Abstract
Background and Aims
The risk of hepatocellular carcinoma (HCC) in patients with hepatitis C (HCV) receiving direct acting antivirals (DAA) has been debated. This study aims to describe the incidence of HCC among patients listed for liver transplantation (LT) in the DAA era.
Methods
Individuals with cirrhosis listed for LT from January 2003 to December 2015 were identified using the Scientific Registry for Transplant Recipients database. Patients with HCC at listing or HCC exception within 180 days were excluded. Patients were divided into 3 eras based on listing date: Eras 1 (2003–2010), 2 (2011–2013), and 3 (2014–2015). Incidence rates of HCC were calculated by era and compared using incident rate ratios (IRR). The association between HCC and listing era was evaluated using Cox regression and competing risk analyses, the latter considering death and LT as competing events.
Results
Of 48,158 eligible waitlist registrants, 3,112 (6.5%) received HCC exceptions after a median of 493 days. In 20,039 individuals with HCV, the incidence of HCC was 49% higher in Era 3 vs. 1 (IRR 1.49, 95% CI 1.24–1.79). In multivariate analysis, those in Era 3 had a higher hazard of HCC compared to Era 1 (HR 1.22, 95% CI 1.01–1.48). However, in multivariable competing risks analysis with death and LT considered as competing events for de novo HCC, era was no longer associated with HCC (sHR 0.83, 95% CI 0.69–1.00).
Conclusion
In this large population-based cohort of LT registrants, the incidence of HCC among HCV patients has increased in the DAA era. Competing risks analysis suggests that this may be explained by changes in rates of LT and waitlist mortality in the HCV population during this time.
Keywords: Sustained virologic response, direct acting antiviral therapy, hepatocellular carcinoma, hepatitis C virus, liver transplantation
Chronic hepatitis C virus (HCV) infection has been the leading indication for liver transplantation (LT) in the United States.1, 2 In late 2013, the approval of sofosbuvir, a potent and safe direct-acting antiviral (DAA), heralded a revolutionary era in the treatment of chronic HCV, and there are now multiple effective DAA-based regimens available for the treatment of HCV. The efficacy and safety of DAA regimens in patients with decompensated cirrhosis have been demonstrated, with the majority of patients exhibiting improvement in Model for End-Stage Liver Disease (MELD) scores and other symptoms of decompensation after sustained virologic response (SVR).3–6 Reduced rates of transplant listing and LT have been observed among patients with HCV, and patients with HCV listed for LT have demonstrated the potential to be removed from the LT waitlist among candidates after viral eradication.7–10 Therefore, there has been widespread optimism that the advent of DAAs for the treatment of HCV would also lead to a decrease in the burden of HCV-related hepatocellular carcinoma (HCC).11, 12
Several recent observational studies have described an incidence and recurrence of HCC in DAA-treated cirrhotics who have achieved SVR that are higher than expected.13–17 This phenomenon has been postulated to be due to a persistent disruption of immunological integrity or failure of immune surveillance, leading to a more aggressive tumor profile.18, 19 An increased rate of HCC incidence or recurrence after DAA therapy has not been observed, however, in other cirrhotic cohorts, which are largely comprised of patients with compensated liver disease.20–24 Overall, data to date have been conflicting, and the association between DAA therapy and HCC in cirrhotic patients after SVR remains unclear, especially in those with more advanced disease.
In this work, we describe the incidence of HCC in a population of patients with HCV with advanced cirrhosis, using data from the US transplant registry. We hypothesized that if DAA-induced SVR increases the risk of HCC meaningfully, there would be a detectable signal in HCC incidence among patients with end-stage liver disease, who are at the highest risk. The aim of the study was to compare the incidence of HCC before and after the availability of DAA among LT candidates, while adjusting for other variables that could affect incidence.
Methods
Patients and Data Acquisition
This is a retrospective cohort study using data from the Scientific Registry of Transplant Recipients (SRTR), which includes data regarding all liver transplants performed in the United States, as submitted by members of the Organ Procurement and Transplantation Network (OPTN).25 All adult patients aged ≥ 18 years listed for primary liver transplantation from January 1, 2003 to December 31, 2015 were included in the analysis, with follow-up until December 15, 2016.
Patients were included if the underlying cause of cirrhosis based on their primary listing diagnosis (based on SRTR diagnostic codes or manually entered text) was HCV, hepatitis B virus (HBV), alcoholic liver disease, nonalcoholic steatohepatitis (NASH), cryptogenic cirrhosis, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis, alpha-1 antitrypsin deficiency, hereditary hemochromatosis, or Wilson disease. Following prior convention, patients listed with a diagnosis of cryptogenic cirrhosis with a body mass index (BMI) ≥ 30, were classified as NASH.26, 27 Patients with combined diagnoses of HCV and any other cause were classified as having liver disease due to HCV.
Patients were excluded if they lacked a unique identifier or listing diagnosis, if they had a listing diagnosis not specified in the inclusion criteria or were listed as Status 1, if they had a history of prior LT, or if their follow-up time was incomplete or < 180 days. To exclude prevalent cases of HCC in the cohort, all patients with a primary or secondary listing diagnosis of HCC, as well as those granted MELD exception application for HCC within 180 days of initial listing, were excluded.
Demographic and clinical data at the time of listing for LT were extracted from the SRTR database. The development of incident HCC was determined by the initial date of a MELD-HCC application, which was prospectively entered into the SRTR database. Since 2002, patients with HCC have been eligible to receive MELD exception points and thus higher priority on the transplant list. This has been based on generally accepted histologic or imaging criteria, which was further standardized by OPTN in 2011.28 Under the current policy, lesions are classified as HCC if there is enhancement during the late arterial phase, with features of washout during later phases, peripheral rim enhancement, or growth by 50% or more on serial imaging obtained ≤ 6 months apart. To qualify for MELD exception points, the burden of HCC must be within Milan criteria (up to three OPTN class 5A or 5B lesions, each larger than 1 cm and smaller than 3 cm, or one OPTN class 5B lesion measuring larger than 2 cm or smaller than 5 cm), prior to any locoregional therapy.
Follow-up time started 180 days after initial listing and ended at (1) the date of HCC application for those with de novo HCC, (2) December 15, 2016 for those still awaiting LT, or (3) the date of removal from the waitlist for any cause, e.g. LT, death or clinical deterioration, or clinical improvement.
Data Analysis
Patients were divided into three cohorts according to the time of initial registration: Era 1, corresponding to the period when interferon (IFN) was the mainstay for HCV therapy (2003–2010); Era 2, when protease inhibitors (PI) were available (2011–2013); and Era 3, following the introduction of sofosbuvir and other DAAs (2014–2015).
Annual HCC incidence rates (IR) were calculated for each etiology of liver disease, and an incidence rate ratio (IRR) comparing the HCC incidence by era of LT wait-listing was calculated using the Mantel-Haenszel method. Univariate and multivariate Cox proportional hazards analyses were performed to describe the association between era of LT wait-listing and de novo HCC, after adjusting for risk factors known to be associated with HCC in this population.29 The proportional hazards assumption was assessed using log-log plots and Schoenfeld residuals. Competing risks regression was performed to evaluate the association between era of LT wait-listing and de novo HCC, adjusted for the same variables as above, and with LT and death treated as competing events. The competing risks analysis considers that waitlist candidates are at risk not only for development of de novo HCC, but also for LT and death, which are considered as events rather than censored as would be in conventional survival analysis. Thus, multivariable competing risk models consider the subdistribution hazard of the cumulative incidence function — in this case, the subdistribution hazard ratio (sHR) represents the hazard of de novo HCC in the presence of these competing events.
Statistical analyses were performed using Stata (Version 12.1, College Station, TX). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki protocol and was approved by the Queen’s University Health Sciences Research Ethics Board (DMED-1688-14).
Results
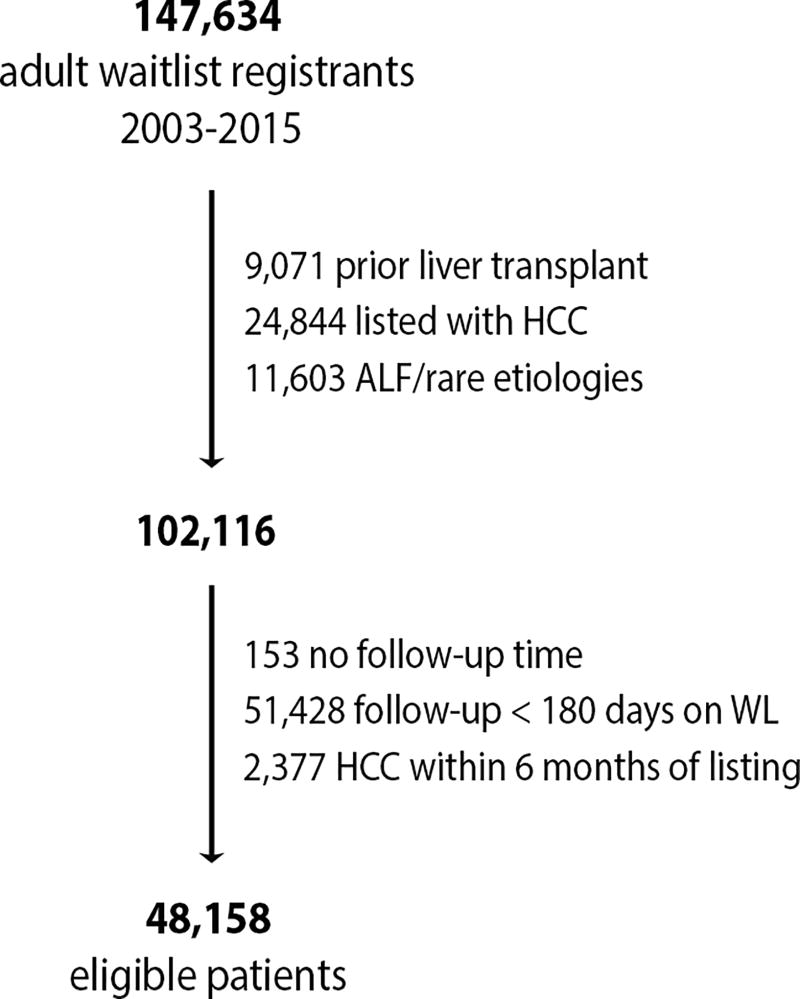
Of 147,634 waitlist registrants for liver transplantation between January 1, 2003 and December 31, 2015 in the SRTR database, 48,158 met the eligibility criteria (Figure 1). Table 1 describes the characteristics of the patients included in the study. The most common etiologies of liver disease were HCV (41.6%), alcohol (20.6%), and NASH/cryptogenic (19.8%). There were 30,028 (62.4%) patients registered in Era 1 (IFN era, 2003–2010), 11,361 (23.6%) in Era 2 (PI era, 2011–2013), and 6,769 (14.1%) in Era 3 (DAA era, 2014–2015). Over time, the proportions of candidates with HCV and those with de novo HCC decreased, whereas those with diabetes and those with NASH, cryptogenic, and alcoholic liver disease increased.
Figure 1.
Cohort recruitment diagram.
Table 1.
Demographic characteristics for the overall cohort (n=48,158).
| Overall 2003–2015 n = 48,158 |
Era 1 2003–2010 n = 30,028 |
Era 2 2011–2013 n = 11,361 |
Era 3 2014–2015 n = 6,769 |
p* | |
|---|---|---|---|---|---|
| Age (IQR) | 55 (49–60) | 54 (48–59) | 56(50–61) | 56 (50–62) | <0.001 |
| Male (%) | 61.9 | 61.9 | 62.3 | 60.1 | 0.19 |
| Race (%) | |||||
| Caucasian non-Hispanic | 73.6 | 73.8 | 73.4 | 73.0 | <0.001 |
| Hispanic/Latino | 16.0 | 16.1 | 16.1 | 15.3 | |
| African American | 6.6 | 6.4 | 6.7 | 7.4 | |
| Asian | 3.0 | 3.0 | 2.8 | 3.1 | |
| Other/Missing | 0.8 | 0.7 | 1.0 | 1.2 | |
| Etiology (%) | |||||
| HCV | 41.6 | 45.8 | 38.6 | 27.9 | <0.001 |
| NASH/CC/EtOH | 40.4 | 35.9 | 44.1 | 54.2 | |
| HBV | 2.5 | 2.8 | 1.9 | 2.0 | |
| Autoimmune† | 14.2 | 14.2 | 13.9 | 14.4 | |
| Other¶ | 1.3 | 1.3 | 1.5 | 1.5 | |
| Diabetes (%) | 26.8 | 25.7 | 27.7 | 30.0 | <0.001 |
| CTP score (%) | |||||
| 5–6 | 13.4 | 13.5 | 13.3 | 13.2 | <0.001 |
| 7–9 | 40.9 | 40.3 | 39.5 | 46.4 | |
| 10–15 | 45.6 | 46.2 | 47.2 | 40.5 | |
| Median follow-up time, days (IQR) | 493 (189–1083) | 600 (206–1358) | 482 (176–1013) | 305 (156–515) | <0.001 |
| De novo HCC (%) | 6.5 | 7.5 | 5.7 | 3.1 | <0.001 |
comparing Era 1 vs. 2 vs. 3
includes autoimmune hepatitis, PSC, PBC
includes hemochromatosis, A1AT and Wilson
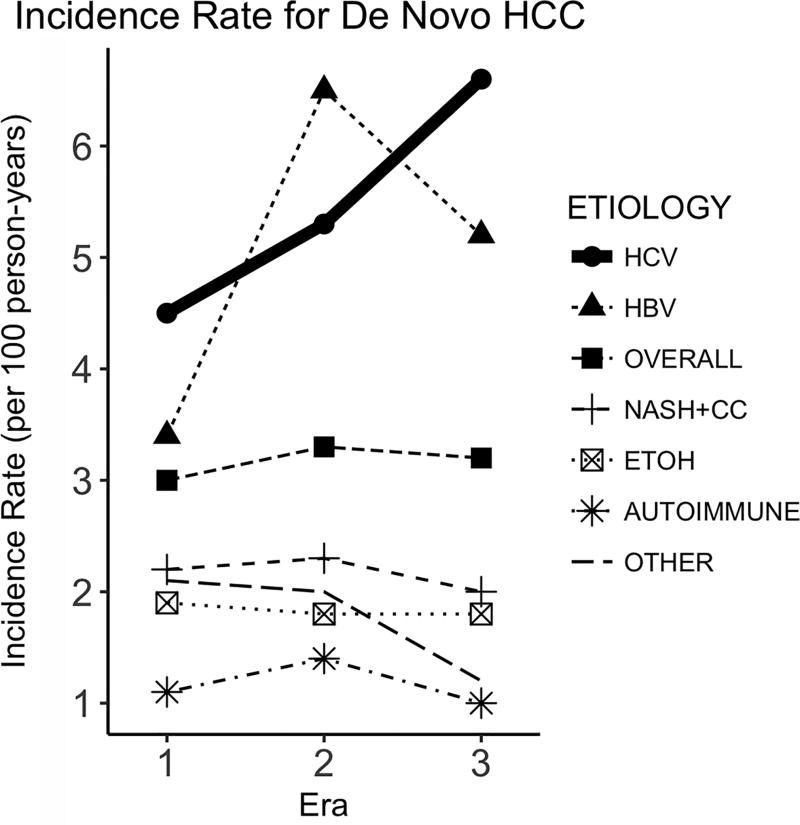
The median duration of observed follow-up on the LT waitlist was 493 days (IQR [interquartile range], 189–1083 days), during which 3,112 (6.5%) developed de novo HCC. The incidence rate of HCC is described in Table 2 and Figure 2. In Table 2, the incidence of HCC did not seem to change substantially over time in the overall registrant population — it increased modestly from 3.0 per 100 person-years (py) in Era 1 to 3.3 per 100 py in Era 2 and 3.2 per 100 py in Era 3. When these rates are formally compared with rate ratios, there was a 11% increase (IRR 1.11, 95% confidence interval [CI] 1.02–1.21) from Era 1 to Era 2, followed by a non-significant decrease (IRR 0.98, 95% CI 0.84–1.15) from Era 2 to Era 3, summating to a non-significant increase between Eras 1 and 3 (IRR 1.09, 95% CI 0.95–1.25).
Table 2.
Average incidence rate of HCC per 100 person-years (rate ratios calculated based on Mantel-Haenszel method).
| Incidence Rate per 100 person years (95% CI) |
Rate Ratio (95% CI, P value) |
|||||
|---|---|---|---|---|---|---|
| 1: IFN 2003–2010 |
2: PI 2011–2013 |
3: DAA 2014–2015 |
PI v. IFN | DAA v. PI | DAA v. IFN | |
| Overall (n= 3,112) | 3.0 (2.9–3.1) | 3.3 (3.1–3.6) | 3.2 (2.8–3.7) | 1.11 (1.02–1.21) P = 0.02 | 0.98 (0.84–1.15) P = 0.82 | 1.09 (0.95–1.25) P = 0.24 |
| HCV (n = 1,982) | 4.5 (4.2–4.7) | 5.3 (4.8–5.8) | 6.6 (5.6–7.9) | 1.19 (1.06–1.33) P = 0.002 | 1.26 (1.03–1.54) P = 0.03 | 1.49 (1.24–1.79) P <0.001 |
| HBV (n = 129) | 3.4 (2.7–4.1) | 6.5 (4.5–9.3) | 5.2 (2.5–10.9) | 1.93 (1.27–2.92) P = 0.002 | 0.80 (0.35–1.82) P = 0.59 | 1.54 (0.71–3.31) P = 0.27 |
| NASH + CC (n =399) | 2.2 (1.9–2.5) | 2.3 (1.8–2.8) | 2.0 (1.4–2.8) | 1.05 (0.82–1.32) P = 0.72 | 0.88 (0.59–1.31) P = 0.524 | 0.92 (0.64–1.32) P = 0.64 |
| EtOH (n = 406) | 1.9 (1.7–2.1) | 1.8 (1.5–2.3) | 1.8 (1.3–2.6) | 0.99 (0.78–1.25) P = 0.91 | 0.99 (0.67–1.48) P = 0.97 | 0.98 (0.68–1.40) P = 0.91 |
| Autoimmune* (n=165) | 1.1 (0.88–1.3) | 1.44 (1.1–2.0) | 1.02 (0.53–1.96) | 1.37 (0.96–1.98) P = 0.09 | 0.71 (0.34–1.46) P = 0.34 | 0.97 (0.49–1.91) P = 0.93 |
| Other† (n = 31) | 2.1 (1.8–2.6) | 2.0 (1.3–3.0) | 1.2 (0.4–3.2) | 0.94 (0.59–1.49) P = 0.79 | 0.59 (0.21–1.72) P = 0.33 | 0.56 (0.21–1.52) P = 0.25 |
CC=cryptogenic cirrhosis
includes patients with AIH, PBC and PSC etiologies
includes patients with A1AT, Wilson, hemochromatosis
Figure 2.
Average incidence rate of de novo HCC (per 100 person-years) for waitlist registrants in Eras 1, 2 and 3.
As shown in Figure 2, however, there was marked variability in the pattern of HCC incidence when stratified by diagnostic category. HCC incidence was highest for HBV and HCV patients, particularly in Eras 2 and 3. Among HCV patients, HCC incidence increased from 4.5 per 100 py in Era 1, to 5.3 per 100 py in Era 2, and to 6.6 per 100 py in Era 3. Incidence rate ratios indicate significant increases for both transitions, with IRR of 1.19 (95% CI 1.06–1.33) between Eras 1 and 2, and 1.26 (95% CI 1.03–1.54) between Eras 2 and 3. For HBV patients, the rise in incidence was limited to the transition between Eras 1 and 2 (IRR 1.93, 95% CI 1.27–2.92), which disappeared when Eras 2 and 3 were compared (IRR 0.80, 95% CI 0.35–1.82). For other non-viral diagnoses, HCC incidence was lower than HBV or HCV, without an apparent trend over time (all p>0.05 in pairwise comparisons of the eras).
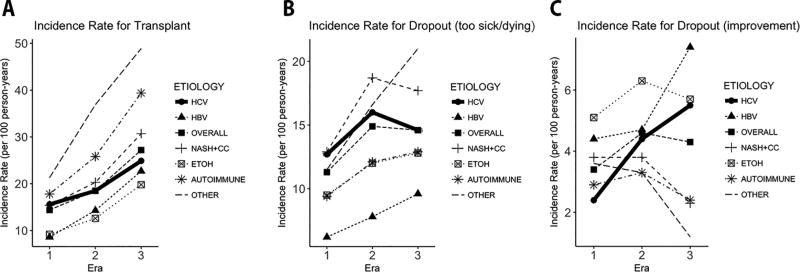
Figure 3 summarizes waitlist outcome other than development of HCC. In Figure 3A, incidence of transplantation increased for all diagnoses over time — however, it was in general lower for HBV and HCV as well as alcoholic liver disease. In fact, HCV was associated with the smallest increase in the transplant rate between Eras 2 and 3 (IRR 1.35, compared to IRR 1.47 for all diagnoses combined) (Supplementary Table 1). Figure 3B shows a trend toward reduced waitlist de-registration for death or being too sick among HCV patients in the DAA era compared to the PI era, though this did not meet statistical significance (IRR 0.91, 95% CI 0.80–1.04) (Supplementary Table 2). Conversely, HCV patients experienced higher incidence of de-registration for improved condition in the DAA era (Figure 3C), with IRR 1.23 (95% CI 0.98–1.54) compared to the PI era and IRR 2.24 (95% CI 1.82–2.75) compared to the IFN era (Supplementary Table 3).
Figure 3.
Average incidence rate (per 100 person-years) of waitlist removal for (A) Transplant, for (B) Death or being too sick to transplant, and for (C) Improved condition.
In light of these results, we performed univariate and multivariable Cox regression analyses in patients with HCV and HBV to take into account confounding variables that may potentially explain the rising trends in HCC incidence. In univariate analyses, the eras, age, race, sex, and CTP score at baseline were significantly associated with increased hazard of developing HCC while on the waiting list, whereas diabetes was not (Table 3). In the multivariable Cox proportional hazards analysis, the hazard ratio for development of de novo HCC in HCV patients in the DAA era, in comparison to the IFN era, was 1.22 (95% CI 1.01–1.48). There was a non-significant 6% increase in the risk of de novo HCC (hazard ratio [HR] 1.06, 95% CI 0.94–1.18) between the IFN and PI eras. For the 1,178 patients listed with HBV, the waitlist registration era was not significantly associated with the hazards of HCC (Supplementary Table 4). In both HBV and HCV patients, age, higher Child-Pugh score, and non-white race were associated with incident HCC.
Table 3.
Univariate and multivariable Cox proportional hazards analysis for time from registration to development of de novo HCC, in patients with HCV (n=20,039).
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
| ||||
| HR | 95% CI | HR | 95% CI | |
| Era of waitlisting | ||||
| 1: 2003–2010 | Ref | - | Ref | - |
| 2: 2011–2013 | 1.15 | 1.03 – 1.29 | 1.06 | 0.94 – 1.18 |
| 3: 2014–2015 | 1.36 | 1.13 – 1.64 | 1.22 | 1.01 – 1.48 |
| Male (vs female) | 1.74 | 1.57 – 1.94 | 1.86 | 1.67 – 2.07 |
| Age (per year increase) | 1.04 | 1.03 – 1.04 | 1.04 | 1.04 – 1.05 |
| Diabetes | 1.10 | 0.99 – 1.22 | 1.03 | 0.92 – 1.14 |
| CPT score (per unit increase) | 1.07 | 1.05 – 1.09 | 1.07 | 1.05 – 1.09 |
| White race (vs. other) | 0.88 | 0.80 – 0.96 | 0.85 | 0.77 – 0.94 |
In addition to the confounding factors, competing events that occur while waiting for LT may also affect the observed incidence of HCC. Table 4 summarizes the competing risk regression analysis in the 20,039 patients with HCV, with LT and death considered as competing risks. When adjusted for the same variables as in the Cox regression models, there was no association between era of waitlisting and the development of de novo HCC, with subdistribution HR (sHR) of 0.89 (95% CI 0.79–0.99) for Era 2 and sHR of 0.83 (95% CI 0.69–1.00) for Era 3, when compared to Era 1. On the other hand, male sex, older age, and non-white race remained predictive for incident HCC.
Table 4.
Univariate and multivariable competing risks regression analysis for time from registration to development of de novo HCC, in patients with HCV (n=20,039). Death and liver transplantation are considered as competing events.
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
| ||||
| sHR | 95% CI | sHR | 95% CI | |
| Era of wait listing | ||||
| 1: 2003–2010 | Ref | - | Ref | - |
| 2: 2011–2013 | 0.99 | 0.89–1.10 | 0.89 | 0.79–0.99 |
| 3: 2014–2015 | 0.98 | 0.82–1.18 | 0.83 | 0.69–1.00 |
| Male (vs female) | 1.62 | 1.46–1.79 | 1.77 | 1.58–1.95 |
| Age (per year increase) | 1.03 | 1.02–1.04 | 1.04 | 1.03–1.04 |
| Diabetes | 1.04 | 0.94–1.16 | 0.96 | 0.86–1.06 |
| CPT score (per unit increase) | 0.95 | 0.94–0.97 | 0.95 | 0.93–0.97 |
| White race (vs. other) | 0.86 | 0.79–0.95 | 0.83 | 0.76–0.92 |
Discussion
In this work incorporating a large, population-based, observational cohort, we found an increased incidence of de novo HCC among waitlist registrants with end-stage liver disease and HCV in the DAA era. However, when other factors, including biological risk factors for HCC and transplant-related competing risks, are taken into account, patients in the DAA era were no longer at a higher risk of HCC than those in previous eras. The unadjusted comparison between the IFN and DAA eras showed 49% higher incidence of HCC for the latter (IRR 1.49, 95% CI 1.24–1.79, Table 2), which compares to a 22% higher risk in the multivariable Cox regression incorporating relevant covariates (multivariable HR 1.22, 95% CI 1.01–1.48, Table 3). Eventually, the era effect disappeared altogether in the multivariable competing risk analysis (sHR 0.83, 95% CI 0.69–1.00, Table 4).
Following earlier reports that DAA-treated HCV patients who achieve SVR after initial therapy for HCC experience more frequent and aggressive recurrence, the impact of SVR on the risk of de novo and recurrent HCC in the DAA era has been fiercely debated.30 Proponents of the association theorize that elimination of viral antigens may result in disruption in immune regulation, eventually resulting in a change in the tumor behavior. It is postulated that SVR induced by IFN could have less of an impact on HCC growth because of the immune-modulating effects of IFN.
Historical data from the IFN era estimate the incidence of HCC at around 1% after viral eradication, with an estimated risk reduction of 76% in SVR patients compared to non-SVR cirrhotic controls.31, 32 In contrast, studies in the DAA era report a 3–7% annual incidence of de novo HCC after successful DAA therapy,14, 17, 33 which is similar to untreated controls or non-responders to DAA. Indeed, a recent meta-analysis of 26 studies reported an increased risk of de novo HCC in the DAA era, with a relative risk (RR) of 2.77 (95% CI 1.46–5.25) compared to the IFN era.21 However, this difference disappeared after adjustment for age and follow-up time (RR 0.68, 95% CI 0.18–2.55). A similar effect was seen in an analysis of the Scottish HCV clinical database, with attenuation of the HR for de novo HCC from 2.48 to 1.15 after adjustment for several relevant co-variates, including age, sex, and Child-Pugh score.34 Our data show a similar pattern, in that covariates included in the multivariable Cox model explain in part the rise in HCC incidence in the DAA era. As the HCV-infected birth cohort ages, waitlist patients are older and present with more advanced liver disease, driving the risk of HCC higher.
The results of our competing risk analysis lend further insight about the higher observed incidence of HCC in the DAA era. As shown in Figure 3, recent HCV patients were less frequently removed from the waiting list for death or being too sick to undergo transplant and were more likely to be withdrawn from the list for an improved condition. It is worth noting that the frequency of waitlist removal was much lower for improvement than for death or being too sick. Finally, while the rate of transplantation increased for all diagnoses, HCV patients experienced the lowest increase in the transplant rate. When taken together, these point to a scenario in which many HCV patients in the DAA era achieved SVR — and their condition did not deteriorate as much compared to HCV patients in previous eras, when only interferon-based therapies were available, and many would have remained untreated. During this period, a greater proportion of HCV patients remained on the waiting list, exposing them to longer periods of at-risk time to develop HCC.
Whether and how rapidly the risk of de novo HCC is altered by SVR in patients with established cirrhosis remains to be determined. It stands to reason that elimination of viral replication, hepatic inflammation and fibrogenesis following SVR should diminish the carcinogenic risk in HCV cirrhosis. Recent analyses from the Veterans Affairs national healthcare system have demonstrated a reduction in the risk of HCC among DAA-treated cirrhotic patients.22, 23 Accordingly, as more patients with HCV cirrhosis are treated with antiviral therapy or undergo LT, rates of incident HCC on the waitlist may in fact decrease.
Based on these findings, we believe that our results help to reconcile the conflicting data about the incidence and risk of HCC in the DAA era. These data corroborate a rising incidence of HCC among recent HCV patients; however, they also indicate that the observation is confounded by the changes in the demographic and clinical profile of HCV patients, as well as changes in the course of their disease progression. These insights could not have been obtained from prior studies, as they tended to be limited by small sample size, potential selection bias, and short follow-up times. In addition, there is significant variation with regard to regional practices and baseline clinical characteristics. With 20,039 patients with decompensated HCV cirrhosis in our overall cohort, 6,769 (33.8%) of whom entered the waitlist in the DAA era, our investigation is one of the largest observational studies to date regarding the effect of DAA on incident HCC, particularly within the transplant population.
Our findings reiterate the importance of HCC in the natural history of patients with end-stage liver disease due to HCV. We observed high incidence rates of HCC (4.5–6.6 per 100 py) in our cohort, even after excluding potentially prevalent cases by omitting patients who applied for HCC exception points within 180 days of listing. Previous analyses have estimated incidence rates of HCC among DAA-treated patients with advanced liver disease due to HCV to be approximately 1.8–3.6 per 100 py in the DAA era.21–23, 35 The high incidence of HCC observed in our study may reflect the severity of cirrhosis and decompensated liver disease and thus high risk of HCC in our US waitlist population, as well as the fact that DAA therapy is not consistently prescribed to all LT candidates. In addition, compared to non-transplant candidates, patients on the liver transplant waitlist may be more closely monitored for the development of HCC.36, 37 Consistent with prior evidence, older age, male sex, and non-white race remained independent risk factors for de novo HCC in our multivariable competing risk model.29 Diabetes was not predictive in this cohort, adding to more recent evidence that patients with HCV cirrhosis and diabetes may not be at added risk for de novo HCC, compared to those without diabetes.38 Together, these data strongly advocate for close surveillance for HCC in patients with advanced liver disease, and particularly those remaining on the LT waitlist. Though the risk of HCC in individual patients may be reduced with DAA therapy, the absolute number of patients developing HCC may increase, particularly when there are a larger number of patients under observation, and for longer periods of time.
Our study also allows comparison of the evolving incidence of HCC in patients with HCV versus other etiologies. An increased incidence of HCC was observed only among patients with HCV in Era 3 compared to Era 2 and Era 1. Among HBV patients, an increase in incident HCC was seen in Era 2 compared to Era 1, but not in Era 3, which could be related to the now widespread availability of HBV therapy and effective long-term viral suppression — however, the total number of HBV patients without pre-existing HCC exceptions on the LT waitlist was small, and so the confidence intervals for these estimates are wide. There was no significant association between era of waitlisting and de novo HCC in patients with non-alcoholic steatohepatitis or alcohol-related cirrhosis.
We do acknowledge several limitations of our study, which stem from its ecological nature in linking DAA availability with HCC and other waitlist outcomes. Patients’ viremia status and pharmaceutical usage were not reported in the SRTR data, which prevent us from being able to distinguish whether the patient received DAA therapy and did in fact achieve SVR. It is worth pointing out in this context that in the most recent era, up to 95% of patients with HCV cirrhosis, even with hepatic decompensation, can achieve SVR with DAA therapy, making these patients no longer considered “difficult-to-treat.”39 Clearly, not all waitlist registrants are treated for their HCV, as some are too sick to receive therapy, while for others, DAAs may be withheld for fear of “MELD purgatory,” a scenario in which successful treatment of HCV infection may attenuate disease progression and prevent the MELD score from escalating to the level needed to receive organ offers, without substantial improvement in symptoms or quality of life. An SRTR linkage with the Symphony database, an integrated data source of prescription and pharmacy coverage, showed that 211 (19%) of 1150 new waitlist registrants with HCV in 2014 received sofosbuvir.40 The proportion of liver transplant candidates receiving anti-viral therapy while on the waitlist is now likely higher, considering the wider availability and safety of DAA-based regimens approved for use in patients with decompensated disease. When only interferon-based regimens were available, decompensated cirrhosis was a relative contraindication to treatment. In our study, 87% those listed with HCV prior to 2014 had either CTP B or C disease; therefore, the proportion of waitlist registrants who would have received HCV treatment in the pre-DAA eras was likely much lower than the observed 19% in 2014. Ultimately, we submit that the lack of treatment details in our data would tend to dilute the observation being made, and that had these specific data been available, the contrast between unadjusted and multivariable analyses may have been even more dramatic.
Our definition of de novo HCC was based on MELD exceptions and may not have captured patients who did not qualify or apply for exception, e.g. if they exceeded transplantable criteria or if their natural MELD obviated the need for exception. In addition, changes to the HCC exception policy occurred during the study time period, which lowered the initial exception points granted for HCC.41 While these factors could have led us to underestimate the true incidence of de novo HCC, it seems unlikely that they occurred frequently overall or differentially across the eras to have affected the trends seen in this analysis. Finally, the “HCC delay” policy, which implements a 6-month waiting period before granting HCC exception points, took effect in October 2015 and would not have tangibly affected our cohort, as we excluded patients applying for HCC exception within 180 days of listing, and recruitment for our study ended on December 31, 2015.
In summary, the incidence and risk of HCC among patients with advanced HCV cirrhosis registered for LT in the most recent era remain high. While mechanistic studies could eventually reveal a link between DAA therapy and the evolution of HCC in cirrhotic HCV patients, our Cox regression and competing risks analyses indicate that the observed increase in incident HCC on the US transplant waitlist may be attributable to the shift in the characteristics of HCV patients over time and changes in waitlist dynamics. As safer and more effective HCV therapies becomes available for patients with decompensated cirrhosis, more HCV patients on the LT waitlist will have achieved SVR. Though new registrations and transplants for HCV in the US are declining, there will remain a population of waitlist registrants with HCV who nonetheless require LT despite having achieved SVR, for complications of liver disease including HCC.8, 42 While more data are being generated to guide DAA treatment in patients with decompensated HCV cirrhosis, the need for close surveillance for HCC in patients with advanced liver disease, and particularly those remaining on the LT waitlist, may not be overemphasized.43–45
Supplementary Material
Acknowledgments
Financial support:
This work was supported in part by a grant from the National Institute of Diabetes, Digestive and Kidney Disease (DK-34238, DK-92336, T32 DK-007056) and a KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR-001083). The funding organization played no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Abbreviations
- HCV
Hepatitis C virus
- LT
liver transplantation
- DAA
direct-acting antiviral
- MELD
Model for End-Stage Liver Disease
- SVR
sustained virologic response
- HCC
hepatocellular carcinoma
- SRTR
Scientific Registry of Transplant Recipients
- OPTN
Organ Procurement and Transplantation Network
- HBV
hepatitis B virus
- NASH
nonalcoholic steatohepatitis
- BMI
body mass index
- IFN
interferon
- PI
protease inhibitors
- IR
incidence rates
- IRR
incidence rate ratio
- py
person-years
- CI
confidence interval
- HR
hazard ratio
- sHR
subdistribution hazard ratio
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant. 2017;17(Suppl 1):174–251. doi: 10.1111/ajt.14126. [DOI] [PubMed] [Google Scholar]
- 2.Fayek SA, Quintini C, Chavin KD, et al. The Current State of Liver Transplantation in the United States: Perspective From American Society of Transplant Surgeons (ASTS) Scientific Studies Committee and Endorsed by ASTS Council. Am J Transplant. 2016;16:3093–3104. doi: 10.1111/ajt.14017. [DOI] [PubMed] [Google Scholar]
- 3.Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649–59. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Manns M, Samuel D, Gane EJ, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 5.Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493–505. doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster GR, Irving WL, Cheung MC, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224–31. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Pascasio JM, Vinaixa C, Ferrer MT, et al. Clinical outcomes of patients undergoing antiviral therapy while awaiting liver transplantation. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Flemming JA, Kim WR, Brosgart CL, et al. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65:804–812. doi: 10.1002/hep.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg D, Ditah IC, Saeian K, et al. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152:1090–1099.e1. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belli LS, Berenguer M, Cortesi PA, et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol. 2016;65:524–31. doi: 10.1016/j.jhep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Nahon P, Bourcier V, Layese R, et al. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology. 2017;152:142–156.e2. doi: 10.1053/j.gastro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB, Kanwal F, Richardson P, et al. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64:130–7. doi: 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reig M, Mariño Z, Perelló C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719–26. doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–33. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Yang JD, Aqel BA, Pungpapong S, et al. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C-associated hepatocellular carcinoma. J Hepatol. 2016;65:859–60. doi: 10.1016/j.jhep.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Kozbial K, Moser S, Schwarzer R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol. 2016;65:856–8. doi: 10.1016/j.jhep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso H, Vale AM, Rodrigues S, et al. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J Hepatol. 2016;65:1070–1071. doi: 10.1016/j.jhep.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Reig M, Boix L, Mariño Z, et al. Liver Cancer Emergence Associated with Antiviral Treatment: An Immune Surveillance Failure? Semin Liver Dis. 2017 doi: 10.1055/s-0037-1601349. [DOI] [PubMed] [Google Scholar]
- 19.Hengst J, Falk CS, Schlaphoff V, et al. Direct-Acting Antiviral-Induced Hepatitis C Virus Clearance Does Not Completely Restore the Altered Cytokine and Chemokine Milieu in Patients With Chronic Hepatitis C. J Infect Dis. 2016;214:1965–1974. doi: 10.1093/infdis/jiw457. [DOI] [PubMed] [Google Scholar]
- 20.ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER COCaCCcEaspaf. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65:734–40. doi: 10.1016/j.jhep.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 21.Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanwal F, Kramer J, Asch SM, et al. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996–1005.e1. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Li DK, Ren Y, Fierer DS, et al. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology. 2017 doi: 10.1002/hep.29707. [DOI] [PubMed] [Google Scholar]
- 25.Levine GN, McCullough KP, Rodgers AM, et al. Analytical methods and database design: implications for transplant researchers, 2005. Am J Transplant. 2006;6:1228–42. doi: 10.1111/j.1600-6143.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 26.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–95. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 27.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 28.Wald C, Russo MW, Heimbach JK, et al. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–82. doi: 10.1148/radiol.12121698. [DOI] [PubMed] [Google Scholar]
- 29.Flemming JA, Yang JD, Vittinghoff E, et al. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer. 2014;120:3485–93. doi: 10.1002/cncr.28832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llovet JM, Villanueva A. Liver cancer: Effect of HCV clearance with direct-acting antiviral agents on HCC. Nat Rev Gastroenterol Hepatol. 2016;13:561–2. doi: 10.1038/nrgastro.2016.140. [DOI] [PubMed] [Google Scholar]
- 31.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 32.Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–37. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 33.Cheung MC, Walker AJ, Hudson BE, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741–7. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 35.Backus LI, Belperio PS, Shahoumian TA, et al. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatology. 2017 doi: 10.1002/hep.29408. [DOI] [PubMed] [Google Scholar]
- 36.Joshi K, Mendler M, Gish R, et al. Hepatocellular carcinoma surveillance: a national survey of current practices in the USA. Dig Dis Sci. 2014;59:3073–7. doi: 10.1007/s10620-014-3256-6. [DOI] [PubMed] [Google Scholar]
- 37.Singal AG, Yopp A, S Skinner C, et al. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27:861–7. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang JD, Mohamed HA, Cvinar JL, et al. Diabetes Mellitus Heightens the Risk of Hepatocellular Carcinoma Except in Patients With Hepatitis C Cirrhosis. Am J Gastroenterol. 2016;111:1573–1580. doi: 10.1038/ajg.2016.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terrault NA, McCaughan GW, Curry MP, et al. International Liver Transplantation Society Consensus Statement on Hepatitis C Management in Liver Transplant Candidates. Transplantation. 2017;101:945–955. doi: 10.1097/TP.0000000000001708. [DOI] [PubMed] [Google Scholar]
- 40.Kim WRDB, Wang JH, Peng Y, Sexter A, Israni A. Sofosbuvir-based therapy is associated with higher incidence of de-registration for clinical improvement in hepatitis C patients awaiting liver transplantation: Analysis of SRTR-Symphony database. Hepatology. 2017;66:S872A. [Google Scholar]
- 41.Rahimi RS, Trotter JF. Liver transplantation for hepatocellular carcinoma: outcomes and treatment options for recurrence. Ann Gastroenterol. 2015;28:323–330. [PMC free article] [PubMed] [Google Scholar]
- 42.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant. 2018;18(Suppl 1):172–253. doi: 10.1111/ajt.14559. [DOI] [PubMed] [Google Scholar]
- 43.El-Sherif O, Jiang ZG, Tapper EB, et al. Baseline Factors Associated with Improvements in Decompensated Cirrhosis After Direct-acting Antiviral Therapy for HCV Infection. Gastroenterology. 2018 doi: 10.1053/j.gastro.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Cortesi PA, Belli LS, Facchetti R, et al. The optimal timing of hepatitis C therapy in liver transplant-eligible patients: Cost-effectiveness analysis of new opportunities. J Viral Hepat. 2018 doi: 10.1111/jvh.12877. [DOI] [PubMed] [Google Scholar]
- 45.Chhatwal J, Samur S, Bethea ED, et al. Transplanting HCV-positive livers into HCV-negative patients with preemptive antiviral treatment: A modeling study. Hepatology. 2017 doi: 10.1002/hep.29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.