Abstract
Parkinson’s disease (PD) is a common neurodegenerative disease characterized pathologically by the selective loss of dopaminergic neurons in the substantia nigra and the intracellular accumulation of α-synuclein in the Lewy bodies. While the pathogenic mechanisms of PD are poorly understood, many lines of evidence point to a role of altered autophagy and membrane trafficking in the development of the disease. Emerging studies show that connections between the deregulation of autophagy and synaptic vesicle (SV) trafficking may contribute to PD. Here we review the evidence that many PD related-genes have roles in both autophagy and SV trafficking and examine how deregulation of these pathways contributes to PD pathogenesis. This review also discusses recent studies aimed at uncovering the role of PD-linked genes in autophagy-lysosome function.
Keywords: Parkinson’s disease, autophagy, synaptic trafficking, LRRK2
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease with more than 10 million people living with the disease worldwide. PD is characterized by the loss of dopaminergic neurons in the substantia nigra overtime, which leads to a variety of motor deficits including tremor and rigidity. While the majority of cases are sporadic, around 5% of cases result from a clear genetic cause [1], and recent advances in genome-wide analyses have uncovered a number of risk factors relevant for sporadic PD [2–7].
A major pathological hallmark of PD is the accumulation of aggregated proteins, mainly α-synuclein, in Lewy bodies. This neuropathological characteristic of PD has prompted great interest in understanding the relevance of protein homeostasis pathways in disease pathogenesis. Autophagy is a major degradation pathway for protein aggregates and plays a critical role in the maintenance of protein homeostasis. Important experimental evidence for a role of autophagy in PD and neurodegeneration came from observations in genetic animal models including autophagy-deficient animals. Multiple mouse models lacking a key autophagy gene (e.g., Atg5, Atg7, Ulk1/2) show neurodegeneration typically preceded by axonal dystrophy suggesting an essential role of autophagy in regulating axon homeostasis and preventing neurotoxicity [8–14]. Moreover, animals lacking the core autophagy gene, Atg7 in dopamine neurons display dystrophic neurites, progressive neuronal loss, locomotor deficits, and presynaptic accumulation of α-synuclein and LRRK2 [8]. Results from many genome-wide association studies have also linked autophagy and lysosomal function to PD pathogenesis and a recent meta-analysis has identified 17 additional risk loci, many of which are associated with the autophagy-lysosome pathway [2].
In addition to the autophagy-lysosomal pathway, insights from genetic studies of PD implicate dysfunction of synaptic vesicle (SV) cycling, another neuronal membrane trafficking pathway, as an underlying mechanism of disease development [15, 16]. While previously thought to be independent pathways, recent studies suggest autophagy and SV trafficking converge at presynaptic terminals [17–21]. Here, we review studies linking dysfunction in the autophagy-lysosomal pathway to PD and highlight how the intersection of SV trafficking and autophagy is emerging as an important mechanism of PD pathogenesis.
Disruption of SV endocytosis and macroautophagy by PD mutants
Recent studies suggest the importance of two neuronal membrane trafficking pathways, SV cycling and autophagy, in the development of PD [22–28]. Without proper maintenance of SV cycling, synapses may become dysfunctional over time, leading to a loss of neuronal communication and ultimately to neurodegeneration [29, 30]. A number of PD-linked proteins, including LRRK2, EndophilinA (EndoA), synaptojanin1 (synj1), dynamin, and auxilin, have well-defined roles in SV endocytosis (Figure 1) [31, 32] Intriguingly, more recent evidence suggests that many of these proteins have additional roles in autophagy. Together, these findings suggest that there is an extensive interaction between SV trafficking and autophagy at the presynapse. Alternatively, there may be neuronal synapse-specific autophagy machinery that is particularly compromised in PD. As the endocytic pathway also merges with autophagy [33], it remains unclear whether these proteins are modified in a way that dictates their function in one pathway or another or if these proteins directly control the intersection of SV cycling and autophagy. Here, we discuss these proteins and how their PD-linked mutations lead to the deregulation of these two pathways.
Figure 1. PD linked genes have roles in SV trafficking.
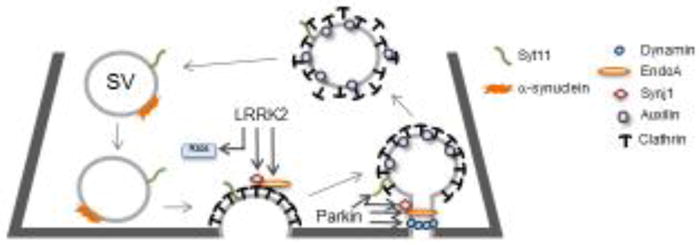
Synaptic vesicles release neurotransmitters through highly regulated and tightly coupled rounds of exo- and endocytosis. Many PD-linked genes including α-synuclein, LRRK2, EndoA, synj1, auxilin, syt11, and dynamin are involved in SV cycling, particularly in SV endocytosis. LRRK2 and Parkin, associated with familial PD, are known to post-translationally modify a number of SV endocytosis genes. LRRK2 phosphorylates the SV endocytosis proteins: EndoA, synj1 as well as multiple Rab GTPases that may be involved in SV cycling. Parkin ubiquitinates EndoA, synj1, syt11, and dynamin though more studies are necessary to determine how modification of these proteins influence their function in SV cycling.
LRRK2
Missense mutations of LRRK2 are linked to the most common inherited forms of PD [34, 35]. LRRK2 protein is reportedly localized to synaptic compartments where it interacts with a variety of SV endocytic proteins and appears to regulate SV trafficking and distribution [36–38]. Multiple studies have shown that the loss of LRRK2 or the expression of PD mutant G2019S slows the kinetics of SV endocytosis, but these same studies find differential effects of LRRK2 on SV exocytosis [36, 39–41]. Additionally, loss of function or hyperactivation of LRRK2 kinase function leads to deregulation of autophagy [42–46]. Multiple PD-linked pathogenic mutations of LRRK2 result in the hyperactivation of its kinase function, suggesting that chronic or improper phosphorylation of LRRK2 substrates may underlie PD development through alterations in SV cycling and autophagy [47].
Despite a variety of studies linking LRRK2 to autophagy, two questions remain unanswered. First, it remains unclear, whether LRRK2 positively or negatively regulates autophagy. Second, LRRK2 has been implicated in regulating multiple steps of autophagy, including initiation and termination, resulting in uncertainty as to the precise role of LRRK2 in autophagy. Major attempts to address the function of LRRK2 have utilized pharmacological inhibitors as well as manipulations that increase LRRK2 activity, namely the overexpression of LRRK2 G2019S, a PD-linked mutation that leads to hyperactivation of LRRK2 kinase activity. Adding to the complexity, alterations in the activity of LRRK2 in either direction result in similar outcomes. Specifically, both the silencing of LRRK2 through gene knockdown and the overexpression of LRRK2 hyperactive kinase mutant result in impaired macroautophagy-mediated degradation in cell lines [48, 49]. Identification of direct LRRK2 targets that mediate autophagy regulation may provide insight into these unsolved issues.
EndophilinA
While associated with PD through its interaction LRRK2, EndoA (encoded by SH3GL2) was also recently identified as a novel risk factor for sporadic PD [2]. EndoA is a protein critical for clathrin-mediated endocytosis at nerve terminals [50, 51]. Studies performed in Drosophila have shown that EndoA phosphorylation is increased upon LRRK2 G2019S expression. Interestingly, both the LRRK2 kinase loss-of-function and gain-of-function impair synaptic endocytosis [36]. Subsequent studies have found that LRRK2-mediated phosphorylation of EndoA biases the membrane-deforming capabilities of this protein to form high curvature membranes that recruit ATG3 and thus initiate autophagosome formation. The loss of EndoA results in degeneration of the fly eye which, importantly, is not rescued by either non-phosphorylatable or phosphomimetic EndoA [20]. These results again suggest that a balance in EndoA phosphorylation state is necessary to maintain healthy cells. This balance would be altered by either the loss of LRRK2, resulting in non-phosphorylated EndoA, or overactivation of LRRK2 kinase resulting in chronic phosphorylation of EndoA. This finding may help explain how alterations in the activity of LRRK2 in either direction disrupt dynamic phosphorylation, which may underlie defects in autophagy and other pathways. While these studies require future validation in mammalian systems, they strongly argue that a careful examination of the effect of chronic phosphorylation of verified LRRK2 substrates is necessary to understand the various roles of LRRK2 more completely.
Past studies of EndoA have shown that the triple knock-out of all three EndoA isoforms in mice results in neurodegeneration. RNA-SEQ analysis revealed changes in pathways including synaptic transmission and protein homeostasis. The most striking finding was an increase in the ubiquitin-proteasome system (UPS) E3 ligase, FBXO32. Studies revealed a direct interaction of FBXO32 and EndoA on autophagosomes. EndoA triple knockout mice showed a reduction in LC3B, Atg5, and autophagosomes while the loss of FBXO32 in flies caused defective autophagosome formation, mimicking EndoA loss. The authors suggest that a disruption in either EndoA-autophagy or FBXO32-UPS overburdens the remaining pathway, disrupting protein homeostasis, and initiating neurodegeneration [19].
Synaptojanin1
Several studies have identified SYNJ1/PARK20 as an early onset Parkinsonian gene [52–54]. As discussed above, synj1 is an EndoA binding partner that acts in SV endocytosis [55–58]. One study examining changes in the proteome and phosphoproteome of brains from LRRK2 hyperactive kinase mutation R1441C expressing flies revealed major changes in SV proteins [59]. Further investigation into these alterations identified synj1 as a putative LRRK2 substrate [59]. Additional studies revealed a distinct phosphorylation site in synj1 (T1205) in vitro using purified LRRK2 G2019S and showed this phosphosite is critical for the interaction of synj1 with EndoA [39]. Aside from its interaction with LRRK2, mutations in the SAC1 and 5′phosphatase domain of synj1 are also linked to hereditary early-onset PD [52–54, 60]. While synj1 is known for its role in SV endocytosis, recent studies have found an additional role of synj1 in autophagy. One PD linked mutation, R258Q, in the synj1 SAC1 domain leads to an imbalance in lipid production that results in the accumulation of WIPI2/Atg18a, a PI(3)P/PI(3,5)P2 binding protein. This accumulation blocks autophagosome maturation at the Drosophila neuromuscular junction and in patient-derived neurons. Interestingly, SV endocytosis remains intact in synj1 RQ mutant flies [61]. However, other investigations in RQ knock-in mice have found a disruption in SV endocytosis, an accumulation of clathrin-coated intermediates, and dystrophic terminal changes [62]. Prior to this study, the role of the SAC1 domain in endocytosis was not well documented; though, it was known that the 5′phoshatase domain is necessary for SV endocytosis [56]. Whether there is a direct role of the SAC1 domain of synj1 in autophagy or if these deficiencies are due to an endocytic defect needs further study. Additionally, recent studies in zebrafish show that a 5′ phosphatase synj1 mutation, D732A, blocks autophagy in photoreceptor neurons [18]. Again, the contribution of disrupted endocytosis to the deregulation of autophagy remains uncertain in this system and requires further study to parse apart the exact role of synj1 in endocytosis versus autophagy. Nonetheless, these studies show synj1 PD-linked mutations disrupt autophagy and, together with the EndoA findings, raise an interesting potential of synapse-specific autophagy machinery that is disrupted in PD.
Dynamin
The dynamin family of GTPases is critical for membrane scission during endocytosis events including SV endocytosis [63]. Recent studies have shown genetic and biochemical interaction between dynamin isoforms and LRRK2. For example, LRRK2 has been shown to interact with dynamins 1–3 in HEK cells and, more specifically, with dynamin 1 in neuronal cultures [64]. Additionally, genetic variability within dynamin 3 was shown to modify the age of onset of PD in LRRK2 G2019S carriers [65]. While the role of the dynamin family in autophagy remains unclear, one recent study has shown dynamin 2 plays a role in the activation of mTORC1 through the endocytosis of amino acids in HEK cells [66]. This again highlights the interconnectivity of endocytosis and autophagy and underpins the need for additional studies to understand the role of this important endocytic protein in neuronal autophagy and to determine the functional role of its interaction with LRRK2.
Rab GTPases
A recent landmark study has shown that multiple members of the Rab family of small GTPases are physiological substrates of LRRK2 [23]. As critical regulators of membrane trafficking, Rab GTPases are likely candidates to regulate neuronal autophagy and SV cycling [67–69]. Interestingly, mouse models harboring LRRK2 hyperactive kinase mutations display defects in synaptic and autophagic function, suggesting chronic phosphorylation of Rab GTPases may underlie these alterations [36, 42, 43]. Indeed, the Rab GTPases identified as LRRK2 substrates, Rab8, 10, and 12, all have putative roles in autophagy and endocytic recycling, particularly in the context of neurological disease conditions [22, 24–28, 67, 70–76]. Further, all three of these Rab proteins have been shown to associate with SVs through mass spectrometry analysis of the protein composition of purified SVs [69]. The exact role of these proteins in neurons and the effect of LRRK2-mediated phosphorylation on their function remains a topic of great interest, which is expected to garner considerable insight into how alterations in neuronal trafficking pathways contribute to PD pathogenesis.
Auxilin
Aside from LRRK2 and its interactors, additional genes implicated in PD have roles in SV trafficking. Specifically, mutations in DNAJC6 have been associated with juvenile Parkinsonism and early-onset PD [54, 77, 78]. DNAJC6 encodes auxilin, a protein enriched in nerve terminals where it functions in clathrin uncoating during endocytosis [79–82]. The effects of its PD-linked mutations on synaptic endocytosis are currently unknown and warrant further study.
Parkin
Mutations in Parkin, an E3 ubiquitin ligase, result in juvenile-onset PD [83]. While the role of Parkin in mitophagy has been extensively studied [84, in this issue], the role of Parkin in regulating synaptic proteins remains under investigation Several synaptic proteins have been shown to be Parkin substrates and/or interactors [85–90]. In particular, Parkin has been shown to ubiquitinate EndoA, synj1, and dynamin, proteins critical in regulating SV endocytosis, as well as another PD-risk factor, Synaptotagmin XI (Syt11) that will be discussed later in this review [87, 90]. The effect of Parkin ubiquitination of these proteins is yet to be investigated; however, marked Parkin upregulation is seen in the brains of both EndoA triple KO mice and synj1-RQ knock-in mice [62, 90]. One potential explanation for this finding is that Parkin upregulation functions as a compensatory mechanism to counteract autophagy defects in these mutant mice [19]. Altogether, these findings suggesting a complex interplay between Parkin and SV endocytic proteins and further corroborate a link between SV endocytosis, autophagy, and PD pathogenesis.
α-synuclein
Mutations or copy number variations of SNCA, the gene encoding α-synuclein, are associated with familial PD [91, 92] while the accumulation of α-synuclein in Lewy bodies is a pathological hallmark of sporadic and many genetically linked PD cases. While wild-type α-synuclein is degraded by chaperone-mediated autophagy (CMA), mutated α-synuclein impairs CMA [93]. Additionally, aggregates of α-synuclein impair autophagosome clearance during macroautophagy [94] and overexpression of α-synuclein inhibits Rab1a activation, which is necessary for macroautophagy [95]. However, knockdown of α-synuclein also disrupts macroautophagy [96], suggesting that α-synuclein plays a role in macroautophagy. As α-synuclein was shown to be associated with SVs, many studies have investigated its potential role in SV trafficking; however, the exact function of α-synuclein in SV cycling is still under debate. Many early studies pointed to a role of this protein in regulating SV exocytosis, while more recent studies also implicate α-synuclein in SV endocytosis; this point of controversy was recently reviewed elsewhere [97].
Summary
While the many proteins described here have strong connections to both autophagy and SV cycling, the majority of the studies performed have investigated the role of these proteins in the two pathways independently. While limited, recent evidence has just begun to reveal how SV proteins modulate autophagy activity at the presynapse [98]. A detailed investigation of the link between these two pathways will allow for a greater understanding of PD pathogenesis and facilitate the targeted correction of PD-related disruptions in neuronal membrane trafficking.
Lysosomal dysfunction in PD
Evidence suggests that many PD-linked genes may affect the degradative capacity of autophagy by impairing lysosomal function. In this section, we review recent investigations into the role of LRRK2 and other PD-linked genes in regulating lysosomal function. Although LRRK2 is expressed highly in astrocytes, few studies have investigated its role in these cells. Recently, Henry et al. showed the expression of various LRRK2 PD mutants (G2019S, R1441C, or Y1699C) results in enlarged lysosomes with decreased degradative capacity. Although the study is carried out in astrocytes, the high expression of LRRK2 in these cells has drawn interest in examining the role of LRRK2 in astrocytes. Ultrastructural analysis of cortical neurons transfected with LRRK2 G2019S show enlarged electron-dense structures reminiscent of swollen lysosomes suggesting a general role of LRRK2 in regulating lysosomal function across neural cell types [99]. Further mechanistic investigation into this finding suggested that G2019S expression reduces lysosomal pH and results in increased expression of another PD gene, ATP13A2. The application of LRRK2 kinase inhibitors blocks this effect suggesting these results are kinase-dependent. Intriguingly, both G2019S and sporadic PD patient brains show decreased LAMP2 intensity in the prefrontal cortex while only G2019S patients show enhanced ATP13A2 expression [100]. ATP13A2 is a lysosomal ATPase linked to a juvenile form of Parkinsonism with dementia [101]. Expression of mutant ATP13A2 in cells results in lysosomal impairment, decreased cathepsin D activity, and α-synuclein accumulation [102]. Intriguingly, ATP13A2 has recently been linked to another PD risk factor gene, Syt11 [103]. Knockdown of Syt11 phenocopied the lysosomal defects observed upon loss of ATP13A2 while double knockdown of these two genes did not further impair functionality suggesting they act in the same pathway [17]. Further studies suggest Syt11 may control the clearance of autophagosomes by regulating autophagosome-lysosome fusion [17]. Syt11 has also been linked to SV endocytosis in dorsal root ganglion and hippocampal neurons [104]. Knockdown studies suggest Syt11 ensures precision in synaptic vesicle endocytosis by limiting membrane retrieval sites [104]. Overall, these findings suggest a network of PD genes that converge on SV trafficking and the autophagy-lysosome pathway.
Additional genes linked to PD have roles in regulating lysosomal function. These include β-glucocerebrosidase (GBA), LAMP2A, and sphingomyelin phosphodiesterase-1 (SMPD1). Mutations in these genes tend to reduce lysosomal function and lead to increased α-synuclein levels. Aside from increasing levels of α-synuclein through a lack of degradation, lysosomal dysfunction has also been linked to an increase in the propagation of α-synuclein [105]. Indeed, decreased enzymatic activity of GBA has been shown to increase the propagation of α-synuclein aggregates [106]. While homozygous mutations in GBA are known to cause Gaucher’s disease, a lysosomal storage disorder, heterozygous mutations in GBA were found to greatly increase the risk of PD [107]. As LAMP2A is the major receptor for CMA, it is not surprising that decreased levels of LAMP2A result in impaired clearance of α-synuclein, a known CMA substrate [105]. LAMP2A was found as a risk factor in sporadic PD [7], and mistrafficking of LAMP2A has been implicated as a pathogenic mechanism in VPS35 linked familial PD [108]. VPS35 is associated with retromer trafficking from endosomes to the trans-golgi network. Mutations in VPS35 result not only in the mistrafficking of LAMP2A but also of Atg9A, which leads to impaired autophagy [109]. Intriguingly, a recent study in Drosophila has shown that the loss of VPS35 results in SV cycling defects that are not rescued by PD-associated mutants [110]. While this finding awaits confirmation in mammalian systems, it adds to the emerging evidence of dysfunction in the coordination of SV cycling and autophagy in PD. Another PD-risk factor, SMPD1 is typically associated with Niemann-Pick lysosomal storage disorder and has been shown to be relevant for lysosomal homeostasis [111]. More recently, a role of sphingolipids in autophagy has been recognized, suggesting yet another link between PD and autophagy/lysosomal function [112].
Conclusion
Recent years have seen an advancement in linking PD pathogenesis to dysfunction within the autophagy-lysosomal system. Now, emerging evidence connects PD to SV cycling, an important neuronal membrane trafficking pathway at presynaptic terminals. The studies over the past few years have shown that these two pathways are not entirely independent and may, in fact, share a number of regulatory proteins. The implication of many of these proteins in PD suggests dysfunction in neuronal membrane trafficking pathways as a key contributor to PD pathogenesis (Figure 2). Although most of these current studies do not directly explain how dysfunctional membrane trafficking leads to neuronal cell death, studies from animal models of PD and other neurodegenerative diseases suggest that changes in SVs and axons represent the earliest detectable phenotypes [44, 99, 113–115]. Additionally, functional imaging in PD patients suggests early changes in dopaminergic terminals in the striatum occur many years before cell loss is observable in the substantia nigra [116]. These findings suggest that the dystrophic changes that occur in axon terminals following defects in autophagy and SV cycling likely represent the initiation of degeneration at the nerve terminal that leads to the “dying-back” or retrograde degeneration of these neurons. This process and its implications in neurodegeneration, especially related to PD, have recently been reviewed elsewhere [117]. As these changes may occur early in the disease, greater understanding of these proteins in regulating autophagy and SV cycling and determination of how deregulation in these membrane trafficking pathways leads to neuronal dysfunction will facilitate the development of interventional therapies aimed at halting PD progression.
Figure 2. Involvement of PD linked genes in SV trafficking and autophagy degradation pathways.
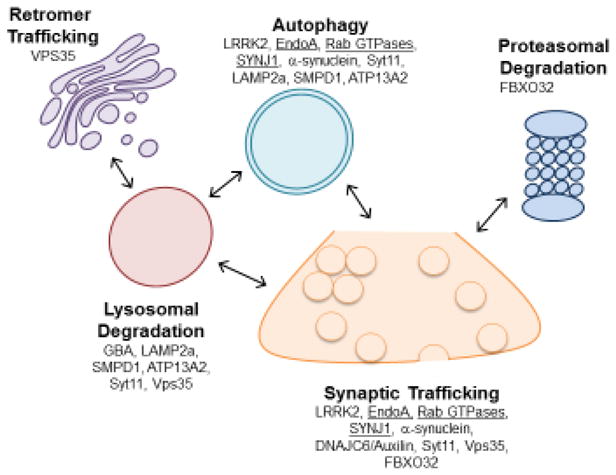
Many genes implicated in PD have roles in multiple neuronal trafficking and degradation pathways. For example, LRRK2 and its substrate, EndoA and synj1, are implicated in the dual regulation of SV trafficking and autophagy. The Rab GTPases, also LRRK2 substrates, are likely to regulate trafficking in a number of these pathways but further study of their roles in neurons is necessary. The other PD genes shown here have been implicated in various pathways and require further study to determine their roles in these pathways and how PD-related mutations alter their function. Putative LRRK2 substrates are underlined.
Highlights.
Multiple PD related genes are known to regulate membrane trafficking pathways.
Autophagy and synaptic vesicle cycling share regulatory proteins.
Mutations within these common regulators are implicated in PD.
Disruption in neuronal trafficking pathways may underlie PD pathogenesis.
Acknowledgments
We are thankful to all members of Yue laboratory for the critical reading and discussion of the manuscript.
Role of funding source
This work was partially supported by NIH grant R01 NS060123 and R01 NS060809 (ZY) and F32 NS105334 (PS).
Footnotes
Author contributions
PS and ZY wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, Burn D, Halliday GM, Bezard E, Przedborski S, Lehericy S, Brooks DJ, Rothwell JC, Hallett M, DeLong MR, Marras C, Tanner CM, Ross GW, Langston JW, Klein C, Bonifati V, Jankovic J, Lozano AM, Deuschl G, Bergman H, Tolosa E, Rodriguez-Violante M, Fahn S, Postuma RB, Berg D, Marek K, Standaert DG, Surmeier DJ, Olanow CW, Kordower JH, Calabresi P, Schapira AHV, Stoessl AJ. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord. 2017;32:1264–1310. doi: 10.1002/mds.27115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang D, Nalls MA, Hallgrimsdottir IB, Hunkapiller J, van der Brug M, Cai F, Kerchner GA, Ayalon G, Bingol B, Sheng M, Hinds D, Behrens TW, Singleton AB, Bhangale TR, Graham RR International Parkinson’s Disease Genomics C, T 23andMe Research. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet. 2017;49:1511–1516. doi: 10.1038/ng.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung HC, Scholz S, Matarin M, Simon-Sanchez J, Hernandez D, Britton A, Gibbs JR, Langefeld C, Stiegert ML, Schymick J, Okun MS, Mandel RJ, Fernandez HH, Foote KD, Rodriguez RL, Peckham E, De Vrieze FW, Gwinn-Hardy K, Hardy JA, Singleton A. Genome-wide genotyping in Parkinson’s disease and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol. 2006;5:911–916. doi: 10.1016/S1474-4422(06)70578-6. [DOI] [PubMed] [Google Scholar]
- 4.Maraganore DM, de Andrade M, Lesnick TG, Strain KJ, Farrer MJ, Rocca WA, Pant PV, Frazer KA, Cox DR, Ballinger DG. High-resolution whole-genome association study of Parkinson disease. Am J Hum Genet. 2005;77:685–693. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, Sveinbjornsdottir S, Stefansson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, Ikram MA, Ioannidis JP, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB I.P.s.D.G. Consortium, G.I. Parkinson’s Study Group Parkinson’s Research: The Organized, 23andMe, GenePd, C. NeuroGenetics Research, G. Hussman Institute of Human, A.J.D. Investigator, H. Cohorts for, E. Aging Research in Genetic, N.A.B.E. Consortium, U.K.B.E. Consortium, G.P.s.D. Consortium, A.G.A. Group. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman LG, Lachenmayer ML, Wang J, He L, Poulose SM, Komatsu M, Holstein GR, Yue Z. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of alpha-synuclein and LRRK2 in the brain. J Neurosci. 2012;32:7585–7593. doi: 10.1523/JNEUROSCI.5809-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 10.Komatsu M, Wang QJ, Holstein GR, Friedrich VL, Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A. 2007;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishiyama J, Miura E, Mizushima N, Watanabe M, Yuzaki M. Aberrant membranes and double-membrane structures accumulate in the axons of Atg5-null Purkinje cells before neuronal death. Autophagy. 2007;3:591–596. doi: 10.4161/auto.4964. [DOI] [PubMed] [Google Scholar]
- 12.Joo JH, Wang B, Frankel E, Ge L, Xu L, Iyengar R, Li-Harms X, Wright C, Shaw TI, Lindsten T, Green DR, Peng J, Hendershot LM, Kilic F, Sze JY, Audhya A, Kundu M. The Noncanonical Role of ULK/ATG1 in ER-to-Golgi Trafficking Is Essential for Cellular Homeostasis. Mol Cell. 2016;62:491–506. doi: 10.1016/j.molcel.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang CC, Wang C, Peng X, Gan B, Guan JL. Neural-specific deletion of FIP200 leads to cerebellar degeneration caused by increased neuronal death and axon degeneration. J Biol Chem. 2010;285:3499–3509. doi: 10.1074/jbc.M109.072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orosco LA, Ross AP, Cates SL, Scott SE, Wu D, Sohn J, Pleasure D, Pleasure SJ, Adamopoulos IE, Zarbalis KS. Loss of Wdfy3 in mice alters cerebral cortical neurogenesis reflecting aspects of the autism pathology. Nat Commun. 2014;5:4692. doi: 10.1038/ncomms5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunn BH, Cragg SJ, Bolam JP, Spillantini MG, Wade-Martins R. Impaired intracellular trafficking defines early Parkinson’s disease. Trends Neurosci. 2015;38:178–188. doi: 10.1016/j.tins.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Huang T, Bu G, Xu H. Dysregulation of protein trafficking in neurodegeneration. Mol Neurodegener. 2014;9:31. doi: 10.1186/1750-1326-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bento CF, Ashkenazi A, Jimenez-Sanchez M, Rubinsztein DC. The Parkinson’s disease-associated genes ATP13A2 and SYT11 regulate autophagy via a common pathway. Nat Commun. 2016;7:11803. doi: 10.1038/ncomms11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George AA, Hayden S, Stanton GR, Brockerhoff SE. Arf6 and the 5’phosphatase of Synaptojanin 1 regulate autophagy in cone photoreceptors. Inside Cell. 2016;1:117–133. doi: 10.1002/icl3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murdoch JD, Rostosky CM, Gowrisankaran S, Arora AS, Soukup SF, Vidal R, Capece V, Freytag S, Fischer A, Verstreken P, Bonn S, Raimundo N, Milosevic I. Endophilin-A Deficiency Induces the Foxo3a-Fbxo32 Network in the Brain and Causes Dysregulation of Autophagy and the Ubiquitin-Proteasome System. Cell Rep. 2016;17:1071–1086. doi: 10.1016/j.celrep.2016.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soukup SF, Kuenen S, Vanhauwaert R, Manetsberger J, Hernandez-Diaz S, Swerts J, Schoovaerts N, Vilain S, Gounko NV, Vints K, Geens A, De Strooper B, Verstreken P. A LRRK2-Dependent EndophilinA Phosphoswitch Is Critical for Macroautophagy at Presynaptic Terminals. Neuron. 2016;92:829–844. doi: 10.1016/j.neuron.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Deng HX, Shi Y, Yang Y, Ahmeti KB, Miller N, Huang C, Cheng L, Zhai H, Deng S, Nuytemans K, Corbett NJ, Kim MJ, Deng H, Tang B, Yang Z, Xu Y, Chan P, Huang B, Gao XP, Song Z, Liu Z, Fecto F, Siddique N, Foroud T, Jankovic J, Ghetti B, Nicholson DA, Krainc D, Melen O, Vance JM, Pericak-Vance MA, Ma YC, Rajput AH, Siddique T. Identification of TMEM230 mutations in familial Parkinson’s disease. Nat Genet. 2016;48:733–739. doi: 10.1038/ng.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Schulze RJ, Weller SG, Krueger EW, Schott MB, Zhang X, Casey CA, Liu J, Stockli J, James DE, McNiven MA. A novel Rab10-EHBP1-EHD2 complex essential for the autophagic engulfment of lipid droplets. Sci Adv. 2016;2:e1601470. doi: 10.1126/sciadv.1601470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, Baptista MA, Fiske BK, Fell MJ, Morrow JA, Reith AD, Alessi DR, Mann M. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife. 2016;5 doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Zhang X, Liu G, Chang D, Liang X, Zhu X, Tao W, Mei L. Intracellular Trafficking Network of Protein Nanocapsules: Endocytosis, Exocytosis and Autophagy. Theranostics. 2016;6:2099–2113. doi: 10.7150/thno.16587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babbey CM, Ahktar N, Wang E, Chen CC, Grant BD, Dunn KW. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CC, Schweinsberg PJ, Vashist S, Mareiniss DP, Lambie EJ, Grant BD. RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol Biol Cell. 2006;17:1286–1297. doi: 10.1091/mbc.E05-08-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilherme A, Soriano NA, Furcinitti PS, Czech MP. Role of EHD1 and EHBP1 in perinuclear sorting and insulin-regulated GLUT4 recycling in 3T3-L1 adipocytes. J Biol Chem. 2004;279:40062–40075. doi: 10.1074/jbc.M401918200. [DOI] [PubMed] [Google Scholar]
- 28.Glodowski DR, Chen CC, Schaefer H, Grant BD, Rongo C. RAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathway. Mol Biol Cell. 2007;18:4387–4396. doi: 10.1091/mbc.E07-05-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010;30:8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garden GA, La Spada AR. Intercellular (mis)communication in neurodegenerative disease. Neuron. 2012;73:886–901. doi: 10.1016/j.neuron.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol. 2012;4:a005645. doi: 10.1101/cshperspect.a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. doi: 10.1038/nature20414. [DOI] [PubMed] [Google Scholar]
- 33.Tooze SA, Abada A, Elazar Z. Endocytosis and autophagy: exploitation or cooperation? Cold Spring Harb Perspect Biol. 2014;6:a018358. doi: 10.1101/cshperspect.a018358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Matta S, Van Kolen K, da Cunha R, van den Bogaart G, Mandemakers W, Miskiewicz K, De Bock PJ, Morais VA, Vilain S, Haddad D, Delbroek L, Swerts J, Chavez-Gutierrez L, Esposito G, Daneels G, Karran E, Holt M, Gevaert K, Moechars DW, De Strooper B, Verstreken P. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron. 2012;75:1008–1021. doi: 10.1016/j.neuron.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Cirnaru MD, Marte A, Belluzzi E, Russo I, Gabrielli M, Longo F, Arcuri L, Murru L, Bubacco L, Matteoli M, Fedele E, Sala C, Passafaro M, Morari M, Greggio E, Onofri F, Piccoli G. LRRK2 kinase activity regulates synaptic vesicle trafficking and neurotransmitter release through modulation of LRRK2 macro-molecular complex. Front Mol Neurosci. 2014;7:49. doi: 10.3389/fnmol.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccoli G, Condliffe SB, Bauer M, Giesert F, Boldt K, De Astis S, Meixner A, Sarioglu H, Vogt-Weisenhorn DM, Wurst W, Gloeckner CJ, Matteoli M, Sala C, Ueffing M. LRRK2 controls synaptic vesicle storage and mobilization within the recycling pool. J Neurosci. 2011;31:2225–2237. doi: 10.1523/JNEUROSCI.3730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan PY, Li X, Wang J, Powell J, Wang Q, Zhang Y, Chen Z, Wicinski B, Hof P, Ryan TA, Yue Z. Parkinson’s disease associated LRRK2 hyperactive kinase mutant disrupts synaptic vesicle trafficking in ventral midbrain neurons. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.0964-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin N, Jeong H, Kwon J, Heo HY, Kwon JJ, Yun HJ, Kim CH, Han BS, Tong Y, Shen J, Hatano T, Hattori N, Kim KS, Chang S, Seol W. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314:2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Arranz AM, Delbroek L, Van Kolen K, Guimaraes MR, Mandemakers W, Daneels G, Matta S, Calafate S, Shaban H, Baatsen P, De Bock PJ, Gevaert K, Vanden Berghe P, Verstreken P, De Strooper B, Moechars D. LRRK2 functions in synaptic vesicle endocytosis through a kinase-dependent mechanism. J Cell Sci. 2015;128:541–552. doi: 10.1242/jcs.158196. [DOI] [PubMed] [Google Scholar]
- 42.Ramonet D, Daher JP, Lin BM, Stafa K, Kim J, Banerjee R, Westerlund M, Pletnikova O, Glauser L, Yang L, Liu Y, Swing DA, Beal MF, Troncoso JC, McCaffery JM, Jenkins NA, Copeland NG, Galter D, Thomas B, Lee MK, Dawson TM, Dawson VL, Moore DJ. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One. 2011;6:e18568. doi: 10.1371/journal.pone.0018568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez-Danes A, Richaud-Patin Y, Carballo-Carbajal I, Jimenez-Delgado S, Caig C, Mora S, Di Guglielmo C, Ezquerra M, Patel B, Giralt A, Canals JM, Memo M, Alberch J, Lopez-Barneo J, Vila M, Cuervo AM, Tolosa E, Consiglio A, Raya A. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plowey ED, Cherra SJ, 3rd, Liu YJ, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higashi S, Moore DJ, Yamamoto R, Minegishi M, Sato K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Hino H, Kosaka K, Emson PC, Wada K, Dawson VL, Dawson TM, Arai H, Iseki E. Abnormal localization of leucine-rich repeat kinase 2 to the endosomal-lysosomal compartment in lewy body disease. J Neuropathol Exp Neurol. 2009;68:994–1005. doi: 10.1097/NEN.0b013e3181b44ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong Y, Coombes CE, Kilaru A, Li X, Gitler AD, Bowers WJ, Dawson VL, Dawson TM, Moore DJ. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet. 2010;6:e1000902. doi: 10.1371/journal.pgen.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biskup S, West AB. Zeroing in on LRRK2-linked pathogenic mechanisms in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:625–633. doi: 10.1016/j.bbadis.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schapansky J, Nardozzi JD, Felizia F, LaVoie MJ. Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum Mol Genet. 2014;23:4201–4214. doi: 10.1093/hmg/ddu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bang Y, Kim KS, Seol W, Choi HJ. LRRK2 interferes with aggresome formation for autophagic clearance. Mol Cell Neurosci. 2016;75:71–80. doi: 10.1016/j.mcn.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Ringstad N, Gad H, Low P, Di Paolo G, Brodin L, Shupliakov O, De Camilli P. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron. 1999;24:143–154. doi: 10.1016/s0896-6273(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 51.Ringstad N, Nemoto Y, De Camilli P. The SH3p4/Sh3p8/SH3p13 protein family: binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc Natl Acad Sci U S A. 1997;94:8569–8574. doi: 10.1073/pnas.94.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krebs CE, Karkheiran S, Powell JC, Cao M, Makarov V, Darvish H, Di Paolo G, Walker RH, Shahidi GA, Buxbaum JD, De Camilli P, Yue Z, Paisan-Ruiz C. The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum Mutat. 2013;34:1200–1207. doi: 10.1002/humu.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quadri M, Fang M, Picillo M, Olgiati S, Breedveld GJ, Graafland J, Wu B, Xu F, Erro R, Amboni M, Pappata S, Quarantelli M, Annesi G, Quattrone A, Chien HF, Barbosa ER, Oostra BA, Barone P, Wang J, Bonifati V International Parkinsonism Genetics N. Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism. Hum Mutat. 2013;34:1208–1215. doi: 10.1002/humu.22373. [DOI] [PubMed] [Google Scholar]
- 54.Olgiati S, De Rosa A, Quadri M, Criscuolo C, Breedveld GJ, Picillo M, Pappata S, Quarantelli M, Barone P, De Michele G, Bonifati V. PARK20 caused by SYNJ1 homozygous Arg258Gln mutation in a new Italian family. Neurogenetics. 2014;15:183–188. doi: 10.1007/s10048-014-0406-0. [DOI] [PubMed] [Google Scholar]
- 55.McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- 56.Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 57.Harris TW, Hartwieg E, Horvitz HR, Jorgensen EM. Mutations in synaptojanin disrupt synaptic vesicle recycling. J Cell Biol. 2000;150:589–600. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- 59.Islam MS, Nolte H, Jacob W, Ziegler AB, Putz S, Grosjean Y, Szczepanowska K, Trifunovic A, Braun T, Heumann H, Heumann R, Hovemann B, Moore DJ, Kruger M. Human R1441C LRRK2 regulates the synaptic vesicle proteome and phosphoproteome in a Drosophila model of Parkinson’s disease. Hum Mol Genet. 2016;25:5365–5382. doi: 10.1093/hmg/ddw352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirola L, Behari M, Shishir C, Thelma BK. Identification of a novel homozygous mutation Arg459Pro in SYNJ1 gene of an Indian family with autosomal recessive juvenile Parkinsonism. Parkinsonism Relat Disord. 2016;31:124–128. doi: 10.1016/j.parkreldis.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 61.Vanhauwaert R, Kuenen S, Masius R, Bademosi A, Manetsberger J, Schoovaerts N, Bounti L, Gontcharenko S, Swerts J, Vilain S, Picillo M, Barone P, Munshi ST, de Vrij FM, Kushner SA, Gounko NV, Mandemakers W, Bonifati V, Meunier FA, Soukup SF, Verstreken P. The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals. EMBO J. 2017;36:1392–1411. doi: 10.15252/embj.201695773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao M, Wu Y, Ashrafi G, McCartney AJ, Wheeler H, Bushong EA, Boassa D, Ellisman MH, Ryan TA, De Camilli P. Parkinson Sac Domain Mutation in Synaptojanin 1 Impairs Clathrin Uncoating at Synapses and Triggers Dystrophic Changes in Dopaminergic Axons. Neuron. 2017;93:882–896 e885. doi: 10.1016/j.neuron.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raimondi A, Ferguson SM, Lou X, Armbruster M, Paradise S, Giovedi S, Messa M, Kono N, Takasaki J, Cappello V, O’Toole E, Ryan TA, De Camilli P. Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron. 2011;70:1100–1114. doi: 10.1016/j.neuron.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stafa K, Tsika E, Moser R, Musso A, Glauser L, Jones A, Biskup S, Xiong Y, Bandopadhyay R, Dawson VL, Dawson TM, Moore DJ. Functional interaction of Parkinson’s disease-associated LRRK2 with members of the dynamin GTPase superfamily. Hum Mol Genet. 2014;23:2055–2077. doi: 10.1093/hmg/ddt600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trinh J, Gustavsson EK, Vilarino-Guell C, Bortnick S, Latourelle J, McKenzie MB, Tu CS, Nosova E, Khinda J, Milnerwood A, Lesage S, Brice A, Tazir M, Aasly JO, Parkkinen L, Haytural H, Foroud T, Myers RH, Sassi SB, Hentati E, Nabli F, Farhat E, Amouri R, Hentati F, Farrer MJ. DNM3 and genetic modifiers of age of onset in LRRK2 Gly2019Ser parkinsonism: a genome-wide linkage and association study. Lancet Neurol. 2016;15:1248–1256. doi: 10.1016/S1474-4422(16)30203-4. [DOI] [PubMed] [Google Scholar]
- 66.Shibutani S, Okazaki H, Iwata H. Dynamin-dependent amino acid endocytosis activates mechanistic target of rapamycin complex 1 (mTORC1) J Biol Chem. 2017 doi: 10.1074/jbc.M117.776443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ao X, Zou L, Wu Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014;21:348–358. doi: 10.1038/cdd.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pavlos NJ, Jahn R. Distinct yet overlapping roles of Rab GTPases on synaptic vesicles. Small GTPases. 2011;2:77–81. doi: 10.4161/sgtp.2.2.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 70.Matsui T, Fukuda M. Rab12 regulates mTORC1 activity and autophagy through controlling the degradation of amino-acid transporter PAT4. EMBO Rep. 2013;14:450–457. doi: 10.1038/embor.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsui T, Itoh T, Fukuda M. Small GTPase Rab12 regulates constitutive degradation of transferrin receptor. Traffic. 2011;12:1432–1443. doi: 10.1111/j.1600-0854.2011.01240.x. [DOI] [PubMed] [Google Scholar]
- 72.Esseltine JL, Ribeiro FM, Ferguson SS. Rab8 modulates metabotropic glutamate receptor subtype 1 intracellular trafficking and signaling in a protein kinase C-dependent manner. J Neurosci. 2012;32:16933–16942a. doi: 10.1523/JNEUROSCI.0625-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pilli M, Arko-Mensah J, Ponpuak M, Roberts E, Master S, Mandell MA, Dupont N, Ornatowski W, Jiang S, Bradfute SB, Bruun JA, Hansen TE, Johansen T, Deretic V. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity. 2012;37:223–234. doi: 10.1016/j.immuni.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sirohi K, Chalasani ML, Sudhakar C, Kumari A, Radha V, Swarup G. M98K-OPTN induces transferrin receptor degradation and RAB12-mediated autophagic death in retinal ganglion cells. Autophagy. 2013;9:510–527. doi: 10.4161/auto.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu J, Fotouhi M, McPherson PS. Phosphorylation of the exchange factor DENND3 by ULK in response to starvation activates Rab12 and induces autophagy. EMBO Rep. 2015;16:709–718. doi: 10.15252/embr.201440006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rydell GE, Renard HF, Garcia-Castillo MD, Dingli F, Loew D, Lamaze C, Romer W, Johannes L. Rab12 localizes to Shiga toxin-induced plasma membrane invaginations and controls toxin transport. Traffic. 2014;15:772–787. doi: 10.1111/tra.12173. [DOI] [PubMed] [Google Scholar]
- 77.Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim YI, Zenvirt S, Jalas C, Lesage S, Brice A, Taraboulos A, Kaestner KH, Greene LE, Elpeleg O. A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS One. 2012;7:e36458. doi: 10.1371/journal.pone.0036458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koroglu C, Baysal L, Cetinkaya M, Karasoy H, Tolun A. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Parkinsonism Relat Disord. 2013;19:320–324. doi: 10.1016/j.parkreldis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 79.Ahle S, Ungewickell E. Auxilin, a newly identified clathrin-associated protein in coated vesicles from bovine brain. J Cell Biol. 1990;111:19–29. doi: 10.1083/jcb.111.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirst J, Sahlender DA, Li S, Lubben NB, Borner GH, Robinson MS. Auxilin depletion causes self-assembly of clathrin into membraneless cages in vivo. Traffic. 2008;9:1354–1371. doi: 10.1111/j.1600-0854.2008.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eisenberg E, Greene LE. Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis. Traffic. 2007;8:640–646. doi: 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 82.Yim YI, Sun T, Wu LG, Raimondi A, De Camilli P, Eisenberg E, Greene LE. Endocytosis and clathrin-uncoating defects at synapses of auxilin knockout mice. Proc Natl Acad Sci U S A. 2010;107:4412–4417. doi: 10.1073/pnas.1000738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 84.Chu CT. NeurosciLett. 2018 [Google Scholar]
- 85.Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fallon L, Moreau F, Croft BG, Labib N, Gu WJ, Fon EA. Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain. J Biol Chem. 2002;277:486–491. doi: 10.1074/jbc.M109806200. [DOI] [PubMed] [Google Scholar]
- 87.Huynh DP, Scoles DR, Nguyen D, Pulst SM. The autosomal recessive juvenile Parkinson disease gene product, parkin, interacts with and ubiquitinates synaptotagmin XI. Hum Mol Genet. 2003;12:2587–2597. doi: 10.1093/hmg/ddg269. [DOI] [PubMed] [Google Scholar]
- 88.Fallon L, Belanger CM, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, Voortman J, Haber M, Rouleau G, Thorarinsdottir T, Brice A, van Bergen En Henegouwen PM, Fon EA. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006;8:834–842. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- 89.Joch M, Ase AR, Chen CX, MacDonald PA, Kontogiannea M, Corera AT, Brice A, Seguela P, Fon EA. Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels. Mol Biol Cell. 2007;18:3105–3118. doi: 10.1091/mbc.E05-11-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao M, Milosevic I, Giovedi S, De Camilli P. Upregulation of Parkin in endophilin mutant mice. J Neurosci. 2014;34:16544–16549. doi: 10.1523/JNEUROSCI.1710-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 92.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 93.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 94.Tanik SA, Schultheiss CE, Volpicelli-Daley LA, Brunden KR, Lee VM. Lewy body-like alpha-synuclein aggregates resist degradation and impair macroautophagy. J Biol Chem. 2013;288:15194–15210. doi: 10.1074/jbc.M113.457408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O’Kane CJ, Rubinsztein DC. alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol. 2010;190:1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu WH, Dorado B, Figueroa HY, Wang L, Planel E, Cookson MR, Clark LN, Duff KE. Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. Am J Pathol. 2009;175:736–747. doi: 10.2353/ajpath.2009.080928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lautenschlager J, Kaminski CF, Kaminski Schierle GS. alpha-Synuclein - Regulator of Exocytosis, Endocytosis, or Both? Trends Cell Biol. 2017;27:468–479. doi: 10.1016/j.tcb.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 98.Vijayan V, Verstreken P. Autophagy in the presynaptic compartment in health and disease. J Cell Biol. 2017;216:1895–1906. doi: 10.1083/jcb.201611113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.MacLeod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 100.Henry AG, Aghamohammadzadeh S, Samaroo H, Chen Y, Mou K, Needle E, Hirst WD. Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum Mol Genet. 2015;24:6013–6028. doi: 10.1093/hmg/ddv314. [DOI] [PubMed] [Google Scholar]
- 101.Park JS, Blair NF, Sue CM. The role of ATP13A2 in Parkinson’s disease: Clinical phenotypes and molecular mechanisms. Mov Disord. 2015;30:770–779. doi: 10.1002/mds.26243. [DOI] [PubMed] [Google Scholar]
- 102.Matsui H, Sato F, Sato S, Koike M, Taruno Y, Saiki S, Funayama M, Ito H, Taniguchi Y, Uemura N, Toyoda A, Sakaki Y, Takeda S, Uchiyama Y, Hattori N, Takahashi R. ATP13A2 deficiency induces a decrease in cathepsin D activity, fingerprint-like inclusion body formation, and selective degeneration of dopaminergic neurons. FEBS Lett. 2013;587:1316–1325. doi: 10.1016/j.febslet.2013.02.046. [DOI] [PubMed] [Google Scholar]
- 103.Beilina A, Rudenko IN, Kaganovich A, Civiero L, Chau H, Kalia SK, Kalia LV, Lobbestael E, Chia R, Ndukwe K, Ding J, Nalls MA, Olszewski M, Hauser DN, Kumaran R, Lozano AM, Baekelandt V, Greene LE, Taymans JM, Greggio E, Cookson MR International Parkinson’s Disease Genomics C, North American Brain Expression C. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc Natl Acad Sci U S A. 2014;111:2626–2631. doi: 10.1073/pnas.1318306111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang C, Wang Y, Hu M, Chai Z, Wu Q, Huang R, Han W, Zhang CX, Zhou Z. Synaptotagmin-11 inhibits clathrin-mediated and bulk endocytosis. EMBO Rep. 2016;17:47–63. doi: 10.15252/embr.201540689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bae EJ, Yang NY, Song M, Lee CS, Lee JS, Jung BC, Lee HJ, Kim S, Masliah E, Sardi SP, Lee SJ. Glucocerebrosidase depletion enhances cell-to-cell transmission of alpha-synuclein. Nat Commun. 2014;5:4755. doi: 10.1038/ncomms5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schapira AH. Glucocerebrosidase and Parkinson disease: Recent advances. Mol Cell Neurosci. 2015;66:37–42. doi: 10.1016/j.mcn.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang FL, Erion JR, Tian Y, Liu W, Yin DM, Ye J, Tang B, Mei L, Xiong WC. VPS35 in Dopamine Neurons Is Required for Endosome-to-Golgi Retrieval of Lamp2a, a Receptor of Chaperone-Mediated Autophagy That Is Critical for alpha-Synuclein Degradation and Prevention of Pathogenesis of Parkinson’s Disease. J Neurosci. 2015;35:10613–10628. doi: 10.1523/JNEUROSCI.0042-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zavodszky E, Seaman MN, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat Commun. 2014;5:3828. doi: 10.1038/ncomms4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Inoshita T, Arano T, Hosaka Y, Meng H, Umezaki Y, Kosugi S, Morimoto T, Koike M, Chang HY, Imai Y, Hattori N. Vps35 in cooperation with LRRK2 regulates synaptic vesicle endocytosis through the endosomal pathway in Drosophila. Hum Mol Genet. 2017;26:2933–2948. doi: 10.1093/hmg/ddx179. [DOI] [PubMed] [Google Scholar]
- 111.Kirkegaard T, Roth AG, Petersen NH, Mahalka AK, Olsen OD, Moilanen I, Zylicz A, Knudsen J, Sandhoff K, Arenz C, Kinnunen PK, Nylandsted J, Jaattela M. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature. 2010;463:549–553. doi: 10.1038/nature08710. [DOI] [PubMed] [Google Scholar]
- 112.Perrotta C, Cervia D, De Palma C, Assi E, Pellegrino P, Bassi MT, Clementi E. The emerging role of acid sphingomyelinase in autophagy. Apoptosis. 2015;20:635–644. doi: 10.1007/s10495-015-1101-9. [DOI] [PubMed] [Google Scholar]
- 113.Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, Burke RE, Li C. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tagliaferro P, Kareva T, Oo TF, Yarygina O, Kholodilov N, Burke RE. An early axonopathy in a hLRRK2(R1441G) transgenic model of Parkinson disease. Neurobiol Dis. 2015;82:359–371. doi: 10.1016/j.nbd.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Overk CR, Masliah E. Pathogenesis of synaptic degeneration in Alzheimer’s disease and Lewy body disease. Biochem Pharmacol. 2014;88:508–516. doi: 10.1016/j.bcp.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sossi V, de la Fuente-Fernandez R, Holden JE, Schulzer M, Ruth TJ, Stoessl J. Changes of dopamine turnover in the progression of Parkinson’s disease as measured by positron emission tomography: their relation to disease-compensatory mechanisms. J Cereb Blood Flow Metab. 2004;24:869–876. doi: 10.1097/01.WCB.0000126563.85360.75. [DOI] [PubMed] [Google Scholar]
- 117.Tagliaferro P, Burke RE. Retrograde Axonal Degeneration in Parkinson Disease. J Parkinsons Dis. 2016;6:1–15. doi: 10.3233/JPD-150769. [DOI] [PMC free article] [PubMed] [Google Scholar]


