Abstract
BACKGROUND
Opioid dependence is a major public health issue without optimal therapeutics. This study investigates the potential therapeutic effect of dezocine, a nonaddictive opioid, in opioid dependence in rat models.
METHODS
Dezocine was administered intraperitoneally to a morphine-dependent rat model to investigate its effect on withdrawal and conditioned place preference (CPP). Effect of dezocine on morphine withdrawal syndrome and CPP was analyzed using 2-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Buprenorphine and vehicle solution containing 20% (v/v) dimethyl sulfoxide were used for positive and negative control, respectively. The astrocytes activation in nucleus accumbens was assessed by immunofluorescence assay of glial fibrillary acidic protein. Effect of dezocine and buprenorphine on the internalization of κ opioid receptor (KOR) was investigated using Neuro2A expressing KOR fused to red fluorescent protein tdTomato (KOR-tdT). Buprenorphine and dezocine were screened against 44 G-protein–coupled receptors, ion channels, and transporter proteins using radioligand-binding assay to compare the molecular targets.
RESULTS
The mean withdrawal score was reduced in rats treated with 1.25 mg·kg−1 dezocine compared to vehicle-treated control animals starting from the day 1 (mean difference: 7.8; 95% confidence interval [CI], 6.35–9.25; P < .0001 by 2-way ANOVA). Significance was observed at all treatment days, including day 7 (mean difference: 2.13; 95% CI, 0.68–3.58; P < .001 by 2-way ANOVA). Furthermore, dezocine inhibited the reinstatement of morphine-induced CPP (mean difference: 314; 95% CI, 197.9–430.1; P < .0001 by 2-way ANOVA) compared to the control group. Chronic morphine administration induced astrocytes activation in nucleus accumbens, which was attenuated by dezocine. Dezocine blocked the agonist-induced KOR internalization in vitro, 1 of the mechanisms involved in the downstream signaling and development of opioid dependence. Dezocine had affinity to norepinephrine and serotonin transporters and sigma-1 receptor, whereas buprenorphine showed no activity against these targets.
CONCLUSIONS
Dezocine could potentially be used to alleviate opioid dependence. Due to the unique molecular target profile different from buprenorphine, it might have important value in studying the mechanisms of morphine dependence and developing novel therapeutic approaches.
Opioid dependence, a growing clinical and social problem, is characterized by tolerance, withdrawal, and relapse, yet its precise management remains challenging. Increasing evidence indicates that κ opioid receptor (KOR) plays an important role in the development of μ opioid receptor (MOR)–mediated opioid dependence, tolerance, and withdrawal.1–3 Activation of KOR relates to the opioid withdrawal, as morphine-dependent KOR knockout mice displayed fewer withdrawal symptoms than wild-type mice.4 Importantly, KOR undergoes qualitative and quantitative changes including elevated levels of mRNA in nucleus accumbens (NAcc), ventral tegmental area, and locus coeruleus—the brain regions associated with drug dependence,5–8 as well as agonist-induced activation and internalization of the receptor for signal transduction cascades.9,10 Although accumulating evidence suggests that KOR agonists attenuate drug reward and demonstrate potent analgesic effects, these agonists have also been shown to accelerate drug relapse,11 establish conditioned place aversions,12,13 and generate aversive mood, such as dysphoria14,15 and depression-like behaviors.16 Moreover, stimulation of KORs with selective agonists can result in a dynorphin–KOR–dependent reinstatement of extinguished cocaine-conditioned place preference (CPP) or self-administration.17 Considering that dynorphin–KOR system activation is likely to play a pivotal role in withdrawal, selective KOR antagonism may be a powerful therapeutic strategy for the treatment of opioid dependence.
Pharmacological replacement therapy with buprenorphine, a partial MOR agonist/KOR antagonist18 or in combination with naltrexone or naloxone,19 has demonstrated advantages over a full μ agonist methadone in individuals with opioid dependence. However, buprenorphine has high affinity to μ receptors, it can cause mild to moderate dependence, and it precipitated withdrawal effects, along with the evident buprenorphine-induced hepatitis. Thus, novel medication for opioid dependence is needed.
Different from buprenorphine, dezocine, a mixed partial MOR agonist and KOR antagonist, is not categorized as a controlled substance and has been used for postoperative analgesia for more than a decade. We recently demonstrated that dezocine is also an inhibitor of the norepinephrine (NET) and serotonin transporters (SERT) and sigma-120 receptor, all of which are associated with pain21,22 and addiction.23 Such a unique multitarget pharmacological profile of dezocine indicates that it might have the potential to manage opioid dependence. In this study, we tested the hypothesis that dezocine could be an alternative medication for the management of opioid dependence using rat models.
METHODS
Materials
Pharmaceutical grade dezocine was obtained from Yangtze River Pharmaceutical Group (Jiangsu, China). Morphine sulfate, buprenorphine hydrochloride, and naloxone hydrochloride were obtained from the pharmacy of the University of Pennsylvania. For animal experiments, dezocine and buprenorphine were dissolved in 20% (v/v) dimethyl sulfoxide (DMSO) solution. The rest of the chemicals used were obtained from Sigma (St. Louis, MO).
Animals
Animal experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Ten-week-old male Sprague–Dawley rats weighing 200–250 g each were housed individually on a12/12-hour light/dark cycle in pathogen-free conditions with food and water available ad libitum. The cage and room conditions and the health condition of the animals were monitored regularly.
Experiment 1: Dezocine on Withdrawal in Morphine-Induced Dependence
The morphine-dependent rat model was established by subcutaneous administration of ascending doses (5, 10, 20, 40, 50, and 60 mg·kg−1) of morphine 3 times per day for 6 consecutive days in all animals except those in the naïve group.24 Normal saline solution was injected subcutaneously into the naive control group. After the establishment of the model, rats were divided randomly into 4 groups (n = 15 per group). After the last dosage of morphine, the solution of normal saline (naive group), 20% (v/v) DMSO (vehicle control), dezocine 1.25 mg·kg−1 (dezocine group), and buprenorphine 0.3 mg·kg−1 (buprenorphine group, positive control) were injected intraperitoneally (i.p.). Intraperitoneal naloxone 2 mg·kg−1 injection was used to induce withdrawal syndrome. Injection volumes were kept constant (0.5 mL) in all experiments. The withdrawal syndrome was assessed within 30 minutes after naloxone injection. The mean withdrawal symptom scores were determined as described previously.25,26 The scores for wet dog shakes, writhing, teeth chattering, jumping, rearing, and body grooming were assigned as 1 (1–3 times), 2 (4–6 times), and 3 (≥7 times); the scores for ptosis were assigned as 1 (1–4 times), 2 (5–8 times), and 3 (≥9 times).
Experiment 2: Assessment of Withdrawal With Various Doses of Dezocine
To evaluate the effect of administration (on days 1 and 7) of various doses of dezocine and buprenorphine in opioid withdrawal syndrome, the rat model was injected either dezocine (vehicle, 0.18, 0.37, 0.625, 1.25, 2.5 and 5 mg·kg−1, i.p.; n = 10 per group) or buprenorphine (vehicle, 0.1, 0.3, and 1 mg·kg−1, i.p.; n = 10 per group). Normal saline was administered to the naive group. Naloxone 2 mg·kg−1 injection was used to induce withdrawal syndrome as described in experiment 1. The scores of morphine withdrawal syndrome were assessed as described above on days 1 and 7 and were plotted as a function of drug concentration.
CPP Experiment
CPP was used to test the reinstatement as described previously.27 The CPP apparatus (JL Behv-CPPG, Shanghai Jiliang Software Technology Co Ltd, Shanghai, China) consisted of 2 compartments of equal size (15 × 15 × 37 cm3) with a 5 × 7 cm2 door in the center. The 2 compartments had different colors (black or white) and floor textures (mesh or grid). The time spent on each side and the number of crossings between the compartments were recorded by video and analyzed by DigBehv-CPP Video Analysis System (Shanghai Jiliang Software Technology Co Ltd).28 CPP was established as follows: before morphine administration (pretest), rats were allowed to move between 2 compartments (1 white and 1 black) freely for 15 minutes to determine the baseline preferences. For the conditioning training, rats were treated once a day for 4 consecutive days with 2 cycles of i.p. injections of morphine in the white compartment and then saline in the black compartment. After injection, rats were immediately confined for 30 minutes to either the white or black compartment. Rats that received morphine injections were confined in the white compartment, while rats that received normal saline were in the black compartment. To test CPP scores, the door separating the black and white compartments was left open, and rats were allowed to freely access both compartments for 15 minutes after ceasing injection. The time that the animal spent in the white compartment was considered as scores of CPP.29 The extinction training was similar to the conditioning training but not reinforced with morphine.30 All rats received a saline injection in both compartments, training for 30 minutes with the center door closed after injection. Extinction was confirmed after the scores of CPP returned to the baseline. Morphine 2 mg·kg−1 was administered for reinstatement of CPP after the extinction training. The same volumes of normal saline were given as a negative control. The rats were then tested for the scores of CPP for 15 minutes.
Rats of CPP were randomly assigned to 3 groups using computerized random numbers generated by GraphPad software (10 rats in each group): DMSO group (morphine 2 mg·kg−1 + 20% [v/v] DMSO), dezocine group (morphine 2 mg·kg−1 + dezocine 1.25 mg·kg−1), and buprenorphine group (morphine 2 mg·kg−1 + buprenorphine 0.3 mg·kg−1). A naive group was used as negative control (no drug administration). After the extinction, dezocine and buprenorphine were administered i.p. 3 times a day for 4 consecutive days. In the DMSO group, the same volumes of 20% (v/v) DMSO were given as control. We picked a dose of dezocine (1.25 mg·kg−1) that is about 4-fold of a dose of buprenorphine because the affinity of dezocine is 4-fold weaker than that of buprenorphine, and it is known to our group that this dose has positive pharmacological (analgesia) effects.
Immunofluorescence Assay for Determination of Astrocytes Activation
The astrocytes activation was assessed by immunofluorescence assay of the glial fibrillary acidic protein (GFAP), a major component of the cytoskeleton of astrocytes. Animals from each group (n = 6) were euthanized with CO2 on day 3 of medication administration. The brain tissues were collected and fixed. Transverse brain sections were obtained and processed as described previously for immunofluorescence assay.31 Sections were incubated in 0.3% Triton X-100 containing 2% goat serum over 1 hour at room temperature and then over 48 hours at 4°C with anti-GFAP antibody (1:250; Santa Cruz Biotechnology, Inc, Santa Cruz, CA), followed by incubation for 1 hour with Alexa 488-conjugated secondary antibody (1:300; Santa Cruz Biotechnology, Inc) at room temperature. Tissue sections were imaged for green fluorescence by Olympus microscope (Olympus Co, Tokyo, Japan) and analyzed by pixel intensity profile using ImageJ 1.48v software (National Institutes of Health, Bethesda, MD). After subtracting the background signal, the ratio of mean pixel intensity (arbitrary units) of each group to naive group was calculated.
KOR Internalization Assay
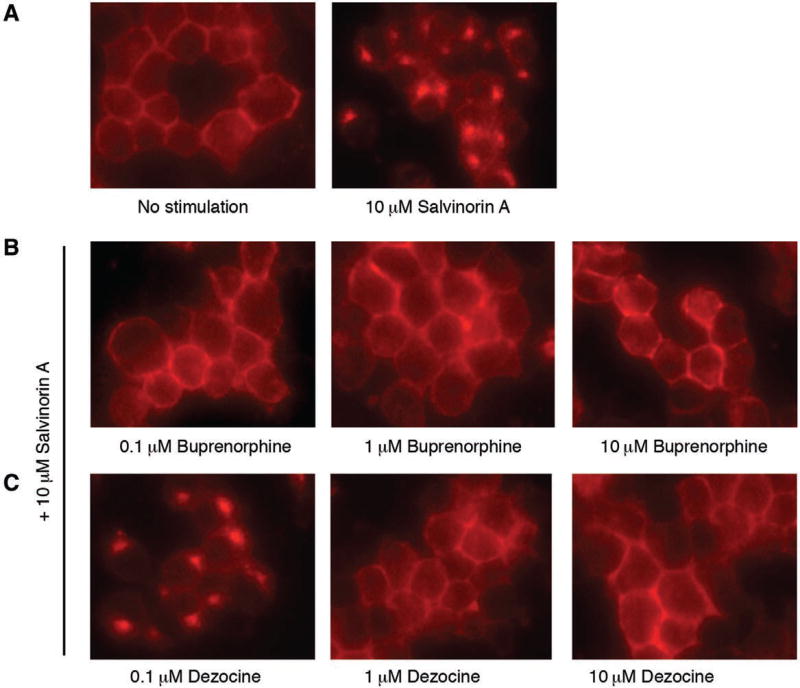
This experiment is designed to determine whether dezocine and buprenorphine could block KOR activation as KOR antagonists. Neuro2A cells stably transfected with KOR-tdT fusion protein were generated as described previously32 and maintained in minimum essential media supplemented with 10% fetal bovine serum, 2% G418, 100 U·mL−1 penicillin, and 100 µg·mL−1 streptomycin in a humidified environment with 5% CO2 at 37°C. Cells were plated onto poly-d-lysine–coated 8-well Lab-Tek chambered cover glasses. Forty-eight hours later, cells were stimulated with salvinorin A (10 µM) for 30 minutes and imaged under an Olympus IX70 microscope (Olympus, Melville, NY). To study the effect of dezocine or buprenorphine on salvinorin A–induced KOR trafficking, dezocine or buprenorphine (0.1, 1, or 10 µM final concentration) were added to the culture medium 30 minutes before the salvinorin A treatment.
Radioligand-Binding Assay and Affinity Determination
Radioligand-binding assay was conducted to screen the binding of dezocine and buprenorphine on 44 available receptors, including G-protein–coupled receptors, transporter proteins, and ion channels as described previously.20 The secondary binding assay was conducted to determine the affinity for selected receptors when the inhibition was >50% in the primary binding assay. Affinities for the receptors are expressed as pKi (−logKi).
Statistical Analysis
All data are shown as mean ± standard deviation unless otherwise indicated. For the in vivo studies of the effect of dezocine on morphine withdrawal syndrome (experiment 1), 2-way analysis of variance (ANOVA) was used to analyze the effects among the groups (DMSO, dezocine, buprenorphine, and naive) and time period, including the interaction between group and time factors. For CPP experiment, effect of the drug was analyzed using 2-way ANOVA with factors conditioning (pretest, posttest, extinction, and reinstatement) and drug treatment and interaction of these 2 factors. For the studies of the effect of administration of dezocine and buprenorphine on withdrawal syndrome in experiment 2, individual dose effects were analyzed using 1-way ANOVA. The data from immunofluorescence experiments were analyzed for statistical significance using 1-way ANOVA. Post hoc analyses were performed after 1- and 2-way ANOVA using Tukey’s test. Statistical power and sample size analyses were performed using G*Power 3.1.33 A sample size in each experiment was estimated based on our previous laboratory experience with this type of studies and indicated that 15 animals per group (10 in the case of the CPP experiment) would give a power from 0.8 to 0.9 at an α level of .05 for detecting a group difference of at least 5 and 200 units on withdrawal and CPP scale, respectively. For the studies of withdrawal syndrome, the assumed standard deviations were 3.7 and 2.9 in the drug-treated and control groups, respectively, to detect an effect size of 1.5, while they were 140 and 52 for CPP to detect an effect size of 1.9. According to the results of G*Power 3.1, the sample size in each group for withdrawal and CPP experiments should be >12 and 7, respectively. Therefore, based on the sample size justification and our preliminary studies, we chose a sample size of 15 in withdrawal and 10 in CPP studies. Statistical analyses were performed with GraphPad Prism version 7 (GraphPad Software, Inc).
RESULTS
Dezocine Alleviates the Morphine Withdrawal Syndrome Comparable to That of Buprenorphine
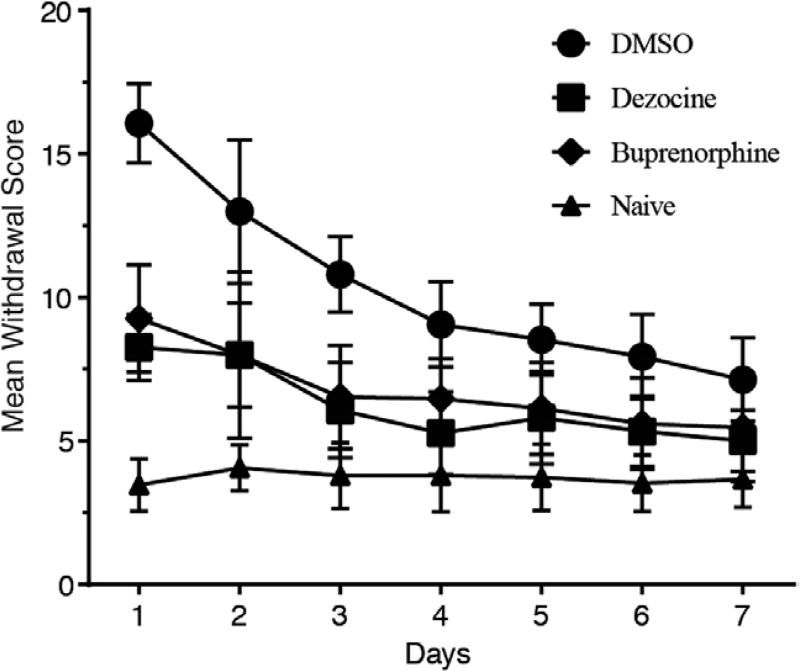
After administration of naloxone 2 mg·kg−1, the morphine-dependent group that received vehicle solution of DMSO (20% v/v) demonstrated significantly higher scores of withdrawal symptoms on day 1 compared to that in the naive group (mean difference: 12.6; 95% confidence interval [CI], 11.15–14.05; P < .0001 by 2-way ANOVA). Treatment with dezocine significantly reduced withdrawal scores starting from the first day compared to the vehicle control group (mean difference: 7.8; 95% CI, 6.35–9.25; P < .0001 by 2-way ANOVA). Significance was observed on all treatment days, including day 7 (mean difference: 2.13; 95% CI, 0.68–3.58; P < .001 by 2-way ANOVA; Figure 1). Significant decrease of withdrawal score was also present in the control buprenorphine group from the first day of administration compared to the vehicle group (mean difference: 6.8; 95% CI, 5.35–8.25; P < .0001 by 2-way ANOVA). The 2-way ANOVA of these data revealed significant effects for group (F0.05,3,392 = 331.9; P < .0001) and time period (F0.05,6,392 = 54.3; P < .0001), as well as statistically significant group and time interaction (F0.05,18,392 = 11.24; P < .0001). Results indicated that dezocine alleviated the morphine withdrawal syndrome in morphine-dependent rats.
Figure 1.
Dezocine alleviates morphine withdrawal syndrome comparable to that of buprenorphine. Data represented as mean ± standard deviation (n = 15 in each group). P < .0001 in both dezocine versus dimethyl sulfoxide (DMSO) and buprenorphine versus DMSO groups by 2-way analysis of variance with Tukey’s post hoc test.
Dezocine Alleviates Morphine Withdrawal Syndrome With Various Doses
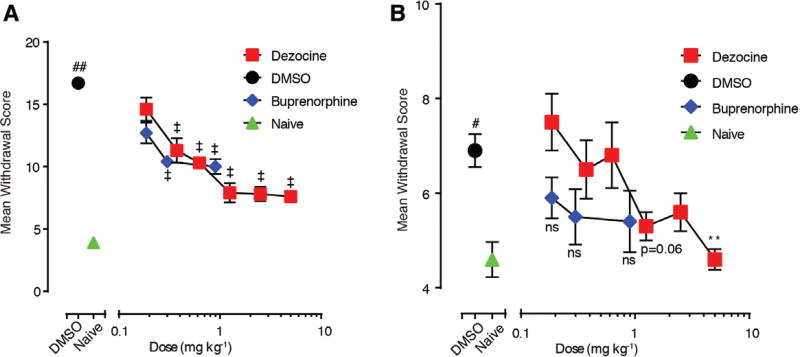
To assess the effects of i.p. injection of dezocine on precipitated withdrawal behavior, morphine-dependent rats were injected with various doses of dezocine ranging from 0.18 to 5 mg·kg−1 10 minutes before the naloxone 2 mg kg−1 injection on days 1 and 7 and were evaluated for 30 minutes. Naloxone administration induced significantly higher scores of withdrawal symptoms in the morphine-dependent group that received vehicle solution of DMSO (20% v/v) on day 1 (mean difference: 12.8; 95% CI, 10.2–15.4; P < .0001 by 1-way ANOVA) and day 7 (mean difference: 2.3; 95% CI, 0.42–4.18; P < .01 by 1-way ANOVA) compared to that in the naive group. Administration of dezocine starting from lower doses decreased mean withdrawal score on day 1 (0.37 mg·kg−1: mean difference: −5.4; 95% CI, −7.68 to −3.12; P < .0001; 0.625 mg·kg−1: mean difference: −6.4; 95% CI, −8.09 to −4.70; P < .0001; 1.25 mg·kg−1: mean difference: −8.8; 95% CI, −10.89 to −6.72; P < .0001; 2. 5 mg·kg−1: mean difference: −8.9; 95% CI, −10.75 to −7.05; P < .0001; 5 mg·kg−1: mean difference: −9.1; 95% CI, −10.8 to −7.4; P < .0001) as analyzed by 1-way ANOVA (Figure 2A). However, on day 7, animals administered with the 5 mg·kg−1 dose of dezocine showed significant reduction in withdrawal signs (mean difference: −2.3; 95% CI, −3.93 to −0.66; P < .01 by 1-way ANOVA) compared to vehicle-treated controls (Figure 2B). The effect of 0.1–1 mg·kg−1 doses of buprenorphine injections was not significantly different compared to that in the DMSO group on day 7 (Figure 2B).
Figure 2.
Dezocine treatment alleviates morphine withdrawal syndrome with various doses. The morphine withdrawal syndrome decreased with the elevation of the dosage of dezocine on day 1 (A) and day 7 (B). Each data point represents mean ± standard error of the mean (n = 10). **P < .01 and ‡P < .0001, a significant difference from the dimethyl sulfoxide (DMSO) group in both (A) and (B). #P < .01 and ##P < .0001, a significant difference from the naive group by 1-way analysis of variance with Tukey’s post hoc test.
Dezocine Alleviates the Reinstatement of CPP
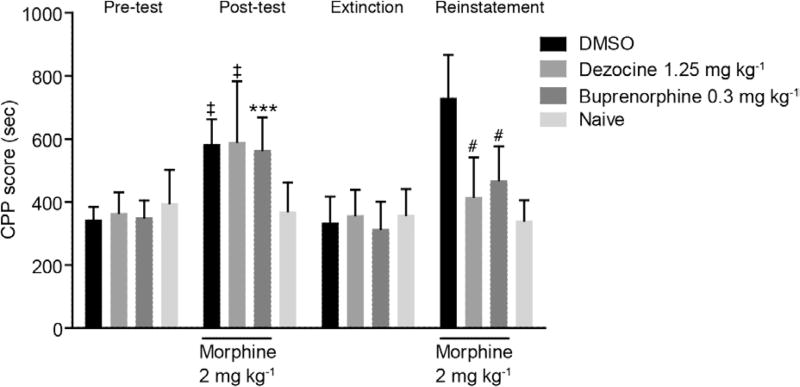
CPP scores showed no difference in the 4 groups at baseline. Two-way ANOVA revealed that after receiving morphine injection, CPP scores in the DMSO (mean difference: 213.6; 95% CI, 97.53–140.9; P < .0001), buprenorphine (mean difference: 195.5; 95% CI, 79.38–311.5; P < .001), and dezocine groups (mean difference: 220.3; 95% CI, 104.2–336.4; P < .0001) significantly increased with morphine administration compared to the naive group before drug intervention. In the extinction stage, there was no significant difference in CPP scores in the 4 groups, which implied that the dependence went away in the DMSO, buprenorphine, and dezocine groups. After reinstatement by morphine (Figure 3), CPP scores increased in the DMSO, buprenorphine, and dezocine groups. Compared to the DMSO group, scores in the dezocine group (mean difference: 314; 95% CI, 197.9–430.1; P < .0001 by 2-way ANOVA) and the buprenorphine control group (mean difference: 261.4; 95% CI, 145.4–377.5; P < .0001 2-way ANOVA) markedly decreased. In the rats treated with dezocine, there were significant main effects of both group and conditioning, F0.05,3,144 = 11.61, P < .0001 and F0.05,3,144 = 33.59, P < .0001, respectively, as well as statistically significant group and conditioning interaction, F0.05,9,144 = 9.729, P < .0001. No significant difference was observed between the dezocine and buprenorphine groups. The data indicated that dezocine significantly alleviated the morphine reinstatement effect after dependent effects were extinct, suggesting that dezocine could be used for the prevention of opioid relapse.
Figure 3.
Dezocine treatment reduced reinstatement of morphine-induced conditioned place preference (CPP). The CPP score was expressed as time spent in the drug-associated compartment during a period of 15 min. Results are presented as the mean ± standard deviation from experiments conducted on 10 mice per group. ‡P < .0001 and ***P < .001, a significant difference from the naive group; #P < .0001 a significant difference from the dimethyl sulfoxide (DMSO) group according to 2-way analysis of variance (ANOVA) with Tukey’s post hoc test.
Dezocine Inhibits Astrocytes Activation in NAcc
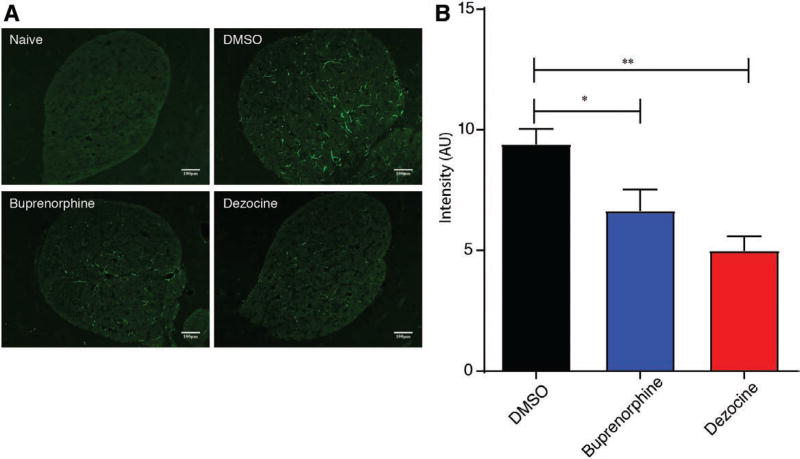
Astrocytes of NAcc were a in resting state in animals in the naive group (Figure 4A). Immunohistochemistry NAcc sections showed that chronic morphine administration resulted in the activation of astrocytes in a vehicle control group characterized by the overexpression of GFAP and enhancement in fluorescence intensity (Figure 4B). Expression of GFAP was significantly reduced on the third day of dezocine (mean difference: 4.4; 95% CI, 2.45–6.36; P < .01 by 1-way ANOVA) and buprenorphine (mean difference: 2.7; 95% CI, 0.34–5.19; P < .05 by 1-way ANOVA) administration compared to the DMSO control group (Figure 4B).
Figure 4.
Dezocine inhibits morphine-induced astrocytes activation in nucleus accumbens. A, Immunofluorescence staining for glial fibrillary acidic protein (GFAP) in the nucleus accumbens slices of rats in treatment groups. No GFAP overexpression was detected in the naive group. However, the number of GFAP-positive cells was significantly increased in the vehicle control group of the morphine-dependent model compared with those in the naive group. GFAP expression was reduced after 3 d of dezocine and buprenorphine administration. B, Summary plot of the effect of drugs on fluorescence showing the significant decrease of astrocytes activation in dezocine- and buprenorphine-administered animal models compared to the vehicle control. Data represented as the ratio of mean pixel intensity of each group (arbitrary units [AU]) to the naive group and shown as mean ± standard error of the mean, n = 6; *P < .05, **P < .01.
Dezocine Inhibits Agonist-Induced Internalization of KOR
Whereas dezocine was originally considered as a KOR agonist, we20 and others34 recently have shown that dezocine acts as a KOR antagonist. Therefore, it is important to determine whether there are similarities between the effects of dezocine and other antagonists, such as buprenorphine in the agonist-induced KOR trafficking. As shown in Figure 5, KOR internalization was induced by 10 µM salvinorin A in the Neuro2A cells expressing KOR-tdT fluorescent fusion protein. This internalization was blocked by buprenorphine pretreatment at concentrations of 0.1, 1, and 10 µM. Similarly, pretreatment of cells with 1 or 10 µM dezocine blocked the salvinorin A–induced KOR internalization. The effect of dezocine was concentration dependent, as at ≤0.1 µM it did not alter KOR internalization. The result further confirmed that dezocine acts as a KOR antagonist, which blocks agonist-induced receptor internalization.
Figure 5.
Dezocine inhibits agonist-induced κ opioid receptor (KOR) internalization. KOR internalization was visualized by fluorescence microscopy in control cells either untreated or treated with salvinorin A (A) and in cells pretreated for 30 min with buprenorphine (B) or dezocine (C) at indicated doses for 30 min before stimulation with salvinorin A.
Dezocine Has Different Molecular Targets Compared to That of Buprenorphine
We have previously demonstrated that dezocine showed >50% of the inhibition in μ, κ, and δ opioid receptors with Ki values of 3.7, 31.9, and 527 nM, respectively.20 Radioligand-binding assay against tested receptors (Supplemental Digital Content, Table 1, http://links.lww.com/AA/C329) revealed the affinity of buprenorphine to μ, κ, and δ receptors with Ki values of 1.0, 0.7, and 1.9 nM, respectively. While dezocine interacts with NET (382 nM), SERT (82 nM), and sigma-1 (1223 nM) as we reported previously,20 buprenorphine has no interaction with these receptors.
DISCUSSION
In this study, we demonstrate that dezocine attenuates the symptoms of naloxone-precipitated morphine withdrawal syndrome and inhibits associated astrocyte activation in NAcc in a morphine-dependent rat model. The decrease in the overall withdrawal score produced by dezocine is comparable to that by buprenorphine. We also show that dezocine alleviated the reinstatement of morphine-induced CPP in rats. This study further confirms that both dezocine and buprenorphine are KOR antagonists because both of them blocked KOR agonist-induced receptor internalization, the process that has been linked to κ agonists–induced behavioral tolerance.10 Microinjections of KOR agonists directly into the NAcc demonstrated that KOR activation produces stress and depressive-like symptoms such as anhedonia.35 Prolonged heroin administration significantly increases KOR activity in NAcc, which is associated with manifestation of withdrawal-related negative behavioral states in drug addiction.36 Consequently, although KOR agonists can inhibit the reinforcing effects of abused drugs, by decreasing dopamine concentrations, the use of KOR antagonists may be beneficial in treating opioid dependence by suppressing withdrawal symptoms, thereby reducing relapse to opioid use, a major symptom of opioid addiction.
Astrocytes of NAcc undergo morphological changes and an increase of GFAP expression on exposure to the opioid of abuse, that is, activation. Within the NAcc, differently from MORs, which are located on the bodies of GABAergic interneurons, KORs are expressed primarily on efferent terminals of inputs from the mesolimbic system. Thus, 1 potentially important site for the actions of dezocine for morphine withdrawal is at least, in part, in the NAcc. Given that activation of astrocytes in NAcc has been implicated in the development of chronic morphine- and cocaine-induced biochemical and behavioral changes,37 the effectiveness of dezocine in inhibiting astrocyte activation in NAcc may indicate its clinically relevant efficacy. We previously demonstrated that dezocine inhibits norepinephrine and serotonin reuptake via the interaction with NET and SERT transporters, both of which are important targets for neurodegenerative diseases, addiction, depression, and pain treatment.20 The interaction of dezocine with SERT was also demonstrated in a recent in vivo study.38 Competitive binding assay indicated that buprenorphine did not interact with these receptors. Compared to buprenorphine, dezocine showed the affinity to sigma-1 receptor in addition to the interaction with opioid receptors. The interaction of dezocine with sigma-1 receptor might contribute to its nonaddictive or antidependence property. It is unclear whether dezocine can activate or inhibit this receptor. Various studies have suggested that sigma-1 receptor activation plays a critical role in plasticity related to reinforcement and addiction processes. Sigma-1 receptor as a receptor chaperone modulates the activities of G-protein–coupled receptors, ion channels, and signaling molecules under pathophysiological conditions, including addiction, pain, and depression.39 The sigma-1 receptor gene and protein expression were upregulated in brain regions related to addiction and reward. CPP induced by cocaine can be blocked by a sigma-1 receptor antagonism, suggesting that targeting sigma-1 receptors might provide a potential approach in managing addition.40 Taken together, the findings from this study suggest that the interaction of dezocine with KOR, NET, SERT, and sigma-1 proteins could be the possible mechanisms that underlie its nonaddictive property and the antiwithdrawal effects of dezocine and provides potential strategies for opioid dependence research and therapy. However, more studies are needed to investigate the intracellular events occurring after dezocine administration and to reveal pharmacological significance of aforementioned targets in the reward pathways at the molecular level.
In summary, we demonstrated that dezocine, a nonaddictive opioid, significantly reduced opioid withdrawal syndrome in a morphine-dependent rat model comparable to that of buprenorphine, indicating that dezocine could be a potential alternative medication for the management of withdrawal syndrome in opioid dependence. The similarity of dezocine and buprenorphine as opioids is partial MOR agonism and KOR antagonism. Both of them can inhibit NAcc astrocytes activation. The differences in molecular targets between buprenorphine and dezocine clearly indicate that dezocine might have important value in studying the mechanisms of opioid dependence and developing novel therapeutics.
Supplementary Material
KEY POINTS.
Question: Can dezocine, a nonaddictive opioid, be used to alleviate opioid dependence and study-related mechanisms?
Findings: Dezocine significantly reduced morphine withdrawal syndrome in a morphine-dependent rat model comparable to that of buprenorphine; similarities and differences in molecular targets between dezocine and buprenorphine were identified.
Meaning: Dezocine could potentially be used to manage opioid dependence; its unique molecular targets provide a valuable tool for studying mechanisms of opioid dependence and developing novel therapeutics.
Acknowledgments
Funding: This study is supported by National Institutes of Health Grant R01 (1R01GM111421) (to R. Liu) and the Department of Anesthesiology and Critical Care at the University of Pennsylvania (to R. Liu). This study is also supported by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract #HHSN-271-2008-00025-C (NIMH PDSP), which is directed by Bryan L. Roth, MD, PhD, at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at National Institute of Mental Health, Bethesda, MD.
The authors appreciate the technical support from Jingyuan Ma and Qingcheng Meng, PhD, at the Department of Anesthesiology and Critical Care at the University of Pennsylvania.
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
DISCLOSURES
Name: Fei-xiang Wu, MD, PhD.
Contribution: This author helped perform the experiments, analyze the data, and write the article. This author participated in the data interpretation, approved the final version, and is accountable for the study.
Name: Hasan Babazada, PhD.
Contribution: This author helped analyze the data and interpretation and is the key person who wrote the article. This author participated in the data interpretation, approved the final version, and is accountable for the study.
Name: Hao Gao, MD, PhD.
Contribution: This author helped perform conditioned place preference experiments and write the article. This author participated in the data interpretation, approved the final version, and is accountable for the study.
Name: Xi-Ping Huang, PhD.
Contribution: This author helped perform the receptor-screening studies. This author participated in the data interpretation, approved the final version, and is accountable for the study.
Name: Chun-hua Xi, MD, MS.
Contribution: This author helped perform the receptor internalization studies. This author participated in the data interpretation, approved the final version, and is accountable for the study.
Name: Chun-hua Chen, MD, PhD.
Contribution: This author helped perform the immunochemistry studies. This author participated in the data interpretation, approved the final version, and is accountable for the study.
Name: Jin Xi, MS.
Contribution: This author helped perform all the experiments except the conditioned place preference studies. This author participated in the data interpretation, approved the final version, and is accountable for the study.
Name: Wei-feng Yu, MD, PhD.
Contribution: This author helped in funding for the conditioned place preference studies and designed that portion of the study. This author participated in the data interpretation, approved the final version, and is accountable for the study.
Name: Renyu Liu, MD, PhD.
Contribution: This author helped design the experiments, provided funding, and supervised the project. This author participated in the data interpretation, approved the final version, and is accountable for the study.
This manuscript was handled by: Jianren Mao, MD, PhD.
References
- 1.Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- 2.Spanagel R, Almeida OF, Bartl C, Shippenberg TS. Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berl) 1994;115:121–127. doi: 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- 3.Wang YH, Sun JF, Tao YM, Chi ZQ, Liu JG. The role of kappa-opioid receptor activation in mediating antinociception and addiction. Acta Pharmacol Sin. 2010;31:1065–1070. doi: 10.1038/aps.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonin F, Valverde O, Smadja C, et al. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansour A, Fox CA, Burke S, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 6.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 7.Turchan J, Przewłocka B, Lasoń W, Przewłocki R. Effects of repeated psychostimulant administration on the prodynorphin system activity and kappa opioid receptor density in the rat brain. Neuroscience. 1998;85:1051–1059. doi: 10.1016/s0306-4522(97)00639-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang XM, Zhou Y, Spangler R, Ho A, Han JS, Kreek MJ. Acute intermittent morphine increases preprodynorphin and kappa opioid receptor mRNA levels in the rat brain. Brain Res Mol Brain Res. 1999;66:184–187. doi: 10.1016/s0169-328x(99)00021-2. [DOI] [PubMed] [Google Scholar]
- 9.Jordan BA, Cvejic S, Devi LA. Kappa opioid receptor endocytosis by dynorphin peptides. DNA Cell Biol. 2000;19:19–27. doi: 10.1089/104454900314672. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin JP, Xu M, Mackie K, Chavkin C. Phosphorylation of a carboxyl-terminal serine within the kappa-opioid receptor produces desensitization and internalization. J Biol Chem. 2003;278:34631–34640. doi: 10.1074/jbc.M304022200. [DOI] [PubMed] [Google Scholar]
- 11.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- 14.Bruchas MR, Land BB, Aita M, et al. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 16.Carlezon WA, Jr, Béguin C, DiNieri JA, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 17.Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerra G, Borella F, Zaimovic A, et al. Buprenorphine versus methadone for opioid dependence: predictor variables for treatment outcome. Drug Alcohol Depend. 2004;75:37–45. doi: 10.1016/j.drugalcdep.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Gerra G, Fantoma A, Zaimovic A. Naltrexone and buprenorphine combination in the treatment of opioid dependence. J Psychopharmacol. 2006;20:806–814. doi: 10.1177/0269881106060835. [DOI] [PubMed] [Google Scholar]
- 20.Liu R, Huang XP, Yeliseev A, Xi J, Roth BL. Novel molecular targets of dezocine and their clinical implications. Anesthesiology. 2014;120:714–723. doi: 10.1097/ALN.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartrick CT. Noradrenergic reuptake inhibition in the treatment of pain. Expert Opin Investig Drugs. 2012;21:1827–1834. doi: 10.1517/13543784.2012.731393. [DOI] [PubMed] [Google Scholar]
- 22.Sindrup SH, Gram LF, Brøsen K, Eshøj O, Mogensen EF. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain. 1990;42:135–144. doi: 10.1016/0304-3959(90)91157-E. [DOI] [PubMed] [Google Scholar]
- 23.Filip M, Frankowska M, Zaniewska M, Gołda A, Przegaliński E. The serotonergic system and its role in cocaine addiction. Pharmacol Rep. 2005;57:685–700. [PubMed] [Google Scholar]
- 24.Xiao H, Zhai DX, Yan BB, et al. A role for the parafascicular thalamic nucleus in the development of morphine dependence and withdrawal. Brain Res. 2009;1271:74–82. doi: 10.1016/j.brainres.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Wu FX, Miao XR, et al. Suppression of acute morphine withdrawal syndrome by adenovirus-mediated β-endorphin in rats. Brain Res. 2011;1422:13–19. doi: 10.1016/j.brainres.2011.07.063. [DOI] [PubMed] [Google Scholar]
- 26.Maldonado R, Negus S, Koob GF. Precipitation of morphine withdrawal syndrome in rats by administration of mu-, delta- and kappa-selective opioid antagonists. Neuropharmacology. 1992;31:1231–1241. doi: 10.1016/0028-3908(92)90051-p. [DOI] [PubMed] [Google Scholar]
- 27.Han WY, Du P, Fu SY, et al. Oxytocin via its receptor affects restraint stress-induced methamphetamine CPP reinstatement in mice: involvement of the medial prefrontal cortex and dorsal hippocampus glutamatergic system. Pharmacol Biochem Behav. 2014;119:80–87. doi: 10.1016/j.pbb.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Li T, Yan CX, Hou Y, et al. Cue-elicited drug craving represses ERK activation in mice prefrontal association cortex. Neurosci Lett. 2008;448:99–104. doi: 10.1016/j.neulet.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 29.Meng S, Quan W, Qi X, Su Z, Yang S. Effect of baclofen on morphine-induced conditioned place preference, extinction, and stress-induced reinstatement in chronically stressed mice. Psychopharmacology (Berl) 2014;231:27–36. doi: 10.1007/s00213-013-3204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conrad KL, Louderback KM, Milano EJ, Winder DG. Assessment of the impact of pattern of cocaine dosing schedule during conditioning and reconditioning on magnitude of cocaine CPP, extinction, and reinstatement. Psychopharmacology (Berl) 2013;227:109–116. doi: 10.1007/s00213-012-2944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu F, Miao X, Chen J, et al. Inhibition of GAP-43 by propentofylline in a rat model of neuropathic pain. Int J Clin Exp Pathol. 2013;6:1516–1522. [PMC free article] [PubMed] [Google Scholar]
- 32.Huang P, Chiu YT, Chen C, Wang Y, Liu-Chen LY. A G protein-coupled receptor (GPCR) in red: live cell imaging of the kappa opioid receptor-tdTomato fusion protein (KOPR-tdT) in neuronal cells. J Pharmacol Toxicol Methods. 2013;68:340–345. doi: 10.1016/j.vascn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 34.Gharagozlou P, Hashemi E, DeLorey TM, Clark JD, Lameh J. Pharmacological profiles of opioid ligands at kappa opioid receptors. BMC Pharmacol. 2006;6:3. doi: 10.1186/1471-2210-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muschamp JWa. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci. 2011;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solecki W, Ziolkowska B, Krowka T, Gieryk A, Filip M, Przewlocki R. Alterations of prodynorphin gene expression in the rat mesocorticolimbic system during heroin self-administration. Brain Res. 2009;1255:113–121. doi: 10.1016/j.brainres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 38.Wang YX, Mao XF, Li TF, Gong N, Zhang MZ. Dezocine exhibits antihypersensitivity activities in neuropathy through spinal μ-opioid receptor activation and norepinephrine reuptake inhibition. Sci Rep. 2017;7:43137. doi: 10.1038/srep43137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamanillo D, Romero L, Merlos M, Vela JM. Sigma 1 receptor: a new therapeutic target for pain. Eur J Pharmacol. 2013;716:78–93. doi: 10.1016/j.ejphar.2013.01.068. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. Cocaine up-regulates Fra-2 and sigma-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmacol Exp Ther. 2005;314:770–779. doi: 10.1124/jpet.105.084525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.