The major surface glycoprotein of Pneumocystis was unable to activate dendritic cells despite being able to bind to macrophage mannose receptor and DC-SIGN, which recognize mannoproteins, suggesting that the loss of genes in Pneumocystis for high mannosylation facilitates immune evasion.
Keywords: Pneumocystis, major surface glycoprotein, Msg, cytokine, dendritic cell
Abstract
The major surface glycoprotein (Msg) is the most abundant surface protein among Pneumocystis species. Given that Msg is present on both the cyst and trophic forms of Pneumocystis and that dendritic cells play a critical role in initiating host immune responses, we undertook studies to examine activation of bone marrow–derived myeloid dendritic cells by Msg purified from Pneumocystis murina. Incubation of dendritic cells with Msg did not lead to increased expression of CD40, CD80, CD86, or major histocompatibility complex class II or to increased secretion of any of 10 cytokines. Microarray analysis identified very few differentially expressed genes. In contrast, lipopolysaccharide-activated dendritic cells had positive results of all of these assays. However, Msg did bind to mouse mannose macrophage receptor and human DC-SIGN, 2 C-type lectins expressed by dendritic cells that are important in recognition of pathogen-associated high-mannose glycoproteins. Deglycosylation of Msg demonstrated that this binding was dependent on glycosylation. These studies suggest that Pneumocystis has developed a mechanism to avoid activation of dendritic cells, potentially by the previously identified loss of genes that are responsible for the high level of protein mannosylation found in other fungi.
Pneumocystis jirovecii is a common opportunistic pathogen in human immunodeficiency virus (HIV)–infected patients and is being seen with increased frequency in other immunosuppressed patients [1–3]. P. jirovecii causes severe pneumonia (Pneumocystis pneumonia [PCP]) in these populations, with mortality rates that can reach 25%–30% among patients without HIV infection [4, 5]. Underlying conditions that are risk factors for PCP include hematologic and solid tumor malignancies, transplantation, congenital immune deficiencies (eg, hyper–immunoglobulin M syndrome), HIV infection, and autoimmune disorders that require immunosuppressive therapy. Although animals are infected with different species of Pneumocystis, animal models of PCP, especially mouse (Pneumocystis murina) and rat (Pneumocystis carinii) models, have been critical to developing therapies for infection, as well as to understanding disease pathophysiology and host immunity. These models have clearly demonstrated the importance of CD4+ T cells [6–8] and, more recently, B cells [9] in the control of Pneumocystis infection.
During host responses to infection, the first step is recognition of organisms and initiation of an immune response, often by dendritic cells, which are professional antigen-presenting cells that connect innate and adaptive immune responses. Because of their location in the lung, dendritic cells are one of the cells that interact with inhaled invaders. Immature dendritic cells capture pathogens for efficient antigen presentation to naive CD4 T cells, and direct their differentiation into effector cells (eg, T-helper type 1 [Th1], Th2, and Th17 effector T cells) and regulatory T cells [10]. Immature dendritic cells use pattern-recognition receptors, such as Toll-like receptors and C-type lectin receptors (CLRs), to recognize conserved molecular patterns in lipids, nucleic acids, and carbohydrates that are components of microorganisms but not the host [11]. Following exposure, dendritic cells mature, leading to secretion of cytokines and induction of adaptive immune responses. Studies to date that have examined the interaction of Pneumocystis with dendritic cells have focused on whole-organism preparations or β-1,3 glucans as the challenge antigen [12–14]. While Pneumocystis glucans were found to activate dendritic cells and lead to T-cell activation, whole organisms did not activate dendritic cells, although interleukin 4 secretion was increased, and pulsed dendritic cells could generate a Pneumocystis-specific response.
Major surface glycoprotein (Msg; also known as gpA) is the most abundant surface protein of Pneumocystis and is encoded by a multicopy gene family [15–17]. Msg is a mannoprotein that may be involved in the attachment of Pneumocystis to alveolar epithelial cells and may help avoid host-adaptive immune responses through antigenic variation. Msg is present on both trophic forms and cysts; in the latter, Msg, together with other proteins, appears to mask β-glucans, which have been shown to activate dendritic cells [18]. Msg is glycosylated, but recent genomic and biochemical data suggest that N-linked glycosylation in Msg is limited to the core structure of high-mannose glycans [19], which may limit activation of dendritic cells through interactions with CLRs [20].
Given that Msg is broadly exposed on different stages of the organism and likely provides the initial contact with host cells, we undertook the current study to examine the ability of purified Msg to activate naive dendritic cells, as measured by costimulatory molecule expression, cytokine production, and, via microarrays, RNA expression. We also examined Msg binding to the macrophage mannose receptor (MMR) and dendritic cell–specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN), CLRs that play important roles in the recognition of mannoproteins by dendritic cells and macrophages [21–24].
METHODS
Generation of Immature Dendritic Cells
Immature dendritic cells were generated following published methods [25, 26]. Briefly, normal bone marrow (BM) precursors were isolated from C57BL/6 mice by flushing femurs and washing cells in PBS. Bone marrow cells were cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamine (Invitrogen), 50 µM 2-ME (Sigma), and 30 ng/mL granulocyte-macrophage colony-stimulating factor (Peprotech), which was replaced every 3 days. After 7–8 days, the loosely adherent or nonadherent cells were collected as immature dendritic cells. All animal work for this study was performed under an National Institutes of Health Clinical Center Animal Care and Use Committee–approved protocol.
Msg and Crude Antigen Preparation
Native Msg was purified using 2 methods. For the first (lyticase-Msg), partially purified P. murina organisms from heavily infected CD40L knockout (KO) mice were digested with lyticase (Sigma-Aldrich) overnight at 37°C [15]. After centrifugation, the supernatant was collected, and Msg was purified using the Glycoprotein Isolation Kit (Concanavalin A) according to the manufacturer’s instructions (Thermo Scientific). For the second (sodium dodecyl sulfate–Msg [SDS-Msg]), partially purified Pneumocystis organisms were boiled 2 times for 10 minutes each in 2% SDS in Tris–ethylenediaminetetraacetic acid buffer. Following centrifugation, SDS was removed from the supernatant by using the SDS-Out SDS Precipitation Reagent (Thermo Scientific), and Msg was purified using the Glycoprotein Isolation Kit (Concanavalin A) as described above. For both preparations, the buffer was changed to PBS, using a Microcon centrifugal filter concentrator (Millipore). Endotoxin was removed by extraction with 1% Triton X-114 (Sigma) [27]. The protein concentration was determined by the bicinchoninic acid protein assay or by measuring the absorbance at 280 nm, using a NanoDrop spectrophotometer. P. carinii Msg was purified by affinity chromatography using monoclonal antibody RA-E7 (a kind gift of Drs Walzer and Linke) as previously described [19]. Msg purity was evaluated by SDS polyacrylamide gel electrophoresis (PAGE; Figure 1). Experiments used P. murina Msg (referred to hereafter as “Msg”) unless they are specifically noted to have used P. carinii Msg. Crude Pneumocystis antigens were prepared as previously reported by homogenization of partially purified organisms (obtained from a heavily infected animal) in PBS (0.25 g/mL), using a TissueLyser (Qiagen) followed by sonication [28]. The homogenate was centrifuged for 10 minutes at 20000 ×g, and the supernatant was used as the crude antigen.
Figure 1.
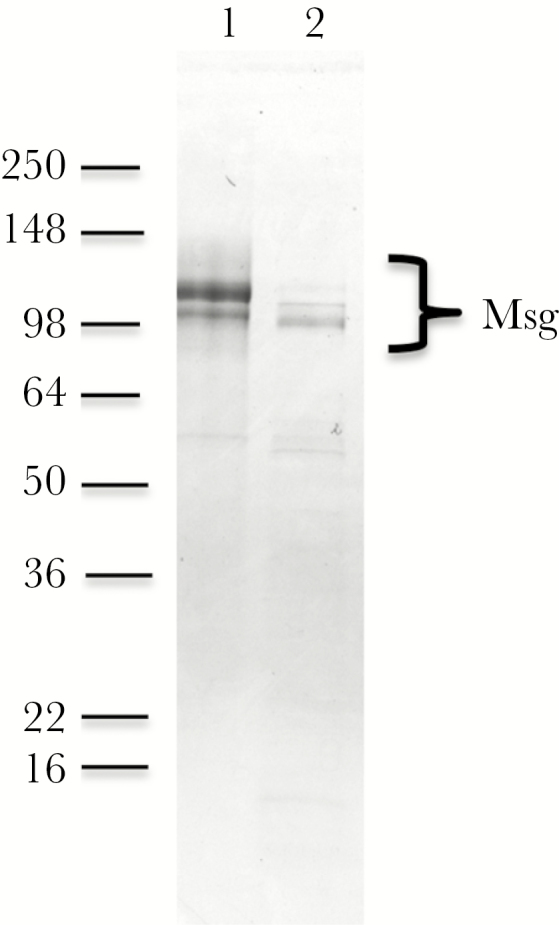
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of purified Pneumocystis murina major surface glycoprotein (Msg). Msg was purified by either SDS extraction or lyticase treatment followed by affinity purification using a concanavalin A column, as described in the methods, after which the samples were analyzed by SDS-PAGE and staining with Coomassie blue. Lane 1, SDS-Msg; lane 2, lyticase-Msg. Lyticase treatment leads to a small decrease in molecular weight of the major band, as previously reported [15].
In Vitro Stimulation and Flow Cytometry
Immature dendritic cells were plated in a 24-well plate (106 cells/well) and were incubated with the following: medium alone, lipopolysaccharide (LPS; 300 ng/mL; Sigma), SDS-Msg (2–5 µg/mL), or lyticase-Msg (2–5 µg/mL). The concentration of Msg used has previously been shown to induce proliferation of splenocytes of immunocompetent mice that had cleared Pneumocystis infection or that were immunized with crude Pneumocystis antigens. After 24 hours, the cells were collected and stained with Live/Dead Fixable Near-IR Dead Cell Stain Kit (Invitrogen) according to the manufacturer’s instructions. After washing with PBS, the cells were costained with phycoerythrin hamster anti-mouse CD11c and either allophycocyanin (APC) or fluorescein isothiocyanate (FITC) hamster anti-mouse CD80, APC or FITC rat anti-mouse CD86, APC or FITC rat anti-mouse CD40, or FITC mouse anti-mouse major histocompatibility complex (MHC) class II (I-Ab; all antibodies from BD Biosciences). Flow cytometry was performed using a FACSCanto BD instrument, and data were analyzed with FlowJo (Tree Star software). Experiments were repeated 4 times.
Cytokine Production
Dendritic cells were positively selected by magnetic separation using CD11c magnetic-activated cell-sorting (MACS) beads (Milteny Biotec). Briefly, after dendritic cells were generated by GM-CSF differentiation as described above, the cells were incubated with 100 µL of anti-mouse CD11c MicroBeads and 400 µL of MACS bovine serum albumin (BSA) solution for 15 minutes at 4oC. Samples were washed and magnetically purified using LS columns according to the manufacturer’s instructions. The purified CD11c+ cells were washed, resuspended in fresh medium, and cultured in a 24-well plate (106 cells/well) with the following: medium alone, LPS (300 ng/mL), crude P. murina antigen (20 µg/mL), β-1,3-glucan (300 ng/ml; β-1,3-glucan from Euglena gracilis; Sigma), SDS-Msg (5 µg/mL), or lyticase-Msg (5 µg/mL). Supernatants were collected after 48 hours and analyzed using the Proinflammatory Panel 1 (mouse) Kit (Meso Scale Discovery), according to the manufacturer’s instructions, for the quantitative determination of the following 10 cytokines: interleukin 12p70, tumor necrosis factor α, interferon γ, interleukin 1β, interleukin 2, interleukin 4, interleukin 5, interleukin 6, Cxcl1 (KC/GRO), and interleukin 10. The experiment was repeated 3 times; reported values are the average ± SD of duplicate wells.
Microarray Study
Immature dendritic cells were incubated in a 24-well plate (106 cells/well) in triplicate with the following: medium alone, LPS (500 ng/mL), SDS-Msg (5 µg/mL), or lyticase-Msg (5 µg/mL). Cells were collected after 24 hours, total RNA was extracted from each well by using the RNeasy Mini Kit (Qiagen) and was quantified using a NanoDrop spectrophotometer. The quality was measured by the Bioanalyzer system (Agilent Technologies), and gene expression was evaluated by microarray analysis, performed as previously reported using the Mouse Gene 1.0 ST Array (Affymetrix); each replicate was analyzed separately [18]. Ingenuity Pathway Analysis (Qiagen) was used to identify canonical pathways associated with genes that were differentially expressed, following incubation with LPS or Msg. The microarray data have been deposited in the National Center for Biotechnology Information (National Library of Medicine, Bethesda, MD) Gene Expression Omnibus (GEO; available at: http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE112229.
Lectin Binding Assays
Purified P. murina Msg (1 µg/mL) or P. carinii Msg (1 µg/mL) were coated in 0.1 M sodium carbonate buffer, pH 9.6, to duplicate wells in a 96-well plate at room temperature overnight. After washing 3 times (with 0.05% Tween 20 in PBS), the wells were blocked with 3% BSA-PBS for 2 hours at 37°C, incubated with mouse C-terminal 6-His tag MMR (10 µg/mL; R&D System) or human DC-SIGN Fc chimera (10 µg/mL; R&D System) in Tris buffer containing 10 mM CaCl2 for 2 hours at 37°C, and then washed. For competitive-binding studies, MMR and DC-SIGN Fc were preincubated for 30–40 minutes with 100 µg/mL of the following: mannan, mannose (Sigma), fucose, galactose, sialic acid, N-acetylglucosamine, or N-acetylgalactosamine (all from Vector Labs). The wells with MMR were incubated with anti-His tag biotinylated monoclonal antibody (R&D Systems) in binding buffer, and after 2 hours at room temperature, the wells were washed and incubated with streptavidin–horseradish peroxidase (HRP; R&D Systems; 1:200 in binding buffer) for 30 minutes at room temperature. The wells with DC-SIGN were incubated with Fc-specific, HRP-conjugated anti-human immunoglobulin G (IgG; Jackson ImmunoLabs) diluted 1:1000 in binding buffer. After wells were washed 3 times, 100 µL of substrate color reagents A and B (R&D Systems) were added to each well. After 20–30 minutes at room temperature, stop solution (2 N sulfuric acid; R&D Systems) was added, and the OD450 was read. Wells with no lectin receptor served as a background control. We verified that Msg was coated and able to bind lectins by using biotinylated concanavalin A (2 µg/mL; VectorLabs) followed by streptavidin-HRP (1:200 in binding buffer) and detection as described above.
Deglycosylation of Msg and Immunoblot Studies
P. murina lyticase-Msg and SDS-Msg were treated enzymatically to remove N-linked and O-linked glycans, using the Protein Deglycosylation Mix (New England BioLabs) according to the manufacturer’s instruction. Untreated and deglycosylated Msg were separated by 4%–20% SDS-PAGE gel (3 µg/lane), and stained with Coomassie blue or transferred to a nitrocellulose membrane (Invitrogen). After blocking with 5% milk in PBS, the membrane were incubated at 4oC overnight with MMR, DC-SIGN Fc, or biotinylated concanavalin A, followed by the same reagents as for the plate binding assays, except that bands were detected using SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific). The presence of Msg in each lane was verified after stripping the membrane and incubating with a hyperimmune mouse anti-P. murina Msg serum, followed by peroxidase-conjugated anti-mouse immunoglobulin G antibody (Jackson ImmunoResearch Laboratories).
Statistical Analysis
For the microarray study, selection criteria for the comparison of endotoxin or Msg exposed to unexposed control dendritic cells included an absolute log2 fold change of ≧1.0 and a P value of ≤.05. Because a minimal number of genes were selected for the Msg-exposed samples by using these criteria, we also examined those with an absolute log2 fold change of ≥0.4 and a P value of ≤.05. Microarray data were analyzed through the use of Ingenuity Pathway Analysis (Qiagen, Redwood City, CA). For comparison of cytokine production by different stimuli to medium alone and for lectin binding and inhibition studies, the ratio paired t test was used (GraphPad Prism, version 7.0). P values of <.05 were considered statistically significant.
RESULTS
Costimulatory Molecule Expression in Murine Dendritic Cells
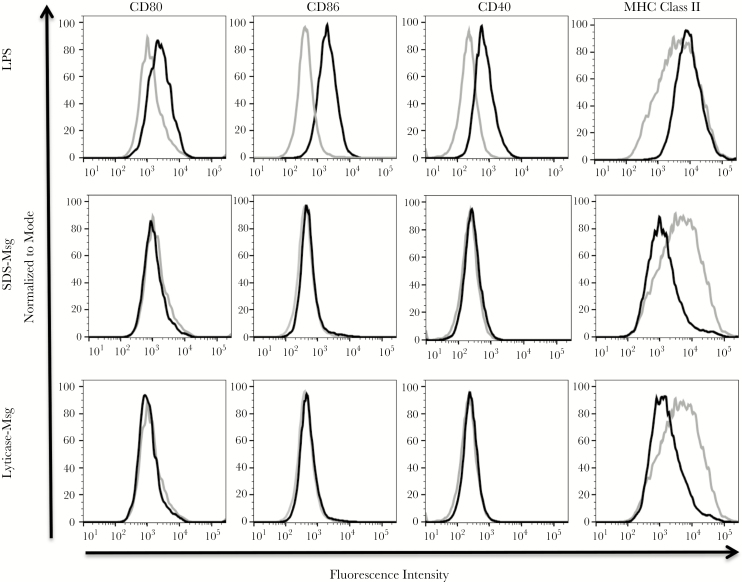
Using immature dendritic cells generated in vitro from bone marrow precursors, we first investigated the expression of costimulatory molecules CD80, CD86, CD40, and MHC class II following 24-hour incubation with LPS, SDS-Msg, or lyticase-Msg, compared with medium alone (Figure 2). The expression of surface activation markers increased upon LPS stimulation, which induced the maturation of dendritic cells. In contrast, neither Msg preparation induced an increase of any costimulatory molecule, with expression levels that were similar to untreated dendritic cells; no significant differences were seen (by the Mann-Whitney U test) in mean fluorescence intensity for any of the 4 markers when comparing unstimulated cells to either SDS-Msg– or lyticase-Msg–stimulated cells (Table 1).
Figure 2.
Flow cytometry of dendritic cell activation by lipopolysaccharide (LPS) and major surface glycoprotein (Msg). Immature mouse dendritic cells were generated in vitro, using granulocyte-macrophage colony-stimulating factor, and were either unstimulated or stimulated with LPS (300 ng/mL), sodium dodecyl sulfate (SDS)–Msg (5 µg/mL), or lyticase-Msg (5 µg/mL), for 24 hours. Dendritic cells were identified on the basis of CD11c expression, and the surface expression of CD80, CD86, CD40, and major histocompatibility complex (MHC) class II were measured by flow cytometry. The expression of activation markers was analyzed by FlowJo and compared to expression for the untreated-dendritic cells. The light gray line represents untreated dendritic cells, and the black line represents the stimulated population. The x-axis is fluorescence intensity, and the y-axis is normalized to mode, using FlowJo software. LPS (top row) led to increased expression of activation markers, while neither Msg preparation led to increased expression of any activation marker. Results are for 1 representative experiment of 4 experiments, all with similar results.
Table 1.
Expression Levels of Dendritic Cell Costimulatory Proteins Following Stimulation With Lipopolysaccharide (LPS) or Major Surface Glycoprotein (Msg)
| Condition | Dendritic Cell Protein Expression, MFI, Median (IQR) | |||
|---|---|---|---|---|
| CD80 | CD86 | CD40 | HLA-DR | |
| Unstimulated | 1648 (562–2059) | 532 (221–652) | 247 (169–2007) | 4647 (3528–6276) |
| LPS | 5134a (2986–6821) | 1480a (818–6.399) | 2071 (1011–2797) | 8722 (5300–34065) |
| SDS-Msg | 1642 (468–2.274) | 491 (223–502) | 282 (209–1869) | 2603 (1364–6614) |
| Lyticase-Msg | 1536 (410–2031) | 430 (206–495) | 274 (175–1900) | 3431 (1996–6512) |
Abbreviations: IQR, interquartile range; SDS, sodium dodecyl sulfate.
a P < .05 by the Mann-Whitney test, compared with unstimulated cells.
Cytokine Secretion
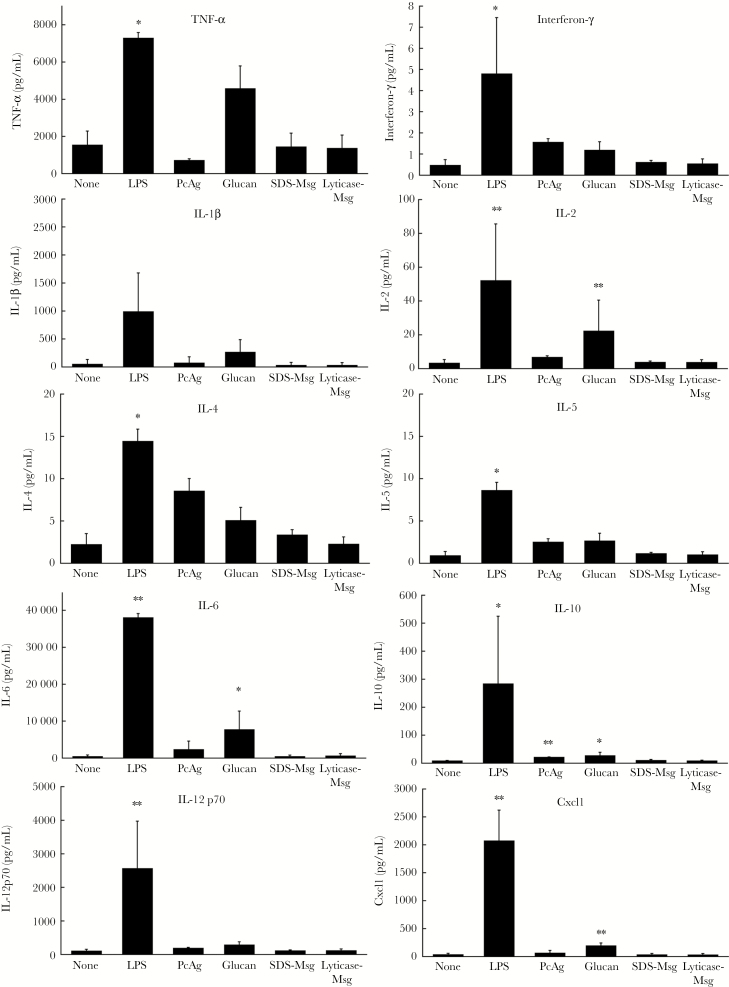
We next investigated the activation of immature dendritic cells by quantifying secretion of 10 different cytokines. Immature dendritic cells were magnetically purified using CD11c microbeads and then incubated for 48 hours with LPS, crude P. murina antigen, β-glucan, SDS-Msg, or lyticase-Msg. After 48 hours, we examined the cytokine levels in the supernatants by using a multiplex assay specific 10 different cytokines (Figure 3). After exposure to LPS, dendritic cells showed significantly increased secretion (P < .05) of nearly all tested cytokines, compared with unstimulated dendritic cells. β-glucan resulted in significantly increased secretion of 4 tested cytokines, although not to the same extent as LPS. Crude P. murina antigen, which contains a variety of different components derived from P. murina, including proteins and β-glucans, showed a significant increase only in interleukin 10 secretion, although there was a trend toward increased secretion for other cytokines. In contrast, dendritic cells exposed to either of the 2 Msg preparations showed no increase in secretion of any tested cytokine, with levels comparable to those of untreated dendritic cells.
Figure 3.
Cytokine production by dendritic cells following activation. Positively selected (CD11c+) immature dendritic cells (106 cells/well) were either unstimulated or stimulated with lipopolysaccharide (LPS; 300 ng/mL), crude Pneumocystis murina antigen (20 µg/mL), β-glucan (300 ng/mL), sodium dodecyl sulfate (SDS)–major surface glycoprotein (Msg; 5 µg/mL), or lyticase-Msg (5 µg/mL) for 48 hours. Supernatants were collected, and levels of 10 cytokines were analyzed as described in Methods. Significant increases were seen for multiple cytokines following stimulation with LPS and, to a lesser extent, with β-glucan but not with either of the Msg preparations. Graphed values are the average ± SD of 3 independent experiments. The ratio paired t test was used to compare each stimulated sample with the unstimulated control. *P < .05 and **P < .01. IL-1β, interleukin 1β; IL-2, interleukin 2; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IL-10, interleukin 10; IL-12p70, interleukin 12p70; TNF-α, tumor necrosis factor α.
Microarray Analysis
Given the lack of activation of dendritic cells following exposure to Msg, as determined by both flow cytometry and cytokine secretion, we further investigated the functional activation of dendritic cells by microarray analysis. When dendritic cells were incubated with endotoxin (500 ng/mL), there was robust activation, with significant upregulation of 703 genes and downregulation of 472 genes when using a P value of ≤.05 and an absolute log2 fold change of >1.0 as selection criteria; for 1025 of these genes, the P values were <10–5. Using the same selection criteria for Msg, only 1 gene (Serpinb3b, which encodes a protease inhibitor) was upregulated and 1 (Mterfd3, which encodes a protein that plays a role in mitochondrial transcription termination) was downregulated for lyticase-Msg, and none were upregulated and 2 (Mterfd3 and Gm16848, which encodes a region of the immunoglobulin κ chain) were downregulated for SDS-Msg. Use of less stringent selection criteria (ie, an absolute log2 fold change of ≥0.4), 115 and 79 genes were upregulated and downregulated, respectively (Supplementary Table 1). Using Ingenuity Pathway Analysis, the 5 top canonical pathways associated with LPS exposure, which included 17–31 genes per pathway, were all related to activation of innate immunity, with P values of <10–9. In contrast, the top canonical pathways for Msg identified when using the less stringent criteria, representing 1–3 genes per pathway, included diverse, nonimmune related functions, most with limited statistical support (Table 2).
Table 2.
Top 5 Canonical Pathways Identified by Ingenuity Pathways for the Dendritic Cell Genes Differentially Expressed Following Exposure to Lipopolysaccharide (LPS), Lyticase Major Surface Glycoprotein (Msg), or Sodium Dodecyl Sulfate (SDS)–Msg, as Determined by Microarray Analysis
| Canonical Pathway | Percentage Overlap | Selected Genes/Total in Pathway | P |
|---|---|---|---|
| LPS a | |||
| Role of pattern-recognition receptors in recognition of bacteria and viruses | 25.2 | 29/115 | 2.98 × 10–12 |
| Crosstalk between dendritic cells and natural killer cells | 30.9 | 21/68 | 4.71 × 10–11 |
| Dendritic cell maturation | 21.2 | 31/146 | 6.54 × 10–11 |
| Activation of interferon regulatory factor by cytosolic pattern-recognition receptors | 34.0 | 17/50 | 6.11 × 10-10 |
| Communication between innate and adaptive immune cells | 30.2 | 19/63 | 6.21 × 10-10 |
| Lyticase-Msg | |||
| Dolichyl-diphosphooligosaccharide biosynthesis | 10.0 | 1/10 | 3.26 × 10–2 |
| Role of tissue factor in cancer | 1.8 | 2/110 | 5.15 × 10–2 |
| Extrinsic prothrombin activation pathway | 6.2 | 1/16 | 5.17 × 10–2 |
| Chondroitin sulfate degradation (Metazoa) | 6.2 | 1/16 | 5.17 × 10–2 |
| Dermatan sulfate degradation (Metazoa) | 5.9 | 1/17 | 5.48 × 10–2 |
| SDS-Msg | |||
| Coagulation system | 8.6 | 3/35 | 5.65 × 10–6 |
| α-tocopherol degradation | 25.0 | 1/4 | 4.02 × 10–3 |
| Myo-inositol biosynthesis | 25.0 | 1/4 | 4.02 × 10–3 |
| Citrulline–nitric oxide cycle | 20.0 | 1/5 | 5.03 × 10–3 |
| LXR/RXR activation | 1.7 | 2/121 | 6.53 × 10–3 |
aGenes selected by the following criteria were included in the LPS analysis: an absolute log2 fold change of ≥1.0 and a P value of ≤ .05. Because very few genes were selected for either Msg preparation by using these criteria, genes selected using the following, less stringent, criteria were included in the Msg analysis: an absolute log2 fold change of ≥0.4 and a P value of ≤ .05.
Binding of CLRs to Msg
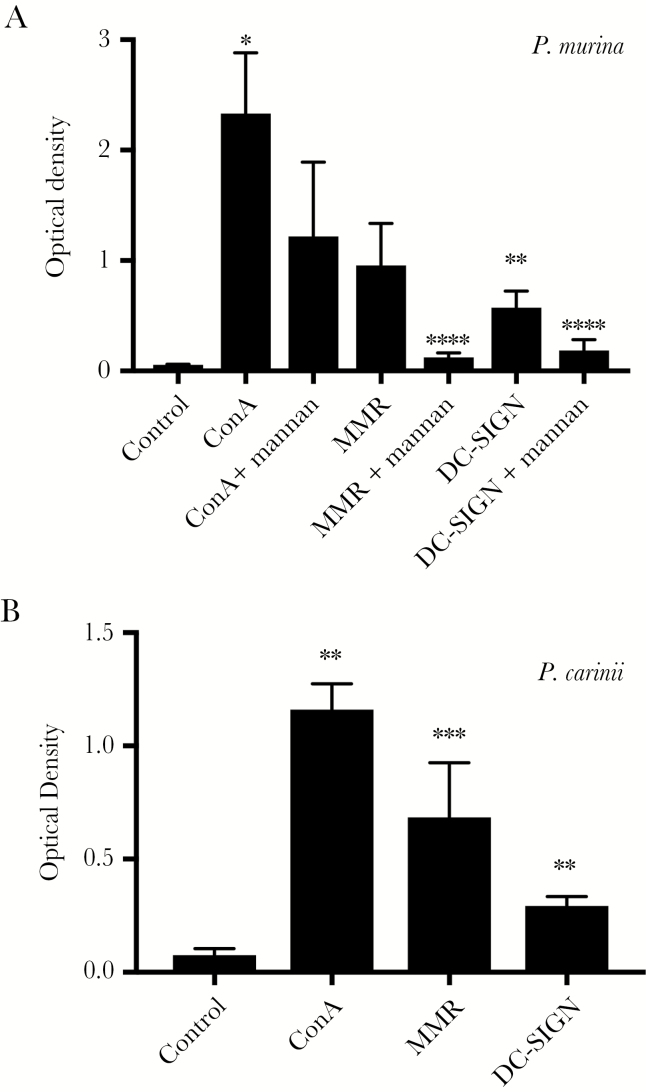
Since Msg is a mannoprotein and CLRs plays an important role in dendritic cell recognition of such proteins, we investigated the ability of mouse MMR and human DC-SIGN, which are expressed on the surface of dendritic cells, to bind to Msg, using an enzyme-linked immunosorbent assay (ELISA) format. We used human DC-SIGN rather than a mouse homologue since the function of human DC-SIGN, which includes recognition of high-mannose glycans, has been well characterized, while mice have 8 homologous genes, none of which appear to be true orthologs [29]. We initially verified that both P. murina Msg and P. carinii Msg bound to the plates, using biotinylated concanavalin A (Figure 4). Both MMR and DC-SIGN bound to P. murina Msg (Figure 4A), although MMR binding was of borderline significance (P = .053); binding of both was inhibited by mannan. In limited studies, other sugars, including, mannose, fucose, galactose, sialic acid, N-acetylglucosamine, and N-acetylgalactosamine, did not show consistent inhibition. Because the concanavalin A–purified P. murina Msg preparations may contain other Pneumocystis or host proteins, we repeated the binding experiments by using preparations of P. carinii Msg that were purified with a monoclonal antibody column. As shown in Figure 4B, MMR and DC-SIGN were both able to bind to rat Msg, although the ODs were in general lower than seen with P. murina Msg.
Figure 4.
Enzyme-linked immunosorbent assay (ELISA) evaluating CLR binding to major surface glycoprotein (Msg). Attachment of concanavalin A (ConA), mouse macrophage mannose receptor (MMR) or human DC-SIGN to Pneumocystis murina or Pneumocystis carinii Msg was evaluated by an ELISA format as described in Methods. Wells were coated with P. murina (top) or P. carinii (bottom) SDS-Msg (1 µg/mL of each) and, after blocking, were incubated with concanavalin A (ConA; 2 µg/mL), MMR (10 µg/mL), or DC-SIGN (10 µg/mL). For P. murina, inhibition with mannan (100 µg/mL) was evaluated in parallel wells. All 3 lectins bound to both Msgs, although the binding of MMR to P. murina was of borderline statistical significance (P = .053). Mannan significantly inhibited binding of both MMR and DC-SIGN to P. murina Msg. Results represent the mean of 2–4 individual experiments for each condition. The ratio paired t test was used to compare lectin binding to control (no lectin) and to compare samples incubated with mannan plus lectin to those incubated with lectin alone. *P < .05, **P < .01, and ***P < .001, for comparison of lectin to control; and ****P < .05, for comparison of lectin alone to lectin plus mannan.
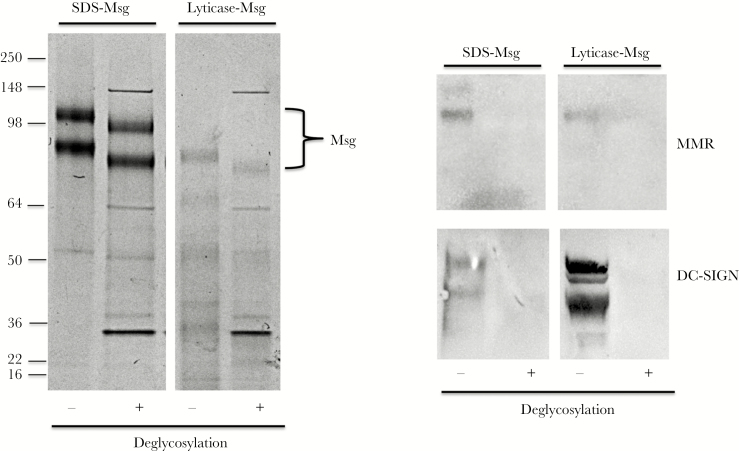
We next used immunoblots to verify that MMR and DC-SIGN were binding specifically to Msg and not to other potentially contaminating proteins. As shown in Figure 5, both MMR and DC-SIGN bound specifically to Msg. To further verify that these lectins were binding through carbohydrate moieties, we deglycosylated Msg by using a kit that deglycosylates both N- and O-linked glycans. Deglycosylation led to a small reduction in the molecular weight of Msg, as previously shown [15]. After deglycosylation, neither MMR nor DC-SIGN bound to Msg, demonstrating that both CLRs bound to carbohydrate moieties (Figure 5).
Figure 5.
Immunoblot and deglycosylation studies of C-type lectin receptor binding to major surface glycoprotein (Msg). Pneumocystis murina sodium dodecyl sulfate (SDS)–Msg and lyticase-Msg were deglycosylated as described in Methods, and untreated or deglycosylated preparations were separated by SDS polyacrylamide gel electrophoresis and stained with Coomassie blue (left) or transferred to a nitrocellulose membrane and incubated with mouse macrophage mannose receptor (MMR) or DC-SIGN followed by detection reagents (right). As seen in the left panel, deglycosylation leads to a small decrease in the apparent molecular weight of both Msg bands (indicated by the bracket). As seen in the right panel, both MMR and DC-SIGN bind to untreated Msg but no longer bind following deglycosylation.
DISCUSSION
In the current study, we demonstrated that P. murina Msg, the most abundant surface protein of Pneumocystis, does not activate murine bone marrow–derived dendritic cells. We used 2 different methods to purify Msg, with essentially identical results. Msg may thus play an important role in helping Pneumocystis evade host innate immune responses, which would be critical to helping the organism survive in its host environment; our recent genome-sequencing studies have suggested that the latter is the exclusive niche in which Pneumocystis exists and proliferates [19].
This lack of activation of dendritic cells was demonstrated using multiple approaches. No changes in surface expression of activation markers, including CD40, CD80, CD86, and MHC class II, were detected by flow cytometry; production of multiple inflammatory cytokines did not change following exposure to Msg; while, as expected, LPS, which served as a positive control, showed activation, based on both of these parameters. Moreover, microarray analysis of dendritic cells following LPS exposure demonstrated robust differential expression of >1000 genes, many of which are related to immune functions and serve as signatures of dendritic cell activation, while only 1 gene was upregulated and 2 were downregulated, using the same selection criteria following Msg exposure. Using less stringent selection criteria for the latter, more genes were selected, but Ingenuity Pathway Analysis did not identify immune-related gene sets.
Immune cells such as dendritic cells can recognize fungi through binding of surface receptors to pathogen-associated molecular patterns (PAMPs), molecular motifs that are conserved among classes of microorganisms. Common PAMPs of fungi include β-1,3 glucans, mannoproteins with high-mannose N-linked glycosylation, and chitin [30, 31]. We have recently shown that, in Pneumocystis, β-1,3 glucans, which are present in only the cyst form of the organism and not the more numerous trophic form, are largely masked by surface proteins, the most abundant of which is Msg [18]. Further, our Pneumocystis genome studies demonstrated loss of genes needed to synthesize or break down chitin, and absence of chitin was experimentally verified [19]. These genome studies also demonstrated loss of genes needed to synthesize the outer-chain mannans of high-mannose glycosylation, while those needed to synthesize the inner core structure were retained. Our current study demonstrates a potential benefit to Pneumocystis from the latter adaptation, since mannoproteins are key activators of dendritic cells. In Candida albicans, mutations that prevent outer-chain synthesis markedly diminish binding to and phagocytosis by dendritic cells, as well as interleukin 6 production [20]. Absence of outer-chain mannans at Msg glycosylation sites may thus explain the lack of activation of dendritic cells by Msg and contribute to evasion of host immune responses by Pneumocystis.
Although Msg does not activate dendritic cells, we were able to demonstrate binding of Msg to DC-SIGN and macrophage mannose receptor, 2 C-type lectins that are important in recognizing pathogen-specific mannoproteins and subsequently triggering activation of dendritic cells [32, 33]. By ELISA or immunoblot analysis, we demonstrated that this binding could be inhibited by mannan or by deglycosylating Msg, suggesting that the binding is through the lectin activity of these receptors. Presumably the lack of activation of dendritic cells results from absence of signal transduction following binding, potentially due to the reduced affinity of these lectins for Msg as compared to highly glycosylated mannoproteins.
One possible explanation for the lack of dendritic cell activation is that the solubilized Msg preparations are not accessible to dendritic cells. However, the same Msg preparation at the same concentration was able to stimulate proliferation in splenocytes from immunized animals following incubation with Msg (data not shown), clearly demonstrating accessibility to antigen-presenting cells.
We chose to examine Msg because it is the most abundant surface protein of Pneumocystis, based on SDS-PAGE analysis, and because native protein could be purified in sufficient quantities for conducting these studies. However, it is likely that other surface proteins of Pneumocystis that may be exposed to immune cells are similarly limited in the level of glycosylation, given the absence of glycosylation-related genes noted above. Thus, dendritic cell responses to Msg are likely representative of responses to other Pneumocystis surface proteins.
Multiple groups including ours have demonstrated that immunization with Msg can induce antibody and proliferative responses and that infection with Pneumocystis induces proliferation and cytokine production, including interleukin 17, by splenocytes (presumably CD4+ T cells) incubated with Msg, indicative of an adaptive cellular and humoral response to Msg [28, 34, 35]. Thus, some antigen-presenting cells must be able to process Msg appropriately. Additional studies are needed to better define the cells and mechanisms responsible for this aspect of the immune response.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Rene Costello, for providing animal care, and Peter Walzer and Michael Linke, for providing antibody RA-E7.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by the Intramural Research Program, National Institutes of Health (NIH) Clinical Center; National Cancer Institute, NIH (contract HHSN261200800001E).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA 2009; 301:2578–85. [DOI] [PubMed] [Google Scholar]
- 2. Maini R, Henderson KL, Sheridan EA, et al. Increasing Pneumocystis pneumonia, England, UK, 2000–2010. Emerg Infect Dis 2013; 19:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol 2007; 5:298–308. [DOI] [PubMed] [Google Scholar]
- 4. Roux A, Canet E, Valade S, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France. Emerg Infect Dis 2014; 20:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bienvenu AL, Traore K, Plekhanova I, Bouchrik M, Bossard C, Picot S. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis 2016; 46:11–7. [DOI] [PubMed] [Google Scholar]
- 6. Harmsen AG, Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med 1990; 172:937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roths JB, Sidman CL. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J Clin Invest 1992; 90:673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shellito J, Suzara VV, Blumenfeld W, Beck JM, Steger HJ, Ermak TH. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Invest 1990; 85:1686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Opata MM, Hollifield ML, Lund FE, et al. B lymphocytes are required during the early priming of CD4+ T cells for clearance of Pneumocystis infection in mice. J Immunol 2015; 195:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baharom F, Rankin G, Blomberg A, Smed-Sörensen A. Human lung mononuclear phagocytes in health and disease. Front Immunol 2017; 8:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Vliet SJ, Garcia-Vallejo JJ, van Kooyk Y. Dendritic cells and C-type lectin receptors: coupling innate to adaptive immune responses. Immunol Cell Biol 2008; 86:580–7. [DOI] [PubMed] [Google Scholar]
- 12. Carmona EM, Kottom TJ, Hebrink DM, et al. Glycosphingolipids mediate Pneumocystis cell wall beta-glucan activation of the IL-23/IL-17 axis in human dendritic cells. Am J Respir Cell Mol Biol 2012; 47:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carmona EM, Vassallo R, Vuk-Pavlovic Z, Standing JE, Kottom TJ, Limper AH. Pneumocystis cell wall beta-glucans induce dendritic cell costimulatory molecule expression and inflammatory activation through a Fas-Fas ligand mechanism. J Immunol 2006; 177:459–67. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi H, Worgall S, O’Connor TP, Crystal RG. Interaction of Pneumocystis carinii with dendritic cells and resulting host responses to P. carinii. J Immunother 2007; 30:54–63. [DOI] [PubMed] [Google Scholar]
- 15. Lundgren B, Lipschik GY, Kovacs JA. Purification and characterization of a major human Pneumocystis carinii surface antigen. J Clin Invest 1991; 87:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kovacs JA, Powell F, Edman JC, et al. Multiple genes encode the major surface glycoprotein of Pneumocystis carinii. J Biol Chem 1993; 268:6034–40. [PubMed] [Google Scholar]
- 17. Keely SP, Stringer JR. Complexity of the MSG gene family of Pneumocystis carinii. BMC Genomics 2009; 10:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kutty G, Davis AS, Ferreyra GA, et al. β-glucans are masked but contribute to pulmonary inflammation during Pneumocystis pneumonia. J Infect Dis 2016; 214:782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma L, Chen Z, Huang da W, et al. Genome analysis of three Pneumocystis species reveals adaptation mechanisms to life exclusively in mammalian hosts. Nat Commun 2016; 7:10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cambi A, Netea MG, Mora-Montes HM, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem 2008; 283:20590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ezekowitz RA, Williams DJ, Koziel H, et al. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature 1991; 351:155–8. [DOI] [PubMed] [Google Scholar]
- 22. Tachado SD, Zhang J, Zhu J, Patel N, Cushion M, Koziel H. Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2. J Leukoc Biol 2007; 81:205–11. [DOI] [PubMed] [Google Scholar]
- 23. Swain SD, Lee SJ, Nussenzweig MC, Harmsen AG. Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo. Infect Immun 2003; 71:6213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drummond RA, Gaffen SL, Hise AG, Brown GD. Innate defense against fungal pathogens. Cold Spring Harb Perspect Med 2015; 5:a019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 1999; 223:77–92. [DOI] [PubMed] [Google Scholar]
- 26. Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J, Wainstok R. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J Immunol 2003; 171:5940–7. [DOI] [PubMed] [Google Scholar]
- 27. Liu S, Tobias R, McClure S, Styba G, Shi Q, Jackowski G. Removal of endotoxin from recombinant protein preparations. Clin Biochem 1997; 30:455–63. [DOI] [PubMed] [Google Scholar]
- 28. Bishop LR, Helman D, Kovacs JA. Discordant antibody and cellular responses to Pneumocystis major surface glycoprotein variants in mice. BMC Immunol 2012; 13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garcia-Vallejo JJ, van Kooyk Y. The physiological role of DC-SIGN: a tale of mice and men. Trends Immunol 2013; 34:482–6. [DOI] [PubMed] [Google Scholar]
- 30. Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol 2012; 13:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elieh Ali Komi D, Sharma L, Dela Cruz CS. Chitin and its effects on inflammatory and immune responses. Clin Rev Allergy Immunol 2018; 54:213–23. doi:10.1007/s12016-017-8600-0. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cambi A, Gijzen K, de Vries lJ, et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol 2003; 33:532–8. [DOI] [PubMed] [Google Scholar]
- 33. Hole C, Wormley FL Jr. Innate host defenses against Cryptococcus neoformans. J Microbiol 2016; 54:202–11. [DOI] [PubMed] [Google Scholar]
- 34. Gigliotti F, Wiley JA, Harmsen AG. Immunization with Pneumocystis carinii gpA is immunogenic but not protective in a mouse model of P. carinii pneumonia. Infect Immun 1998; 66:3179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Theus SA, Smulian AG, Steele P, Linke MJ, Walzer PD. Immunization with the major surface glycoprotein of Pneumocystis carinii elicits a protective response. Vaccine 1998; 16:1149–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.