Abstract
Congestive hepatopathy (CH) arises from chronically elevated right sided heart pressures transmitted to the liver by passive venous congestion. Over time CH can lead to hepatic bridging fibrosis, decompensated cirrhosis, and hepatocellular carcinoma. Currently there are no evidence-based guidelines to direct appropriate screening or management of patients with CH partly because of the inability of current clinical tools (serum tests, imaging studies, liver stiffness measurements, and liver biopsy) to accurately estimate hepatic fibrosis or the risk for hepatic decompensation. The Model for end-stage liver disease excluding international normalized ratio (MELD-XI) score is the only validated serum-based test to predict clinical outcomes in CH, and non-invasive liver stiffness measurements are proving to be of minimal utility as all patients with CH have elevated values that currently cannot differentiate between congestion and fibrosis. In addition, fibrosis staging by liver biopsy is difficult to standardize because of heterogeneous collagen deposition in CH, and liver biopsy results have little predictive value for post-heart transplant hepatic outcomes in patients with CH. Evaluating liver nodules and masses is also complicated in CH, as the finding of delayed venous washout in nodules is not specific for HCC in the background of a congested liver, and these lesions may require biopsy to confirm the diagnosis. The lack of effective clinical tools for predicting liver fibrosis and liver function suggests the need for the development of novel biomarkers in patients with CH to assist in the management of this complicated disease.
Keywords: Fontan Associated Liver Disease, Non-Invasive Biomarkers, Clinical Risk Calculators, Liver Stiffness Scores, Combined Heart and Liver Transplantation
Congestive hepatopathy (CH) arises from chronically elevated hepatic venous pressures secondary to right sided heart failure. High pressures from biventricular or isolated right sided heart failure are transmitted to the central veins of the liver resulting in increased sinusoidal pressure. This pressure increase causes shear stress on the sinusoidal endothelial cells leading to decreased nitrous oxide production and in turn, vasoconstriction. Vasoconstriction results in zone 3 sinusoidal ischemia and fibrosis deposition resulting in bridging fibrosis, cirrhosis, and even hepatocellular carcinoma. In addition, low cardiac output itself may also lead to low circulating blood flow to the liver accelerating fibrosis pathways(1–4).
The burden of CH is not well understood and there is a lack of epidemiologic study in CH outside of academic medical centers. This may be due to the heterogeneous causes of CH (e.g., ischemic cardiomyopathy, valvular disease, constrictive pericarditis, congenital heart disease) and the limited validated techniques available to diagnosis and stage this disease. Most cases of CH are sub-clinical with presenting symptoms and morbidity primarily driven by cardiac disease. Only the most severe cases typically manifest hepatic complications.(5, 6) A growing population of patients with CH are adults with a history of congenital heart disease who develop Fontan associated liver disease (FALD). The Fontan procedure is an operation to connect a single working heart ventricle to the systemic vascular system while allowing passive venous return to the pulmonary arteries (Figure 1).(7) Thus, patients exposed to decades of high pressures within the liver serve as a model for CH, and belong to an orphaned group in need of research to better understand and care for FALD.
Figure 1.
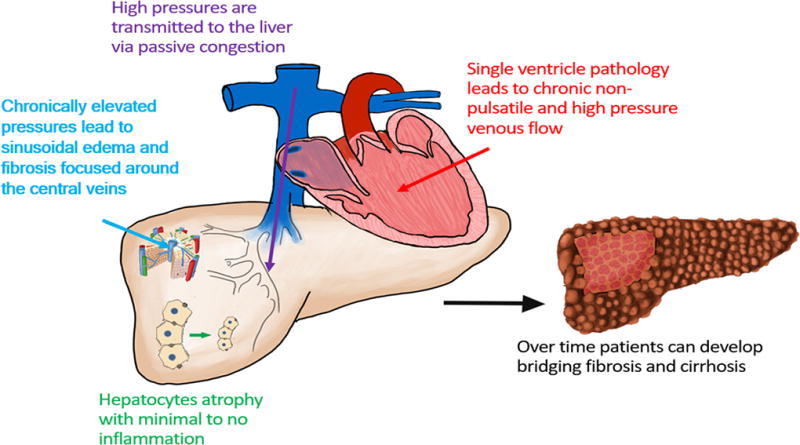
Accurate staging of liver fibrosis is a key factor in reliably determining hepatic reserve and function in CH. This information is critical to determine a specific patient’s prognosis, screening and management strategy, and ultimately candidacy for isolated heart versus combined heart-liver transplant as cardiac disease worsens. Unlike the inflammatory hepatopathies (e.g., viral, alcoholic, and nonalcoholic fatty liver disease (NAFLD)) which have validated biomarkers of fibrosis and clinical outcomes (serum tests, fibrosis calculators, and liver stiffness assessments), there is a growing awareness that these tools are unreliable in CH.(2, 3, 8) There is also evidence suggesting that even liver biopsy, which is touted as the gold standard for fibrosis assessment, may be unable to accurately stage fibrosis and predict clinical outcomes in CH.(2) This review examines the current evidence supporting the utility of available diagnostic tools in predicting clinical outcomes in patients with CH.
Noninvasive Biomarkers: Can We Estimate Liver Fibrosis in Congestive Hepatopathy?
Non-invasive biomarkers are increasingly used to estimate the severity of hepatic fibrosis in almost all etiologies of liver disease. Serologic tests, radiographic modalities, and liver stiffness assessments have excellent predictive value for advanced fibrosis when compared to liver biopsy, particularly in hepatitis C and NAFLD.(9, 10) However, the current repertoire of non-invasive biomarkers is unreliable in predicting advanced fibrosis in patients with CH.
Serologic Markers
The standard serum markers including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, bilirubin, and prothrombin time do not correlate with the degree of fibrosis seen on biopsy in CH (Table 1).(8, 11–15) Initial small studies (max n=13) suggested that a low platelet count or an elevated gamma glutamyl transferase (GGT) level may correlate with fibrosis stage on biopsy.(13, 16, 17) However, in a multi-center cross-sectional investigation of 71 patients with biopsy-proven CH there was no correlation between the number or degree of abnormal serum markers (low platelet count, elevated GGT level or aminotransferase levels) and fibrosis stage.(15) In addition, two retrospective studies with a combined 112 post-Fontan patients showed no difference in platelet count or GGT level between patients with low and high stage fibrosis on biopsy.(11, 14)
Table 1.
Summary of Correlation Between Non-Invasive Biomarkers and Fibrosis on Liver Biopsy
| Biomarker | Author | Number of Subjects | Population Studied | Effect Estimate - (P Value) |
|---|---|---|---|---|
| AST | Surrey9 | 74 | Post-Fontan | R2 = 0.08 (0.5) |
| AST | Wu, FM10 | 68 | Post-Fontan | R2 = No Significant Correlation* |
| ALT | Surrey9 | 74 | Post-Fontan | R2 = −0.06 (0.6) |
| ALT | Wu, FM10 | 68 | Post-Fontan | R2 = No Significant Correlation* |
| Alkaline Phosphatase | Surrey9 | 74 | Post-Fontan | R2 = 0.03 (0.8) |
| Alkaline Phosphatase | Wu, FM10 | 68 | Post-Fontan | R2 = No Significant Correlation* |
| GGT | Surrey9 | 74 | Post-Fontan | R2 = 0.14 (0.25) |
| GGT | Wu, FM10 | 68 | Post-Fontan | R2 = No Significant Correlation* |
| GGT | Goldberg6 | 67 | Post-Fontan | R2 = 0.21 (0.09) |
| Total Bilirubin | Surrey9 | 74 | Post-Fontan | R2 = 0.19 (0.12) |
| Total Bilirubin | Wu, FM10 | 68 | Post-Fontan | R2 = No Significant Correlation* |
| Albumin | Surrey9 | 74 | Post-Fontan | R2 = 0.08 (0.51) |
| Albumin | Wu, FM10 | 68 | Post-Fontan | R2 = Value Not Reported But Significant (0.05) |
| Platelet Count | Surrey9 | 74 | Post-Fontan | R2 = 0.004 (0.97) |
| Platelet Count | Wu, FM10 | 68 | Post-Fontan | R2 = No Significant Correlation* |
| Platelet Count | Goldberg6 | 67 | Post-Fontan | R2 = −0.04 (0.75) |
| Platelet Count | Schwartz11 | 13 | Post-Fontan | Odds Ratio = 0.84 (0.04) |
| Prothrombin Time | Surrey9 | 74 | Post-Fontan | R2 = 0.25 (0.034) |
| Prothrombin Time | Wu, FM10 | 68 | Post-Fontan | R2 = No Significant Correlation* |
| Prothrombin Time | Goldberg6 | 67 | Post-Fontan | R2 = 0.15 (0.25) |
| MELD | Louie2 | 21 | Pre-Heart Transplant | R2 = No Significant Correlation* |
| MELD-XI | Louie2 | 21 | Pre-Heart Transplant | R2 = No Significant Correlation* |
| MELD-XI | Evans14 | 70 | Post-Fontan | R2 = 0.4 (0.003) |
| MELD-XI | Wu, FM10 | 68 | Post-Fontan | R2 = No Significant Correlation* |
| FibroSURE | Wu, FM13 | 27 | Post-Fontan | PPV = 33.3%, NPV =52.6% |
| Hyaluronic Acid | Wu, FM13 | 27 | Post-Fontan | PPV = 33.3%, NPV = 38.5% |
Author does not report value, but reports non significant correlation in text
 indicates statistical significance
indicates statistical significance
More complex serum fibrosis markers and clinical risk calculators, including the FibroSURE test, hyaluronic acid levels, APRI Score (AST to platelet ratio index), AST/ALT ratio, Forns index (GGT, platelet count, age, and cholesterol), and FIB4 (AST, ALT, platelet count, and age) are either poorly studied in CH or have evidence demonstrating no correlation to fibrosis stage on biopsy.(18) Given the underlying pathophysiology of CH and limited correlation of individual serum markers with fibrosis stage, clinical risk calculators are unlikely to effectively predict fibrosis risk in CH.(8, 19) For example, several models heavily weight aminotransferase values as markers of liver necroinflammation, but CH is not an inflammatory disease.(1–3) Among 27 patients with FALD, the positive predictive value (PPV) and negative predictive value (NPV) of FibroSURE for identifying evolving or established cirrhosis when compared to liver biopsy were 33.3% (PPV) and 52.6% (NPV), respectively. The PPV and NPV of hyaluronic acid levels were 33.3% and 38.5%, respectively (Table 1).(18)
The model for end-stage liver disease excluding international normalized ratio (MELD-XI) score shows some promise in estimating hepatic fibrosis.(19, 20) In a retrospective study of 73 post-Fontan patients, elevated MELD-XI values correlated with increasing total fibrosis scores (r=0.4, p=0.003).(19) However, the clinical application of this finding should be interpreted with caution since the MELD-XI only moderately correlates with fibrosis score and other small studies have failed to demonstrate this correlation (Table 1).(2, 15) In contrast, traditional MELD score (which includes INR) does not reflect true liver function in CH given the high prevalence of anticoagulation use.
In summary, current evidence suggests that standard serum markers, FibroSURE testing, hyaluronic acid levels, and most clinical risk calculators, do not correlate with biopsy determined fibrosis staging in patients with CH. The MELD-XI may have some utility in predicting fibrosis staging, but even it has conflicting evidence suggesting that further studies are needed to validate these results.
Imaging Modalities and Liver Stiffness Assessment Tools
In many forms of liver disease, advanced fibrosis is often suggested radiographically by the presence of a shrunken nodular liver, heterogeneous enhancement of the hepatic parenchyma, and segmental atrophy and hypertrophy by ultrasound, computer tomography (CT), or magnetic resonance imaging (MRI). However, in CH there is minimal evidence correlating these findings to fibrosis grade by biopsy or clinical hepatic decompensation. For example, approximately 70% of patients post-Fontan with nodular appearance of the liver on cross-sectional imaging and who underwent heart transplantation had no lasting signs of liver dysfunction post-transplant(2, 21–23). Heterogeneous hepatic enhancement is likely the radiographic manifestation of passive hepatic congestion itself. This is supported by the fact that heterogeneous hepatic enhancement is present in 67–90% of patients post-Fontan, the vast majority of whom have no evidence of clinically significant liver dysfunction.(21, 23) Overall there is a paucity of evidence validating imaging characteristics that are typically associated with advanced fibrosis with biopsy determined fibrosis grade or clinical outcomes in CH. Thus, clinicians should use caution when using these imaging findings alone for establishing the diagnosis of cirrhosis in CH.
Radiographic liver stiffness assessments (e.g., transient elastography (TE), acoustic radiation force impulse (ARFI), MR elastography (MRE)) can accurately assess advanced fibrosis in many forms of liver disease.(24, 25) However, the accuracy of these tools is limited in CH where increased blood volume within the liver results in at least modestly elevated liver stiffness measures.(20, 26–29) Elevated liver stiffness scores are common in post-Fontan patients and an increased duration of time since the Fontan operation correlates with higher liver stiffness score (Tables 2 and 3).(7, 20, 27, 29) Thus, distinguishing congestion from underlying fibrosis is very difficult in CH. This concept is well-demonstrated in a prospective study of 32 patients with valvular heart disease who underwent TE before and after valvular repair. Ninety days post-operatively patients demonstrated a significant decline in liver stiffness (8.4 –> 6.0 kPA, p=0.026) suggesting improvement in congestion rather than reversal of fibrosis given the short time frame.(30)
Table 2.
Average Liver Stiffness Scores in Congestive Hepatopathy Compared to Healthy Controls
| Liver Stiftness Tool | Author | Number of Subjects | Average Liver Stiffness Score (CI) | Upper Limit Values of Healthy Controls* |
|---|---|---|---|---|
| Transient Elastography | Wu, FM22 | 45 | 21.4 kPa (10.6-32.2) | 7 kPa |
| Transient Elastography | Yoo24 | 46 | 21.1 kPa (13.1–29.1) | 7 kPa |
| ARFI | Melero-Ferrer4 | 21 | 1.86 m/s (1.36–2.36) | 1.44 m/s |
| MR Elastography | Poterucha21 | 50 | 5.5 kPa (4.1–6.9) | 3.44 kPa |
Cutoffs obtained from UpToDate®
Table 3.
Correlation Between Time Since Fontan Procedure and Liver Stiffness Scores
Only one small study (n=10) exists comparing liver stiffness determined by TE to fibrosis stage by biopsy, and no studies exist for ARFI. In the TE study, liver stiffness values overestimated the amount of fibrosis by at least one stage in seven out of ten subjects and overestimated the level of fibrosis by at least two stages in five out of ten subjects. In no cases did liver stiffness underestimate the level of fibrosis seen on biopsy.(27) These results are certainly discouraging for using TE in clinical practice to diagnose advanced fibrosis. Whether normal stiffness scores on TE can effectively rule out advanced fibrosis requires a larger sample size.
In contrast, magnetic resonance elastography (MRE) liver stiffness values show more promise for predicting fibrosis in CH. In a small study (n=8), MRE liver stiffness values correlated with fibrosis grade on biopsy (R=.74, p=.02).(26) Interestingly, a more novel concept of spleen stiffness measurements by MRE may have the most promising evidence for predicting liver fibrosis in CH. In the same study, there was a very strong correlation between spleen stiffness and liver fibrosis grade on biopsy (R=.97; p=.002).(26) This is potentially encouraging data for the use of MRE, but should be applied cautiously to clinical practice given the small sample size and the method by which patients were selected to receive biopsies, as the selection process was not randomized. In addition, animal models do not support MRE for predicting biopsy-proven fibrosis.(28)
Unlike MRE, MRI with diffusion-weighted imaging (DWI) has shown some promise in differentiating congestion from fibrosis, particularly among patients with FALD. In non-congestive liver disease, the calculated value of the apparent diffusion coefficient (ADC) by DWI imaging correlates with fibrosis stages as determined by biopsy.(31, 32) In FALD, the ADC is significantly lower than in healthy volunteers and continues to worsen as time from the operation increases.(33) However, Fontan patients with worsening cardiac parameters (such as increasing inferior vena cava diameter) also demonstrate low ADC. Thus, it is unclear if ADC reflects hepatic fibrosis and function or merely cardiac congestion of the liver. In contrast, DWI with intravoxel incoherent motion (IVIM) may be able to separate congestion from fibrosis. Compared to ADC, which does not take into account changes in tissue microstructure, IVIM is able to separate microperfusion parameters from molecular diffusion parameters. Microperfusion parameters are hypothesized to decrease in the setting of poor hepatic blood flow whereas molecular diffusion parameters are hypothesized to decrease with the formation of hepatic fibrosis.(34) This concept was recently illustrated in a single study of patients with FALD in which microperfusion parameters were lower than controls early after the Fontan operation, thus reflective of immediate alterations in hepatic blood flow, but molecular diffusion scores were similar to controls. However, FALD patients who were twenty to thirty years post-Fontan had stable microperfusion parameters, but worsening molecular diffusion scores (and thus worsening overall ADC) suggesting the formation of hepatic fibrosis.(34) MRI with DWI has not been validated against liver fibrosis scores by biopsy or clinical hepatic outcomes, but the potential clinical utility of MRI with DWI in this difficult population warrants further investigation. It is important to note that the clinical feasibility of MRI testing in patients with CH, and certainly in the post-Fontan patient, may be limited as many of these patients have non-MR compatible cardiac devices (e.g., percutaneous pacemaker).
Prediction of Hepatic Decompensation in Congestive Hepatopathy and Determining Candidacy for Heart Transplantation
Predicting hepatic function and reserve in CH when patients are undergoing assessment for heart transplant or combined heart-liver transplant is a challenging question that hepatologists at academic centers are facing with increasing frequency. The two critical questions that usually arise are whether 1) the liver can tolerate the hypotensive and low flow environment of the heart transplant itself, and 2) if successfully transplanted with a functional heart will the liver recover or demonstrate ongoing signs of hepatic dysfunction and portal hypertension. This task can be extremely difficult given the aforementioned paucity of tools to predict hepatic fibrosis and function, and the growing concern that even isolated liver biopsy may have limited utility in predicting hepatic function and reserve in patients with CH.
Clinical prediction tools, including the MELD score, are highly utilized in most forms of liver disease to estimate the risk of hepatic decompensation and mortality in patients with liver disease. However, MELD is not an accurate prediction tool for hepatic decompensation in CH due to its reliance on the INR which frequently does not reflect true liver function among patients with CH who have a high prevalence of anticoagulation use. For example, in a study of 21 patients with CH who received a heart transplant, the six patients with pre-transplant MELD scores >20 were either still alive at follow-up after a year without significant signs of liver dysfunction or died of causes unrelated to liver failure, suggesting poor correlation of the MELD with true underlying function and reserve.(2)
In concordance with its correlation with liver fibrosis scores, the MELD-XI score appears to be more useful in predicting clinically significant patient outcomes in the CH population than other prediction tools.(19, 20, 35–39) One prospective study of 18 post-Fontan patients found that higher MELD-XI scores independently correlated to the composite endpoint of increased incidence of sudden cardiac death, death from congestive heart failure, and need for cardiac transplantation (Hazard Ratio (HR)=7.76, 95% CI 2.05–29.33, p=0.008).(35) Elevated MELD-XI scores prior to adult and pediatric heart transplantation also predicted adverse post-cardiac transplant outcomes, including one year mortality in multiple studies.(37–39) In a recent investigation, patients with a MELD-XI ≥ 19 had higher odds of all-cause mortality (HR=1.17, p=0.013).(36) However, although the MELD-XI appears to be a useful tool in predicting cardiac morbidity and mortality in CH, whether or not MELD-XI is useful to predict hepatic outcomes is unknown.
Growing awareness of the lack of accurate non-invasive surrogates for liver fibrosis and hepatic decompensation in CH have led many clinicians to rely heavily on liver biopsy to estimate hepatic reserve and candidacy for isolated heart transplantation. However, evidence suggests that in CH fibrosis on liver biopsy is heterogeneous, advanced hepatic fibrosis is potentially reversible when normal cardiac function is restored, and most importantly pre-transplant liver biopsies may not predict post-heart transplant hepatic outcomes.(2, 40) In a retrospective study including six patients with CH who received a combined heart-liver transplant and twenty one patients with CH who received a heart transplant alone, pre-transplant laboratory values and liver biopsies were analyzed to determine their ability to predict post-transplant outcomes. Five out of six explanted livers from the combined heart-liver transplant group demonstrated evidence of bridging fibrosis with sinusoidal dilation, but the fibrosis was extremely heterogeneous, with three of the six livers (50%) having significant areas of the liver that if biopsied would have appeared normal.(2) Also, although all six patients received a liver transplant, one explant did not show any evidence of bridging fibrosis.(2) In the twenty one heart alone recipients, only one of thirteen patients who had bridging fibrosis on liver biopsy died in the perioperative period, and the presence of bridging fibrosis did not significantly correlate with survival at one year post transplant.(2) Additionally, three of twenty patients whose biopsies demonstrated only stage 2 or 3 fibrosis showed prolonged liver failure postoperatively and all three patients died within twelve months following heart transplant.(2) No other known studies exist investigating the prognostic value of pre-heart transplant liver biopsies in patients with CH, but this provocative data suggests that biopsy determined fibrosis scores do not accurately predict hepatic complications in heart transplant candidates. Regardless, liver biopsy certainly still plays an important role in patients with hepatic dysfunction and heart failure, as it may be needed to rule out alternative etiologies of liver disease (i.e., NASH) and for evaluation for nodules that are suspicious for hepatocellular carcinoma.(40)
Diagnostic tools with the capability of estimating the summative fibrosis burden and function of the liver, rather than an isolated sample such as a liver biopsy, are attractive in CH given the heterogeneous nature of the fibrosis deposition. Transjugular measurement of hepatic venous pressure gradient (HVPG) is an invasive test gaining favor at select academic medical centers as some experts believe it aids in distinguishing whether portal hypertension is caused by heart failure or hepatic fibrosis. The HVPG is the difference between the wedged hepatic vein pressure (surrogate for portal vein pressure) and the free hepatic vein pressure (surrogate for inferior vena cava pressure).(41) In all patients with Fontan circulation it is expected that the free hepatic vein pressure and wedged hepatic vein pressure will be elevated due to chronically elevated right sided heart pressures. However, in theory, if the HVPG >6mm Hg (normal is <5mm Hg) then the portal vein pressure is significantly more elevated than the inferior vena cava pressure, suggesting that intrinsic liver fibrosis is significant enough alone to cause portal hypertension. Some academic transplant centers use a HVPG value of <12 mm Hg as a cutoff for offering isolated heart transplantation rather than requiring a combined heart liver transplant, however no validation studies exist demonstrating this cutoff carries clinical significance.(41)
Clearly, there are limited objective measures by which one can predict hepatic decompensation in patients with CH, and no absolute criteria exist for determining whether a patient with CH is a candidate for isolated heart transplant or may need to be listed for combined heart-liver transplant based on our current repertoire of diagnostic tests. Ultimately, this critical decision still must be made on a case-by-case basis mainly guided by expert opinion.
Hepatocellular Carcinoma in Congestive Hepatopathy – The Difficulty in Distinguishing Regenerative Nodules from Malignancy
In all forms of liver disease one of the most feared outcomes is the development of hepatocellular carcinoma (HCC). The prevalence of HCC in CH appears to be highest in the post-Fontan population at approximately 5% and the greatest risk factor for HCC in this population is time since the Fontan procedure (median 22 years post-Fontan, IQR: 10–29 years).(42) Distinguishing HCC from other atypical nodules found on hepatic imaging can be difficult in CH. Evidence shows that the radiographic finding of delayed venous washout within a liver nodule is not specific for HCC in post-Fontan patients, likely due to retained contrast by the background parenchyma and not necessarily the nodule itself.(43) Significant change in appearance of a nodule over 24 months, a heterogeneous appearing mass, a portal thrombus, and elevated AFP are more specific for HCC in CH.(43) No guidelines currently exist for screening for HCC or diagnosing HCC in CH or FALD. However, based on limited available evidence our expert opinion is that in post-Fontan patients it may be reasonable to begin screening for HCC at 15–20 years after the operation. In addition, in all CH patients with atypical appearing nodules with delayed venous washout these lesions may require biopsy to confirm the diagnosis if no other frank evidence of malignancy is present on imaging given the lack of specificity of this finding in a congested liver.
Summary and Future Directions
Identifying validated markers for the presence of liver fibrosis and predicting clinical outcomes in CH is necessary to provide evidence-based guidelines on the initiation of screening for complications of cirrhosis including HCC, and for predicting a patient’s risk of hepatic decompensation from a major surgery such as heart-transplantation. After an extensive review of the available literature it appears that almost all standard serum markers and clinical risk calculators do not reproducibly correlate with the stage of liver fibrosis on biopsy or meaningfully predict risk of future hepatic decompensation or reserve. The MELD-XI may have the most utility in predicting liver fibrosis staging by biopsy and in predicting important cardiac outcomes in patients with CH including one-year survival post-heart transplantation. However, the correlation with liver fibrosis appears modest at best and no studies have validated the MELD-XI for hepatic specific clinical outcomes.
Radiographically, the appearance of a nodular or heterogeneous liver on standard imaging is not sufficient to diagnosis cirrhosis in CH. Liver stiffness scores seem to provide little value in CH due to the uniform elevation in liver stiffness in all patients without differentiation between congestion and fibrosis, although a small body of evidence suggests that liver stiffness and spleen stiffness calculated specifically by MRE may have some correlation to the stage of liver fibrosis on biopsy. New advances in imaging techniques, such as MRI with DWI, may have the potential to differentiate fibrosis from congestion but require further study. Invasive testing such as transjugular measurement of HVPG may be needed in patients with CH. However, although an elevated HVPG >6mmHg theoretically represents intrinsic liver fibrosis rather than cardiac induced portal hypertension, this threshold has not been evaluated for prediction of hepatic decompensation events and survival after heart transplantation, so its clinical utility remains unknown in CH. Finally, patients with CH and especially FALD are at significant risk of HCC. Delayed venous washout within a hepatic nodule is not necessarily specific for HCC in CH and a guided biopsy may be needed to confirm a diagnosis of HCC. A high index of suspicion for HCC is needed in these patients for accurate and early diagnosis.
The most significant revelation from this review is that liver biopsy may not accurately predict post-heart transplant hepatic outcomes and mortality, suggesting that isolated liver biopsy may not be the gold standard for determining irreversible fibrosis and hepatic reserve that it represents in other forms of liver disease. This discovery suggests that future studies of serum, imaging, and invasive tools within the CH population may only be clinically relevant if they are validated against clinical outcomes rather than histologic fibrosis scores. Thus, there is a need for large multi-center studies with adequate power to detect significant associations between potential biomarkers and clinical outcomes in CH. Given the lack of currently effective tools to accurately estimate clinical outcomes in patients suffering from CH, it seems paramount to investigate novel biomarkers of fibrosis, liver reserve, and liver function to help fill the gaps in our understanding of this complicated disease.
Acknowledgments
Funding Source: Dr. Lemmer and Dr. Ganger are supported by the Digestive Health Foundation Research Grant. This is an institutional grant affiliated with Northwestern University Division of Gastroenterology and Hepatology.
Dr. VanWagner is supported by the National Institutes of Health's National Center for Advancing Translational Sciences (KL2TR001424).
Dr. VanWagner is on the speaker’s bureau for Salix and receives research grant support from Novartis and Advanced Magnetic Resonance Analytics (AMRA) outside the submitted work. Dr. Daniel Ganger is on the speaker’s bureau for Gilead and Merck and receives grant support from the NIDDK as part of the Acute Liver Failure Study Group outside the submitted work.
List of Abbreviations (in order of appearance)
- CH
Congestive hepatopathy
- FALD
Fontan associated liver disease
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- GGT
Gamma glutamyl transferase
- PPV
Positive predictive value
- NPV
negative predictive value
- APRI
AST to platelet ratio index
- FIB4
Fibrosis-4 score
- MELD-XI
Model for End-Stage Liver Disease without INR
- MELD
Model for End-Stage Liver Disease
- DWI
Diffusion-weighted imaging
- IVIM
intravoxel incoherent motions
- ADC
Apparent diffusion coefficients
- TE
Transient elastography
- ARFI
Acoustic radiation force impulse elastography (ARFI)
- MRE
Magnetic resonance elastography
- HVPG
Hepatic venous pressure gradient
Footnotes
Disclosures
The authors have no conflicts of interest pertinent to this study.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institutes of Health.
References
- 1.Wells ML, Fenstad ER, Poterucha JT, Hough DM, Young PM, Araoz PA, Ehman RL, et al. Imaging Findings of Congestive Hepatopathy. Radiographics. 2016;36:1024–1037. doi: 10.1148/rg.2016150207. [DOI] [PubMed] [Google Scholar]
- 2.Louie CY, Pham MX, Daugherty TJ, Kambham N, Higgins JP. The liver in heart failure: a biopsy and explant series of the histopathologic and laboratory findings with a particular focus on pre-cardiac transplant evaluation. Mod Pathol. 2015;28:932–943. doi: 10.1038/modpathol.2015.40. [DOI] [PubMed] [Google Scholar]
- 3.Ford RM, Book W, Spivey JR. Liver disease related to the heart. Transplant Rev (Orlando) 2015;29:33–37. doi: 10.1016/j.trre.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014;61:912–924. doi: 10.1016/j.jhep.2014.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maleki M, Vakilian F, Amin A. Liver diseases in heart failure. Heart Asia. 2011;3:143–149. doi: 10.1136/heartasia-2011-010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J. 2013;34:2804–2811. doi: 10.1093/eurheartj/eht246. [DOI] [PubMed] [Google Scholar]
- 7.Melero-Ferrer JL, Osa-Saez A, Buendia-Fuentes F, Ballesta-Cunat A, Flors L, Rodriguez-Serrano M, Calvillo-Batlles P, et al. Fontan Circulation in Adult Patients: Acoustic Radiation Force Impulse Elastography as a Useful Tool for Liver Assessment. World J Pediatr Congenit Heart Surg. 2014;5:365–371. doi: 10.1177/2150135114530172. [DOI] [PubMed] [Google Scholar]
- 8.Bradley E, Hendrickson B, Daniels C. Fontan Liver Disease: Review of an Emerging Epidemic and Management Options. Curr Treat Options Cardiovasc Med. 2015;17:51. doi: 10.1007/s11936-015-0412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crossan C, Tsochatzis EA, Longworth L, Gurusamy K, Davidson B, Rodriguez-Peralvarez M, Mantzoukis K, et al. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: systematic review and economic evaluation. Health Technol Assess. 2015;19:1–409. v–vi. doi: 10.3310/hta19090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papastergiou V, Tsochatzis E, Burroughs AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol. 2012;25:218–231. [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg DJ, Surrey LF, Glatz AC, Dodds K, O'Byrne ML, Lin HC, Fogel M, et al. Hepatic Fibrosis Is Universal Following Fontan Operation, and Severity is Associated With Time From Surgery: A Liver Biopsy and Hemodynamic Study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaulitz R, Haber P, Sturm E, Schafer J, Hofbeck M. Serial evaluation of hepatic function profile after Fontan operation. Herz. 2014;39:98–104. doi: 10.1007/s00059-013-3811-5. [DOI] [PubMed] [Google Scholar]
- 13.Ofei SY, Gariepy C, Hanje J, Sisk T, Daniels CJ, Zaidi AN. Liver fibrosis in adults with Fontan palliation: Do common screening studies predict disease severity? Int J Cardiol. 2015;181:174–175. doi: 10.1016/j.ijcard.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Surrey LF, Russo P, Rychik J, Goldberg DJ, Dodds K, O'Byrne ML, Glatz AC, et al. Prevalence and characterization of fibrosis in surveillance liver biopsies of patients with Fontan circulation. Hum Pathol. 2016;57:106–115. doi: 10.1016/j.humpath.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Wu FM, Kogon B, Earing MG, Aboulhosn JA, Broberg CS, John AS, Harmon A, et al. Liver health in adults with Fontan circulation: A multicenter cross-sectional study. J Thorac Cardiovasc Surg. 2017;153:656–664. doi: 10.1016/j.jtcvs.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MC, Sullivan LM, Glatz AC, Rand E, Russo P, Goldberg DJ, Rome JJ, et al. Portal and sinusoidal fibrosis are common on liver biopsy after Fontan surgery. Pediatr Cardiol. 2013;34:135–142. doi: 10.1007/s00246-012-0402-9. [DOI] [PubMed] [Google Scholar]
- 17.Vasconcelos LA, de Almeida EA, Bachur LF. Clinical evaluation and hepatic laboratory assessment in individuals with congestive heart failure. Arq Bras Cardiol. 2007;88:590–595. doi: 10.1590/s0066-782x2007000500015. [DOI] [PubMed] [Google Scholar]
- 18.Wu FM, Earing MG, Aboulhosn JA, Johncilla ME, Singh MN, Odze RD, Ukomadu C, et al. Predictive value of biomarkers of hepatic fibrosis in adult Fontan patients. J Heart Lung Transplant. 2017;36:211–219. doi: 10.1016/j.healun.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Evans WN, Acherman RJ, Ciccolo ML, Carrillo SA, Galindo A, Rothman A, Winn BJ, et al. MELD-XI Scores Correlate with Post-Fontan Hepatic Biopsy Fibrosis Scores. Pediatr Cardiol. 2016;37:1274–1277. doi: 10.1007/s00246-016-1428-1. [DOI] [PubMed] [Google Scholar]
- 20.Friedrich-Rust M, Koch C, Rentzsch A, Sarrazin C, Schwarz P, Herrmann E, Lindinger A, et al. Noninvasive assessment of liver fibrosis in patients with Fontan circulation using transient elastography and biochemical fibrosis markers. J Thorac Cardiovasc Surg. 2008;135:560–567. doi: 10.1016/j.jtcvs.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Bae JM, Jeon TY, Kim JS, Kim S, Hwang SM, Yoo SY, Kim JH. Fontan-associated liver disease: Spectrum of US findings. Eur J Radiol. 2016;85:850–856. doi: 10.1016/j.ejrad.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Baek JS, Bae EJ, Ko JS, Kim GB, Kwon BS, Lee SY, Noh CI, et al. Late hepatic complications after Fontan operation; non-invasive markers of hepatic fibrosis and risk factors. Heart. 2010;96:1750–1755. doi: 10.1136/hrt.2010.201772. [DOI] [PubMed] [Google Scholar]
- 23.Wallihan DB, Podberesky DJ. Hepatic pathology after Fontan palliation: spectrum of imaging findings. Pediatr Radiol. 2013;43:330–338. doi: 10.1007/s00247-012-2531-y. [DOI] [PubMed] [Google Scholar]
- 24.Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, Valasek MA, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453–461. doi: 10.1002/hep.28337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee MS, Bae JM, Joo SK, Woo H, Lee DH, Jung YJ, Kim BG, et al. Prospective comparison among transient elastography, supersonic shear imaging, and ARFI imaging for predicting fibrosis in nonalcoholic fatty liver disease. PLoS One. 2017;12:e0188321. doi: 10.1371/journal.pone.0188321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poterucha JT, Johnson JN, Qureshi MY, O'Leary PW, Kamath PS, Lennon RJ, Bonnichsen CR, et al. Magnetic Resonance Elastography: A Novel Technique for the Detection of Hepatic Fibrosis and Hepatocellular Carcinoma After the Fontan Operation. Mayo Clin Proc. 2015;90:882–894. doi: 10.1016/j.mayocp.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu FM, Opotowsky AR, Raza R, Harney S, Ukomadu C, Landzberg MJ, Valente AM, et al. Transient elastography may identify Fontan patients with unfavorable hemodynamics and advanced hepatic fibrosis. Congenit Heart Dis. 2014;9:438–447. doi: 10.1111/chd.12159. [DOI] [PubMed] [Google Scholar]
- 28.Yin M, Glaser KJ, Manduca A, Mounajjed T, Malhi H, Simonetto DA, Wang R, et al. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology. 2017:160622. doi: 10.1148/radiol.2017160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo BW, Choi JY, Eun LY, Park HK, Park YH, Kim SU. Congestive hepatopathy after Fontan operation and related factors assessed by transient elastography. J Thorac Cardiovasc Surg. 2014;148:1498–1505. doi: 10.1016/j.jtcvs.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Chon YE, Kim SU, Park JY, Kim DY, Ahn SH, Han KH, Chon CY, et al. Dynamics of the liver stiffness value using transient elastography during the perioperative period in patients with valvular heart disease. PLoS One. 2014;9:e92795. doi: 10.1371/journal.pone.0092795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakan AA, Inci E, Bakan S, Gokturk S, Cimilli T. Utility of diffusion-weighted imaging in the evaluation of liver fibrosis. Eur Radiol. 2012;22:682–687. doi: 10.1007/s00330-011-2295-z. [DOI] [PubMed] [Google Scholar]
- 32.Lewin M, Poujol-Robert A, Boelle PY, Wendum D, Lasnier E, Viallon M, Guechot J, et al. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology. 2007;46:658–665. doi: 10.1002/hep.21747. [DOI] [PubMed] [Google Scholar]
- 33.Wolff D, van Melle JP, Dijkstra H, Bartelds B, Willems TP, Hillege H, van den Berg AP, et al. The Fontan circulation and the liver: A magnetic resonance diffusion-weighted imaging study. Int J Cardiol. 2016;202:595–600. doi: 10.1016/j.ijcard.2015.09.088. [DOI] [PubMed] [Google Scholar]
- 34.Dijkstra H, Wolff D, van Melle JP, Bartelds B, Willems TP, Oudkerk M, Hillege H, et al. Diminished liver microperfusion in Fontan patients: A biexponential DWI study. PLoS One. 2017;12:e0173149. doi: 10.1371/journal.pone.0173149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assenza GE, Graham DA, Landzberg MJ, Valente AM, Singh MN, Bashir A, Fernandes S, et al. MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart. 2013;99:491–496. doi: 10.1136/heartjnl-2012-303347. [DOI] [PubMed] [Google Scholar]
- 36.Berg CJ, Bauer BS, Hageman A, Aboulhosn JA, Reardon LC. Mortality Risk Stratification in Fontan Patients Who Underwent Heart Transplantation. Am J Cardiol. 2017;119:1675–1679. doi: 10.1016/j.amjcard.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Deo SV, Al-Kindi SG, Altarabsheh SE, Hang D, Kumar S, Ginwalla MB, ElAmm CA, et al. Model for end-stage liver disease excluding international normalized ratio (MELD-XI) score predicts heart transplant outcomes: Evidence from the registry of the United Network for Organ Sharing. J Heart Lung Transplant. 2016;35:222–227. doi: 10.1016/j.healun.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Farr M, Mitchell J, Lippel M, Kato TS, Jin Z, Ippolito P, Dove L, et al. Combination of liver biopsy with MELD-XI scores for post-transplant outcome prediction in patients with advanced heart failure and suspected liver dysfunction. J Heart Lung Transplant. 2015;34:873–882. doi: 10.1016/j.healun.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimm JC, Magruder JT, Do N, Spinner JA, Dungan SP, Kilic A, Patel N, et al. Modified Model for End-Stage Liver Disease eXcluding INR (MELD-XI) Score Predicts Early Death After Pediatric Heart Transplantation. Ann Thorac Surg. 2016;101:730–735. doi: 10.1016/j.athoracsur.2015.06.063. [DOI] [PubMed] [Google Scholar]
- 40.Greenway SC, Crossland DS, Hudson M, Martin SR, Myers RP, Prieur T, Hasan A, et al. Fontan-associated liver disease: Implications for heart transplantation. J Heart Lung Transplant. 2016;35:26–33. doi: 10.1016/j.healun.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Hilscher MB, Johnson JN, Cetta F, Driscoll DJ, Poterucha JJ, Sanchez W, Connolly HM, et al. Surveillance for liver complications after the Fontan procedure. Congenit Heart Dis. 2017;12:124–132. doi: 10.1111/chd.12446. [DOI] [PubMed] [Google Scholar]
- 42.Nandwana SB, Olaiya B, Cox K, Sahu A, Mittal P. Abdominal Imaging Surveillance in Adult Patients After Fontan Procedure: Risk of Chronic Liver Disease and Hepatocellular Carcinoma. Curr Probl Diagn Radiol. 2018;47:19–22. doi: 10.1067/j.cpradiol.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Wells ML, Hough DM, Fidler JL, Kamath PS, Poterucha JT, Venkatesh SK. Benign nodules in post-Fontan livers can show imaging features considered diagnostic for hepatocellular carcinoma. Abdom Radiol (NY) 2017;42:2623–2631. doi: 10.1007/s00261-017-1181-9. [DOI] [PubMed] [Google Scholar]


