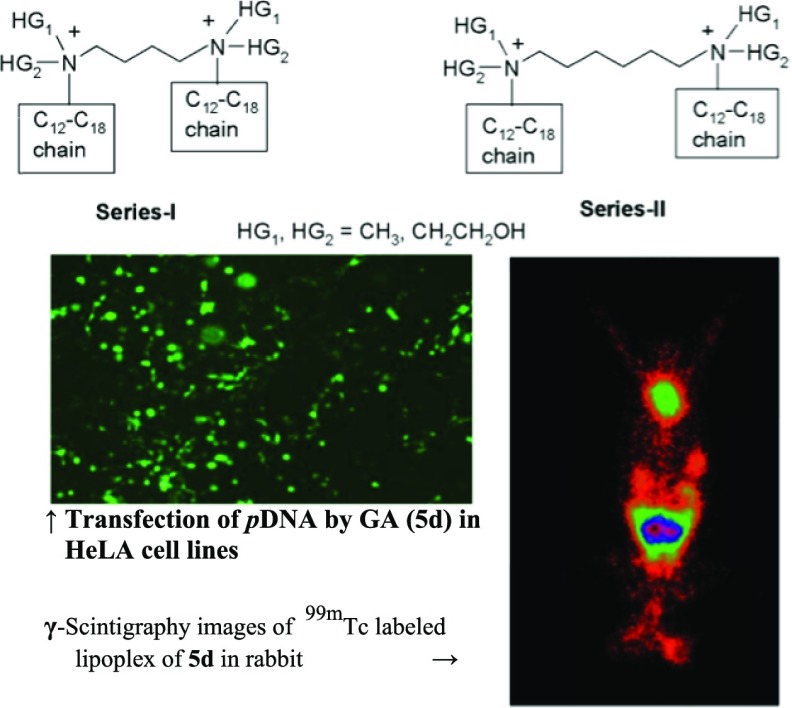
Abstract
Some quaternary gemini amphiphiles (GAs) were synthesized as nonviral gene delivery carriers. The critical miceller concentration values of these amphiphiles are indicative of their superior surface-active properties. All of the synthesized GAs, alone or along with lipids like cholesterol and/or dioleoylphosphatidyl ethanolamine (DOPE), were formulated as liposomes. Formulations of GAs with DOPE showed average particle diameters of 326–400 nm with positive ζ-potential (30.1–46.4 mV). The lipoplexes of theses formulations showed complete pDNA retention at the base at a N/P ratio higher than 1.0 in gel retardation study. The GAs were effective in condensing pDNA into a ψ-phase, as indicated by circular dichroism study, and provided complete protection of the pDNA against the enzyme DNase at a N/P ratio more than 1. In vitro cell line studies showed that GA liposomal formulations caused β-gal expression and offered a higher transfection efficiency than that of liposomes prepared with the help of N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP)/DOPE and dicyclocarbodiimide (DCC)/DOPE but comparable to those of Lipofectamine 2000 in A549 and HeLa cell lines. Modulation of head group polarity significantly affected the transfection efficacy of GAs. The cell viabilities of almost all of the formulations were comparable to those of the standards (DCC/DOPE and DOTAP/DOPE liposomes). Incorporation of cholesterol [GA/DOPE/cholesterol in the ratio of 1:1:1] further improved the serum compatibility of the formulations and improved the transfection efficacy when evaluated in A549 and HeLa cell lines. Fluorescence-assisted cell sorting studies showed comparable number of transfected cells to Lipofectamine 2000 in the HeLa cell line. Intracellular trafficking studies using confocal microscopy indicated transfection of the HeLa cells with the reporter gene within 30 min of lipoplex treatment. γ-Scintigraphy using 99mTc-labeled lipoplexes showed higher concentrations of the lipoplexes in vital tissues like liver, spleen, lungs, and kidneys.
1. Introduction
Gene therapy, a unique therapeutic approach, has been exploited for the treatment of inherited as well as acquired diseases.1 In gene therapy, there is importance of the availability of (i) the therapeutic gene that could be expressed at the targeted location and (ii) the delivery system that should be safe and efficient and having the ability to deliver the therapeutic gene to the targeted organ.2,3 A formulation is always desirable having the ability to deliver a therapeutic gene (trans-gene) to the selected cells where expression of that gene is intended, through intravenous administration.
Delivery of the naked DNA to the targeted site is notably inhibited by the factors like the size, shape, and polyanionic charge of the DNA and its susceptibility to the serum nucleases.3 Both viral and nonviral vectors have been utilized to solve the problems associated with the delivery of the naked DNA. The clinical applications of viral vectors are hampered due to factors like immunogenicity, mutagenicity, host rejection, inability to transfect the nondividing cells, possible oncogenicity, and limited DNA cargo carrying capacity of the viral vectors.4−7 Nonviral vectors including physical and chemical techniques have been developed to solve the problems associated with viral vectors. Physical techniques involve the implementation of physical forces for gene delivery, whereas the chemical techniques involve the utilization of polymeric, lipidic, and other amphiphilic carrier systems. Polymeric carriers such as poly[l-lysine], polyethylenimine, chitosan, dextran, poly(β-aminoesters), poly[2-(dimethyalamino)ethyl methacrylate], polyesters, poloxamers, poly(d,l-lactide-co-glycolide), etc. and lipophilic carriers such as quaternary ammonium lipids, lipoamines, and amidinium lipids are utilized for gene delivery applications.8−12
Among the amphiphilic carrier systems, bisquaternary gemini amphiphiles (GAa) is a category of cationic gene delivery carriers, which has been studied extensively.1 GAs offer better surfactant properties in comparison with the corresponding single-chain-, single-head-containing monovalent compounds. This can make them useful for biomedical applications demanding a better safety profile.13 The initial optimization step for such compounds is to reduce their in vivo concentration in the living system. Using lesser amount of a compound to get the same level of effect also has economic advantage. Moreover, bisquaternary GAs have the advantage of being prepared with ease with a variety of possible structural modifications as they are composed of three basic units, namely, the head, the spacer, and the hydrocarbon chain, allowing greater flexibility in the design of GAs, possessing properties like high stability in biological fluids, less toxicity, low immunogenicity, and biodegradability, which are the essential requirements for any gene delivery system.14,15 The bivalent positive charge in the head group of GAs allows efficient complexation and compaction of polyanionic DNA into particles (lipoplexes) of small sizes that can easily be endocytosed by the target cells.16 The nature, length, and stereochemistry of the spacer also have noteworthy effect on the transfection efficacy of GAs.17,18 Optimization of the desired characteristics of a GA can be done by modulating the hydrophobic group, the head group, and the linker chain. These lipophilic amphiphiles can have a variety of structural features in terms of the type of the lipophilic hydrocarbon chain, the head group linker, and the cationic head group. Therefore, the modular approach is useful for planning and designing new amphiphile vectors for gene delivery.
Chemical alterations in the head group in the structure of the cationic amphiphiles show significant enhancement in the transfection efficiency. For instance, hydroxyethylation of the cationic head group has proven to substantially improve the transfection efficiency.19 The gene transfection efficiency of the lipids, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride and N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP), was further enhanced by adding a hydroxyethyl group to get vectors like 1,2-dioleoyl-3-dimethylhydroxyethylammonium bromide and 1,2-dioleoyloxypropyl-3-dimethylhydroxyethylammonium chloride, respectively.20,21 The literature showed that both monohydroxylation and dihydroxylation of the head group resulted in higher transfection compared to that from the corresponding nonhydroxyethylated amphiphiles. Monohydroxylated lipid N,N-di-n-hexadecyl-N-methyl-N-(2-hydroxyethyl)ammonium chloride22 and dihydroxylated lipid N,N-di-n-hexadecyl-N,N-dihydroxyethylammonium bromide23 were found to impart high levels of gene transfection efficacy, compared with a structurally similar dioctadecyldimethylammonium bromide (DDAB). The hydroxyethyl group can also be derived from lactic acid saccharides. The lipid N,N-myristyl-N-(1-hydroxyprop-2-yl)-N-methylammonium chloride, in which the hydroxyethyl portion has been derived from lactic acid, has been observed to be doubly efficient when compared with DDAB/dioleoylphosphatidyl ethanolamine (DOPE) in the in vitro studies.24 In the lipids 1-deoxy-1-[methyl(ditetradecyl)ammonio]-D-arabinitol and 1-deoxy-1-[dihexadecyl(methyl)ammonio]-D-xylitol, which are derivatives of arabinose and xylose, respectively, addition of more than two hydroxyethyl moieties in the head group was found to be responsible for higher transfection levels and decreased toxicity.25
Apart from the modular design of the amphiphiles for the required structural features for efficient gene delivery, the formulation factors are equally important. Dioleoylphosphatidyl ethanolamine (DOPE) and cholesterol have shown promising results when formulated along with the cationic carriers including gemini amphiphiles.26 These helper components of the formulation favor endosomal escape of the lipoplexes, which is one of the decisive steps in the gene delivery by different mechanisms.27,28
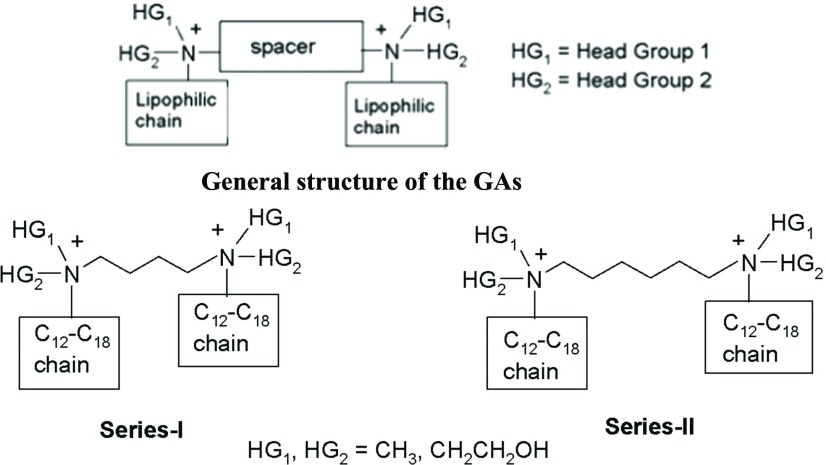
Keeping the above points in mind and taking advantage of the modular structure of the gemini amphiphiles, it was decided to synthesize two series of amphiphiles having different sizes of the spacer by varying the distance between the two cationic heads. In each series, the nature of the head group was systematically changed from nonpolar (i.e., methyl) to polar [i.e., monohydroxyethyl and di(hydroxyethyl)] and the lipophilicity of the cationic head was also varied by attaching hydrocarbon chains of different lengths (from C12 to C18). The idea was to see the effect of changes in polarity of the head group and lipophilicity of the cationic heads of the GAs of the two series (having different lengths of the spacer) on the transfection efficacy and cytotoxicity of the resulting GAs (Figures 1 and 2).
Figure 1.
General structures of the designed GAs.
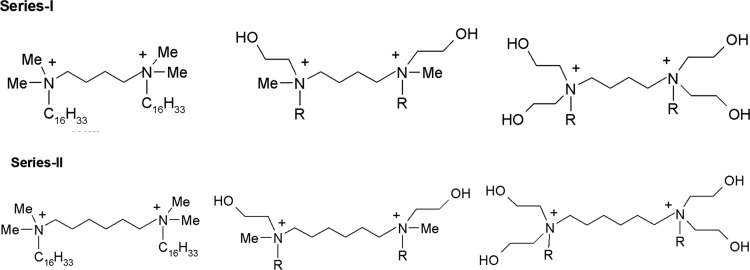
Figure 2.
Structures of the synthesized GAs (5a–5h, 6a–6g).
2. Materials and Methods
2.1. Materials
Plasmid pCMV·SPORT-β-gal [(β-galactosidase)pDNA] was procured from IICT, Hyderabad, India. The pDNA (pCMV·SPORT-β-gal and green fluorescent protein (GFP) plasmid) was transformed into Escherichia coli DH5α by the TransformAid bacterial transformation kit and isolated and purified with the QIAGEN plasmid purification kit. The purity of pDNA was ascertained by gel electrophoresis and also by a UV spectrophotometer (Schimadzu 1700) by finding out the A260/A280 ratio (1.8–2.0).29 HeLa and A549 cell lines were obtained from the National Centre for Cell Sciences (NCCS), Pune, India. Cells were cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) with fetal bovine serum (10%) and penicillin–streptomycin–amphotericin B (1%) solution in a humidified atmosphere containing CO2 (5%). N-Hexadecyl-N,N-dimethylamine, N-methylethanolamine, diethanolamine, 1,4-dibromobutane, DMEM, β-galactosidase (140 U/mg) enzyme, o-nitrophenol-β-galactopyranoside (ONPG), 4′,6-diamidino-2-phenylindole (DAPI), YOYO 1, diethylenetriaminepentaacetic acid, and stannous chloride dihydrate (SnCl2·2H2O) were purchased from Sigma-Aldrich, St. Louis, M.O. Fetal bovine serum (FBS), trypsin–ethylenediaminetetraacetic acid (EDTA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), phosphate buffer saline (PBS), Nonidet P-40 (NP-40), and antibiotic cocktail (penicillin–streptomycin–amphotericin B) were purchased from Hi-media, Mumbai. 99mTc in the form of sodium pertechnetate, separated from molybdenum-99 (99Mo) by the solvent extraction method, was provided by the Regional Center for Radiopharmaceutical Division (Northern Region), Board of Radiation and Isotope Technology (BRIT, Delhi, India). Instant thin layer chromatography ITLC-SG plates were purchased from Gelman Science Inc., Ann Arbor, MI.
2.2. Methods
2.2.1. Synthesis and Characterization of GAs
Completion of the reactions and purity of the compounds were monitored by thin layer chromatography (TLC) on silica gel plates (60 F254; Merck), visualizing with iodine vapors. A Veego make silicon oil bath-type melting point apparatus was used to determine the melting points and are uncorrected. The IR spectra were recorded using attenuated total reflectance and KBr disc methods for liquid and solid samples, respectively, on a Bruker FT-IR spectrometer, model alpha. The PMR spectra were recorded using a Bruker 300 MHz spectrometer in deuterated solvents (CDCl3 and DMSO-d6) (chemical shifts in δ ppm; s is used for a singlet; m, for a multiplet; t, for a triplet; bs, for a broad singlet; bm, for a broad multiplet; and bt, for a broad triplet). Mass spectral data were obtained on a scientific mass spectrometer (Thermo, DSQ II). Elemental analyses were performed on a Thermo Fisher FLASH 2000 organic elemental analyzer. All of the final GAs offered results within ±0.4% of the calculated values of carbon, hydrogen, and nitrogen elements.
2.2.1.1. Series-I
2.2.1.1.1 N-Hexadecyl-N-methylethanolamine (3b): Procedure A:N-Methylethanolamine (1b) (3.55 mL, 44 mmol), 1-bromohexadecane (2a) (12.26 mL, 40 mmol), and anhydrous sodium carbonate (2.32 g, 22 mmol) in dry ethanol (100 mL) were refluxed for 12–14 h under anhydrous conditions. The reaction mixture was cooled to room temperature and filtered, and the solvent was recovered under vacuum from the filtrate to yield a crude oily product. The crude product was dissolved in dichloromethane/ether and washed with brine four to five times. The organic layer was dried, and the solvent was removed under vacuum to yield the desired product (3b) as a yellowish oil (11.4 g, 95%); TLC: Rf 0.7 (10% MeOH in CHCl3). IR: 3386, 1040 cm–1.
2.2.1.1.2 N-Dodecyl-N-methylethanolamine (3c): Procedure A:N-Methylethanolamine (1b) (3.55 mL, 44 mmol) and 1-bromododecane (2b) (9.67 mL, 40 mmol) offered a yellowish liquid product (3c) (8.9 g, 95%). TLC: Rf 0.65 (10% MeOH in CHCl3), IR: 3393, 1038 cm–1.
2.2.1.1.3 N-Methyl-N-tetradecylethanolamine (3d): Procedure A:N-Methylethanolamine (1b) (3.55 mL, 44 mmol) and 1-bromotetradecane (2c) (10.97 mL, 40 mmol) yielded an oily product (3d) (10 g, 94%); TLC: Rf 0.7 (10% MeOH in CHCl3); IR: 3337, 1038 cm–1.
2.2.1.1.4 N-Methyl-N-octadecylethanolamine (3e): Procedure A:N-Methylethanolamine (1b) (3.55 mL, 44 mmol) and 1-bromooctadecane (2d) (13.65 mL, 40 mmol) afforded a yellowish waxy solid (3e) (12.3 g, 92%) (mp 58–60 °C). TLC: Rf 0.75 (10% MeOH in CHCl3). IR: 3337, 1038 cm–1.
2.2.1.1.5 N-Hexadecyl-N,N-di(2-hydroxyethyl)amine (3f): Procedure A: Diethanolamine (1c) (4.23 mL, 44 mmol) and 1-bromohexadecane (2a) (12.26 mL, 40 mmol) afforded the desired amine (3f) as a yellow waxy solid (11.8 g, 90%) (mp 54–56 °C). TLC: Rf 0.65 (10% MeOH in CHCl3). IR: 3342, 1150, 1044 cm–1.
2.2.1.1.6 N,N-Di(2-hydroxyethyl)-N-tetradecylamine (3g): Procedure A: Diethanolamine (1c) (4.23 mL, 44 mmol) and 1-bromotetradecane (2c) (10.97 mL, 40 mmol) afforded an oily product (3g) (10.6 g, 90%). TLC: Rf 0.60 (10% MeOH in CHCl3). IR: 3346, 1150, 1043 cm–1.
2.2.1.1.7 N-Dodecyl-N,N-di(2-hydroxyethyl)amine (3h): Procedure A: Diethanolamine (1c) (4.23 mL, 44 mmol) and 1-bromododecane (2b) (9.7 mL, 40 mM) yielded the desired product (3h) as an oil (9.9 g, 94%). TLC: 0.55 (10% MeOH in CHCl3). IR: 3337, 1150, 1041 cm–1.
2.2.1.1.8 1,4-Di(n-hexadecyldimethylammonium)butane Dibromide (5a): Procedure B:N-Hexadecyl-N,N-dimethylamine (3a) (2.96 mL, 8.8 mmol) and 1,4-dibromobutane (4a) (0.49 mL, 4.0 mmol) in dry acetone (20 mL) were reacted in a sealed tube at about 80 °C for 2–3 days till the reaction got completed. The solvent was removed under vacuum, and the residue was washed with a mixture of hexane and ethyl acetate or dry ether. The crude solid so obtained was crystallized from methanol–ethyl acetate to afford the desired compound (5a) as a white solid (2.2 g, 71%) (mp 232–34 °C); TLC: Rf 0.4 (10% MeOH in CHCl3). IR: 3353, 1099 cm–1. PMR: δ 0.88 (t, 6H; (N(CH2)15CH3)), 1.25–1.36 (bm, 52H; (NCH2CH2(CH2)13CH3)2), 1.75–1.77 (bm, 4H; (N–CH2CH2(CH2)13CH3)2), 2.18 (t, 4H; (NCH2CH2)2), 3.26 (s, 12H; (N(CH3)2)2), 3.37–3.42 (t, 4H; (N–CH2(CH2)14CH3)2) and 4.00 (t, 4H; (NCH2CH2)2).
2.2.1.1.9 1,4-Di[n-hexadecyl(2-hydroxyethyl)methylammonium]butane Dibromide (5b): Procedure B:N-Hexadecyl-N-methylethanolamine (3b) (2.64 g, 8.8 mmol) and 1,4-dibromobutane (4a) (0.49 mL, 4.0 mmol) yielded the desired compound (5b) as a white solid (2.1 g, 65%), mp 223–25 °C, TLC: Rf 0.4 (10% MeOH in CHCl3) IR: 3318, 1087, 1046 cm–1 PMR: δ 0.85 (t, 6H; (N–(CH2)15CH3)2), 1.25–1.35 (bm, 52H (NCH2CH2(CH2)13CH3)2), 1.74–2.05 (m, 8H; (CH2CH2(CH2)13CH3)2 and (NCH2CH2)2), 3.26 (s, 6H (NCH3)2), 3.40 (t, 4H; (NCH2(CH2)14 CH3)2), 3.61 (t, 4H (NCH2CH2)2), 3.82 (t, 4H (NCH2CH2OH)2), 4.10 (t, 4H (NCH2CH2OH)2) and 5.08 (bs, 2H; (N–CH2CH2OH)2). Mass spectrometry (MS): m/z 735.9, (M+).
2.2.1.1.10 1,4-Di[n-dodecyl(2-hydroxyethyl)methylammonium]butane Dibromide (5c): Procedure B:N-Dodecyl-N-methylethanolamine (3c) (2.16 g, 8.8 mmol) and 1,4-dibromobutane (4a) (0.49 mL, 4.0 mM) afforded a white solid product (5c) (2.0 g, 70%) (mp 217–19 °C). TLC: Rf 0.35 (10% MeOH in CHCl3). IR: 3316, 1132, 1045 cm–1. PMR: δ 0.85 (t, 6H; (N–CH2CH2(CH2)9CH3)2), 1.25–1.34 (bm, 36H; (N–CH2CH2(CH2)9CH3)2), 1.73–2.05 (m, 8H; (NCH2CH2(CH2)9CH3)2 and (NCH2CH2−)2), 3.26 (s, 6H; (NCH3)2), 3.40 (t, 4H; (N–CH2CH2(CH2)9CH3)2), 3.62 (t, 4H; (N–CH2CH2−)2), 3.78 (t, 4H; (N–CH2CH2OH)2), 4.09 (t, 4H; (N–CH2CH2OH)2) and 5.08 (bs, 2H; (NCH2CH2OH)2). MS: m/z 621.8, (M+).
2.2.1.1.11 1,4-Di[(2-hydroxyethyl)methyl-n-tetradecylammonium]butane Dibromide (5d): Procedure B:N-Methyl-N-tetradecylethanolamine (3d) (2.40 g, 8.8 mmol) and 1,4-dibromobutane (4a) (0.49 mL, 4.0 mmol) afforded the desired compound (5d) as a white solid (2.2 g, 72%) (mp 231–33 °C), TLC: Rf 0.35 (10% MeOH in CHCl3). IR: 3352, 1132, 1045 cm–1. PMR: δ 0.86 (t, 6H; (N–(CH2)13CH3)2), 1.25–1.35 (bm, 44H; (NCH2CH2(CH2)11CH3)2), 1.75–2.07 (m, 8H; (NCH2CH2(CH2)11CH3)2 and (NCH2CH2−)2), 3.27 (s, 6H; (NCH3)2), 3.44 (t, 4H; (N–CH2CH2(CH2)11CH3)2), 3.63 (t, 4H; (N–CH2CH2−)2), 3.79 (t, 4H; (N–CH2CH2OH)2), 4.10 (t, 4H; (N–CH2CH2OH)2) and 5.07 (bs, 2H; (NCH2CH2OH)2).
2.2.1.1.12 1,4-Di[(2-hydroxyethyl)methyl-n-octadecylammonium]butane Dibromide (5e): Procedure B:N-Methyl-N-octadecylethanolamine (3e) (2.88 g, 8.8 mmol) and 1,4-dibromobutane (4a) (0.49 mL, 4.0 mmol) yielded the product (5e) as a white solid (2.3 g, 65%) (mp 230–32 °C), TLC: Rf 0.4 (10% MeOH in CHCl3). IR: 3223, 1092, 1048 cm–1. PMR: δ 0.85 (t, 6H; (N(CH2)17CH3)2), 1.25–1.35 (bm, 60H; (NCH2CH2(CH2)15CH3)2), 1.75–2.11 (m, 8H; (NCH2CH2(CH2)15CH3)2 and (NCH2CH2−)2), 3.24 (s, 6H; (NCH3)2), 3.42 (t, 4H; (N–CH2CH2(CH2)15CH3)2), 3.58 (t, 4H; (N–CH2CH2−)2), 3.89 (t, 4H; (N–CH2CH2OH)2), 4.10 (t, 4H; (N–CH2CH2OH)2) and 5.05 (bs, 2H; (NCH2CH2OH)2).
2.2.1.1.13 1,4-Di[n-hexadecyldi(2-hydroxyethyl)ammonium]butane Dibromide (5f): Procedure B:N,N-Di(2-hydroxyethyl)-N-hexadecylamine (3f) (2.92 g, 8.8 mmol) and 1,4-dibromobutane (4a) (0.49 mL, 4.0 mmol) on reaction yielded the titled compound (5f) (2.3 g, 67%) (mp 228–30 °C). TLC: 0.3 (10% MeOH in CHCl3). IR: 3335, 1098, 1068 cm–1. PMR: δ 0.88 (t, 6H; (N(CH2)15CH3)2), 1.07–1.26 (bm, 52H; (NCH2CH2(CH2)13CH3)2), 1.70–1.84 (bm, 8H; (NCH2CH2(CH2)13CH3)2 and (NCH2CH2−)2), 3.35 (bt, 8H; (NCH2(CH2)14CH3)2 and ((NCH2CH2−)2), 3.52 (bt, 8H; ((NCH2CH2OH)2)2), 3.91 (bt, 8H; (NCH2CH2OH)2)2) and 5.26 (bs, 4H; ((NCH2CH2OH)2)2).
2.2.1.1.14 1,4-Di[di(2-hydroxyethyl)-n-tetradecylammonium]butane Dibromide (5g): Procedure B:N,N-Di(2-hydroxyethyl)-N-tetradecylamine (3g) (2.64 g, 8.8 mmol) and 1,4-dibromobutane (4a) (0.49 mL, 4.0 mmol) afforded a white solid as the desired product (5g) (1.9 g, 58%) (mp 223–25 °C), TLC: 0.3 (10% MeOH in CHCl3). IR: 3337, 1071, 1036 cm–1. PMR: δ 0.85 (t, 6H; (N(CH2)13CH3)2), 1.25 (bm, 44H; (NCH2CH2(CH2)11CH3)2), 1.67 (bm, 8H; (NCH2CH2(CH2)11CH3)2 and (NCH2CH2−)2), 3.28 (bt, 8H; (NCH2(CH2)12CH3)2 and (NCH2CH2−)2), 3.42 (bt, 8H; (N–(CH2CH2OH)2)2), 3.8 (bt, 8H; (N–(CH2CH2OH)2)2) and 5.23 (bs, 4H; ((NCH2CH2OH)2)2).
2.2.1.1.15 1,4-Di[n-dodecyldi(2-dihydroxyethyl)ammonium]butane Dibromide (5h): Procedure B:N-Dodecyl-N,N-di(2-hydroxyethyl)amine (3h) (2.4 g, 8.8 mmol) and 1,4-dibromobutane (4a) (0.49 mL, 4.0 mmol) afforded the product (5h) as a white solid (1.8 g, 62%) (mp 160–62 °C); TLC: 0.25 (10% MeOH in CHCl3) IR: 3338, 1071, 1037 cm–1. PMR: δ 0.85 (t, 6H; (N(CH2)11CH3)2), 1.25 (bm, 36H; (NCH2CH2(CH2)9CH3)2), 1.67 (bm, 8H; (NCH2CH2(CH2)9CH3)2 and (NCH2CH2−)2), 3.34 (bt, 8H; (NCH2(CH2)10CH3)2 and (NCH2CH2−)2), 3.42 (bt, 8H; ((N–CH2CH2OH)2)2), 3.8 (bt, 8H; ((NCH2CH2OH)2)2) and 5.21 (bs, 4H; ((NCH2CH2OH)2)2).
2.2.1.2. Series-II
2.2.1.2.1 1,6-Di(n-hexadecyldimethylammonium)hexane Dibromide (6a): Procedure B:N-Hexadecyl-N,N-dimethylamine (3a) (2.96 mL, 8.8 mmol) and 1,6-dibromohexane (4b) (0.61 mL, 4.0 mmol) yielded the desired white solid compound (6a) (2.1 g, 68%) (mp 238–40 °C), TLC: 0.5 (10% MeOH in CHCl3). IR: 3423, 1099 cm–1. PMR: δ 0.88 (t, 6H; (N(CH2)15CH3)2), 1.25–1.35 (bm, 52H; (NCH2CH2(CH2)13CH3)2), 1.61–1.72 (bm, 12H; (NCH2(CH2)4(CH2N) and (NCH2CH2CH2)13CH3)2), 2.06 (t, 4H; (NCH2CH2(CH2)13CH3)2, 3.46 (s, 12H; (NCH3)2)2) and 3.76 (t, 4H; (N–CH2CH2CH2−)2).
2.2.1.2.2 1,6-Di(n-hexadecyl(2-hydroxyethyl)methylammonium)hexane Dibromide (6b): Procedure B:N-Hexadecyl-N-methylethanolamine (3b) (2.64 g, 8.8 mmol) and 1,6-dibromohexane (4b) (0.61 mL, 4.0 mmol) offered the desired product (6b) as a white solid (2.0 g, 62%) (mp 228–30 °C), TLC: Rf 0.4 (10% MeOH in CHCl3). IR: 3290, 1090, 1048 cm–1. PMR: δ 0.85 (t, 6H; (N(CH2)15CH3)2), 1.25–1.34 (bm, 52H; (NCH2CH2(CH2)13CH3)2), 1.56–1.71 (m, 8H; (NCH2 CH2 (CH2)13CH3)2 and (NCH2CH2CH2−)2), 1.97 (m, 4H; (NCH2CH2CH2−)2), 3.29 (s, 6H; (NCH3)2), 3.49 (t, 4H; (N–CH2(CH2)14CH3)2), 3.71 (bt, 8H; (NCH2CH2CH2−)2 and (NCH2CH2OH)2), 4.10 (t, 4H; (NCH2CH2OH)2) and 5.07 (bs, 2H; (NCH2CH2OH)2).
2.2.1.2.3 1,6-Di[n-dodecyl(2-hydroxyethyl)methylammonium]hexane Dibromide (6c): Procedure B:N-Dodecyl-N-methylethanolamine (3c) (2.16 g, 8.8 mmol) and 1,6-dibromohexane (4b) (0.61 mL, 4.0 mM) offered the desired product (6c) (1.7 g, 60%) (mp 195–98 °C), TLC: Rf 0.35 (10% MeOH in CHCl3). IR: 3299, 1090, 1048 cm–1. PMR: δ 0.85 (t, 6H; (N(CH2)11CH3)2), 1.25–1.34 (bm, 36H; (NCH2CH2(CH2)9CH3)2), 1.55–1.70 (m, 8H; (NCH2CH2(CH2)2CH2CH2)N and (NCH2CH2(CH2)9CH3)2), 1.93 (m, 4H; (NCH2CH2CH2−)2), 3.29 (s, 6H; (NCH3)2), 3.47 (t, 4H; (NCH2(CH2)10CH3)2), 3.62 (t, 4H; (NCH2CH2CH2−)2), 3.70 (t, 4H; (NCH2CH2OH)2), 4.09 (t, 4H; (NCH2CH2OH)2) and 5.06 (bs, 2H; (NCH2CH2OH)2).
2.2.1.2.4 1,6-Di[(2-hydroxyethyl)methyl-n-tetradecylammonium]hexane Dibromide (6d): Procedure B:N-Methyl-N-tetradecylethanolamine (3d) (2.40 g, 8.8 mmol) and 1,6-dibromohexane (4b) (0.61 mL, 4.0 mmol) afforded the desired product (6d) (2.0 g, 65%) (mp 231–33 °C). TLC: Rf 0.35 (10% MeOH in CHCl3). IR: 3286, 1090, 1056 cm–1. PMR: δ 0.88 (t, 6H; (N(CH2)13CH3)2), 1.25–1.34 (bm, 44H; (NCH2CH2(CH2)11CH3)2), 1.56 (m, 4H; (NCH2CH2CH2−)2), 1.71 (m, 4H; (NCH2CH2(CH2)11CH3)2), 1.98 (t, 4H; (NCH2CH2CH2−)2), 3.29 (s, 6H; (NCH3)2), 3.48 (t, 4H; (NCH2(CH2)12CH3)2), 3.67–3.76 (t, 4H; (NCH2CH2OH)2), 4.09 (t, 4H; (NCH2CH2OH)2) and 5.06 (bs, 2H; (NCH2CH2OH)2).
2.2.1.2.5 1,6-Di[(2-hydroxyethyl)methyl-n-octadecylammonium]hexane Dibromide (6e): Procedure B:N-Methyl-N-octadecylethanolamine (3e) (2.88 g, 8.8 mmol) and 1,6-dibromohexane (4b) (0.61 mL, 4.0 mmol) yielded the desired product (6e) (2.2 g, 61%) (mp 230–32 °C). TLC: Rf 0.45 (10% MeOH in CHCl3). IR: 3289, 1090, 1048 cm–1. PMR: δ 0.85 (t, 6H; (N–(CH2)17CH3)2), 1.25–1.35 (bm, 60H; (NCH2CH2(CH2)15CH3)2), 1.56–1.73 (m, 8H; (NCH2CH2(CH2)15CH3)2 and (NCH2CH2CH2−)2), 1.98 (t, 4H; (NCH2CH2CH2)2), 3.28 (s, 6H; (NCH3)2), 3.46 (t, 4H; (NCH2(CH2)16CH3)2), 3.71 (bt, 8H; (NCH2CH2CH2−)2 and (NCH2CH2OH)2), 4.10 (t, 4H; (NCH2CH2OH)2) and 5.04 (bs, 2H; (NCH2CH2OH)2).
2.2.1.2.6 1,6-Di[n-hexadecyldi(2-dihydroxyethyl)ammonium]hexane Dibromide (6f): Procedure B:N,N-Di(2-hydroxyethyl)-N-hexadecylamine (3f) (2.92 g, 8.8 mmol) and 1,6-dibromohexane (4b) (0.61, 4.0 mmol) afforded the desired white solid product (6f) (2.0 g, 57%) (mp 229–31 °C); TLC: 0.3 (10% MeOH in CHCl3). IR: 3308, 1071, 1044 cm–1. PMR: δ 0.85 (t, 6H; (N(CH2)5CH3)2), 1.24 (bm, 56H; (NCH2CH2(CH2)13CH3)2) and (NCH2CH2CH2−)2, 1.66 (m, 8H; (NCH2CH2(CH2)13CH3)2 and (NCH2CH2CH2)2), 3.33 (bm, 8H; (NCH2(CH2)14CH3)2 and (NCH2CH2CH2−)2), 3.42 (bm, 8H; ((NCH2CH2OH)2)2), 3.8 (bt, 8H; ((NCH2CH2OH)2)2) and 5.10 (bs, 4H; ((NCH2CH2OH)2)2).
2.2.1.2.7 1,6-Di[di(2-dihydroxyethyl)-n-tetradecylammonium]hexane Dibromide (6g): Procedure B:N,N-Di(2-hydroxyethyl)-N-tetradecylamine (3g) (2.64 g, 8.8 mmol) and 1,4-dibromohexane (4b) (0.61 mL, 4.0 mmol) offered the desired product (6g) (1.9 g, 60%) (mp 229–31 °C). TLC: 0.3 (10% MeOH in CHCl3). IR: 3224, 1071, 1049 cm–1. PMR: δ 0.85 (t, 6H; (N(CH2)13CH3)2), 1.16–1.34 (bm, 44H; (NCH2CH2(CH2)11CH3)2), 1.53 (m, 4H; (NCH2CH2CH2−)2), 1.66 (bm, 8H; (NCH2CH2CH2−)2 and (NCH2CH2(CH2)11CH3)2), 3.37–3.70 (bm, 16H; ((NCH2CH2OH)2)2, (NCH2CH2CH2−)2 and (NCH2(CH2)12CH3)2), 4.07 (bt, 8H; ((NCH2CH2OH)2)2) and 4.97 (bs, 4H; ((NCH2CH2OH)2)2).
2.2.2. Determination of Critical Miceller Concentration (cmc)
Conductance was measured as a function of GA concentration using digital conductivity meter 306 (Equiptronic, Mumbai) having a cell constant of 1.01 cm–1 S at 30 ± 0.2 °C. Serial dilutions of GAs covering the range of 10–3–10–6 mM were prepared in double-distilled water, and conductance of the solutions so prepared was measured. The value of specific conductance of the solutions was plotted against the concentrations. The inflection point in the graph yielded the “cmc” values for the GAs under study.30,31
2.2.3. Preparation of GA Formulations (Liposomes)
Two types of formulations were prepared, one using the GAs alone and the second type consisting of a GA in combination with the helper lipid DOPE in molar ratios of 1:1, 1:2, and 1:3 (GA/DOPE). Required quantities of GAs and DOPE were dissolved in a mixture of solvent (chloroform/methanol, 1:1) in glass vials such that the total lipid quantity (GA plus DOPE) remained constant. The solvent was evaporated under a stream of nitrogen. Residual amounts of the solvent were removed from the samples by applying vacuum overnight. The resulting dry films were hydrated using 20 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) buffer (pH 7.4) and incubated for 30 min at ∼70 °C, followed by vigorous vortexing and repeated freeze–thawing (ice cold water to ∼70 °C) with intermittent vortexing to ensure hydration. The resulting suspensions were sonicated for 3 min and passed through polycarbonate filters (0.22 μm) 2–3 times.
For the purpose of observing the transfection efficacy of the synthesized GAs in the presence of serum, a third type of formulation containing cholesterol was prepared. Cholesterol was also included as a helper lipid in the optimized GA/DOPE formulations such that the final formulations contained GA/DOPE/cholesterol in molar ratios of 1:1:1 and 1:1:0.5.18,32
2.2.4. Preparation of Lipoplexes
Lipoplexes were prepared by mixing the freshly prepared formulations of the cationic GAs with plasmid DNA under gentle vortexing in DMEM at different N/P ratios (0.5, 1, 1.5, 2.0, 2.5, 3.0, 4.0, and 6.0) and then incubating the mixtures at 37 °C for 15 min. The N/P ratio is defined as the molar ratio of the nitrogen contents in the cationic GAs to the phosphorus contents of the anionic pDNA. All of the complexes (lipoplexes) were prepared keeping the quantity of pDNA constant and varying the quantity of the formulations. However, the volumes of the cationic formulations and the pDNA dilutions were kept constant during lipoplex preparation. Characterizations and the experiments for transfection were performed immediately after incubation of the lipoplexes.32,33
2.2.5. Assessment of Degree of Complexation of pDNA with the GAs
Agarose gel was used for assessing the DNA-binding ability of the cationic GAs by the gel retardation assay (1%, prestained with ethidium bromide, 0.1%) across varying N/P ratios from 0.25 to 8. pDNA (pCMV·SPORT-β-gal, 300 ng) was complexed with GA formulations containing different amounts of cationic GAs in HEPES buffer (pH 7.4, total volume 20 μL) and incubated for 20–25 min at room temperature. A loading buffer (4 μL) (0.25% bromophenol blue in 40% w/v sucrose in water) was added, and the resulting solution (24 μL) was loaded on each well. Electrophoresis of the samples was performed with Tris–acetate buffer at 80 V for 40 min. DNA bands were visualized in the gel documentation unit.34,35
2.2.6. Evaluation of pDNA Protection Capacity of Lipoplexes Against DNase I Enzyme
To obtain a suitable N/P ratio for the GAs that would protect the pDNA from degradation, GA formulations containing different amounts of GAs were complexed with pDNA (1000 ng) in HEPES buffer (pH 7.4, 30 μL total volume). The complexes were incubated at room temperature for 30 min on a rotary shaker. The lipoplexes so prepared were given a treatment of DNase I (1 μg/mL, 30 μL, 10 μL) and MgCl2 (20 mM) and incubated for 20 min at 37 °C. The hydrolytic reaction was then stopped by adding EDTA (50 mM), and the containers were incubated for 10 min at 60 °C in a water bath. The aqueous layer was washed with a phenol/chloroform/isoamyl alcohol mixture (25:24:1 v/v 50 μL) and centrifuged at 10 000 rpm for 5 min. The aqueous supernatants were separated, loaded (25 μL) on a 1% agarose gel (prestained with ethidium bromide), and electrophoresed at 100 V for 1 h.35
2.2.7. Assessment of Condensation of pDNA in the Lipoplexes Using Circular Dichroism (CD) Studies
CD experiments were performed to assess the conformational changes occurring in DNA upon binding with the GAs using a circular dichroism spectrometer (JASCO-J815), in the range of 320–200 nm with a scanning speed of 50 nm/min, band width of 1 nm, and response of 1 s using a quartz cuvette of cell length 0.2 cm.36,37
Serial dilution of pDNA and GAs were prepared in HEPES buffer (pH 7.4) and mixed to achieve different N/P ratios. The CD spectra were obtained at T = 303 K immediately after addition of pDNA to the liposomal suspension (t = 0) and after 30 min. A positive band near 277 nm and a negative band near 245 nm indicate a typical B-form of DNA in the CD spectrum in the absence of the GAs.18,38
2.2.8. In Vitro Cell Line Studies
2.2.8.1. Transfection Studies for Evaluating Transfection Efficacy of the Synthesized GAs Using β-Gal Reporter Gene Assay in the Absence of Serum
To evaluate the transfection efficiency of the cationic GAs alone and their formulations to induce gene expression in HeLa and A549 cells, pCMV·SPORT-β-gal (300 ng) was used to form lipoplexes at various N/P ratios (1–6).23,39 The cells were seeded in 96-well plates at a density of 5000 cells/well in DMEM (200 μL) growth medium and penicillin–streptomycin–amphotericin B (1%) solution. After 18–24 h, the cells were treated with the diluted lipoplexes in 200 μL plain DMEM per well. After 4 h of incubation of the formulation, the culture media were removed, cells were washed with PBS (pH 7.4), and complete growth medium (200 μL) was added to each well. Culture media were removed after a time span of 48 h, and cells were washed with PBS (pH 7.4) and lysed (using lysis buffer 0.5% Nonidet P-40 in Tris buffer, pH 8.0, 50 μL). The cells were further treated with ONPG solution (2×, 50 μL), a substrate for β-galactosidase. The intensity of yellow color was recorded after 15 min of incubation at 37 °C in an enzyme-linked immunosorbent assay plate reader (Biorad, Model 680 XR) at 405 nm. Naked DNA transfected cells were used as the negative control in all of the experiments.22,24
2.2.8.2. Transfection Studies in the Presence of Serum
To evaluate the serum compatibility of lipoplexes containing the optimized GA/DOPE ratio, transfection studies were performed in the presence of FBS (10%), whereas other variables were kept constant, as described in the case of transfection studies in the absence of serum.
2.2.8.3. MTT Assay for Cytotoxicity Evaluation
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay was used to assess the cytotoxicity of all of the GA formulations. The ratio of the number of cells to the quantum of GA formulation was maintained constant in the cytotoxicity assay, which was performed in 96-well plates in the transfection experiments. After 4 h of incubation of the formulation treatment, the culture media were removed, cells were washed with PBS (pH 7.4), and complete growth medium (200 μL) was added to each well. After 48 h, the culture medium was removed, cells were washed with PBS (pH 7.4), MTT solution (50 μL, 1 mg/mL in plain DMEM) was added to each well, and the plate was incubated at 37 °C for 4 h. After incubation, DMEM was removed and DMSO (100 μL) was added to each well. The purple formazan crystals formed in the plate were dissolved using mechanical stirring. The optical density was measured at 570 nm keeping the reference at 650 nm using a plate reader. The percent of cell viability was calculated using the formula given below32,40,41
2.2.8.4. Fluorescence-Assisted Cell Sorting (FACS) Studies
Twenty four-well plates were used for seeding the cells at a density of 50 000 cells/well in DMEM growth medium (1 mL) supplemented with FBS (10%) and penicillin–streptomycin–amphotericin B (1%) solution. After 18–24 h, the cells were treated with diluted lipoplexes in plain DMEM (500 μL) per well. After 4 h of incubation of the lipoplex formulation treatment, the culture medium was removed, cells were washed with PBS (pH 7.4), and complete growth medium (1 mL) was added to each well. The cells were processed for FACS analysis using the protocol reported by Soboleski et al. in the absence of light to prevent quenching of fluorescence.42,43
2.2.8.5. Intracellular Trafficking Study Using Confocal Microscopy
Cells were cultured in complete growth media in 6-well plates containing a glass cover slip and seeded with 2 × 105 cells per well. After attaining the required confluency, cells were transfected with transfection medium (lipoplex of optimized formulation at an optimized N/P ratio with YOYO-tagged pDNA). After different time intervals (10, 20, and 30 min), cells were washed with 1× PBS (1 mL) (three times) and treated with paraformaldehyde (1 mL, 4%) for 10 min to fix the cells. Paraformaldehyde was removed, and the cells were washed with 1× PBS (1 mL) (three times), treated with nuclear staining dye DAPI (0.7 mL) for 1 h, and washed with 1× PBS (1 mL) (three times). Cover slips were mounted on glass slides, and fluorescence was viewed and photographed on a confocal microscope (Zeiss, LSM-510 META, Germany) using an argon laser at an excitation wavelength of 488 nm and emission wavelength of 520 nm.44,45
2.2.9. In Vivo Studies
2.2.9.1. Optimization of Radiolabeling of the Lipoplexes by Direct Labeling Procedure
The radiolabeling of the lipoplexes at the optimized N/P ratios was carried out using a direct labeling procedure with 99mTc by the simple reduction method using stannous chloride. Lipoplexes were prepared by mixing the required amount of liposomal suspension in Milli-Q water (0.25 mL) and pDNA (15 μg) in Milli-Q water (0.25 mL). After 20 min, reduced 99mTc (in saline) was added to achieve a final concentration of 2.5 mCi/mL. Stannous chloride (60 μg) solution was used to reduce 99mTc followed by bicarbonate buffer (0.1 mL, 0.5 M, pH 9.0) to maintain the final pH to 6.0–6.5.
The labeling was carried out by mixing the reagents for 10–15 min at ambient temperature. Radiolabeling efficiency/radiochemical purity of the labeled complex was estimated by the ITLC chromatography technique. To achieve stable labeling with higher yields, the labeling protocol was standardized by varying reagent concentrations.46,47
2.2.9.2. Biodistribution Studies
Biodistribution studies of 99mTc-labeled lipoplexes were carried out according to the method approved by local IAEC of the Institute of Nuclear Medicine and Allied Sciences, Ministry of Defence, Government of India, India. Balb/C mice were injected with 99mTc-labeled lipoplexes (0.2 mL per animal) by tail vein. Blood was withdrawn by cardiac puncture after different time intervals, and the animals were sacrificed by cervical dislocation. Subsequently, brain and other tissues (stomach, intestine, spleen, liver, lungs, kidney, and tail) were dissected and washed twice using normal saline to remove the adhering tissue/fluid and weighed. The radioactivity present in each organ was measured using a γ-scintillation counter (Capintec). Three animals were used for each time point (1, 6, and 24 h) for every formulation. Radioactivity uptake in each organ was measured as a fraction of the dose of the radiopharmaceutical administered by using the following equation46,48
2.2.9.3. γ-Scintigraphic Study in Rabbits
For γ-scintigraphic studies, a rabbit was administered with 0.3 mL of the optimized 99mTc-lebelled lipoplex of the formulation intravenously through ear vein. The animal was anesthetized using diazepam (0.5 mL of 10 mg/mL) intramuscular injection. SPECT, LC 75-005 (Diacam, Siemens AG, Erlanger, Germany) was used for the purpose of imaging after 30 min of administration of the radiolabeled formulation to the animals.46,48
3. Results and Discussion
3.1. Chemical Aspects
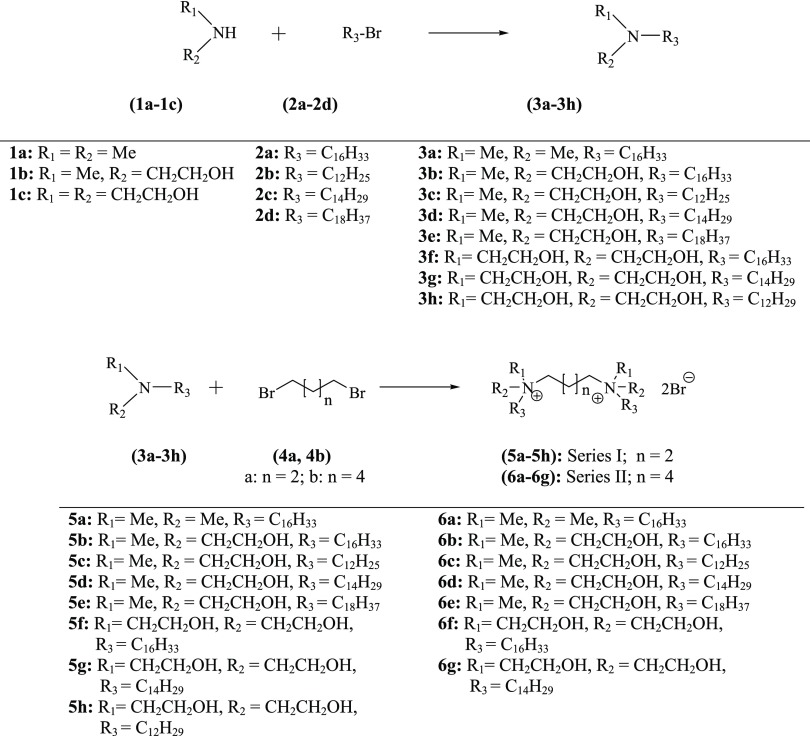
The chemical structure of a cationic surfactant affects not only the formulation aspects but also the transfection efficacy in the gene delivery systems. Along with the double positive charge, the modular structure offers the opportunity to design GAs in a rational way. In the current work, it was planned to synthesize two series of GAs. In series-I, the spacer chain length was kept as four methylene carbons, whereas in series-II, the chain length was increased to six methylene carbons. In both the series, it was decided to initially synthesize a limited number of GAs in which head groups’ polarity was increased stepwise while maintaining the length of the cationic hydrocarbon to C16 (series-I: 5a, 5b, and 5f; series-II: 6a, 6b, and 6f). PMR spectra of 5a–6g are shown in Figures 1S–15S, Supporting Information. After assessing the transfection efficacy of the reporter gene in the isolated cell culture, of the formulations containing the initially synthesized GAs, it was observed that the monohydroxyethyl head group (5b and 6b) offered the best results followed by the di(hydroethyl) heads (5f and 6f). Considering these results, the head group polarity was kept constant (monohydroxyethyl) and the chain length of the lipophilic hydrocarbon was varied from C12 to C18, leading to the synthesis of all four compounds of the hydroxyethyl group in each series while some selected additional compounds were also synthesized for the more polar di(hydroxyethyl) head group in both the series. A total of 15 GAs were synthesized for the present work, as given in Figure 3. The intermediate tertiary amines were either procured commercially (3a) or synthesized (3b–3h) as per the scheme given in Figure 3. The four/six carbon chain linkers were attached by reacting the tertiary amines (3b–3h) with the respective dibromoalkanes (4a and 4b) to obtain compounds of both of the series (series I and II).
Figure 3.
Synthetic scheme for the preparation of compounds of series I and II.
3.2. Formulation Aspects
3.2.1. Determination of Critical Miceller Concentration (cmc) of the Synthesized GAs
The cmc values (Table 1S, Supporting Information) of the synthesized GAs were determined by the conductometric method30,49 by plotting specific conductance vs the concentrations of the GAs (Figure 16S, Supporting Information). cmc is affected by all of the three structural variables, i.e., polarity of the head group, chain length of the spacer, and the length of the nonpolar hydrocarbon. An increase in the chain length of the spacer causes an increase in the cmc, whereas an increase in the length of the nonpolar hydrocarbon from C12 to C18 causes a decrease in cmc of the resulting GAs. An increase in the polarity of the head group from methyl to hydroxyethyl causes an increase in cmc, but a further increase in the polarity by attaching two hydroxyethyl groups causes a decrease in cmc. Overall, cmc of the synthesized GAs (1.1 × 10–6–3.6 × 10–5) was found to be much superior to that of the monomeric counterparts like cetyltrimethylammonium bromide (1.3 × 10–3 M).
3.2.2. Preparation of Formulations (Liposomal) of GAs
All of the synthesized GAs were formulated as liposomes all alone (plain GAs) and in combination with the helper lipid DOPE in different molar ratios (1:1, 1:2, and 1:3) in a buffer system (HEPES, 20 mM, pH 7.4). DOPE was selected as the helper lipid due to its fusogenic property at the endosomal stage.27 Different molar ratios of DOPE were tried to get the best results in terms of transfection efficacy. The buffer system (HEPES, 20 mM) was selected to maintain the pH of the formulations to 7.4. The nonionic nature of HEPES did not provide any hindrance during lipoplex preparation with pDNA.
Serum is known to decrease the transfection efficacy of a gene delivery carrier, whereas cholesterol is known to impart serum compatibility to gene delivery carriers.50 For improving the transfection efficacy of the synthesized GAs in serum, cholesterol was also incorporated in the GA formulations in two different molar ratios, i.e., GA/DOPE/cholesterol in the ratios of 1:1:1 and 1:1:0.5. Three standard formulations, DOTAP/DOPE (1.37 mU), DCC/DOPE (1.68 mU), and Lipofectamine 2000 (2.07 mU), alone were used as positive controls.
3.2.3. Preparation and Characterization of Lipoplexes
Lipoplexes of GA formulations were prepared with plasmid DNA (pCMV·SPORT-β-gal) at different N/P ratios (0.25–6.0). The N/P ratio is defined as the molar ratio of the nitrogen contents in the carbonic GA to the phosphorus contents of the anionic pDNA. All of the complexes were prepared keeping the quantity of the pDNA constant and varying the quantity of GAs in the formulations. The volumes of the cationic formulations and pDNA dilutions were also kept constant during the lipoplex preparation.32,33
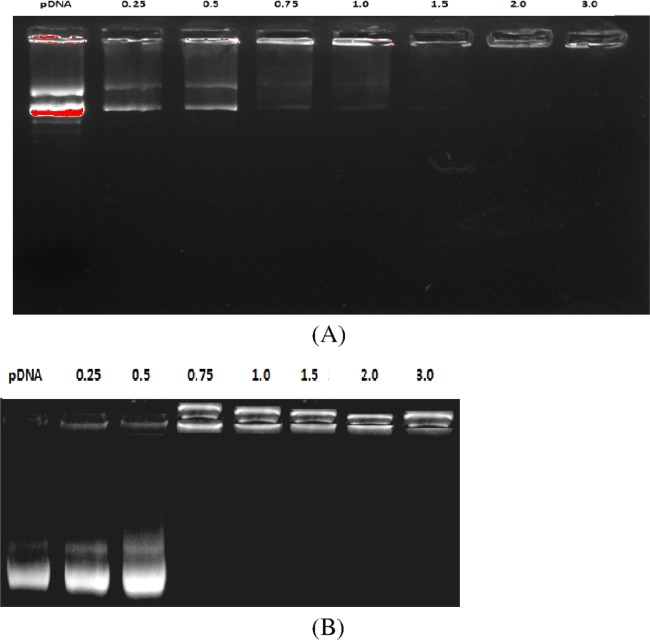
Electrostatic interactions between the negatively charged pDNA and cationic liposomes as a function of N/P ratios were assessed by the electrophoretic gel retardation assay. In this study, the uncomplexed pDNA would move out of the well, whereas the complexed pDNA would remain inside the well.35 The typical electrostatic patterns for formulations of two GAs (5a, 5f) have been shown in Figure 4. Lane 1 showed plain pDNA that moved out of the well under the influence of electrostatic force. Other lanes exhibited different levels of retardation of pDNA as the N/P ratio increased from 0.25 to 3.0. Complete 100% pDNA retardation has been observed for all of the GA formulations at N/P of 1.0 and higher. No effect of head group polarity, spacer chain length, or hydrocarbon chain length was observed in the gel retardation pattern at similar N/P ratios for different GA formulations.
Figure 4.
Complexation behavior of the synthesized GAs using different N/P ratios under gel electrophoresis: (A) 5a and (B) 5f. Lane 1: plasmid DNA alone; lanes 2–8: plasmid DNA with GAs (N/P ratio of 0.25–3.0).
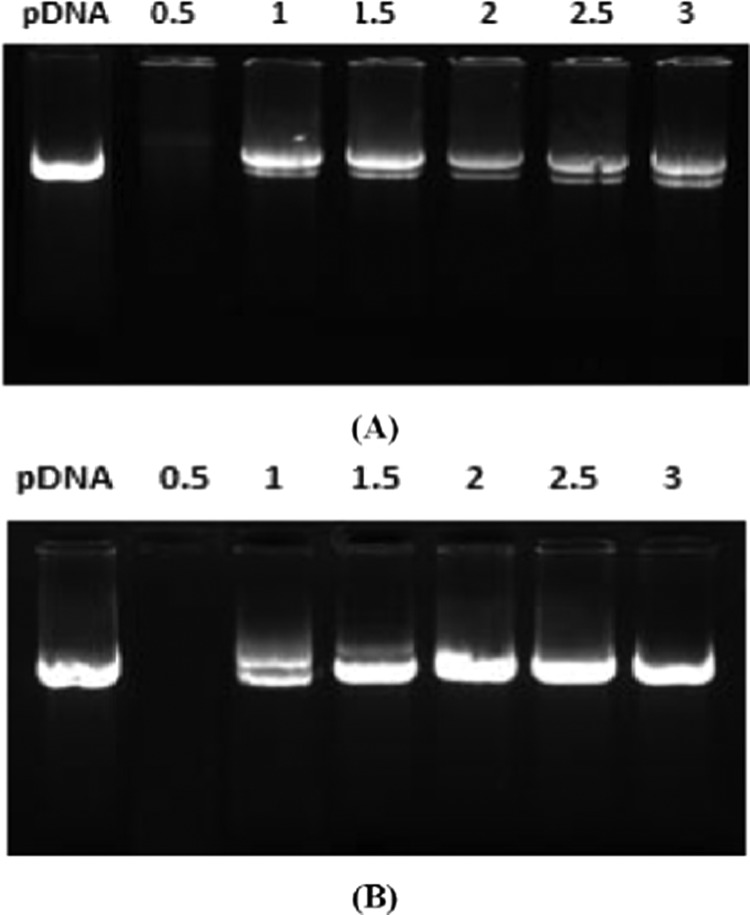
DNase I digestion studies were performed to assess the pDNA protection behavior of the GA formulations in the lipoplexes. A crucial point for obtaining high transfection efficacy is to safeguard the therapeutic genes undamaged.51,52 The results have been shown in Figure 5. Lane 1 shows the naked pDNA without the DNase I treatment. At a N/P ratio of 0.5, all of the lipolexes formulated with GA formulations have shown complete degradation of pDNA. At a N/P ratio of 1, the GAs could protect about 90% of pDNA, whereas complete protection of the pDNA was observed with N/P ratio of more than 1. Treatment of the naked pDNA with DNase I caused its complete degradation. No effect of head group polarity and chain length of the spacer or the hydrocarbon was observed in this study.
Figure 5.
DNase-I digestion study for the assessment of pDNA protection by the GAs: (A) 5a and (B) 5b. First lane shows pDNA without DNase treatment, and rest of the lanes show DNase-treated lipoplexes with varying N/P ratios.
Circular dichroism (CD) spectra provide information about the double-stranded DNA helical conformation. Cationic amphiphiles bind to the native β-form of the DNA and induce changes in the secondary structure in such a way that the number of base pairs/turns is reduced from 10 to 9.33.37 Positive and negative bands near 277 and 245 nm, respectively, are exhibited by the pDNA in HEPES buffer. Condensation of DNA into a chiral ψ-phase is indicated by an overall shift of the spectrum to higher wavelengths, an enhanced negative band, and flattening of the positive band.36 Addition of GA formulations causes change in the β-form to ψ-form, as can be visualized from Figure 17S (Supporting Information). An increase in the N/P ratio causes more condensation. An effective complexation of DNA and liposomes would cause compaction of the nucleic acid that would help in the penetration of the DNA into the target cell due to reduction in its size. Simberg et al. demonstrated that formulation of the compact ψ-DNA helped in efficient delivery of genes.39 Results of the CD experiment demonstrate clearly that all of the three initially synthesized GAs (5a, 5b, and 5f) are effective in condensing pDNA into the ψ-phase. Under the same experimental conditions, 5b showed more effective condensation than the remaining two.
3.3. In Vitro Cell Line Studies
3.3.1. Assessment of Transfection Efficacy of the Lipoplexes [in the Absence of Fetal Bovine Serum (FBS)]
The transfection efficacy of the lipoplexes prepared from different GA formulations was evaluated in A549 and HeLa cell lines, using the β-gal reporter plasmid. There were two variables used for each GA formulation. One variable was the absence or presence of DOPE as a helper lipid in the ratios of GA/DOPE as 1:1, 1:2, or 1:3 respectively. The second variable was the use of GA formulations in N/P ratios of 0.5, 1, 2, 3, 4, and 6. The amount of β-galactosidase enzyme expressed in the cells transfected with the β-gal reporter plasmid is directly proportional to the transfection efficacy of the concerned GA used as the gene delivery carrier.25 The results showed that all of the GAs exhibited β-gal expression when formulated along with DOPE (Figures 18S–32S; Supporting Information). This may be due to early release of the lipoplex at the endosomal stage inside the cells.26 It is evident from Table 1 that higher β-gal expression has taken place in HeLa than in A549 cell lines. This may be due to higher propensity of HeLa cells for transfection in comparison to that of A54 cells. GAs of series I caused better expression of the reporter protein than the GAs of series-II.
Table 1. Transfection Efficacy of Various GA Formulations and Standard Carrier Formulations Using the Best N/P Ratio for A549 and HeLa Cell Lines.
| highest transfection efficacy in the cell lines (A549 and HeLa) shown by the formulations |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| in absence of FBS (10%) |
in presence of FBS (10%) |
||||||||||
| without DOPE |
with DOPE |
with DOPE |
|||||||||
| GA | N/P ratioa | in A549 cells | in HeLa cells | GA/DOPE molar ratio | N/P ratioa | in A549 cells | in HeLa cells | GA/DOPE molar ratio | N/P ratioa | in A549 cells | in HeLa cells |
| Series-I | |||||||||||
| 5a | 1 (1) | 0.94 | 1.02 | 1:1 | 4 (4) | 1.60 | 1.80 | ||||
| 5c | 1 (2) | 0.29 | 0.48 | 1:2 | 1 (1) | 0.95 | 1.13 | ||||
| 5d | 1 (2) | 1.32 | 1.56 | 1:1 | 2 (3) | 1.84 | 3.44 | 1:1 | 2 (3) | 1.40 | 2.37 |
| 5b | 4 (2) | 1.11 | 1.83 | 1:1 | 1 (1) | 1.79 | 2.76 | 1:1 | 1 (1) | 1.37 | 2.18 |
| 5e | 1 (2) | 0.16 | 0.48 | 1:1 | 1 (1) | 0.78 | 0.96 | ||||
| 5h | 2 (1) | 0.33 | 0.40 | 1:2 | 2 (2) | 0.99 | 1.05 | ||||
| 5g | 3 (3) | 0.60 | 0.96 | 1:1 | 2 (2) | 1.81 | 2.06 | 1:1 | 2 (2) | 1.18 | 1.44 |
| 5f | 2 (3) | 1.02 | 1.04 | 1:1 | 2 (2) | 1.98 | 2.22 | 1:1 | 2 (2) | 1.16 | 1.64 |
| Series-II | |||||||||||
| 6a | 2 (1) | 0.88 | 1.05 | 1:2 | 2 (3) | 1.26 | 1.65 | ||||
| 6c | 2 (2) | 0.22 | 0.26 | 1:1 | 3 (3) | 0.44 | 0.64 | ||||
| 6d | 1 (3) | 0.79 | 1.40 | 1:2 | 1 (1) | 1.74 | 2.31 | 1:2 | 1 (1) | 1.18 | 1.62 |
| 6b | 2 (1) | 1.01 | 1.24 | 1:2 | 2 (2) | 1.45 | 2.60 | 1:2 | 2 (2) | 1.17 | 1.88 |
| 6e | 1 (2) | 0.27 | 0.45 | 1:2 | 2 (2) | 0.75 | 1.00 | ||||
| 6g | 2 (2) | 0.59 | 0.98 | 1:1 | 1 (1) | 1.64 | 1.93 | 1 (1) | 1.09 | 1.35 | |
| 6f | 2 (2) | 0.93 | 0.97 | 1:1 | 2 (2) | 1.70 | 1.84 | 2 (2) | 0.72 | 1.33 | |
| highest transfection efficacy in cell lines |
||||
|---|---|---|---|---|
| in absence
of serum |
in presence of serum (10% FBS) |
|||
| formulations of standard carriers | A549 | HeLa | A549 | HeLa |
| DCC/DOPE | 1.68 | 2.47 | 1.11 | 1.58 |
| DOTAP/DOPE | 1.37 | 1.94 | 0.92 | 1.38 |
| Lipofectamine 2000 | 2.07 | 3.12 | ||
Values outside parentheses are for A549 and within parentheses are for HeLa cell lines
3.3.1.1. Effect of Head Group Variations in GAs on the Transfection Efficacy
Modulation in head group polarity affects the transfection efficacy of the GAs significantly. For instance, incorporation of an hydroxyethyl group in place of a methyl group in the head group region (HG1 and HG2) of the GAs having the C4 spacer and C16 hydrocarbon chain increases the transfection efficacy [e.g., 5a (no hydroxyethyl group), 5b (one hydroxyethyl group), and 5f (two hydroxyethyl groups) showed the expression of 0.94, 1.11, and 1.02 in A549 and 1.02, 1.83, and 1.04 in HeLa cell lines, respectively]. However, there was a little decrease in transfection efficacy on moving from one hydroxyethyl to two hydroxyethyl groups per quaternary nitrogen while maintaining the hydrocarbon chain length constant (C16). Same observations were made in formulations made with GAs having the C6 spacer. The transfection efficacy of all of the GA formulations was greatly increased by incorporation of DOPE into the formulations as the helper liquid. Incorporation of DOPE greatly reduced the molar concentrations (low N/P) of the GAs in the formulations to achieve the highest transfection efficacies, in general.
3.3.1.2. Effect of Chain Length Variation of the Spacer
Changes in the second variable in the GA structures, i.e., length of the spacer, showed a significant effect on the transfection efficacy of the GA formulations. Change of the spacer from (CH2)4 to (CH2)6 caused a decrease in transfection efficacies of all of the GA formulations in the absence as well as in the presence of DOPE (Table 2).
Table 2. Transfection Efficacy of Formulations [5b(chol) and 5d(chol)] of the Two GAs Containing Cholesterol and DOPE as Helper Lipids in the Absence/Presence of Serum in the Two Cell Lines.
| transfection
efficacy in cell lines |
|||||||
|---|---|---|---|---|---|---|---|
| in absence of serum |
in presence of serum (10% FBS) |
||||||
| GA used | formulation | molar ratios of GA/DOPE/cholesterol | best N/P ratio | A549 | HeLa | A549 | HeLa |
| 5b | 5b(chol) | 1:1:1 | 1 | 1.96 | 2.95 | 1.44 | 2.45 |
| 1:1:0.5 | 1 | 1.81 | 2.74 | 1.36 | 2.38 | ||
| 5d | 5d(chol) | 1:1:1 | 2 | 1.94 | 3.61 | 1.72 | 3.39 |
| 1:1:0.5 | 2 | 1.73 | 3.07 | 1.68 | 2.92 | ||
3.3.1.3. Effect of Variations in Hydrocarbon Chain Length
Hydrocarbon chain length, the third variable in the structures of the GAs, also exhibited noticeable effects on the transfection efficacies of the GA formulations. In the (CH2)4 series (series I) with the same head group (monohydroxyethyl) (5b, 5c, 5d, and 5e), the order of transfection efficacy remained as 5d > 5b > 5c > 5e in the A549 cell line and 5b > 5d > 5c ≥ 5e in the HeLa cell lines with values of 1.32 > 1.11 > 0.29 > 0.11 and 1.83 > 1.56 > 0.48 ≥ 0.48, respectively, whereas the order remained as 6b > 6d > 6e > 6c in the 549 cell line and 6d > 6b > 6e > 6c in HeLa cell lines with the values of 1.01 > 0.79 > 0.27 > 0.22 and 1.40 > 1.24 > 0.45 > 0.26, respectively, in the formulations of (CH2)6 series when the GAs were used all alone. When DOPE was included in the formulations as a helper lipid, the order of efficacy for the (CH2)4 series of GAs was observed as 5d > 5b > 5c > 5d in both the cell lines and for the (CH2)6 series the order was 6d > 6b > 6e > 6c in A549 cell lines and 6b > 6d > 6e > 6c in the HeLa cell lines. This finding suggested that the transaction efficacy of the GAs having different size hydrocarbon chain length followed the order C14 ≈ C16 > C18 > C12 when the head group and the spacer were kept constant.
GA formulations containing optimum ratios of the helper lipid DOPE were compared with the expression of naked β-gal plasmid (pDNA) (without making lipolex) as negative control and commercially available transfection reagents (Lipofectamine 2000; DCC/DOPE; and DOTAP/DOPE liposomes) as positive controls. Naked pDNA showed negligible β-gal expression of 0.04 and 0.077 mU in A549 and HeLA cell lines, respectively. Various formulations of the synthesized GAs showed good expression. They exhibited higher or comparable levels of expression to DOTAP/DOPE liposomes (1.37 and 1.94 in A549 and HeLa cell lines, respectively) and to DCC/DOPE liposomes (1.68 and 1.94 in A549 and HeLA cell lines, respectively). However, some formulations (5d, 5b, and 5f) exhibited higher or comparable β-gal expression to Lipofectamine 2000 (2.07 and 3.12 in A549 and HeLa cell lines, respectively).
3.3.2. Evaluation of Transfection Efficacy of Some Selected Formulations in Serum
Serum is known to decrease the transfection efficacy of gene delivery carriers. Transfection efficacies of the optimized GA formulations showing the best result in A459 and HeLa cell lines in the absence of serum in the previous experiments were evaluated in the presence of 10% FBS. A decrease in transfection efficacy was noted in both the cell lines in the presence of serum for the selected GA formulations (Table 2). The standard formulations (DCC/DOPE and DOTAP/DOPE) also showed significant reductions in their transfection efficacies in the presence of serum.
Cholesterol is known to impart serum compatibility to gene delivery carriers.50 In our quest to improve transfection efficacies in the presence of serum, GAs exhibiting the best results (5b and 5d) were reformulated to include cholesterol. New formulations were prepared to include two different concentrations of cholesterol in the ratios such as GA/DOPE/cholesterol of 1:1:1 and 1:1:0.5. The transfection efficacies were checked for both of these formulations in the absence and presence of 10% FBS in both the cell lines (Table 2).
It could be observed that reduction in the efficacy of the enzyme expression did take place in the presence of serum even in the new formulations containing cholesterol in both the cell lines. However, addition of cholesterol in the ratio of 1:1:1 (GA/DOPE/cholesterol) definitely improved the transfection efficacy of the new formulations [1.72 vs 1.40 mU for 5d in the A549 cell line and 3.39 vs 2.37 mU in the HeLa cell line; 1.44 vs 1.37 mU for 5b in the A549 cell line and 2.45 vs 2.18 in the Hela cell line; Tables 1 and 2].
3.3.3. Assessment of Cell Toxicity Due to GAs Using MTT Assay
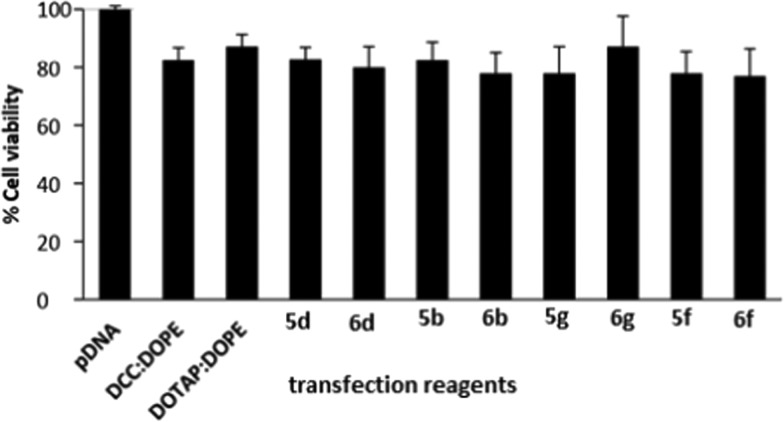
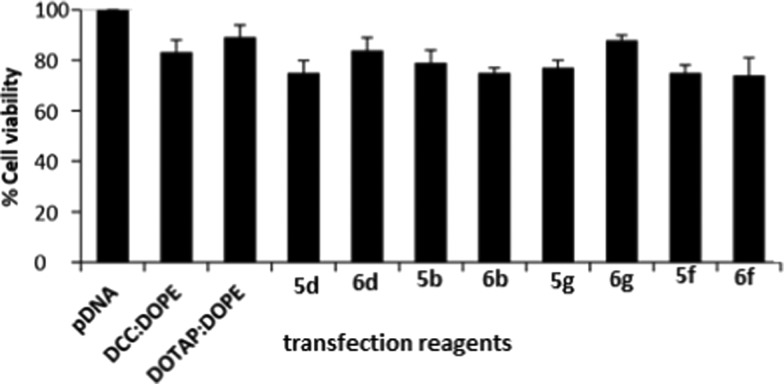
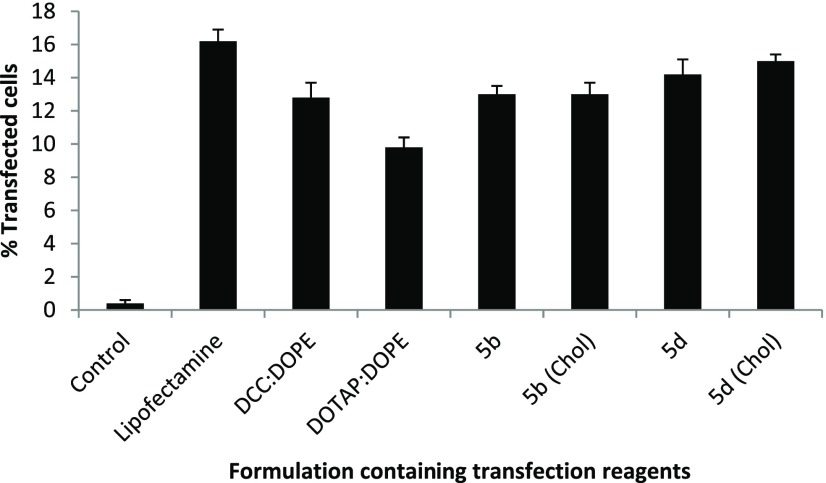
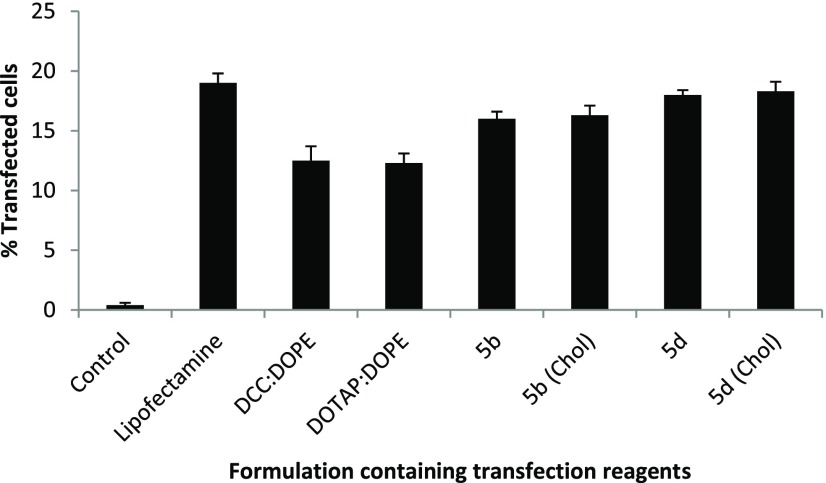
Cell safety (or toxicity) is an important parameter in selecting a suitable GA for gene delivery. A good GA should exhibit not only a high transfection efficacy but also minimal level of toxicity. Some selected GAs in formulations having DOPE in ratios 1:1, 1:2, and 1:3 (DOPE/GA) at different N/P ratios were evaluated for their safety profile in A549 and HeLa cell lines using the MTT assay. Cell viability of the control cells without GA treatment was considered as 100%. As the N/P ratio of the lipoplexes increased from 0.5 to 6, cell viability decreased in both of the cell lines for all of the formulations. Cell viabilities of all of the optimized GA formulations have been evaluated and compared with those of the standard (DCC/DOPE and DOTAP/DOPE liposomes) in A549 and HeLa cell lines in Figures 6 and 7. The cell viabilities of almost all of the formulations were comparable to those shown by the two standard formulations. DCC/DOPE and DOTAP/DOPE liposomes showed cell viabilities of 82.6 and 87.2 and 83.38 and 89.2% in A549 and HeLa cell lines, respectively. The cell viabilities were found to be independent of the chain length of the hydrocarbon tails, polarity of the cationic head, or the length of spacer of the GAs.
Figure 6.
Compiled results of percent cell viability of the optimized GA formulations (showing the best N/P results in transfection studies) in A549 cells, of the GAs showing the highest transfection efficacies.
Figure 7.
Compiled results of percent cell viability of the optimized GA formulations (showing the best N/P results in transfection studies) in HeLa cells, of the GAs showing the highest transfection efficacies.
3.4. Fluorescence-Assisted Cell Sorting (FACS) Studies
The amount of β-galactosidase protein expressed in cells is a measure of the transfection efficacy of the gene delivery carriers. However, the number of cells transfected by the transfection reagent is also important. The percentage of cells transfected during the transfection efficacy experiments by the formulations showing promising results was evaluated using FACS studies in a 24-well format.42 Fluorescence of the GFP expressed by the transfected GFP gene was observed and captured under the fluorescence microscope (Figures 33S and 34S (Supporting Information)). Figures 8 and 9 show the percentage of cells transfected with GFP using these formulations in comparison to that of the naked GPF as a negative control and the two standards as positive controls in A545 and HeLa cells. It was observed that formulations containing cholesterol showed a higher number of transfected cells in comparison with the formulations without cholesterol.
Figure 8.
FACS studies of the optimized formulations of 5b and 5d without cholesterol and with cholesterol [5b(chol) and 5d(chol)] using GFP expression in A549 cells without FBS.
Figure 9.
FACS studies of optimized formulations of 5b and 5d without cholesterol and with cholesterol [5b(chol) and 5d(chol)] using GFP expression in HeLa cells without FBS.
The optimized formulation of 5d containing cholesterol 5d(chol) showed almost comparable percentage (14.99%) of transfected cells to Lipofectamine 2000 (16.8%) in the A549 cell line and 18.78% [5d(chol)] to 19.32% (Lipofectamine 2000) in the HeLa cell line. Cholesterol-containing formulations [5d(chol) and 5b(chol)] showed a higher percentage of transfected cells in comparison to their respective formulations without cholesterol (5d and 5b).
All of the data presented above indicates the mean ± standard deviation from three independent measurements and was analyzed using descriptive statistics and single-factor analysis of variance.
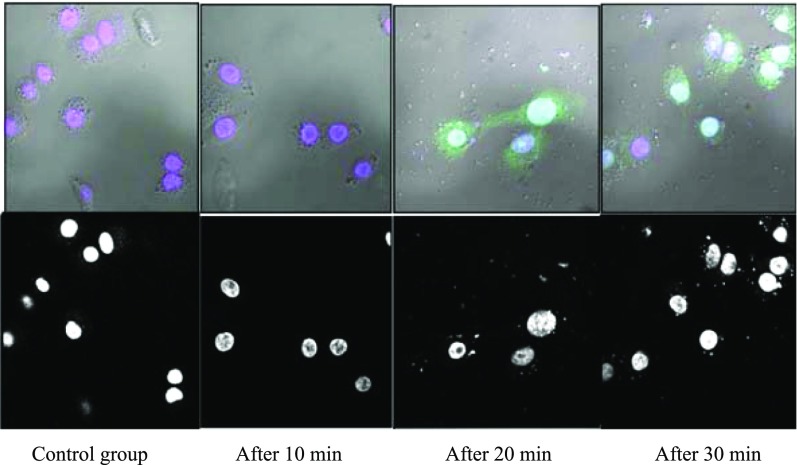
3.5. Intracellular Trafficking Study Using Confocal Microscopy
Confocal microscopy was performed to track the intracellular trafficking of the lipoplex [obtained on treatment of the reporter plasmid with the best GA formulation containing cholesterol 5d(chol) showing the best results in the in vitro transfection and FACS studies].45 The lipoplex was tagged with the green fluorescent dye (YOYO 1) by incubating with it overnight. The cells (A549 and HeLa) were treated with the tagged lipoplex in a 6-well format. The cells were harvested at 10, 20, and 30 min. The first column of Figure 10 shows the control group of cells without any lipoplex treatment. The second-, third-, and fourth-column images show blue nuclei of the cells, green fluorescent dye-tagged plasmid in the cells, and merged images after 10, 20, and 30 min of treatment, respectively. The images clearly reveal the journey of pDNA to nucleus inside the HeLa cells at different time intervals after treatment of the cells with the labeled lipoplex (Figures 10 and 35S (Supporting Information)).
Figure 10.
Confocal microscopic images of A549 cells treated with the lipolex formed from formulation 5d(chol): first column shows the control group (cells without any lipoplex treatment), second column after 10 min, third column after 20 min, and fourth column after 30 min of lipoplex treatment of the cells. The bottom row images represent phase contrast images of the cells, whereas the upper ones are merged images showing blue-stained nucleus and the green dots surrounding the cytoplasm and entering the nucleus, at different time intervals.
3.6. Biodistribution and γ-Scintigraphy Studies
The lipoplex of the optimized GA formulation containing cholesterol [5d(chol)] showing the best results in the in vitro studies was chosen for biodistribution studies in rats.46,47 Radiolabeling of the lipolex was performed with 99mTc by the direct labeling procedure. The 99mTc-labeled lipoplex of 5d(chol) formulation containing pDNA (15 μg) was injected in the tail vein of the rats. Radioactivity in various organs was detected quantitatively at predetermined time points (1, 6, and 24 h), and the presence of radioactivity per gram of the tissue was plotted as given in Figure 11. At an initial point (1 h), maximum of the lipoplex accumulated in vital organs like liver, spleen, lungs, kidneys, etc., and with the passage of time (6 h), accumulation of the lipoplex increased in vital organs. After 24 h, spleen was found to contain the maximum concentration of lipoplex followed by liver.
Figure 11.
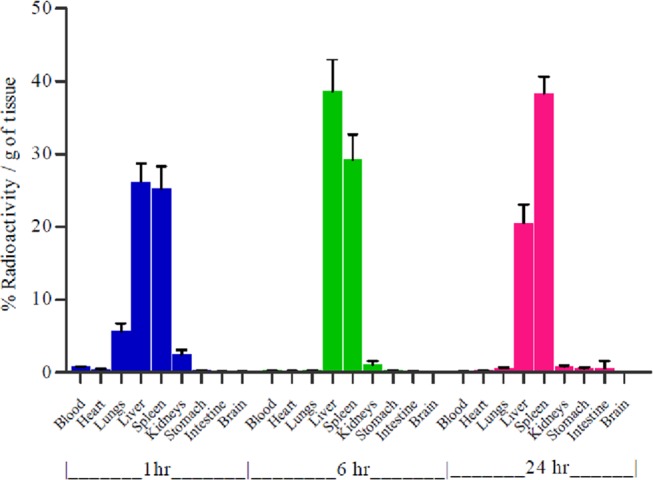
Quantitative biodistribution of 99mTc-labeled 5d(chol) lipolex in rats.
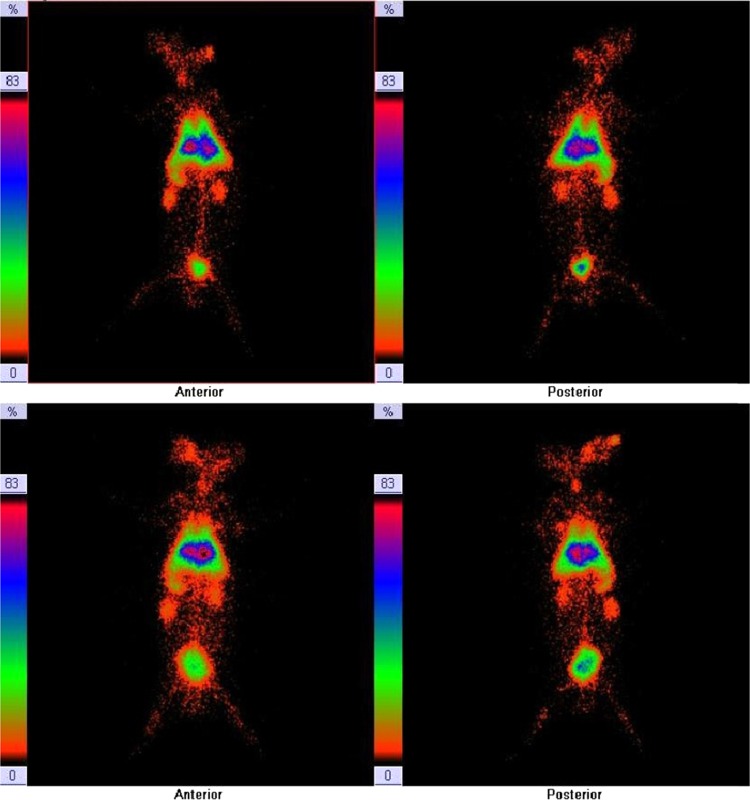
γ-Scintigraphic study was performed on rabbit for qualitative assessment of the optimized 99mTc-labeled lipoplex (5d(chol) formulation).46−48 The scintigraphic images have been shown in Figure 12 after 1 h of administration. The images show the accumulation of 99mTc-labeled lipoplex in liver, spleen, lungs, and kidneys.
Figure 12.
γ-Scintigraphy images of 99mTc-labeled lipoplex formulations administered in rabbits, of 5d (without cholesterol as helper lipid) (first row: anterior and posterior view) and of 5d(chol) (second row: anterior and posterior view).
4. Conclusions
Two series of gemini amphiphiles differing in the size of the chain length of the linker, polarity of the head groups, and number of hydrocarbons in the hydrophobic moieties were synthesized as carriers for gene delivery. The synthesized GAs were formulated all alone and in combination with helper lipids like DCC, DOTAP, and cholesterol. The GA formulations were used as carriers of the reporter gene to obtain lipoplexes with different N/P ratios, and their transfection efficacies were assessed in two cell lines, A549 and HeLa, in the absence of serum. The best N/P ratios for various GA formulations without cholesterol and with cholesterol as the helper lipid were also assessed in these two cell lines in the presence of bovine serum albumin (BSA). The DNA stability against DNase-I in the lipoplexes was also assessed. Compaction of the reporter gene was found out using CD spectrometry. The best N/P ratios of the GA formulations were also evaluated for their cytoprotective/toxic effects using the MTT assay in both of these cell lines wherein almost all of the GAs showed comparable cytoprotective effect to the commercially available DNA carriers like DCC/DOPE and DOTAP/DOPE. FACS studies for the two best formulations [5b(chol) and 5d(chol)] containing DOPE and cholesterol as helper lipids of the GAs exhibited almost equal transfection efficacies to Lipofectamine 2000 in the presence of BSA. Confocal microscopy showed intracellular trafficking of the reporter gene to the nucleus within 30 min of exposure of the cells to the lipoplexes made from the best GA formulation [5d(chol)] containing DOPE and cholesterol as the helper lipids. The two best lipoplex formulations [5d(chol)] of the GA were labeled with 99mTc and used for studying biodistribution in rat and γ-scintigraphy in rabbit animal models. The labeled lipoplexes remained localized in vital tissues like liver and spleen for 24 h. The GA formulation [5d(chol)] containing DOPE and cholesterol as the helper lipids offered the best results in the overall study. GA (5d) has shown the potential to be developed as a synthetic carrier for the delivery of therapeutic genes.
Acknowledgments
The authors acknowledge the crucial role of Dr Pradeep Kumar, Scientist, Nucleic Acid Chemistry, Institute of Genomics and Integrative Biology, New Delhi, for providing permission to use all required facilities to complete FACS and confocal studies. Financial grant from the University Grants Commission (UGC) during this work in the form of SRF to M.K. is duly acknowledged. M.R.Y. is thankful to UGC, New Delhi, for awarding UGC-BSR Faculty Fellowship [No. F.18-1/2011(BSR)].
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01014.
PMR spectrum of 5a–5h and 6a–6g; mass spectra of 5b and 5c; representative plots for cmc value determination; circular dichroism spectra of 5a, 5b, and 5f; transfection efficacies of GAs (5a–5h; 6a–6g); fluorescence images of GFP expression; confocal images for uptake study in HeLa cells; and CMC values of the GAs (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ledley F. D. Pharmaceutical approach to somatic gene therapy. Pharm. Res. 1996, 13, 1595–1614. 10.1023/A:1016420102549. [DOI] [PubMed] [Google Scholar]
- Vacik J.; Dean B. S.; Zimmer W. E.; Dean D. A. Cell specific nuclear import of plasmid DNA. Gene Ther. 1999, 6, 1006–1014. 10.1038/sj.gt.3300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton M.; Wagner E. Non-viral approaches to gene therapy. Curr. Opin. Biotechnol. 1993, 4, 705–710. 10.1016/0958-1669(93)90053-Y. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Nunes F. A.; Berencsi K.; Gonczol E.; Engelhardt J. F.; Wilson J. M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat. Genet. 1994, 7, 362–369. 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Nunes F. A.; Berencsi K.; Gonczol E.; Engelhardt J. F.; Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. U.S.A. 1994, 91, 4407–4411. 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. R.; Hohneker K. W.; Zhou Z.; Olsen J. C.; Noah T. L.; Hu P. C.; Leigh M. W.; Engelhardt J. F.; Edwards L. J.; Jones K. R. A controlled study of adenoviral vector mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N. Engl. J. Med. 1995, 333, 823–831. 10.1056/NEJM199509283331302. [DOI] [PubMed] [Google Scholar]
- Crystal R. G.; Mc Elvaney N. G.; Rosenfeld M. A.; Chu C. S.; Mastrangeli A.; Hay J. G.; Brody S. L.; Jaffe H. A.; Eissa N. T.; Daneil C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat. Genet. 1994, 8, 42–51. 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- Puchkov P. A.; Kartashova I. A.; Shmendel E. V.; Luneva A. S.; Morozova N. G.; Zenkova M. A.; Maslov M. A. Spacer structure and hydrophobicity influences transfection activity of novel polycationic gemini amphiphiles. Bioorg. Med. Chem. Lett. 2017, 27, 3284–3288. 10.1016/j.bmcl.2017.06.026. [DOI] [PubMed] [Google Scholar]
- Peña L. C.; Argarañá M. F.; De Zan M. M.; Giorello A.; Antuña S.; Prieto C. C.; Veaute C. M.; Müller D. M. New Amphiphilic Amino Acid Derivatives for Efficient DNA Transfection in Vitro. Adv. Chem. Eng. Sci. 2017, 7, 191–205. 10.4236/aces.2017.72014. [DOI] [Google Scholar]
- Kirby A. J.; Camilleri P.; Engberts J. B.; Feiters M. C.; Nolte R. J.; Soderman O.; Bergsma M.; Bell P. C.; Fielden M. L.; Garcia Rodriguez C. L.; Guedat P.; Kremer A.; McGregor C.; Perrin C.; Ronsin G.; van Eijk M. C. Gemini surfactants: New synthetic vectors for gene transfection. Angew. Chem., Int. Ed. 2003, 42, 1448–1457. 10.1002/anie.200201597. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Jinturkar K.; Yadav M. R.; Misra A. N. Gemini amphiphiles: A novel class of non-viral gene delivery vectors. Crit. Rev. Ther. Drug Carrier Syst. 2010, 27, 237–278. 10.1615/CritRevTherDrugCarrierSyst.v27.i3.20. [DOI] [PubMed] [Google Scholar]
- Bombelli C.; Giansanti L.; Luciani P.; Mancini G. Gemini surfactant based carriers in gene and drug delivery. Curr. Med. Chem. 2009, 16, 171–183. 10.2174/092986709787002808. [DOI] [PubMed] [Google Scholar]
- Cardoso A. M.; Morais C. M.; Silva S. G.; Marques F. E.; Pedrosode C. M.; Amália S. Bis-quaternary gemini surfactants as components of nonviral gene delivery systems: A comprehensive study from physicochemical properties to membrane interactions. Int. J. Pharm. 2014, 474, 57–69. 10.1016/j.ijpharm.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Rosenzweig H. S.; Rakhmanova V. A.; MacDonald C. Diquaternary ammonium compounds as transfection agents. Bioconjug. Chem. 2001, 12, 258–263. 10.1021/bc000099z. [DOI] [PubMed] [Google Scholar]
- Badea I.; Wettig S.; Verrall R.; Foldvari M. Topical non-invasive gene delivery using gemini nanoparticles in interferon-gamma-deficient mice. Eur. J. Pharm. Biopharm. 2007, 65, 414–422. 10.1016/j.ejpb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Badea I.; Verrall R.; Baca-Estrada M.; Suresh T.; Rosenberg A.; Kumar P.; Marianna F. In vivo cutaneous interferon-gamma gene delivery using novel dicationic (gemini) surfactant plasmid complexes. J. Gene Med. 2005, 7, 1200–1214. 10.1002/jgm.763. [DOI] [PubMed] [Google Scholar]
- Luciani P.; Bombelli C.; Colone M.; Giansanti L.; Ryhänen S. J.; Säily V. M. J.; Mancini G.; Kinnunen P. K. J. Influence of the spacer of cationic gemini amphiphiles on the hydration of lipoplexes. Biomacromolecules 2007, 8, 1999–2003. 10.1021/bm070202o. [DOI] [PubMed] [Google Scholar]
- Bombelli C.; Faggioli F.; Luciani P.; Giovanna M.; Grazia Sacco M. Efficient transfection of DNA by liposomes formulated with cationic gemini amphiphiles. J. Med. Chem. 2005, 48, 5378–5382. 10.1021/jm050477r. [DOI] [PubMed] [Google Scholar]
- Yoshiyuki H.; Wu-xiao D.; Maitani Y. Yoshie Maitan. Highly efficient cationic hydroxyethylated cholesterol-based nanoparticle-mediated gene transfer in vivo andin vitro in prostate carcinoma PC-3 cells. J. Controlled Release 2007, 120, 122–130. 10.1016/j.jconrel.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Bennett M. J.; Aberle A. M.; Balasubramaniam R. P.; Malone J. G.; Malone R. W.; Nantz M. H. Cationic lipid-mediated gene delivery to murine lung: Correlation of lipid hydration with in vivo transfection activity. J. Med. Chem. 1997, 40, 4069–4078. 10.1021/jm970155q. [DOI] [PubMed] [Google Scholar]
- Felgner J. H.; Kumar R.; Sridhar C. N.; Wheeler C. J.; Tsai Y. J.; Border R.; Ramsey P.; Martine M.; Felgner P. L. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem. 1994, 269, 2550–2561. [PubMed] [Google Scholar]
- Srilakshmi G. V.; Sen J.; Chaudhuri A.; Ramadas Y.; Madhusudhana N. Anchor dependent lipofection with non-glycerol based cytofectins containing single 2-hydroxyethyl head groups. Biochim. Biophys. Acta, Biomembr. 2002, 1559, 87–95. 10.1016/S0005-2736(01)00442-4. [DOI] [PubMed] [Google Scholar]
- Banerjee R.; Das P. K.; Srilakshmi G. V.; Chaudhuri A.; Rao N. M. Novel series of non-glycerol based cationic transfection lipids for use in liposomal gene delivery. J. Med. Chem. 1999, 42, 4292–4299. 10.1021/jm9806446. [DOI] [PubMed] [Google Scholar]
- Laxmi A. A.; Vijayalakshmi P.; Balagopala; Kaimal T. N.; Chaudhari A.; Ramadas Y.; Rao N. M. Novel non-glycerol based cytofectins with lactic acid-derived head groups. Biochem. Biophys. Res. Commun. 2001, 289, 1057–62. 10.1006/bbrc.2001.6065. [DOI] [PubMed] [Google Scholar]
- Banerjee R.; Mahidhar Y. V.; Chaudhuri A.; et al. Design, synthesis, and transfection biology of novel cationic glycolipids for use in liposomal gene delivery. J. Med. Chem. 2001, 44, 4176–4185. 10.1021/jm000466s. [DOI] [PubMed] [Google Scholar]
- Hui S. W.; Langner M.; Zhao Y.; Ross P.; Hurley E.; Chan K. The role of helper lipids in cationic liposome-mediated gene transfer. Biophys. J. 1996, 71, 590–599. 10.1016/S0006-3495(96)79309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P.; Epand R. M. The mechanism of lamellar to inverted hexagonal phase transitions in phosphatidylethanolamine: Implications for membrane fusion mechanisms. Biophys. J. 1997, 73, 3089–3111. 10.1016/S0006-3495(97)78336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida P. F.; Vaz W. L. C.; Thompson T. E. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: A free volume analysis. Biochemistry 1992, 31, 6739–6747. 10.1021/bi00144a013. [DOI] [PubMed] [Google Scholar]
- Manchester K. L. Value of A260/A280 ratios for measurement of purity of nucleic acids. BioTechniques 1995, 19, 208–210. [PubMed] [Google Scholar]
- Ralston A. W.; Eggenberger D. N.; Harwood H. J.; Dubrow P. J. The electrical conductivities of long chain quaternary ammonium chlorides containing hydroxyl alkyl group. J. Am. Chem. Soc. 1947, 69, 2095–2097. 10.1021/ja01201a004. [DOI] [Google Scholar]
- Zana R.; Benrroau M.; Rueff R. Alkanediyl-alpha,omega-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 1991, 7, 1072–1075. 10.1021/la00054a008. [DOI] [Google Scholar]
- Bajaj A.; Kondiah P.; Bhattacharya S. Design, synthesis, and in vitro gene delivery efficacies of novel cholesterol-based gemini cationic lipids and their serum compatibility: A structure-activity investigation. J. Med. Chem. 2007, 50, 2432–2442. 10.1021/jm0611253. [DOI] [PubMed] [Google Scholar]
- Lonez C.; Lensink M. F.; Kleiren E.; Vanderwinden J. M.; Ruysschaert J. M.; et al. Fusogenic activity of cationic lipids and lipid shape distribution. Cell. Mol. Life Sci. 2010, 67, 483–493. 10.1007/s00018-009-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calladine C. R.; Collis C. M.; Drew H. R.; Mott M. R. A study of electrophoretic mobility of DNA in agarose and polyacrylamide gels. J. Mol. Biol. 1991, 221, 981–1005. 10.1016/0022-2836(91)80187-Y. [DOI] [PubMed] [Google Scholar]
- Jinturkar K. A.; Anish C.; Kumar M. K.; Bagchi T.; Panda A. K.; Mishra A. R. Liposomal formulations of etoposide and docetaxel for p53 mediated enhanced cytotoxicity in lung cancer cell lines. Biomaterials 2012, 33, 2492–2507. 10.1016/j.biomaterials.2011.11.067. [DOI] [PubMed] [Google Scholar]
- Keller D.; Bustamante C. Theory of the interaction of light with large inhomogeneous molecular aggregates. II. Psi-type circular dichroism. J. Chem. Phys. 1986, 84, 2972–2979. 10.1063/1.450278. [DOI] [Google Scholar]
- Zhang Z.; Huang W.; Tang J.; Wang E.; Dong S. Conformational transition of DNA induced by cationic lipid vesicle in acidic solution: Spectroscopy investigation. Biophys. Chem. 2002, 97, 7–16. 10.1016/S0301-4622(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Nakanishi H.; Tsuchiya K.; Okubo T.; Sakai H.; Abe M. Cationic surfactant changes the morphology of DNA molecules. Langmuir 2007, 23, 345–347. 10.1021/la051443p. [DOI] [PubMed] [Google Scholar]
- Simberg D.; Danino D.; Talmon Y.; Minsky A.; Ferrari M. E.; et al. Phase behavior, DNA ordering and size instability of cationic lipoplexes: Relevance to optimal transfection activity. J. Biol. Chem. 2001, 276, 47453–47459. 10.1074/jbc.M105588200. [DOI] [PubMed] [Google Scholar]
- Van Meerloo J.; Kaspers G. J.; Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol. Biol. 2011, 731, 237–245. 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- Sylvester P. W. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol. Biol. 2011, 716, 157–168. 10.1007/978-1-61779-012-6_9. [DOI] [PubMed] [Google Scholar]
- Soboleski M. R.; Oaks J.; Halford W. P. Green fluorescent protein is a quantitative reporter of gene expression in individual eukaryotic cells. FASEB J. 2005, 19, 440–442. 10.1096/fj.04-3180fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovala A. T.; Harvey K. A.; Mcgynn P.; Boguslawski G.; Garcia J. G.; English D. High efficiency transient transfection of endothelial cells for functional analysis. FASEB J. 2000, 14, 2486–2494. 10.1096/fj.00-0147com. [DOI] [PubMed] [Google Scholar]
- Davda J.; Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. Int. J. Pharm. 2002, 233, 51–59. 10.1016/S0378-5173(01)00923-1. [DOI] [PubMed] [Google Scholar]
- Elouahabi A.; Ruysschaert J. M. Formation and intracellular trafficking of lipoplexes and polyplexes. Mol. Ther. 2005, 11, 336–347. 10.1016/j.ymthe.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Pathak A.; Kumar P.; Chuttani K.; Jani S.; Mishra A.; Vyas S. P.; Gupta K. C. Gene expression, biodistribution, and pharmacoscintigraphic evaluation of chondroitin sulfate–PEI nanoconstructs mediated tumor gene therapy. ACS Nano 2009, 3, 1493–1505. 10.1021/nn900044f. [DOI] [PubMed] [Google Scholar]
- Shi J.; Chou B.; Choi J. L.; Ta A. L.; Pun S. H. Investigation of Polyethylenimine/DNA polyplex transfection to cultured cells using radiolabeling and subcelluler fractionation methods. Mol. Pharm. 2013, 10, 2145–2156. 10.1021/mp300651q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhajosula S.; Killeen R. P.; Osborne J. R. Altered biodistribution of radiopharmaceuticals: role of radiochemical/pharmaceutical purity, physiological, and pharmacologic factors. Semin. Nucl. Med. 2010, 40, 220–241. 10.1053/j.semnuclmed.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Ciani L.; Casini A.; Gabbiani C.; Ristori S.; Messori L.; Martini G. DOTAP/DOPE and DC- Chol/DOPE lipoplexes for gene delivery studied by circular dichroism and other biophysical technique. Biophys. Chem. 2007, 127, 213–220. 10.1016/j.bpc.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Lenssen K.; Jantscheff P.; von Kiedrowski G.; Massing U. Combinatorial synthesis of new cationic lipids and high-throughput screening of their transfection properties. Chem. Bio. Chem. 2002, 3, 852–858. . [DOI] [PubMed] [Google Scholar]
- Polozov I. V.; Polozova A.; Mishra V.; Anantharamaiah G.; Segrest J.; Epand R. Biochim. Biophys. Acta 1998, 1368, 343–354. 10.1016/S0005-2736(97)00210-1. [DOI] [PubMed] [Google Scholar]
- Simberg D.; Danino D.; Talmon Y.; Minsky A.; Ferrari M.; Wheeler C.; Barenholz Y. J. Biol. Chem. 2001, 276, 47453–47459. 10.1074/jbc.M105588200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.