Abstract
cGMP-independent nitric oxide (NO) signaling occurs via S-nitrosylation. We evaluated whether aberrant S-nitrosylation operates in the penis under conditions of cavernous nerve injury and targets proteins involved in regulating erectile function. Adult male Sprague-Dawley rats underwent bilateral cavernous nerve crush injury (BCNI) or sham surgery. Rats were given a denitrosylation agent N-acetylcysteine (NAC, 300 mg/kg/day) or vehicle in drinking water starting 2 days before BCNI and continuing for 2 weeks following surgery. After assessment of erectile function (intracavernous pressure), penes were collected for measurements of S-nitrosylation by Saville-Griess and TMT-switch assays and PKG-I function by immunoblotting of phospho (P)-VASP-Ser-239. Erectile function was decreased (P<0.05) after BCNI, and it was preserved (P<0.05) by NAC treatment. Total S-nitrosothiols and total S-nitrosylated proteins were increased (P<0.05) after BCNI, and these were partially prevented by NAC treatment. S-nitrosylation of sGC was increased (P<0.05) after BCNI, and it was prevented (P<0.05) by NAC treatment. S-nitrosylation of eNOS was increased (P<0.05) after BCNI, and showed a trend towards decrease by NAC treatment. Protein expression of P-VASP-Ser-239 was decreased (P<0.05) after BCNI, and showed a trend towards increase by NAC treatment. In conclusion, erectile dysfunction following BCNI is mediated in part by S-nitrosylation of eNOS and its downstream signaling mediator GC, while denitrosylation protects erectile function by preserving the NO/cGMP signaling pathway.
INTRODUCTION
Erectile dysfunction (ED) is a common consequence of radical prostatectomy, despite advances in surgical techniques, such as nerve-sparing and robot-assisted surgery (1). The rates of ED range from 12 to 96% in men undergoing radical prostatectomy (2). This complication is associated with cavernous nerve injury with effects observed in the penis at cellular and molecular levels. Cellular changes include apoptosis of smooth muscle and endothelial cells, decreased number of blood vessels, and increased fibrosis of the corporal tissue (3–8). At the molecular level, oxidative stress and RhoA/ROCK pathway are increased (9–12), while expressions of endothelial nitric oxide (NO) synthase (eNOS) and neuronal NO synthase (nNOS) mRNA and protein, and NO production, are decreased (13–16).
NO signaling in the penis is mediated through a well recognized signal transduction pathway involving activation of soluble guanylyl cyclase (sGC) and 3′,5′-cyclic guanosine monophosphate (cGMP)-induced activation of protein kinase G (PKG, 17, 18). It is increasingly recognized that NO signaling is also mediated by S-nitrosylation, an alternative signaling pathway for NO that mediates cGMP-independent effects (19, 20). S-nitrosylation, the covalent attachment of a NO group to the thiol side chain of cysteine on proteins and peptides to form S-nitrosothiols (SNOs), has emerged as an important mechanism for dynamic, post-translational regulation of many proteins, including NOS enzymes themselves. The levels of SNO proteins are regulated by denitrosylation and transnitrosylation (21). Denitrosylation is achieved by the action of enzymes such as the thioredoxin family of proteins and S-nitrosoglutathione reductase (22). Transnitrosylation refers to the reaction of an S-nitrosothiol with a neighboring cysteine residue within the same or an adjacent protein, or with glutathione, and is the major mechanism for generating SNO proteins in vivo (23).
We recently demonstrated the importance of transnitrosylation mechanisms in the penis in physiologic NO signaling, such that unchecked nitrosylation decreases NO bioactivity and increases oxidative/nitrosative stress (24). Whether S-nitrosylation is involved in pathologic effects in the penis and exerts deleterious effects with respect to erection preservation under conditions of penile neuropathy is unknown. In this study, we hypothesized that increased S-nitrosylation of key mediators of penile erection in the penis is induced by cavernous nerve injury, contributing to erectile impairment, while denitrosylation in the face of cavernous nerve injury protects erectile function by preserving NO/cGMP signaling pathway function.
MATERIALS AND METHODS
Animals
Adult male Sprague–Dawley rats (325–350 g; Charles River Breeding Laboratories, Wilmington, MA) were used. All experiments were conducted in accordance with the ethical standards of the Johns Hopkins University School of Medicine Guidelines for the Care and Use of Animals.
Bilateral cavernous nerve crush injury
To perform bilateral cavernous nerve crush injury (BCNI), rats were anesthetized with Isofluorine inhalation (Oxygen flow rate 0.8–1.5 l/min, Isofluorine 2.5%). Prostate and bilateral major pelvic ganglia were identified via a midline lower abdominal incision, and left and right cavernous nerves were isolated. Both nerves were crushed 1–2 mm distal to the major pelvic ganglia. To limit variability, all surgeries were completed by the same trained investigator. BCNI was induced by crushing nerves with a fine-grade hemostat for 2 minutes (25). Sham surgeries were completed by exposing the cavernous nerves but not manipulating them.
N- acetyl- L-cysteine (NAC) treatment
Rats were randomly divided into 4 groups (n = 9–12/group): Sham+Vehicle, Sham+ N-acetyl-cysteine (NAC), BCNI+Vehicle, and BCNI+NAC. NAC, an antioxidant and a denitrosylating agent (Sigma-Aldrich, St. Louis, MO), was given to rats in drinking water starting 2 days before BCNI or sham injury and continuing for 2 weeks after the surgery. NAC was prepared fresh daily and its pH was adjusted to 7. NAC was given at 300 mg/kg/day (5 g/l), and the dose was monitored by measuring consumption of water/day/rat (26).
In vivo erection studies
Erectile function was measured two weeks following BCNI or sham injury by monitoring intracavernous pressure (ICP) in anesthetized rats (50 mg/kg ketamine/5 mg/kg xylazine, intraperitoneal injections), as described previously (9, 10, 25). Briefly, cavernous nerves were isolated via a midline abdominal incision, and the crura of the penis were identified. ICP was monitored and recorded via a 25-gauge needle inserted into the left crus connected via PE-50 tubing to a pressure transducer (DI-190; Dataq Instruments, Akron, OH). For electrically stimulated penile erections, a bipolar electrode attached to a Grass Instruments S48 stimulator (Quincy, MA) was placed around the cavernous nerve at 1–2 mm distal to the crush site. Stimulation parameters were 1, 2, and 4 Volts at a frequency of 16 Hz with square-wave duration of 5 msec for 1 min. Mean arterial pressure (MAP) was continuously monitored after cannulation of the right carotid artery with polyethylene-60 tubing. Response parameters (maximal ICP above baseline, indicating maximum pressure that is reached during cavernous nerve electrical stimulation, and ICP area above baseline, indicating the ICP response for the duration of cavernous nerve electrical stimulation) were calculated using MATLAB software (Mathworks, Natick, MA). NAC dosing and erectile function measurement were performed in a blinded fashion, by 2 persons. Following measurement of ICP on a group of rats (n=5–8), penes were snapped frozen for molecular analyses of S-nitrosylaion. A separate group of rats (n=4), which did not undergo erectile function measurement, was used for penes collection for measurement of P-VASP (Ser-239), in order to avoid confounding effect of repeated electrical stimulation of the cavernous nerve on this measurement.
Saville-Griess assay
Saville-Griess assay, a colorimetric method based on the breakdown of SNO by mercury, was used to measure total S-nitrosothiols and total S-nitrosylated proteins (27). Rat penis was pulverized by mortar-pestle in liquid nitrogen. Lysates were prepared in Griess lysis buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl KCl, 5 mM KCl, 1% Triton-X100, 1 mM PMSF, 1 mM bathocuproinedisulfonic acid, 1 mM EDTA and 10 mM N-ethymaleimide) and centrifuged at 12,000 × g at 4 C for 15 minutes. Protein concentrations in lysates were measured with BCA protein assay reagent kit (Pierce Biotechnology, Rockford, lL). For assay of total S-nitrosothiol, 1 mg of lysate was incubated with 1% sulfanilamide and 0.1% N-(1-naphthyl) ethylenediamine with or without 0.4 mM HgCl2 for 20 min at room temperature in a microplate. The RSNO content was measured photometrically at 550 nm (Microplate reader, Model 680, BioRad), and the data were calculated as optical density / mg proteins. For assay of total S-nitrosylated proteins, 5 mg of lysate was passed through Hitrap desalting columns (GE Health Care, Piscataway, NJ) pre-equilibrated with Griess lysis buffer. Total protein-SNOs were separated from low molecular weight S-NO by desalting with 150 mM NaCl (27, 28), and the content of total nitrosylated proteins was measured after incubation with 1% sulfanilamide and 0.1% N-(1-naphthyl) ethylenediamine in the presence or absence of 0.4 mM HgCl2 at room temperature for 20 min. Data were calculated as optical density at 550 nm / mg proteins loaded on the column.
TMT switch assay
TMT switch assay, which indirectly detects Cysteine-SNO through their reduction by ascorbate into free thiols for subsequent labeling with TMT and detection by Western blot with anti-TMT antibody, was performed using a commercially available kit (Pierce S-Nitrosylation Western Blot Kit, Thermo Scientific, Rockford, lL). Penis lysates were prepared in HENS buffer (50 mM Tris-HCl, pH 8.0, 150 mM KCl, 1% Nonidet-P40, 1 mM PMSF, 1 mM bathocuproinedisulfonic acid, 1 mM EDTA and 10 mM N-ethymaleimide) as described (29) with some modifications. Briefly, 7 mg DTT-free samples were treated with methyl methanethiosulfonate (20 mM MMTS) in a buffer (250 mm HEPES, pH 7.7, 10 mm EDTA, 0.1 M neocuproine, 2.5% SDS) to block free thiols. Unreacted MMTS were removed by protein precipitation in acetone at −20 C, followed by resuspending acetone precipitated samples in HENS buffer. Sodium ascorbate was added to samples (except for negative controls) to convert the cysteine residues that had been S-nitrosylated to free thiols, which were then tagged with TMT. Tagged proteins were immunoprecipitated using anti-eNOS, anti-nNOS, or anti-sGC alpha1 antibody (rabbit polyclonal from Cell Signaling Technology, Beverly, MA, #9572 and #4234, and Sigma-Aldrich Chemical, St. Louis, MO, #G-4280, respectively) conjugated with protein- A/G agarose (Santa Cruz Biotechnology Inc.), according to manufacturer’s protocol (Abcam, Cambridge, MA). Briefly, diluted solution of antibody was prepared in 1 mg/ml BSA/PBS (0.2 µg antibody/100ul dilution buffer). Protein A/G bead slurry (50%/PBS) and diluted antibody solution were gently mixed at 1:1 ratio and rotated for 1 hr at 4 C. After centrifugation at 2,000 × g for 5 min, the pellet was resuspended in a dilution buffer after repeated washing. Western blot was performed against TMT. Because of high protein content required for the assay, total of 3–4 measurements were performed.
Western blot
Minced penile tissue was homogenized as described (30). Penile homogenates (50 µg) were resolved on 4–20% Tris gels and transferred to polyvinylidene difluoride membrane. Membranes were probed with polyclonal rabbit anti-phospho (P)-vasodilator-stimulated-protein (VASP) (Ser-239) antibody (Cell Signaling Technology, #3114) at 1:500 dilution. Membranes were then stripped and probed with rabbit anti-VASP (Cell Signaling Technology, #3132) at 1:1,000 dilution (31). For TMT-switch assay, TMT-tagged eNOS, nNOS, and sGC were immunoprecipitated and Western blotted against mouse anti-TMT antibody (1:1,000, Pierce S-Nitrosylation Western Blot Kit). A separate set of penile homogenates (30 µg) was run on a gel for β-actin (Sigma-Aldrich) at 1:5,000 dilution as a loading control for S-nitrosylated proteins (26). Bands were detected by horseradish peroxidase conjugated anti-rabbit or anti-mouse antibodies (GE Healthcare, UK), and analyzed using National Institutes of Health Image software. Results were expressed relative to vehicle-treated sham rats.
Statistical analyses
The number of animals was chosen to achieve a power of test of 0.80 to 0.85 with a probability of a Type I error to 0.05. The program GraphPad Prism (GraphPad Software) was used for statistical analysis. For ICP and Saville-Griess analyses, statistical analyses were performed using one-way analysis of variance, followed by Newman–Keuls multiple comparison test. The data were expressed as the mean ± standard error of the mean (SEM). For comparison of Western blot data between treatment groups, a non-parametric Kruskal-Wallis test was used, due to a small number of samples. For comparison of Western blot data between Sham and each treatment group, one-sided Mann-Whitney test was used to compare the experimental groups with the normalized control ratio (32). A value of P< 0.05 was considered to be statistically significant.
RESULTS
NAC treatment did not affect body weights
NAC treatment did not affect body weight gain (P>0.05) in sham (water treated rats: + 27.3 ± 3.1 g vs NAC treated rats: + 28.5 ± 1.8 g) or BCNI (water treated rats: + 23.3 ± 3.1 g vs NAC treated rats: + 24.8 ± 2.4 g) rats.
Cavernous nerve injury induces ED, which is prevented by NAC treatment
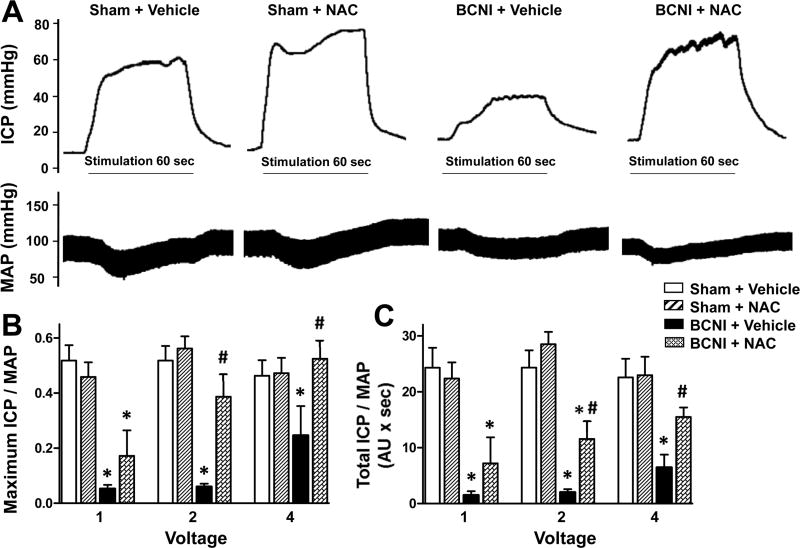
Cavernous nerve injury resulted in significantly (P<0.05) decreased erectile function (measured as maximal ICP/MAP and total ICP/MAP) compared to sham surgery (Fig. 1). At a low stimulation intensity (2 volts), both maximal ICP/MAP and total ICP/MAP were significantly (P<0.05) increased in BCNI rats treated with NAC compared to vehicle treatment, but total ICP/MAP was still significantly (P<0.05) lower than that of sham animals. At a higher stimulation intensity (4 volts), NAC significantly (P<0.05) increased erectile function relative to that of vehicle treated BCNI rats to levels comparable to sham animals. NAC did not affect erectile response in sham rats. These results evince NAC treatment in the prevention of ED following BCNI.
Fig. 1.
Erectile function is decreased in rats with cavernous nerve injury, which is prevented by treatment with a denitrosylating agent NAC. Sham-injured and BCNI-rats were treated with vehicle or NAC (300 mg/kg/day) starting 2 days before BCNI or sham injury and continuing for 2 weeks after the surgery. Electrical stimulation of the cavernous nerve was performed as voltage response (1, 2, and 4 V) at 16 Hz with square-wave duration of 5 msec for 1 min. Representative ICP and MAP responses to 4 V electrical stimulation (A). The stimulus interval is indicated by a solid bar. Erectile response to electrical stimulation of the cavernous nerve is indicated by maximal ICP/MAP (B) and total ICP/MAP (C). Each bar represents the mean ± SEM of 5-8 rats. *P < 0.05 vs. Sham + Vehicle; #P < 0.05 vs. BCNI + Vehicle.
Total S-nitrosothiols and total S-nitrosylated proteins are increased in the penis after cavernous nerve injury, which is partially prevented by NAC treatment
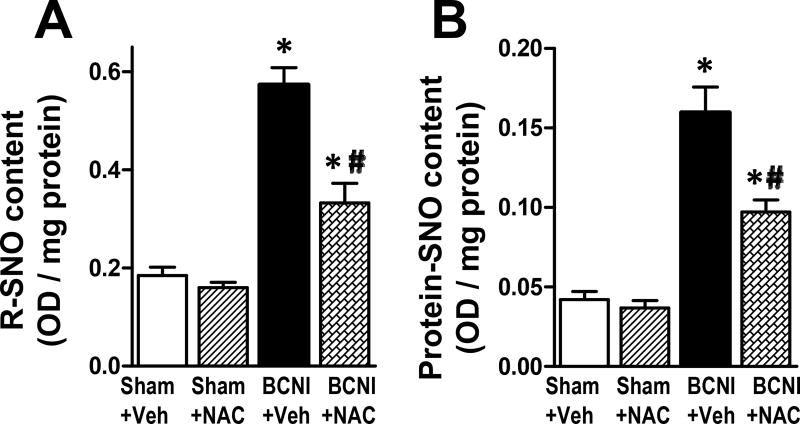
Cavernous nerve injury resulted in significantly (P<0.05) increased content of total S-nitrosothiols (Fig. 2A) and total S-nitrosylated proteins (Fig. 2B) in the penis, as measured by Saville-Griess assay. NAC treatment of BCNI rats significantly (P<0.05) reduced both measurements, but they were still significantly (P<0.05) higher than that of sham animals. NAC treatment did not affect the content of total S-nitrosothiols and total S-nitrosylated proteins in the penis of sham rats. These results indicate that NAC treatment partially prevented BCNI-induced increases in total S-nitrosothiols and total S-nitrosylated proteins in the penis.
Fig. 2.
Total S-nitrosothiol and total S-nitrosylated proteins are increased in the penis after cavernous nerve injury, which is partially prevented by treatment with a denitrosylating agent NAC. Saville-Griess assay was performed without (A, for total S-nitrosothiol) or with (B, for total S-nitrosylated proteins) desalting to eliminate low molecular weight nitrosylated thiols. Each bar represents the mean ± SEM of 6 rats. *P < 0.05 vs. Sham + Vehicle; #P < 0.05 vs. BCNI + Vehicle.
S-nitrosylation of eNOS is increased in the penis after cavernous nerve injury, and decreased by NAC treatment
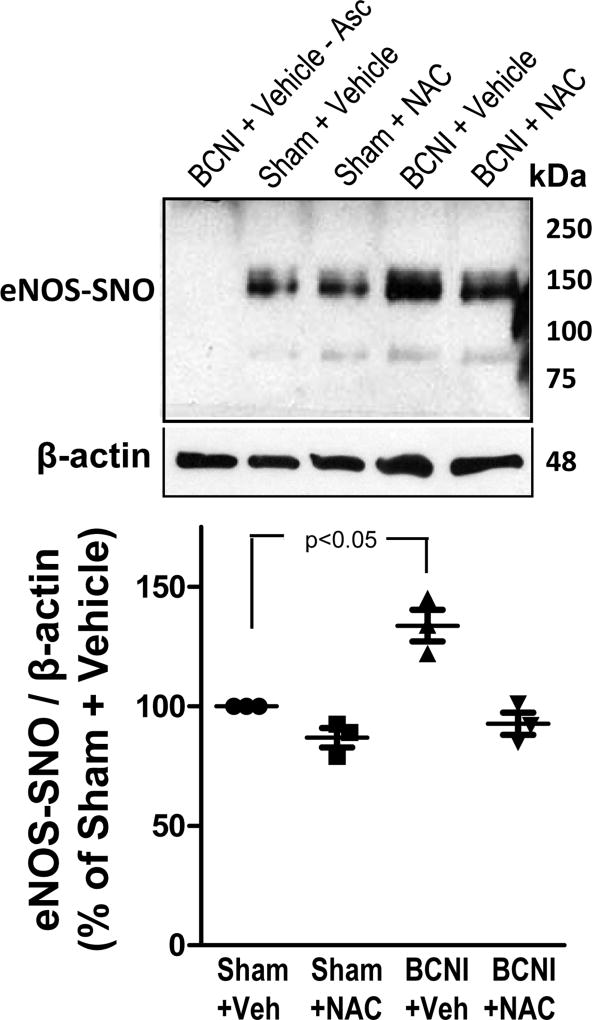
Cavernous nerve injury resulted in significantly (P<0.05) increased eNOS S-nitrosylation in the penis, as measured by TMT-switch assay (Fig. 3). NAC treatment of BCNI rats reduced eNOS S-nitrosylation approximately 1.5 fold, but it did not reach statistical significance.
Fig. 3.
S-nitrosylation of eNOS is increased in the penis after cavernous nerve injury, and is decreased by treatment with a denitrosylating agent NAC. eNOS S-nitrosylation was measured by TMT-switch assay consisting of immunoprecipitation of TMT-tagged proteins with anti-eNOS antibody, followed by Western blot against TMT. Upper panels are representative Western immunoblots of eNOS-SNO and β-actin in penes of Sham+Vehicle, Sham+NAC, BCNI+Vehicle, and BCNI+NAC rats (lanes 2–5). Lower panel represents quantitative analysis of eNOS-SNO/β-actin in the same treatment groups. No S-nitrosylation of eNOS could be detected in penile tissue of BCNI+Vehicle rats in the absence of ascorbate (lane 1). n=3
NAC treatment did not affect eNOS S-nitrosylation in the penis of sham rats. These results show that NAC treatment showed a trend towards prevention of BCNI-induced increases in eNOS S-nitrosylation in the penis.
nNOS is not S-nitrosylated in the rat penis
TMT-switch assay did not reveal nitrosylated nNOS in the penis of rats with or without BCNI and NAC treatment (data not shown).
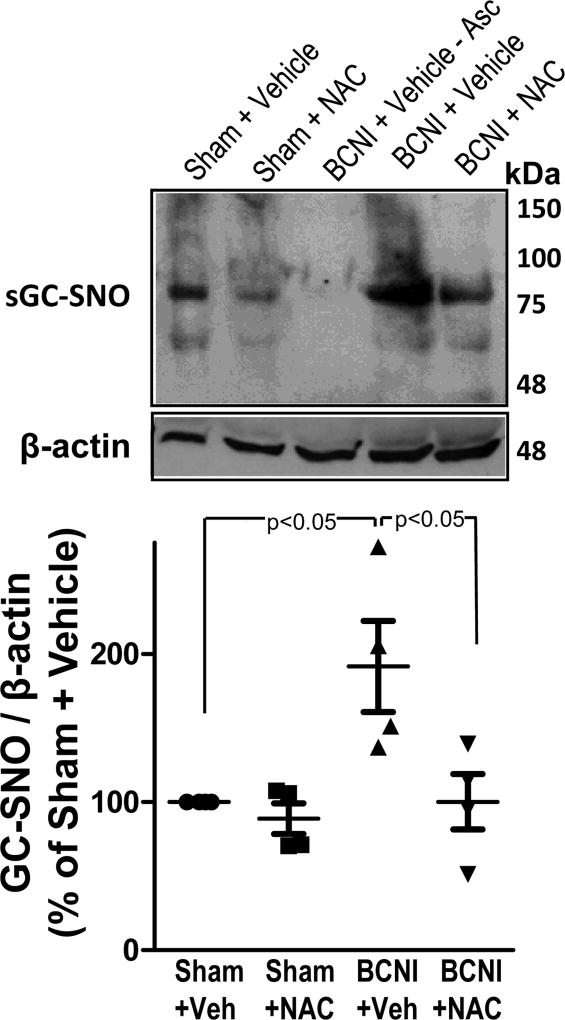
S-nitrosylation of sGC is increased in the penis after cavernous nerve injury, which is prevented by NAC treatment
Cavernous nerve injury resulted in significantly (P<0.05) increased sGC S-nitrosylation in the penis, as measured by TMT-switch assay (Fig. 4). NAC treatment significantly (P<0.05) decreased sGC S-nitrosylation in penes of BCNI rats to levels comparable to sham levels, and it had no effect on sGC S-nitrosylation in the penis of sham rats. These results demonstrate that NAC treatment prevented BCNI-induced increases in sGC S-nitrosylation in the penis.
Fig. 4.
S-nitrosylation of sGC is increased in the penis after cavernous nerve injury, which is prevented by treatment with a denitrosylating agent NAC. sGC S-nitrosylation was measured by TMT-switch assay consisting of immunoprecipitation of TMT-tagged proteins with anti-sGC antibody, followed by Western blot against TMT. Upper panels are representative Western immunoblots of sGC-SNO and β-actin in penes of Sham+Vehicle, Sham+NAC, BCNI+Vehicle, and BCNI+NAC rats (lanes 1, 2, 4, 5). Lower panel represents quantitative analysis of sGC-SNO/β-actin in the same treatment groups. No S-nitrosylation of sGC could be detected in penile tissue of BCNI+Vehicle rats in the absence of ascorbate (lane 3). n=4.
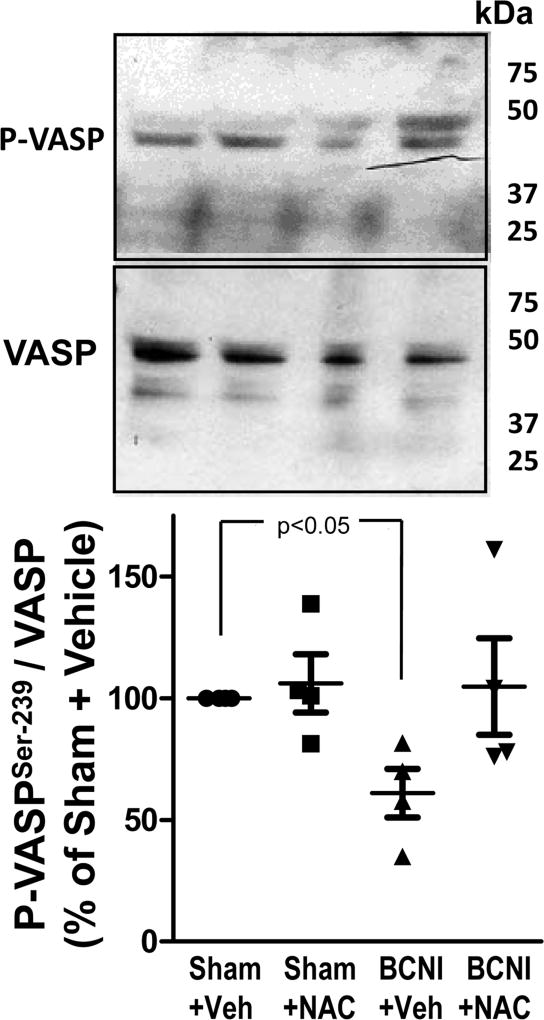
Protein expression of P-VASP (Ser-239) is decreased in the penis after cavernous nerve injury, and increased by NAC treatment
The phosphorylation state of VASP on Ser-239, a substrate of PKG-I, is a surrogate parameter of PKG-I function and represents the integrity of the NO/cGMP signaling pathway (33). Cavernous nerve injury resulted in significantly (P<0.05) decreased levels of P-VASP (Ser-239) in the penis, as measured by Western blot (Figure 5). NAC treatment increased P-VASP (Ser-239) expression in penes of BCNI rats approximately 2-fold, but it did not reach statistical significance. NAC treatment had no effect on P-VASP (Ser-239) expression in the penis of sham rats. These results suggest that NAC treatment showed a trend towards prevention of BCNI-induced decrease in PKG function in the penis.
Fig. 5.
Protein expression of P-VASP (Ser-239) is decreased in the penis after cavernous nerve injury, and is increased by treatment with a denitrosylating agent NAC. Upper panels are representative Western immunoblots of P-VASP (Ser-239)/VASP in penes of Sham+Vehicle, Sham+NAC, BCNI+Vehicle, and BCNI+NAC rats. Lower panel represents quantitative analysis of P-VASP/VASP in the same treatment groups. n=4.
DISCUSSION
Our results show that ED under conditions of penile neuropathy induced by cavernous nerve injury is mediated in part by S-nitrosylation of eNOS and its downstream signaling mediator sGC. We also show that denitrosylation in the face of cavernous nerve injury may protect erectile function by preserving NOS signaling pathway function.
The structure and function of many proteins are dynamically regulated by S-nitrosylation. NOS enzymes themselves can be self-nitrosylated, and thus inhibited, leading to altered NO signaling responses (34, 35). In endothelial cells, S-nitrosylated eNOS exhibits reduced catalytic activity and indicates the loss of the enzyme’s functional coupled state (34, 36, 37). Furthermore, S-nitrosylation of eNOS is inversely related to eNOS phosphorylation on Ser-1177, a site associated with eNOS activation (34). Here, we found that total S-nitrosothiols and total S-nitrosylated proteins are increased in the penis following cavernous nerve injury, in association with ED, suggesting the involvement of S-nitrosylation in cavernous nerve injury-induced ED. More importantly, we found that S-nitrosylation of eNOS was also increased in the penis after cavernous nerve injury, implying that this posttranslational modification of eNOS is a novel mechanism that may reduce the enzyme’s activity in the face of cavernous nerve injury. These findings are in line with previous studies showing decreased eNOS activity by decreased eNOS phosphorylation on Ser-1177 in the penis of rats and mice following cavernous nerve injury (7, 38). Furthermore, we show that S-nitrosylation of sGC is also increased in the penis following cavernous nerve injury, conceivably attenuating the function of the NO downstream signaling mediator PKG. Indeed, phosphorylation of VASP (Ser-239), a target of PKG, was decreased in the neuropathic penis, supporting our hypothesis. S-nitrosylation of sGC has previously been shown to inhibit its enzymatic activity in human umbilical vein endothelial cells, aortic smooth muscle cells, and cardiomyocytes, resulting in attenuation of NO-induced sGC activation and subsequent PKG activation (39–42). Interestingly, VASP, a constituent of focal adhesions with the main role in maintaining vascular barrier integrity, may itself be S-nitrosylated by proinflammatory agents, resulting in the disruption of barrier integrity and increased endothelial permeability (43). Further studies will delineate whether eNOS-mediated S-nitrosylation of VASP, or changes in VASP protein expression, are linked to ED in response to cavernous nerve injury.
To confirm that cavernous nerve injury induced ED through S-nitrosylation of eNOS and sGC, we treated rats with NAC starting 2 days prior to nerve injury. NAC is a physiologic precursor of glutathione, which may act by scavenging superoxide or by facilitating the reversion of S-nitrosylation due to thiol depletion (26, 39, 44). Within cells, glutathione and L-cysteine are important sources of thiol groups, which are able to accept and transfer NO by transnitrosylation. Our results, showing that NAC prevented cavernous nerve injury-induced erectile dysfunction and S-nitrosylation of sGC, and showed a trend towards preventing cavernous nerve injury-induced S-nitrosylation of eNOS and PKG dysfunction, support the idea that cavernous nerve injury-dependent S-nitrosylation of main mediators of erectile function is a mechanism of ED. However, because NAC is an effective scavenger of free radicals as well as a major contributor to maintenance of the cellular GSH status and thus a denitrosylating agent, we cannot exclude that both effects of NAC may be involved in its protective effect observed in this animal model.
In this study, we could not detect nNOS S-nitrosylation in the penis. S-nitrosylation of nNOS and the consequent decrease in this enzyme’s activity has been documented in primary cortical and hippocampal neurons (35, 45) and brainstem (46). Our findings imply that S-nitrosylation mainly affects eNOS and its downstream signaling in the neuropathic penis induced by cavernous nerve injury, while other modifications of nNOS, such as decreased synthesis (8, 13, 14, 47, 48) may be primarily responsible for neurogenic ED in this condition. It is also possible that nNOS is S-nitrosylated in the neuropathic penis but to a smaller extent than eNOS, making this posttranslational modification hard to detect with available techniques.
The mechanism underlying cavernous nerve injury-induced increased S-nitrosylation of target proteins in the penis is not known. S-nitrosylation depends on the intracellular redox state, and this posttranslational modification occurs in the presence of increased oxidative and nitrosative stress (49). Increased oxidative/nitrosative stress leads to a decreased intracellular glutathione pool, which may favor SNO formation by attenuating transnitrosylation or denitrosylation (22). Increased oxidative stress and decreased expression of antioxidant factors have been described in the penis following cavernous nerve injury (9–11), suggesting altered redox state as a cause of increased S-nitrosylation. Increased NO production via the inducible NOS (50) and overactive, but dysfunctional nNOS (35), has also been shown to increase S-nitrosylation levels. It is possible that iNOS expressed in infiltrated immune cells in the corpus cavernosum under conditions of cavernous nerve injury may have played a role in the observed protein S-nitrosylation. S-nitrosylation is mainly dependent upon a dynamic denitrosylation process such as that regulated by the thioredoxin system and S-nitrosoglutathione reductase (22). As such, it is conceivably that once induced, protein S-nitrosylation may be propagated in the absence of nitrosylating agents (NO and reactive oxygen/nitrogen species).
We acknowledge that several additional research areas require investigation. The assessment of functional integrity of the corpus cavernosum with and without NAC treatment could be reinforced by quantitating eNOS and nNOS content and oxidative stress in the corpus cavernosum. Future experiments will be required to determine the dose-dependency of NAC, as well as effects of other denitrosylating agents, to further substantiate our finding. Furthermore, for this study we initiated treatment with NAC before cavernous nerve injury to evaluate the preventive action of this denitrosylating agent. Further studies may determine the effectiveness of NAC given post cavernous nerve injury short- and long term in reversing S-nitrosylation and producing functional outcomes in this model.
Our study is the first to show that nitrosylation occurs in the penis after cavernous nerve injury and contributes to erectile dysfunction; as such, many questions remain unanswered and should be topics of further investigations. For example, the use of specific NOS inhibitors and mice lacking eNOS, nNOS, iNOS, or all NOS isoforms, would be crucial to determine which NOS isoform contributes to nitrosylation in the setting of cavernous nerve injury. Experimental manipulation of specific enzymatic sources of oxidative/nitrosative stress, such as uncoupling of a specific NOS isoform, NADPH oxidase, or xanthine oxidase, would also further our knowledge of the sources of oxidative stress aiding to nitrosylation in animal models of cavernous nerve injury. In addition, using mice deficient in denitrosylation mechanisms, such as GSNOR-deleted mice, would specifically implicate the significance of denitrosylation in the cavernous nerve injury-induced ED.
In summary, we describe S-nitrosylation of eNOS and sGC in the penis to be a novel mechanism for the derangement of NO signaling pathway that promotes ED under conditions of cavernous nerve injury. We also determine that denitrosylation in the face of cavernous nerve injury may protect erectile function by preserving NO/cGMP signaling pathway function.
Acknowledgments
This work was supported by a grant from the National Institutes of Health, USA (NIH/NIDDK grant R01DK067223 to ALB)
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Facio F, Jr, Burnett AL. Penile rehabilitation and neuromodulation. Scientific World Journal. 2009;9:652–664. doi: 10.1100/tsw.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulhall JP. Defining and reporting erectile function outcomes after radical prostatectomy: challenges and misconceptions. J Urol. 2009;181:462–471. doi: 10.1016/j.juro.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Angeloni NL, Bond CW, McVary KT, Podlasek CA. Sonic hedgehog protein is decreased and penile morphology is altered in prostatectomy and diabetic patients. PLoS One. 2013;14:e70985. doi: 10.1371/journal.pone.0070985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacono F, Giannella R, Somma P, Manno G, Fusco F, Mirone V. Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005;173:1673–1676. doi: 10.1097/01.ju.0000154356.76027.4f. [DOI] [PubMed] [Google Scholar]
- 5.Hannan JL, Kutlu O, Stopak BL, Liu X, Castiglione F, Hedlund P, et al. Valproic acid prevents penile fibrosis and erectile dysfunction in cavernous nerve-injured rats. J Sex Med. 2014;11:1442–1451. doi: 10.1111/jsm.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, Lue TF. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song KM, Jin HR, Park JM, Choi MJ, Kwon MH, Kwon KD, et al. Intracavernous delivery of stromal vascular fraction restores erectile function through production of angiogenic factors in a mouse model of cavernous nerve injury. J Sex Med. 2014;11:1962–1973. doi: 10.1111/jsm.12597. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Matheu MP, Sun F, Wang L, Sanford MT, Ning H, et al. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. J Sex Med. 2016;13:22–32. doi: 10.1016/j.jsxm.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Lagoda G, Jin L, Lehrfeld TJ, Liu T, Burnett AL. FK506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med. 2007;4:908–916. doi: 10.1111/j.1743-6109.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 10.Lagoda G, Xie Y, Sezen SF, Hurt KJ, Liu L, Musicki B, et al. FK506 neuroprotection after cavernous nerve injury is mediated by thioredoxin and glutathione redox systems. J Sex Med. 2011;8:3325–3334. doi: 10.1111/j.1743-6109.2011.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Ding XG, Li SW, Zheng H, Zheng XM, Navin S, Li L, Wang XH. Role of oxidative stress in surgical cavernous nerve injury in a rat model. J Neurosci Res. 2015;93:922–929. doi: 10.1002/jnr.23545. [DOI] [PubMed] [Google Scholar]
- 12.Hannan JL, Matsui H, Sopko NA, Liu X, Weyne E, Albersen M, et al. Caspase-3 dependent nitrergic neuronal apoptosis following cavernous nerve injury is mediated via RhoA and ROCK activation in major pelvic ganglion. Sci Rep. 2016;6:29416. doi: 10.1038/srep29416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CH, Kim HS, Goo MJ, Kang KK, Ahn BO, Kim SH, et al. Chronic administration of udenafil, a selective phosphodiesterase type 5 inhibitor, promotes erectile function recovery in an animal model of bilateral cavernous nerve crush injury. J Sex Med. 2011;8:1330–1340. doi: 10.1111/j.1743-6109.2011.02228.x. [DOI] [PubMed] [Google Scholar]
- 14.Shindel AW, Xin ZC, Lin G, Fandel TM, Huang YC, Banie L, et al. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (Epimedium spp.) in vitro and in vivo. J Sex Med. 2010;7:1518–1528. doi: 10.1111/j.1743-6109.2009.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannan JL, Albersen M, Kutlu O, Gratzke C, Stief CG, Burnett AL, et al. Inhibition of Rho-kinase improves erectile function, increases nitric oxide signaling and decreases penile apoptosis in a rat model of cavernous nerve injury. J Urol. 2013;189:1155–1161. doi: 10.1016/j.juro.2012.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aliperti LA, Lasker GF, Hagan SS, Hellstrom JA, Gokce A, Trost LW, et al. Efficacy of pioglitazone on erectile function recovery in a rat model of cavernous nerve injury. Urology. 2014;84:1122–1127. doi: 10.1016/j.urology.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 17.Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- 18.Burnett AL. Nitric oxide in the penis: physiology and pathology. J Urol. 1997;157:320–324. [PubMed] [Google Scholar]
- 19.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulton DJ. Transcriptional and Posttranslational Regulation of eNOS in the Endothelium. Adv Pharmacol. 2016;77:29–64. doi: 10.1016/bs.apha.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Anand P, Stamler JS. Enzymatic mechanisms regulating protein S-nitrosylation: implications in health and disease. J Mol Med (Berl) 2012;90:233–244. doi: 10.1007/s00109-012-0878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–296. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura T, Lipton SA. Emerging role of protein-protein transnitrosylation in cell signaling pathways. Antioxid Redox Signal. 2013;18:239–249. doi: 10.1089/ars.2012.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musicki B, Lagoda G, Goetz T, La Favor JD, Burnett AL. Transnitrosylation: a factor in nitric oxide-mediated penile erection. J Sex Med. 2016;13:808–814. doi: 10.1016/j.jsxm.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burnett AL, Sezen SF, Hoke A, Caggiano AO, Iaci J, Lagoda G, et al. GGF2 is neuroprotective in a rat model of cavernous nerve injury-induced erectile dysfunction. J Sex Med. 2015;12:897–905. doi: 10.1111/jsm.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fioramonti X, Deak A, Deshpande S, Carneiro L, Zhou C, Sayed N, et al. Hypothalamic S-nitrosylation contributes to the counter-regulatory response impairment following recurrent hypoglycemia. PLoS One. 2013;8:e68709. doi: 10.1371/journal.pone.0068709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann J, Haendeler J, Zeiher AM, Dimmeler S. TNFalpha and oxLDL reduce protein S-nitrosylation in endothelial cells. J Biol Chem. 2001;276:41383–41387. doi: 10.1074/jbc.M107566200. [DOI] [PubMed] [Google Scholar]
- 28.Eu JP, Liu L, Zeng M, Stamler JS. An apoptotic model for nitrosative stress. Biochemistry. 2000;39:1040–1047. doi: 10.1021/bi992046e. [DOI] [PubMed] [Google Scholar]
- 29.Murray CI, Uhrigshardt H, O'Meally RN, Cole RN, Van Eyk JE. Identification and quantification of S-nitrosylation by cysteine reactive tandem mass tag switch assay. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.013441. M111.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci USA. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musicki B, Liu T, Lagoda GA, Strong TD, Sezen SF, Johnson JM, et al. Hypercholesterolemia-induced erectile dysfunction: endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J Sex Med. 2010;7:3023–3032. doi: 10.1111/j.1743-6109.2010.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludbrook J. Should we use one-sided or two-sided P values in tests of significance? Clin Exp Pharmacol Physiol. 2013;40:357–361. doi: 10.1111/1440-1681.12086. [DOI] [PubMed] [Google Scholar]
- 33.Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, et al. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res. 2000;87:999–1005. doi: 10.1161/01.res.87.11.999. [DOI] [PubMed] [Google Scholar]
- 34.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005;280:19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 35.Qu ZW, Miao WY, Hu SQ, Li C, Zhuo XL, Zong YY, et al. N-methyl-D-aspartate receptor-dependent denitrosylation of neuronal nitric oxide synthase increase the enzyme activity. PLoS ONE. 2012;7:e52788. doi: 10.1371/journal.pone.0052788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erwin PA, Mitchell DA, Sartoretto J, Marletta MA, Michel T. Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J Biol Chem. 2006;281:151–157. doi: 10.1074/jbc.M510421200. [DOI] [PubMed] [Google Scholar]
- 37.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci U S A. 2004;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulhall JP, Müller A, Donohue JF, Mullerad M, Kobylarz K, Paduch DA, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med. 2008;5:1126–1136. doi: 10.1111/j.1743-6109.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 39.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci U S A. 2007;104:12312–12317. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sayed N, Kim DD, Fioramonti X, Iwahashi T, Durán WN, Beuve A. Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res. 2008;103:606–614. doi: 10.1161/CIRCRESAHA.108.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beuve A, Wu C, Cui C, Liu T, Jain MR, Huang C, et al. Identification of novel S-nitrosation sites in soluble guanylyl cyclase, the nitric oxide receptor. J Proteomics. 2016;138:40–47. doi: 10.1016/j.jprot.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajagopal S, Nalli AD, Kumar DP, Bhattacharya S, Hu W, Mahavadi S, et al. Cytokine-induced S-nitrosylation of soluble guanylyl cyclase and expression of phosphodiesterase 1A contribute to dysfunction of longitudinal smooth muscle relaxation. J Pharmacol Exp Ther. 2015;352:509–518. doi: 10.1124/jpet.114.221929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamorano P, Marín N, Córdova F, Aguilar A, Meininger C, Boric MP, et al. S-nitrosylation of VASP at cysteine 64 mediates the inflammation-stimulated increase in microvascular permeability. Am J Physiol Heart Circ Physiol. 2017;313:H66–H71. doi: 10.1152/ajpheart.00135.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu HM, Xu J, Li C, Zhou C, Zhang F, Han D, et al. Coupling between neuronal nitric oxide synthase and glutamate receptor 6-mediated c-Jun N-terminal kinase signaling pathway via S-nitrosylation contributes to ischemia neuronal death. Neuroscience. 2008;155:1120–1132. doi: 10.1016/j.neuroscience.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 46.Palmer LA, Kimberly deRonde, Brown-Steinke K, Gunter S, Jyothikumar V, Forbes MS, et al. Hypoxia-induced changes in protein s-nitrosylation in female mouse brainstem. Am J Respir Cell Mol Biol. 2015;52:37–45. doi: 10.1165/rcmb.2013-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao CH, Wu YN, Chen BH, Lin Y, Ho HO, Chiang HS. Neuroprotective effect of docosahexaenoic acid nanoemulsion on erectile function in a rat model of bilateral cavernous nerve injury. Sci Rep. 2016;6:33040. doi: 10.1038/srep33040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Yang Q, Zheng T, Bian J, Sun X, Shi Y, et al. Neurotrophic effect of adipose tissue-derived stem cells on erectile function recovery by pigment epithelium-derived factor secretion in a rat model of cavernous nerve injury. Stem Cells Int. 2016;2016:5161248. doi: 10.1155/2016/5161248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forrester MT, Foster MW, Stamler JS. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J Biol Chem. 2007;282:13977–13983. doi: 10.1074/jbc.M609684200. [DOI] [PubMed] [Google Scholar]
- 50.Nakazawa H, Chang K, Shinozaki S, Yasukawa T, Ishimaru K, Yasuhara S, et al. iNOS as a driver of inflammation and apoptosis in mouse skeletal muscle after burn injury: possible involvement of Sirt1 S-Nitrosylation-mediated acetylation of p65 NF-κB and p53. PLoS One. 2017;12:e017039. doi: 10.1371/journal.pone.0170391. [DOI] [PMC free article] [PubMed] [Google Scholar]