Abstract
Autogenous tissue grafting remains the gold standard in the treatment of critical sized bone and certain cartilage defects, while the translation of tissue engineered osteogenesis or chondrogenesis from the lab bench into clinical practice, utilizing natural or synthetic biomimetic devices, remains challenging. One of the crucial underestimated reasons for non-translatability could be the imprecision and inconsistency of generated gene expression profiles, utilizing improperly optimized and standardized quantitative gene assays. Utilizing GeNorm for downstream qRT-PCR applications, the stability of reference genes in relation to optimal cDNA amounts was assessed on human bone marrow-derived mesenchymal and adipose-derived stem cells neat and made to differentiate into chondrocytes including normal human derived chondrocytes and muscle tissue from rats. Results showed that reference genes can vary substantially across separately and/or combined cell lines and/or tissue types including treatment parameters. The recommendations to all bone and cartilage tissue engineers utilizing qRT-PCR is not to assume that reference gene stability and quantity remain conserved across cell lines or tissue types but to always determine, for each new experiment, the stability and normalization quantity of reference genes anew.
Introduction
After more than a century, clinical bone regenerative procedures still rely on autogenous bone grafting1–3 to heal and regenerate large bone defects in human. Similarly, damaged cartilage caused either by osteoarthritis or intensive sports are still being treated by varying surgically invasive procedures with limited regenerative techniques being unable to heal critical defects4–9. Whilst some progress has been made in our understanding in some of the molecular mechanisms and how bone and cartilage formation can be induced experimentally the concept of either regenerating defects or complete re-growth of the damaged osteogenic or chondrogenic material remains problematic10–20. One factor not often considered is the variety of analytical methodologies used to monitor gene expression patterns and their subsequent translation, which are deciding factors for generating realistic mechanistic molecular insights in how regenerative processes function and how to modulate them.
Real-time reverse transcription quantitative polymerase chain reaction (qRT-PCR) has become the leading analytic technique, and is an economical, jet simple and highly effective method for monitoring gene transcription, which over the last two decades has made considerable progress at improving the accuracy of gene expression data21–31. Quantitative real-time PCR remains the best alternative to new emerging techniques such as digital droplet PCR and Next Generation Sequencing that are often very expensive to perform. Whilst some bone and cartilage research groups have already used the new standards as set out by Bustin et al.27 pioneering “Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines”20,27,32–34, a considerable number of research groups still rely on outdated and highly insufficient qRT-PCR techniques; when combined with insufficient experimental detail, these techniques render replication of many published findings challenging35 and questionable. The lack of accurate and reproducible gene expression data could be one of the factors contributing to the failing or weak translatability of the in vitro to in vivo to the clinical context, thereby preventing regenerative therapies from replacing the gold standards currently employed to treat bone and cartilage damage and defects.
Results
cDNA amount dependent reference genes expression
In all cell lines and tissue included in the present study, the mean Cq of RNA28S was the lowest (Table 1). For human bone-derived mesenchymal stem cells (hBMSCs), the mean Cq of RPL13a was consistent with complementary DNA (cDNA) quantity ranging from 2.5 ng to 40 ng (20.03 ± 0.68); the mean Cq of SDHA, TBP, RNA28S4, GAPDH, and RPLP0 decreased as the amount of cDNA increased; the mean Cq of POLR2e and ACTB increased as the amount of cDNA increased (Table 1). For human adipose-derived mesenchymal stem cells (hADSCs) and human chondrocytes, the mean threshold cycle of all eight candidate reference genes decreased as the amount of cDNA increased. The two reference genes with the highest mean Cq were SDHA and TBP (Table 1). Interestingly, for the rat rectus abdominis muscle, the mean threshold cycle of TBP and GAPDH remained constant independently of the cDNA quantity utilized (26.46 ± 0.96, and 26.64 ± 0.24 respectively); the mean Cq of POLR2e and RNA28S4 decreased as the amount of cDNA increased for the muscle tissue with the mean Cq of SDHA, ACTB, RPL13a, and RPLP0 increasing as the amount of cDNA increased (Table 1). Optimum cDNA quantity, to use for all other qRT-PCR applications, was determined to be between 2.5 ng to 10 ng which permits for maximum usage of reference genes and test genes to be used during qRT-PCR assays without waste of RNA or subsequent cDNA.
Table 1.
Mean threshold cycle (Cq) of reference genes in a 2x dilution series of cDNA quantities from chondrogenic hMSCs (Chond.), hADSCs (Chond.), normal human chondrocytes (hChond.) and muscle tissue from rat.
| Reference Gene | Cell/Tissue Type | Cq at cDNA quantity | ||||
|---|---|---|---|---|---|---|
| 2.5 ng | 5 ng | 10 ng | 20 ng | 40 ng | ||
| SDHA | hMSCs (Chond.) | 25.52 | 24.45 | 23.72 | 22.94 | 22.66 |
| hADSCs (Chond.) | 27.07 | 26.09 | 24.98 | 24.03 | 23.07 | |
| hChond. | 26.94 | 25.82 | 24.63 | 23.51 | 22.74 | |
| Muscle tissue (rat) | 22.63 | 21.51 | 20.77 | 21.18 | 25.89 | |
| POLR2e | hMSCs (Chond.) | 25.13 | 23.90 | 23.03 | 22.21 | 29.57 |
| hADSCs (Chond.) | 27.75 | 26.37 | 25.49 | 24.42 | 24.23 | |
| hChond. | 23.60 | 22.98 | 21.38 | 20.42 | 19.88 | |
| Muscle tissue (rat) | 23.91 | 22.92 | 22.34 | 21.61 | 22.35 | |
| TBP | hMSCs (Chond.) | 28.02 | 26.73 | 26.02 | 25.34 | 25.77 |
| hADSCs (Chond.) | 28.07 | 27.10 | 26.10 | 25.21 | 24.04 | |
| hChond. | 26.06 | 25.09 | 24.05 | 22.91 | 21.9 | |
| Muscle tissue (rat) | 27.40 | 26.44 | 25.70 | 25.33 | 27.43 | |
| ACTB | hMSCs (Chond.) | 17.10 | 16.02 | 15.48 | 16.51 | 23.83 |
| hADSCs (Chond.) | 25.45 | 23.96 | 22.73 | 21.81 | 21.19 | |
| hChond. | 16.94 | 15.83 | 14.86 | 13.82 | 12.80 | |
| Muscle tissue (rat) | 22.26 | 21.07 | 20.93 | 20.89 | 23.59 | |
| RPL13a | hMSCs (Chond.) | 20.72 | 19.88 | 20.22 | 18.94 | 20.39 |
| hADSCs (Chond.) | 18.26 | 17.03 | 15.90 | 14.99 | 13.85 | |
| hChond. | 19.83 | 18.71 | 17.45 | 16.68 | 15.82 | |
| Muscle tissue (rat) | 20.83 | 20.10 | 20.04 | 19.99 | 22.18 | |
| RNA28S4 | hMSCs (Chond.) | 12.63 | 11.38 | 10.63 | 9.76 | 8.93 |
| hADSCs (Chond.) | 13.95 | 12.84 | 11.93 | 10.82 | 11.30 | |
| hChond. | 12.70 | 11.70 | 10.62 | 9.81 | 8.98 | |
| Muscle tissue (rat) | 12.60 | 11.63 | 10.26 | 8.93 | 8.98 | |
| GAPDH | hMSCs (Chond.) | 19.48 | 18.38 | 17.62 | 17.07 | 16.66 |
| hADSCs (Chond.) | 23.04 | 22.02 | 20.55 | 19.60 | 18.53 | |
| hChond. | 18.31 | 17.12 | 16.02 | 15.08 | 14.10 | |
| Muscle tissue (rat) | 26.78 | 26.49 | 26.31 | 26.93 | 26.69 | |
| RPLP0 | hMSCs (Chond.) | 20.70 | 19.56 | 19.06 | 18.59 | 18.75 |
| hADSCs (Chond.) | 25.32 | 24.07 | 22.87 | 21.93 | 21.39 | |
| hChond | 19.07 | 17.26 | 16.37 | 15.40 | 14.81 | |
| Muscle tissue (rat) | 26.80 | 25.99 | 25.77 | 26.35 | 29.82 | |
Stability and optimal number of reference gene(s) expression
Cells were assessed separately, in combination for a single cell or tissue type, differentiated or undifferentiated, or as a total combination of all cells and tissue types. The different analyses represent different hypothetical experimental scenarios that could come about during a study. Scenarios are divided into: hBMSCs normal and differentiated into chondrocytes; hADSCs normal and differentiated into chondrocytes; normal chondrocytes and normal chondrocytes treated (made apoptotic); hADSCs/differentiated hADSCs with hBMSCs/differentiated hBMSCs with normal/treated chondrocytes with the final scenario being rat muscle tissue normal and osteogenic medium treatment.
hBMSCs and chondrogenic hBMSCs
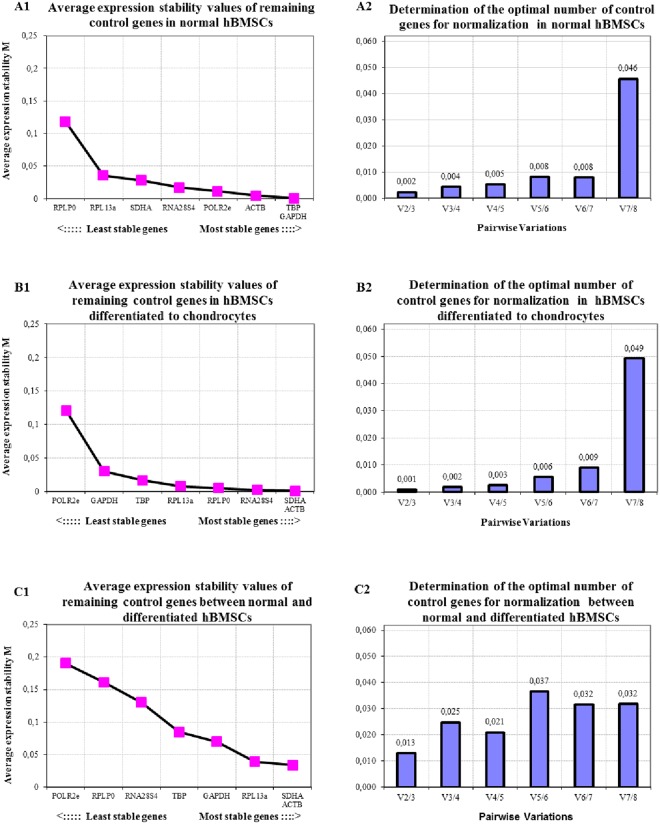
GeNorm analysis was carried out singly for hBMSCs and hBMSCs differentiated into chondrocytes including both types of cell types combined (Fig. 1A–C). For undifferentiated hBMSCs the most stable expressed reference primers were TBP, GAPDH, ACTB and POLR2e (Fig. 1A1), with SDHA, ACTB, RNA28S4 and RPLP0 being the reference genes with the best stability in hBMSCs that had undergone chondrogenic differentiation (Fig. 1B1). When both cell types were assessed in combination, using GeNorm, SDHA, ACTB, RPL13a and GAPDH were most stably expressed (Fig. 1C1). With respect to the optimal number of reference genes necessary for normalization, results showed that 2 to 3 reference genes were sufficient for both single and combined GeNorm analysis sets (Fig. 1A2–C2).
Figure 1.
Average expression stability (A1,B1,C1) and optimal number of reference genes for normalization (A2,B2,C2) for qRT-PCR assays, utilizing the GeNorm algorithm, for (A) normal hBMSCs and (B) chondrogenic differentiated hBMSCs separately or (C) both cell lines combined.
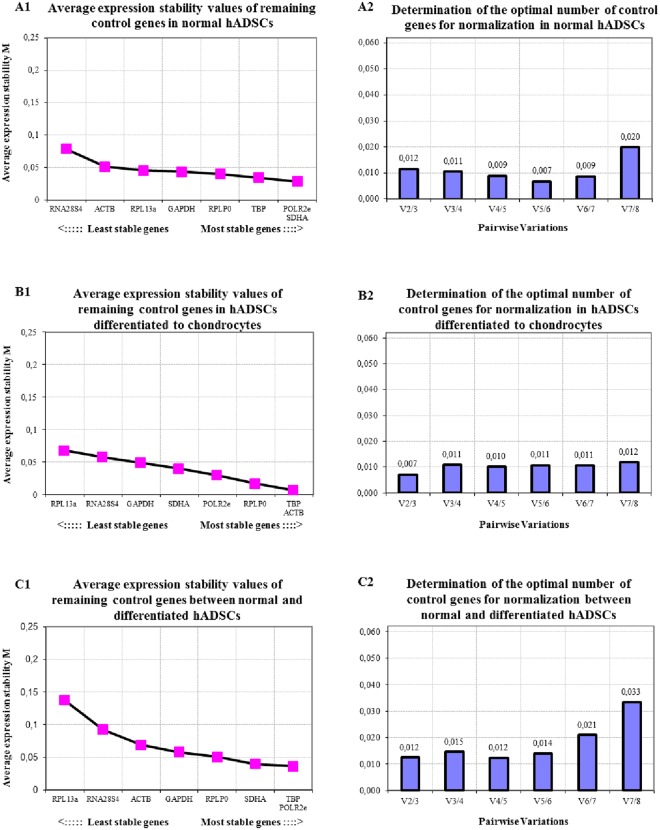
hADSCs and chondrogenic hADSCs
As shown in Fig. 2, the optimal number of reference genes to use for normalization during a qRT-PCR analysis was 5 to 6 for the hADSCs group singly, 2 to 3 in chondrogenic hADSCs singly and 4 to 5 when both hADSCs normal/differentiated were analyzed together utilizing GeNorm, respectively (Fig. 2A2–C2). The reference gene expression stability of untreated hADSCs, chondrogenic hADSCs or combined GeNorm analysis sets generated similar results, in which TBP, SDHA, POLR2e, RPLP0 and ACTB/GAPDH were among the most stable reference genes expressed (Fig. 2A1–C1). The order of these reference genes on the stability scale changed. In general, the highest six reference genes in the ranking of stability were POLR2e, SDHA, TBP, RPLP0, GAPDH, and RPL13a in untreated hADSCs; TBP, ACTB, RPLP0, POLR2e, SDHA, and GAPDH in chondrogenic hADSCs; TBP, POLR2e, SDHA, RPLP0, GAPDH, and ACTB in combined hADSCs sets.
Figure 2.
Average expression stability (A1,B1,C1) and optimal number of reference genes for normalization (A2,B2,C2) for qRT-PCR assays, utilizing the GeNorm algorithm, for (A) normal hADSCs and (B) chondrogenic differentiated hADSCs separately or (C) both untreated and treated hADSCs cell lines combined.
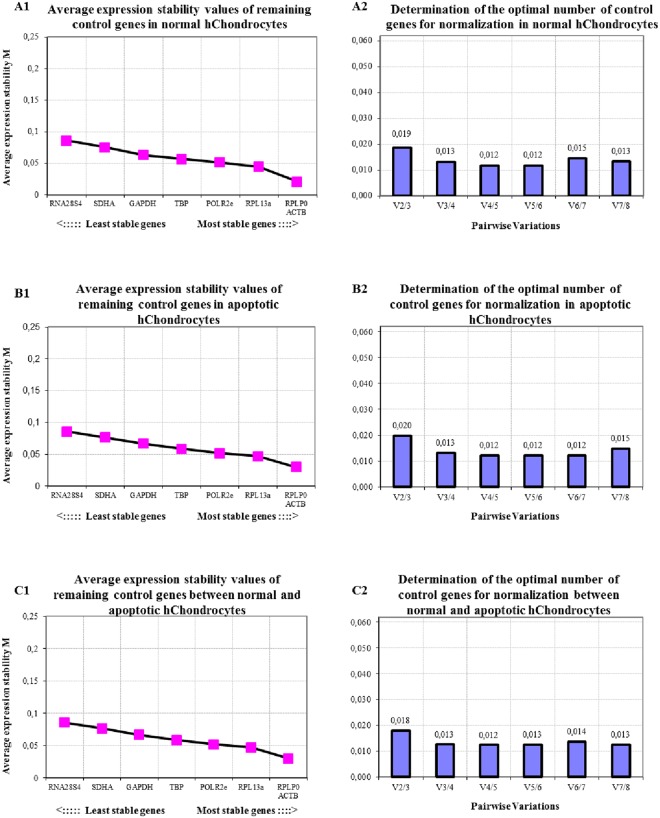
Normal and treated (apoptotic) chondrocytes
ATDC5 human chondrocytes untreated or made apoptotic (treatment), show that the optimal number of reference genes to use for normalization in qRT-PCR, after GeNorm assessment, is 5 to 6 for untreated chondrocytes, 6 to 7 for the treated chondrocyte group and 4 to 5 when both untreated and apoptotic chondrocyte groups are assed together (Fig. 3A2–C2). The reference gene expression stability order between the different groups remained consistent (Fig. 3A1–C1). The highest seven reference genes in the ranking of stability from most to least stable were RPLP0, ACTB, RPL13a, POLR2e, TBP, GAPDH, and SDHA.
Figure 3.
Average expression stability (A1,B1,C1) and optimal number of reference genes for normalization (A2,B2,C2) for qRT-PCR assays, utilizing the GeNorm algorithm, for (A) normal human Chondrocytes (hChondrocytes) and (B) hChondrocytes undergoing apoptosis or (C) both normal and apoptotic chondrocyte cell lines combined.
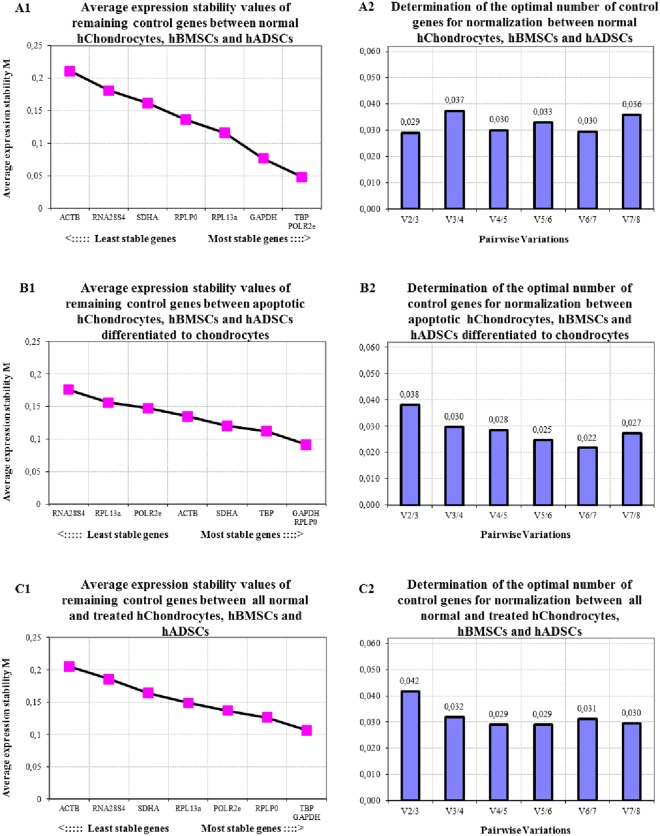
Chondrocytes, hBMSCs and hADSCs
For all normal, treated or differentiated cell types, results showed that the optimal number of reference genes to use for normalization during a qRT-PCR analysis are 2 to 3 when chondrocytes, hBMSCs and hADSCs were looked at in GeNorm, 6 to 7 when treated chondrocytes (apoptotic), chondrogenic hBMSCs and chondrogenic hADSCs were assessed with GeNorm and 5 to 6 when all cell groups were assessed, respectively (Fig. 4A2–C2). For reference gene expression stability between these different scenario groups TBP, POLR2e, GAPDH and RPL13a were the most stable genes in the chondrocyte, hBMSCs and hADSCs group, GAPDH, RPLP0, TBP and SDHA were the most stable in treated chondrocytes, chondrogenic hBMSCs and hADSCs with TBP, GAPDH, RPLP0 and POLR2e being the most stable between all cell groups (Fig. 4A1–C1).
Figure 4.
Average expression stability (A1,B1,C1) and optimal number of reference genes for normalization (A2,B2,C2) for qRT-PCR assays, utilizing the GeNorm algorithm. (A) GeNorm results between Control cell groups with normal hChondrocytes, hBMSCs and hADSCs. (B) GeNorm results between treated cell groups, i.e. hChondrocytes undergoing apoptosis, chondrogenic differentiated hBMSCs and hADSCs. (C) GeNorm results between all untreated and treated cell lines and types.
Rattus novericus rectus abdominis muscle normal and osteogenic
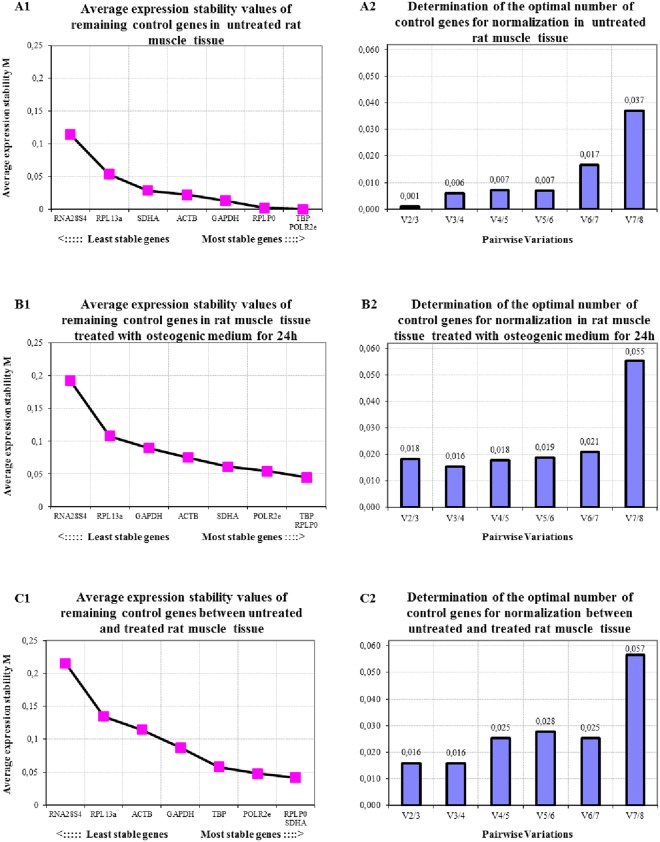
Untreated muscle tissue, including muscle tissue that had been stimulated to undergo osteogenic differentiation utilizing osteogenic medium within an in vitro culture system, were analyzed separately and combined using GeNorm assessment. Results show that that reference stability changes considerably when untreated, treated and combined experimental groups were assessed (Fig. 5A1–C1), where ranking of the reference gene is concerned on the stability curve. The highest four most stable reference genes in untreated rat muscle were TBP, POLR2e, RPLP0, and GAPDH. TBP, RPLP0, POLR2e, and SDHA were most stable in the osteogenic treated rat muscle group, whereas RPLP0, SDHA, POLR2e, and TBP were in combined rat muscle groups. As for the optimal number of reference genes needed for normalization, the results show that 2 to 3 are needed in untreated rat muscle group with 3 to 4 in osteogenic rat muscle or both untreated and treatment groups combined (Fig. 5A2–C2). The overall gene expression stability of untreated rat muscle was the highest, while osteogenic rat muscle ranked second with the combined groups coming last.
Figure 5.
Average expression stability (A1,B1,C1) and optimal number of reference genes for normalization (A2,B2,C2) for qRT-PCR assays, utilizing the GeNorm algorithm, for (A) untreated rat rectus abdominis muscle tissue, (B) rat rectus abdominis muscle tissue treated with osteogenic medium, and (C) both normal and treated rat muscle tissue.
Discussion
The results from the present study clearly show that reference genes in many relative qRT-PCR applications are inappropriately used, as the housekeeping gene expression changes between cell and tissue types including how these are utilized under various experimental conditions. Reference gene(s) stability varies together with the appropriate number of candidate reference genes to use during qRT-PCR gene expression analyses. This can significantly alter the gene expression results and subsequent downstream interpretations, consequently reducing the accuracy of gene transcription patterns that are necessary to make appropriate deductions for molecular mechanisms in tissue engineering fields.
Regeneration of bone and cartilage tissue without the need for autografting is a promising aspect in medicine. However, the translation from in vitro to in vivo and finally into the clinical setting remains problematic and challenging36. Results from numerous studies are difficult to interpret or compare with each other as there is a considerable heterogeneity in experimental designs. Whether it is different stem cell or cell lines, tissue types, varying time points of analysis during experimental setups or the plethora of different treatments, there is currently no standardized concept in the field of bone and cartilage tissue engineering in the way of methodologies. This however is a crucial criterion to ensure that generated data remains consistent and reproducible. One critical aspect in the “standardization debate” is the generation of accurate gene expression data, as genes control life. Understanding the patterns of modulation is critical to successfully regenerate bone, cartilage or any tissue type in vivo37. However, few groups do conform to new standards in qRT-PCR applications. The use of validated techniques and the quantity of reference genes for normalization remains problematic but are an important criterion without which, the generation of accurate, comparable and reproducible gene expression results cannot be obtained, thereby making the generated genetic expression profile of cells or tissue during bone or cartilage regenerative procedures results questionable. Whilst more modern techniques exist, they are prohibitively expensive but at the same time require validation through qRT-PCR assays, to determine the validity of generated results38,39. As such, if used properly, qRT-PCR remains the best alternative. As of now, variation still exists as few studies conform to the new and proven standards by Bustin, Hellemans and Vandesompele27–31. This might explain why bone and cartilage regeneration still appear to fail clinically. The present study attempted to optimize and validate necessary reference genes for specific cell and tissue types that are most commonly used in bone and cartilage tissue engineering studies. What we discovered was a critical aspect in the validation process of reference genes to utilize during qRT-PCR experiments and generating the corresponding relative quantitative gene expression data of test genes, which has to be considered to ensure that future gene expression results would become more exact, but more importantly comparable and reproducible.
Reference genes are considered to be genes that are constantly expressed by cells, individually or or within tissue40. Various studies have shown that this is not to be taken as a rule as these genes can also be affected by treatment modalities41.The recommendations by Bustin et al.27 are to use a minimum of at least 2 reference genes during a qRT-PCR run, as the original reference gene of choice could be affected depending on the experiment. Our study clearly re-iterated this fact for the GAPDH gene, a very common utilized reference gene in bone and cartilage qRT-PCR assays42–44. This gene is considered to be the most “stably” expressed reference gene, with many presented experimental publications and groups never deviating or questioning the validity of this concept. However, from the results of the present study it is clear that whilst GAPDH can be one of the stably expressed reference genes, its position on the stability scale can greatly deviate. This, depending on the quantity of reference genes necessary for normalization, can considerably alter the gene expression profile of selected analytical genes that an experiment is trying to determine.
GeNorm25, NormFinder45, and BestKeeper46 are currently the three most used algorithms for determining the gene expression stability and the quantity of reference genes to use for normalization in qRT-PCR. Given that the GeNorm algorithm is highly dependent on the assumption that the reference genes being analyzed are not co-regulated45, in the present study an analysis to judge whether co-regulation existed between reference genes, was conducted. This way, it can be predicted that if two genes indeed co-regulated each other, when analyzed with GeNorm, they would occupy adjacent positions in the ranking47. As such an internal control was performed where different reference genes were removed (data not shown). Results validated that the ranking of reference genes related to stability and optimal number was not altered, meaning that there was no co-regulation between the different genes selected. These findings are supported by previous results where NormFinder and BestKeeper algorithms were utilized instead of GeNorm48. Although there have been suggestions to use both GeNorm in conjunction with BestKeeper or NormFinder or to only use BestKeeper with NormFinder45–47, the present results clearly indicate that irrelevant of the gene stability and quantity determining reference gene programs utilized, these will not impact on which reference genes are stable or the optimum number to use in an experiment. The GeNorm algorithm can be considered a reliable and very simple method for analyzing gene expression stability and determining the optimal number of reference genes for a selected study. Yet, what is of critical importance is to not assume that, once a certain reference gene set has been determined for a cell or tissue type within an experiment, such reference genes will always remain stably expressed, when utilizing this same cell or tissue type in consecutive experiments. This notion of using reference genes like this49–51 is dangerous and should be evaluated carefully in light of the present study’s findings.
Conducting qRT-PCR experiments in light of the new recommendations by Bustin et al.31 sometimes relies on optimized kits from various scientific companies or reference genes based on the recommendations from other research groups50–53. Care must however be made regarding the methodological steps present in the current or past literature, as these are often not detailed enough to allow reproducing experiments. This is particularly critical when reporting how the qRT-PCR sections are constructed. A good example, for the tissue engineering bone and cartilage fields are the publications by Klar et al.32, Klar et al.20, Ripamonti et al.54, where the qRT-PCR sections describe in great detail each step and how the material was optimized20,32,54. If publications do not offer this amount of detail, then the material must be carefully considered before use. This is made very clear when comparing the GeNorm results, from the present study, to the literature where similar designed studies utilizing similar cell types make recommendations to reference gene usage in qRT-PCR based experiments55. Interestingly, we discovered that even for a certain cell line with slightly different cell sources, for instance isolated cells from human tissue directly versus commercially available cell lines, results were considerably different. Compared to the study by Ragni et al.55, in which the study also included TBP, ACTB, RPLP0, GAPDH, and RPL13A as the candidate reference genes for chondrogenic human stem cells, the optimal number of reference genes to use was 9 to10 in their study, whereas our results suggested a minimum of 4 to a maximum of 5. Subsequently to this, the ranking of the reference genes expression stability also varied considerably between these two datasets with the sequence from most stable to least stable reference genes being TBP, RPL13A, GAPDH, RPLP0 and ACTB from the chosen literature source, whereas in the present studies results it was ACTB, RPLP0, GAPDH, RPL13A and TBP. Furthermore, we found that when we put all the different cell groups together, be they untreated or treated groups, and assessed these in GeNorm analysis (Fig. 4C1,C2), the generated results of the reference gene expression stability versus optimal number of candidate reference genes to use for qRT-PCR applications varied substantially from those where only untreated or treated samples were assessed with each other separately (Fig. 4A1,A2,B1,B2). With respect to inductive tissues used for bone or cartilage regeneration, there are also considerable differences between in vitro versus in vivo based tissue gene expression patterns51,56–59. For the rectus abdominis muscle tissue from rats, since the tissue is comprised of a plethora of different cell layers and cell types, the stability of gene expression could be compensated for (Fig. 5), which is not the case for cells in vitro as these are often single cell lines lacking the presence of other cell types. However, similar to the patterns shown in cell lines of the present study, decreased stability of reference gene expression was also observed in the tissue when both untreated and treatment samples were combined in the GeNorm analysis. Similar to cells in vitro, there seems to be a pattern in the GeNorm analysis, where the moment the various groups are co-analyzed, the amount of reference genes to use to for the normalization varies. As such, it can be implied that subtle differences between different datasets can produce considerable variations in the gene expression stability and the optimal number of reference genes. From this, we thus make the recommendation not to assume that reference gene stability and quantity remains conserved for cell lines or tissue types between experiments but that there is a need to determine, for each new experiment, the stability and normalization quantity of reference genes anew. We believe that failing to do so might result in deviations in gene expression accuracy patterns during downstream analytical processes and consequently lead to difficulty in the translation of approaches to a clinical environment.
Of course, it must be said that the reference genes used in the current study are not all the reference genes presently available. There are a variety of reference genes produced by cells in addition to the 8 selected for this study60. As such we cannot definitively state that the presented reference genes are the most stable reference genes for cell types or tissue types in bone or cartilage research. To compensate for this, digital droplet PCR or Next Generation Sequencing could be utilized as these techniques eliminate the necessity for utilizing reference genes. However, since such technologies for gene analysis are very expensive to acquire and perform38,39, if normal qRT-PCR is used, it appears crucial that studies account for all know reference genes and be based on reference primers that are optimized specifically for each experiment rather than relying on risky assumptions that reference genes used in a similar study also be adequate for this specific experiment.
A subsequent important limitation is RNA quality. This could be one of the reasons why our results in terms of stability and normalization quantity deviates from other groups results55,61,62. Research has established that there is a direct link between quality of RNA and qRT-PCR data. Is the quality poor, results can deviate considerably in gene expression patterns63. In the present study, we utilized a modified Trizol extraction procedure64 out of economic reasons, whose outcomes in RNA quantities are user-dependent. Since the quality of RNA was good for the present study with RINs being between 6 to 8, the variation in quality cannot as such be based on the method but rather the user who is doing the extraction65. This is a further limitation that possibly prevented consistency in generated results with other groups66,67. Four different users performed the method separately, for each of the cell lines and the tissue type, as these results are part of other experiments. We believe that more accurate data might have emerged from experiments conducted by a single user, and might have yielded results that would be similar to literature55,61,62,68.
In conclusion, qRT- PCR remains the best validated technique to economically generate accurate relative gene expression results, which, when properly standardized, are required to develop effective treatments for healing bone or cartilage defects. Attempts at translating from cell to animal and ultimately to human in most cases are probably failing because of inaccurate gene expression results from outsourced and non-viable qRT-PCR methods, as these require a lot of pre-optimizations. This is supported by the present results. However, reference genes are also genes and can be affected by experimental conditions. We therefore suggest not to use reference genes from other study groups, even if the same cell line or tissue type is utilized, as the reference genes can differ between experiments which can lead to inaccuracies in gene expression results. Taken in account together with the RNA quality, we recommend that for all future studies involving qRT-PCR, in bone and cartilage research, laboratories should ensure that their RNA quality is consistently good and before doing qRT-PCR according the guidelines of Bustin et al.31, determine which reference genes are suitable for their given experiments. This muct be done by utilizing all known reference genes and defining which are the most stable and how many reference genes are necessary to generate accurate results. Future research can then establish a general standard, with respect to stable reference genes for cell and tissue types in bone and cartilage regenerative research. This would enable future scientists in the field to always generate the most consistent, accurate and reproducible gene expression data. If these steps are not performed, we fear that bone and cartilage regeneration will remain problematic clinically.
Materials and Methods
Cell and Tissue Specimens
Cell and tissue types chosen are commonly utilized for studying chondrogenic or osteogenic differentiation or regeneration. Human ADSCs, hBMSCs, chondrocytes and muscle tissue from Rattus novericus where utilized in this optimization and standardization study. A total of 12 specimens per cell and tissue type were utilized with 6 specimens acting as the untreated cell or tissue group with the remaining 6 specimens being treated to either undergo chondrogenic differentiation (all stem cells) or osteogenic differentiation (tissue type). For hADSCs, cells were donated to our lab by Maryna et al.69, whereas chondrocytes and hBMSCs were obtained from Lonza (Lonza, Basel, Switzerland).
Rattus novericus rectus abdomins muscle tissue was obtained by killing a F-344 adult male rat (Charles River Sulzbach, Germany) with an overdose of isoflurane (Abbot, Chicago, U.S.A.). This was done in accordance to the rules and regulations of the Animal Protection Laboratory Animal Regulations (2013), European Directive 2010/63/EU and approved by the Animal ethics research committee (AESC) of the Ludwig Maximillian’s University of Munich (LMU), Bavaria, Germany Tierschutzgesetz §1/§4/§17 (https://www.gesetze-im-internet.de/tierschg/TierSchG.pdf) with respect to animal usage for pure tissue or organ harvest only. Utilising 4 mm dermal biopsy punches (PFM medical, Cologne, Germany), 12 muscle tissue fragments were harvested. Six of the muscle fragments were treated to undergo osteogenesis utilising osteogenic medium (treatment) for 24 h, with the remaining six tissue fragments left untreated in DMEM. All tissue specimens were subsequently flash frozen in liquid nitrogen after which they were stored at −80 °C to be used for downstream molecular applications.
Chondrogenic differentiation
Human BMSCs and hADSCs were stimulated to undergo chondrogenic differentiation, to determine if reference genes remain the same within and between the two stem cell types or are differentially expressed. Briefly, after stem cells had entered the fourth passage, stem cell cultures were supplemented with chondrogenic differentiation medium comprised of DMEM–high glucose (DMEM-hg), 10% fetal bovine serum, 100 units/ml penicillin, and 100 mg/ml streptomycin, 1x insulin-transferrin-selenium supplement (ITS + 1, Sigma-Aldrich), 50 µg/ml ascorbate 2-phosphate (Sigma-Aldrich), 40 µg/ml L-proline (Sigma-Aldrich),100 nm dexamethasone (Sigma-Aldrich), and 10 ng/ml rhTGF-β3 (R&D Systems, Minneapolis, U.S.A.). Every two days medium was changed, and cells cultured for 14 days to permit for differentiation. Chondrogenic differentiation was then confirmed via ACAN gene marker test, which is expressed only by differentiated chondrocytes. Differentiated cells as well as untreated control cells were then flash frozen in liquid nitrogen to be used for downstream RNA and qRT-PCR standardisation protocols.
RNA extraction and cDNA synthesis
Harvested cells were disrupted under frozen conditions at 3000 rpm for 1 min, using a Micro-Dismembrator S (Sartorius Stedim Biotech, Göttingen, Germany). Tissue specimens were ground to powder utilising an Eppendorf mortar and pestle system in the presence of liquid Nitrogen. Total RNA was then isolated using a modified RNA Trizol extraction procedure70. Briefly, 1 ml Trizol (Invitrogen, San Diego, CA, USA) was added to the tissue or cell material after which chloroform (Sigma-Aldrich) was added to permit for the separation of the RNA from the proteinaceous material. After centrifugation, the aqueous RNA containing phase was transferred to a fresh tube where the RNA was then precipitated out by adding Isopropanol (Sigma-Aldrich). After incubation at RT for 10 min samples were centrifuged at 20000 rpm over-night at 4 °C, upon which RNA pellets were then washed with 75% ethanol (Merck, Billerica MA, U.S.A.) and permitted to dry thoroughly, to prevent alcohol contamination. After drying, total RNA was resuspended in 32 μl RNase free water (Gibco, California, U.S.A.) after which the concentration and purity of the RNA was determined using a NanoDropTMLite (Thermo Scientific, Waltham, U.S.A.) and quality assessed with a Bioanalyzer 2100 (Agilent Technologies, CA, U.S.A.). After RNA extraction, approximately 1 µg of RNA was reverse transcribed into complementary DNA (cDNA) utilising the QuantiTect Reverse Transcription cDNA Synthesis Kit (Qiagen, Hilden, Germany). CDNA was stored at −20 °C.
Primer design and optimisation
Candidate reference genes were selected out of a gene library pool, known to be suitable reference genes optimal for the normalization of RNA qRT-qPCR expression studies, which focus on those with a standard deviation of the average amplification threshold cycles (Cq) of less than 1 across 35 in human, rat, mouse cell and tissues60,71. Whilst a considerable number of different reference genes are known, for the present study 8 potential reference genes were selected; TATA-binding protein (TBP), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), RNA 28S ribosomal 4 (RNA28S4), RNA polymerase II subunit e (POLR2e), ribosomal protein lateral stalk subunit P0 (RPLPO), succinate dehydrogenase complex flavoprotein subunit A (SDHA), actin beta (ACTB), ribosomal protein L13a (RPL13a) (Table 2); as generally these are commonly and stably expressed in most cell and tissue types, taken into consideration with modern qRT-PCR techniques, where more than four reference genes is a rarity for generating accurate relative gene expression data.
Table 2.
Reference gene primers with amplicon size and annealing temperature ranges for Rattus norvegicus and Homo sapiens.
| Animal | Reference Gene | Accession Number | 5′-3′ sequence | 3′-5′ sequence | Amplicon Length (bp) | Tm range (°C) |
|---|---|---|---|---|---|---|
| Rattus noverigus | SDHA | NM_130428.1 | GCGGTATGAGACCAGTTATT | CCTGGCAAGGTAAACCAG | 239 | 56–64 |
| POLR2e | BC158787.1 | GACCATCAAGGTGTACTGC | CAGCTCCTGCTGTAGAAAC | 151 | 55–64 | |
| TBP | BC081939.1 | TAACCCAGAAAGTCGAAGAC | CCGTAAGGCATCATTGGA | 185 | 55–64 | |
| ACTB | NM_031144.3 | AGCTATGAGCTGCCTGA | GGCAGTAATCTCCTTCTGC | 243 | 55–64 | |
| RPL13a | NM_173340.2 | TTTCTCCGAAAGCGGATG | AGGGATCCCATCCAACA | 159 | 55–62 | |
| RNA28S4 | NR_145822.1 | GCGGCCAAGCGTTCATA | CCTGTCTCACGACGGTCTAA | 143 | 56–65 | |
| GAPDH | BC083511.1 | CATGGGTGTGAACCATGA | TGTCATGGATGACCTTGG | 104 | 55–63 | |
| RPLP0 | BC001834.2 | CAACCCAGCTCTGGAGA | CAGCTGGCACCTTATTGG | 116 | 55–62 | |
| Homo sapiens | SDHA | NM_001330758.1 | CTTCCTTGCCAGGACCTA | GGCGTATCGCTCCATAAAC | 117 | 55–65 |
| POLR2e | J04965.1 | CTATCTGGTGACCCAGGA | CTGCAGAAACTGCTCCA | 322 | 55–61 | |
| TBP | XX000000.0 | CACTTCGTGCCCGAAAC | GCCAGTGTGGACTGTTCT | 121 | 55–63 | |
| ACTB | NM_001101.3 | CTGCCCTGAGGCACTC | GTGCCAGGGCAGTGAT | 197 | 55–64 | |
| RPL13a | NM_012423.3 | CTTTCCTCCGCAAGCGG | GTCCGCCAGAAGATGCG | 159 | 55–62 | |
| RNA28S4 | NR_145822.1 | GCGGCCAAGCGTTCATA | CCTGTCTCACGACGGTCTAA | 143 | 56–65 | |
| GAPDH | BC083511.1 | CATGGGTGTGAACCATGA | TGTCATGGATGACCTTGG | 104 | 55–63 | |
| RPLP0 | BC001834.2 | CAACCCAGCTCTGGAGA | CAGCTGGCACCTTATTGG | 116 | 55–62 |
The relevant sequences of all candidate reference genes (from Homo sapiens and Rattus novericus) were obtained from GenBank (http://www.ncbi.nlm.nih.gov/genbank/). Reference gene primer sequences were designed utilising PrimeQuest in conjunction with OligoAnalyzer 3.1 on the IDT website (https://eu.idtdna.com/site) with specificity of the designed primer being confirmed through the use of the Basic Local Alignment Search Tool program on Pubmed Central (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Primers were optimised using a standard temperature gradient run to determine the optimal range of primer annealing temperatures, utilising 25 ng cDNA (from control cell or tissue samples), with 2x FastStart Essential DNA Green Master (Roche, Basel, Switzerland) and 10 µM of each primer (Table 2) in a final reaction volume of 10 µl. Runs were performed using a LightCycler® 96 thermocycler (Roche, Basel, Swiss), with thermocycling parameters including a pre-incubation of 3 min at 95 °C, followed by a three-step amplification programme of 40 cycles consisting of a denaturation, annealing and extension step set at 95 °C for 10 s, 55 to 65 °C for 15 s and 72 °C for 30 s, respectively. A melt curve was included in each run to confirm amplification of a single product. After PCR amplification wells identified with positive amplicons were purified with the Mini Elute PCR Purification Kit (Qiagen, Crawley, UK), according to the manufacturer’s instructions and analysed, after Sanger sequencing (GATC Biotech, Cologne, Germany) utilising BLAST against the GenBank database to validate primer reference gene sequence amplification specificity.
Standardisation of cDNA quantity for qRT-PCR
In order to determine optimum cDNA quantity to be used per qRT-PCR reaction in cells and tissue samples a standard curve was utilised. A 2x dilution gradient of cDNA amounts, specifically 40 ng, 20 ng, 10 ng, 5 ng, 2.5 ng and 0 ng, was utilised to generate the standard curve in relation to the Cq. PCR reactions were carried out in 96-well plates in duplicate. The PCR reactions were performed using a qRT-PCR LightCycler® 96 Instrument (Roche, Basel, Swiss), where the total volume per reaction was 10 μl, containing 10 μM of each reference primer (Table 2), the corresponding diluted cDNA and 2x FastStart Essential DNA Green Master (Roche). Standardisation runs were performed using a LightCycler® 96 thermocycler (Roche, Basel, Swiss), with thermocycling parameters possessing a pre-incubation of 3 min at 95 °C, followed by a three step amplification programme of 40 cycles consisting of a denaturation, annealing and extension step set at 95 °C for 10 s, 60 °C for 15 s and 72 °C for 30 s, respectively. A melt curve was included in each run to validate product amplification. Standard curve results were summarised into a table showing the [cDNA] dilution in relation to the Cq value.
Reference primer expression stability and quantity
All reference primers were tested in hBMSCs and hADSCs undergoing chondrogenic differentiation, normal chondrocytes (with and without treatment) or rat muscle tissue to determine reference primer stability and how many reference primers were needed to generate accurate gene expression data. PCR reactions were carried out in 96-well plates in duplicate utilising a qRT-PCR LightCycler® 96 Instrument (Roche) with a total reaction volume of 10 μl, that contained 10 μM of each reference primer (Table 2), 10 ng of cDNA and 2x FastStart Essential DNA Green Master (Roche). Thermocycling parameters had a pre-incubation step of 3 min at 95 °C, followed by a three step amplification programme of 40 cycles consisting of a denaturation, annealing and extension step set at 95 °C for 10 s, 60 °C for 15 s and 72 °C for 30 s, respectively. A melt curve was included in each run to validate product amplification. Generated data was then inputted into GeNorm (http://medgen.ugent.be/wjvdesomp/genorm/) using the relative quantities based on comparative Cq method72. The statistical tools used by GeNorm were used to assess the expression stability of the candidate reference gene using the M-value, which refers to the average pairwise variation between each reference gene and the other reference genes. A gene with M <1.5 is considered as a stable reference gene. Subsequently, the pairwise variation (V-score) was determined which indicates the optimal number of reference genes to use for the cell or tissue type to generate realistic and accurate relative quantitative gene expression data27,72,73. The value of Vn/n + 1 under 0.15 indicates that no additional reference genes are required for normalization72.
Acknowledgements
This work was funded from the annual funding budget from the Department of Orthopaedics provided by Ludwig Maximillian’s University of Munich support funding structure for Scientific Establishments, including the Friedrich Bauer Foundation in Bavaria and from China Scholarship Council (#201606230235). We would like to thank Mrs. Barbara Berens and Ms. Bärbel Schmitt, our Biological and Medical Technicians, who helped in primer design and ensure that high cell and tissue culturing standards were maintained throughout the experiments. The senior author would also like to thank Dr. Yan Chevalier who helped proof read the manuscript and who is a continued supporter in helping the senior author to make total limb regeneration a reality for the future.
Author Contributions
Study design: R.M.K. Study conduct: T.H., Y.H. and J.C.C. Data collection: T.H., Y.H. and J.C.C. Data analysis: T.H. and Y.H. Data interpretation: R.M.K. and T.H. Drafting manuscript: T.H., Y.H., and J.C.C. Revising manuscript content: R.M.K. and T.H. Approving final version of manuscript: R.M.K. and T.H. R.M.K. takes responsibility for the integrity of the data analysis.
Data and Code Availability
The necessary algorithmic codes of the program GeNorm are readily available at (http://medgen.ugent.be/wjvdesomp/genorm/). All data, raw and processed, is readily available from the corresponding author on request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tao He and Yijiang Huang contributed equally
References
- 1.Havers, C. & Geuder, M. F. Osteologia nova: sive, Novae quaedam observationes de ossibus, et partibus ad illa pertinentibus (Apud Georgium Wilhelmum Kühnium, 1692).
- 2.Ollier, L. Traité expérimental et clinique de la régénération des os et de la production artificielle du tissu osseux (Victor Masson, Paris, 1867).
- 3.Senn N. On the healing of aseptic bone cavities by implantation of antiseptic decalcified bone. Am J Med Sci. 1889;98:219–247. doi: 10.1097/00000441-188909000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J. Preplanned correction of enophthalmos using diced cartilage grafts. Br J Plast Surg. 2000;53:17–23. doi: 10.1054/bjps.1999.3244. [DOI] [PubMed] [Google Scholar]
- 5.Koshino T, et al. Regeneration of degenerated articular cartilage after high tibial valgus osteotomy for medial compartmental osteoarthritis of the knee. Knee. 2003;10:229–36. doi: 10.1016/S0968-0160(03)00005-X. [DOI] [PubMed] [Google Scholar]
- 6.Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13:213–221. doi: 10.1007/s00167-004-0499-3. [DOI] [PubMed] [Google Scholar]
- 7.Uematsu K, et al. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials. 2005;26:4273–4279. doi: 10.1016/j.biomaterials.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Chiang H, Jiang CC. Repair of articular cartilage defects: review and perspectives. J Formos Med Assoc. 2009;108:87–101. doi: 10.1016/S0929-6646(09)60039-5. [DOI] [PubMed] [Google Scholar]
- 9.Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huggins C. The formation of bone under the influence of epithelium of the urinary tract. Arch Surg. 1931;22:377–408. doi: 10.1001/archsurg.1931.01160030026002. [DOI] [Google Scholar]
- 11.Lavender G. A study of bone regeneration. Surg Gynec Obstet. 1938;67:705–748. [Google Scholar]
- 12.Lacroix P. Recent investigations on the growth of bone. Nature. 1945;156:576. doi: 10.1038/156576a0. [DOI] [Google Scholar]
- 13.Urist MR. Science. 1965. Bone: formation by autoinduction; pp. 893–899. [DOI] [PubMed] [Google Scholar]
- 14.Wang EA, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci USA. 1990;87:2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampath TK, Reddi AH. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci USA. 1981;78:7599–7603. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ripamonti U. The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium carbonate exoskeletons of coral. J Bone Joint Surg Am. 1991;73:692–703. doi: 10.2106/00004623-199173050-00007. [DOI] [PubMed] [Google Scholar]
- 17.Reddi AH. Symbiosis of biotechnology and biomaterials: applications in tissue engineering of bone and cartilage. J Cell Biochem. 1994;56:192–195. doi: 10.1002/jcb.240560213. [DOI] [PubMed] [Google Scholar]
- 18.Ripamonti U, et al. Induction of endochondral bone formation by recombinant human transforming growth factor-β2 in the baboon (Papio ursinus) Growth Factors. 2000;17:269–285. doi: 10.3109/08977190009028971. [DOI] [PubMed] [Google Scholar]
- 19.Dhinsa BS, Adesida AB. Current clinical therapies for cartilage repair, their limitation and the role of stem cells. Curr Stem Cell Res Ther. 2012;7:143–148. doi: 10.2174/157488812799219009. [DOI] [PubMed] [Google Scholar]
- 20.Klar RM, et al. The induction of bone formation by the recombinant human transforming growth factor-β3. Biomaterials. 2014;35:2773–2788. doi: 10.1016/j.biomaterials.2013.12.062. [DOI] [PubMed] [Google Scholar]
- 21.Mullis KB. The unusual origin of the polymerase chain reaction. Sci Am. 1990;262(56-61):64–5. doi: 10.1038/scientificamerican0490-56. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi R, et al. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology (NY). 1993;11:1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- 23.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 24.Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- 25.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15:155–166. [PMC free article] [PubMed] [Google Scholar]
- 27.Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 28.Bustin SA, et al. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol. 2010;11:74. doi: 10.1186/1471-2199-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bustin SA, et al. Primer sequence disclosure: a clarification of the MIQE guidelines. Clin Chem. 2011;57:919–921. doi: 10.1373/clinchem.2011.162958. [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen J, et al. Measurable impact of RNA quality on gene expression results from quantitative PCR. Nucleic Acids Res. 2011;39:e63. doi: 10.1093/nar/gkr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bustin SA, et al. The need for transparency and good practices in the qPCR literature. Nat Methods. 2013;10:1063–1067. doi: 10.1038/nmeth.2697. [DOI] [PubMed] [Google Scholar]
- 32.Klar RM, et al. Calcium ions and osteoclastogenesis initiate the induction of bone formation by coral-derived macroporous constructs. J Cell Mol Med. 2013;17:1444–1457. doi: 10.1111/jcmm.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ripamonti U, et al. The synergistic induction of bone formation by the osteogenic proteins of the TGF-β supergene family. Biomaterials. 2016;104:279–296. doi: 10.1016/j.biomaterials.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Ripamonti U, et al. Cementogenesis and osteogenesis in periodontal tissue regeneration by recombinant human transforming growth factor-β3: a pilot study in Papio ursinus. J Clin Periodontol. 2017;44:83–95. doi: 10.1111/jcpe.12642. [DOI] [PubMed] [Google Scholar]
- 35.Sanders R, et al. Considerations for accurate gene expression measurement by reverse transcription quantitative PCR when analysing clinical samples. Anal Bioanal Chem. 2014;406:6471–6483. doi: 10.1007/s00216-014-7857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klar RM. Bone Induction: Regeneration through Chaos. Front Physiol. 2011;2:21–22. doi: 10.3389/fphys.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett J, Hondred D, Register JC. Keeping qRT-PCR rigorous and biologically relevant. Plant Cell Rep. 2015;34:1–3. doi: 10.1007/s00299-014-1692-6. [DOI] [PubMed] [Google Scholar]
- 38.Robinson S, et al. Droplet digital PCR as a novel detection method for quantifying microRNAs in acute myocardial infarction. Int J Cardiol. 2018;257:247–254. doi: 10.1016/j.ijcard.2017.10.111. [DOI] [PubMed] [Google Scholar]
- 39.Podnar J, et al. Next-Generation Sequencing RNA-Seq Library Construction. Curr Protoc Mol Biol. 2014;106:1–19. doi: 10.1002/0471142727.mb0421s106. [DOI] [PubMed] [Google Scholar]
- 40.Chapman JR, Waldenstrom J. With reference to reference genes: a systematic review of endogenous controls in gene expression studies. PLoS One. 2015;10:e0141853. doi: 10.1371/journal.pone.0141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Y, et al. Validation of potential reference genes for qPCR in maize across abiotic stresses, hormone treatments, and tissue types. PLoS One. 2014;9:e95445. doi: 10.1371/journal.pone.0095445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma H, et al. Validation of suitable reference genes for quantitative polymerase chain reaction analysis in rabbit bone marrow mesenchymal stem cell differentiation. Mol Med Rep. 2015;12:2961–2968. doi: 10.3892/mmr.2015.3776. [DOI] [PubMed] [Google Scholar]
- 43.Zhou ZJ, et al. Selection of suitable reference genes for normalization of quantitative real-time polymerase chain reaction in human cartilage endplate of the lumbar spine. PLoS One. 2014;9:e88892. doi: 10.1371/journal.pone.0088892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maccoux LJ, et al. Identification of new reference genes for the normalisation of canine osteoarthritic joint tissue transcripts from microarray data. BMC Mol Biol. 2007;8:62. doi: 10.1186/1471-2199-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 46.Pfaffl MW, et al. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 47.Tong Z, et al. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol Biol. 2009;10:71. doi: 10.1186/1471-2199-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, et al. Stability of endogenous reference genes in postmortem human brains for normalization of quantitative real-time PCR data: comprehensive evaluation using geNorm, NormFinder, and BestKeeper. Int J Legal Med. 2012;126:943–952. doi: 10.1007/s00414-012-0774-7. [DOI] [PubMed] [Google Scholar]
- 49.Li T, et al. Selection and validation of appropriate reference genes for qRT-PCR analysis in isatis indigotica fort. Front Plant Sci. 2017;8:1139. doi: 10.3389/fpls.2017.01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batra A, Maier HJ, Fife MS. Selection of reference genes for gene expression analysis by real-time qPCR in avian cells infected with infectious bronchitis virus. Avian Pathol. 2017;46:173–180. doi: 10.1080/03079457.2016.1235258. [DOI] [PubMed] [Google Scholar]
- 51.Zhang WX, et al. Selection of suitable reference genes for quantitative real-time PCR normalization in three types of rat adipose tissue. Int J Mol Sci. 2016;17:e968. doi: 10.3390/ijms17060968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ceelen L, De Craene J, De Spiegelaere W. Evaluation of normalization strategies used in real-time quantitative PCR experiments in HepaRG cell line studies. Clin Chem. 2014;60:451–454. doi: 10.1373/clinchem.2013.209478. [DOI] [PubMed] [Google Scholar]
- 53.Jacob F, et al. Careful selection of reference genes is required for reliable performance of RT-qPCR in human normal and cancer cell lines. PLoS One. 2013;8:e59180. doi: 10.1371/journal.pone.0059180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ripamonti U, et al. Tissue segregation restores the induction of bone formation by the mammalian transforming growth factor-β3 in calvarial defects of the non-human primate Papio ursinus. Biomaterials. 2016;86:21–32. doi: 10.1016/j.biomaterials.2016.01.071. [DOI] [PubMed] [Google Scholar]
- 55.Ragni E, et al. What is beyond a qRT-PCR study on mesenchymal stem cell differentiation properties: how to choose the most reliable housekeeping genes. J Cell Mol Med. 2013;17:168–180. doi: 10.1111/j.1582-4934.2012.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puech C, et al. Design and evaluation of a unique SYBR Green real-time RT-PCR assay for quantification of five major cytokines in cattle, sheep and goats. BMC Vet Res. 2015;11:65. doi: 10.1186/s12917-015-0382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas KC, et al. Evidence based selection of commonly used RT-qPCR reference genes for the analysis of mouse skeletal muscle. PLoS One. 2014;9:e88653. doi: 10.1371/journal.pone.0088653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plain KM, et al. High-throughput direct fecal PCR assay for detection of Mycobacterium avium subsp. paratuberculosis in sheep and cattle. J Clin Microbiol. 2014;52:745–757. doi: 10.1128/JCM.03233-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pettengill EA, Parmentier-Line C, Coleman GD. Evaluation of qPCR reference genes in two genotypes of Populus for use in photoperiod and low-temperature studies. BMC Res Notes. 2012;5:366. doi: 10.1186/1756-0500-5-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warrington JA, et al. Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol Genomics. 2000;2:143–147. doi: 10.1152/physiolgenomics.2000.2.3.143. [DOI] [PubMed] [Google Scholar]
- 61.Su X, et al. Optimization of reference genes for normalization of reverse transcription quantitative real-time polymerase chain reaction results in senescence study of mesenchymal stem cells. Stem Cells Dev. 2016;25:1355–1365. doi: 10.1089/scd.2016.0031. [DOI] [PubMed] [Google Scholar]
- 62.Rauh J, Jacobi A, Stiehler M. Identification of stable reference genes for gene expression analysis of three-dimensional cultivated human bone marrow-derived mesenchymal stromal cells for bone tissue engineering. Tissue Eng Part C Methods. 2015;21:192–206. doi: 10.1089/ten.tec.2014.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caboux E, et al. Impact of delay to cryopreservation on RNA integrity and genome-wide expression profiles in resected tumor samples. PLoS One. 2013;8:e79826. doi: 10.1371/journal.pone.0079826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ren B, et al. Gene-activated tissue grafts for sustained bone morphogenetic protein-2 delivery and bone engineering: Is muscle with fascia superior to muscle and fat? J Tissue Eng Regen Med. 2018;12:1002–1011. doi: 10.1002/term.2575. [DOI] [PubMed] [Google Scholar]
- 65.Chadderton T, et al. Evaluation of three rapid RNA extraction reagents: relevance for use in RT-PCR’s and measurement of low level gene expression in clinical samples. Cell Mol Biol (Noisy-le-grand). 1997;43:1227–1234. [PubMed] [Google Scholar]
- 66.Brown RAM, et al. Total RNA extraction from tissues for microRNA and target gene expression analysis: not all kits are created equal. BMC Biotechnol. 2018;18:16. doi: 10.1186/s12896-018-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villa-Rodriguez E, Ibarra-Gamez C, de Los, Santos-Villalobos S. Extraction of high-quality RNA from Bacillus subtilis with a lysozyme pre-treatment followed by the Trizol method. J Microbiol Methods. 2018;147:14–16. doi: 10.1016/j.mimet.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 68.Roth R, Madhani HD, Garcia JF. Total RNA isolation and quantification of specific RNAs in fission yeast. Methods Mol Biol. 2018;1721:63–72. doi: 10.1007/978-1-4939-7546-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bondarava M, et al. Osseous differentiation of human fat tissue grafts: From tissue engineering to tissue differentiation. Sci Rep. 2017;7:39712. doi: 10.1038/srep39712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chomczynski P, Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- 71.Dheda K, et al. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37:112–114. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- 72.VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619–626. doi: 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- 73.Dolgin E. The most popular genes in the human genome. Nature. 2017;551:427–431. doi: 10.1038/551S15a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The necessary algorithmic codes of the program GeNorm are readily available at (http://medgen.ugent.be/wjvdesomp/genorm/). All data, raw and processed, is readily available from the corresponding author on request.