Abstract
Purpose
To evaluate the prevalence of novel candidate Sjogren’s Syndrome (SS) autoantibodies (salivary protein-1 (SP-1), parotid secretory protein (PSP), carbonic anhydrase 6 (CA-6)), in the DRy Eye Assessment and Management (DREAM©) cohort, a study evaluating the effectiveness of omega-3 fatty acid supplements for the treatment of dry eye.
Methods
Participants underwent ocular surface examinations and serological testing for traditional and novel SS autoantibodies. DREAM© participants were categorized into the following 3 groups: 1) no history of SS or other autoimmune disease and negative traditional SS autoantibodies (n=352); 2) no history of SS but a history of other autoimmune disease (n=66); and 3) those who met the 2012 American College of Rheumatology SS classification criteria (n=52).
Results
Eleven percent had a history of SS and 6% of those without a history of SS most likely had undiagnosed SS. The SS group had a higher prevalence of SP-1 autoantibodies than the group without SS or other autoimmune disease (33% vs. 19%; p=0.02), but had no difference in CA-6 (p=0.31) or PSP autoantibodies (p=0.33). Participants who were positive for the traditional autoantibodies alone, or positive for both traditional and novel autoantibodies had the highest scores for corneal (p=0.002) and conjunctival staining (p<0.001).
Conclusion
Data from this multi-center, prospective study demonstrated that one of the novel candidate autoantibodies, SP-1, is associated with underlying SS and that novel autoantibodies may be associated with worse ocular surface disease. Future longitudinal studies are needed to evaluate their utility in screening dry eye patients for SS.
Keywords: Sjogren’s syndrome, novel antibodies, dry eye
Introduction
Sjogren’s syndrome (SS) is one of the most common autoimmune diseases, affecting 2–4 million people in the United States alone.1,2 SS is characterized by lymphocytic infiltration of the exocrine glands, e.g. lacrimal glands and salivary glands, leading to symptoms of dry eye and dry mouth. Patients with SS also have increased autoantibody production and a higher risk of lymphoma.3 Signs and symptoms of SS span the domains of ophthalmology, endocrinology, and rheumatology and the diagnostic criteria are complex.4–11 The diversity of signs and symptoms are barriers to early diagnosis and many patients with SS are undiagnosed.4, 12, 13 Early detection of SS is important so that treatments can be implemented that may relieve symptoms and also to enable monitoring for systemic complications. In addition, patients who are started on a biological agent treatment within the first 5 years of disease onset may be more likely to respond to treatment than those with delayed initiation of therapy.14–16
More specific and sensitive markers for SS are needed to allow for earlier diagnosis and timely management of patients. The traditional autoantibodies to Sjogren’s Syndrome related antigen A (SSA/Ro) and Sjogren’s Syndrome related antigen B (SSB/La) 1, 17 are present in only 50–70% of SS patients.17, 18 In addition, because traditional SS autoantibodies appear late in the course of disease,19 patients with early SS are often negative for these antibodies, thereby contributing to delays in diagnosis.
Recently, the novel autoantibodies salivary protein 1 (SP-1), carbonic anhydrase 6 (CA-6), and parotid secretory protein (PSP) have been identified as early markers of disease in a mouse model of SS.20 However, there are limited data regarding the expression and clinical significance of these antibodies in humans. These novel markers have been shown to be present in some dry eye patients with or without SS in a few small studies21–25, but studies examining the prevalence of these antibodies in large, well-characterized cohorts are needed to understand the clinical significance of these autoantibodies. In addition, information on how the expression of these autoantibodies changes over time is needed.
The Dry Eye Assessment and Management (DREAM©) Study is a multi-center clinical trial funded by the National Eye Institute, National Institute of Health to examine the efficacy and safety of oral omega-3 fatty acid supplement for the treatment of dry eye. Both dry eye patients with SS and patients without SS were enrolled in DREAM©. The data from the DREAM© Study present a unique opportunity to assess the prevalence of these novel candidate SS autoantibodies and any associated ocular surface phenotypic features in a well-characterized cohort of SS and non-SS dry eye patients.
Methods
Subjects
The DREAM© Study was a prospective, randomized, double-masked, superiority clinical trial (clinicaltrials.gov: NCT02128763) involving an active supplement group and a placebo group. Participants were enrolled from 27 centers in 17 states throughout the United States. Institutional Review Board (IRB)/Ethics Committee approval was obtained. In addition, the study adhered to the tenets of the Declaration of Helsinki and was HIPAA-compliant. After written informed consent was obtained, participants who had at least one eye meeting the DREAM© criteria for dry eye were enrolled. Inclusion criteria included age greater than or equal to 18 years; dry eye related ocular symptoms for at least 6 months before the screening visit and the use or desire to use artificial tears on average 2 times per day in the 2 weeks prior to the screening visit. Participants also had to demonstrate the presence of at least 2 of the 4 following signs in the same eye at the screening visit and eligibility confirmation visits: 1) Conjunctival staining present ≥ 1 (out of possible score of 6 per eye); 2) Corneal fluorescein staining present ≥ 4 (out of a possible score of 15 per eye); 3) Tear break up time (TBUT) ≤ 7 seconds; and 4) anesthetized Schirmer’s test ≥ 1 to ≤ 7 mm/5min. In addition, participants had to report symptoms of dry eye with an Ocular Surface Disease Index (OSDI) score of at least 25 (≥ 25 to ≤ 80) at the screening visit and at least 21 (≥ 21 to ≤ 80) at the baseline randomization visit. Finally, participants had to demonstrate compliance with taking placebo softgels as directed during a two-week run-in period (≥ 90% taken, by pill count).
Major exclusion criteria included the following: the presence of acute allergic conjunctivitis, infection, or inflammation; history of ocular herpes keratitis; ocular surgery within 6 months; history of previous LASIK or other corneal surgery; use of glaucoma medication or history of filtering surgery for glaucoma; eyelid abnormalities or extensive ocular surface scarring; anticoagulation therapy; contact lens wear within 30 days of screening visit; current use of EPA/DHA supplements greater than 1200 mg/day; and history of allergy to ingredients of supplements (active or placebo).
During the eligibility confirmation visit, clinical coordinators asked patients about their medical history, including specific items on SS and rheumatoid arthritis. Participants provided information regarding diagnoses of other autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus, fibromyalgia, CREST(calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome, antiphospholipid syndrome, Raynaud’s disease, scleroderma, graft-versus-host-disease (GVHD), and sarcoidosis) when answering review of systems questions. Patients provided a 4–10 cc blood sample that was sent to a central laboratory for masked analysis of traditional and novel SS autoantibodies (Sjo test, Immco Diagnostics, Inc. (Buffalo, NY)). A standard Enzyme Linked Immunosorbent Assay (ELISA) for antibodies to SS antigens was used to detect immunoglobulin G (IgG), immunoglobulin A (IgA) and immunoglobulin M (IgM) antibodies in the human serum extract reactive to recombinant SP-1, CA-6 and PSP proteins expressed and purified from E. Coli. Results were expressed in ELISA units per milliliter (EU/ml) and were reported as positive or negative as defined by the manufacturer.25 Results of testing were made available to the patient and the treating physician after they exited the DREAM© Study.
Designation of Sjogren’s syndrome status
We used the 2012 American College of Rheumatology (ACR) criteria for SS 10 as the basis for classifying DREAM patients. The ACR criteria require that at least 2 of the following 3 criteria be met: 1) positive for the traditional SS antibodies (positive for SSA or positive for SSB or (positive for rheumatoid factor and ANA ≥1:320)); 2) ocular staining system (OSS) score from the cornea and conjunctiva of 3 or more in the worse eye, 3) labial salivary gland biopsy exhibiting focal lymphocytic sialadenitis with a focus score of 1 focus/4mm2. Labial salivary gland biopsy results were not available for DREAM patients. The OSS was not used in the DREAM study, however, for each eye, the corneal fluorescein staining score (NEI scale; scores 0 to 15) was added to the conjunctival lissamine green staining score (modified Oxford scale; scores 0 to 6). We estimated that a total sum of corneal and conjunctival staining of 3 or more was equivalent to an OSS score of 3 or more. DREAM patients were classified as: 1) Group 1 (Control group): those with an autoantibody profile that did not fulfill ACR criteria and without a reported history of SS or other autoimmune disease; 2) Group 2: those with an antibody profile that did not meet ACR criteria, without a reported history of SS but with a history of other autoimmune disease; and 3) Group 3: those with an antibody profile that met ACR criteria and with a score of ≥3 on DREAM ocular surface staining tests(SS group).
Data Analysis
The primary analysis compared the SS group (Group 3) and the control group (Group 1) on the baseline characteristics and prevalence of each of the novel autoantibodies using the two-sample t-test for means and the Fisher exact test for proportions. Secondary analyses compared the autoimmune disease group (Group 2) and the control group for their baseline characteristics and prevalence of antibodies.
To evaluate whether SS antibodies were associated with more severe dry eye disease, dry eye signs and symptoms were compared among the following 4 groups of participants based on their traditional and novel autoantibody status: 1) positive for the traditional autoantibodies only; 2) positive for the novel autoantibodies only; 3) positive for both traditional and novel autoantibodies; and 4) negative for both traditional and novel autoantibodies. All statistical analyses were performed in SAS v9.4 (SAS Institute Inc., Cary, NC) and a p-value <0.05 was considered statistically significant.
Results
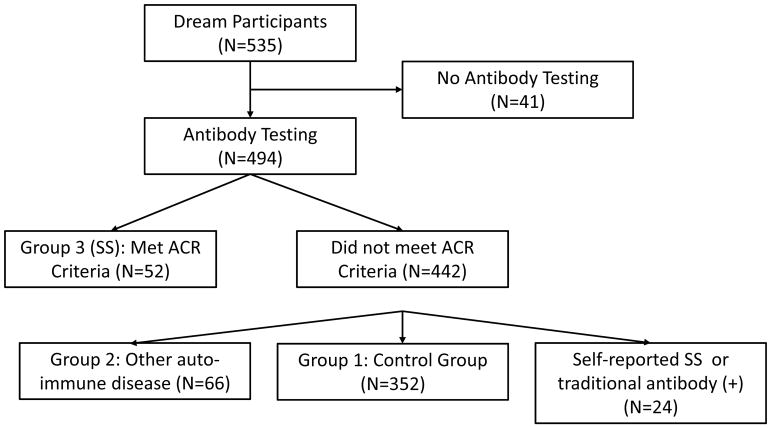
Among 535 patients randomized into the DREAM© study, 494 had antibody testing (Figure 1). Antibody testing was not performed when a licensed phlebotomist was unavailable during the patient visit, the patient refused, or the appropriate shipping materials were not available. Among those with antibody testing, 52 (10.5%) patients met the ACR criteria for inclusion in Group 3 with SS, 66 (13.4%)reported an autoimmune disease to qualify for Group 2, and 352 (71.3%) reported no history of SS or autoimmune disease and were included in the control group (Group 1). Twenty-four patients (4.9%) either reported a history of SS or had an antibody profile meeting ACR SS criteria, but did not meet the full ACR criteria and were considered indeterminate.
Figure 1.
Flow chart for the analysis of DREAM© Study participants regarding Sjogren’s syndrome (SS) and autoantibody testing
Baseline characteristics
The baseline characteristics of the participants with SS (Group 3) compared to those without SS or other autoimmune disease (Group 1) are shown in Table 1. SS participants were predominantly female (92%) and predominantly Caucasian. There was no significant difference in mean OSDI score between these two groups. However, the 4 key signs of dry eye, tear break-up, Schirmer test, and corneal and conjunctival staining, were significantly worse in SS participants than in those without SS or other autoimmune disease (all p≤0.02). Also, SS participants used artificial tears (p=0.004) or ointments (p=0.01) more frequently than those without SS or other autoimmune disease participants.
Table 1.
Baseline characteristics of DREAM© study participants with or without Sjogren’s syndrome (SS)
| Baseline Characteristics | Group 1: No SS and no other autoimmune disease* (n=352) | Group 3: SS (N=52)** | P-value |
|---|---|---|---|
| Age (years): Mean (SD) | 58.8 (13.6) | 56.6 (12.5) | 0.91 |
| Gender: Male (%) | 76 (22%) | 4 (8%) | 0.02 |
| Race | 0.63 | ||
| Caucasian | 274 (78%) | 42 (81%) | |
| African-American | 39 (11%) | 4 (8%) | |
| Asian | 13 (4%) | 1 (2%) | |
| American Indian or Alaskan Native | 2 (1%) | 0 (0%) | |
| Mixed | 4 (1%) | 2 (4%) | |
| Unknown | 20 (6%) | 3 (6%) | |
| OSDI score: mean (SD) | 41.4 (15.1) | 42.8 (15.6) | 0.55 |
| Tear Break-up Time (seconds): mean (SD) ┼ | 2.7 (1.4) | 2.2 (1.1) | 0.001 |
| Schirmer test (mm): mean (SD)┼ | 8.4 (6.3) | 6.3 (5.3) | 0.02 |
| Fluorescein staining of cornea: mean (SD) ┼ | 4.2 (2.9) | 6.0 (3.6) | 0.001 |
| Lissamine green staining of conjunctiva: mean (SD)┼ | 3.2 (1.4) | 4.4 (1.5) | <0.0001 |
| Frequency of using artificial tears or gel use in last week | 0.004 | ||
| 0 | 80 (23%) | 5 (10%) | |
| 1–2 times | 160 (45%) | 19 (37%) | |
| 3–4 times | 65 (19%) | 11 (21%) | |
| 5–10 times | 32 (9%) | 11 (21%) | |
| Greater than 10 times | 15 (4%) | 6 (12%) | |
| Regularly use lubricating ointment (%) | 29 (8%) | 10 (19%) | 0.01 |
| Ever used steroid eye drops or ointment (%) | 74 (21%) | 17 (33%) | 0.06 |
Group 1: Participants without a history of SS or other autoimmune disease, and negative in SSA, SSB, and not positive for both RF and ANA
Group 3: Participants who would likely have met the 2012 American College of Rheumatology SS classification criteria based on serology and ocular surface staining
SD= Standard deviation; SS=Sjogren’s syndrome
From the worse eye of a specific ocular dry eye measurement
Novel candidate SS Antibodies
Participants with SS had a higher prevalence (46%) of expressing at least 1 novel autoantibody compared to those without SS or other autoimmune disease (31%; p=0.02)(Table 2). In particular, SS participants had a higher prevalence (33%) of SP-1 autoantibodies than those without SS or other autoimmune disease (19%; p=0.02). However there was no significant difference in prevalence of CA-6 autoantibodies (21% versus 15%; p=0.31) or in PSP autoantibodies (9.4% versus 13.5%, p= 0.33).
Table 2.
Antibody testing results of DREAM© Study participants with or without Sjogren’s syndrome (SS)
| Baseline Antibodies | Group 1: No SS and no other autoimmune disease* (n=352) | Group 3: SS ** (N=52) | Fisher exact P-value*** |
|---|---|---|---|
| Traditional SS antibodies: | |||
| SS-A(Ro) >25 EU/ml | 0 (0.0%) | 48 (92.3%) | |
| SS-B(La) >25 EU/ml | 0 (0.0%) | 15 (28.9%) | |
| Positive tests for SS-A(Ro) and SS-B(La) | |||
| 0 | 352 (100%) | 2 (3.9%) | |
| 1 | 0 (0.0%) | 37 (71.2%) | |
| 2 | 0 (0.0%) | 13 (25.0%) | |
| Anti-nuclear antibody ≥1:320§ | 13 (3.7%) | 23 (44.2%) | |
| Rheumatoid factor: any positive§ | 81 (23.1%) | 32 (61.5%) | |
| Positive tests for traditional antibodies | |||
| 0 | 258 (73.3%) | 0 (0.0%) | |
| 1 | 94 (26.7%) | 14 (26.9%) | |
| 2 | 0 (0.0%) | 20 (38.5%) | |
| 3 | 0 (0.0%) | 8 (15.4%) | |
| 4 | 0 (0.0%) | 10 (19.2%) | |
| Novel SS antibodies§: | |||
| Salivary protein 1 (SP-1): any positive | 65 (18.5%) | 17 (32.7%) | 0.02 |
| Carbonic anhydrase VI (CA VI): any positive | 53 (15.1%) | 11 (21.2%) | 0.31 |
| Parotid specific protein (PSP): any positive | 33 (9.4%) | 7 (13.5%) | 0.33 |
| Positive in any novel antibody | 107 (30.5%) | 24 (46.2%) | 0.02 |
| Positive tests for novel antibodies | 0.03┼ | ||
| 0 | 244 (69.3%) | 28 (53.9%) | |
| 1 | 66 (18.8%) | 16 (30.8%) | |
| 2 | 38 (10.8%) | 5 (9.6%) | |
| 3 | 3 (0.9%) | 3 (5.8%) |
Group 1: Participants without a history of SS or other autoimmune disease, and negative in SSA, SSB, and not positive for both RF and ANA)
Group 3: Participants who would likely have met the 2012 ACR SS classification criteria based on serology and ocular surface staining
No p-values provided for traditional SS antibodies as these values were used to define the two comparison groups.
For test of linear trend.
One patient in the non-SS group had missing data for antibody testing.
Comparison by antibody groups
Among 4 groups based on testing results of the traditional and novel candidate SS autoantibodies, demographic and ocular characteristics were similar (Table 3). Participants who were positive for the traditional autoantibodies alone, or positive for both the traditional and novel autoantibodies had the highest scores for corneal staining (p=0.002) and conjunctival staining (p<0.001) (Table 3).
Table 3.
Comparison of demographics, dry eye symptoms, signs and treatment among Sjogren’s syndrome (SS) autoantibody groups of participants in the DREAM© Study*
| Demographics | Negative for traditional and novel SS antibodies (n=214) | Positive for novel SS antibodies only (n=85) | Positive for traditional SS antibodies only (n=86) | Positive for traditional and novel SS antibodies (n=63) | P-value |
|---|---|---|---|---|---|
| Age (years); mean (SD) | 58.8 (13.3) | 59.0 (12.7) | 58.6 (12.7) | 59.5 (15.3) | 0.98 |
| Gender: male (%) | 45 (21.0%) | 16 (18.8%) | 17 (19.8%) | 8 (12.7%) | 0.53 |
| Race, n(%) | 0.03 | ||||
| Caucasian | 169 (79.0%) | 67 (78.8%) | 54 (62.8%) | 53 (84.1%) | |
| African-American | 19 (8.9%) | 13 (15.3%) | 13 (15.1%) | 9 (14.3%) | |
| Asian | 7 (3.3%) | 1 (1.2%) | 8 (9.3%) | 0 (0.0%) | |
| American Indian or Alaskan Native | 2 (0.9%) | 0 (0.0%) | 1 (1.2%) | 0 (0.0%) | |
| Mixed | 3 (1.4%) | 2 (2.4%) | 2 (2.3%) | 1 (1.6%) | |
| Unknown | 14 (6.5%) | 2 (2.4%) | 8 (9.3%) | 3 (3.9%) | |
| OSDI score: mean (SD) | 42.1 (15.0) | 42.7 (16.6) | 38.8 (14.6) | 42.6 (15.3) | 0.30 |
| Tear break-up time (seconds): mean (SD) ┼ | 2.8 (1.4) | 2.7 (1.4) | 2.6 (1.4) | 2.3 (0.8) | 0.09 |
| Schirmer test (mm): mean (SD)┼ | 8.5 (6.5) | 8.0 (6.0) | 7.4 (5.7) | 7.6 (6.3) | 0.49 |
| Staining of cornea: mean (SD) ┼ | 4.1 (2.9) | 4.3 (2.8) | 4.9 (3.2) | 5.7 (3.6) | 0.002 |
| Staining of conjunctiva: mean (SD)┼ | 3.0 (1.4) | 3.1 (1.5) | 4.0 (1.5) | 3.7 (1.6) | <0.001 |
| Frequency of using artificial tears or gel use in last week, n(%) | 0.70 | ||||
| 0 | 49 (22.9%) | 18 (21.2%) | 20 (23.3%) | 11 (17.5%) | |
| 1–2 times | 83 (38.8%) | 43 (50.6%) | 34 (39.5%) | 30 (47.6%) | |
| 3–4 times | 47 (22.0%) | 12 (14.1%) | 20 (23.3%) | 14 (22.2%) | |
| 5–10 times | 23 (10.8%) | 10 (11.8%) | 6 (7.0%) | 5 (7.9%) | |
| Greater than 10 times | 12 (5.6%) | 2 (2.4%) | 7 (7.0%) | 3 (4.8%) | |
| Regularly use lubricating ointment, n(%) | 16 (7.5%) | 14 (16.5%) | 12 (14.0%) | 7 (11.1%) | 0.11 |
| Ever used steroid eye drops or ointment, n(%) | 36 (16.8%) | 23 (27.1%) | 23 (26.7%) | 19 (30.2%) | 0.047 |
One participant without novel antibody results was excluded.
SD= Standard deviation. SS=Sjogren’s syndrome.
From the worse eye of a specific ocular dry eye measurement.
Comparison of non-SS patients with or without other autoimmune disease
In a secondary analysis, the baseline characteristics and the autoantibody status of 66 participants without SS but with a history of another autoimmune disease (Group 2) were examined and compared to those without SS or other autoimmune disease (Group 1). (Supplemental Digital Content: Tables 1 and 2). Approximately half (53%) of participants with another autoimmune disease (Group 2) reported having ongoing rheumatoid arthritis. Participants in both of these groups (Groups 1 and 2) were of similar age and gender. There was a higher proportion of African-American patients in Group 2 than in Group 1 (21% vs. 11%, p=0.04). The mean OSDI score was similar between groups (p=0.39). The mean score for each of the signs of dry eye was worse in Group 2 (those with other autoimmune disease) than in Group 1 (no SS or other autoimmune disease), but none of the differences was statistically significant (all p≥0.08) (Supplemental Digital Content: Table 1). There was a significantly higher prevalence of autoantibodies to CA-6 (25% vs. 15%; p=0.047) and PSP (18% vs. 9%; p=0.049) in Group 1 compared to Group 2. However, there was no difference in the prevalence of anti-SP-1 antibodies (Group 1: 19% vs. Group 2: 23%; p=0.4)(Supplemental Digital Content: Table 2).
Correlation between traditional and novel SS antibodies
Among all participants (Groups 1–3) who had testing for each autoantibody (n=492), there was a weak correlation (Pearson correlation coefficient =0.17; p=0.0002) between the number of participants who were traditional autoantibody positive and the number who were novel autoantibody positive (Supplemental Digital Content: Table 3).
Discussion
In the present study, we found that SS participants had a significantly higher prevalence of SP-1 autoantibodies compared to those without SS or other autoimmune disease. However, the prevalence of the novel autoantibodies in both SS patients and non-SS participants in our study differed from previous reports.20–22, 24–26 There are a few possible explanations for the different prevalence rates of the novel autoantibodies in our study compared to previously published studies in both SS and non-SS dry eye patients. Factors such as differences in the classification criteria used to define SS, duration of disease, as well as age, gender, and/or race and ethnicity could account for the varying prevalence rates seen across studies.
We also found that participants who were positive for the traditional SS autoantibodies alone, or for both traditional and novel autoantibodies, had worse corneal and conjunctival staining than those who were not positive for any of these autoantibodies. These novel autoantibodies may be a marker of more severe ocular surface disease in those positive for the traditional SS antibodies. Future longitudinal studies are needed to examine the time course for both traditional and novel SS autoantibodies and to determine whether or not ocular surface damage progresses more quickly among those with specific subtypes of autoantibodies.
This study has certain limitations. First, the assignment of case status of SS was based on a combination of traditional SS antibody status and the ocular surface exam. However, we did not have information on previous SS work-ups and some participants had never undergone lip biopsies. This could have resulted in some misclassification bias in that some patients in the non-SS dry eye group may have had undiagnosed SS, while some in the SS group may not have truly had SS. This potential misclassification would have diluted any real difference in the prevalence of autoantibodies between the two groups. However, we found that the prevalence of traditional SS autoantibodies in our SS participants was similar to the prevalence reported in well-characterized groups of SS patients, which supports our classification of SS and non-SS groups.20, 27 In addition, we defined our SS group as only those who would have met the 2012 ACR classification criteria for SS.
An additional limitation is that our SS group was comprised of 52 participants. Therefore, our finding that there was no significant difference in novel autoantibody prevalence between groups could be due to the fact that there is no true association or from having low statistical power to detect a difference. Future larger studies would be helpful in confirming our results. Another limitation is that we did not have information about the duration of SS. Because the novel candidate SS autoantibodies were detected early in the course of disease in a mouse model, these antibodies may be more likely to be present in patients with early SS.20 If many of the SS patients in our cohort had longstanding SS, then it is possible that this would cause a lower proportion of them to express these novel autoantibodies. Longitudinal studies that assess the impact of duration of SS are needed. Finally, our cohort did not include any participants without dry eye. Future studies that include this subgroup would be helpful in comparing the prevalence of the novel autoantibodies in those with or without dry eye.
The traditional SS autoantibodies have limitations in specificity and sensitivity and therefore there is a need for better biomarkers for SS. For example, recently it was shown that SSB antibodies, in the absence of SSA antibodies, was not associated with key phenotypic features of SS28 and as a result anti-SSB is not included in the latest set of classification criteria for SS.11 We found that there was a weak correlation between traditional SS antibody positive results and novel candidate SS antibody positive results. While the novel candidate SS autoantibodies have shown promise in a mouse model for SS, their meaning and clinical utility in humans need to be studied further, including the sensitivity and specificity of these antibodies for the early diagnosis of SS in humans. In addition, the meaning of positivity of the different isotypes of each novel autoantibody is unknown. Finally, it is important to remember that these novel candidate SS autoantibodies are not currently part of any of the classification criteria sets for SS.9–11
We also found that approximately 11% of our dry eye patient cohort reported having a history of SS, which is consistent with previous reports.27 However, 6% of dry eye patients without a history of SS most likely had undiagnosed SS based on the 2012 ACR criteria, underscoring the need for improved screening methods and referrals for timely systemic evaluations for SS.
In conclusion, the DREAM© clinical trial provides the largest dataset to date that allows for the examination of the prevalence of novel candidate SS autoantibodies in dry eye patients with or without SS. We found that dry eye patients with SS had a significantly higher prevalence of SP-1 autoantibodies compared to those without SS or other autoimmune disease. In addition, among those with traditional SS autoantibodies, the concomitant presence of the novel autoantibodies may be a marker of more severe ocular surface disease. Longitudinal data regarding autoantibody expression over time will be useful in further examining the patterns of expression in SS and non-SS dry eye patients and correlations with clinical disease.
Supplementary Material
Baseline characteristics of non-Sjogren’s syndrome DREAM© Study participants with or without other autoimmune disease.
Antibody testing results of non-Sjogren’s syndrome DREAM© Study participants by history of other autoimmune disease
Correlation between the number of traditional antibody positive versus the number of novel antibody positive participants in the DREAM© Study
Acknowledgments
Source of Funding:
The DREAM© Study is supported by the National Eye Institute grants: U10EY022879 and U10EY022881.
Bausch & Lomb/Immco Diagnostics, Inc, provided the antibody testing for this study.
VYB is currently receiving a grant from the National Eye Institute (R01 EY026972); and a grant from Bausch & Lomb for a separate research study. She also receives funding from Research to Prevent Blindness.
GSY is currently receiving grants from the National Eye Institute (U10EY022879, R01 EY026972). He also receives personal fees from Chengdu Kanghong Biotech Co. Ltd and from Ziemer Ophthalmic Systems AG outside the submitted work.
MGM is currently receiving grants from the National Eye Institute and from the Office of Dietary Supplements, National Institute of Health (U10EY022879).
EK is currently receiving grants from the National Eye Institute and Office of Dietary Supplements, National Institutes of Health (U10EY022881). He also receives grants from MC2 Therapeutics outside the submitted work.
MCL is currently receiving grants from CooperVision Inc, Johnson& Johnson, Essilor USA, Orinda Pharma, LeoLens for studies outside the submitted work.
EP is currently receiving grants from the National Eye Institute and from the Office of Dietary Supplements, National Institute of Health (U10EY022879).
PAA is currently receiving grants from the National Eye Institute and from the Office of Dietary Supplements, National Institute of Health (U10EY022881). She also receives the following (outside the submitted work): personal fees from Santen, personal fees from Shire, grants and personal fees from Novartis; personal fees from Medscape; grants and personal fees from MC2 Therapeutics; grants and personal fees from Valeant, Bausch& Lomb; personal fees from Allergan; personal fees from Scientia; CME, grants and personal fees from Rtech, personal fees from Oculus; grants and personal fees from Miotech; and personal fees from Kao.
Lakshmanan Suresh DDS, MS, PhD and Long Shen, PhD reviewed the manuscript and provided useful comments.
Please see the Credit Roster in the Appendix for a list of clinical center personnel for the DREAM© study.
Footnotes
Conflict of Interest: For the remaining authors none were declared.
References
- 1.Tincani A, Andreoli L, Cavazzana I, et al. Novel aspects of Sjogren’s syndrome in 2012. BMC Med. 2013;11:93. doi: 10.1186/1741-7015-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165:2337–44. doi: 10.1001/archinte.165.20.2337. [DOI] [PubMed] [Google Scholar]
- 4.Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjogren syndrome. Arch Intern Med. 2004;164:1275–84. doi: 10.1001/archinte.164.12.1275. [DOI] [PubMed] [Google Scholar]
- 5.Akpek EK, Klimava A, Thorne JE, et al. Evaluation of patients with dry eye for presence of underlying Sjogren syndrome. Cornea. 2009;28:493–7. doi: 10.1097/ICO.0b013e31818d3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Gomes PS, Juodzbalys G, Fernandes MH, Guobis Z. Diagnostic Approaches to Sjogren’s syndrome: a Literature Review and Own Clinical Experience. J Oral Maxillofac Res. 2012;3:e3. doi: 10.5037/jomr.2012.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Molina G, Sanchez-Hernandez T. Clinimetric methods in Sjogren’s syndrome. Semin Arthritis Rheum. 2013;42:627–39. doi: 10.1016/j.semarthrit.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Beckman KA, Luchs J, Milner MS. Making the diagnosis of Sjogren’s syndrome in patients with dry eye. Clin Ophthalmol. 2016;10:43–53. doi: 10.2147/OPTH.S80043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjogren’s syndrome: a data-driven, expert consensus approach in the Sjogren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012;64:475–87. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017;69:35–45. doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruszka P, O’Brian RJ. Diagnosis and management of Sjogren syndrome. Am Fam Physician. 2009;79:465–70. [PubMed] [Google Scholar]
- 13.Manthorpe R, Asmussen K, Oxholm P. Primary Sjogren’s syndrome: diagnostic criteria, clinical features, and disease activity. J Rheumatol Suppl. 1997;50:8–11. [PubMed] [Google Scholar]
- 14.Meiners PM, Vissink A, Kroese FG, et al. Abatacept treatment reduces disease activity in early primary Sjogren’s syndrome (open-label proof of concept ASAP study) Ann Rheum Dis. 2014;73:1393–6. doi: 10.1136/annrheumdis-2013-204653. [DOI] [PubMed] [Google Scholar]
- 15.Meijer JM, Meiners PM, Vissink A, et al. Effectiveness of rituximab treatment in primary Sjogren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:960–8. doi: 10.1002/art.27314. [DOI] [PubMed] [Google Scholar]
- 16.Pijpe J, van Imhoff GW, Spijkervet FK, et al. Rituximab treatment in patients with primary Sjogren’s syndrome: an open-label phase II study. Arthritis Rheum. 2005;52:2740–50. doi: 10.1002/art.21260. [DOI] [PubMed] [Google Scholar]
- 17.Fayyaz A, Kurien BT, Scofield RH. Autoantibodies in Sjogren’s Syndrome. Rheum Dis Clin North Am. 2016;42:419–34. doi: 10.1016/j.rdc.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Molina G, Leal-Alegre G, Michel-Peregrina M. The meaning of anti-Ro and anti-La antibodies in primary Sjogren’s syndrome. Autoimmun Rev. 2011;10:123–5. doi: 10.1016/j.autrev.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Mavragani CP, Tzioufas AG, Moutsopoulos HM. Sjogren’s syndrome: autoantibodies to cellular antigens. Clinical and molecular aspects. Int Arch Allergy Immunol. 2000;123:46–57. doi: 10.1159/000024423. [DOI] [PubMed] [Google Scholar]
- 20.Shen L, Suresh L, Lindemann M, et al. Novel autoantibodies in Sjogren’s syndrome. Clin Immunol. 2012;145:251–5. doi: 10.1016/j.clim.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Matossian C, Micucci J. Characterization of the serological biomarkers associated with Sjogren’s syndrome in patients with recalcitrant dry eye disease. Clin Ophthalmol. 2016;10:1329–34. doi: 10.2147/OPTH.S106973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen L, Kapsogeorgou EK, Yu M, et al. Evaluation of salivary gland protein 1 antibodies in patients with primary and secondary Sjogren’s syndrome. Clin Immunol. 2014;155:42–6. doi: 10.1016/j.clim.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Suresh L, Malyavantham K, Shen L, Ambrus JL., Jr Investigation of novel autoantibodies in Sjogren’s syndrome utilizing Sera from the Sjogren’s international collaborative clinical alliance cohort. BMC Ophthalmol. 2015;15:38. doi: 10.1186/s12886-015-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Everett S, Vishwanath S, Cavero V, et al. Analysis of novel Sjogren’s syndrome autoantibodies in patients with dry eyes. BMC Ophthalmol. 2017;17:20. doi: 10.1186/s12886-017-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Langhe E, Bossuyt X, Shen L, et al. Evaluation of Autoantibodies in Patients with Primary and Secondary Sjogren’s Syndrome. Open Rheumatol J. 2017;11:10–5. doi: 10.2174/1874312901711010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karakus S, Baer AN, Agrawal D, et al. Utility of Novel Autoantibodies in the Diagnosis of Sjogren’s Syndrome Among Patients With Dry Eye. Cornea. 2018;37:405–11. doi: 10.1097/ICO.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 27.Liew MS, Zhang M, Kim E, Akpek EK. Prevalence and predictors of Sjogren’s syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol. 2012;96:1498–503. doi: 10.1136/bjophthalmol-2012-301767. [DOI] [PubMed] [Google Scholar]
- 28.Baer AN, McAdams DeMarco M, Shiboski SC, et al. The SSB-positive/SSA-negative antibody profile is not associated with key phenotypic features of Sjogren’s syndrome. Ann Rheum Dis. 2015;74:1557–61. doi: 10.1136/annrheumdis-2014-206683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of non-Sjogren’s syndrome DREAM© Study participants with or without other autoimmune disease.
Antibody testing results of non-Sjogren’s syndrome DREAM© Study participants by history of other autoimmune disease
Correlation between the number of traditional antibody positive versus the number of novel antibody positive participants in the DREAM© Study