are limitations, conventional breeding has a major role to play.
If we are to succeed in doubling global agricultural production for both crop and animal food commodities we need to be able to reduce the production timelines for both plant and animal breeding programs and to introduce new sources of genetic variation that improve yield potential, nutrition, and lower yield losses from disease and environmental factors such as changing climate and soil depletion. This is where biotechnology and the development of Genetically-Modified Organisms comes into its own and along with conventional breeding, molecular and genomic assisted crop/livestock improvement and novel genetic modification technologies, may be the vital tools that gets us to the critical goal of sustainable global food security. GMO technologies offer more rapid crop improvement, novel genetic strategies for crop improvement, and the ability to use genes from all sources regardless of origin from within the tree of life.
For the remainder of this review I will be concentrating the narrative on GM crops rather than the more universal use of the term GMO. For information regarding GM farm animals and fish I refer the reader to the recent review by Forabosco et al.14
What Are GMOs?
The term Genetically-Modified Organism is amorphous and somewhat imprecise. All of our crops and livestock are GMOs in that their genetics have been manipulated and designed by man over the last 10,000 years or more. This has occurred to such an extent that most barely resemble their wild progenitors. The majority could not compete or survive long outside of an agricultural setting. The FAO and the European Commission define a GMO, and the products thereof, as being plants or animals that are produced through techniques in which the genetic material has been altered in a way that does not occur naturally by mating and/or natural recombination. Although this is a closer description of what is meant in the general usage of the term GMO, it would also encompass several crops that have long been accepted as conventional, e.g., Triticale. Triticale is a grain crop commonly used in bread and pasta that was developed to offer a more nutritious food source (higher protein and low gluten). It is totally “man-made.” It was first developed in the laboratory in 1884 by crossing wheat with rye to form a sterile hybrid which would not survive in nature. To produce the crop, fertility had to be restored, and this was achieved by chromosome doubling to form a stable polyploid plant with two copies of each of the parental genomes (rye and wheat).15 This was achieved in the late 1930s using in vitro culture technology and treatment of embryos with the chemical colchicine, which interferes with the normal process of cell division (mitosis) to generate polyploid cells. Clearly, this is a crop that would fit the FAO definition of a GMO but it is not designated as such. Perhaps a better definition would be a modification to The Cartagena Protocol16 definition for “living modified organisms,” which would then read, “Genetic Modified Organism” means any living organism that possesses a novel combination of genetic material obtained through the use of modern biotechnology.
I would suggest something new and less obtuse such as Biotechnologically Modified Organism (BMO) but for the sake of convention I will stick to the use of GMO as defined in the aforementioned modified Cartagena Protocol definition.
How Do We Produce GM Crops?
To transfer genes into a crop plant to generate a GMO, (a transgenic plant) generally requires a two-step process:
Successful delivery of the gene into a plant cell(s), called transformation, and
The regeneration of a transgenic plant, primarily in tissue culture, from the transformed cell(s).
The transferred gene, termed a transgene, is usually engineered to control when and in what tissue it is expressed so that the maximum benefit can be realized. Gene delivery into plant cells is generally achieved in one of two ways: either direct transfer of “naked” DNA or indirectly using a bacterial vehicle, the “natural genetic engineer,” Agrobacterium tumefaciens. Efficient regeneration protocols have been developed for almost all crop species but they will not be discussed here.
The direct delivery of DNA into plant cells leading to transformation has been achieved in several ways, electroporation into protoplasts (plant cells minus the rigid cell wall), microinjection, chloroplast transformation, silicon-carbide slivers (coated in DNA), mesophorus silica nanoparticles, and microparticle bombardment.17 By far the most common and widely used technique for direct DNA transfer is particle bombardment. Microparticle bombardment, also known as biolistics or the “gene gun”, was first developed by Sanford in the late 1980s18 using pressurized helium to fire gold or tungsten microparticles (diameter between .5 and 1.0 μm) coated with the engineered gene of interest as naked DNA into the plant tissue at high velocities. The pressure used to project the microparticles varied depending upon the target tissues but could go up as high as 2,200 psi: the higher the velocity of the particles, the deeper the penetration into the target tissue. The primary targeted tissues were embryonic tissues from the seed or meristems. The engineered gene was delivered as a high copy number plasmid (a circle of DNA capable of replicating in a bacterial host during the engineering process) and once in the cell was capable of integrating into the plant genome, often in multiple copies. Although the equipment has become more sophisticated and the microprojectiles have changed with time, microparticle bombardment still operates on the same principles as the original Sanford “gene gun”. Microparticle bombardment has been successfully used to produce transgenic plants in a wide-range of crops including all of the cereals, some tuber crops, and trees. It has the advantage over other methods in that it can be used to transfer large DNA fragments and has even been used to transfer whole chromosomes and multiple independently engineered genes at the same time.19
Although micropartical bombardment has been successful, its use is greatly surpassed by the use of Agrobacterim tumefaciens in the commercial realm. A. tumefaciens is a soil bacterium that infects plants, generally where stem and roots meet (known as the crown in gardening terms), and alters the genome of the plant by inserting genes that cause cell proliferation. The cell proliferation forms a mass of cells (a gall or tumour) within which the bacteria live. Not only does A. tumefaciens “instruct” the plant to form a gall (crown-gall disease), it also provides the cells with genetic information to make opines, modified amino acids that the bacteria use as a nutrient source. It accomplishes this natural genetic engineering via a large tumour-inducing plasmid (Ti plasmid) that contains a section of DNA known as T-DNA (for transfer DNA). The T-DNA is flanked by two small (25 base pairs) direct repeats, known as the right (RB) and left border (LB) sequences, that act as insertion signals for the T-DNA transfer into the plant genome. The T-DNA contains genes that encode enzymes that synthesize plant hormones, auxin and cytokinin, that cause cell proliferation and tumour formation along with the genes that encode the enzymes for opine metabolism. The rest of the Ti plasmid, along with the bacterial chromosome, contains the virulence genes that control the ability of the bacteria to infect the plant tissue and to transfer the T-DNA into the nucleus of the target plant cell. A complete description of the mechanism by which A. tumefaciens infects plant tissues, transfers the T-DNA into the cell, and incorporates it into the plant genome can be found in Barampuram and Zhang.17
The exploitation of A. tumefaciens as a possible means to insert a novel gene in to plants was first recognized in the late 1970s, 20 but the real explosion in the field occurred in the early 1980s with the advent of binary vectors. Binary vectors separated the T-DNA region onto a separate smaller plasmid away from the rest of the Ti plasmid, which remains as a separate vector within the Agrobacterium cell. The smaller binary vector was engineered so that it could replicate in both A. tumefaciens and E. coli, which greatly facilitated the construction and insertion of the target genes of interest between the RB and LB of the T-DNA.17 Replacement of the T-DNA with engineered genes capable of expression in plant cells, “disarmed” the binary vector so that the infection and transfer of DNA to the plant by the bacteria no longer resulted in cell proliferation and opine biosynthesis.
Little has changed in this part of the process since these early days and we still use only a limited number of binary vector systems, more focus has been placed on increasing the efficiency of the transformation and new ways of introducing Agrobacteria to plant tissues. For the former the most successful development has been the introduction of explant wounding techniques that deliver A. tumefaciens deep into a wound site to promote closer contact of the bacteria to transformable plant cells. The “dip-wounding” technique for soybeans where explants are wounded with a blade covered in A. tumefaciens prior to placing them in a suspension of A. tumefaciens for cocultivation in tissue culture, increased transformation efficiency ten-fold.17 The most promising of the latter efforts has been the development of methods that target the delivery of A. tumefaciens to transform developing ovules in situ, as a means of avoiding the need for regeneration of whole transgenic plants in tissue culture. Such an approach, known as the floral-dip method, has long been used to transform the model plant Arabidopsis thaliana for research purposes. More recently variations on this established method have been used to transform wheat, pakchoi and rape/canola by vacuum infiltration of flower buds, sorghum, corn, cotton, and wheat by application of A. tumefaciens directly to the pistil.21
In generating transgenic plants (GMOs) the binary vector systems contained selectable markers, genes whose products allowed for the selection of transformed cells of the target tissue, and the tissues that were regenerated from them, away from non-transformed cells/tissues. These selectable marker genes were located along side the gene of interest within the T-DNA. The most commonly used selectable marker genes are those that infer antibiotic resistance or herbicide tolerance.17 The inclusion of these selectable marker genes within the transferred DNA was considered to pose an unacceptable added environmental risk22, 23 and their elimination became a commercial priority.
There are a multitude of techniques, or in development, that have been mobilized in this effort.23 The most effective and successful of these has been the use of site-specific recombinases. Site-specific recombinases are enzymes, common in bacterial and some eukaryotes such as yeast, which catalyze recombination of DNA between two enzyme specific recognition sites (short inverted repeat sequences). The two most common site-specific recombinases in use are the CRE-LOX and the FLP-FRT systems (the first triplet of letters designates the enzyme and the second triplet the recognition sites). The recombination activity that these enzymes catalyze is guided by the orientation of the recognitions sites, if they are oriented in tandem (in the same direction) the DNA between the two sites is excised, and if in opposite directions (facing each other) the DNA is inverted. The strategy for using these recombinases in producing marker-free GM crops (See Figure 3), in this example using CRE-LOX, involves:
Figure 3.
The CRE-LOX system for removal of antibiotic marker genes from GM plants.
Each block represents the strands of the genomic DNA of the plant. The checkered boxes represent the recognition sites (LOX) for the CRE recombinase.
engineering LOX sites (in tandem) either side of the marker gene within the binary vector gene construct;
expression of the CRE recombinase enzymes in the transgenic plant or tissue after selection; and
segregation of the marker free transgenic plants from the progeny for development of the GM crop.
These and other technologies to remove the antibiotic marker genes have been deployed even though there is no evidence that such genes pose a risk to either animals, including us, or microbial communities within the soil. The amount of DNA encoding the antibiotic gene that is ingested by an animal or us is an extremely small component (approximately 0.00005%) of the 0.1 to 1g of DNA a day consumed in a normal diet24, 25 and is subject to degradation by the same digestive processes as all DNA. The perceived risk for ingested antibiotic maker genes is that they might be incorporated either into the DNA of the animal ingesting the DNA or by microbes in the gut thus rendering them antibiotic-resistant. DNA is generally cleaved into very small fragments during digestion (and food processing) making it even less likely that a whole gene remains intact. This is true for all ingested DNA and so the likelihood that any gene is incorporated into the genome of the animal or the microbes in the gut is highly unlikely.26 It is also well documented that the antibiotic resistance marker genes pose no health risk to humans or livestock, and that they are naturally present in environment and in gut flora,27 so should such an unlikely gene transfer event into the genome of a gut microbe occur it would be of little consequence. To date there is no evidence that DNA absorbed through the intestines following ingestion can be integrated into the germ line of either humans or livestock.
Impact and Safety of GM Crops
It has been thirty years since the first genetically engineered plants were generated, and it has been eighteen years since the first introduction of a transgenic crop into U.S. agriculture. Since their emergence the acreage planted with GM crops has steadily increased such that in 2013, 433 million acres (175.2 hectares) of land were dedicated to their production, 56% of which were grown in developing countries.28 As of 2013, a total more than four billion acres of GMA crops have been grown in 27 countries world-wide, primarily in corn, soybean and cotton, although new crops are being introduced at an increasing rate. The economic benefits of the deployment of these crops have been substantial. Mannion and Morse29 report that on a global level, from 1996 to 2006, GM crops increased farm income by $40.7 billion, occurring in both developed (47%) and developing agricultures (53%). In the following six years (as of 2012) the global increase in farm income from GM crops had almost tripled that of the previous 10 years to reach $116 billion.28, 30 Both studies estimate that 42% of this economic gain is derived from the increased yield associated with lower weed and pest damage as well as superior genetics. The remaining 58% accrued from a decrease in production costs (decreased herbicide and pesticide costs and a reduction in tillage). These figures indicate that the underlying agronomic benefits derived from GM crops are equally impressive: with a global yield increase of 377 million tons from 1996 to 2012. In 2012 the increase in yield attributed to GM crops for the U.S. was 47 million tons.28, 30 Brooks and Barfoot30 estimate that to attain an equal yield increase to that delivered by GM crops between 1996 and 2012, an additional 303 million acres (123 million hectares) of conventional crops would have been required. As James28 postulates that to attain this extra land industrial nations would have to use marginal lands that are generally characterized by poor soils (requiring substantial inputs such as fertilizer and irrigation) and developing countries would primarily target tropical forests. Certainly such an added conversion of land to agricultural purposes would have serious ecological and environmental impacts regardless of what part of the world it is acquired.
It is well documented that the use of biotechnology is having an impact on the alleviation of poverty and to hunger in those developing countries, especially China and India (if one can still classify these two as developing), where development and deployment of GM crops has been adopted.28, 29, 31 Economic gains are being translated into improving agriculture-based economies and higher and more stable yields are alleviating some of the concerns about food security.
GM crop production is a vital tool in the “agricultural toolbox” and along with advances in the development of the new genomics based genetic technologies that improve conventional crop production it may be realistic to expect to meet the aforementioned lofty goals. Organic crop production technologies, although generally delivering lower yields than conventional crops,32 have an important role in yield improvement and stability efforts in areas where these technologies are optimal. To abandon any one of these efforts would be unwise and potentially catastrophic, especially without sound scientific reason, as agricultural production systems are complex and changing, more so now than ever before, as global climate change alters the “farming landscape.”
There are those that are adamantly opposed to the adoption of GM technologies in agriculture (though not in medicine) as a means of increasing yields and improving nutrition, and thus removing this key tool from the toolbox. The reasons for this opposition are complex and multifaceted, but from what is articulated and communicated by those who oppose GMOs, they are based on the perception that such crops pose an unacceptable risk to both human health and the environment. Such sentiment exists even though there have been no adverse health or environmental affects from the almost four billion acres of GMO crops grown since their introduction in 1996. Several National Research Council committees and European Commissions (as well as joint commissions) have concluded that with the extensive scientific inquiry into the safety issues surrounding the adoption of GM crops, genetic engineering using biotechnology is no different from conventional breeding in terms of unintended consequences to the environment or animal and human health.33 The European Commission funded more 50 research programs from 2001–2010 to address concerns regarding the use of GM crops to reach this same determination.34 Nicolia et al.24 constructed a database of 1,783 scientific original research papers, reviews, relevant opinion articles, and reports published between 2002 and October of 2010 on GMO safety issues, and reviewed the contents to generate a comprehensive overview of the accumulated knowledge. The overall conclusion of this mammoth undertaking was that “the scientific research conducted so far has not detected any significant hazards directly connected with the use of GM crops.
At the present time, two types of GM crops dominate GMO crop plantings:30
herbicide-tolerant crops, primarily glyphosate (RoundupTM) resistant, that express enzymes that are unaffected by the herbicide and thus bypass the native susceptible protein (5-enolpyruvoyl-shikimate-3-phosphate synthetase (EPSPS) in the case of glyphosate) or enzymes that degrade the herbicide; and
insect-resistant crops, almost exclusively crops expressing the insecticidal crystal proteins (CRY) produced by Bacillus thuringiensis (Bt) a soil bacterium.
In both cases, the aim was to improve yields by limiting losses due to competition from weeds and damage from insect pests, reduce input costs for the farmer by better crop management, and to reduce both herbicide (by reducing the need for multiple sprayings) and insecticide use. As much of the debate as to the safety and impact of GMOs is focused on these two classifications, a detailed look at the adoption of these technologies and associated outcomes will serve to highlight some of the issues that fuel the ongoing debate.
Herbicide Tolerance
Herbicide-tolerant GM crops have been widely adopted in the U.S., such that >90% of corn, soybeans, and cotton are GM and herbicide-tolerant28 and as other countries adopt GM technologies, the amount of acreage planted with herbicide-tolerant GM crops will continue to grow. In Canada 98% of the canola crop is GM. The perceived issues with herbicide-tolerant crops relate to the development of herbicide-resistant weeds (so called “superweeds”), transgene transfer (gene flow) to wild relatives or non-GMO crops close by, and environmental/ecological concerns that relate to biodiversity and chemical usage. All of these issues actually predate the adoption of GM crops. Herbicide resistant weeds have long been an issue for countries that rely on herbicides for weed control.35 Gene transfer is not a unique property of GM crops and is equally an issue with herbicide tolerance (or any other trait) that is developed through conventional breeding methods.36 Herbicide use, as is true for agriculture in general, has environmental and ecological consequences even for crops derived by conventional breeding programs. These considerations should be taken into account in any risk assessment for GMO crops.
Gene flow between closely related plant species (wild or cultivated) is a natural phenomenon that has been difficult to document and study but is known to occur in both directions.37 It is somewhat ironic that the transgenes inserted into GM crops are ideal markers for documenting and studying this process.38 The transfer of a transgene from a GMO crop to a wild relative depends on several factors: the reproductive strategy of the crop (open or self-pollination), the proximity of sexually compatible wild relatives, and the fertility/fecundity and fitness of the resultant hybrid. The fertility/fecundity and fitness of the hybrid is the controlling factor in establishing the presence of the transgene in the population of the wild relative of the GMO crop. To date, even with the large acreage of GM crops, this has only been observed in a small number of cases and only in the U.S. and Canada.37 The glyphosate tolerance transgene from GM grasses grown in a field trial in Oregon escaped and has been incorporated into native creeping bentgrass populations39 and the establishment of GM canola (rapeseed in the U.S.) along trucking routes in North Dakota has led to transgene transfer into non-GMO “feral” canola in these locations.38 As far as can be determined there is no evidence that the establishment of the herbicide tolerance gene in these populations has had a detrimental effect and mitigation strategies have been identified.40
Transgene flow from a GMO crop into a neighbouring field of an identical non-GMO crop is a problem for organic farming where registration as a non-GMO crop relies upon the lack of a transgene. This is also true for conventional farming operations that wish to take advantage of the non-GMO market. There are strategies to prevent this from occurring but as of yet they have not been deployed.41, 42, 43 Prevention of this occurrence remains a crop management problem. Coexistence strategies for many crops have been investigated and deployment is driven not only by a scientific or social compulsion but also by economic feasibility factors.24 These strategies include separation by a distance that negates pollen flow from one crop to another, harvesting practices that reduce residual seed accumulation, and transportation and other post-harvest containment practices. All of these present an economic challenge for producers where coexistence is desired.
Although herbicide tolerance in weeds resulting from transgene flow from a crop is rare and limited to a small number of crops and related weeds (and does not occur when the crop and weed are sexually incompatible), the development of herbicide tolerant weeds in agricultural settings is becoming a problem. The widespread adoption of glyphosate resistant GM crops in the U.S. and the reliance of upon the use a single herbicide for weed control established a strong selection pressure for weeds that have natural herbicide tolerance genes. This would occur whether or not the herbicide tolerance in the crop is GM or conventionally bred: as documented for the toxic herbicide atrazine for which GM derived resistance has not been employed.44 The over reliance on a single herbicide as the main strategy for weed control will eventually limit the usefulness of both the herbicide and the tolerant GM crop.45 There is a broad consensus in the agricultural scientific community that over reliance on a lone herbicide strategy is not sustainable. The problems associated with the evolution of herbicide tolerance in weeds can be mitigated or solved if GM herbicide tolerance is part of a broader integrated weed management program that incorporates crop rotation, herbicide tolerance gene-stacking technologies and field management technologies.29, 44–47
The adoption of GM herbicide tolerant crops does alter the biodiversity of plant populations (weeds) in agricultural ecosystems and some of the insects and other organisms that rely upon them but this is related to weed management and herbicide use not the GM crop. Alterations in biodiversity also occur in conventional agriculture where weed management strategies are employed.48 Nevertheless there is great deal of evidence that the adoption of GM herbicide tolerant crops has had a beneficial impact on the environment. The conversion of natural habitat and ecosystems to urban development and agriculture is clearly the most detrimental aspect of human activity as it relates to environmental impact and loss of biodiversity. As yields increase with the adoption of GM crops, as discussed previously, the need to dedicate land for agriculture decreases. Apart from the reduced conversion of land to agricultural use the emergence of GM herbicide tolerant crops has accelerated and enabled the adoption of conservative tillage (no-till and reduced-till) practices.30, 45, 48 Such practices enhance soil quality, reduce water run-off, conserves nutrients, increases water infiltration, and contributes to a reduction in greenhouse gases.
The GM herbicide tolerant crops have also been developed to enable the use of less toxic and more environmentally-friendly chemicals. Glyphosate and glufosinate (another GM targeted herbicide resistance), for example are both Class III herbicides (EPA), which are only slightly toxic and have low persistence in the soil and environment, averaging approximately 40 days. These herbicides have replaced herbicides either more toxic or that are known to contaminate and persist in groundwater.33 In Argentina, glyphosate replaced several Class II herbicides (significantly toxic) by over 83% deployed on herbicide tolerant soybeans.48 Thus the impact of targeting less toxic herbicides is a reduction in human exposure and a positive impact on environmental and human health.
With the global increase in acreage of GM herbicide tolerant crops there has been some concern that overall herbicide use would increase and thus the possible environmental impact would negate the value of planting GM crops and ultimately render them a detriment. Initially, at least for the U.S., the overall quantity of herbicide deployed in the environment was reduced but by 2010, when USDA stopped collecting usage data, the amount of herbicide used was approaching pre-GM levels. However, the quantity of herbicide used in an agricultural endeavour is not a satisfactory indicator of environmental impact as the new herbicides substituted for older more toxic chemicals. Kovach et al. 49 developed a metric entitled the Environmental Impact Quotient (EIQ) that utilizes toxicity and exposure data for each herbicide, pesticide, or fungicides to derive a single value that is effectively the average risk/impact of the farm worker, consumer, and ecological components of the agricultural production system. Brooke and Barfoot30 describe a field EIQ that is derived by multiplying the EIQ for a herbicide (or a pesticide or fungicide) by the amount of the active ingredient applied per hectare and thus conventional herbicide (or pesticide or fungicide) usage can be directly compared usage in a GM crop production field. They point out that the EIQ is a useful, but not comprehensive indicator, for environmental impact but it is more informative than simply recording and comparing the quantity of a chemical used. Using the available data Brookes and Barfoot30 report that both the amount of active ingredient used and the environmental load (EIQ) has been significantly reduced for all of the major GM crops (maize, soybean, and cotton) in all of the GM adopting countries between 1997 and 2012.
Insect Resistance
Insect-resistant GM crops have also been widely adopted in the U.S., over 90% of corn, soybeans, and cotton are GM for insect resistance,28 and like herbicide-tolerant GM crops the insect-resistant GM crops are rapidly growing in acreage globally. As mentioned above the primary transgene used in the production of insect resistant GM crops is one that allows the synthesis of a CRY protein toxin from the bacteria Bacillus thurengensis (Bt). This toxin is relatively specific to key agronomic caterpillar and beetle pests that feed on the crop plants, affecting the gut cells of the insect and preventing digestion. The CRY toxins are specific to their target insects and are innocuous to vertebrates, including humans, and have no impact on the plant. They are also biodegradable and thus do not persist in the environment.50, 51 This made them ideal targets for GM technology to combat insect pests and the damage and the resultant yield reductions they cause. The recognition that these proteins were useful pesticides predated GMOs. Sprayable formulations, of both the crystal proteins and bacteria preparations (a microbial pest control agent [MCPA]), have long been used in agriculture.51 It is one of the few pesticidal treatments available to an organic farmer. It is widely used today, making up over 90% of the MCPA market (http://www.bt.ucsd.edu/organic_farming.html).
The use of the same pesticide in GM crops that has long been used in organic and conventional agriculture took advantage of the wealth of EPA and FDA testing data for this toxin. This allowed government agencies worldwide to conclude that Bt GM crops are as safe for both human and animal consumption as well as the environment as conventional/organic crops that have been sprayed with the CRY protein or bacterial preparations.33 In fact, because the Bt GM crop only delivers the CRY toxin to those insects that eat the crop, whether directly or in crop residue, it was considered less likely to cause environmental issues than spraying or dusting plants with the toxin or bacterial preparations. Nevertheless, as with herbicide-tolerant GM crops, concerns remain and for Bt GM crops these relate to the development of Bt-resistant insects, transgene transfer (gene flow) to wild relatives or non-GMO crops close by and environmental/ecological concerns that relate to biodiversity.
The concern for Bt GM crops in regards to gene flow is that unlike herbicide tolerant GM plants the transfer of insect-resistance to wild relatives theoretically could offer a selective advantage to the recipient from increased seed production as a result of reduced loss of vegetative tissue from herbivory. The transfer of insect resistance from a crop to a wild sexually compatible relative is not dependent on the transgene but would occur in conventional insect resistant crops, as discussed previously. After almost twenty years of cultivation there have not been any negative effects of gene transfer from a Bt GM crop to a wild relative.29, 45 In a review of the literature of gene flow, Chandler and Dunwell37 uncovered reports of Bt gene transfer between Bt-canola (Brassica napus) and a related wild species Brassica rapa that indicated that plants that have the Bt gene are less fit than those that do not in the absence of the herbivorous insects but survived better than the non-Bt plants under heavy infestations. Other studies using similar populations did not see an increase in fitness in the hybrids. Snow et al.52 crossed Bt-sunflowers with a weedy relative and demonstrated that the GM hybrids and offspring produced more seed than the non-GM siblings but as this was in a controlled experimental system, and it was not clear if fitness would be enhanced in an agricultural setting. The transfer of the Bt transgene from a GMO crop into a neighbouring field of the non-GM crop counterpart is, as described for herbicide tolerant GM crops, a specific concern for organic farming and requires specific management strategies to negate its occurrence.
As with all insecticides, insect populations that are resistant to the pesticide arise, and Bt crystal proteins are no exception. Long before Bt-GM crops emerged on the scene, the diamondback moth (or cabbage moth), an important pest of cruciferous crops developed resistance to Bt preparations repeatedly applied to fields of conventional crops.53 Although the Bt toxin is only contained within the tissues of a Bt-GM crop and not applied as a spray in the field, it is not surprising that resistance to Bt-GM crops has emerged in the target insects; most recently in western corn root worm.54 The strategy to combat the development of resistance to Bt in the targeted pests has been to establish Bt-GM crop free refuges, either within or adjacent to the Bt-GM crop. The refuge strategy works on the theory that if there is a large population of susceptible target insects close to the Bt-GM crop then the rare insect that survives feeding on the crop will, in all likelihood, mate with a susceptible insect that is feeding on the non-GM plants nearby. As most resistance genes tend to be recessive the hybrid offspring of such a mating would be susceptible to the Bt in the GM crop and would die. This has delayed the evolution of Bt-resistant pests.33 This is not a perfect system and Bt-resistant insects have evolved. In some cases this has arisen because the level of Bt in the GM plants is not sufficient to kill the hybrid insects or because of the significant costs associated with establishing and maintaining refuges some producers fail to provide them at all or limit the size. Strategies to ensure that refuges are established and maintained are being implemented, including increasing the dose of CRY protein that the plant delivers, economic incentives, and “refuge in the bag” (adding non-GM seed to the bag of Bt-GM seed to ensure refuge establishment) may help further delay widespread resistance. Recently GM crops containing “stacked” Bt genes, more than one CRY protein gene, have been developed in the hope of eliminating or slowing insect resistance. New emerging technologies that utilize more than one mode of action (Bt plus another insecticide) as well as maintaining sufficiently large refuge areas may also help prevent or severely delay the development if pest resistance.
The deployment of Bt-GM crops has resulted in a significant decrease in the use of chemical pesticides in all countries where they have been adopted, along with the reduction in environmental impact and associated human exposure.29–31 The reductions are both in quantities of active ingredient and the overall field EIQs for each major crop. In the U.S. the use of Bt-GM maize reduced the amount of pesticide used on corn to target corn borers and root-worms by 80% and the field EIQ load by 54%. Since 1966 the overall decrease in pesticide use on corn was 45% with a reduction of 38% for the field IEQ load. Where data is available, the reductions in total pesticide use and EIQ in all countries that have adopted Bt-maize cultivation. Similar figures are also available for Bt-cotton and other crops.30 The beneficial economic, environmental, and human health effects resulting from a reduction in pesticide use (and reduced need for toxic pesticide alternatives) can be directly attributed to the ability of GM technologies to contain the pesticide within the plant that is targeted by specific insects (or other invertebrate pests) and to deliver the pesticide only to those pests that ingest the tissues of the plant. The reduction in the need to expose the environment and workers to chemical sprays is clearly a positive outcome of the deployment of GM crops.
The widespread use of broad-spectrum pesticides to combat agricultural pests has significant and negative effects on biodiversity at all levels in the agricultural ecosystem, from mammals to soil microbes and is well documented.55, 56 This is not the case for Bt-GM crops, where the consensus is that the effects on biodiversity have been positive. The debate on the possible impact of Bt-GM crops on biodiversity was fueled by early reports that laboratory-feeding experiments using Bt-pollen indicated that Bt-GM corn posed a serious threat to the conservation of monarch butterflies in the U.S. These reports spawned a series of field-based ecological impact studies that concluded that commercial large-scale cultivation of Bt-maize did not pose a significant threat to monarch populations and that the lab-based studies were flawed.57 This initial flurry of environmental impact assessments of Bt-GM crops on biodiversity of both beneficial organisms (non-targeted) and the targeted pests has continued and the data collected is substantial.48 The analysis of the literature and data leads to the following conclusions:
The deployment of Bt-GM crops has had little or no effect on the biodiversity of soil organisms. There are some reports of changes in soil organisms, primarily soil microbes but these changes are indistinguishable or can be explained by the effect of temperature, soil type, or other unrelated parameters.
No significant adverse effects of Bt-GM crops on the non-target organisms or beneficials have been detected in the field.
Controlled lab or greenhouse studies only observe an effect of Bt-GM crops on the natural predators or parasites of the targeted pest if they are fed (or use as a host) an insect which is damaged by feeding on Bt-GM plants but not dead and that in the field these effects are not observed.
In some areas where Bt-GM crops predominate the landscape, the populations of the targeted pests decline to levels that benefit nearby farms that grow non-GM crops of a reduction in the level and frequency of insecticide deployment.
In a more recent study of the impact of Bt-GM crops, using data collected between 1990 and 2010 at 36 sites across northern China, Lu et al.58 demonstrated that with the adoption of Bt-cotton and the resulting decrease in insecticide use there was a major reduction in the target insect, the cotton bollworm, and an increase in abundance of several generalist predators (ladybugs, spiders, and lacewings). With the increase in the generalist predators they also saw a decrease in the cotton aphid populations that damage the plants but are not controlled by the Bt toxin. As reported by others they conclude that the impact on beneficial predators (generalists) provides a measure of biocontrol of plant pests that affect neighboring crops that are not necessarily GM.
Substantial Equivalence
A major paradigm in the risk assessment of GM crops, particularly for human consumption, is the concept of “substantial equivalence” which is based on the idea that a GM crop is directly comparable (within normal levels of variation) to its non-GM counterpart to ensure that there are no unintended hazards associated with the insertion of the transgene. The GM and non-GM plants are compared with regard to their agronomic and morphological characteristics prior to an in depth compositional analysis. The compositional comparisons encompass “those components in a particular food that may have a substantial impact in the overall diet”59 present in the food/feed products that are derived from the GM crop. The analysis can include macro- and micronutrients, anti-nutrients, secondary metabolites, and toxins. The non-GM crop that is used as a point of comparison is presumed to be safe, as it will have had a history of successful and safe use as food or feed. Any difference in the composition of the GM crop must fall within the normal range of variability for the non-GM counterpart for it to be considered safe. If the differences fall outside the normal range then the GM crop must be further assessed for its safety. All of the GM crops adopted so far have been fully tested for substantial equivalence, and all have been graded as equivalent to their non-GM counterpart, and thus, safe.60 The approaches to assess equivalence are constantly improving and there is a movement towards non-targeted approaches including “omics” based analyses (genomics, proteomics, metabolomics, etc.). In a recent review, Ricroch et al.61 concluded that GM crop plants more closely resemble the parental line from which they were generated than do their conventional bred (or mutagenized) counterparts. The “omics” analyses would support the conclusion that the insertion of a transgene into a plant to generate a GM crop is neither inherently risky and nor does it present novel or greater sources of risk than conventional breeding. The use of “omics” in the normal testing for substantial equivalence is not yet part of a standard approach.24
GM crops are more rigorously tested for safety than any conventionally bred crop (which are not tested), even though the genetic changes that are made in the production of GM crops are precisely assessed and minimal, and none have yet failed to pass this intense scrutiny, including golden rice.
On the Horizon
The focus of the discussion so far has been on the first generation of GM crops that are primarily targeted to two agronomic traits. At the present time the only other agricultural crop grown commercially is the GM papaya, the first GM tree crop, with resistance to the devastating papaya ringspot virus that threatened to wipeout the papaya industry in Hawaii.62 This was the pioneering public institution driven (Cornell University, USDA-ARS) development of a GM crop, with cooperation from private industry, to combat a national crisis. The safety of GM papaya can be attested to by the fact that they have been approved for direct consumption in Japan, a difficult consumer market to penetrate with a GM product. However, with recent advances and the prior establishment of “transgenic pipelines” we are beginning to see other important traits being addressed using GM technologies. These next generation GM crops involve more than just the major crops such as corn, soybeans, and cotton, and have utilized genes from sources other than microbes, including genes that are derived from plant sources that can enable new trait development in our commercial crops. The farthest advanced is the new drought-tolerance technology that uses a bacterial gene (a protein that stabilizes RNA structure) that is in its first year of commercialization in the U.S., and under a public-private partnership in development for deployment in Africa. Those that are still in the pipelines for commercialization address such traits as: pest and disease resistance, photosynthetic efficiency, salinity, nutrient efficiency (nitrogen and phosphorus uptake), nitrogen fixation, modifications for biofuel production, and biofortification. The latter trait, biofortification, is where golden rice is leading the way and other nutrient deficiencies that significantly impact human health, such as vitamin A, iron, and zinc deficiencies, are all in the GM pipeline (see reference 45 for a comprehensive look at new technologies).
Each trait will undergo the rigorous testing that is demanded of commercial or public entities, so that any environmental or health safety issues are addressed and accounted for before release.
The Future of GM Technologies
The future is very promising for GM technologies to enhance our efforts to meet the future global needs for food, feed and fiber in a sustainable and responsible way. Conventional breeding methods, especially with the advent of genome level technologies, that are designed to both generate and exploit genetic variation in order to isolate effective alleles (variants) of genes that generate yield increases, disease resistance, pest resistance etc., also clearly play a role in this effort. Organic farming practices also have a place at the global table63 where such practices make sense. Agriculture is a diverse endeavor, and if we are to be successful we need to embrace that diversity.
Biography
Melvin J. Oliver, PhD, is a Supervisory Research Geneticist (Plants), USDA, Agricultural Research Service, University of Missouri.
Contact: Mel.oliver@ars.usda.gov

Footnotes
Disclosure
None reported.
References
- 1.Alberts B, Beachy RN, Baulcombe D, Blobel G, Datta S, Fedoroff N, Kennedy D, Khush GS, Peacock J, Rees M, Sharp P. Standing up for GMOs. Science. 2013;341:6152. doi: 10.1126/science.1245017. [DOI] [PubMed] [Google Scholar]
- 2.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. British Medical J. 2011;343:d5094. doi: 10.1136/bmj.d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal S. What’s fueling the biotech engine – 2011 to 2012. Nature Biotech. 2012;30:1191–1197. doi: 10.1038/nbt.2437. [DOI] [PubMed] [Google Scholar]
- 4.Gosse ME, Manochia M. The first biopharmaceuticals approved in the United States: 1980–1994. Drug Information Journal. 1996;30:991–1001. [Google Scholar]
- 5.Frewer LJ, van der Lans IA, Fischer ARH, Reinders MJ, Menozzi D, Zhang X, van der Berg I, Zimmermann KL. Public perceptions of agri-food applications of genetic modification: A systematic review and metaanalysis. Trends in Food Science & Technology. 2013;30:142–152. [Google Scholar]
- 6.FAO, WFP and IFAD. Economic growth is necessary but not sufficient to accelerate reduction of hunger and malnutrition. Rome: FAO; 2012. The State of Food Insecurity in the World 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabashnik BE. Evolution of resistance to Bacillus thuringiensis. Annu Rev Entomol. 1994;39:47–79. [Google Scholar]
- 8.Ray DK, Mueller ND, West PC, Foley JA. Yield trends are insufficient to double global crop production by 2050. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0066428. e66428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray DK, Ramankutty N, Mueller ND, West PC, Foley JA. Recent patterns of crop yield growth and stagnation. Nature Communications. 2012;3:1293. doi: 10.1038/ncomms2296. [DOI] [PubMed] [Google Scholar]
- 10.Lobell DB, Schlenker W, Costa-Roberts J. Climate trends and global crop production since 1980. Science. 2011 2011 Jul 29;333(6042):616–620. doi: 10.1126/science.1204531. [DOI] [PubMed] [Google Scholar]
- 11.Alexandratos N, Bruinsma J. World agriculture towards 2030/2050: The 2012 Revision. Food and Agriculture Organization of the United Nations; 2012. www.fao.org/economic/esa. [Google Scholar]
- 12.Long SP, Ort DR. More than taking the heat: crops and global change. Current Opinion in Plant Biology. 2010;2010;13:241–248. doi: 10.1016/j.pbi.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Phillips RL. Norman Ernest Borlaug. 25 March 1914 – 12 September 2009. Biogr Mems Fell R Soc. 2013;59 doi: 10.1098/rsbm.2013.0012. [DOI] [Google Scholar]
- 14.Forabosco F, Lõhmus M, Rydhmer L, Sundström LF. Genetically-modified farm animals and fish in agriculture: a review. Livestock Science. 2013;153:1–9. [Google Scholar]
- 15.Ammar K, Mergoum M, Rajaram S. The history and evolution of triticale. In: Mergoum M, Gómez-Macpherson H, editors. Triticale improvement and production. Food and Agriculture Organization of the United Nations; Rome: 2004. pp. 1–10. [Google Scholar]
- 16.Secretariat of the Convention on Biological Diversity. Montreal: 2000. The Cartagena Protocol on Biosafety to the Convention on Biological Diversity. [Google Scholar]
- 17.Barampuram S, Zhang ZJ. Recent advances in Plant transformation. In: Plant Chromosome Engineering: Methods and Protocols. In: Birchler JA, editor. Methods in Molecular Biology. Vol. 701. Humana Press; 2011. pp. 1–35. [DOI] [PubMed] [Google Scholar]
- 18.Sanford JC. Biolistic plant transformation. Physiol Plant. 1990;79:206–209. [Google Scholar]
- 19.Schmidt MA, Lafayette PR, Artelt BA, Parrott WA. A comparison of strategies for transformation with multiplegenes via microprojectile-mediated bombardment. In Vitro Cell Dev. Biol. Plant. 2008;44:162–168. [Google Scholar]
- 20.Schell J, Van Montagu M. The Ti-plasmid of Agrobacterium tumefaciens, a natural vector for the introduction of nif genes in plants. Basic Life Sci. 1977;9:159–79. doi: 10.1007/978-1-4684-0880-5_12. [DOI] [PubMed] [Google Scholar]
- 21.Chumakov MI, Moiseeva EM. technologies of Agrobacterium plant transformation in planta. Applied Biochemistry and Microbiology. 2012;48:657–666. [Google Scholar]
- 22.Hare PD, Chua NH. Excision of selectable marker genes from transgenic plants. Nature Biotechnology. 2002;20:572–580. doi: 10.1038/nbt0602-575. [DOI] [PubMed] [Google Scholar]
- 23.Yau YY, Stewart CN., Jr Less is more: strategies to remove marker genes from transgenic plants. BMC Biotechnology. 2013;2013;13:36. doi: 10.1186/1472-6750-13-36. http://www.biomedcentral.com/1472-6750/13/36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolia A, Manzo A, Veronesi F, Rosellini D. An overview of the last 10 years of genetically engineered crop safety research. Crit Rev in Biotechnology. 2014;34:77–88. doi: 10.3109/07388551.2013.823595. [DOI] [PubMed] [Google Scholar]
- 25.Parrott W, Chassy B, Ligon J, Meyer L, Petrick J, Zhou J, Herman R, Delaney B, Levine M. Application of food and feed safety assessment principles to evaluate transgenic approaches to gene modulation in crops. Food and Chemical Toxicology. 2010;48:1773–1790. doi: 10.1016/j.fct.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Rizzi A, Raddadi N, Sorlini C, Norgrd L, Nielsen KM, Daffonchio D. The stability and degradation of dietary DNA in the gastrointestinal tract of mammals: Implications for horizontal gene transfer and the biosafety of GMOs. Crit Reviews in Food Sci and Nutrition. 2012;52:142–161. doi: 10.1080/10408398.2010.499480. [DOI] [PubMed] [Google Scholar]
- 27.European Food Safety Authority (EFSA) Guidance for risk assessment of food and feed from genetically-modified plants. EFSA J. 2011;9:2150, 37. doi: 10.2903/j.efsa.2023.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James Clive. ISAAA Brief No. 46. ISAAA; Ithaca, NY: 2013. Global status of commercialized biotech/GM Crops: 2013. [Google Scholar]
- 29.Mannion AM, Morse S. GM crops 1996–2012 a review of agronomic, environmental and socio-economic impacts. University of Surrey, Centre for Environmental Strategy (CES) Working Paper 04/13. 2013. http://www.surrey.ac.uk/ces/activity/publications/index.htm.
- 30.Brookes G, Barfoot P. Economic impact of GM crops: The global income and production effects 1996–2012. GM Crops and Food: Biotechnology in Agriculture and the Food Chain. 2014;5:37–47. doi: 10.4161/gmcr.28098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qaim M. The economics of genetically-modified crops. Annu Rev Resour Econ. 2009;1:665–93. [Google Scholar]
- 32.Suefert V, Ramankutty N, Foley JA. Comparing the yields of organic and conventional agriculture. Nature. 2012;485:229–232. doi: 10.1038/nature11069. [DOI] [PubMed] [Google Scholar]
- 33.Roland P. Plant genetics, sustainable agriculture and global food security. Genetics. 2011;188:11–20. doi: 10.1534/genetics.111.128553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Commission. A decade of EU-funded GMO research. 2010. http://ec.europa.eu/research/biosociety/library/brochures_reports_en.htm.
- 35.Busi R, Vila-Aiub MM, Beckie HJ, Gaines TA, Goggin DE, Kaundun SS, Lacoste M, Neve P, Nissen SJ, Norsworthy JK, Renton M, Shaner DL, Tranel PJ, Wright T, Yu Q, Powles SB. (2013, Herbicide-resistant weeds: from research and knowledge to future needs. Evolutionary Applications. 6:1218–1221. doi: 10.1111/eva.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellstrand NC, Prentice HC, Hancock JF. Gene flow and introgression from domesticated plants into their wild relatives. Annu Rev Ecol Syst. 1999;30:539–563. [Google Scholar]
- 37.Chandler S, Dunwell JM. Gene flow, risk assessment and the environmental release of transgenic plants. Crit Rev Plant Sci. 2008;27:25–49. [Google Scholar]
- 38.Schafer MG, Ross AA, Londo JP, Burdick CA, Lee EH, Travers SE, Van de Water PK, Sagers CL. The Establishment of Genetically Engineered Canola Populations in the US. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0025736. e25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichman JR, Watrud LS, Lee EH, Burdick CA, Bollman MA, Storm MJ, King GA, Mallory-Smith C. Establishment of transgenic herbicide-resistant creeping bentgrass (Agrostis stolonifera L.) in nonagronomic habitats. Molecular Ecology. 2006;15:4243–4255. doi: 10.1111/j.1365-294X.2006.03072.x. [DOI] [PubMed] [Google Scholar]
- 40.Kwit C, Moon HS, Warwick SI, Stewart CN., Jr Transgene introgression in crop relatives: molecular evidence and mitigation strategies. Trends Biotechnol. 2011;29:284–93. doi: 10.1016/j.tibtech.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Kausch AP, Hague J, Deresienski A, Tilelli M, Nelson K. Male sterility and hybrid plant systems for gene confinement. In: Oliver MJ, Li Y, editors. Plant Gene Containment. Wiley-Blackwell Publishing Ltd; Hoboken, NJ: 2012. pp. 85–100. [Google Scholar]
- 42.Oliver MJ, Hake K. Oliver MJ, Li Y. Plant Gene Containment. Wiley-Blackwell Publishing Ltd; Hoboken, NJ: 2012. Seed-based gene containment strategies; pp. 113–124. [Google Scholar]
- 43.Li Y, Duan H, Chen Y, McAvoy R. Gene-Deletor technology and its potential applications in addressing gene flow and food safety concerns over transgenic plants. In: Oliver MJ, Li Y, editors. Plant Gene Containment. Wiley-Blackwell Publishing Ltd; Hoboken, NJ: 2012. pp. 101–112. [Google Scholar]
- 44.Gilbert N. A hard look at GM Crops. Nature. 2013;497:24–26. doi: 10.1038/497024a. [DOI] [PubMed] [Google Scholar]
- 45.Baulcombe D, Dunwell J, Jones J, Pickett J, Puigdomenech P. GM Science Update: A report to the Council for Science and Technology. 2014. https://www.gov.uk/government/publications/genetic-modification-gm-technologies.
- 46.Gibson DJ, Gage KL, Matthews JL, Young BG, Owen MDK, Wilson RG, Weller SC, Shaw DR, Jordan DL. The effect of weed management systems and location on arable weed species communities in glyphosate-resistant cropping systems. Applied Vegetation Science. 2013;16:676–687. [Google Scholar]
- 47.Werth J, Boucher L, Thornby D, Walker S, Charles G. Changes in weed species since the introduction of glyphosate-resistant cotton. Crop and Pasture Science. 2013;64:791–798. [Google Scholar]
- 48.Carpenter JE. Impact of GM crops on biodiversity. GM Crops. 2011;2:7–23. doi: 10.4161/gmcr.2.1.15086. [DOI] [PubMed] [Google Scholar]
- 49.Kovach J, Petzoldt C, Degni J, Tette J. New York’s Food and Life Sciences Bulletin. Geneva, NY: NYS Agricultural Experiment Station, Cornell University; 1992. A method to measure the environmental impact of pesticides. http://www.nysipm.cornell.edu/publications/EIQ.html. [Google Scholar]
- 50.Bravo A, Gill SS, Soberón M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49:423–435. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanchez V. From microbial sprays to insect-resistant transgenic plants: history of the biospesticide Bacillus thuringiensis. A review. Agron Sustain Dev. 2010;2011;31:217–231. [Google Scholar]
- 52.Snow A, Pilson D, Rieseberg H, Paulsen MJ, Pleskac N, Reagon MR, Wolf DE, Selbo SM. A Bt transgene reduces herbivory and enhances fecundity in wild sunflowers. Ecol Applic. 2003;13:279–286. [Google Scholar]
- 53.Tabashnik BE. Evolution of resistance to Bacillus thuringiensis. Annu Rev Entomol. 1994;39:47–79. [Google Scholar]
- 54.Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW. Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0022629. e22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobsen CS, Hjelmsø MH. Agricultural soils, pesticides and microbial diversity. Current Opinion in Biotechnology. 2014;27:15–20. doi: 10.1016/j.copbio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Pimentel D, Burgess M. Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. In: Pimentel David, Peshin Rajinderpp., editors. Integrated Pest Management. Springer; Netherlands: 2014. pp. 47–71. [Google Scholar]
- 57.Gatehouse AMR, Ferry N, Raemaekers RJM. The case of the monarch butterfly: a verdict is returned. Trends in Genetics. 2002;18:249–251. doi: 10.1016/s0168-9525(02)02664-1. [DOI] [PubMed] [Google Scholar]
- 58.Lu Y, Wu K, Jiang Y, Guo Y, Desneux N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature. 2012;487:362–365. doi: 10.1038/nature11153. [DOI] [PubMed] [Google Scholar]
- 59.Codex. Alinorm 03/34: Joint FAO/WHO Food Standard Programme, Codex Alimentarius Commission, Appendix III, Guideline for the conduct of food safety assessment of foods derived from recombinant-DNA plants. Codex Alimentarius Commission; Rome, Italy: 2003. http://www.codexalimentarius.net/download/standards/10021/CXG_045e.pdf. [Google Scholar]
- 60.Parrott W. The nature of change: towards sensible regulation of transgenic crops based on lessons from plant breeding, biotechnology and genomics. Proceedings of the 17th North American Biothecnology Council; Nahville, Tenn. 2005. [Google Scholar]
- 61.Ricroch AE, Berge JB, Kuntz M. Evaluation of genetically engineered crops using transcriptomic, proteomic, and metabolomics profiling techniques. Plant Physiology. 2011;155:1752–176. doi: 10.1104/pp.111.173609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonsalves D, Gonsalves C, Ferreira S, Pitz K, Fitch M, Manshardt R, Slightom J. Transgenic virus resistant papaya: from hope to reality for controlling papaya ringspot virus in Hawaii. APSnet feature story for July 2004. 2004. http://www.apsnet.org/publications/apsnetfeatures/Pages/papayaringspot.aspx.
- 63.Tscharntke T, clough Y, Wanger TC, Jackson L, Motzke I, Perfecto I, Vandermeer J, Whitbread A. Global food security, biodiversity conservation and the future of agricultural intensification. Biological Conservation. 2012;151:53–59. [Google Scholar]