Abstract
Study Design
Repeated measures
Objective
Reports suggest passive limb movement (PLM) could be used as a therapy to increase blood flow and tissue perfusion in the paralyzed lower limbs of those with spinal cord injuries. However, the hyperemic response to PLM appears to be transient, lasting only 30-45 seconds despite continued limb movement. The purpose of this investigation was to determine if the hyperemic response is repeatable across multiple short bouts of passive limb movement.
Setting
Cleveland Veterans Affairs Medical Center
Methods
Nine individuals with paraplegia 46±6 years of age, 17±12 years post injury (range 3-33 years) with complete T3-T11 injuries were subject to 5 × 1 minute bouts of passive knee extension/flexion at 1 Hz with a 1 minute recovery period between each bout. Heart rate (HR), mean arterial pressure (MAP), femoral artery blood flow (FABF), skin blood flow (SBF) and tissue perfusion in the lower limb were recorded during baseline and throughout each bout of PLM.
Results
Despite no increase in HR (p≥0.8) or MAP (p≥0.40) across all 5 bouts of PLM, the average increase in FABF during each bout ranged from 71±87% to 88±93% greater than baseline (p≤0.043). SBF also increased between 465±302% and 582± 309% across the five bouts of PLM (p≤0.005).
Conclusion
Repeated bouts of PLM in those with SCI while in an upright position resulted in a robust and steady increase in FABF and SBF which could have implications for improving vascular health and tissue perfusion in the lower limbs of those with paraplegia.
Introduction
Individuals with spinal cord injury (SCI) are at heightened risk for developing pressure ulcers [1], accelerates the rate of developing cardiovascular disease [2], deep vein thrombosis and poor wound healing below the level of the spinal cord lesion. The increased incidence of these conditions is due to decreased physical activity, prolonged periods of immobility, muscle atrophy, changes in muscle fatty composition and alterations to the structure and function in the peripheral vasculature. For example, previous studies have observed a 30% reduction in femoral artery diameter [3] and decreases in resting femoral artery blood flow [4] in this population. In addition, changes in vascular and muscle physiology can lead to impairments in blood flow, promote hemorrhage, and increase the rate of intravascular thrombosis and vasospasm [5].
To counteract these detrimental effects, previous investigators have sought to examine the efficacy of utilizing passive leg movement (PLM) to invoke an increase in blood flow to the limb. In an able bodied population, PLM elicits a ≈45 second transient hyperemic response in the femoral artery [6]. Both central and peripheral mechanisms are likely responsible for the hyperemia with PLM in able bodied individuals including stimulation of cardiovascular control center by type III afferent feedback [7], increased stroke volume due to skeletal muscle pump [8], as well as nitric oxide [9] and mechanically induced vasodilation [10]. However, due to the nature of a complete spinal cord injury afferent feedback to the cardiorespiratory control center is absent, thus the hyperemic response to a bout of PLM would be attributed solely to peripheral factors in this population. Still some investigators [11-13], but not all [14,15] have reported a significant increase in blood flow to the paralyzed limbs with passive movement. Most recently, Venturelli and colleagues performed continuous blood flow monitoring during a 2-minute bout of PLM (knee extension/flexion at 1 Hz) and reported a twofold transient increase in femoral artery blood flow despite the absence of a concomitant increase in heart rate.
As the hyperemic response appears to be short lived, lasting approximately 45 seconds despite continued PLM, it likely does not provide a significant shear stress stimulus along the vessel walls to maintain or improve vascular and tissue health. However, Hellsten and colleagues [16] reported an increases in VEGF protein, endothelial cell proliferation and eNOS mRNA following a continuous 90 minute bout of PLM potentially due to the repeated mechanical stimulus of lengthening and shortening of the muscle itself. Therefore, if repeated bouts of PLM movement elicit repeated robust hyperemic responses, and subsequently shear stress stimulus, it is likely that the production of pro-angiogenic factors would be augmented and vascular health and tissue perfusion improved. To this end, the primary aim of this study was to evaluate the efficacy of five 1 minute bouts of PLM, with 1 minute recovery periods, to initiate a repeatable and robust hyperemia in the passively moved limb. We hypothesized that each one minute bout of PLM would elicit near identical hyperemic responses.
Methods
Nine individuals with paraplegia participated in the preset study (table 1). All subjects had clinically confirmed complete lesions (American Spinal Injury Association Impairment Scale level A) between the 3rd and 11th thoracic spinal cord segment (T3-11). All procedures were approved by the Institutional Review Board at the Louis Stokes Cleveland Veterans Affairs Medical Center and written informed consent was obtained from all subjects prior to their participation. Subjects were free from known pulmonary, cardiovascular and metabolic disease and had a minimal level of spasticity such that it would not interfere with the experimental protocol. Subjects were asked to maintain their current medication regimen but refrain from exercise for 24 hours before reporting to the laboratory and to report in a fasted state (4 hours) with no consumption of caffeine within the previous 8 hours.
Table 1. Subject characteristics.
| Subject | Level | Post Injury | Gender | Height | Weight | BMI | Age | BP | Medication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | T7 | 25 | F | 1.60 | 54.1 | 21 | 55 | 116/64 | 10 mg bac |
| 2 | T9 | 4 | M | 1.78 | 104.5 | 33 | 44 | 126/80 | 5 mg diaz |
| 3 | T7 | 32 | M | 1.75 | 64.1 | 21 | 53 | 118/64 | 5 mg diaz |
| 4 | T11 | 33 | M | 1.80 | 73.6 | 23 | 53 | 132/76 | |
| 5 | T7 | 16 | M | 1.78 | 88.2 | 28 | 44 | 114/70 | |
| 6 | T8 | 28 | M | 1.75 | 80.5 | 26 | 53 | 130/80 | 5 mg bac |
| 7 | T11 | 14 | M | 1.75 | 105.5 | 34 | 40 | 132/76 | 10 mg bac |
| 8 | T9 | 3 | M | 1.75 | 89.5 | 29 | 49 | 112/76 | |
| 9 | T3 | 3 | F | 1.72 | 79.1 | 27 | 40 | 106/68 | |
| Average | - | 17±12 yr | - | 1.74±0.1 m | 82.1±17.1 kg | 27±5 | 48±6 yr | 121/73 mmHG |
Experimental Protocols
Subjects were assisted into a comfortable seated-upright chair associated with the Biodex system 4 Pro (Biodex Medical Systems, Shirley, NY, USA) and remained in this position for the duration of the study. The Biodex was calibrated to move the subject's right leg through an 80° range of motion (10° to 90° of knee joint flexion) at an angular velocity of 180°/second (approximately 50 extension/flexions per minute). The dynamometer was programmed to cycle at one minute intervals (one minute on, one minute off) for total of 5 bouts of PLM. For the duration of the protocol, the subject's contralateral leg remained stationary.
Central hemodynamics
Heart rate (HR) was determined with the use of a 3 lead electrocardiogram (ECG) streaming into a data acquisition box (Lab Chart 8 Pro, AD Instruments, Denver, CO, USA). Mean arterial pressure (MAP) was determined with the use of a Nexfin (Nexfin, Bmeyecorp, Amsterdam, Netherlands). Before the start of data collection the Nexfin was allowed adequate time for self-calibration (physical) and the finger cuff remained inflated for the entire duration of the protocol. Nexfin's heart reference system was also utilized to correct for hydrostatic pressure differences due to differences in height between the finger and the heart.
Femoral artery blood flow
Measurement of femoral artery vessel diameter and blood velocity were taken distal to the inguinal ligament and at least 3 cm proximal to the deep/superficial femoral bifurcation with the use of a Logic 7 ultrasound system (General Electric Medical Systems, Milwaukee, WI, USA). The ultrasound system was equipped with a M12L transducer operating at a frequency of 14 MHz (ultrasound) and 5 MHz (Doppler). Femoral diameter was measured at a 90° angle along the central axis of the vessel on several images during baseline. These baseline diameter measurements were used to calculate blood flow throughout the entire protocol. Velocity measurements were obtained with the transducer positioned to ensure an insonation angle of 60° or less and maintained constant throughout baseline and all 5 bouts of PLM. Mean blood velocity and arterial diameter were then combined to calculate femoral artery blood flow (FABF) in milliliters per minute with the following equation: mean blood velocity × π(vessel diameter/2)2 × 60. All scanning and analyses were performed by experienced and skilled sonographers.
Tissue oxygenation
Changes in tissue oxy-hemoglobin (Oxy) total hemoglobin (tHb) and normalized tissue hemoglobin index (nTHI) [17], the percent change from initial hemoglobin concentration, were assessed with the use of a NIRS (near infrared spectroscopy) system (NIRO 200, Hamamatsu Phototonics, Hamamatsu, Japan) by placing electrodes on the anterior aspect of the rectus femoris muscle. The laser on the NIRS system penetrates approximately 2cm into the muscle tissue. Total hemoglobin was calculated from alterations in light absorption at each of the transmitter laser frequencies according to the Beer-Lambert law. Rather than utilizing an estimated optical pathlength, the NIRO-200 provided delta values relative to pathlength (ΔμM per cm). Prior to the start of baseline recordings the NIRs unit was ‘zeroset’ allowing us to simply report changes in Oxy, tHb and nTHI from the initial baseline to the end of each bout of PLM.
Skin blood flow
Skin blood flow (SBF) was determined via a Laser Doppler and Perfusion Monitor (moorVMS-LDF2, Moor Instruments, Axminster, UK) with the VP1/7 probe placed on the anterior aspect of rectus femoris muscle over the middle of the muscle belly. Laser Doppler flowmetry is based on red blood cell flux and calculated as product of red blood cell concentration and velocity. This variable is expressed in arbitrary units (PU) and is indicative of superficial skin blood flow [18].
Data analysis
The dependent variables assessed were FABF, SBF, HR, MAP, Oxy, tHb and nTHI. Throughout the protocol all variables went through analog-to-digital conversion and were simultaneously acquired (20 Hz) by commercially available data-acquisition system and supporting software (powerlab 8/36 and Lab Chart Pro 8, AD Instruments, Denver, CO, USA). Due to the different kinetics of each variable, the approach to the analysis was not the same for all variables. With regards to femoral blood flow and skin blood flow the data were averaged into a one-minute baseline value, 1 minute averages for each of the 1 minute bouts of PLM and then 1 minute recovery following the last bout of PLM. In addition, the peak FABF during each bout of PLM was determined. One-way repeated measures ANOVA was used to determine if the main effect of PLM bout was significant. If a main effect of bout was present, paired samples t-tests were used simply compare the baseline value to the individual values for each bout of PLM. To determine if the increase in femoral blood flow was constant throughout each bout of PLM T-tests were utilized to compare blood flow during seconds 12-24 (after initial rise) and 48-60. With regards to Oxy, tHb and nTHI, baseline and recovery values were compared to the values during the last 12 seconds of each bout of PLM. Post-hoc comparisons were adjusted using the Benjamini-Hochberg false discovery rate correction equation. An a priori power analysis indicated a sample size of 8 subjects would be needed to obtain a statistical significance of p<0.05 and power of β>0.80. All data are presented as mean±SD.
Results
Heart Rate and Mean Arterial Pressure
There were no statistical differences in HR or MAP from baseline to each bout of PLM. At baseline, HR was 82±13 bpm while during the 5 bouts of PLM the average heart rates were 81±10, 81±10, 82±11, 80±11 and 83±7 bpm (p≥0.8). At baseline MAP was 99.6±19.2 mmHg, while during the bouts of PLM MAP averaged 101.0±17.1, 104.1±17.7, 103.4±16.4, 101.8±18.6 and 106.7±16.8 mmHG (p≥0.4).
Femoral Artery Blood Flow
Repeated measures ANOVA revealed a main effect of bout (p=0.03) for the average femoral blood flow. At baseline, FABF was 120±91 ml/min and the average during each subsequent one-minute bout of PLM was 189±150 ml/min (85±103%), 182±147 ml/min (71±87%), 188±148 ml/min (79±90%), 180±132 ml/min (76±87%) and 196±142 ml/min (88±93%), respectively (Figure 1A). These values were all significantly greater than the baseline (p≤0.04) and recovery (123±90ml/min; p≤0.031) but were not significantly different from each other (p=0.81). The effect size ranged from 0.25 to 0.30. T-tests revealed that the average blood flow during seconds 12-24 and 48-60 within each bout of PLM were not significantly different from each other (P≥0.32 for all comparisons; Figure 1B).
Figure 1.
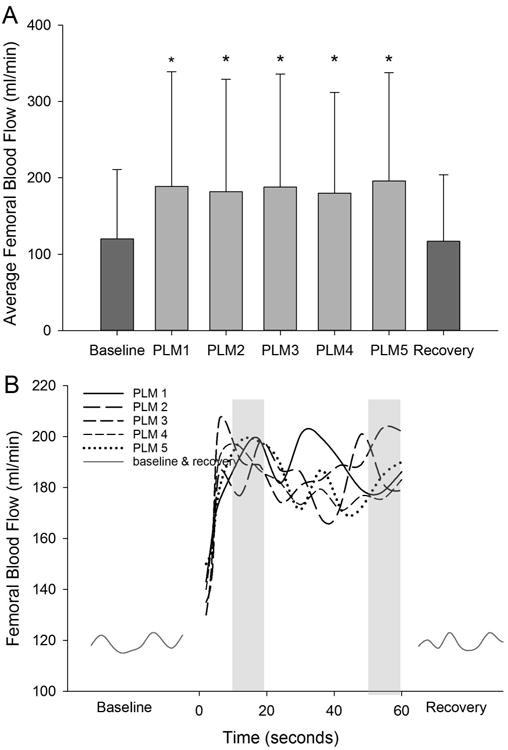
Average femoral blood flow values across the 5 bouts of PLM. * Denotes a significant increase (p<0.05) from baseline and recovery (A). There was no difference in average blood flow between the 5 bouts of PLM. Data presented as mean±SD. Line graphs illustrating the kinetics of femoral blood flow for each bout of PLM, (B). T-tests revealed no significant differences in average blood flow between 12-24 and 48-60 seconds (represented by grey column) within each bout of PLM.
Repeated measures ANOVA also indicated a significant main effect of bout for peak FABF (P<0.001). FABF values for each bout of PLM was 253±196 ml/min (174±224%), 248±204 ml/min (148±175%), 257±206 ml/min (172±208%), 245±178 ml/min (163±190%) and 227±168 ml/min (153±207%), respectively, which were all significantly different from baseline (P≤0.021) but not different from each other (p=0.405). The effect size ranged from 0.37-0.40.
Oxy-Hemoglobin, Total Hemoglobin and Normalized Tissue Hemoglobin Index
At baseline Oxy was -0.57±3.49ΔμM per cm and dropped to -2.79±2.51 (P=0.088), -3.76±3.01 (P=0.042),-3.51±3.30 (P=0.068) -3.15±4.07 (P=0.106) and -3.65±4.37 (P=0.080) during the last 12 seconds of each bout of PLM respectively (Figure 2a). Thus, significant decreases in Oxy were observed only in one bout but strong trends were observed in the rest. The average Oxy over the last 12 seconds of each bout of PLM was significantly lower than the Oxy during the recovery period (-0.10 ΔμM per cm) for all bouts of PLM except the 4th (P=0.054). Baseline tHb was 0.40±4.46ΔμM per cm and dropped to -2.70±3.78 (P=0.113), -4.45±5.47 (P=0.04), -4.00±5.62 (P=0.07), -3.57±6.63(0.108) and -4.08±7.13 (0.086) at the end of each of the 5 bouts of PLM respectively (Figure 2b). During recovery period tHb was 1.24±2.09 which was significantly greater than tHb at the end of bout 2 and 3 (P≤0.38) and had strong trends for bouts 1 (P=0.053), 4 (P=0.078) and 5 (P=0.063). Finally, nTHI at baseline was 0.996±0.046 a.u. and fell to 0.917±0.069, 0.905±0.098, 0.899±0.095, 0.896±0.100 and 0.897±0.106 during the last 12 seconds during the 5 bouts of PLM (Figure 2C). The change from baseline to the end of PLM was significant for all 5 bouts (P≤0.022). Furthermore, nTHI at the end of each bout of PLM was significantly reduced compared to the recovery (1.018±0.036; P≤0.011 for all comparisons).
Figure 2.

Changes in oxygenated hemoglobin (A), total hemoglobin (B) and nTHI (C) values at baseline, across the 5 bouts of PLM and during recovery. * Denotes a significant decrease from the baseline value while # denotes a significant decrease from the recovery period. The numbers next to the symbols indicate which bout of PLM resulted in significant differences (P<0.05). Note, see results as there were strong trends for many of the trials that did not achieve significance (P<0.08).
Skin Blood Flow
Similar to femoral blood flow, repeated measures ANOVA indicated a significant effect of bout for average skin blood flow (p<0.001). At baseline SBF was 18.3±10.0 PU and the average during each subsequent one minute bout of PLM was 83.4±26.0, 85.9±35.7, 94.9±52.2, 101.5±70.8, and 117±87.4 PU which were all significantly greater than baseline (p≤0.005) but were not different from each other (p=0.216) (Figure 2b). The effect size ranged from 0.62-0.85. Skin blood flow during the recovery period was 23.5±30.1 PU which was significantly less than average skin blood flow during each bout of PLM (P≤0.015).
Discussion
Previous investigators have reported a transient hyperemic response to PLM. However, the present study is the first to determine if the hyperemic response can be repeated in those with complete spinal cord injuries when the bouts of PLM are interspaced with short recovery periods. Specifically, we examined if repeated bouts of PLM could result in consistent changes in FABF, SBF and tissue perfusion of the passively moved limb. The present study not only indicates that despite no increase in HR or MAP, repeated one-minute bouts of PLM results in repeated robust increases in FABF, but also that when those with SCI are placed in an upright position the hyperemic response is constant rather than transient. This suggests that utilizing PLM could be used to stimulate greater hyperemic responses and ultimately vascular shear stress in the SCI population. This has implications for the use of passive movement as a rehabilitation modality to promote vascular health and angiogenesis.
Hyperemic Responses
Several investigators have reported there is no increase in FABF during passive movements in the SCI population [14,15] while others have reported passive movements do increase blood flow or blood velocity [11-13]. Specifically, this hyperemic response was not observed by Svensson (1995) and Ter Woerds (2006) as they measured blood flow upon completion of the passive movements rather than during the movements and likely missed the hyperemic response. The results of the current study agree with the studies performed by Ballaz and Venturelli. Specifically, Venturelli (2012 and 2014) reported peak blood flow responses to be nearly double baseline values during a sole bout of PLM. The results of the current study are similar with an average peak blood flow of 162% above baseline values observed across all five bouts of PLM. Interestingly, the hyperemic response between the able-bodied and SCI population are nearly identical after controlling for thigh volume and resting FABF, therefore it was postulated that those with SCI have heightened vascular sensitivity below the level of the lesion [12]. As this hypersensitivity is likely in response to the non-active paralyzed limb being subjected to unaccustomed movements [19] it was unknown whether or not employing repeated bouts of PLM would diminish this sensitivity resulting in a decreased hyperemic response with each subsequent bout of PLM. The results of the present study are promising as the 5 bouts of PLM resulted in nearly identical hyperemic responses. A follow up study is required to determine if this response remains after chronic exposure to repeated bouts of PLM.
Surprisingly this investigation the hyperemic responses to all 5 bouts of PLM were not transient, rather blood flow remained elevated throughout the entire bout of PLM (Figure 1). This is in stark contrast to previous reports on the able-bodied population [6,20,21] and those with spinal cord injuries [12,13]. This discrepancy is likely due to the lack of sympathetic stimulation in the lower legs of our subjects and the upright body position utilized in this investigation. Specifically, Trinity et al reported a prolonged hyperemic response to PLM when subjects were in an upright position rather than a supine position due to the additional influence of gravity on lower limb blood flow [21]. In addition, the lack of sympathetic control of the vessels in the lower limbs results in excessive blood pooling in the lower extremities especially while in the upright position. The onset of PLM likely results in mechanically induced [10] and nitric oxide stimulated [22,23] vasodilation and also engages the skeletal muscle pump, albeit to a lower extent than active exercise facilitating venous return and ultimately blood throughput in the lower limb. Thus, with regards to utilizing PLM as a therapy modality to improve blood flow and tissue perfusion in the lower limbs of those with SCI it appears that circumventing the transient hyperemic response is possible with a more aggressive upright posture rather than a supine position.
Previous reports indicate that at least in the able-bodied population 35% of the hyperemic response is from central factors and 65% is the result of peripheral mechanisms [23]. The robust hyperemic responses observed in this investigation are not facilitated by central factors as heart rate and mean arterial pressure did not to change with PLM. This is in agreement with previous studies that looked at heart rates responses during either passive cycling [11,24] or PLM [12,13] in those with spinal cord injuries and also reported no changes in HR. This absence of central responses in the SCI population, particularly in the AIS A subgroup, is likely due to the lack of afferent feedback from the limbs to the cardiovascular control center. This is supported by Trinity et al 2010 that reported significant decrease in HR response to PLM in able bodied individuals following an intrathecal fentanyl injection that blocks the afferent feedback from the muscles of the lower limb [25].
The repeatable and prolonged hyperemic response could augment the promotion of a pro-angiogenic environment in the skeletal muscle by increasing the physiologic stimulators of angiogenesis leading to increased vessel growth and capillary density [26]. A recent investigation by Liu reported an engineered transcription factor that activates VEGF leads to increased capillary density in spinal cord injured rodent model [27]. This provides support that upregulation of the VEGF in human spinal injured persons could illicit a similar response. An investigation by Hellsten and colleagues reported increases in VEGF protein at 30, 60 and 90 minutes after the start of a 90-minute bout of continuous PLM. This increase was attributed to shear stress due to increased blood flow and mechanical stress due to the passive shortening and lengthening of the muscle [16]. Therefore it is likely that the hyperemic response invoked by the PLM, similar to those performed in the current study, could promote a greater increase in VEGF leading to a more favorable pro-angiogenic environment in the passively moved limb.
Muscle Tissue Perfusion and Skin Blood Flow
Unlike FABF, Oxy, tHb and nTHI did not appear to reach a steady state within the 1-minute bouts of PLM, rather they tended to decrease from baseline over time (Figure 2). Although the decrease in hemoglobin may seem to contradict the FABF response, this could again be explained by the lack of vascular control in the lower limbs in our subjects. Specifically, the inability to constrict the vessels in the lower limb through sympathetic activation [28] can result in significant pooling of venous blood in the lower limbs when in a seated or standing position in this population [28,29]. The rhythmic lengthening and shortening of the hamstrings and quadriceps likely promoted venous return from the lower limbs via the skeletal muscle pump resulting in the tendency for Oxy, tHb and ultimately nTHI to decrease within each bout of PLM. Ultimately venous return was augmented to a greater extent than femoral blood flow reducing hemoglobin concentration but increasing hemoglobin throughput in the muscle. It is likely that if these bouts of PLM continued for longer than 1-minute tissue perfusion would have also reached a steady state. Similar to femoral blood flow, skin blood flow was elevated during each bout of PLM and returned to baseline values during the recovery period. Unlike femoral blood flow and tissue perfusion, skin blood flow didn't appear to remain steady across each bout and was absent the onset kinetics. This is likely due to the fact that the skin is not influenced by the skeletal muscle pump.
Implications
In addition to the benefits to vascular health previously mentioned, the increase in skin blood flow and tissue perfusion could provide other health benefits. Specifically, deep vein thrombosis (DVT) which is three times higher in the SCI population compared to the able bodied population [30] is partially due to stasis of blood flow and endothelial injury [31]. Periodic PLM could reduce blood stasis and improve endothelial function. In addition, PLM could prove beneficial at reducing pressure ulcers and improving skin wound healing in the SCI population. Specifically, an investigation by Sonenblum and colleagues (2014) looked at the effectiveness of pressure relief maneuvers to increase skin blood flow of the buttocks reported comparable increases in SBF to the current investigation [32]. Coggrave (2003) reported the mean duration of a pressure lift needed to achieve pressure relief to allow for adequate SBF and tissue oxygenation above the ischial tuberosity was 1 minute and 51 seconds [33]. Holding pressure lifts for this long of duration could be extremely difficult for persons with paraplegia as they may not possess the upper body strength or motivation required. The prolonged increase in skin and muscle perfusion observed with PLM may help reduce the risk of pressure ulcers. However, the present study measured SBF and tissue oxygenation on the thigh while pressure ulcers most commonly form in areas that are exposed to constant pressure and bony prominences such as the ischial and sacral regions. Thus although these results may not directly translate to pressure ulcer reduction around the ischial tuberosity, this study does provide motivation for future investigations to evaluate changes in SBF around the ischial tuberosities. Such studies should also include movement across the hip joint.
The decreased physical activity and absence of autonomic regulation following a spinal cord injury is a major contributor to cardiovascular disease and subsequent death [34,35]. Although it is well established that increased physical activity improves central cardiovascular health, it is less clear which exercise modalities improve peripheral vascular function in the paralyzed limbs in this population. Previous report indicated that PLM results in a transient hyperemic response. However, the data gathered in this investigation indicate that the hyperemic response is constant in the SCI population when performed in a more upright posture and support the use of PLM to initiate hyperemic responses in the peripheral vasculature which can facilitate improvement [16] or at least maintenance of peripheral vascular function. Due to the passive nature of this modality, benefits typically associated with increased muscle metabolic rate of voluntary exercise, such as improved lipid and glucose metabolism, will likely be absent. Other proposed therapeutic modalities for this population include FES stimulation and upper body exercise. Although FES stimulation might be the optimal strategy as it is associated with increased metabolic rate of the paralyzed muscle, not all individuals respond to FES and it is often cost prohibitive or unavailable to the majority of individuals with SCI. Upper body exercise can also lead to improvements in central cardiovascular health. However reports on the influence of upper body exercise on lower limb blood flow are mixed [4,29,36] likely due to the range of autonomic dysregulation in this population. In addition to the mixed results from FES and upper body exercise investigations, the use of both of these modalities are limited to the availability of equipment which can be costly and difficult to use. A potential benefit to PLM is that no equipment is needed and could be performed on an hourly or daily basis by a care-giver or a therapist and passive nature of the modality may be safer as it has limited chances of evoking autonomic dysreflexia. As previously stated future studies need to focus on tissue changes in tissue perfusion where PU often occur, and include PLM across the hip joint to include those hip extension muscles that are often impacted by pressure ulcers. Furthermore, this study only individuals with SCI between T3 and T12. The inclusion of higher level injuries that result in flaccid paralysis also needs to be investigated. Currently we assume they would have similar hyperemic responses but to a reduced magnitude due to reduced muscle mass [12].
Conclusion
Previous studies have observed that the hyperemic response to PLM is transient in nature lasting less than 45 seconds in the SCI population. Thus the aim of this investigation was to determine if this transient hyperemic response is repeatable when bouts of PLM are interspersed with 1 minute recovery periods. Not only are we the first to report that the response is repeatable, but also the hyperemic response appears to be more constant in this population when individuals are in a more aggressive supine position. This further supports the use of PLM to improve vascular health and tissue perfusion. The bouts of PLM also generated robust increases in SBF and reduced blood pooling which could aid in lessening the impact of pressure ulcers and DVTs. Additional studies should be performed to determine the optimum PLM duration and rest period and measure markers of vascular health following chronic PLM.
Figure 3.
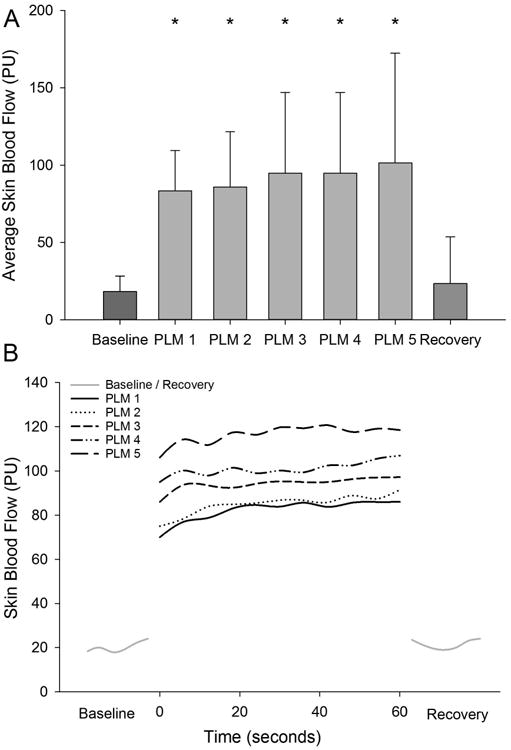
1 Average skin blood flow across the 5 bouts of PLM. * Denotes a significant increase (p<0.05) from baseline and recovery Data presented as mean±SD, (A). Line graphs illustrating the kinetics of skin blood flow for each bout of PLM.
Acknowledgments
This work was supported by SPIRE award 1 121 RX001732-01A1 from the United States Department of Veterans Affairs Rehabilitation Research and Development Service.
List of Abbreviations
- SCI
spinal cord injury
- PLM
passive limb movement
- MAP
mean arterial pressure
- HR
heart rate
- FABF
femoral artery blood flow
- SBF
skin blood flow
- tHb
total hemoglobin
- nTHI
normalized tissue hemoglobin index
- Oxy
oxygenated hemoglobin
- VEGF
vascular endothelial growth factor
Footnotes
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government
Author Disclosure: The authors have no conflict of interest with the work performed in this investigation
References
- 1.Bogie KM, Wang X, Triolo RJ. Long-term prevention of pressure ulcers in high-risk patients: a single case study of the use of gluteal neuromuscular electric stimulation. Archives of Physical Medicine and Rehabilitation. 2006;87(4):585–591. doi: 10.1016/j.apmr.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Phillips WT, Kiratli BJ, Sarkarati M, Weraarchakul G, Myers J, Franklin B, et al. Effect of spinal cord injury on the heart and cardiovascular fitness. Current Problems in Cardiology. 1998;23(11):641–716. doi: 10.1016/s0146-2806(98)80003-0. [DOI] [PubMed] [Google Scholar]
- 3.de Groot PC, Hjeltnes N, Heijboer AC, Stal W, Birkeland K. Effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of spinal cord injured individuals. Spinal Cord. 2003;41(12):673–679. doi: 10.1038/sj.sc.3101534. [DOI] [PubMed] [Google Scholar]
- 4.Hopman MT, Van Asten WNJC, Oeseburg B. Oxygen Transport to Tissue XVII. Springer; 1996. Changes in blood flow in the common femoral artery related to inactivity and muscle atrophy in individuals with long-standing paraplegia; pp. 379–383. [DOI] [PubMed] [Google Scholar]
- 5.Martin TP, Stein RB, Hoeppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. Journal of Applied Physiology. 1992;72(4):1401–1406. doi: 10.1152/jappl.1992.72.4.1401. [DOI] [PubMed] [Google Scholar]
- 6.McDaniel J, Fjeldstad AS, Ives SJ, Hayman MA, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. Journal of Applied Physiology. 2010;108(1):76–84. doi: 10.1152/japplphysiol.00895.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herr MD, Imadojemu V, Kunselman AR, Sinoway LI. Characteristics of the muscle mechanoreflex during quadriceps contractions in humans. Journal of Applied Physiology. 1999;86(2):767–72. doi: 10.1152/jappl.1999.86.2.767. [DOI] [PubMed] [Google Scholar]
- 8.Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. The American journal of physiology. 1987;253(5 Pt 2):H993–1004. doi: 10.1152/ajpheart.1987.253.5.H993. [DOI] [PubMed] [Google Scholar]
- 9.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE, et al. The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure. J Physiol. 2015;593(17):3917–28. doi: 10.1113/JP270195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. The Journal of Physiology. 2006;572(Pt 2):561–7. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballaz L, Fusco N, Crétual A, Langella B, Brissot R. Acute peripheral blood flow response induced by passive leg cycle exercise in people with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2007;88(4):471–476. doi: 10.1016/j.apmr.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Venturelli M, Amann M, Layec G, McDaniel J, Trinity JD, Fjeldstad AS, et al. Passive leg movement-induced hyperaemia with a spinal cord lesion: evidence of preserved vascular function. Acta Physiologica. 2014 doi: 10.1111/apha.12173. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venturelli M, Amann M, McDaniel J, Trinity JD, Fjeldstad AS, Richardson RS. Central and peripheral hemodynamic responses to passive limb movement: the role of arousal. American Journal of Physiology-Heart and Circulatory Physiology. 2012;302(1):H333–H339. doi: 10.1152/ajpheart.00851.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ter Woerds W, De Groot PC, van Kuppevelt DH, Hopman MT. Passive leg movements and passive cycling do not alter arterial leg blood flow in subjects with spinal cord injury. Physical Therapy. 2006;86(5):636–645. [PubMed] [Google Scholar]
- 15.Svensson M, Siösteen A, Wetterqvist H, Sullivan L. Influence of physiotherapy on leg blood flow in patients with complete spinal cord injury lesions. Physiotherapy Theory and Practice. 1995;11(2):97–107. [Google Scholar]
- 16.Hellsten Y, Rufener N, Nielsen JJ, Høier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008;294(3):R975–R982. doi: 10.1152/ajpregu.00677.2007. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Canadian Journal of Applied Physiology. 2004;29(4):463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 18.Bonner RF, Nossal R. Laser-Doppler Blood Flowmetry. Springer; 1990. Principles of laser-Doppler flowmetry; pp. 17–45. [Google Scholar]
- 19.Bentzer P, Nielsen N, Arner M, Danielsen N, Ekblad E, Lundborg G, et al. Supersensitivity in rat micro-arteries after short-term denervation. Acta Physiologica Scandinavica. 1997;161(2):125–133. doi: 10.1046/j.1365-201X.1997.00177.x. [DOI] [PubMed] [Google Scholar]
- 20.McDaniel J, Hayman MA, Ives SJ, Fjeldstad AS, Trinity JD, Wray DW, et al. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. The Journal of Physiology. 2010;588(22):4507–4517. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MA, et al. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: implications for rehabilitative medicine. American Journal of Physiology-Heart and Circulatory Physiology. 2011;300(5):H1885–H1891. doi: 10.1152/ajpheart.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trinity JD, Groot JH, Layec G, Rossman MJ, Ives SJ, Morgan DE, et al. Passive leg movement and nitric oxide-mediated vascular function: The impact of age. American Journal of Physiology-Heart and Circulatory Physiology. 2015 doi: 10.1152/ajpheart.00806.2014. ajpheart. 00806.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, et al. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol. 2012;590(6):1413–25. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muraki S, Ehara Y, Yamasaki M. Cardiovascular responses at the onset of passive leg cycle exercise in paraplegics with spinal cord injury. European Journal of Applied Physiology. 2000;81(4):271–274. doi: 10.1007/s004210050042. [DOI] [PubMed] [Google Scholar]
- 25.Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O'Keefe Z, Runnels S, et al. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. American Journal of Physiology-Heart and Circulatory Physiology. 2010;299(5):H1693–H1700. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature Medicine. 2000;6(4):389–396. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Figley S, Spratt SK, Lee G, Ando D, Surosky R, et al. An engineered transcription factor which activates VEGF-A enhances recovery after spinal cord injury. Neurobiology of disease. 2010;37(2):384–393. doi: 10.1016/j.nbd.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Claydon VE, Steeves JD, Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord. 2005;44(6):341–351. doi: 10.1038/sj.sc.3101855. [DOI] [PubMed] [Google Scholar]
- 29.Kinzer SM, Convertino VA. Role of leg vasculature in the cardiovascular response to arm work in wheelchair-dependent populations. Clinical physiology. 1989;9(6):525–33. doi: 10.1111/j.1475-097x.1989.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 30.Miranda AR, Hassouna HI. Mechanisms of thrombosis in spinal cord injury. Hematology/Oncology Clinics of North America. 2000;14(2):401–416. doi: 10.1016/s0889-8588(05)70141-6. [DOI] [PubMed] [Google Scholar]
- 31.Lensing AWA, Prandoni P, Prins M, Büller HR. Deep-vein thrombosis. The Lancet. 1999;353(9151):479–485. doi: 10.1016/s0140-6736(98)04298-6. [DOI] [PubMed] [Google Scholar]
- 32.Sonenblum SE, Vonk TE, Janssen TW, Sprigle SH. Effects of wheelchair cushions and pressure relief maneuvers on ischial interface pressure and blood flow in people with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2014;95(7):1350–1357. doi: 10.1016/j.apmr.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Coggrave MJ, Rose LS. A specialist seating assessment clinic: changing pressure relief practice. Spinal Cord. 2003;41(12):692–5. doi: 10.1038/sj.sc.3101527. [DOI] [PubMed] [Google Scholar]
- 34.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408–16. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Archives of physical medicine and rehabilitation. 1993;74(3):248–54. [PubMed] [Google Scholar]
- 36.Burkett LN, Chisum J, Pierce J, Pomeroy K, Fisher J, Martin M. Blood flow and lactic acid levels in exercising paralyzed wheelchair bound individuals. Adapted Physical Activity Quarterly. 1988;5:60–73. [Google Scholar]


