Abstract
Genes encoding TRK are oncogenic drivers in multiple tumour types including infantile fibrosarcoma, papillary thyroid cancer and high-grade gliomas (HGG). TRK fusions have a critical role in tumourigenesis in 40% of infant HGG. Here we report the first case of a TRK fusion-driven HGG treated with larotrectinib—the first selective pan-TRK inhibitor in clinical development. This 3-year-old girl had failed multiple therapies including chemotherapy and radiotherapy. Tumour profiling confirmed an ETV6–NTRK3 fusion. Treatment with larotrectinib led to rapid clinical improvement with near total resolution of primary and metastatic lesions on MRI imaging. This is the first report of a TRK fusion glioma successfully treated with a TRK inhibitor.
Subject terms: Targeted therapies, CNS cancer
Introduction
High-grade gliomas (HGG) are highly aggressive brain tumours that affect both adults and children.1 The standard treatment is focal radiation therapy (RT), and the addition of temozolomide may prolong survival by a few months.2 HGG in infants has a better outcome with a survival rate of ~50%.3 Many respond to chemotherapy, however RT is with-held due to the impact of radiation therapy in infants.4 Treatment with RT may be used at recurrence, but with devastating sequelae. Progression following RT leads to a dismal outcome.5
Genes encoding neurotrophin receptor kinases (TRK) have recently been implicated as oncogenic drivers in multiple tumour types including infantile fibrosarcoma, papillary thyroid cancer and, rarely, adult HGG.6,7 Three members of the TRK proto-oncogene family have been described: TRKA, TRKB and TRKC, coded by the NTRK1, NTRK2 and NTRK3 genes, respectively.8 TRK fusions have been identified at varying frequency in paediatric gliomas, including in 3 out of 7 cases of infant HGG in one study, and have a critical role in tumourigenesis in mouse models.9
Larotrectinib is the first selective pan-TRK inhibitor in clinical development. Recent Phase 1 results showed that larotrectinib is well tolerated in children, with very high response rates in solid tumours that harbour TRK fusions.10 Conversely, solid tumours and HGG without TRK fusions were unresponsive to the inhibitor.11 Here we report the first case of a patient with a TRK fusion-driven HGG treated with a TRK inhibitor.
Case report
The patient is a 3-year-old girl who was diagnosed with a brain tumour at 5 months of age. She presented initially with vomiting and seizures and an MRI showed a heterogeneous mass measuring 6 × 3 × 2 cm in the right lateral ventricle. Following gross total resection pathology showed predominance of large epithelioid and spindle-shaped cells with mild pleomorphism, mitotic index of 14 per 10 high power fields and a Ki67 proliferative index of 40%. The tumour showed patchy positivity for GFAP, strong nuclear staining for p53, and was negative for synaptophysin, chromogranin, NeuN, BRAF V600E, H3K27M and ATRX. She was diagnosed with a HGG and was treated with an infant brain tumour protocol with 13 cycles of chemotherapy.5
Four months after completing treatment, she had disease progression in the tumour bed with multiple nodules in the lateral and third ventricles. Further tumour debulking confirmed recurrent HGG. After 6 months, a new mass in the tumour bed was subtotally resected and she received focal radiotherapy of 54 Gy to the tumour bed. The resected tumour was profiled on a pilot personalised medicine study. Three months following completion of radiation therapy, she represented with difficulty walking, drowsiness, vomiting and irritability. MRI showed widespread progressive disease with increased enhancement at the resection site, and enlarging suprasellar and subependymal nodules in the lateral and third ventricles. Dexamethasone was continued at 1.5 mg daily. The parents were told that she was incurable, and she was referred to palliative care for symptom management.
Results
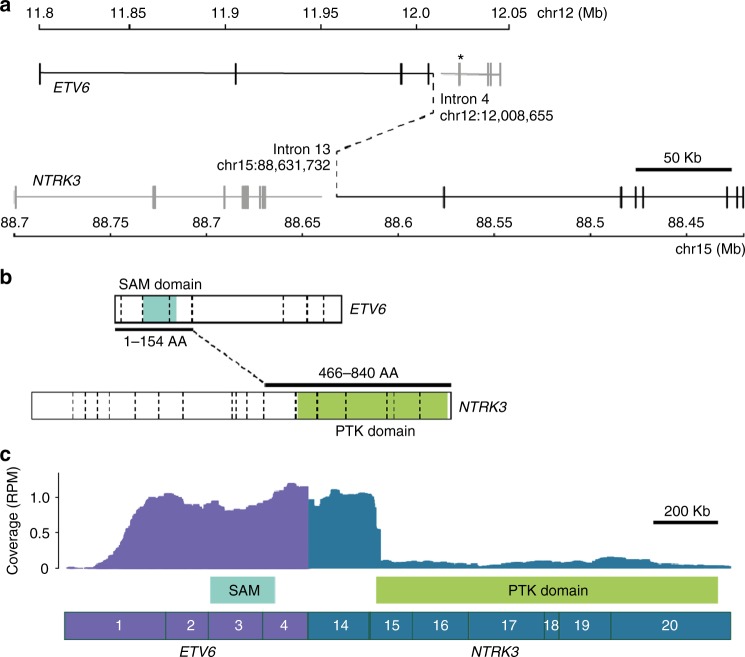
Whole-genome sequencing of fresh-frozen tumour DNA (116× average depth) and matched germline DNA (43× average depth) revealed a t(12;15)(p13.2;q25.3) translocation, resulting in an ETV6–NTRK3 fusion (Fig. 1a). The resulting fusion gene was in-frame, and retained the ETV6 sterile alpha motif (SAM) domain, as well as the NTRK3 protein tyrosine kinase (PTK) domain (Fig. 1b). RNA-Seq from fresh-frozen tumour RNA demonstrated that the ETV6–NTRK3 fusion was robustly expressed (Fig. 1c). Immunohistochemistry staining with a pan-TRK antibody (monoclonal rabbit antibody EPR17341, Abcam) did not detect TRK expression.12 The tumour had a pathogenic TP53 missense variant c.422G>A (p.Cys141Tyr), as well as somatic copy neutral loss of heterozygosity on chromosome 17, resulting in clonal biallelic loss of TP53, plus hemizygous loss of 9p and 18q, three copies of chrX, and biallelic focal deletion of CDKN2A/B. No germline mutations were identified.
Fig. 1.
ETV6–NTRK3 fusion. a Whole-genome sequencing revealed a t(12;15)(p13.2;q25.3) translocation, resulting in an in-frame ETV6–NTRK3 fusion, denoted by black solid and dashed lines. The ETV6-breakpoint differs from the common ETV6-RUNX1 translocation hotspot, which is indicated (*). b The first 154 ETV6, and last 374 NTRK3 amino acids (AA) are fused, retaining ETV6′s sterile alpha motif (SAM) domain, as well as NTRK3′s protein tyrosine kinase (PTK) domain. Exon–exon boundaries are indicated with dashed vertical lines. c RNA-Seq confirmed the expression of the ETV6–NTRK3 fusion, with 62 sequencing reads supporting the breakpoint junction. Exons are numbered. RPM: reads per million mapped reads. ETV6 (NM_001987) and NTRK3 (NM_001012338) isoforms used in all figures
Compassionate access to larotrectinib was obtained and commenced at a dose of 100 mg/m2 bd. After 4 weeks, she had no further lethargy, drowsiness, headaches or vomiting, was eating well, started talking clearly and had been weaned off dexamethasone. After 6 weeks, she was able to walk independently, was speaking in 2–3 word sentences, and had normal energy levels. By week 8, she was running, dancing, and continued to gain new words and language. No adverse events attributable to larotrectinib were observed.
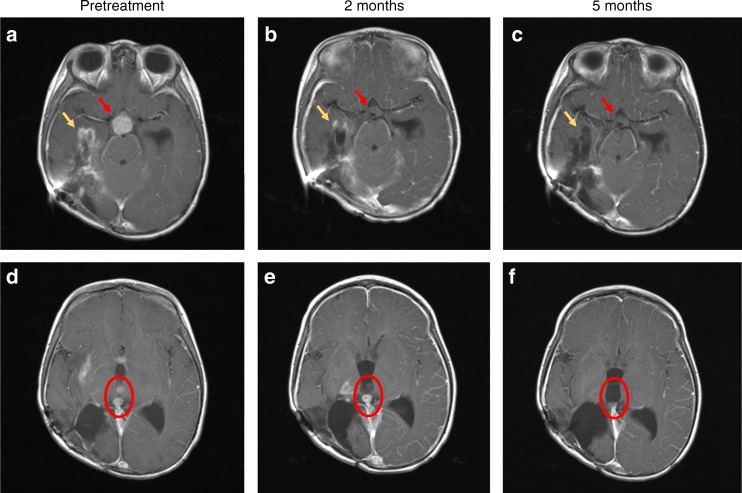
An MRI performed after 8 weeks of therapy showed resolution of the enhancing suprasellar mass, with improvement or resolution of all metastatic ventricular nodules and significantly less enhancement in the surgical bed. MRI at 5 months confirmed the response, with resolution of enhancement in the tumour bed and almost all metastatic lesions (Fig. 2). As of the time of this report, at 9 months from the start of larotrectinib, the patient continues on treatment with no adverse events.
Fig. 2.
T1-weighted brain magnetic resonance imaging (MRI) with contrast images shown pre-treatment a, d, after 2 months of treatment b, e and after 5 months of treatment c, f. The contrast enhancing suprasellar mass (red arrows, a), had resolved after 2 months of treatment b, with sustained response at 5 months (c). Tumour bed enhancement (yellow arrows) improved at 2 months (b), and near complete at 5 months c. Examples of two contrast enhancing intraventricular lesions (red circle) pre-treatment d, improved after 2 months e and with complete resolution at 5 months f. The contrasting enhancing disease in the right thalamus are also visible in e, and had completely resolved after 5 months of treatment f
Discussion
To our knowledge, this is the first reported case of treatment with, and response to, a TRK inhibitor, in a patient with a TRK fusion-driven HGG. The marked response opens a new paradigm in the management of patients with this aggressive brain tumour. While 50% of infants with HGG respond to systemic chemotherapy, progression following chemotherapy and radiotherapy remains incurable, with dismal outcomes.3,5 The response to larotrectinib following failure of multiagent chemotherapy and radiotherapy suggests that TRK inhibitor therapy should be tested in the treatment of other TRK fusion-driven gliomas.
There are several TRK inhibitors currently in development, but no clinical results have till now been published in patients with TRK-driven brain tumours. Entrectinib is a selective tyrosine kinase inhibitor of the TRK kinases, C-Ros oncogene 1 and anaplastic lymphoma kinase. It has been shown to be active in a patient with a TRK-driven non-small cell lung cancer with cerebral metastases, suggesting the potential for this class of drugs to target primary intracranial tumours.13 In the recent report on larotrectinib efficacy in paediatric and adult clinical trials, 75% of patients with a TRK-driven tumour had an objective response to therapy.14 However, no patients were reported who had brain tumours harbouring TRK fusions, and the utility of this inhibitor for these HGG patients has, until now, been unknown. This report indicates that larotrectinib penetrates the BBB and may have potent activity in TRK-driven HGG.
It is unclear whether the activity seen in this case can be translated to adults with HGGs that harbour TRK fusions. TRK fusions in adult HGG are rare,6 however, given the paucity of effective treatment options for these patients, the results here suggest that biomarker-driven trials of TRK inhibitors are warranted in adult HGG patients.
How can TRK inhibitors be incorporated into the treatment of TRK fusion-driven HGG? Most important will be the detection of fusions by immunohistochemistry, FISH or sequenced-based tumour profiling (targeted sequencing, or whole genome and transcriptome sequencing). The latter techniques provide an unbiased approach, and may identify fusions in atypical tumour types, or novel rearrangements which may not be detected with standard tests. Notably, the pan-TRK antibody used here failed to recognise the ETV6–TRK3 fusion in this case, similar to a recent analysis.15 Further assessment of larotrectinib in clinical trials of HGG patients is needed to determine whether genomic profiling should be considered early in patient work-up. More evidence is also required to integrate TRK inhibitors into standard treatment, especially as some infants have durable responses to first-line chemotherapy. TRK inhibitors could be trialled in infants with relapsed or progressive disease, and as a strategy to avoid the damaging effects of high-dose radiation therapy, or combined with chemotherapy at diagnosis. The potential for integration of this molecularly driven, targeted therapy into the treatment for patients with HGG warrants further testing in clinical trials.
Acknowledgements
We thank the Kinghorn Centre for Clinical Genomics and the Murdoch Children’s Research Institute Research Genomics laboratory for assistance with production and processing of Whole Genome, and RNA Sequencing data. We are grateful to Mr Peter Priestley for helpful discussions about whole-genome sequencing analysis. Compassionate access to larotrectinib was provided by Loxo Oncology, Inc. The brain tumour precision medicine programme was funded by the Cure Brain Cancer Foundation. Whole-Genome Sequencing was funded by the Australian Lions Childhood Cancer Research Foundation and Lions Club International Foundation (LCIF) as part of the Lions Kids Cancer Genome Project. A.K. and V.T.’s salaries were supported by the CRC for Cancer Therapeutics, E.M.’s salary was supported by The Kids Cancer Project, L.L.’s and C.M.’s salary were supported by Cancer Institute NSW, and M.J.C.’s salary was supported by NSW Department of Health Early-Mid Career Fellowship. D.K. is the Herman Clinical Fellow at the University of Melbourne. The Zero Childhood Cancer Program has also been supported by the Commonwealth Government of Australia, The NSW State Government, University of NSW, Tour de Cure, The Rich Family Foundation, the Robert Connor Dawes Foundation, and The Lenity Foundation.
Author contributions
M.T., E.M., V.T., D.-A.K.-Q., M.P., V.G., L.M.S.L., M.R., M.J.C., P.G.E., G.M.M. and M.H. carried out the analysis of the data; M.J.C., M.W., C.M., and A.K. performed bio-informatics analysis; R.J.C., L.M.S.L. and D.S.Z. provided clinical data; M.R. and A.G. performed histopathological analysis; M.C.C. and M.R. provided drug, M.J.C., P.G.E. and D.S.Z. wrote the paper. All authors reviewed and approved the final manuscript.
Ethics approval and consent to participate:
Tumour analysis was performed as part of the TARGET study, and was approved by the Sydney Children’s Hospital Network Human Research Ethics Committee in accordance with the Declaration of Helsinki.
Consent for publication:
Consent was provided by the parents for publication of this case.
Competing interests
M.R. is a consultant for Loxo Oncology, Inc and holds a patent 62/318,041 issued to Loxo Oncology, Inc. M.C.C. is an employee of and owns stock in Loxo Oncology, Inc and holds a patent 62/318,041 issued to Loxo Oncology, Inc. The remaining authors declare no competing interests.
Data availability:
All data generated or analysed during this study are included in this published article.
Funding:
The brain tumour precision medicine program was funded by the Cure Brain Cancer Foundation. Whole-Genome Sequencing was funded by the Australian Lions Childhood Cancer Research Foundation and Lions Club International Foundation (LCIF) as part of the Lions Kids Cancer Genome Project. AK and VT’s salaries were supported by the CRC for Cancer Therapeutics, EM’s salary was supported by The Kids Cancer Project, LL’s and CM’s salary were supported by Cancer Institute NSW, and MJC’s salary was supported by NSW Department of Health Early-Mid Career Fellowship. DK is the Herman Clinical Fellow at the University of Melbourne. The Zero Childhood Cancer Program has also been supported by the Commonwealth Government of Australia, The NSW State Government, University of NSW, Tour de Cure, The Rich Family Foundation, the Robert Connor Dawes Foundation, and The Lenity Foundation.
Footnotes
These authors contributed equally: Marie Wong, Chelsea Mayoh, Amit Kumar.
References
- 1.Jones C, et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. NeuroOncology. 2017;19:153–161. doi: 10.1093/neuonc/now101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Duffner PK, et al. Treatment of infants with malignant gliomas: the Pediatric Oncology Group experience. J. Neurooncol. 1996;28:245–256. doi: 10.1007/BF00250203. [DOI] [PubMed] [Google Scholar]
- 4.Espinoza JC, et al. Outcome of young children with high‐grade glioma treated with irradiation‐avoiding intensive chemotherapy regimens: Final report of the Head Start II and III trials. Pediatr. Blood. Cancer. 2016;63:1806–1813. doi: 10.1002/pbc.26118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geyer JR, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J. Clin. Oncol. 2005;23:7621–7631. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 6.Frattini V, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat. Genet. 2013;45:1141. doi: 10.1038/ng.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad ML, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122:1097–1107. doi: 10.1002/cncr.29887. [DOI] [PubMed] [Google Scholar]
- 8.Doebele RC, et al. An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov. 2015;5:1049–1057. doi: 10.1158/2159-8290.CD-15-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu G, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat. Genet. 2014;46:444. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laetsch TW, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19:705–714. doi: 10.1016/S1470-2045(18)30119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong D.S., Dowlati A., Burris H.A., Lee J.J., Brose M.S., Farago A.F., Bauer T.M., Taylor M., Shaw A.T., Smith S., Nanda N., Cruikshank S., Cox M.C., Doebele R.C. Clinical safety and activity from a phase 1 study of LOXO-101, a selective TRKA/B/C inhibitor, in solid-tumor patients with NTRK gene fusions. European Journal of Cancer. 2017;72:S148. doi: 10.1016/S0959-8049(17)30561-0. [DOI] [Google Scholar]
- 12.Hechtman JF, et al. Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am. J. Surg. Pathol. 2017;41:1547–1551. doi: 10.1097/PAS.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farago AF, et al. Durable clinical response to entrectinib in NTRK1-rearranged non-small cell lung cancer. J. Thorac. Oncol. 2015;10:1670–1674. doi: 10.1097/01.JTO.0000473485.38553.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drilon A, et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. New Engl. J. Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatalica, Z., Swensen, J., Kimbrough, J., Xiu J. Abstract A047: Molecular Characterization of the Malignancies with Targetable NTRK Gene Fusions (AACR, Philadelphia, PA, 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.