Abstract
The ability to mount an effective anti-tumor immune response requires coordinate control of CD4 T cell and CD8 T cell function by antigen presenting cells (APCs). Unfortunately, tumors create an immunosuppressive microenvironment that helps protect tumor cells from immune recognition. In many cases this defect can be traced back to a failure of APCs (most importantly dendritic cells (DCs)) to recognize, process, and present tumor antigens to T cells. In this review, we will summarize work addressing the role of different DC subsets in anti-tumor immunity and the various mechanisms used by tumor cells to suppress the ability of APCs to stimulate potent anti-tumor T cell responses.
Keywords: antigen processing and presentation, cancer
1. Introduction
The immune system is composed of a variety of specialized cell types that fight not only pathogen-infected cells but also oncogenic cellular components. To initiate this fight, “foreign” antigens must first be recognized and presented to effector cells of immune system. Although endogenous antigens can be presented on major histocompatibility complexes (MHC) class I (MHC-I) by most nucleated cells, specific immune responses to endo- and exogenous antigens requires naïve T cell priming by APCs [1]. DCs are highly specialized “professional” APCs that link antigen-independent innate immunity (microbe and danger sensing) and antigen-specific adaptive immunity (T cell and B cell priming) [2]. While immunotherapies are now showing great promise in treating cancer patients [3], there is growing evidence that the efficacy of “traditional” therapeutic methods of radiation/chemotherapy largely depend on the host immune system [4, 5]. Whereas T cell priming by DCs is essential for the initial generation of antitumor T cells, it fails in more advance stages of cancer. This review will focus on the antigen presentation properties of DCs in the context of cancer and how the tumor microenvironment impairs antigen presentation, thereby suppressing anti-tumor immune responses. For the purposes of this review “antigen processing and presentation” refers not only to the ability of an APC to process and present antigenic peptides to antigen-specific T cells, but also includes additional signals provided by the APC, which lead to an effective immune response.
2. DC subtypes in cancer
2.1. cDC1 and cDC2
Since their identification by Steinman and Cohn in 1973 [6], DC development and the capacity of DCs to present antigens to naïve T cells has been extensively investigated. DCs originate in bone marrow from macrophage/DC progenitors (MDP) [7] that give rise to common DC progenitors (CDP) that differentiate into two major categories: classical DCs (cDCs) and plasmacytoid DCs (pDCs) [8]. Murine cDCs consist of two subtypes currently described as cDC1 and cDC2 with their human counterparts being BDCA3+ DC and BDCA1+ DC, respectively [9]. These two subtypes of DC differ functionally and phenotypically. cDC1 specialize in presenting internalized antigens bound to MHC-I to CD8 T cells in a process termed cross-presentation [10]. These cells do not express CD11b and reside in both lymphoid tissues (as CD8α+ cDC1) and in non-lymphoid tissues (as CD103+ cDC1) [11].
The differentiation of both CD8α+ and CD103+ cDC1 subsets is driven by a common transcription factor Batf3 [12]. Both cDC1 subsets (as well as the human homologue of CD8α+ DCs [13]) are characterized by surface expression of the chemokine receptor XCR1 that has a unique ligand, XCL1. This chemokine is produced by CD8 T cells and the XCR1-XCL1 axis provides communication between cross-presenting DCs and antigen-specific CD8 T cells [13, 14]. The importance of cross-presenting cDC1 for anti-tumor immunity has been revealed by several groups. CD103+ DCs can produce large amounts of IL-12 and are very efficient for antigen cross-presentation and crucial during initial priming of CD8 T cells [15–17]. Expression of CCR7 regulates the ability of CD103+ DCs to migrate from the tumor environment to the draining lymph node (LN) where they initially prime naïve CD8 T cells [18]. Due in part to their low expression of lysosomal enzymes, CD103+ DCs deliver intact tumor antigens to draining LNs [19, 20] and “hand off” tumor antigens to other DC subsets in LNs (including CD8α+ DCs) [18], further highlighting the importance of this DC subset in tumor immunity. Furthermore, tumor-resident CD103+ DCs play a crucial role in recruiting CD8 effector T cells and CD4 TH1 helper cells to the tumor site by the virtue of their production of the CXCR3 ligands CXCL9 and CXCL10 [21]. Since CD103+ DCs seem to play a role both at the tumor site and in the tumor-draining LN, it has been suggested that CD103+ DCs present in the tumor microenvironment migrate to the LN to prime naïve CD8 T cells, however some of these cells remain at the tumor side and secrete CXCR3 ligands to recruit T cells that were primed in the draining LN [22]. Not surprisingly, expansion of cross-presenting CD103+ DCs in the tumor environment can stimulate anti-tumor immune responses [20] and higher numbers of these DCs in human tumors correlates with improved clinical outcome [23].
Unlike cDC1 cells, lymphoid tissue resident cDC2 express CD11b and these cells play a critical role in presenting internalized exogenous antigens bound to MHC class II (MHC-II) to CD4 T cells [24]. cDC2 are the main APC subtype that prime naïve CD4 T cells in LNs [25], an essential first step in acquired immunity. The importance of these cells in anti-tumor responses has recently been highlighted in a study by Ma et al, in which neutralization of CD11b+ DCs blocked chemotherapy-induced anti-tumor immunity [26].
2.2. inf-DC
Bipotent MDP also gives rise to monocytes that differentiate in peripheral tissues into a special DC subset called “inflammatory DCs” (inf-DC) [27, 28]. As their name implies, these cells are generated by inflammatory stimuli and are present in the tumor microenvironment. Inf-DCs induce TH17 responses that are important not only in autoimmune/inflammatory disorders but also in the tumor-associated immune environment, making them a target of interest in the context of cancer therapy [28, 29]. In addition to promoting antigen-specific CD4 T cell responses, inf-DCs are also able to cross-present antigens to CD8 T cells and their presence has a positive impact on treatment success [30]. In a recent study of adoptive T cell therapy, a novel subset of inf-DCs, termed TIP-DCs, was shown to produce large amounts of TNF-α and nitric oxide (NO), factors that promoted anti-tumor T cell expansion [31].
2.3. pDC
A second major subset of DCs that differentiates from CDP are pDCs. This relatively small cell population specializes in production of type I interferon (IFN) and participates in antiviral immune responses, but these cells can also secrete IL-12, IL-6, TNF-α and other pro-inflammatory cytokines [32, 33]. pDCs are present in the tumor environment as well as in tumor-draining LNs. Although less efficient than cDC as APCs, pDC can present antigens to T cells [34], however their role in cancer is thought to be tolerogenic [35] and high tumor infiltration by pDC is associated with poor prognosis [36]. Through production of the tryptophan-depleting enzyme indoleamine 2,3-dioxygenase (IDO), pDCs contribute to the generation of immunosuppressive regulatory T cells (Tregs) in the cancer environment and in tumor-draining LNs [37, 38]. pDCs can also stimulate Treg generation by their expression of ICOS-L [36, 39]. Unfortunately, ICOS-L and OX40L-expressing pDCs promote tumor-supporting TH2 responses in melanoma patients [40], revealing a deleterious role for pDCs in the host response to tumor cells.
Surprisingly, pDCs have been reported to have the capacity to promote antitumor responses. Activation of pDCs with the TLR7 agonist imiquimod (used for the treatment of skin tumors) results in their expression of TRAIL and acquisition of cytotoxic activity against tumor cells in vitro [41]. A similar finding was shown in a mouse model of melanoma, demonstrating that the cytolytic potential of pDCs in eradicating tumor cells can be independent of adaptive immunity [42]. pDCs can also present melanoma-derived antigens to CD8 T cells and indirectly support anti-tumor responses through type I IFN secretion [43, 44]. It is worth noting that adoptive transfer of tumor-antigen loaded pDCs has been reported to induce anti-tumor T cell responses in patients suffering from melanoma [45], revealing a potent role for this DC subtype in controlling cancer. Given the apparent contradictory role that pDCs play in anti-tumor immune responses, additional studies must be performed to fully understand the importance of this DC subset in modulating anti-tumor immunity.
3. Tumor-antigen processing and presentation by DCs
For immunotherapy to be effective, tumors must be immunogenic, meaning that tumor-associated antigens (TAAs) must be recognized and processed by APCs to stimulate anti-tumor T cell responses. Studies in mouse models of cancer showed that tumor-antigen presentation, particularly by DCs, is essential for induction of anti-tumor immunity [46, 47]. Anti-tumor immune responses are induced in three consecutive steps: a) tumor-antigen processing and presentation, b) priming of tumor-antigen specific T cells by APCs, and c) eradication of tumor cells by effector T cells at the tumor site [48]. Failure of any of these steps suppresses anti-tumor immune responses, and therefore there is intense interest in understanding these processes both under normal and pathophysiological conditions. Different subsets of DCs (summarized above) are equipped to induce different types of T cell responses. In addition, the location and ability to capture tumor antigens also regulates antigen processing by DCs and subsequent T cell responses.
Antigens associated with neoplastic cells are processed and presented as complexes with MHC molecules on the surface of both APCs and transformed cells themselves. Tumor antigens presented by APCs are essential to prime antigen-specific T cells that subsequently recognize MHC-peptide complexes on target neoplastic cells [49]. Cell-associated antigens at the tumor site are captured by tissue-resident DCs that can either present them to T cells in situ, or after migration of the DCs through afferent lymphatics, in tumor-draining LNs [50]. Necrotic or apoptotic tumor cells, or even fragments of tumor cells, are engulfed by DCs by phagocytosis. Phagosomes fuse with lysosomes to become phagolysosomes, compartments in which complexes of tumor antigen-derived peptides bind to MHC-II molecules [48]. Soluble tumor-associated antigens can also directly travel via lymphatic vessels to LNs for uptake by macropinocytosis by DCs, are processed into peptides, and presented by LN-resident DCs to either CD4 or CD8 T cells [25]. cDC2 cells are very efficient in generating pMHC-II from internalized foreign antigens, and this is essential for stimulation of naïve CD4 T cells. As mentioned previously, cDC1 are specially equipped with the machinery required to load internalized exogenous protein antigens on MHC-I for stimulation of CD8 T cells by cross-presentation [51]. This pathway also applies to presentation of antigens derived from apoptotic tumor cells that are engulfed by DCs [48]. Antigen cross-presentation by MHC-I is especially important in eradication of tumors as it induces CD8 T cells that differentiate into cytotoxic T lymphocytes (CTL) that recognize exogenous tumor-derived antigens.
4. Tumor-derived factors suppress DC maturation
A primary mechanism by which tumors escape the immune system is by suppressing antigen presentation [1]. Based on their distinction as “professional” APCs, DCs should be able to promote antitumor immunity. However, as cancer progresses, DCs fail to activate anti-tumor immunity and eventually immune suppression ensues. For antigen presentation to be efficient in stimulating anti-tumor T cell responses, several requirements must be met, including a) the appropriate type of DC acquire and process tumor antigens, b) efficient antigen processing and delivery of pMHC to the DC surface, c) and sufficient enhancement of DC costimulatory/homing molecules to ensure efficient T cell activation [52]. Malignant cells can unfortunately impact DCs at each of these stages by a variety of mechanisms that either disable generation of tumor-associated antigen-specific T cells or promote immune cell tolerance to the tumor [53].
To induce potent T cell responses, antigen-MHC interactions with antigen-specific T cell receptors (TCR) must be accompanied by costimulatory signals provided by the binding of receptors on T cells (such as CD28) with complementary ligands on APCs (such as B7). Resting DCs express low amounts of costimulatory molecules on their surface, however DC activation enhances expression of costimulatory molecules [54]. DC activation can occur by recognition of “classical danger” signals [54] or “sterile/homeostatic danger” signals [55]. TCR stimulation by DCs in the absence of costimulation results either in T cell anergy or induction of Tregs, processes that play critical roles in the suppression of anti-tumor immunity [56–58]. T cell activation is also disrupted when the DC costimulatory molecule B7 interacts with the T cell inhibitory receptor CTLA-4 instead of the stimulatory receptor CD28 [59, 60]. In normal physiology, the B7/CTLA-4 inhibitory pathway is thought to protect from excessive T cell activation, however this pathway is exploited by tumors to escape detection by the immune system.
4.1. Inhibition of DC function by IL-6 and IL-10
An important mechanism of immune escape by tumors is by maintaining DCs in an immature state, thereby rendering these cells unable to stimulate an anti-tumor T cell response. The environment created by tumor-derived factors (including IL-6, IL-10, and VEGF) impairs DC maturation and ultimately their function as APCs. Tumor cells can interfere with the antigen presenting properties of DCs by secreting IL-10, a cytokine that inhibits DC maturation and their ability to secrete IL-12 [56]. IL-10 signaling has been reported to directly suppress DC activation by a variety of mechanisms [61]. IL-6 is also produced by tumor cells and other cell types present in the tumor microenvironment, and in cancer patients high serum level of IL-6 is usually correlated to poor disease outcome [62]. IL-6 is a proinflammatory cytokine that normally supports cell proliferation and inhibits apoptosis. While promoting survival of inflammatory cells is helpful for acute immune responses, dysregulated expression of IL-6 leads to chronic inflammation and oncogenesis [63]. Like IL-10, IL-6 has immunosuppressive effects on DCs including down-regulating expression of both MHC-II [64, 65] and the LN-homing receptor CCR7 [66]. Both IL-10 and IL-6 activate STAT3, a transcription factor that is activated in many types of cancer [67] and whose activation is linked to DC dysfunction [68]. Tumors induce autocrine secretion of IL-6 and IL-10 by DCs themselves, further contributing to immune suppression and revealing cell-intrinsic factors that reduce DC function in cancer [69]. Increased sensitivity to IL-6 and IL-10 is thought to be due to increased expression of IL-6 receptors and IL-10 receptors by activating TLR2 ligands [69], thereby enhancing STAT3 signaling and suppressing DC activation.
4.2. Generation of regulatory DCs by tumors
In addition to maintaining DCs in a non-functional “immature” state, tumors promote the generation of “mature” DCs possessing immunosuppressive instead of immunostimulatory properties. Regulatory DCs, a DC subset dependent on the transcription factor Satb1, are activated not by pattern recognition receptor (PRR) ligands but are activated instead by inflammatory mediators such as IL-1β, TNF-α, type I IFN, and prostaglandin E2 [70]. Activation by these factors, or by ligation of membrane-bound CTLA-4 or CD200, can trigger the expression of the tryptophan catabolizing enzyme IDO in tumor-infiltrating DCs [71], thereby depleting this essential amino acid required for T cell proliferation [72]. Phenotypically mature DCs that inhibit anti-tumor T cell immunity can also be found in the tumor microenvironment and these cells suppress CD8 T cell function by depriving them of the essential amino acid L-arginine [73], however whether these cells are Satb1-dependent regulatory DCs remains to be determined.
4.3. TH skewing alters anti-tumor CD8 CTL function
An additional mechanism used by tumors to subvert immune detection is by influencing the ability of DCs to skew T cell differentiation. Differentiation of naïve CD8 T cell into cytotoxic effector cells and differentiation of CD4 T cells into distinct subsets of T helper cells depends on cytokine signaling during antigen presentation. DCs producing large amounts of IL-12 preferentially induce TH1 cells, low IL-12-producing DCs induce TH2 responses [74], and secretion of TGF-β and IL-6 leads to the generation of TH17 cells [75]. As mentioned previously, immature DCs presenting antigen to CD4 T cells in the absence of costimulation [76] or IDO- or ICOS-L-expressing pDCs [37, 39] can also guide differentiation of Tregs. By altering DC maturation and cytokine secretion, tumors are therefore able to interfere with T cell differentiation. The quality of T cells induced by DCs ultimately modulates the outcome of immune responses to cancer by either supporting tumor growth or anti-tumor immunity.
TH1 T cells promote anti-tumor CD8 T cell responses by supporting their expansion into long-term memory cells [77, 78]. TH17 cells are also recognized as potent anti-tumor cells due to their support of anti-tumor CTL-responses and recruitment of cross-priming DCs to tumor-draining LNs [79]. Unlike TH1 and TH17 cells, TH2 cells actually promote tumor survival by cytokine-dependent suppression of T cell function or by induction of T cell anergy [50]. T cell differentiation towards TH2 is enhanced by several factors present in the tumor microenvironment. For example, tumor-derived cytokine thymic stromal lymphopoietin (TSLP) upregulates expression of OX40L on DCs, thereby instructing them to generate CD4 TH2 cells that produce the tumor-supporting cytokines IL-4 and IL-13 [80, 81]. These cytokines stimulate cancer cell growth both directly and indirectly (by attracting alternatively-activated macrophages that might contribute to angiogenesis) [80]. Tumor-infiltrating DCs modulate the T cell repertoire by their production of IL-6, IL-10, and galectin-1 [82]. As discussed in section 4.1, IL-6 and IL-10 impair the ability of DCs to mature, thereby suppressing DC secretion of IL-12 and leading to the generation of TH2 responses. These immature DCs also promote the generation of pro-tumorigenic Tregs by a TGF-β-dependent mechanism [83]. Production of the immunosuppressive lectin galectin-1 by tumor infiltrating DCs also supports Treg generation and diminishes TH1 and TH17 T cell differentiation, also inhibiting anti-tumor immune responses [84].
5. Dysfunction in antigen presentation machinery in cancer
Tumor cell escape from immune surveillance is primarily based on disabling the process of tumor-antigen presentation. As discussed above, inhibition of DC activation by cytokines present in the tumor microenvironment profoundly reduces tumor-antigen processing and presentation. However, specific alterations of the molecular machinery controlling antigen processing and presentation in tumor cells themselves can also profoundly inhibit anti-tumor immunity. Antigen presentation in anti-tumor immunity is important at two distinct stages: firstly, when naïve T cells are primed by APCs presenting tumor-associated antigens and secondly, when primed cytotoxic effector T cells must find their target antigen presented by MHC-I on tumor cells themselves.
5.1. MHC-II-pathway
As mentioned above, a variety of tumor-derived factors (such as IL-6 and IL-10) impact the function of DCs in cancer but further studies are necessary to explain the mechanisms of these tumor-induced defects. Curiously, intracellular accumulation of lipids has been identified as one of the causes of DC dysfunction in cancer. Upregulation of the scavenger receptor Msr1 by unknown tumor-derived factors leads to increased uptake of extracellular lipids by DCs in the tumor-bearing host. Although their activation status appeared normal, high lipid-laden DCs did not efficiently present soluble protein antigens to antigen-specific CD4 T cells but presented preprocessed antigenic peptides normally, revealing defective MHC-II antigen processing ability in lipid-laden DCs [85]. Therefore, dysfunction of antigen processing caused by lipid accumulation could attribute to antigen presentation defects in immune responses to cancer.
MHC-II expression is controlled by the APC-specific regulator of transcription CIITA [86]. APCs such as B cells, DCs and macrophages can be targets of different types of malignancies. DC neoplasms are extremely rare tumors that include Langerhans cell (LC) histiocytosis, LC sarcoma, interdigitating DC sarcoma and follicular DC sarcoma [87]. We are unaware of any reports demonstrating defects in the antigen presentation properties of DC tumors. Unlike DC neoplasms, dysregulation of antigen presentation has been broadly defined for largely common B cell malignancies. In diffuse large B cell lymphoma, MHC-II expression is reduced by downregulation of CIITA [88] and by mutations within the MHC-II locus itself [89]. In addition to affecting overall MHC-II expression, there have been reports of dysregulation of peptide binding to MHC-II. MHC-II molecules are assembled in ER with invariant chain (Ii) and traffic to endocytic compartments [90]. In endo/lysosomes MHC-II-bound Ii is degraded and ultimately a small Ii-derived “CLIP” peptide is removed from the MHC-II peptide binding groove by HLA-DM [91, 92]. Malignant cells in Hodgkin’s B cell lymphoma have been shown to express large amounts of surface MHC-II still containing the Ii-associated CLIP peptide instead of antigenic peptides [93], suggesting that there is a failure of HLA-DM to remove CLIP from the MHC-II peptide binding groove in these cells.
A common feature of B cell lymphomas is increased activation of the oncogene c-myc. A recent study in Burkitt’s lymphoma cells correlated high levels of c-myc with poor antigen presentation to CD4 T cells [94]. This was reportedly due to alterations in HLA-DM-catalyzed peptide loading onto MHC-II as well as altered expression of the IFN-γ-induced thiol reductase GILT in endo/lysosomal antigen processing compartments [94]. Aberrant MHC-II expression has also been reported in some non-lymphoid cancers [95], however expression of MHC-II in tumor cells themselves seems to actually support tumor eradication and is associated with relatively good prognosis.
5.2. MHC-I pathway
The immune system, especially in the early stages of cancer development, is still able to generate tumor-antigen specific CD8 T cells [96, 97], and therefore tumor cells must clearly use additional approaches to escape immune recognition. Like many viruses, tumor cells attempt to become “invisible” to the immune system by modifying the MHC-I antigen loading and presentation pathway [98]. Tumor cells at early stages of disease express MHC-I, but this is downregulated or lost as cancer progresses. The selective pressure of CD8 T cells on tumor cells themselves, as well as immunoediting by malignant cells, helps limit T cell attack of tumor cells [99, 100]. Tumors can also suppress the function of the proteasome, thereby reducing the supply of antigenic peptides for binding to MHC-I both quantitatively and qualitatively [101]. Downregulation of ER-resident aminopeptidases (ERAP) that shape the repertoire of MHC-I-binding peptides [102] and deficient expression of TAP in tumor cells have also been reported to limit tumor-antigen binding to MHC-I [103, 104]. Tumor-induced changes in the MHC-I machinery can be restored by incubation of tumor cells with cytokines such as IFN-γ, a known transcriptional regulator of the MHC-I peptide binding machinery [105]. Unlike IFN-γ, IL-10 expressed in the tumor microenvironment reduces TAP function on tumor cells [106, 107], thereby diminishing presentation of MHC-I-associated antigens. Whereas disruption of MHC-I function in tumor cells is a common way tumors attempt to subvert T cell recognition, we are not aware of dysregulation of MHC-I expression in DCs in the tumor microenvironment.
Curiously, tumor-derived IL-10 can subvert the immune system by promoting the expression of the non-classical MHC molecule HLA-G on tumor cells themselves [108]. HLA-G is normally expressed during pregnancy where it functions to protect the fetus from rejection by the maternal immune system [109]. Similarly, tumor cell expression of HLA-G induces tolerance when tumor cells interact with T cells or DCs that express ILT receptors, inhibitory receptors that recognize MHC-I [110]. Expression of HLA-G can also be induced on the surface of DCs by IDO, leading to the generation of DCs that promote the development of immunosuppressive CD4 T cells [111, 112].
6. Overcoming the antigen presentation defects for immunotherapy
In recent years, immunotherapy based on DC vaccines has complemented T cell-based cancer therapies. Many approaches of improving immunotherapies have focused on the priming phase of the anti-tumor immune response. For efficient T cell-priming, however, tumor-induced defects in DC function need to be remedied, regardless whether the vaccination strategy is based on in vivo or in ex vivo DC function.
It is important to consider the functional plasticity of DC subsets when considering DC-based therapies. For example, it would be advantageous to route antigens directly to the MHC-I pathway to assure presentation to CD8 T cells [113]. Enhancing cDC1 cross-presentation could be accomplished by specifically recruiting Batf3+ DCs to the tumor environment (e.g. by blocking CSF-1 [17]), thereby stimulating endogenous T cell priming in tumors with poor T cell infiltration [21]. Another strategy to specifically target antigens to cross-presenting DCs in situ would be to take advantage of receptors such as DEC-205 [114], Clec10A [115], or other endocytic receptors specific for cross-presenting DCs such as XCR1 [13]. One caveat of specifically targeting the MHC-I pathway, however, is that CD4 T cell help could be required for the formation of immunological memory, and therefore stimulating CD8 T cells alone might be insufficient for anti-tumor immunity [113].
Defects in MHC-II-dependent antigen processing and presentation must be remedied to make cDC2 cells efficient stimulators of naïve CD4 T cells. To counteract DC dysfunction in initiating anti-tumor response, DC-based vaccines could be improved by manipulating pathways that are dysregulated in DCs in cancer and potentially include normalization of lipid levels by inhibiting fatty acid synthesis using an acetyl-CoA carboxylase inhibitor [85], by using IL-10R antagonists to restore IL-12 production by DCs [17, 116] or by TLR2 blockade with neutralizing antibodies to reduce the autocrine production of IL-6 and IL-10 by DCs [69].
7. Concluding remarks
Efficient immune-mediated elimination of tumor cells is a multi-dimensional problem. Whereas T cells are major effectors of anti-tumor immune responses, activation of both CD4 and CD8 T cells requires tumor antigen presentation by APCs (primarily DCs). Only by understanding the distinct roles different DC subsets play in anti-tumor immunity, how soluble factors present in the tumor microenvironment affect DC function, and how tumor cells can affect MHC-I and MHC-II antigen processing and presentation will be able to manipulate the tumor microenvironment in a way that will foster anti-tumor immunity.
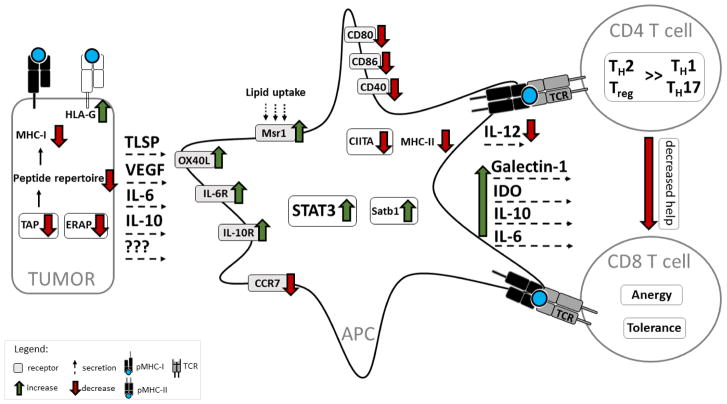
Figure 1. The impact of tumor-derived factors on antigen presentation by DCs.
Immune escape by tumors is based on two main mechanisms. One mechanism is to directly become “invisible” to immune cells. Mutations in MHC-I and downregulation TAP or ERAP lead to reduced surface expression of peptide-loaded MHC-I. Tumor cells can also express the non-classical MHC molecule HLA-G that can lead to T cell tolerance. Another mechanism is by indirectly escaping immune surveillance by impacting APCs activation and function. Tumor-derived IL-6, IL-10, VEGF and other unidentified factors inhibit production of IL-12 and decrease expression of peptide-MHC-II, costimulatory molecules, and the LN homing receptor CCR7 on the DC surface. Tumor-derived IL-6 and IL-10 activate the transcription factor STAT3, which suppresses DC maturation and promotes DC dysfunction. These cytokines also induce autocrine production of IL-6 and IL-10 in DCs, which further augments STAT3 signaling. Tumor-derived TSLP upregulates OX40L, which, together with reduced expression of IL-12, promotes DC-based skewing of T cell differentiation towards the tumor-supporting TH2 phenotype instead of the anti-tumor TH1 or TH17 phenotype that support CD8 T cell expansion. The tumor environment can also induce regulatory DCs, possibly by enhancing expression of the transcription factor Satb1. Tumors also promote Galectin-1, TGF-β, and IDO production in DCs, thereby leading to Treg generation. Finally, upregulation of the scavenger receptor Msr1 leads to enhanced lipid uptake and results in impaired antigen presentation by DCs. Additional defects in MHC-II expression, including downregulation of CIITA and impaired removal of the MHC-II CLIP peptide, have been observed in malignant B cells, however it remains to be determined if these changes also occur in DCs in the tumor microenvironment.
HIGHLIGHTS.
DCs are important regulators of CD4 T cell and CD8 T cell function
Tumor-derived factors suppress the ability of DCs to fully mature
Tumor-derived factors directly suppress the molecular machinery of antigen processing and presentation
Acknowledgments
Our work cited in this review was supported by the Intramural Research Program of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Charette M, Marabelle A, Houot R. Turning tumour cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur J Cancer. 2016;68:134–147. doi: 10.1016/j.ejca.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghiringhelli F, Apetoh L. Chemotherapy and immunomodulation: from immunogenic chemotherapies to novel therapeutic strategies. Future Oncol. 2013;9:469–472. doi: 10.2217/fon.12.207. [DOI] [PubMed] [Google Scholar]
- 5.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 8.Diao J, Winter E, Chen W, Cantin C, Cattral MS. Characterization of distinct conventional and plasmacytoid dendritic cell-committed precursors in murine bone marrow. J Immunol. 2004;173:1826–1833. doi: 10.4049/jimmunol.173.3.1826. [DOI] [PubMed] [Google Scholar]
- 9.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez-Martinez E, Planes R, Anselmi G, Reynolds M, Menezes S, Adiko AC, Saveanu L, Guermonprez P. Cross-Presentation of Cell-Associated Antigens by MHC Class I in Dendritic Cell Subsets. Front Immunol. 2015;6:363. doi: 10.3389/fimmu.2015.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel PM, Gurka S, Kroczek RA. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Guttler S, Hutloff A, Mages HW, Ranke K, Schaefer M, Jack RS, Henn V, Kroczek RA. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity. 2009;31:823–833. doi: 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 16.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S, Van’t Veer LJ, Sperling AI, Wolf DM, Krummel MF. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, Krummel MF. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell. 2016;30:324–336. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, Chakarov S, Rivera C, Hogstad B, Bosenberg M, Hashimoto D, Gnjatic S, Bhardwaj N, Palucka AK, Brown BD, Brody J, Ginhoux F, Merad M. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, Ku AW, Frelinger JG, Odunsi K, Gajewski TF, Luster AD, Evans SS. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. 2015;6:7458. doi: 10.1038/ncomms8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spranger S, Dai D, Horton B, Gajewski TF. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell. 2017;31:711–723 e714. doi: 10.1016/j.ccell.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S, Van’t Veer LJ, Sperling AI, Wolf DM, Krummel MF. Dissecting the Tumor Myeloid Compartment Reveals Rare Activating Antigen-Presenting Cells Critical for T Cell Immunity. Cancer Cell. 2014;26:938. doi: 10.1016/j.ccell.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, Yamamoto K, Suematsu T, Nakamura M, Yui K, Kumatori A. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha-dendritic cell development. Proc Natl Acad Sci U S A. 2004;101:8981–8986. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, Martins I, Schlemmer F, Michaud M, Kepp O, Sukkurwala AQ, Menger L, Vacchelli E, Droin N, Galluzzi L, Krzysiek R, Gordon S, Taylor PR, Van Endert P, Solary E, Smyth MJ, Zitvogel L, Kroemer G. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Segura E, Amigorena S. Identification of human inflammatory dendritic cells. Oncoimmunology. 2013;2:e23851. doi: 10.4161/onci.23851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y, Shen F, Crellin NK, Ouyang W. The IL-17 pathway as a major therapeutic target in autoimmune diseases. Ann N Y Acad Sci. 2011;1217:60–76. doi: 10.1111/j.1749-6632.2010.05825.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn S, Yang J, Ronchese F. Monocyte-Derived Dendritic Cells Are Essential for CD8(+) T Cell Activation and Antitumor Responses After Local Immunotherapy. Front Immunol. 2015;6:584. doi: 10.3389/fimmu.2015.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marigo I, Zilio S, Desantis G, Mlecnik B, Agnellini AH, Ugel S, Sasso MS, Qualls JE, Kratochvill F, Zanovello P, Molon B, Ries CH, Runza V, Hoves S, Bilocq AM, Bindea G, Mazza EM, Bicciato S, Galon J, Murray PJ, Bronte V. T Cell Cancer Therapy Requires CD40-CD40L Activation of Tumor Necrosis Factor and Inducible Nitric-Oxide-Synthase-Producing Dendritic Cells. Cancer Cell. 2016;30:651. doi: 10.1016/j.ccell.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Swiecki M, Colonna M. Accumulation of plasmacytoid DC: Roles in disease pathogenesis and targets for immunotherapy. Eur J Immunol. 2010;40:2094–2098. doi: 10.1002/eji.201040602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faget J, Bendriss-Vermare N, Gobert M, Durand I, Olive D, Biota C, Bachelot T, Treilleux I, Goddard-Leon S, Lavergne E, Chabaud S, Blay JY, Caux C, Menetrier-Caux C. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res. 2012;72:6130–6141. doi: 10.1158/0008-5472.CAN-12-2409. [DOI] [PubMed] [Google Scholar]
- 37.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aspord C, Leccia MT, Charles J, Plumas J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol Res. 2013;1:402–415. doi: 10.1158/2326-6066.CIR-13-0114-T. [DOI] [PubMed] [Google Scholar]
- 41.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, Colonna M, Sibilia M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wenzel J, Bekisch B, Uerlich M, Haller O, Bieber T, Tuting T. Type I interferon-associated recruitment of cytotoxic lymphocytes: a common mechanism in regressive melanocytic lesions. Am J Clin Pathol. 2005;124:37–48. doi: 10.1309/4EJ9KL7CGDENVVLE. [DOI] [PubMed] [Google Scholar]
- 44.Salio M, Cella M, Vermi W, Facchetti F, Palmowski MJ, Smith CL, Shepherd D, Colonna M, Cerundolo V. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–1062. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- 45.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M, Winkels G, Coulie PG, Punt CJ, Figdor CG, de Vries IJ. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 46.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nouri-Shirazi M, Banchereau J, Bell D, Burkeholder S, Kraus ET, Davoust J, Palucka KA. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J Immunol. 2000;165:3797–3803. doi: 10.4049/jimmunol.165.7.3797. [DOI] [PubMed] [Google Scholar]
- 49.Escors D. Tumour immunogenicity, antigen presentation and immunological barriers in cancer immunotherapy. New J Sci. 2014;2014 doi: 10.1155/2014/734515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell. 2016;164:1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 52.Schnurr M, Chen Q, Shin A, Chen W, Toy T, Jenderek C, Green S, Miloradovic L, Drane D, Davis ID, Villadangos J, Shortman K, Maraskovsky E, Cebon J. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood. 2005;105:2465–2472. doi: 10.1182/blood-2004-08-3105. [DOI] [PubMed] [Google Scholar]
- 53.Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. 2017;45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 55.Gallo PM, Gallucci S. The dendritic cell response to classic, emerging, and homeostatic danger signals. Implications for autoimmunity. Front Immunol. 2013;4:138. doi: 10.3389/fimmu.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 57.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 59.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 60.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22–27. doi: 10.1016/j.coi.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 63.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, Murakami M, Hirano T. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 65.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, Murakami M, Hirano T. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 66.Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression. FASEB J. 2004;18:1439–1441. doi: 10.1096/fj.03-0969fje. [DOI] [PubMed] [Google Scholar]
- 67.Haura EB, Zheng Z, Song L, Cantor A, Bepler G. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res. 2005;11:8288–8294. doi: 10.1158/1078-0432.CCR-05-0827. [DOI] [PubMed] [Google Scholar]
- 68.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, Jove R, Pardoll D, Yu H. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 69.Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS. Toll-like Receptor 2 Activation Promotes Tumor Dendritic Cell Dysfunction by Regulating IL-6 and IL-10 Receptor Signaling. Cell Rep. 2015;13:2851–2864. doi: 10.1016/j.celrep.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt SV, Nino-Castro AC, Schultze JL. Regulatory dendritic cells: there is more than just immune activation. Front Immunol. 2012;3:274. doi: 10.3389/fimmu.2012.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Popov A, Schultze JL. IDO-expressing regulatory dendritic cells in cancer and chronic infection. J Mol Med (Berl) 2008;86:145–160. doi: 10.1007/s00109-007-0262-6. [DOI] [PubMed] [Google Scholar]
- 72.Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 73.Norian LA, Rodriguez PC, O’Mara LA, Zabaleta J, Ochoa AC, Cella M, Allen PM. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 2009;69:3086–3094. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–589. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 75.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lo Kuan E, Ziegler SF. Thymic stromal lymphopoietin and cancer. J Immunol. 2014;193:4283–4288. doi: 10.4049/jimmunol.1400864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tesone AJ, Rutkowski MR, Brencicova E, Svoronos N, Perales-Puchalt A, Stephen TL, Allegrezza MJ, Payne KK, Nguyen JM, Wickramasinghe J, Tchou J, Borowsky ME, Rabinovich GA, Kossenkov AV, Conejo-Garcia JR. Satb1 Overexpression Drives Tumor-Promoting Activities in Cancer-Associated Dendritic Cells. Cell Rep. 2016;14:1774–1786. doi: 10.1016/j.celrep.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 85.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, Knight SC, Padhya T, McCaffrey TV, McCaffrey JC, Antonia S, Fishman M, Ferris RL, Kagan VE, Gabrilovich DI. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riley JL, Westerheide SD, Price JA, Brown JA, Boss JM. Activation of class II MHC genes requires both the X box region and the class II transactivator (CIITA) Immunity. 1995;2:533–543. doi: 10.1016/1074-7613(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 87.Kairouz S, Hashash J, Kabbara W, McHayleh W, Tabbara IA. Dendritic cell neoplasms: an overview. Am J Hematol. 2007;82:924–928. doi: 10.1002/ajh.20857. [DOI] [PubMed] [Google Scholar]
- 88.Cycon KA, Rimsza LM, Murphy SP. Alterations in CIITA constitute a common mechanism accounting for downregulation of MHC class II expression in diffuse large B-cell lymphoma (DLBCL) Exp Hematol. 2009;37:184–194. doi: 10.1016/j.exphem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Bushway M, Cycon KA, Mulvaney K, Murphy SP. Coordinate loss of MHC class II expression in the diffuse large B cell lymphoma cell line OCI-Ly2 is due to a novel mutation in RFX-AP. Immunogenetics. 2010;62:109–116. doi: 10.1007/s00251-009-0418-3. [DOI] [PubMed] [Google Scholar]
- 90.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15:203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 92.Kropshofer H, Arndt SO, Moldenhauer G, Hammerling GJ, Vogt AB. HLA-DM acts as a molecular chaperone and rescues empty HLA-DR molecules at lysosomal pH. Immunity. 1997;6:293–302. doi: 10.1016/s1074-7613(00)80332-5. [DOI] [PubMed] [Google Scholar]
- 93.Bosshart H, Jarrett RF. Deficient major histocompatibility complex class II antigen presentation in a subset of Hodgkin’s disease tumor cells. Blood. 1998;92:2252–2259. [PubMed] [Google Scholar]
- 94.God JM, Cameron C, Figueroa J, Amria S, Hossain A, Kempkes B, Bornkamm GW, Stuart RK, Blum JS, Haque A. Elevation of c-MYC disrupts HLA class II-mediated immune recognition of human B cell tumors. J Immunol. 2015;194:1434–1445. doi: 10.4049/jimmunol.1402382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garrido F, Algarra I. MHC antigens and tumor escape from immune surveillance. Adv Cancer Res. 2001;83:117–158. doi: 10.1016/s0065-230x(01)83005-0. [DOI] [PubMed] [Google Scholar]
- 96.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 97.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 98.Hicklin DJ, Wang Z, Arienti F, Rivoltini L, Parmiani G, Ferrone S. beta2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J Clin Invest. 1998;101:2720–2729. doi: 10.1172/JCI498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carretero R, Romero JM, Ruiz-Cabello F, Maleno I, Rodriguez F, Camacho FM, Real LM, Garrido F, Cabrera T. Analysis of HLA class I expression in progressing and regressing metastatic melanoma lesions after immunotherapy. Immunogenetics. 2008;60:439–447. doi: 10.1007/s00251-008-0303-5. [DOI] [PubMed] [Google Scholar]
- 100.Garrido F, Romero I, Aptsiauri N, Garcia-Lora AM. Generation of MHC class I diversity in primary tumors and selection of the malignant phenotype. Int J Cancer. 2016;138:271–280. doi: 10.1002/ijc.29375. [DOI] [PubMed] [Google Scholar]
- 101.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–464. doi: 10.1016/s0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 102.Kamphausen E, Kellert C, Abbas T, Akkad N, Tenzer S, Pawelec G, Schild H, van Endert P, Seliger B. Distinct molecular mechanisms leading to deficient expression of ER-resident aminopeptidases in melanoma. Cancer Immunol Immunother. 2010;59:1273–1284. doi: 10.1007/s00262-010-0856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kamarashev J, Ferrone S, Seifert B, Boni R, Nestle FO, Burg G, Dummer R. TAP1 down-regulation in primary melanoma lesions: an independent marker of poor prognosis. Int J Cancer. 2001;95:23–28. doi: 10.1002/1097-0215(20010120)95:1<23::aid-ijc1004>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 104.Ayalon O, Hughes EA, Cresswell P, Lee J, O’Donnell L, Pardi R, Bender JR. Induction of transporter associated with antigen processing by interferon gamma confers endothelial cell cytoprotection against natural killer-mediated lysis. Proc Natl Acad Sci U S A. 1998;95:2435–2440. doi: 10.1073/pnas.95.5.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seliger B, Hammers S, Hohne A, Zeidler R, Knuth A, Gerharz CD, Huber C. IFN-gamma-mediated coordinated transcriptional regulation of the human TAP-1 and LMP-2 genes in human renal cell carcinoma. Clin Cancer Res. 1997;3:573–578. [PubMed] [Google Scholar]
- 106.Petersson M, Charo J, Salazar-Onfray F, Noffz G, Mohaupt M, Qin Z, Klein G, Blankenstein T, Kiessling R. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J Immunol. 1998;161:2099–2105. [PubMed] [Google Scholar]
- 107.Zeidler R, Eissner G, Meissner P, Uebel S, Tampe R, Lazis S, Hammerschmidt W. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus-encoded interleukin-10. Blood. 1997;90:2390–2397. [PubMed] [Google Scholar]
- 108.Carosella ED, Rouas-Freiss N, Paul P, Dausset J. HLA-G: a tolerance molecule from the major histocompatibility complex. Immunol Today. 1999;20:60–62. doi: 10.1016/s0167-5699(98)01387-5. [DOI] [PubMed] [Google Scholar]
- 109.Hunt JS, Petroff MG, Morales P, Sedlmayr P, Geraghty DE, Ober C. HLA-G in reproduction: studies on the maternal-fetal interface. Hum Immunol. 2000;61:1113–1117. doi: 10.1016/s0198-8859(00)00195-6. [DOI] [PubMed] [Google Scholar]
- 110.Rouas-Freiss N, Moreau P, Menier C, LeMaoult J, Carosella ED. Expression of tolerogenic HLA-G molecules in cancer prevents antitumor responses. Semin Cancer Biol. 2007;17:413–421. doi: 10.1016/j.semcancer.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 111.LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci U S A. 2004;101:7064–7069. doi: 10.1073/pnas.0401922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lopez AS, Alegre E, LeMaoult J, Carosella E, Gonzalez A. Regulatory role of tryptophan degradation pathway in HLA-G expression by human monocyte-derived dendritic cells. Mol Immunol. 2006;43:2151–2160. doi: 10.1016/j.molimm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 113.Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- 114.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lahoud MH, Proietto AI, Ahmet F, Kitsoulis S, Eidsmo L, Wu L, Sathe P, Pietersz S, Chang HW, Walker ID, Maraskovsky E, Braley H, Lew AM, Wright MD, Heath WR, Shortman K, Caminschi I. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J Immunol. 2009;182:7587–7594. doi: 10.4049/jimmunol.0900464. [DOI] [PubMed] [Google Scholar]
- 116.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]