Abstract
The development of smart illumination sources represents a central challenge for current technology. In this context, the quest for novel materials that enable efficient light generation is essential. Metal halide compounds with perovskite crystalline structure (ABX3) have gained tremendous interest in the last five years since they come as easy-to-prepare high performance semiconductors. Perovskite absorbers are driving the power-conversion-efficiencies of thin film photovoltaics to unprecedented values. Nowadays, mixed-cation, mixed-halide lead perovskite solar cells reach efficiencies consistently over 20% and promise to get close to 30% in multijunction devices when combined with silicon cells at no surcharge. Nonetheless, perovskites’ fame extends further since extensive research on these novel semiconductors has also revealed their brightest side. Soon after their irruption in the photovoltaic scenario, demonstration of efficient color tunable—with high color purity—perovskite emitters has opened new avenues for light generation applications that are timely to discuss herein.
Human species developed under sunlight. Consequently, light regulates key physiological functions such as circadian rhythm or visual cycle that allow us to distinguish shapes and colors. Light also drives economic activity and wealth. Thus, artificial lighting represents a significant fraction (∼10%) of the total electrical energy consumed worldwide,1 with traditional illumination sources such as incandescent or fluorescent lamps unarguably being inefficient or ineffective. Aiming at developing energy efficient, environmentally friendly, and versatile light sources, the demonstration of electroluminescent semiconductors during the last part of the 20th century opened the door to the so-called solid-state lighting (SSL) technology. SSL sources are those based on inorganic or organic materials, which emit light when electrical energy is applied to them. Although light-emitting diodes (LEDs) were widely employed as indicators in electronic devices, recent technological advancements have enabled the widespread use of LEDs for illumination. Indeed, LED-based sources are destined to substitute every lamp on earth, displacing conventional technologies in all applications in which visible light is needed to save costs and lower energy consumption. Figure 1 (a) displays a picture of Milan (Italy) at night taken from space that illustrates the lighting technology transition that is occurring in most of our cities, with sodium vapor lamps gradually being replaced by LEDs. However, the development of efficient lamps is bringing about an increase of lighting usage and hence light pollution, which is concerning for living creatures from bacteria to mammals that have evolved according to a day-night cycle.2 Consequently, it is of outmost importance to develop new routes to achieve a finer control of the generated light to make an effective use of resources. Indeed, limited control over brightness, color quality, and directionality of LED light emission that conventional materials such as mirrors and lenses (typically employed as secondary optical elements to guide the generated light) provide is limiting the success of the much-needed SSL transition. Metal halide perovskite materials have emerged as a potential solution to some of these limitations. These easy-to-prepare high-performance semiconductors feature long carrier lifetime, large mobility, high photoluminescence quantum yield (PLQY), and direct bandgap that can be precisely tuned.3 Additionally, perovskites can be processed from solution, which allows the fast screening of a myriad of compositions, and they are compatible with thermal evaporation, which paves the way toward commercialization. The advent of these semiconductors offers new opportunities for the realization of bright emitting materials with which to demonstrate light-emitting devices of improved performance.4
Figure 1.
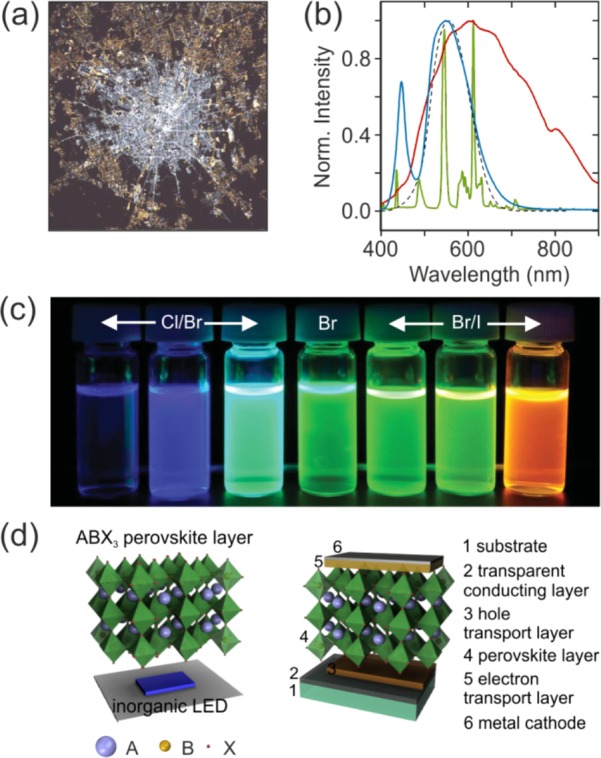
(a) Picture of Milan (Italy) taken from space in 2015 that demonstrates the lighting technology transition from sodium vapor to LED. Reprinted with permission from ref (2). Copyright 2017 American Association for the Advancement of Science. (b) Emission spectrum of different commercial white light sources, i.e., halogen lamp (red curve), fluorescent tube (green curve), and white LED (blue curve). The photopic response of the eye is also shown as a black dashed line. (c) Picture of the photoluminescence exhibited by CsPbX3 perovskite NCs of different size and composition (X= Cl/Br, Br or Br/I). Adapted from ref (16) with permission from John Wiley & Sons Ltd. (d) Sketch of a perovskite downshifter (left) and a perovskite electroluminescent device (right).
The development of the long-sought-after blue LEDs enabled the creation of white light by combining the three primary colors in one chip. However, commercial solid-state white light is generally achieved starting from an electroluminescent blue or ultraviolet (UV) inorganic semiconductor and converting part of its light to longer wavelengths by means of one or more photoluminescent materials.5 Illumination devices, fabricated based on this principle, are generally called phosphor-converted LEDs (pcLEDs), with the chromaticity of the generated light being mainly dictated by the photoluminescence (PL) spectrum of the converter.6 The current leading solution to achieve white light using phosphor-based LEDs involves yttrium aluminum garnet (YAG, Y3Al5O12) doped with Ce3+ (YAG:Ce) as a color-converter (blue curve in Figure 1b). However, the color quality of standard devices is poor owing to the low red content that typical YAG:Ce phosphors offer. Indeed, standard white LEDs feature a significant fraction of blue emission compared with other commercial white light sources as shown in Figure 1b, which gives rise to a significant increase of light pollution when these lamps are employed for outdoor lighting. Additionally, white pcLEDs typically yield cold white light with a color-rendering index (CRI) below 80, which is inadequate, especially for indoor lighting. The combination of YAG:Ce with a red phosphor (e.g., CaAlSiN3:Eu2+) results in light sources able to reveal colors more faithfully at the expense of a reduced luminous efficiency (LE), which brings to light the trade-off between CRI and LE. High CRI sources are based on broadband emission spectra that span throughout the visible, whereas in order to achieve high LE, light emission in spectral regions where the photopic response of the eye reduces (λ<500 nm and λ>620 nm) must be minimized. Consequently, the development of novel emitters with properties suited to LED integration is a subject of in-depth research nowadays. In particular, organic fluorophores or semiconductor nanocrystals (NCs) that exhibit quantum size effects have also been investigated for color conversion (also called downshifting) in LEDs. In fact, red CdSe/CdS core/shell semiconductor NCs have been already combined with green-emitting Eu2+ phosphors in order to improve color saturation in display backlights.7 Complementary technologies like those based on organic molecules, transition metal complexes, or the aforementioned colloidal NCs, offer several advantages over conventional LEDs such as the feasibility to develop thin, large-area, flexible devices with extensive design freedom. In fact, to date, organic LEDs (OLEDs) and quantum dot LEDs (QDLEDs) are central in the development of high-end displays. Although record devices feature external quantum efficiency (EQE) values well above 20%,8,9 critical issues related to their efficiency, stability, lifetime, and manufacturing costs have limited their widespread use for lighting. Although both metal halide perovskite materials and conventional II–VI or III–V semiconductor NCs show vivid emission in narrow spectral ranges that can be tuned throughout the visible spectrum, perovskites feature high defect tolerance, which is in stark contrast to conventional semiconductor NCs, in which electronic surface passivation is central. Additionally, composition control in perovskite materials is more convenient than in many conventional NCs by postsynthetic anion exchange. Metal halide perovskites thus appear as promising candidates to develop a novel generation of light-emitting devices. The brightness of artificial light sources must adapt to the very different light levels required for each particular situation. Notice that illuminance levels of natural light ranges between the more than 10 000 lm·m–2 we perceived under full daylight and the less than 1 lm·m–2 under moonlight. For instance, an illuminance level of 500 lm·m–2 is typically necessary for office lighting and 50 lm·m–2 for the illumination of a living room. Commercial lamps based on inorganic LEDs show efficiencies over 150 lm·W–1, being able to produce over 20 000 lm during more than 100 000 h. Their organic counterparts have reached conversion efficiencies close to 100 lm·W–1 and extended their lifetime at significantly larger production costs, which have hindered their use for general lighting. Display applications, in turn, are far less stringent with respect to the stability or brightness since typical displays require luminance levels of few hundreds of cd·m–2. In this context, perovskite materials arise as newcomers amenable for mass production with close to 100% PLQY and highly saturated colors that promise great potential to achieve displays with wider color gamut or SSL sources with higher CRI.10,11
Intrinsic Material Properties for Efficient Light Generation. In ABX3 perovskites, the A site in the structure is typically occupied by a cation such as methylammonium (MA), formamidinium (FA), or cesium; a divalent metallic cation (lead, tin or germanium) sits in B, whereas a halide (iodide, bromide, or chloride) is in C. Optical quality thin films made of ABX3 have been successfully prepared following both solution-based methods and vacuum deposition processes. Strikingly different to most semiconductors, the performance of perovskite materials is highly tolerant to structural defects.12 However, the presence of halide vacancies and mobile halides has a profound impact on both nonradiative recombination and stability.13,14 MAPbI3 has been the most widely investigated composition, being the Drosophila of metal halide perovskites. The choice of metal and halide mainly determines the position of the electronic bandgap, which is 1.6 eV for MAPbI3. This fact, together with a large extinction coefficient, makes them excellent materials for photovoltaics. More interestingly, these semiconductors present a direct bandgap, and radiative recombination events are mainly dictated by the bimolecular recombination of free electrons and holes.15 The emission of MAPbI3 lies in the near-infrared (NIR) region of the electromagnetic spectrum where our eye is not sensitive. Halide replacement in APbX3 perovskites allows in turn the gradual shift of the electronic bandgap and hence the emission spectrum throughout the visible as shown in Figure 1c.16 Indeed, Tan et al. showed that electroluminescence (EL) could be tuned from the NIR to the green, whereas Sadhanala et al. demonstrated tunable EL from green to blue by controlling the halide composition.17,18 However, light-induced halide segregation in perovskites represents a central challenge that limits the control over the emission color with time. In contrast to solar energy conversion,19 where record devices feature organic–inorganic perovskites in which the monovalent A cation is typically based on a mixture of MA and FA, most widely investigated perovskites for light emission lately are purely inorganic, with Cs in the A site, to improve thermal and chemical stability, which represent the true weak spot of these novel semiconductors.20 Finally, it is worth mentioning that regulations against the use of heavy metals in consumer electronics are increasingly restrictive. For this reason, significant efforts are devoted to the challenging task of developing light-emitting lead-free perovskite NCs.21
Perovskite-Based Emitting Devices. In contrast to standard semiconductor materials employed in SSL devices, perovskites can be processed without using high-temperature or high-vacuum processes, which makes them ideal candidates to contribute to satisfy our voracious appetite for light.22 In particular, perovskite emitters can be integrated into light-emitting devices, as displayed in Figure 2d, such as (i) color-converting layers combined with blue or UV LEDs, or (ii) active layers in electrically driven perovskite LEDs (PeLEDs). Perovskites feature unique properties that render these versatile semiconductors in interesting materials for light generation. In spite of being an incipient technology, results attained so far are so encouraging that it is timely to assess their actual potential to solve limitations that SSL currently presents.
Figure 2.
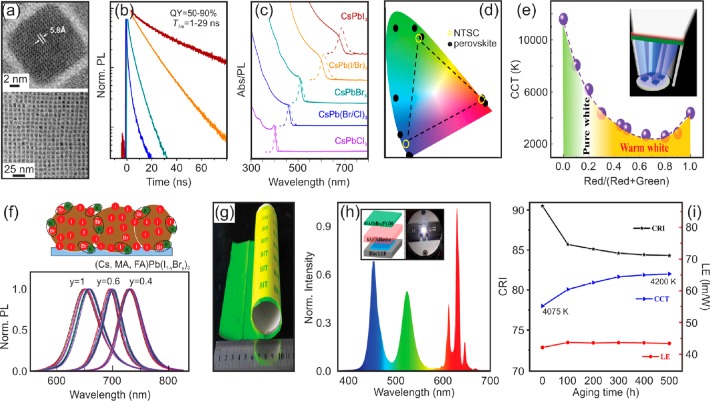
(a) TEM images of CsPbBr3 NCs. (b,c) Time resolved PL intensity (b) and absorption and PL spectra of CsPbX3 NCs (c). Reproduced with permission from ref (25). Copyright 2015 American Chemical Society. (d) Color gamut defined by perovskite NCs compared to the NTSC standard. (e) Color temperatures of perovskite-based emitting devices as a function of the ratio of green-to-red perovskite NCs. Adapted from ref (27) with permission from John Wiley & Sons Ltd. (f) Sketch of the mechanism by which complexing with potassium leads to the immobilization of bromide excess (top). PL of K-passivated perovskite films of different composition measured over time. Adapted from ref (30) with permission from Macmillan Publishers Ltd., copyright 2018. (g) Digital picture of MAPbBr3 NCs embedded in PVDF composite film. (h) Emission spectrum of a white LED that uses as color converter a combination of green emissive MAPbBr3 /PVDF composite films along with a red emissive phosphor. Adapted from ref (35) with permission from John Wiley & Sons Ltd. (i) Color rendering index, luminous efficiency, and color temperature of a perovskite-based white pcLED as a function of the aging time. Reprinted from ref (36), Copyright (2018), with permission from Elsevier.
Perovskite Downshifters. Color converters employed in display backlighting or phosphor LED lamps must feature (i) high PLQY to minimize the energy loss produced by the unavoidable Stokes shift associated with the downshifting process, (ii) high extinction coefficient at the wavelength used for photoexcitation, and (iii) excellent thermal and chemical stability. At the device level, two alternatives are typically considered to assemble the color-converting layer and the LED chip, which are known as “on-chip” and “remote” configurations depending on the relative position of the color converter respect to the LED. The former is simpler from the standpoint of the integration as the emitting layer is casting the LED. However, it presents critical issues associated with heat management due to the high temperatures usually reached in high power LEDs employed for illumination purposes, which may critically affect perovskite converters. This is less of a concern in the remote configuration since the emitting layer and the LED are spatially separated. However, more advanced light management is required in remote pcLEDs since a significant fraction of the emitted light does so toward the LED chip, and therefore scattering materials and mirrors must be included to redirect this light out of the device.
Although PLQY as high as 70% was demonstrated for solution-processed MAPbI3 films,22 perovskite crystal size and defect passivation are critical to enhance the PL emission of these materials. Indeed, the development of small perovskite NCs that may even exhibit quantum confinement effects has opened new avenues for investigation that researchers worldwide are exploring. Exciton confinement in small volumes leads to an increase in exciton binding energy that may result in an enhancement of the radiative recombination mediated by excitons rather than free electron and holes. Schmidt et al. performed the first demonstration of colloidal perovskite NCs in 2014. The authors succeeded to develop stable colloidal dispersions of efficient green-emitting MAPbBr3 NCs (PLQY > 80%) in different organic solvents using a mixture of MABr and alkylammonium bromide chains that reacted with PbBr2 in the presence of oleic acid and octadecene.23,24 In 2015, Protesescu et al. adapted the hot-injection method, typically employed to synthesized chalcogenide colloidal NCs, to demonstrate inorganic CsPbX3 colloidal nanocubes as shown in Figure 2a. The use of Cs+ as inorganic cation improves thermal and chemical stability of the perovskite and provides the material with a shorter lifetime (tens vs hundreds of nanoseconds) because of higher exciton recombination ratio (see Figure 2b). The bandgap of such perovskite NCs is tunable throughout the visible spectrum not only by means of the halide composition but also through the crystal size due to quantum confinement as displayed in Figure 2c. As a result, colloidal dispersions with PLQY as high as 90% and narrow emission line widths (12–42 nm) yielding a wide color gamut were reported.25 In order to improve colloidal stability and achieve larger exciton binding energy, the use of longer ligands and nonstoichiometric synthesis to prepare NCs with Br-rich surfaces that minimize the density of halide defects has been reported.26 The combination of MAPbB3 NCs with saturated green emission, red phosphors, and a standard blue chip yields prototypes as efficient as 48 lm·W1–. However, this technique to prepare perovskite NCs is difficult to scale up in part due to the high temperature, the toxic solvents and the inert conditions generally involved. Aiming to develop robust and high-yield synthetic routes, room-temperature alternatives that make use of environmentally friendly solvents have been also shown.27,28 Casting blue LEDs with NCs dispersed in poly methyl methacrylate (PMMA) enables the fabrication of on-chip devices with a color gamut that cover a significantly larger area than the color space of the National Television Systems Committee (NTSC), which highlights the opportunities these NCs offer for display applications (see Figure 2d). Also, the color temperature can be tuned from warm to cool white (∼2500–11500 K) by controlling the amount of green and red emitting NCs,27 as shown in Figure 2e. On top of this, the use of shorter ligands to passivate the surface of CsPbBr3 NCs allowed the preparation of films with a PLQY of 35%.28 With a similar intent, green emitting films with PLQYs over 95% have been recently demonstrated by spin coating as prepared CsPbBr3 NCs and applying a postsynthetic treatment with PbBr2 that suppresses nonradiative decay channels.29 Although halide exchange reactions enable the preparation of CsPbBr3–xIx and CsPbBr3–xClx NCs that emit across the visible, compositional bandgap tuning brings about the worsening of the efficiency with Br/I (Br/Cl) mixtures displaying a PLQY of 50% (25%) on average,29 which represents a central challenge that must be addressed. In this context, a recent report demonstrates that the integration of passivating potassium halide layers in triple cation mixed halide perovskite films leads to a significant reduction of photoinduced halide migration (see Figure 2f) and nonradiative losses, which gives rise to internal yields close to 100%.30
In fact, in order to produce white light-employing perovskites that emit different colors, it is essential to find reliable ways to tune both the spectral position of the emission and its line width. Halide exchange between mixtures of perovskite NCs of different composition represents a versatile method to adjust the bandgap and thus control the color of the emission. However, in close relation to this mechanism, halide migration depicts a critical issue hampering chromaticity stability in the long term. In fact, the ease of halide exchange between perovskite NCs of different composition seems to be both a blessing and a curse for perovskite materials. Although X-ray irradiation under vacuum has proven effective to inhibit anion-exchange reactions,31 in order to provide perovskite NCs with mechanical, chemical and thermal stability, these nanomaterials are generally incorporated into organic or inorganic hosts. Indeed, mixing NCs based on Cl–, Br– and I– into an insulating and transparent polymer matrix, such as polystyrene or PMMA, allows the preparation of blue-, green- and red-emitting films with PLQYs of 10%, 25%, and 15%, respectively, keeping unaltered the spectral position of the PL spectra over a few hours of continuous illumination. Combining different films with commercial blue LEDs results in the generation of different shades of white light, achieving CRI values as high as 86 for cool white light (5229 K).32 However, the authors did appreciate a gradual reduction of the PL intensity, which highlights the stability concerns provoked by these materials. Indeed, halide exchange jeopardizes not only the long-term stability of perovskite emitters but also the presence of light, oxygen, or moisture. In order to protect CsPbX3 NCs against water, a surface treatment based on a polyhedral oligomeric silsesquioxane has been employed. Passivated perovskite NCs allowed the fabrication of perovskite converters for white LEDs with CRI of 81 and LE of 14.1 lm·W–1.33 Similarly, coating perovskite nanofibrous membranes with a hydrophobic silicone resin yields emitting layers that are more stable against oxygen and moisture. Specifically, the emission intensity of such materials reduces 15% after 120 h of UV light excitation, which represents an improvement with respect to unprotected materials that lessen its initial intensity up to 80% after the same irradiation time.34 In addition, polyvinylidene fluoride (PVDF) demonstrated excellent properties to embed perovskite NCs. Composite films shown in Figure 2g show a ∼10% decrease in the emission intensity after 400 h of UV excitation or water immersion. By contrast, films are not stable at temperatures higher than 70 °C. A white LED for backlighting with LE as high as 109 lm·W–1 was demonstrated by mixing the blue light emitted by a LED chip as displayed in Figure 2h, the green emission of the NC/PVDF composite and the red emission of Mn4+ phosphor.35 In another example, the epoxy resin commonly used as encapsulation material in high power LEDs has been recently employed to provide embedded CsPbX3 NCs with thermal insulation. The combination of a blue LED chip and a YAG:Ce substrate with ER-blended CsPbI2Br NCs result in rather efficient (LE = 42 lum·W–1) warm white light (4067 K) with high CRI (90.5) along with an improved stability since only ∼15% of the initial intensity is lost after 500 h under accelerated aging conditions (85 °C and 85% relative humidity) as can be observed in Figure 2i.36 Another approach to improve the stability consists in the use of inorganic matrices to enclose perovskite NCs. Efficient (50.5 lm·W–1) white LEDs (3674 K) with a CRI of 83.4 were demonstrated at a current of 20 mA using a combination of CsPbBr3 NCs precipitated into phosphosilicate glass and Eu2+ phosphors.37 Also, mesoporous silica particles with small pore size have proven useful to encapsulate green-emitting CsPbBr3 NCs. As a result, the emission intensity decreases to 40% (10% for unprotected NCs) of the initial value when the temperature rises to 100 °C. In addition, the emission decreases to 80% (40% for unprotected NCs) after 4 days of UV irradiation. In another promising example, green- and red-emitting composites formed by perovskite NCs synthesized in a silica matrix with PLQY ∼ 70% were developed. In combination with a blue LED chip, white pcLEDs in the remote configuration were demonstrated with a maximum LE of 61.2 lm·W–1 at an operating current of 300 mA·cm–2.38 Similarly, encapsulated green NCs mixed with red CsPb(Br0.4I0.6)3 NCs in a silicone resin casted a blue LED to yield an on-chip white LED package (30 lm·W–1) for backlighting.39 Finally, it is worth mentioning a novel approach based on the use of porous mesostructures of metal oxide thin films as nanocages to synthesize perovskite NCs that has turned the attention of the community lately.40−43 This approximation represents a simple route to prepare films with optical quality that are loaded with ligand-free NCs of controlled size distribution. It holds promise for developing blue-emitting materials based on only bromide perovskites.
Perovskite Electroluminescent Devices. Developing bright, efficient and stable PeLEDs is far more challenging. Central aspects are related to (i) perovskite thin film morphology, nonradiative decay channels and efficiency, and (ii) device architecture to reduce electron and hole injection barriers. Similarly to OLEDs, PeLEDs may consist of only one perovskite emitting layer sandwiched between two electrodes.44 Simple configurations comprising just injecting layers demonstrate the great potential of PeLEDs.45 However, state-of-the-art devices often contain several additional layers (see Figure 1d). Although a reference architecture has not been established yet, layers such as PolyTPD, TPBi, PEDOT:PSS, or ZnO, are generally used to separate blocking and injection layers in order to facilitate charge transport in the device and to prevent recombination of electron and holes away from the active layer to improve their efficiency and lifetime. A metallic layer such as LiF/Al or MoO3/Ag works as one of the contacts, whereas a layer of a transparent conductive oxide material, usually ITO, is employed as the other contact so that the emitted light can exit the device through the substrate. The whole stack is encapsulated to avoid the reaction of the different materials with oxygen and moisture.
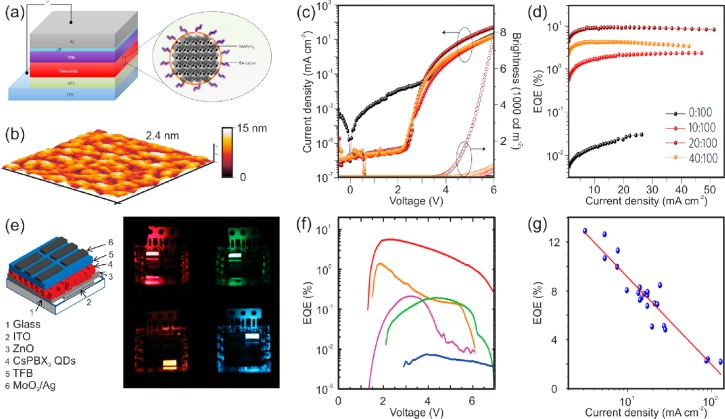
Hybrid Organic–Inorganic Perovskite Layers for Efficient Devices. Although green emission from perovskite-based devices was already proven in the last years of the 20th century,46,47 the first demonstration of bright (L > 100 cd·m–2) but rather inefficient EL (CE = . 0.3 cd·A–1; EQE = 0.1%) using solution-processed MAPbBr3 perovskite polycrystalline films was in 2014.17 Since then, significant efforts have been devoted to improve the efficiency and the stability of these devices. Strategies to develop perovskite films with the right morphology have been of paramount importance. Deficient perovskite film formation leads to pin-holes in the emitting layer through which injected charge carriers may circumvent the emitting layer, hampering the efficiency of the lamp. Indeed, it has been demonstrated that a combination of a fine modification of deposition procedures, precursor solutions and thermal annealing conditions of the films results in better film uniformity and long-term stability,48−53 which yield green emitting devices as efficient as those with CE of 42.9 cd·A–1 at ca. 9 V and EQE of 8.53%.48 The size of perovskite grains turns out to be critical as well, since nanograins allow confining spatially electron and holes, thus improving radiative recombination.54−57 Thus, ligand engineering has proven to be useful to limit crystal formation to achieve ultraflat films that feature nanometer-sized grains (see Figure 3a,b). Devices featuring CE of 17.1 cd·A–1 at ca. 6 V and EQE as high 10.4% have been demonstrated following this route as shown in Figure 3c,d.56 At the device level, layers of dissimilar nature have been employed to favor radiative bimolecular recombination in organic–inorganic perovskite layers by tuning electron and hole injection levels, reducing exciton quenching and exciton diffusion length, and enhancing long-term stability, aiming to improve the efficiency of PeLEDs.58−61
Figure 3.
(a) PeLED architecture along with a sketch of a perovskite grain decorated with butylammonium (BA) cations. (b) AFM image of a perovskite layer with a 20:100 molar ratio of BABr:MAPbBr3. (c,d) Current density versus voltage (c) and EQE versus current density (d) of PeLEDs with active layers prepared with different BABr:MAPbBr3 molar ratios. Adapted from ref (56) with permission from Macmillan Publishers Ltd., copyright 2017. (e) Sketch of a PeLED architecture based on CsPbX3 NCs. (f) Pictures of the electroluminescence with X = Br/Cl (0.5:0.5), Br, Br/I (0.25:0.75) and I. (g) EQE vs voltage curves. Adapted from ref (69) with permission from John Wiley & Sons Ltd. (g) Dependence of the maximum EQE with its corresponding driving current density of PeLEDs based on CsPbBr3 NCs. Adapted with permission from ref (72). Copyright 2018 American Chemical Society.
Purely Inorganic Perovskite Layers. Despite the rapid progress demonstrated in the efficiency of PeLEDs based on organic–inorganic perovskites, stability against water or high temperatures represents a true Achilles heel that is taking the wind out of the sails of these novel semiconductors.62 Aiming to solve this issue, the organic cation (MA or FA) has been replaced by inorganic alternatives such as Cs. Indeed, polycrystalline CsPbBr3 perovskite films were first demonstrated in 2015.63 Besides exhibiting an enhanced chemical and thermal stability, purely inorganic CsPbBr3 shows higher color purity since typical EL spectra feature a full width at half-maximum (fwhm) of ≈17 nm, which is slightly narrower than that of MAPbBr3 (≈20 nm) and significantly narrower than the fwhm of OLEDs or QLEDs. Notice that color saturation or color purity is proportional to the fwhm, so narrow emission yields a wider color gamut. Using nonstoichiometric precursor solutions to prevent EL quenching, pinhole-free CsPbBr3 or CsPbBr3 composite films enable the development of bright green-emitting inorganic-based PeLEDs,44,64−66 which show a CE of 15.6 cd·A–1 at ∼7.5 V and EQE of 4.3%.64 Very recently, Zhang et al. demonstrated that a mixed cation formulation combined with the deposition of a hydrophilic polymer on top of the electron-injection layer improve the performance of perovskite devices by bettering film morphology, reducing nonradiative recombination at interfaces and perovskite grain boundaries, and amelioration charge injection balance. In particular, Cs0.87MA0.13PbBr3 perovskite films yield green-emitting devices with an EQE of 10.4% (CE = 33.9 cd·A–1) and maximum brightness of over 90 000 cd·m–2, which is among the most efficient perovskite devices ever reported.67
Nanoscaled Perovskites. As previously discussed, recent results confirm that it is possible to prepare films with very high PLQY using CsPbBr3 NCs.29 However, protective organic ligands employed to provide NCs with colloidal stability hamper charge injection when the emitters are excited electrically. As a result, devices based on perovskite NCs are typically less bright and feature high turn-on voltage (Von). Despite rather inefficient (CE < 0.5 cd·A–1, EQE ∼ 0.1%, and LE < 0.2 lm·W–1), Song et al. demonstrated in 2015 the first blue-, green-, and yellow-emitting PeLED based on CsPbX3 NCs at Von > 4 V.16 Aiming to improve injection efficiency and thus reduce Von, a layer of perfluorinated ionomer was introduced between the perovskite emitting layer and the hole transport layer. As a result, it was possible to observe green emission under a voltage of 2.5 V, although devices remained inefficient and unstable since the intensity decreases 50% after 10 min in N2 atmosphere.68 In order to improve NC film processing, a cross-linking method based on trimethylaluminum vapor renders NC films insoluble, which improves film coverage and facilitates the processing of subsequent device layers. Following this route, a cross-linked CsPbI3 PeLED with EQE of 5.7% at a current density of 755 mA·cm–2, which represents the most efficient red-emitting device reported (see Figure 3e). However, results attained applying the same method to orange-, green-, or blue-emitting devices are more modest, as displayed in Figure 3f.69 Another route to reduce luminescence quenching in perovskite NCs consists in improving the crystalline structure and thus reducing trap density via the combination of CsPbBr3 NCs with CsPb2Br5 nanoparticles. Such composites give rise to bright devices (L = 3853 cd·m–2) with a CE of ∼9 cd·A–1 and EQE of ∼2.2%.70 Surface ligands in colloidal perovskite NCs are essential to provide surface passivation to yield high PLQY and colloidal stability. However, ligand excess hampers film processing and limits charge injection that deteriorates device performance. Ligand density control through different cycles of solvents has proven effective to achieve a good balance between surface defect density and carrier injection in the NCs. As a result, green-emitting devices with EQE as high as 6.27% (CE = 13.3 cd·A–1, LE = 5.24 lm·W–1; Von = 3.4 V) have been demonstrated.71 Recently, it has been proven to achieve highly efficient (EQE = 12.9% and LE = 26.1 lm·W–1) green-emitting diodes by reducing Auger recombination working at low current densities as shown in Figure 3g. However, we still have a lengthy path ahead of us since device brightness reduces to 50% of its initial value after only 6 min.72
Challenges for Next-Generation Illumination Devices. LED light sources are nowadays ubiquitous but SSL technology should address several key challenges in the next years in order to push the next generation of emitting devices. Perovskite materials can contribute to tackle such challenges aiming at enhancing the efficiency or providing light sources to come with new functionalities.
In terms of efficiency, the biggest challenge in inorganic LEDs based on GaN is the so-called “efficiency droop” or “efficiency roll-off”, i.e., efficiency of the LED increases at low input power density, peaks at few tens of A·cm–2 and then reduces at higher input power values because electrons leak out of the device without emitting any light. Consequently, commercial white pcLEDs comprise several efficient blue LEDs arranged in a single package to increase the output light flux keeping low input power densities. LED devices based on perovskite materials also suffer from efficiency roll-off with EQE values that peak at few tens of mA·cm–2 and then decrease by half when the input current density increases 1 order of magnitude, which has been attributed to luminescence quenching. Additionally, the development of efficient blue PeLEDs remains elusive due to the high defect densities of chloride-based perovskites. In fact, brightest blue PeLEDs based on bromide/chloride NCs developed so far reach 350 cd·m–2 and EQE values are still below 1%.73,74 Moving from three-dimensional to layered perovskites offers larger quantum confinement effects that leads to wider bandgaps and higher stability compared to their bulk counterparts. Indeed, the assembly of PbBr4 monolayer sheets within layered perovskites has enabled violet EL,75 providing new opportunities that researchers are exploring with determination. Ruddlesden–Popper (RP) layered perovskites based on phase-pure materials are attracting considerable attention lately aiming to achieve efficient and stable devices. In fact, nanoscale perovskites are already showing their potential. Indeed, NIR PeLEDs based on multiple quantum wells feature reduced nonradiative recombination rates to yield efficiencies as high as 12.7% while such values remain at 10% of the peak value under 500 mA·cm–2.76 In another example that outlines the way ahead, RP layered pure iodine perovskites shine at peak operation conditions with no droop at current densities of several A·cm–2 for more than 14 h.77 Also, the combination of perovskite bromide microplatelets and bromide NCs has recently yielded one of the most efficient (EQE = 13.4%, LE = 57.6 cd·A–1) green-emitting PeLED developed to date.78 These late examples illustrate that exploiting quantum confinement effects in nanoscale perovskites represents the most promising route to overcome the modest performance exhibited so far by perovskite materials and devices.
Commercial inorganic LEDs of different colors are not equally efficient. Although state-of-the art blue LEDs show electrical-to-optical conversion efficiencies around 50%, red and especially yellow and green LEDs feature efficiencies below 30% or 20%, respectively. For this reason, it is nowadays more convenient to produce green-to-yellow light using pcLEDs. Indeed, it has been recently demonstrated that it is possible to downshift the blue light emitted by a commercial GaN LED using highly efficient (PLQY > 90%) colloidal quantum wells to produce green light with a luminous power efficiency of 90 lm·W–1.79 However, it is highly relevant to develop electroluminescent materials to overcome the so-called “green gap”. Bromide-based perovskites have emerged as efficient green emitters with enormous potential, but a major breakthrough is yet to come so that PeLEDs can compete and outperform standard LEDs. In this regard, it is central to acquire deeper understanding on the mechanisms that govern radiative recombination in order to minimize nonradiative decay channels as a function of the electron energy density. Different preparation methods seem to yield perovskite materials with large variations in defect density. Consequently, it is not always straightforward to transfer conclusions extracted from different studies. Simultaneously, at the device level, balanced charge injection and transport is key to maximize internal quantum efficiency. To achieve this, further research on charge transport layers and contacts based on inexpensive materials that are stable under oxygen or moisture is of great significance. Establishing standard device architectures to benchmark results obtained in different laboratories appears to be of extreme importance in order to tackle these challenges.
Looking beyond efficiency, developing light sources with expanded functionalities will open new avenues for the flourishing of LEDs. Applications range from visible light communication (VLC) to horticulture or healthcare, where perovskite materials may also play a role. VLC uses light sources for both illumination and data transferring without cables, being the maximum bandwidth of the communication system limited by the rate at which LEDs can turn on or turn off. Perovskite NCs show faster radiative lifetimes than standard phosphors, which allows attaining white light sources with rapid response. As a result, a modulation bandwidth of 491 MHz (1 order of magnitude larger than that of conventional pcLEDs) can be achieved, making it possible to transmit information at a rate of 2 Gbit·s–1.80 The spectral composition of light and its duration should also be adapted depending on the specific task or the particular environment since light has a great impact on our mood or productivity. In addition, the quality of light influences crop cultivations, having an effect on the growth of plants from germination to harvest. In this context, the emission color of perovskite materials can be precisely tuned throughout the visible, which offers a myriad of possibilities to accustom the chromaticity of the devices to the specifications required in each situation.
A precise control of the emission properties of devices beyond efficiency connects with the growing interest for the effective generation of light since more than half of the produced light is generally misused. For this reason, it is central to find new ways to generate light of the desired shade exactly where it is required. Nonetheless, conventional optical elements relying on geometrical optics provide limited control over brightness, color quality or directionality of LED emission. The combination of perovskite emitters with photonic nanostructures that provide a devised design of their optical environment will maximize light output and allow a precise control of the radiation shaping in PeLEDs.81
In this Perspective, I have addressed the main opportunities offered by metal halide perovskites materials for next-generation lighting devices. Although the potential of these defect-tolerant semiconductors seems endless, challenges related to nonradiative losses and halide migration and segregation represent the main concerns limiting the development of this technology. Although we have witnessed a rapid progress in terms of device efficiency in the last three years, a major breakthrough, based on a deep understanding of the above-mentioned issues, which could lead to the rational design of perovskite light-emitting devices with the highest efficiency is yet to come.
Key Performance Parameters. To characterize an electroluminescent device, it is generally measured the current density in A·m–2 and the luminance, i.e., eye response weighted radiance assuming photopic conditions, in cd·m–2 as a function of the applied voltage in V. Candela measures the luminous intensity in a particular direction; lumen measures the total quantity of visible light emitted by a source, and lux measures the intensity of visible light with which a specific surface is illuminated. Indeed, 1 lm equals 1 cd·sr–1, which means that a source emitting 1 cd features a luminous intensity of 4π lumen, and 1 lx equals 1 lm·m–2. Additionally, several parameters are typically employed to assess both the efficiency and the appearance of the LED light source. The external quantum efficiency (EQE) is defined as the product of the injection efficiency (ratio of electrons injected into the quantum well related to the ones provided by the power source), internal quantum efficiency (ratio of photons generated to the number of electron–hole recombinations occurred), conversion efficiency (emitted green-to-red photons to absorbed blue photons), and the extraction efficiency (ratio of photons leaving the device to those generated). EQE is the ratio of the number of photons emitted by the device to the number of electrons supplied, being thus dimensionless. The luminous efficiency in cd·A–1 and the luminous power efficiency in lum·W–1 represent the efficiency with which LEDs convert electrical energy into optical power according to the sensitivity of the human eye. Notice that meaningful efficiency values should be always provided together with those of luminance, driving current and voltage. For example, a state-of-the-art white LED is 30% efficient considering that the efficiency of a blue LED is ca. 50%, color conversion and extraction is ca. 70%, and spectral match to the human eye response is ca. 85%.82 Beyond efficiency, the calculation of tristimulus values allows the description of color in terms of widely employed chromaticity diagrams. In a pcLED, the color of the light source is given by the mixing of the nonabsorbed excitation light with the emission of the color converter. For white-light applications, the resultant color point must lie on, or very close to, the locus of points that follows the line of a blackbody radiator, which determines the shade of the white light source given by its correlated color temperature (CCT in K). The CRI quantifies the ability of a light source to reveal the color appearance of an object in comparison with the Sun. Although it depends on the particular application, in general, higher values are required for indoor lighting applications.
Acknowledgments
I thank Mauricio E. Calvo and Miguel Anaya for fruitful discussions. This research has received funding from Spanish Ministry of Economy and Competitiveness under Grant MAT2017-88584-R and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme NANOPHOM (ERC-2016-StG 715832).
Biography
Gabriel Lozano holds a permanent position in the Spanish National Research Council (CSIC), where he works at the Institute of Materials Science of Seville (ICMS, Spain). He received his Ph.D. at the University of Seville (Spain) in 2010. He worked as a postdoc at AMOLF (The Netherlands) from 2011 until 2013 and at the ICMS from 2014 until 2017. Since 2017, he leads an ERC Starting Grant project devoted to develop novel nanostructured optical materials to improve the performance of light-emitting devices.
The author declares no competing financial interest.
References
- Annual Energy Outlook; U.S. Energy Information Administration: Washington, DC, 2018. [Google Scholar]
- Kyba C. C. M.; Kuester T.; Sánchez de Miguel A.; Baugh K.; Jechow A.; Hölker F.; Bennie J.; Elvidge C. D.; Gaston K. J.; Guanter L. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 2017, 3, e1701528. 10.1126/sciadv.1701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Eperon G. E.; Snaith H. J. Metal halide perovskites for energy applications. Nat. Energy 2016, 1, 16048. 10.1038/nenergy.2016.48. [DOI] [Google Scholar]
- Kovalenko M. V.; Protesescu L.; Bodnarchuk M. I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. 10.1126/science.aam7093. [DOI] [PubMed] [Google Scholar]
- Pimputkar S.; Speck J. S.; DenBaars S. P.; Nakamura S. Prospects for LED lighting. Nat. Photonics 2009, 3, 180–182. 10.1038/nphoton.2009.32. [DOI] [Google Scholar]
- Smet P. S.; Parmentier A. B.; Poelman D. Selecting conversion phosphors for white light-emitting diodes. J. Electrochem. Soc. 2011, 158, R37–R54. 10.1149/1.3568524. [DOI] [Google Scholar]
- Abe S.; Joos J. J.; Martin L.; Hens Z.; Smet P. F. Hybrid remote quantum dot/powder phosphor designs for display backlights. Light: Sci. Appl. 2016, 6, e16271. 10.1038/lsa.2016.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuei C.-Y.; Tsai W.-L.; Tong B.; Jiao M.; Lee W.-K.; Chi Y.; Wu C.-C.; Liu S.-H.; Lee G.-H.; Chou P.-T. Phosphorescence and Organic Light-Emitting Diodes with External Quantum Efficiency Exceeding 31%. Adv. Mater. 2016, 28, 2795–2800. 10.1002/adma.201505790. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Chen S.; Sun X. W. Efficient Red/Green/Blue Tandem Quantum-Dot Light-Emitting Diodes with External Quantum Efficiency Exceeding 21%. ACS Nano 2018, 12, 697–704. 10.1021/acsnano.7b07867. [DOI] [PubMed] [Google Scholar]
- Chen H.-W.; Zhu R.-D.; He J.; Duan W.; Hu W.; Lu Y-Q.; Li M.-C.; Lee S.-L.; Dong Y.-J.; Wu S.-T. Going beyond the limit of an LCD’s color gamut. Light: Sci. Appl. 2017, 6, e17043. 10.1038/lsa.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H.-C.; Oh H.; Lee S.; Park J. B.; Do Y. R. Circadian-tunable Perovskite Quantum Dot-based Down- Converted Multi-Package White LED with a Color Fidelity Index over 90. Sci. Rep. 2017, 7, 2808. 10.1038/s41598-017-03063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.; Wang L. W. High Defect Tolerance in Lead Halide Perovskite CsPbBr3. J. Phys. Chem. Lett. 2017, 8, 489–493. 10.1021/acs.jpclett.6b02800. [DOI] [PubMed] [Google Scholar]
- Brandt R. E.; Poindexter J. R.; Gorai P.; Kurchin R. C.; Hoye R. L. Z.; Nienhaus L.; Wilson M. W. B.; Polizzotti J. A.; Sereika R.; Žaltauskas R.; et al. Searching for “Defect-Tolerant” Photovoltaic Materials: Combined Theoretical and Experimental Screening. Chem. Mater. 2017, 29, 4667–4674. 10.1021/acs.chemmater.6b05496. [DOI] [Google Scholar]
- deQuilettes D. W.; Zhang W.; Burlakov V. M.; Graham D. J.; Leijtens T.; Osherov A.; Bulović V.; Snaith H. J.; Ginger D. S.; Stranks S. D. Photo-induced halide redistribution in organic–inorganic perovskite films. Nat. Commun. 2016, 7, 11683. 10.1038/ncomms11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J. M.; Abdi-Jalebi M.; Sadhanala A.; Tabachnyk M.; Rivett J. P. H.; Pazos-Outón L. M.; Godel K. C.; Price M.; Deschler F.; Friend R. H. Enhancing photoluminescence yields in lead halide perovskites by photon recycling and light out-coupling. Nat. Commun. 2016, 7, 13941. 10.1038/ncomms13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; Li J.; Li X.; Xu L.; Dong Y.; Zeng H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. 10.1002/adma.201502567. [DOI] [PubMed] [Google Scholar]
- Tan Z.-K.; Moghaddam R. S.; Lai M. B.; Docampo P.; Higler R.; Deschler F.; Price M.; Sadhanala A.; Pazos L. M.; Credgington D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. 10.1038/nnano.2014.149. [DOI] [PubMed] [Google Scholar]
- Sadhanala A.; Ahmad S.; Zhao B.; Giesbrecht N.; Pearce P. M.; Deschler F.; Hoye R. L. Z.; Gödel K. Z.; Bein T.; Docampo P.; et al. Blue-Green Color Tunable Solution Processable Organolead Chloride–Bromide Mixed Halide Perovskites for Optoelectronic Applications. Nano Lett. 2015, 15, 6095–6101. 10.1021/acs.nanolett.5b02369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith H. J. Present status and future prospects of perovskite photovoltaics. Nat. Mater. 2018, 17, 372–376. 10.1038/s41563-018-0071-z. [DOI] [PubMed] [Google Scholar]
- Cho H.; Kim Y.-H.; Wolf C.; Lee H.-D.; Lee T.-W. Improving the Stability of Metal Halide Perovskite Materials and Light-Emitting Diodes. Adv. Mater. 2018, 1704587. 10.1002/adma.201704587. [DOI] [PubMed] [Google Scholar]
- Sun J.; Yang J.; Lee J. I.; Cho J. H.; Kang M. S. Lead-Free Perovskite Nanocrystals for Light-Emitting Devices. J. Phys. Chem. Lett. 2018, 9, 1573–1583. 10.1021/acs.jpclett.8b00301. [DOI] [PubMed] [Google Scholar]
- Deschler F.; Price M.; Pathak S.; Klintberg L. E.; Jarausch D.-D.; Higler R.; Hüttner S.; Leijtens T.; Stranks S. D.; Snaith H. J.; et al. High Photoluminescence Efficiency and Optically Pumped Lasing in Solution-Processed Mixed Halide Perovskite Semiconductors. J. Phys. Chem. Lett. 2014, 5, 1421–1426. 10.1021/jz5005285. [DOI] [PubMed] [Google Scholar]
- Schmidt L. C.; Pertegás A.; González-Carrero S.; Malinkiewicz O.; Agouram S.; Mínguez Espallargas G.; Bolink H. J.; Galian R. E.; Pérez-Prieto J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. 10.1021/ja4109209. [DOI] [PubMed] [Google Scholar]
- González-Carrero S.; Galian R. E.; Pérez-Prieto J. Maximizing the emissive properties of CH3NH3PbBr3 perovskite nanoparticles. J. Mater. Chem. A 2015, 3, 9187–9193. 10.1039/C4TA05878J. [DOI] [Google Scholar]
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Zhong H.; Chen C.; Wu X.; Hu X.; Huang H.; Han J.; Zou B.; Dong Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. 10.1021/acsnano.5b01154. [DOI] [PubMed] [Google Scholar]
- Li X.; Wu Y.; Zhang S.; Cai B.; Gu Y.; Song J.; Zeng H. CsPbX3 Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. 10.1002/adfm.201600109. [DOI] [Google Scholar]
- Akkerman Q. A.; Gandini M.; Di Stasio F.; Rastogi P.; Palazon F.; Bertoni G.; Ball J. M.; Prato M.; Petrozza A.; Manna L. Strongly emissive perovskite nanocrystal inks for high-voltage solar cells. Nat. Energy 2017, 2, 16194. 10.1038/nenergy.2016.194. [DOI] [Google Scholar]
- Di Stasio F.; Christodoulou S.; Huo N.; Konstantatos G. Near-Unity Photoluminescence Quantum Yield in CsPbBr3 Nanocrystal Solid-State Films via Postsynthesis Treatment with Lead Bromide. Chem. Mater. 2017, 29, 7663–7667. 10.1021/acs.chemmater.7b02834. [DOI] [Google Scholar]
- Abdi-Jalebi M.; Andaji-Garmaroudi Z.; Cacovich S.; Stavrakas C.; Philippe B.; Richter J. M.; Alsari M.; Booker E. P.; Hutter E. M.; Pearson A. J.; et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555, 497–501. 10.1038/nature25989. [DOI] [PubMed] [Google Scholar]
- Palazon F.; Di Stasio F.; Akkerman Q. A.; Krahne R.; Prato M.; Manna L. Polymer-Free Films of Inorganic Halide Perovskite Nanocrystals as UV-to-White Color-Conversion Layers in LEDs. Chem. Mater. 2016, 28, 2902–2906. 10.1021/acs.chemmater.6b00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S.; Sakai N.; Wisnivesky Rocca Rivarola F.; Rivarola R.; Stranks S. D.; Liu J.; Eperon G. E.; Ducati C.; Wojciechowski K.; Griffiths J. T.; et al. Perovskite Crystals for Tunable White Light Emission. Chem. Mater. 2015, 27, 8066–8075. 10.1021/acs.chemmater.5b03769. [DOI] [Google Scholar]
- Huang H.; Chen B.; Wang Z.; Hung T. K.; Susha A. S.; Zhong H.; Rogach A. L. Water resistant CsPbX3 nanocrystals coated with polyhedral oligomeric silsesquioxane and their use as solid state luminophores in all-perovskite white light-emitting devices. Chem. Sci. 2016, 7, 5699–5703. 10.1039/C6SC01758D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai J.; Li H.; Zhao Y.; Chen F.; Peng Y.; Wang B. Designing of blue, green, and red CsPbX3 perovskite-codoped flexible films with water resistant property and elimination of anion-exchange for tunable white light emission. Chem. Commun. 2017, 53, 5400–5403. 10.1039/C7CC01152K. [DOI] [PubMed] [Google Scholar]
- Zhou Q.; Bai Z.; Lu W.; Wang Y.; Zou B.; Zhong H. In Situ Fabrication of Halide Perovskite Nanocrystal-Embedded Polymer Composite Films with Enhanced Photoluminescence for Display Backlights. Adv. Mater. 2016, 28, 9163–9168. 10.1002/adma.201602651. [DOI] [PubMed] [Google Scholar]
- Liu S.; He M.; Di X.; Li P.; Xiang W.; Liang X. CsPbX3 nanocrystals films coated on YAG: Ce3+ PiG for warm white lighting source. Chem. Eng. J. 2017, 330, 823–830. 10.1016/j.cej.2017.08.032. [DOI] [Google Scholar]
- Di X.; Hu Z.; Jiang J.; He M.; Zhou L.; Xiang W.; Liang X. Use of long-term stable CsPbBr3 perovskite quantum dots in phospho-silicate glass for highly efficient white LEDs. Chem. Commun. 2017, 53, 11068–11071. 10.1039/C7CC06486A. [DOI] [PubMed] [Google Scholar]
- Sun C.; Zhang Y.; Ruan C.; Yin C.; Wang X.; Wang Y.; Yu W. W. Efficient and Stable White LEDs with Silica-Coated Inorganic Perovskite Quantum Dots. Adv. Mater. 2016, 28, 10088–10094. 10.1002/adma.201603081. [DOI] [PubMed] [Google Scholar]
- Wang H.-C.; Lin S.-Y.; Tang A.-C.; Singh B. P.; Tong H.-C.; Chen C.-Y.; Lee Y.-C.; Tsai T.-L.; Liu R.-S. Mesoporous Silica Particles Integrated with All- Inorganic CsPbBr3 Perovskite Quantum-Dot Nanocomposites (MP-PQDs) with High Stability and Wide Color Gamut Used for Backlight Display. Angew. Chem., Int. Ed. 2016, 55, 7924–7929. 10.1002/anie.201603698. [DOI] [PubMed] [Google Scholar]
- Kojima A.; Ikegami M.; Teshima K.; Miyasaka T. Highly Luminescent Lead Bromide Perovskite Nanoparticles Synthesized with Porous Alumina Media. Chem. Lett. 2012, 41, 397–399. 10.1246/cl.2012.397. [DOI] [Google Scholar]
- Dirin D. N.; Protesescu L.; Trummer D.; Kochetygov I. V.; Yakunin S.; Krumeich F.; Stadie N. P.; Kovalenko M. V. Harnessing Defect-Tolerance at the Nanoscale: Highly Luminescent Lead Halide Perovskite Nanocrystals in Mesoporous Silica Matrixes. Nano Lett. 2016, 16, 5866–5874. 10.1021/acs.nanolett.6b02688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaya M.; Rubino A.; Rojas T. C.; Galisteo-López J. F.; Calvo M. E.; Míguez H. Strong Quantum Confinement and Fast Photoemission Activation in CH3NH3PbI3 Perovskite Nanocrystals Grown within Periodically Mesostructured Films. Adv. Opt. Mater. 2017, 5, 1601087. 10.1002/adom.201601087. [DOI] [Google Scholar]
- Ghosh J.; Ghosh R.; Giri P. K. Mesoporous Si Nanowire Templated Controlled Fabrication of Organometal Halide Perovskite Nanoparticles with High Photoluminescence Quantum Yield for Light-Emitting Applications. ACS Appl. Nano Mater. 2018, 1, 1551–1562. 10.1021/acsanm.8b00047. [DOI] [Google Scholar]
- Li J.; Shan X.; Bade S. G. R.; Geske T.; Jiang Q.; Yang X.; Yu Z. Single-Layer Halide Perovskite Light-Emitting Diodes with Sub- Band Gap Turn-On Voltage and High Brightness. J. Phys. Chem. Lett. 2016, 7, 4059–4066. 10.1021/acs.jpclett.6b01942. [DOI] [PubMed] [Google Scholar]
- Jaramillo-Quintero O. A.; Sanchez R. S.; Rincon M.; Mora-Sero I. Bright Visible-Infrared Light Emitting Diodes Based on Hybrid Halide Perovskite with Spiro-OMeTAD as a Hole-Injecting Layer. J. Phys. Chem. Lett. 2015, 6, 1883–1890. 10.1021/acs.jpclett.5b00732. [DOI] [PubMed] [Google Scholar]
- Era M.; Morimoto S.; Tsutsui T.; Saito S. Organic-inorganic heterostructure electroluminescent device using a layered perovskite semiconductor (C6H5C2H4NH3)2PbI4. Appl. Phys. Lett. 1994, 65, 676–678. 10.1063/1.112265. [DOI] [Google Scholar]
- Chondroudis K.; Mitzi D. B. Electroluminescence from an Organic-Inorganic Perovskite Incorporating a Quaterthiophene Dye within Lead Halide Perovskite Layers. Chem. Mater. 1999, 11, 3028–3030. 10.1021/cm990561t. [DOI] [Google Scholar]
- Cho H.; Jeong S.-H.; Park M.-H.; Kim Y.-H.; Wolf C.; Lee C.-L.; Heo J. H.; Sadhanala A.; Myoung N.; Yoo S. S.; et al. Overcoming the Electroluminescence Efficiency Limitations of Perovskite Light-Emitting Diodes. Science 2015, 350, 1222–1225. 10.1126/science.aad1818. [DOI] [PubMed] [Google Scholar]
- Yu J. C.; Kim D. W.; Kim D. B.; Jung E. D.; Park J. H.; Lee A.-Y.; Lee B. R.; Di Nuzzo D.; Friend R. H.; Song M. H. Improving the Stability and Performance of Perovskite Light-Emitting Diodes by Thermal Annealing Treatment. Adv. Mater. 2016, 28, 6906–6913. 10.1002/adma.201601105. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Zhang B.; Zhao R.; Yao B.; Liu X.; Liu J.; Xie Z. Simple and Efficient Green-Light-Emitting Diodes Based on Thin Organolead Bromide Perovskite Films via Tuning Precursor Ratios and Postannealing Temperature. J. Phys. Chem. Lett. 2016, 7, 4259–4266. 10.1021/acs.jpclett.6b02160. [DOI] [PubMed] [Google Scholar]
- Yu J. C.; Kim D. B.; Jung E. D.; Lee B. R.; Song M. H. High-performance perovskite light-emitting diodes via morphological control of perovskite films. Nanoscale 2016, 8, 7036–7042. 10.1039/C5NR05604G. [DOI] [PubMed] [Google Scholar]
- Chih Y.-K.; Wang J.-C.; Yang R.-T.; Liu C.-C.; Chang Y.-C.; Fu Y.-S.; Lai W.-C.; Chen P.; Wen T.-C.; Huang Y.-C.; et al. NiO x Electrode Interlayer and CH3NH2/CH3NH3PbBr3 Interface Treatment to Markedly Advance Hybrid Perovskite-Based Light-Emitting Diodes. Adv. Mater. 2016, 28, 8687–8694. 10.1002/adma.201602974. [DOI] [PubMed] [Google Scholar]
- Chen P.; Xiong Z.; Wu X.; Shao M.; Ma X.; Xiong Z.; Gao C. Highly Efficient Perovskite Light-Emitting Diodes Incorporating Full Film Coverage and Bipolar Charge Injection. J. Phys. Chem. Lett. 2017, 8, 1810–1818. 10.1021/acs.jpclett.7b00368. [DOI] [PubMed] [Google Scholar]
- Li G.; Tan Z.-K.; Di D.; Lai M. L.; Jiang L.; Lim J. H.-W.; Friend R. H.; Greenham N. C. Efficient Light-Emitting Diodes Based on Nanocrystalline Perovskite in a Dielectric Polymer Matrix. Nano Lett. 2015, 15, 2640–2644. 10.1021/acs.nanolett.5b00235. [DOI] [PubMed] [Google Scholar]
- deQuilettes D. W.; Koch S.; Burke S.; Paranji R. K.; Shropshire A. J.; Ziffer M. E.; Ginger D. S. Photoluminescence Lifetimes Exceeding 8 μs and Quantum Yields Exceeding 30% in Hybrid Perovskite Thin Films by Ligand Passivation. ACS Energy Lett. 2016, 1, 438–444. 10.1021/acsenergylett.6b00236. [DOI] [Google Scholar]
- Xiao Z.; Kerner R. A.; Zhao L.; Tran N. L.; Lee K.; Koh T.-W.; Scholes G. D.; Rand B. P. Efficient perovskite light-emitting diodes featuring nanometre-sized crystallites. Nat. Photonics 2017, 11, 108–116. 10.1038/nphoton.2016.269. [DOI] [Google Scholar]
- Kim Y.-H.; Leed G.-H.; Kim Y.-T.; Wolf C.; Yun H. J.; Kwon W.; Park C. G.; Lee T.-W. High efficiency perovskite light-emitting diodes of ligand-engineered colloidal formamidinium lead bromide nanoparticles. Nano Energy 2017, 38, 51–58. 10.1016/j.nanoen.2017.05.002. [DOI] [Google Scholar]
- Kim Y.-H.; Cho H.; Heo J. H.; Kim T.-S.; Myoung N.; Lee C.-L.; Im S. H.; Lee T.-W. Multicolored Organic/Inorganic Hybrid Perovskite Light-Emitting Diodes. Adv. Mater. 2015, 27, 1248–1254. 10.1002/adma.201403751. [DOI] [PubMed] [Google Scholar]
- Hoye R. L. Z.; Chua M. R.; Musselman K. P.; Li G.; Lai M.-L.; Tan Z.-K.; Greenham N. C.; MacManus-Driscoll J. L.; Friend R. H.; Credgington D. Enhanced Performance in Fluorene-Free Organometal Halide Perovskite Light-Emitting Diodes using Tunable, Low Electron Affinity Oxide Electron Injectors. Adv. Mater. 2015, 27, 1414–1419. 10.1002/adma.201405044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L.; Yao E.-P.; Hong Z.; Chen H.; Sun P.; Yang Z.; Li G.; Yang Y. Pure Formamidinium-Based Perovskite Light-Emitting Diodes with High Efficiency and Low Driving Voltage. Adv. Mater. 2017, 29, 1603826. 10.1002/adma.201603826. [DOI] [PubMed] [Google Scholar]
- Chen P.; Xiong Z.; Wu X.; Shao M.; Meng Y.; Xiong Z.; Gao C. Nearly 100% Efficiency Enhancement of CH3NH3PbBr3 Perovskite Light-Emitting Diodes by Utilizing Plasmonic Au Nanoparticles. J. Phys. Chem. Lett. 2017, 8, 3961–3969. 10.1021/acs.jpclett.7b01562. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Park N.-G. Stability Issues on Perovskite Solar Cells. Photonics 2015, 2, 1139–1151. 10.3390/photonics2041139. [DOI] [Google Scholar]
- Yantara N.; Bhaumik S.; Yan F.; Sabba D.; Dewi H. A.; Mathews N.; Boix P. P.; Demir H. V.; Mhaisalkar S. Inorganic Halide Perovskites for Efficient Light-Emitting Diodes. J. Phys. Chem. Lett. 2015, 6, 4360–4364. 10.1021/acs.jpclett.5b02011. [DOI] [PubMed] [Google Scholar]
- Ling Y.; Tian Y.; Wang X.; Wang J. C.; Knox J. M.; Perez-Orive F.; Du Y.; Tan L.; Hanson K.; Ma B.; Gao H. Enhanced Optical and Electrical Properties of Polymer-Assisted All-Inorganic Perovskites for Light-Emitting Diodes. Adv. Mater. 2016, 28, 8983–8989. 10.1002/adma.201602513. [DOI] [PubMed] [Google Scholar]
- Wei Z.; Perumal A.; Su R.; Sushant S.; Xing J.; Zhang Q.; Tan S. T.; Demir H. V.; Xiong Q. Solution-processed highly bright and durable cesium lead halide perovskite light-emitting diodes. Nanoscale 2016, 8, 18021–18026. 10.1039/C6NR05330K. [DOI] [PubMed] [Google Scholar]
- Cho H.; Wolf C.; Kim J. S.; Yun H. J.; Bae J. S.; Kim H.; Heo J.-M.; Ahn S.; Lee T.-W. High-Efficiency Solution-Processed Inorganic Metal Halide Perovskite Light-Emitting Diodes. Adv. Mater. 2017, 29, 1700579. 10.1002/adma.201700579. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Yang X.; Jiang Q.; Wang P.; Yin Z.; Zhang X.; Tan H.; Yang Y.; Wei M.; Sutherland B. R.; et al. Ultra-bright and highly efficient inorganic based perovskite light-emitting diodes. Nat. Commun. 2017, 8, 15640. 10.1038/ncomms15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Lin H.; Huang H.; Reckmeier C.; Zhang Y.; Choy W. C. H.; Rogach A. L. Enhancing the Brightness of Cesium Lead Halide Perovskite Nanocrystal Based Green Light-Emitting Devices through the Interface Engineering with Perfluorinated Ionomer. Nano Lett. 2016, 16, 1415–1420. 10.1021/acs.nanolett.5b04959. [DOI] [PubMed] [Google Scholar]
- Li G.; Rivarola F. W. R.; Davis N. J. L. K.; Bai S.; Jellicoe T. C.; de la Peña F.; Hou S.; Ducati C.; Gao F.; Friend R. H.; et al. Highly Efficient Perovskite Nanocrystal Light-Emitting Diodes Enabled by a Universal Crosslinking Method. Adv. Mater. 2016, 28, 3528–3534. 10.1002/adma.201600064. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Xu B.; Zhang J.; Gao Y.; Zheng Y.; Wang K.; Sun X. W. All-Inorganic Perovskite Nanocrystals for High-Efficiency Light Emitting Diodes: Dual-Phase CsPbBr3 -CsPb2Br5 Composites. Adv. Funct. Mater. 2016, 26, 4595–4600. 10.1002/adfm.201600958. [DOI] [Google Scholar]
- Li J.; Xu L.; Wang T.; Song J.; Chen J.; Xue J.; Dong Y.; Cai B.; Shan Q.; Han B.; Zeng H. 50-Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control. Adv. Mater. 2017, 29, 1603885. 10.1002/adma.201603885. [DOI] [PubMed] [Google Scholar]
- Yan F.; Xing J.; Xing G.; Quan L.; Tan S. T.; Zhao J.; Su R.; Zhang L.; Chen S.; Zhao Y.; et al. Highly Efficient Visible Colloidal Lead-Halide Perovskite Nanocrystal Light-Emitting Diodes. Nano Lett. 2018, 18, 3157–3164. 10.1021/acs.nanolett.8b00789. [DOI] [PubMed] [Google Scholar]
- Yao E.-P.; Yang Z.; Meng L.; Sun P.; Dong S.; Yang Y.; Yang Y. High-Brightness Blue and White LEDs based on Inorganic Perovskite Nanocrystals and their Composites. Adv. Mater. 2017, 29, 1606859. 10.1002/adma.201606859. [DOI] [PubMed] [Google Scholar]
- Gangishetty M. K.; Hou S.; Quan Q.; Congreve D. N. Reducing Architecture Limitations for Efficient Blue Perovskite Light-Emitting Diodes. Adv. Mater. 2018, 30, 1706226. 10.1002/adma.201706226. [DOI] [PubMed] [Google Scholar]
- Liang D.; Peng Y.; Fu Y.; Shearer M. J.; Zhang J.; Zhai J.; Zhang Y.; Hamers R. J.; Andrew T. L.; Jin S. Color-Pure Violet-Light-Emitting Diodes Based on Layered Lead Halide Perovskite Nanoplates. ACS Nano 2016, 10, 6897–6904. 10.1021/acsnano.6b02683. [DOI] [PubMed] [Google Scholar]
- Zou W.; Li R.; Zhang S.; Liu Y.; Wang N.; Cao Y.; Miao Y.; Xu M.; Guo Q.; Di D.; et al. Minimising efficiency roll-off in high-brightness perovskite light-emitting diodes. Nat. Commun. 2018, 9, 608. 10.1038/s41467-018-03049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.; Nie W.; Blancon J. C.; Stoumpos C. C.; Soe C. M. M.; Yoo J.; Crochet J.; Tretiak S.; Even J.; Sadhanala A.; et al. Stable Light-Emitting Diodes Using Phase-Pure Ruddlesden–Popper Layered Perovskites. Adv. Mater. 2018, 30, 1704217. 10.1002/adma.201704217. [DOI] [PubMed] [Google Scholar]
- Chin X. Y.; Perumal A.; Bruno A.; Yantara N.; Veldhuis S. A.; Martínez-Sarti L.; Chandran B.; Chirvony V.; Lo A. S.-Z.; So J.; et al. Self-assembled hierarchical nanostructured perovskites enable highly efficient LEDs via an energy cascade. Energy Environ. Sci. 2018, 10.1039/C8EE00293B. [DOI] [Google Scholar]
- Jagielski J.; Kumar S.; Wang M.; Scullion D.; Lawrence R.; Li Y.-T.; Yakunin S.; Tian T.; Kovalenko M. V.; Chiu Y.-C.; et al. Aggregation-induced emission in lamellar solids of colloidal perovskite quantum wells. Sci. Adv. 2017, 3, eaaq0208. 10.1126/sciadv.aaq0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun I.; Shen C.; Parida M. R.; Pan J.; Sarmah S. P.; Priante D.; Alyami N.; Liu J.; Saidaminov M. I.; Alias M. S.; et al. Perovskite Nanocrystals as a Color Converter for Visible Light Communication. ACS Photonics 2016, 3, 1150–1156. 10.1021/acsphotonics.6b00187. [DOI] [Google Scholar]
- Jimenez-Solano A.; Galisteo-Lopez J. F.; Míguez H. Absorption and Emission of Light in Optoelectronic Nanomaterials: The Role of the Local Optical Environment. J. Phys. Chem. Lett. 2018, 9, 2077–2084. 10.1021/acs.jpclett.8b00848. [DOI] [PubMed] [Google Scholar]
- Tsao J. Y.; Crawford M. H.; Coltrin M. E.; Fischer A. J.; Koleske D. D.; Subramania G. S.; Wang G. T.; Wierer J. J.; Karlicek R. F. Jr Toward Smart and Ultra-efficient Solid-State Lighting. Adv. Opt. Mater. 2014, 2, 809–836. 10.1002/adom.201400131. [DOI] [Google Scholar]