Abstract
Objectives
The literature about the frequency of metabolic syndrome in patients with multinodular goitre and a new onset of metabolic syndrome after total thyroidectomy is limited. The aim of this study was to investigate the effects of total thyroidectomy and thyroid hormones on a new onset of metabolic syndrome in patients who underwent total thyroidectomy and have received thyroid hormone replacement.
Material and Methods
Fifty-nine patients who underwent total thyroidectomy for multinodular goitre were included in this prospective study. Patients’ height, weight, and waist circumference were measured, and the body mass index was calculated. Peripheral blood samples were obtained preoperatively and at the 12th and 24th month after total thyroidectomy to examine the lipid profile, glucose homeostasis, and thyroid function tests.
Results
The lipid profile and blood pressure parameters deteriorated, and the mean body mass index and waist circumference with the metabolic syndrome rates significantly increased at the 12th and 24th months follow-up. Preoperative body mass index (Exp[B] 1.60; p=0.003) was independently associated with metabolic syndrome at the 2nd year after total thyroidectomyin a multivariate regression analysis.
Conclusion
The frequency and severity of MetS is high in adult patients with non-toxic multinodular goitre after total thyroidectomy. The frequency of metabolic syndrome increased in patients with a high body mass index after total thyroidectomy.
Keywords: Metabolic syndrome, total thyroidectomy, subclinical hypothyroidism, body mass index
INTRODUCTION
In the United States, according to the Centers for Disease Control and Prevention report on the National Hospital Discharge Survey 2010, surgical procedures involving thyroid disorders (303.000/year) rank second among the most frequently performed surgical procedures (1). The surgical practice has changed in favor of total thyroidectomy (TT) for the management of benign nodular disease in the last decade. The adherents of TT emphasize its potential advantages of a lower risk for recurrent nodular disease and a lower rate of thyroidectomy for incidental thyroid carcinoma (2–4). On the other hand, when the nodular disease relapse is considered as the primary endpoint in the treatment of multinodular goitre (MNG), two current prospective randomized studies have reported extremely good results with the Dunhill procedure (hemithyroidectomy plus subtotal resection) and demonstrated the safety and efficacy of leaving a small remnant of thyroid tissue without rendering the patient to total thyroid ablation (5, 6).
The prevention of metabolic complications after TT is as important as surgery-related complications, and it necessitates a close follow-up and treatment. In general, thyroid hormones regulate the basal metabolism, and by acting directly on carbohydrate and lipid metabolism, they determine the basal metabolic rate and energy expenditure, thus regulating thermogenesis (7–9). Thyroid hormones influence cardiac contractility, the heart rate, and systemic vascular resistance. Triiodothyronine (T3) via its direct effect on the vascular smooth muscle cells leads to vascular relaxation (10). Thyroid hormones increase the activity of lipoprotein-lipase with eventual degradation and utilization of triglyceride substrates. Severe hypothyroidism is usually associated with an increased serum concentration of total cholesterol, low-density lipoprotein-cholesterol (LDL-C), and atherogenic lipoprotein profile represented by increased triglyceride (TGC) and decreased high-density lipoprotein-cholesterol (HDL-C) levels (11, 12).
With its growing importance, metabolic syndrome (MetS) is an epidemic public health issue (13). The frequency varies by country, but it ranges between 15% and 30% in Europe (14). The main components of MetS are systemic disorders such as insulin resistance-mediated abdominal obesity, glucose intolerance or diabetes mellitus (DM), and dyslipidemia and hypertension. Thyroid hormones and thyroid dysfunction play an active role in nearly all of these components (15). The effect of hypothyroidism on MetS is unclear, and the impact of total thyroidectomy has not been previously examined. In this respect, the thyroxine (T4) replacement therapy to prevent hypothyroidism and to obviate MetS after total TT is a major concern in surgical practice.
In this study, considering the side-effects of hypothyroidism mentioned above, we investigated the TT and thyroid hormons effects on a new onset of MetS in patients who underwent TT and have received a thyroid hormone replacement therapy. In addition, we aimed to draw attention to the new onset metabolic derangements following the operation.
MATERIAL AND METHODS
Patients and study protocol
This prospective study included patients with MNG who had been treated with TT. Sixty-five patients were allocated between October 2011 and July 2014 at the Department of University of Health Sciences, Izmir Bozyaka Research and Training Hospital, Department of General Surgery. The study protocol was approved by the local ethical committee. Informed consent was obtained from all patients. Six patients were excluded from the study for failing to attend regular visits. Analysis of the study was based on the laboratory, demographic and anthropometric data obtained during the preoperative period and at 1 and 2 years after the operation. The T4 replacement therapy was arranged to keep the thyroid-stimulating hormone (TSH) level within the normal range. It was considered that the patients with a TSH value below 4.24 mIU/mL at the 1st and 2nd year after TT had received effective treatment.
Patients’ height (H), weight (W), and waist circumference (WC) were measured and recorded, and the body mass index (BMI) was calculated to assess the patients’ body fat. A BMI from 20 to 25, from 25 to 30, and over 30 is considered normal, overweight, and obese, respectively. The definition of MetS is based on the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (16). The metabolic syndrome severity score (MetSSS) was calculated using the calculator available at http://publichealth.hsc.wvu.edu/biostatistics/metabolic-syndrome-severity-calculator (17). Patients were considered hypertensive based on double high reading of a systolic blood pressure (SBP) ≥140 mm Hg and/or a diastolic blood pressure (DBP) ≥90 mmHg (18). A clinical diagnosis of hypertension is established if the patient has already been under a treatment program or complied with the above criteria.
The criteria for the diagnosis of diabetes are based on the American Diabetes Association guidelines. Thus, patients with fasting plasma glucose level (FPG) ≥126 mg/dL (7.0 mmol/L) or HbA1C ≥6.5%, and those with a previous diagnosis of diabetes and/or regularly using antidiabetic drugs were considered to have overt diabetes (19). The homeostatic model assessment (HOMA-IR) test was used to assess the insulin resistance. A fasting insulin test less than 10 IU/mL was considered optimal. The test was calculated by the following formula: HOMA-IR=(fasting insulin [mU/l]xfasting plasma glucose [mg/dL])/405.
The diagnosis of atherogenic dyslipidemia (AD) required elevated TGC ≥150 mg/dL and low concentrations of HDL-C (<40 mg/dL in men and <50mg/dL in women) in a routine lipoprotein profile (20, 21).
Postoperative subclinical hypothyroidism is defined as an elevated TSH (above the upper limit of reference range) with normal FT4 and FT3 concentrations. Normal ranges for TSH, FT3, and FT4 were 0.41–4.24 mIU/mL, 2.5–3.9 pg/mL, and 0.61–1.06 ng/dL, respectively.
Peripheral blood samples were obtained after 12 hours of fasting, and low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C), total cholesterol (TC), triglycerides (TGC), fasting blood glucose (FBG), fasting insulin (FI), thyroid stimulating hormone (TSH), free T3 (FT3), free T4 (FT4), highly sensitive C-reactive protein (hsCRP), and hemoglobin A1c (HbA1c) levels were analyzed. Patients were invited to the hospital for 12- and 24-month visits, and the previous steps were repeated. The data and concomitant medication of the patients were recorded.
Laboratory Technology
A Roche cobas c 311 photometric analyzer was used for the measurement of substrates (albumin, total protein, Urea/Bun), enzymes (ALP, ALT, AST, GGT), the cardiovascular risk profile (TC, HDL-C, LDL-C, TGC), and hsCRP. The UniCel DxI 800; Beckman CoulterNyon, Switzerland, system was used for the determination of the FT3, FT4, and TSH levels. Applied FT3 and FT4 assays were paramagnetic particles; chemiluminescent immunoassays for the quantitative determination of free thyroxine and T3 levels in human serum and plasma. A hypersensitive hTSH/fast TSH Reagent (third-generation assay) made up of paramagnetic particles coated with goat anti-mouse IgG/anti-hTSH MAb complexes was used for the TSH measurement. The analytical sensitivity of the assay was 0.003 μIU/mL.
Statistical Analysis
Data were expressed as the mean±standard deviation of the mean. Proportions were compared by x2 analysis. The mean values of two groups (with or without MetS) were compared by the Student’s T-test or by a nonparametric test if the data were not normally distributed. Comparisons of parameters with more than 2 groups (preoperative and postoperative 12th and 24th month values) were analyzed by the analysis of variance. Stepwise logistic regression analysis was used to determine independent predictors of MetS. p<0.05 was considered statistically significant. All statistical analyses were performed using the SPSS, version 15 (SPSS Inc.; Chicago, IL, USA).
Compared to patients with preexisting MetS before the operation, we assumed a 10% increase at the end of the 1st year after total thyroidectomy. Thus, determining the statistical power as 0.8 and alpha error level of 5%, we found that the required number of patients was 136. In our study, the percentage of patients with metabolic syndrome increased by approximately 12% in the first year after surgery. However, this analysis was based on 59 patients, and the statistical power was 0.64.
RESULTS
The mean age of the patients was 51.9±12.7 (23–75) years. The mean BMI and the average WC were 28.3±4.21 kg/m2 (20.7–40.7) and 100.2±10.1 (76–123) cm. The mean systolic and diastolic BP were 120.2±11.4 mmHg (100–150) and 76.8±8.6 mmHg (60–90), respectively.
There was a female preponderance (50 pts. or 83.3% for females vs. 9 pts. or 16.6% for males for MNG). With respect to pre-existing (preoperative) co-morbid diseases, 18 patients had hypertension (30.5%), nine had DM (15.3%), three had coronary artery disease (5.1%), and three had hyperlipidemia (5.1%). Of the hypertensive patients, 8.5% was on ss-a blocker treatment (n=5), 15.3% were using angiotensin-converting-enzyme inhibitors or angiotensin II receptor antagonists (n=9), 8.5% of them received thiazide diuretics (n=5), and 5.1% were using calcium-channel blockers (n=3). Seven patients with diabetes were using oral anti-diabetic drugs, and 2 were on insulin treatment.
The mean TSH, FT3, and FT4 values were 1.58±2.72 mIU/mL (0.01–19), 3.11±0.41 pg/mL (2.54–4.69) and 0.84±0.16 ng/dL (0.43–1.30). All but 1 patient had normal FT3 and FT4 values before the operation. A female patient presented with a FT4 level of 1.3 ng/dL and TSH value of 0.06 mIU/mL.
The indication for surgery was compressive symptoms causing neck discomfort, progressive growth of the dominant nodule or recurrence of cysts after aspiration in all, and additionally nodule(s) suspicious for malignancy in 2 patients. The final pathological diagnosis was MNG in 49 patients (83%), MNG with micropapillary carcinoma in 8 cases (13.6%), and MNG with papillary thyroid carcinoma in 2 (3.4%) patients. Postoperative replacement therapy with L-thyroxine is not implemented in any case until the final pathology result is obtained. This corresponded to an average period of 2 weeks.
Fifty-seven patients completed the 1st year, and 42 cases already completed the 2nd year controls, and the results were statistically evaluated.
Comparison of Pre-Operative Period vs. Post-Operative 1st Year
A preoperative TSH value of 1.58±2.72 μIU/mL increased to 2.13±3.64 (p=0.06), and preoperative FT4 value of 0.84±0.16 ng/dL improved significantly to 0.99±0.20 ng/dL (p<0.001) during the postoperative 12 months, indicating the adequacy of thyroxine replacement therapy. Indeed, the evaluation of the 12-month laboratory results revealed normal FT4 levels in all cases (Table 1).
Table 1.
Comparison of laboratory and metabolic parameters in patients according to the follow-up period
| Pre-operative (N:59) (Mean±SD) | Post-operative 12 Months (N:58) (Mean±SD) | Post-operative 24 Months (N:42) (Mean±SD) | p | |
|---|---|---|---|---|
| Free T3 (pg/mL) (N:2.5–3.9) | 3.11±0.40 | 2.84±0.32 | 3.35±4.42 | 0.02 |
| Free T4 (ng/dL) (N:0.6–1.1) | 0.84±0.16 | 0.99±0.20 | 1.02±0.23 | <0.001 |
| TSH (mIU/mL) (N:0.4–4.24) | 1.58±2.72 | 2.13±3.64 | 4.63±7.46 | 0.06 |
| Subclinical hypothyroid patient rate (%) | 10 | 19 | 31 | 0.46 |
| Fasting glucose (mg/dL) | 109±31 | 108±25 | 109±28 | 0.93 |
| HOMA-IR | 3.24±3.93 | 2.89±3.58 | 3.08±4.63 | 0.74 |
| HgbA1c (%) | 5.8±0.7 | 6.0±0.6 | 5.8±0.6 | 0.4 |
| hsCRP (mg/dL) | 2.55±3.51 | 0.55±0.62 | 2.72±3.78 | <0.001 |
| Total cholesterol (mg/dL) | 190±31 | 208±36 | 216±34 | 0.179 |
| HDL-C (mg/dL) | 54±12 | 53±11 | 54±13 | 0.37 |
| LDL-C (mg/dL) | 118±26 | 139±28 | 143±28 | 0.002 |
| Triglyceride (mg/dL) | 116±53 | 146±88 | 149±77 | 0.004 |
| Systolic BP (mmHg) | 118±11 | 128±13 | 130±12 | 0.001 |
| Diastolic BP (mmHg) | 75±8.8 | 83±11 | 80±10 | 0.01 |
| Weight (kg) | 75±12 | 78±12 | 79±12 | 0.001 |
| BMI (kg/m2) | 28.2±4.2 | 30.0±4.2 | 30.3±4.3 | 0.001 |
| Waist circumference (mm) | 100±10 | 105±11 | 106±11 | <0.001 |
| Obesity (%) | 34 | 39.7 | 47.7 | 0.78 |
| Metabolic syndrome (%) | 39 | 51.7 | 61.9 | 0.01 |
| Metabolic syndrome severity score | 0.92±0.75 | 0.96±0.60 | 1.04±0.72 | 0.85 |
SD: standard deviation; TSH: thyroid stimulanting hormone; HDL-C: high-density lipoproteins cholesterol; LDL-C: low-density lipoprotein cholesterol; BP: blood pressure; BMI: body mass index
Preoperative values of BMI, WC, and the ratio of patients with metabolic syndrome increased significantly statistically during the 1st year after the operation. With respect to BMI, 15 patients (25.4%) were normal weight, 24 (40.6%) were overweight, and 20 (34%) were obese before the operation. At the 1st year follow-up, the number of patients with normal weight markedly decreased, and the number of obese patients increased (Figure 1).
Figure 1.
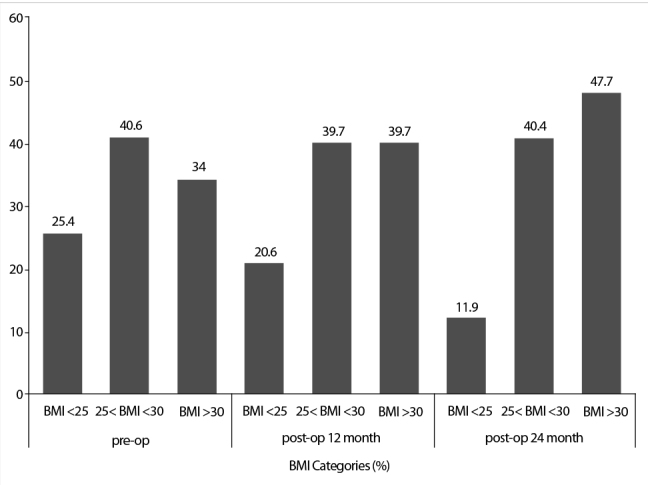
Body mass index (BMI) change over time
Similarly, SBP and DBP increased, and the TG and LDL values worsened significantly (Table 1). In addition, 1 patient had newly diagnosed hypertension, and 2 cases manifested with uncontrolled hypertension requiring a second drug. At the first year, 11 patients (19%) had TSH values higher than normal (>4.24 ml/mL). The comparison of patients with normal and high TSH levels at 1 year after the operation revealed no statistically significant difference in terms of the proportion of cases with MetS (55% vs. 36%; p=0.265), the mean BMI (30±5 vs. 29±4; p=0.44), and mean WC (105±12cm vs. 103±11cm; p=0.52). At the postoperative first year, the number of patients with MetS was 30 (51.7%) and their MetSSS was 0.96±0.60. Seven of them were newly diagnosed MetS (Figure 2).
Figure 2.
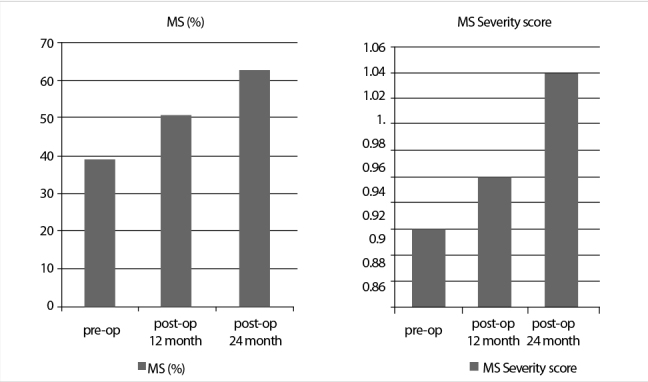
Metabolic syndrome and the severity score change over time
The metabolic syndrome severity score of patients with former MetS (23 pts.) increased from 1.05±0.76 to 1.13±0.67; p=0.56. Compared to other patients, patients with MetS were in elderly, with higher baseline BMI and more often had a history of diabetes and hypertension, but they did not have significant difference with regard to the thyroid function test values (Table 2). Among the variables such as age, gender, the TSH level, basal BMI, history of DM and hypertension; the presence of DM (Exp[B] 14.34; p=0.03) and hypertension (Exp[B] 6.57; p=0.02) independently associated with the development of MetS in multivariate logistic regression analysis.
Table 2.
Comparison of patients with or without metabolic syndrome in three periods
| Pre-op MS(+) [(n:23),39%] (Mean±SD) | Pre-op MS(−) [(N:36),61%] (Mean±SD) | p | 12 months MS (+) [(n:30),51.7%] (Mean±SD) | 12 months MS (−) [(n:28),48.3%] (Mean±SD) | P | 24 months MS (+) [(n:26),61.9%] (Mean±SD) | 24 months MS(−) [(n:16),38.1%] (Mean±SD) | P | |
|---|---|---|---|---|---|---|---|---|---|
| Age* (years) | 57±10 | 49±13 | 0.01 | 56±10 | 48±13 | 0.01 | 58±10 | 49±14 | 0.02 |
| Gender (female/male) | 19/4 | 31/5 | 0.72 | 26/4 | 23/5 | 0.64 | 23/3 | 13/3 | 0.52 |
| BMI* (kg/m2) (basal) | 30.5±4.3 | 26.8±3.5 | 0.001 | 29.8±3.96 | 26.7±3.78 | 0.004 | 30.9±3.89 | 25.7±3.10 | <0.001 |
| BMI* (kg/m2) (Last control) | 30.6±4.13 | 27.5±3.88 | 0.005 | 31.9±3.8 | 27.1±4.0 | <0.001 | |||
| Obesity# (basal) | 11(47.8) | 9(25) | 0.08 | 13 (43) | 7 (25) | 0.14 | 16 (62) | 2(13) | 0.001 |
| Obesity# (Last control) | 14(46.6) | 9(32) | 0.26 | 17(65) | 3(19) | 0.002 | |||
| Hypertansion# | 14(60.5) | 4(11,1) | <0,001 | 16(53) | 3(11) | 0.001 | 12(46) | 2(12.5) | 0.01 |
| Hyperlipidemia# | 3(11.5) | 0(0) | 0.08 | 10(33.3) | 0(0) | 0.08 | 3(11.5) | 0(0) | 0.08 |
| Diabetes# | 6(26) | 3(8.3) | 0.09 | 8(27) | 1(4) | 0.01 | 8(30.7) | 1 (6.2) | 0.03 |
| HOMA-IR* | 4. 20±5.69 | 2.45±3.61 | 0.154 | 3.91±5.42 | 2.37±3.46 | 0.20 | 3.82±4.33 | 1.60±0.61 | 0.08 |
| Fasting Glucose* (mg/dL) | 116±31 | 99±23 | 0.01 | 114±31 | 98±20 | 0.02 | 116±33 | 97±9.4 | 0.009 |
| HbA1c* (%) | 6.08±0.87 | 5.62±0.68 | 0.02 | 6.0±0.7 | 5.5±0.3 | 0.01 | 5.97±0.62 | 5.39±0.41 | 0.002 |
| HsCRP* (mg/dL) | 1.36±1.99 | 1.81±3.16 | 0.546 | 2.20±3.50 | 1.08±1.53 | 0.12 | 2.56±3.12 | 3.20±3.58 | 0.54 |
| FT3* (pg/mL) | 2.98±0.30 | 3.18±0.44 | 0.06 | 2.89±0.35 | 2.91±0.31 | 0.12 | 3.70±5.55 | 2.73±0.51 | 0.49 |
| FT4* (ng/dL) | 0.86±0.18 | 0.81±0.13 | 0.25 | 1.05±0.21 | 0.93±0.16 | 0.19 | 1.01±0.27 | 1.03±0.17 | 0.78 |
| TSH* (mIU/mL) | 2.22±3.97 | 1.10±1.21 | 0.20 | 1.95±3.03 | 4.78±1.95 | 0.10 | 6.59±8.54 | 1.36±2.97 | 0.007 |
| Hypothyroid Patients Rate# | 3(11.5) | 3(8.3) | 0.56 | 4(13) | 7(25) | 0.27 | 12 (46) | 1(6) | 0.002 |
Pre-op: preoperative; MS: metabolic syndrome; SD: standard deviation; FT3: free-T3; FT4: free-T4;
TSH: thyroid stimulanting hormone; BMI: body mass index
Datas are presented as
mean±SD
n (%)
Comparison of Pre-Operatıve vs. Post-Operative 2nd Year
Forty-two patients have completed the 2nd year after BTT. Of the patients, the mean TSH, FT4, and FT3 values were 4.63±7.46 mIU/mL, 1.02±0.23 ng/dL, and 3.35±4.42pg/mL., respectively. The lipid profile and blood pressure parameters deteriorated, and the mean BMI and WC with MetS rates significantly increased at the 2nd year follow-up of patients (Table 1). The increase in the MetS rates was accompanied by an increase in MetSSS (Figure 2).
Subclinic hypothyroidism was observed in 13 cases (30.9%) at the 24th month after surgery. Eight of them (61.5%) had obesity, and 12 (92%) had MetS. Of the remaining 29 patients with normal TSH values, 12 (41%) were obese, and 14 (48%) had developed MetS. The difference between the MetS rates of patients with normal and high TSH levels was statistically significant (92% vs. 48%; p=0.001).
Those who had MetS were elderly, often had subclinic hypothyroidism, a higher basal and surveillance BMI, and had more frequently DM and hypertension (Table 2). Among the variables such as age, gender, baseline BMI, the history of DM and hypertension, and the TSH level; the baseline BMI (Exp[B] 1.60; p=0.003) independently associated with MetS in multivariate logistic regression analysis was identified.
DISCUSSION
In this study; despite the LT4 replacement therapy in patients who underwent TT, we observed worsening of metabolic parameters and an increase in the prevalence of MetS during the 2-year follow-up period. Indeed, the incidence of MetS increased from 39% to 52% at the postoperative 1st year and rose up to 62% at the 2nd year follow-up with simultaneous increase in MetSSS. Moreover, MetS rate rose to 92% in patients with the TSH level >4.2 mIU/mL at the 2nd year control.
The relationship between thyroid dysfunction and MetS has been shown in several studies. In these patients, the most common thyroid dysfunction is subclinical hypothyroidism (22–24). However, the literature about the frequency of MetS in patients with multinodular goitre is limited. In a study of 1422 Caucasian patients with thyroid disease, the presence of multinodular non-toxic goitre independently predicted the development of MetS (25). In our study, metabolic syndrome was present in 39% of patients referred for surgery because of MNG. More importantly, we detected a significant increase in the rate and severity of MetS after the operation. This indicates the importance of the choice of operation (total or subtotal thyroidectomy) and a close follow-up in these patients with high cardiovascular risk.
In our study group, we observed a significant increase in the prevalence of MetS at the 1st year follow-up despite the LT-4 replacement therapy. Several factors may be responsible for this process. First of all, keeping the target TSH levels lower for these specific patients can lead to a reduction in the development and progression of MetS. A significant relationship between the prevalence of MetS and the TSH levels existed in a large-scale cohort study including 7270 patients and by offsetting a normal TSH value between 0.35 and 1.38 mIU/mL; the prevalence of MetS increased 1.25-fold in patients with the TSH levels between 2.42 and 3.44 mIU/mL), and the MetS risk is increased 1.92-fold in cases with TSH>4.48 mIU/mL (26). In our study, the average TSH value at 1 year was in the normal range (2.13±3.64 mIU/mL); however, it was higher than the reference TSH value of the previously mentioned study. Relatively high TSH values may contribute to the deterioration of metabolic profile of patients after TT.
Two important features of this study were the progression of central obesity reflected by elevated body weight, BMI, and WC after surgery and the increasing rate of patients with MetS (from 39% to 51.7%) at the postoperative 1st year (Table 1). The FT3 levels significantly decreased within the 1st year following the operation but improved thereafter. In our opinion, the most rationalistic explanation of the increase in WC appears to be diminished 3.3’-triiodothyronine-responsive energy expenditure and corresponding slow basal metabolic rate in these patients with the FT3 hormone levels within the lower limit of normal value (27, 28). It is very-well known that thyroid hormones affect the synthesis and particularly the degradation of lipids. Thus, both the utilization of lipid substrates and the mobilization of triglycerides stored in the adipose tissue should be reduced in these patients (29). Lipid disorders in our series were consistent with a Colorado study, which was reflected by the increased mean total cholesterol and low-density lipoprotein cholesterol levels. In the Colorado study, impaired lipid profile was observed particularly in subjects with subclinical hypothyroidism (TSH values between 5.1 and 10 mIU/L); however, in our study, a progressively deteriorating TC and LDL profile was present in the clear majority of patients independently from the TSH levels (30). Similarly, in a separate study, total thyroidectomy seriously deteriorated the lipid profile. By an effective thyroxine replacement, the patients have become euthyroid at least for 3 months after TT. However, compared with the the preoperative serum total cholesterol levels, the deteriorated TC values after TT remained unchanged at the 6th month follow-up (31). What measures to be taken to counteract the cardiometabolic adverse effects of impaired lipid profile and increased (W, BMI, WC) is a matter of concern in TT patients. An association between subclinical hypothyroidism and an increased risk of the coronary heart disease mortality has been shown particularly in those with a TSH level of 10 mIU/L or greater (32). None of the patients with hypertension and coronary heart disease required cardiac intervention or experienced myocardial infarction in our series. This may be attributed to shorter follow-up despite a high (31%) prevalance of subclinic hypothyroidism (13 cases at the 24th month after TT).
This study provides important data of various biochemical parameters and body composition and their effects in patients undergoing TT. However, it lacks a control group in terms of an optimal medical treatment after surgery; therefore, a controlled study is necessary to confirm our hypothesis that TT renders patients to metabolic complications that could not be managed by the levothyroxine treatment alone. This analysis is based on 59 patients, and the statistical power is 0.64. Although the statistical power is low, our results were noteworthy that especially preoperative BMI was statistically significant in predicting the postoperative MetS.
CONCLUSION
MetS is a major problem in adult patients with non-toxic multinodular goiter undergoing surgery. In these patients, an increased frequency of MetS can be observed even after the administration of levothyroxine replacement therapy following TT. Given the increased frequency of MetS after surgery in patients with preexisting DM, hypertension, and high BMI, if possible, a subtotal thyroidectomy and/or alternative thyroid replacement therapy may be considered.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (amended in October 2013).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - İ.Z., A.U.; Design - A.U.; Supervision - V.S., A.U.; Materials - B.Z., G.O.; Data Collection and/or Processing - B.Z., G.O., A.A.; Analysis and/or Interpretation - A.D. E.T.; Literature Search - E.T., A.U.; Writing Manuscript - İ.Z., A.U., E.T.; Critical Reviews - V.S., E.T.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.FASTSTATS – Inpatient Surgery. Available from: http://www.cdc.gov/nchs/fastats/insurg.htm.
- 2.Barczyński M, Konturek A, Stopa M, Cichoń S, Richter P, Nowak W. Total thyroidectomy for benign thyroid disease: is it really worthwhile? Ann Surg. 2011;254:724–729. doi: 10.1097/SLA.0b013e3182360118. [DOI] [PubMed] [Google Scholar]
- 3.Tezelman S, Borucu I, Senyurek Giles Y, Tunca F, Terzioglu T. The change in surgical practice from subtotal to near-total or total thyroidectomy in the treatment of patients with benign multinodular goiter. World J Surg. 2009;33:400–405. doi: 10.1007/s00268-008-9808-1. [DOI] [PubMed] [Google Scholar]
- 4.Musholt TJ. Total thyroidectomy for multinodular goiter. Chirurg. 2010;81:603–606. 608–611. doi: 10.1007/s00104-009-1880-z. [DOI] [PubMed] [Google Scholar]
- 5.Rayes N, Steinmüller T, Schröder S, Klötzler A, Bertram H, Denecke T, et al. Bilateral subtotal thyroidectomy versus hemithyroidectomy plus subtotal resection (Dunhill procedure) for benign goiter: long-term results of a prospective, randomized study. World J Surg. 2013;37:84–90. doi: 10.1007/s00268-012-1793-8. [DOI] [PubMed] [Google Scholar]
- 6.Sancho JJ, Prieto R, Due-as JP, Ribera C, Ripollés J, Larrad A, et al. A randomized trial of hemithyroidectomy versus Dunhill for the surgical management of asymmetrical multinodular goiter. Ann Surg. 2012;256:846–851. doi: 10.1097/SLA.0b013e318272df62. [DOI] [PubMed] [Google Scholar]
- 7.Bernal J, Refetoff S. The action of thyroid hormone. Clin Endocrinol (Oxf) 1977;6:227–249. doi: 10.1111/j.1365-2265.1977.tb03319.x. [DOI] [PubMed] [Google Scholar]
- 8.Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord. 2000;24( Suppl 2):109–112. doi: 10.1038/sj.ijo.0801292. [DOI] [PubMed] [Google Scholar]
- 9.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–144. doi: 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- 10.Klein I, Ojamaa K. Thyroid hormone targeting the vascular smooth muscle cell. Circ Res. 2001;88:260–261. doi: 10.1161/01.RES.88.3.260. [DOI] [PubMed] [Google Scholar]
- 11.Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab. 2012;97:326–333. doi: 10.1210/jc.2011-2532. [DOI] [PubMed] [Google Scholar]
- 12.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 13.Poyrazoglu S, Bas F, Darendeliler F. Metabolic syndrome in young people. Curr Opin Endocrinol Diabetes Obes. 2014;21:56–63. doi: 10.1097/01.med.0000436414.90240.2c. [DOI] [PubMed] [Google Scholar]
- 14.Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K DECODE Study Group. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066–1076. doi: 10.1001/archinte.164.10.1066. [DOI] [PubMed] [Google Scholar]
- 15.Iwen KA, Schröder E, Brabant G. Thyroid hormones and the metabolic syndrome. Eur Thyroid J. 2013;2:83–92. doi: 10.1159/000351249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism. 2014;63:218–225. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36:11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 21.Quispe R, Manalac RJ, Faridi KF, Blaha MJ, Toth PP, Kulkarni KR, et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: The Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis. 2015;242:243–250. doi: 10.1016/j.atherosclerosis.2015.06.057. [DOI] [PubMed] [Google Scholar]
- 22.Meher LK, Raveendranathan SK, Kota SK, Sarangi J, Jali SN. Prevalence of hypothyroidism in patients with metabolic syndrome. Thyroid Res Pract. 2013;10:60–64. doi: 10.4103/0973-0354.110583. [DOI] [Google Scholar]
- 23.Lai CC, Tang SH, Pei D, Lai CC, Chen YL, Wu CZ, et al. The prevalence of subclinical thyroid dysfunction and its association with metabolic syndrome in Taiwanese elderly. Int J Gerontol. 2011;5:25–29. doi: 10.1016/j.ijge.2011.01.005. [DOI] [Google Scholar]
- 24.Uzunlulu M, Yorulmaz E, Oguz A. Prevalence of subclinical hypothyroidism in patients with metabolic syndrome. Endocr J. 2007;54:71–76. doi: 10.1507/endocrj.K06-124. [DOI] [PubMed] [Google Scholar]
- 25.Rendina D, De Filippo G, Mossetti G, Zampa G, Muscariello R, Benvenuto G, et al. Relationship between metabolic syndrome and multinodular non-toxic goiter in an inpatient population from a geographic area with moderate iodine deficiency. J Endocrinol Invest. 2012;35:407–412. doi: 10.3275/7842. [DOI] [PubMed] [Google Scholar]
- 26.Lee YK, Kim JE, Oh HJ, Park KS, Kim SK, Park SW, et al. Serum TSH level in healthy Koreans and the association of TSH with serum lipid concentration and metabolic syndrome. Korean J Intern Med. 2011;26:432–439. doi: 10.3904/kjim.2011.26.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–144. doi: 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- 28.Weetman AP. Whose thyroid hormone replacement is it anyway? Clin Endocrinol (Oxf) 2006;64:231–233. doi: 10.1111/j.1365-2265.2006.02478.x. [DOI] [PubMed] [Google Scholar]
- 29.Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord. 2000;24( Suppl 2):109–112. doi: 10.1038/sj.ijo.0801292. [DOI] [PubMed] [Google Scholar]
- 30.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 31.Erbil Y, Özbey N, Giriş M, Salmaslıoğlu A, Özarmağan S, Tezelman S. Effects of thyroxine replacement on lipid profile and endothelial function after thyroidectomy. Br J Surg. 2007;94:1485–1490. doi: 10.1002/bjs.5915. [DOI] [PubMed] [Google Scholar]
- 32.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. for the Thyroid Studies. Subclinical Hypothyroidism and the Risk of Coronary Heart Disease and Mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]


