Abstract
Biofilms are ubiquitous in the natural and man-made environment. They are defined as microbes that are encapsulated in an extracellular, self-produced, biofilm matrix. Growing evidence from the genetic and biochemical analysis of single species biofilms has linked the presence of fibrous proteins to a functional biofilm matrix. Some of these fibers have been described as functional amyloid or amyloid-like fibers. Here we provide an overview of the biophysical and biological data for a wide range of protein fibers found in the biofilm matrix of Gram-positive and Gram-negative bacteria.
Keywords: functional amyloid fibers, biofilm matrix, curli, TasA, PSM
Abbreviations: eDNA, extracellular DNA; ThT, thioflavin T; CD, circular dichroism; TEM, transmission electron microscopy; MTP, M. tuberculosis pili; HR, hypersensitivity response
Graphical abstract
Highlights
-
•
Biofilms are complex communities of bacteria encased in an extracellular matrix.
-
•
The biofilm matrix frequently contains fibrous proteins.
-
•
Many of the proteins have been designated amyloid-like fibers.
-
•
We review the designation in light of biochemical and biophysical data.
Biofilms and Fiber-forming Proteins of the Matrix
Biofilms are communities of microorganisms attached to a surface and encompassed by a self-produced extracellular matrix. The biofilm matrix is dynamic and fulfils multiple functions including nutrient sequestration and water adsorption, shielding the resident cells from environmental stress and competition [1], and acting as a signaling facilitator for cells both within and outside the biofilm [2]. There is great diversity in composition of the biofilm matrix across polymicrobial and between single-species biofilms; however, commonly occurring constituent parts include polysaccharides, extracellular DNA (eDNA), lipids and proteins, some of which are fibrous in nature [1], [3]. Growing evidence suggests that fiber-forming proteins provide structural integrity to many biofilms [4], [5], [6], which ultimately provides protection for the bacteria, for example, from phage predation [7].
Amyloid fibers have serious negative medical implications and are predominately associated with neuropathic, single-organ and systemic diseases that are characterized by extracellular insoluble protein deposition [8]. In contrast with these disease-associated fibers, an emerging field of study concerns the “functional” amyloid fold, with proposed examples occurring in mammals, fungi and bacteria [9], [10], [11]. Many of the fiber-forming proteins in the biofilm matrix have been described as “amyloid” or “amyloid-like.” Here, however, we posit that, in the context of the biofilm matrix, the term “functional amyloid fiber” has been broadened to include a diverse range of protein structures. For example, the term amyloid-fiber has been associated with both β-helix [12], [13] and α-helical fiber structures [14]. We overview the historical significance of the term “amyloid” and its medical origins and significance. We then describe the structure and function of fibers that have been previously identified as “amyloid-like” in biofilms from a broad range of species.
Amyloid Fibers in Disease
Mammalian systems depend on complex regulatory networks to deal with misfolded proteins appropriately, and dysregulation of these processes can cause disease. When a native protein misfolds and deposits as insoluble fibers with a cross-β structure, the resulting disease is termed an amyloidosis [8]. The site of deposition can be in single organs, such as the pancreas in type 2 diabetes [15], in multiple organs in systemic amyloidosis, or neuropathic as in Alzheimer's and Parkinson's disease [16]. In all of these diseases, the insoluble structure of the amyloid fiber has devastating consequences on homeostasis.
This same insolubility long hindered structural elucidation of the amyloid fiber, but awareness of the extracellular deposits by histopathology can be traced back to the 18th century [17]. Accordingly, medical notes are full of archaic descriptors like “lardaceous,” which stemmed from the waxy deposits in diseased kidneys, spleens and livers resembling bacon in the eyes of French pathologists [17]. The name “amyloid” was introduced in 1854 by Virchow, due to the blue-black color change of bound iodine in tissue samples from the brain [18]. During the mid-20th century, the publication of an amyloid fiber extraction technique (the Pras method [19]) allowed the molecular structure of amyloid fibers to be probed in vitro. This involved homogenizing amyloid-laden tissue and removing the soluble fraction of protein, and then isolating the insoluble amyloid-containing fraction by repeated centrifugation. This also removes any endogenous fiber-associated factors and possibly alters the structure of the amyloid fiber in the process. In the next section, we overview the methods used to analyze fiber-forming proteins.
Methodologies for Understanding the Amyloid Fiber
Physical characteristics of amyloid fibers
Amyloid fibers are insoluble in SDS and are resistant to proteolytic cleavage. This is due to the “cross-β” structure common to all amyloid fibers where β-sheets run parallel to the fiber axis composed of β-strands stacked vertically, like the rungs of a ladder (Fig. 1a). The β-sheets, which are typically in-register, are close enough to be governed by Van der Waals forces, and in some cases [21], [22], the sidechains interdigitate to form a “steric zipper,” thereby excluding water from the interior of the protofilament. The individual β-strands of the protofilament can be arranged in a parallel or antiparallel fashion, each extensively hydrogen-bonded via amide and carboxyl groups in the backbone of the polypeptide chain. These protofilaments stack to give the quaternary structure. It is this combination of Van der Waals interactions and an extensive hydrogen-bonded network that gives amyloid fibers their extraordinary stability.
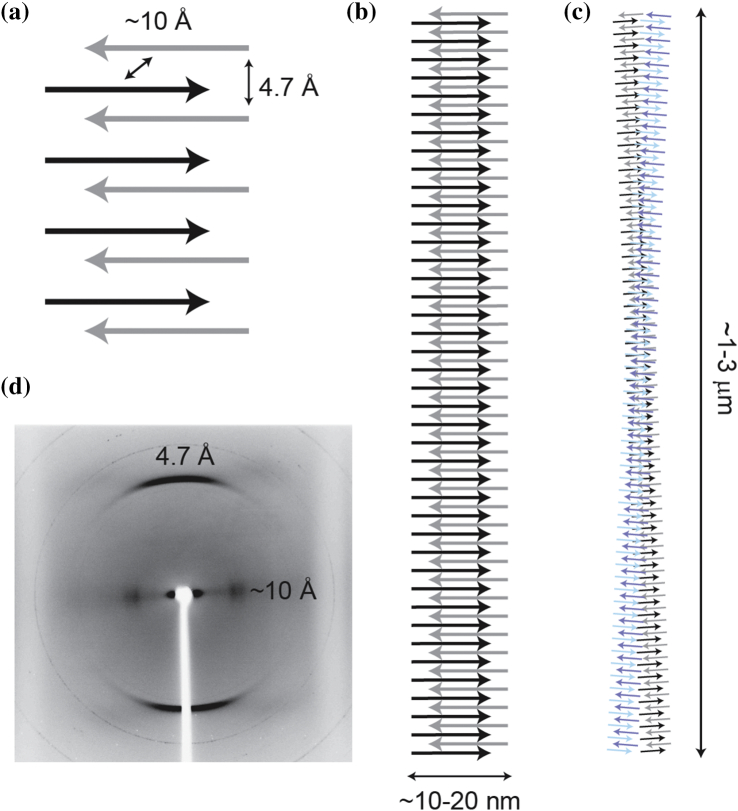
Fig. 1.
Characteristic structure of an amyloid-like fibril. In amyloid-like fibers, proteins adopt a predominantly β-sheet conformation (a). β-Strands are arranged orthogonally to the fiber axis, held together by interactions between sidechains in the fiber core, and by an extensive network of hydrogen bonds running parallel to the fiber axis (b, c). The distance between β-strands parallel to the fiber axis is a characteristic 4.7 Å, reflecting the hydrogen bond array, and the intersheet distance ranges between 6 and 12 Å. These distances, and therefore this “cross-β” arrangement, are confirmed by X-ray fiber diffraction, which reveals two orthogonal reflections (d). Reprinted with permission from Ref. [20]. Copyright 2008 American Chemical Society.
Direct dyes
In the early 20th century, it was discovered that the extracellular deposits bound the dye Congo red and exhibited birefringence: the bound dye emits a range of colors, typically “apple-green,” under crossed polarizers [23]. The common pitfalls that should be considered when using Congo red are extensively reviewed elsewhere [24], but crucially, this dye binds many polymeric substances including DNA and cellulose. It is common to see Congo red used in conjunction with another dye, thioflavin T (ThT). First introduced in 1959, ThT has long been used to diagnose amyloidosis [25] and to follow the kinetics of amyloid polymerization in vitro [26], but like Congo red, ThT has been demonstrated to exhibit false-positive binding to other polymers, and also proteins and protein aggregates that are not in the amyloid conformation [27], [28], [29]. The exact molecular mechanism that underpins ThT binding is still being investigated, but current consensus holds that the increase in fluorescence intensity is dependent on the restriction of the internal molecular rotation when the dye monomer is bound, or as viscosity of the microenvironment increases [30], [31].
Immunolabeling
An additional technique used to identify amyloid species is immunolabeling. There are antibodies available that have been differentially raised against fibrillar (e.g., WO1/WO2 [32]) and soluble oligomeric amyloid species (e.g., A11 [33]). Despite showing sensitivity toward the fibrillar and oligomeric amyloid fold, to the best of our knowledge, there has been no systematic testing of the specificity of these antibodies for false-positive cross-reactivity with other bacterial filamentous structures such as flagella or with other β-rich structures, for example, β-solenoid domains.
Structural characterization
Circular dichroism
Circular dichroism (CD) spectroscopy measures the difference in absorption of right and left polarized light by chiral molecules. The CD spectra at far-UV wavelength of proteins are influenced by the three-dimensional structure of the amide backbone, rather than the individual chiral amino acids, and therefore can be used to investigate polypetide secondary structure [34]. In the far-UV spectrum, the ratio of different secondary structural features can be identified; for amyloid structures, this is predominately β-sheet (Fig. 1a). When first introduced, it was thought that the technique could not provide adequate information on the arrangement of β-sheet secondary structure elements, but advancements have been made in the field [35].
X-ray diffraction
Additional structural information can be obtained by analyzing the molecular arrangement of the protofilament (Fig. 1b). Through observing the diffraction pattern of a beam of electrons (giving small angle patterning) or more commonly X-rays (allowing a wider range) through the insoluble fiber, it is possible to resolve the tertiary structure [36]. To perform this analysis, a droplet of a protein sample is suspended on a stretch frame apparatus and allowed to dry. As it dries, proteins fibrils (Fig. 1c) within the sample ideally become aligned. Since, in the case of amyloid fibers, the cross-β structure of the fiber is constant along the fiber axis, the patterning shows two reflections, characteristic of the inter-strand (4.7 Å) and inter-sheet (6–12 Å) distances. Thus, the term “cross-β” arises from the fact that these two reflections are orthogonal: the inter-strand distance is seen on the meridian, and the inter-sheet distance on the equator (Fig. 1d).
Atomic resolution
More recent technological advancements have enabled the atomic structure of fibers to be elucidated, albeit in a relatively limited number of cases (reviewed in Ref. [37]). Through a combination of solid-state nuclear magnetic resonance and mass/length estimates, the structure of protofilaments assembled from the Aβ peptide and a transthyretin peptide have been mapped [38], and cryo-electron microscopy has been used to determine the structure of native tau filaments extracted from a patient with Alzheimer's disease [39]. Interestingly, the tau fibers form an elaborate mixed architecture comprising two canonical cross-β structures, connected by a β-helix motif. Other atomic resolution studies include that of the Het-S fungal prion-forming domain, where the dry interface has three sides in a triangular β-solenoid [40]. The use of small internal peptides derived from full-length proteins as model systems for amyloid fiber formation is further complicated by the possibility of conformational variants. For example, for the hexapeptide “GNNQQNY” derived from the N-terminal region of the yeast prion protein Sup35, different conformations of the cross-β backbone between microcrystalline and fibrous assemblies have been detected by magic angle spinning solid-state NMR [41]. Whether this represents true heterogeneity in the cross-β motif is debatable; small-angle X-ray scattering studies show a single backbone conformation [42]. Furthermore, variation between the hexapeptide and the full-length Sup35 assembly has been noted, the latter reported to form amyloid fibers of out-of-register parallel structure [43]. Overall, the diversity that has emerged from atomic resolution studies has presented many questions regarding sample preparation and whether legitimate comparison can be made between in silico, in vitro, and in vivo derived structures.
Considerations
As the structural investigations have progressed, so has the understanding of the mechanisms that result in globular, folded proteins adopting the misfolded cross-β structure. By introducing environmental stress in the form of temperature or pH changes, it is possible to induce the amyloid fiber fold in any polypeptide chain [44], [45]; even those consisting of a single repeating amino acid. These are biophysically indistinguishable from the disease-causing amyloid fibers [44]. This is an important consideration when extracting novel proteins, ensuring that neutral conditions are used so as not to influence the energy landscape of the folded protein, which might otherwise bias toward inadvertent protein self-assembly.
Protein Fibers in Biofilms
A survey investigating the abundance of amyloid fibers in natural biofilms was undertaken in sewage filtration plants and by using the amyloid fiber WO1 antibody, coupled with phyla-specific oligonucleotide probe-based fluorescence in situ hybridization revealed that 10%–40% of bacteria produced elements that bound to the WO1 antibody [46]. However, the identification of a novel functional fiber protein typically begins with an initial observation of an extracellular fiber network by electron microscopy. This is then followed by either native extraction or production of the recombinant protein in another species, commonly Escherichia coli, to allow a closer examination of its biophysical properties. If the protein proves to be insoluble in common solvents and therefore cannot be analyzed by SDS-PAGE, it may be necessary to expose it to harsh conditions. However, it is worth noting that this treatment alone may induce amyloid fiber conversion of the target protein regardless of prior conformation [44]. After extraction of the protein fibers, subsequent structural analysis needs to be undertaken to ascertain the molecular structure of the protein and to ultimately link this with its role in the biofilm matrix.
In the following sections, we will examine matrix-associated fibers that have been classified as amyloid-like, highlighting the variety of fiber structures and functions. We collate the properties of these fibers that have been collected through biophysical means and through associated traits such as resistance to SDS and susceptibility to proteolytic cleavage (Table 1).
Table 1.
Biochemical and biophysical properties of bacteria protein fibers
| Species | Proteina | Physiological functionb | Source | Birefringence | Congo red | ThT | Antibodies | Secondary structure (XRD, NMR) | Secondary structure (CD, FTIR) | Ultrastructure (EM) | Soluble in SDS | Protease resistant | Isolated fiber biological activity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli and S.typhimurium | Curli/Tafi [47] | Structural biofilm matrix molecule [47] | Native, extracted by sequential centrifugation | Green/yellow [48] | Red shift [49] | Enhanced fluorescence [49] | No data | No data | β-sheet [49], [50] | fiber network [47] | No [51] | Yes [51] | Genetic complementation [52] |
| CsgA Full-length recombinant His-tagged | No data | Red shift [49] | Enhanced fluorescence [53] | A11 + ve intermediates [53] | Not in-register; diffraction rings at 4.8 Å, 9 Å [12] | β-sheet [49] | Needle-like fibers [49] | No [49] | Yes [12] | Polymerized by csgA − cells [52] | |||
| CsgB Recombinant Δ18 aa from C-term | No data | Red shift | Enhanced fluorescence [54] | No data | No data | β-sheet [54] | Needle-like fibers [54] | No [54] | No Data | Can nucleate CsgA in vitro, mislocalizes in vivo[54] | |||
| CsgB full-length recombinant | No data | No data | Enhanced fluorescence [12] | No data | Not in-register; diffraction rings at 4.7 Å, 9 Å [12] | Mixed β-sheet/β-turn/α-helical [12] | Fibers [12] | No [12] | No data | No data | |||
| Pseudomonas sp. | Fap [55] | Structural biofilm matrix molecule [55] | Native, extracted by boiling in SDS | Data not shown [55] | No data | Enhanced fluorescence [55] | WO1 + ve fibers [56] | No data | β-Sheet post-formic acid [55] | Fibers [55] | No [55] | No data | No data |
| Whole fapA-H operon Recombinant | No data | No data | No data | No data | Diffraction rings at 4.15 Å, 4.34 Å, 4.61 Å, 6.32 Å, 11.53 Å [55] | β-Sheet [55] | Fibers [55] | No [57] | No data | Increased aggregation in vivo[57] | |||
| Bacillus sp. | TasA [58] | Structural biofilm matrix molecule [59] | Native extracted by salting | No data | No red shift [59] | Enhanced fluorescence [59] | A11 + ve intermediates [59] | No data | α-Helical [59] | Extracellular fibers [59] | Yes [59] | No data | Yes [59] |
| Recombinant Δ22 aa from C-term | No data | No data | No data | No data | Jellyroll [60] | No data | No data | No data | No data | N/A | |||
| M. tuberculosis | MTP [61] | Structural biofilm matrix molecule [61] | Native | No data | Data not shown [61] | No data | No data | No data | No data | Extracellular fibers [61]; Disputed [62] | Yes [61] | No data | Yes [63]; Disputed [62] |
| S. mutans | P1 [64] | Structural biofilm matrix molecule | His-tagged Recombinant P1 (stirred) | Green/yellow [48] | No red shift [48] | Enhanced fluorescence [48] | No data | No data | No data | Needle-like fibers [48] | No data | No data | No data |
| WapA (Antigen A) | Cell-wall associated | Produced by curli system recombinant | Green/Yellow [65] | No data | Enhanced fluorescence [65] | No data | No data | α-Helical to β-sheet at pH 4 [65] | Dark extracellular aggregates [65] | Yes [65] | No data | No data | |
| SMU_63c [65] | Putative competence involvement [65] | Produced by curli system recombinant | Green/Yellow [65] | No data | Enhanced fluorescence [65] | No data | No data | α-Helical to β-sheet at pH 4 [65] | Dark extracellular aggregates [65] | Yes [65] | No data | No data | |
| Staphylococcus species | Phenol-soluble modulins [66] | Putative biofilm matrix molecule [67] | Native | No data | Red shift by synthetic internal peptide [67] | Enhanced fluorescence by internal peptide [67] | No data | No data | β-Sheet [67] | Extracellular fibers [67]; Disputed [68] | No [67] | Yes [67] | Biofilm [67]; Disputed [68] |
| PSMα3 [14] | Synthetic PDB:5155 | No data | No data | Enhanced fluorescence [14] | No data | Diffraction rings at 32 Å, 11.5 Å, 9.6 Å, 4.5 Å [14] | Fiber and monomer α-helical [14] | Twisted bundles of fibers [14] | No data | No data | Cytotoxicity [14] | ||
| SuhB | Putative biofilm matrix molecule [69] | His-tagged recombinant | No data | Red shift [70] | Enhanced fluorescence [70] | No data | Diffraction rings at 3.8 Å, 4.7 Å, 10–11 Å [70] | β-Sheet [70] | Sheet-like fiber network [70] | Yes [70] | No data | No data | |
| Bap | Adhesion during biofilm formation [71] | Recombinant Internal peptide | No data | Red shift at pH 4.5 [71] | Enhanced fluorescence at pH 4.5 [71] | No data | No data | β-Sheet; 205 nm minima of internal peptide [71] | Compares peptides to native by TEM [71] | No [71] | No [71] | Cell clumping [71] | |
| Gallibacterium anatis | Tu Elongation factor | Adhesion during biofilm formation [72] | His-tagged recombinant | No data | No data | No data | No data | No data | No data | Dark extracellular aggregates [72] | Yes [72] | No data | No data |
| Xanthomonas anxidopidos | HpaG [73] | Plant pathogenicity [73] | His tagged Recombinant | Green/yellow [74] | Red shift [74] | No data | No data | No data | α-Helical to β-sheet [74] | Network of fibers [74] | Yes [74] | Limited resistance [74] | No data |
| Legionella pneumophila | No data | Biofilm-associated structural [75] | Native | Red binding [75] | No data | Enhanced fluorescence [75] | WO2 + ve fibers [75] | No data | No data | No data | No data | No data | No data |
| Solibacillus silvestris AM1 | BE-AM1 [76] | Bioemulsfier [76] | Native | Green/Yellow [76] | No data | No data | No data | No data | β-Sheet; Mixed α-helical and β-sheet [76] | [76] | No data | No data | No data |
| Klebsiella pneumoniae | Microcin E492 [77] | Modulation of cytotoxic active monomer [78] | MccE492 full-length recombinant with immunity determinant | No data | Red-shift [78] | Enhanced fluorescence [78] | A11 + ve intermediates [79] | Diffraction rings at 4.71 Å, 9.92 Å [80] | β-Sheet [78] | Fibers polymerize after 220 rpm shaking 15 h [78] | Yes [78] | No [78] | No data |
| Streptomyces coelicolor | Rodlins [81]; Chaplins [82] | Formation of rodlet layer [83] | ChpA-F Native extracts | No data | No data | Enhanced fluorescence [84] | No data | No data | Random coil to β-sheet [84] | 4- to 6-nm wide lines shadowing on formvar grids [84] | No [84] | No data | Aerial hyphae formation [84] |
| Synthetic ChpA-F peptides | No data | No data | Enhanced fluorescence [85] | No data | Diffraction rings at 4.7 Å and 10 Å [85] | β-Sheet [85] | Fibers [85] | No data | No data | Aerial hyphae formation [85] | |||
| His-tagged RdlB recombinant | No data | No data | Enhanced fluorescence [86] | No data | Diffraction rings at 4.7 Å, 10.2 Å and 30.9 Å [86] | Random coil to β-sheet [86] | Twisted bundles of fibers [86] | No data | No data | Aerial hyphae formation [86] |
The reference indicated is the first identification of the protein.
Physiological function assigned.
Amyloid-like Curli Fibers of E. coli and Related Species
The foremost studied biofilm-associated functional amyloid-like fibers are the curli fibers from Enterobacteriaceae. Highly conserved between the Gram-negative E. coli and Salmonella species, curli extracellular fiber networks were first identified by transmission electron microscopy (TEM) and noted for their insolubility [47], [51]. The red, dry and rough (rdar) colony morphology observed on agar plates supplemented with Congo red dye is curli dependant in both species [87]. The red coloration provided a simple way of screening for mutations in associated genes, leading to the mapping of the large curli-related gene network [88]. Curli expression is dependent on the starvation response resulting from stratification of the biofilm and thus is more prominent where there are non-dividing cells furthest from nutrients (Fig. 2a) [89]. The cells in this zone become curliated and form a network of “cell-moulded baskets” throughout the intercellular space providing essential structure to the colony [89] (Fig. 2B). Production of curli is also critical for initial adhesion to both biotic and abiotic surfaces and is linked to environmental resistance and pathogenesis [90], [91], [92], [93]. Most recently, in addition to aiding cell adhesion, curli have been shown to both sequester and hinder diffusion of a predatory phage, preventing it from reaching the interior of the biofilm community [7] (Fig. 2c, d), directly demonstrating the protection that is provided to the cells residing in a biofilm. In addition to the curli, some strains of E. coli also (or only in some cases) produce the polysaccharide cellulose carrying a phosphoethanolamine modification [94], which together with the protein fibers produce a nanocomposite matrix material [95].
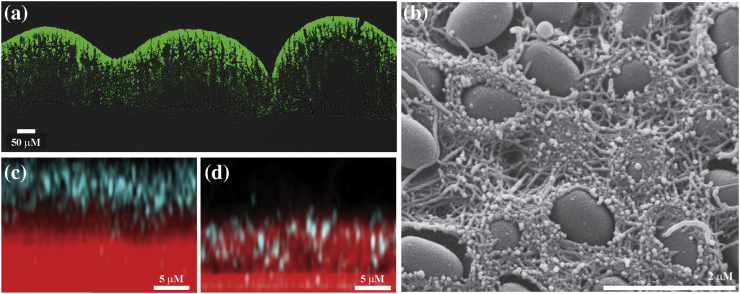
Fig. 2.
A structural and protective role for curli in the E. coli biofilm matrix. (a) The localization of the curli in a vertical cross section of a 7-day-old E. coli biofilm stained with thioflavin S (ThS). Merged bright-field and false-colored fluorescence for ThS. (b) Scanning electron micrograph in high vacuum mode of the top view of a 7-day-old K-12 W3110 cellulose-free E. coli biofilm. The cells are round and the curli baskets are visible. (c, d) Maximum intensity z-projections of phage localization (cyan) after 8-h exposure of 72 h E. coli wild-type (c) and curli-deficient (d) biofilm. Cells are in red. Parts a and b [89] and c and d [7] are reproduced with permission along with the corresponding legend.
Curli have a dedicated secretion system, chaperone proteins and inhibitors, all encoded by the csgBAC and csgDEFG operons, with regulation and polymerization being tightly controlled [96], [97]. CsgA is the major protein subunit of curli; the encoding gene csgA is co-transcribed with csgB, and both gene products are translocated across the inner membrane by the Sec pathway and outer membrane by the CsgGFE secretion complex [21], [98]. The current model proposed has CsgB embedded in the outer membrane where it acts as a nucleator for the polymerization of the CsgA unstructured monomers into the fiber (along with a minority of CsgB) [99], [100]. When extracted by sequential differential centrifugation, the native fibers were found to be insoluble in SDS and had binding affinity for the dyes Congo red and ThT [49]. Analysis of the curli fibers by CD revealed a single minimum at 218 nm, typical of β-sheet structure. In combination, this led to the categorization of curli as amyloid fibers [49]. Recombinant CsgA and CsgB spontaneously polymerize, and it is these in vitro fibers that have been used for structural characterization by solid-state NMR and X-ray diffraction [12], [53]. Structural analysis of recombinant CsgA fibers by solid state-NMR revealed interatomic distances consistent with a β-helix architecture, but the data are not yet sufficient to rule out other architectures [12]. X-ray fiber diffraction [50] suggests a structure consistent with a β-arrangement, but either the recombinant fibrils were not sufficiently aligned within the fiber to clearly demonstrate the cross-β architecture, or an alternative β-structure is adopted. The relevance of these studies to the in vivo environment is unclear as it is possible there are modifications to the atomic structure that are driven by the nucleating role of CsgB [50]. Despite in vivo production of curli being linked to both the rdar colony morphotype and adhesion of the biofilm to abiotic and biotic surfaces, there is currently no direct evidence for functional activity of the recombinant CsgA or CsgB fibers assembled in vitro. The closest available data for functionality of recombinant CsgA in vivo are the nucleation of purified CsgA monomers at the cell surface of a csgA mutant, likely to be mediated by CsgB at the membrane [52].
The Fap Fibers, an Emerging Story in Pseudomonas Species
Biofilm formation represents a survival strategy of Pseudomonas aeruginosa in the lung environment, as well as in wounds and on catheters [101]. The biofilm matrix components have been well characterized and include the capsule and aggregative polysaccharides, eDNA and appendages such as type IV pili and flagella (these matrix components have been reviewed extensively elsewhere [102]). In addition, there are the Fap fibers (that comprised the FapC subunit) that were initially identified in the Pseudomonas fluorescens biofilm matrix [55]. Evidence for the Fap fiber being functional comes from complementation of a ΔfapABCDEF strain with the complete operon encoded on a plasmid leading to increased aggregation compared with wild-type [103]. Moreover, recombinant expression of the fap operon is correlated with increased aggregation in P. aeruginosa, P. putida, P. fluorescens and E. coli [55], [57]. Native extracts of the fibers were described as having similar amyloid-like properties to the curli, displaying positive Congo red and ThT binding, coupled with insolubility in SDS and resistance to proteinase-K degradation [55]. Solution CD spectra collected from natively extracted Fap fibers reveals a 217-nm minima indicative of β-sheet secondary structure [55]. Similar characteristics were seen for recombinant FapC, and additional X-ray diffraction analysis of the fibers structure gave a range of reflections: 4.15, 4.34, 4.61, 6.32 and 11.53 Å [55]. The fibrils within the fiber employed for X-ray diffraction were not sufficiently aligned to provide definitive evidence for a cross-β arrangement.
Phenol-Soluble Modulins by Staphylococci—A “Cross-α” Helix
Staphylococcus epidermidis and Staphylococcus aureus are the predominant colonizers of medical implants in the hospital setting and are often identified as opportunistic wound pathogens [104]. When examining staphylococci biofilms, the matrix composition is diverse and can be dependent or independent of polysaccharide internal adhesins [104]. Polysaccharide internal adhesins-independent biofilms rely on proteinaceous components and are especially prevalent in S. epidermidis infections [105]. The phenol-soluble modulins (PSMα1–4, PSMβ1–2 and γ-toxin) were first identified as a complex secreted by S. epidermidis acting as cytotoxic virulence factors involved in cytotoxicity and immunomodulation [66]. Investigations of the role played by these inflammatory proteins during biofilm formation began when fibrous material extracted from S. aureus was identified to contain αPSM and γPSM protein by mass spectrometric analysis [67]. Subsequent structural analysis was performed on synthetic αPSM1–4, βPSM1–2 and γ-toxin, which spontaneously assembled [67]. The CD spectra collected from each of the synthetic PSM peptides displayed β-sheet secondary structural features [67]. A role for eDNA in triggering PSM fiber polymerization has been explored [106], but it is unclear if formation of these fibers is biologically relevant, and an alternative hypothesis has been postulated where the PSM form a protective barrier on the eDNA [68]. Evidence that the synthetic αPSM3 fibers are not amyloid-like fibers was derived from X-ray diffraction of a microcrystalline form, where a “cross-α” structure was revealed [14]. Despite adhering to a general patterning reminiscent of the cross-β hallmark, this protein has a very different structure and can be considered a novel fiber form in bacteria if it is found natively in the biofilm. Recent proteomic analysis of an S. aureus in vivo infection model did not identify any PSM in the implant-associated biofilm matrix [107], highlighting the variation that can be generated when comparing in vitro and in vivo models.
Non-amyloidogenic TasA Fibers Formed by Bacillus subtilis
Bacillus subtilis is a Gram-positive, spore-forming bacteria found ubiquitously in the soil and is a model organism for collective behaviors and differentiation [108], [109]. B. subtilis (strain NCIB3610) forms rugose biofilm colonies on agar and floating pellicles at air-liquid interfaces that are characterized by a dependency on matrix production [110]. The biofilm matrix contains a fiber-forming protein TasA that is essential for biofilm rugosity and is encoded as part of a three-gene operon: tapA–sipW–tasA [59], [111]. TapA has been reported as an accessory protein needed for TasA function, possibly as a nucleating species on the cell surface [112], and SipW is a dedicated signal peptidase, responsible for processing both the TapA and TasA proteins [58].
Structural and biochemical analysis of TasA has been performed using protein extracted from B. subtilis [59], [113] and with recombinant protein generated in E. coli [60]. Initial experiments were performed using TasA oligomers extracted from an exopolysaccharide negative, matrix-enriched B. subtilis strain [59], [113]. Here, solution CD spectra identified α-helical elements and a sensitivity to SDS depolymerization was observed [59], [113]. There was no red shift in Congo red absorbance, but the extracted protein did enhance the fluorescence of ThT [59]. Structural analysis of a truncated recombinant TasA monomer (amino acids 28–239 of the 261-amino-acid protein) by X-ray crystallography revealed that two antiparallel β-sheets are flanked by helices and loop regions (also known as a jellyroll conformation) [60]. Crucially, structural evidence of amyloid-like fibers is absent [59], [60] until the protein is exposed to acidic pH when the β-content increases [59], and cross-β X-ray diffraction patterns are recorded [60]. Although both natively extracted [59] and recombinant fibers [60] have been shown to be biologically active, the biological activity of the acid-treated form has not been shown. Finally, the requirement for TapA in the formation of TasA fibers that are functional in vivo has been refuted [114]. The significance of the mischaracterization of functional fibers as amyloid-like should not be understated, as TasA has been used in anti-amyloid drug screens [115], [116]. In toto, while the ability of TasA to form functional fibers associated with the biofilm matrix is not in doubt, these fibers are not amyloid-like in nature.
Mycobacterium tuberculosis Pili
Biofilm formation by mycobacteria has been suggested to play a role in the environmental persistence and pathogenesis of non-tuberculous Mycobacterium opportunistic pathogens [117]. Currently, there is only limited evidence linking biofilm formation by the human pathogen M. tuberculosis with pathogenesis [117], although biofilm formation is speculated to enhance extracellular survival and antibiotic resistance, especially within the inflammation-driven granuloma [118]. Investigations into the matrix composition of M. tuberculosis pellicles predominately identified free mycolic acids [119]. This is perhaps unsurprising as mycobacteria are characterized by a three-layer cell envelope; peptidoglycan is covalently linked to the inner plasma membrane and the outer arabinogalactan and mycolic acid, and surrounded by a free-form capsule [120]. It has been reported that M. tuberculosis forms curli-like M. tuberculosis pili (MTP) that also contribute to the biofilm matrix. MTP are insoluble, being found in the chloroform–methanol fraction during purification, with the monomer not detected using SDS-PAGE [61]. Positive Congo red binding to MTP pili was also reported [61]. In vivo α-MTP antibodies can be identified in sera collected from patients infected with M. tuberculosis [61], and deletion of mtp was reported to impact negatively on pellicle formation and biomass in vitro [63]. Although MTP insolubility and dye binding are compatible with the hypothesis of an amyloid-like fiber, further studies showed that the binding of Congo red to M. tuberculosis biofilms occurred in the absence of the mtp gene, and when screened by TEM, it was highlighted that there were not always obvious phenotypic differences between the wild-type and mtp mutants [62]. It is possible that some of the fibrous morphology initially identified [61] could be due to the capsule and/or matrix mycolic acids collapsing into rope-like bundles [121]. During in vivo infection models, the mtp mutant was neither sensitized to antibiotics nor attenuated for extracellular survival [62]. Thus, whether MTP are required for biofilm formation, and if they are amyloid-like fibers in form, requires further investigation.
Protease-Sensitive Fibers in Xanthomonas Species
Xanthomonas axonopodis pv. citri (hereafter X. citri) is a phytopathogen responsible for citrus canker [122], large necrotic lesions that develop on the leaves and fruits of citrus trees. This bacterium can develop structured biofilms in vitro and on the leaf surface [122] and switches from an epiphytic to a pathogenic lifestyle in a process dependent on the size of the colony on the plant surface [123]. Biofilm formation of X. citri is reliant on an active type III-secretion system [124], which injects effector proteins into the plant host. Among these proteins are the harpins, a heat stable family of proteins encoded by the hypersensitivity-response-and-pathogenicity (hrp) locus [73]. When introduced to the plant cytoplasm, harpins generally initiate an immune defence known as the hypersensitivity response (HR) that is similar to programmed cell death and designed to prevent further spread of the pathogen [73]. HpaG is a three-domain harpin protein with two α-helical domains linked by a putative prion domain that was identified in silico [74]. Recombinant poly-histidine tagged HpaG is biologically functional, in that it induces HR in non-host tobacco leaves, and when freshly purified, size exclusion chromatography indicates that the protein forms a tetramer. When this fraction is collected, TEM reveals ring-shaped structures and the CD spectrum is predominately α-helical [74]. Over the course of 10 days, the purified protein formed an obvious gel and TEM analysis shows the formation of fibers, 5–7 nm wide, with a 220-nm minima observed by CD [74]. These fibers bound Congo red but were sensitive to proteinase-K degradation, indicating that they are not amyloid-like [74], and although It was speculated that this capacity to form fibrous aggregates may be linked to HR induction, further investigation is required.
SDS-Sensitive Recombinant Fibers of S. aureus SuhB
The fibers observed to form during recombinant over-expression of the poly-histidine tagged S. aureus suhB gene (SaSuhB) in E. coli are large. With a 4-mm length, the macroscale assemblies are visible to the naked eye and can be separated from the cell culture with a 0.22-μM cheesecloth. The recombinant E. coli strain displays a red phenotype when grown on agar supplemented with Congo red, a phenotype that is not seen either when the empty vector or when the E. coli native suhB gene is used [120]. The authors further speculated that the protein is secreted by the curli-specific secretion system.
The recombinant fibers are sensitive to SDS degradation and appear to be extremely sticky, with E. coli cells remaining on the TEM grid even after extensive washing. When the polyhistidine-tagged SaSuhB is purified from crude cell lysate by Nickel-NTA affinity and subjected to size exclusion chromatography, there is a void volume peak that has been attributed to protofilaments and a peak corresponding to dimeric protein [70]. When the CD spectra of both species are compared, the protofilaments and dimers have mixed α-helical and random coil structural elements, with some β-sheet enrichment upon polymerization but no major conformational changes [70]. The secondary structure arrangement by X-ray diffraction pattern of the fibers exhibits three strong bands at 3.8, 4.7 and 10–11 Å [70]. In light of the solubility of the fibers, it seems unlikely that the protofilaments have a cross-β structure, and currently, there are no in vivo data available for the functionality.
Bap Fibers by S. aureus
The gene encoding the biofilm-associated protein (Bap) in S. aureus was first identified in a transposon mutagenesis screen looking for biofilm-attenuated mutants [125]. Bap was subsequently demonstrated to play a role in the colonization of surfaces in vitro and in an in vivo infection model [125]. The cell wall fraction of S. aureus, grown in the presence of glucose, was shown to contain SDS-insoluble aggregates which reacted with α-Bap antibodies [71]. Bap was categorized as an amyloid-like fiber based on the binding of Congo red and ThT dyes by a synthetic internal peptide of the protein (named rBap_B). Consistent with this designation, fibrous aggregates were observed by TEM, although these experiments were performed at a low pH. As S. aureus is capable of glucose fermentation, the authors suggested the accumulation of acidic by-products may lower the pH in a biologically relevant fashion. The rBap_B peptide was found to constitute the minimal functional unit that is capable of inducing cell clumping in a Δbap mutant at pH 4.5, with faint, needle-like fibers being visible in the extracellular space by TEM [71]. The role of Bap in vivo needs further study; for example, there was no difference in the adhesion capacity of wild-type and Δbap strains in an implanted catheter mouse model at 4 days but the absence of bap did reduce persistence over 10 days [71]. Further structural characterization is necessary to determine if these fibers contain the cross-β hallmark structure, and whether the low pH form is of biological relevance in clinical settings.
The Surface Adhesins of Streptococcus mutans
S. mutans has historically been considered responsible for dental caries, although recent data examining the oral microbiome have raised doubts of any single causative organism [126]. Within the polymicrobial oral biofilm, S. mutans is capable of producing an extracellular polysaccharide called glucan by metabolising sucrose, and utilizing it to attach to tooth enamel and other microorganisms [127], [128]. S. mutans also has cell surface expressed proteins that can mediate adhesion. The best-studied antigen is the P1 (or AgI/II) antigen, which binds to the glycoprotein salivary agglutinin secreted by the salivary gland [129]. Initial TEM imaging of immunogold-labeled α-P1 with S. mutans cells revealed a fibrillar “fuzzy” layer [64]. Subsequent characterization reveals a complex multidomain tertiary structure capable of aggregation [129], [130]. The crystal structure of the three N-terminal domains reveals an elongated 50-nm stalk on which a hypervariable head is extended with a C-terminal region anchoring to the cell [129], [130]. The S. mutans surface-expressed antigen P1, the antigen precursor protein WapA and the secreted SMC_63c protein have all been reported as forming amyloid fibers [48], [65]; however, their designation as amyloid fibers relies heavily on Congo red and ThT binding. In vitro analysis of recombinant P1 showed positive Congo red and ThT binding after a period of agitation [48], but positive Congo red binding is also shown for three wild-type strains isolated from dental caries as well as in a P1 deletion mutant [48]. This is perhaps unsurprising considering the dyes' non-specific binding to other polymers [131]. The P1, WapA and SMC_63c proteins are all sensitive to SDS depolymerization [48], [65], and no molecular structural information has been collected to suggest formation of the cross-β amyloid structure. CD spectra of the P1 C-terminal domain indicates it is predominately β-sheet [65], and SMC_63c undergoes a conformational change when exposed to pH 4 from an α-helical to predominately β-sheet composition [65]. As discussed previously, the conformational changes induced at this pH may not be biologically relevant particularly if low pH exposes regions of the protein that initiate misfolding into an amyloid cross-β architecture, although in the case of P1, WapA and SMC_63c proteins, there is currently no evidence of such tertiary structures.
Concluding Remarks
Since the identification of curli as amyloid-like fibers present in the biofilm matrix [49], it became common to compare proteinaceous fibers from other single species biofilms to the curli. However, as highlighted here, the biophysical characterization of a cross-β structure has not been directly linked to biological activity for many of the examples in the literature (Table 1). Moreover, secondary structure data (e.g., CD spectra) do not provide evidence of a cross-β arrangement, which requires either X-ray fiber diffraction performed on aligned fibrils or atomistic methods such as solid-state NMR. Furthermore, some of the fibers discussed here are unstable when exposed to SDS and/or proteinase-K [59], [61], [65], [70], [72], [74], which is suggestive of a non-amyloid fiber structure. Finally, if additional variables such as extremely low pH or high temperatures are introduced, these can induce misfolding into an amyloid-like fibril form, which may or may not have relevance in the native biofilm. Thus, an evaluation of structural evidence available for the fiber-forming proteins present in single species bacterial biofilms reveals that currently very few meet the criteria of possessing the cross-β hallmark structure of amyloid fibers. In the absence of this, it appears logical to conclude that many of the functional fibers found in the biofilm matrix adopt forms that may not be amyloid in nature. It is important to note that this does not alter their importance to biofilm formation or the interest in understanding the mode of formation. It simply highlights the great variety of mechanisms bacteria have evolved to form protein fibers to support life in a biofilm.
Acknowledgments
Work was supported by the Biotechnology and Biological Sciences Research Council (BB/P001335/1, BB/R012415/1, BB/N022254/1). EE is supported by the Wellcome Institutional Strategic Support Fund (Award No. 097818/Z/11).
Declarations of interest: None.
Edited by Antoine Loquet
Footnotes
We confirm that this work has not been published previously or under consideration somewhere else.
Contributor Information
Cait E. MacPhee, Email: cait.macphee@ed.ac.uk.
Nicola R. Stanley-Wall, Email: n.r.stanleywall@dundee.ac.uk.
References
- 1.Flemming H.-C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 2.Dragoš A., Kovács Á.T. The peculiar functions of the bacterial extracellular matrix. Trends Microbiol. 2017;25:257–266. doi: 10.1016/j.tim.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Costerton J.W., Cheng K.J., Geesey G.G., Ladd T.I., Nickel J.C., Dasgupta M., Marrie T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 4.Taglialegna A., Lasa I., Valle J. Amyloid Structures as Biofilm Matrix Scaffolds. J. Bacteriol. 2016;198:2579–2588. doi: 10.1128/JB.00122-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero D., Kolter R. Functional amyloids in bacteria. Int. Microbiol. 2014;17:65–73. doi: 10.2436/20.1501.01.208. [DOI] [PubMed] [Google Scholar]
- 6.Blanco L.P., Evans M.L., Smith D.R., Badtke M.P., Chapman M.R. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 2012;20:66–73. doi: 10.1016/j.tim.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidakovic L., Singh P.K., Hartmann R., Nadell C.D., Drescher K. Dynamic biofilm architecture confers individual and collective mechanisms of viral protection. Nat. Microbiol. 2018;3:26–31. doi: 10.1038/s41564-017-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiti F., Dobson C.M. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu. Rev. Biochem. 2017;86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- 9.Otzen D., Nielsen P.H. We find them here, we find them there: functional bacterial amyloid. Cell. Mol. Life Sci. 2008;65:910–927. doi: 10.1007/s00018-007-7404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson M.P., Hewitt E.W. Why are functional amyloids non-toxic in humans? Biomolecules. 2017;7 doi: 10.3390/biom7040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saupe S.J., Jarosz D.F., True H.L. The Fungal Kingdom. American Society of Microbiology; 2016. Amyloid prions in fungi; pp. 673–685. [Google Scholar]
- 12.Shewmaker F., McGlinchey R.P., Thurber K.R., McPhie P., Dyda F., Tycko R., Wickner R.B. The functional curli amyloid is not based on in-register parallel β-sheet structure. J. Biol. Chem. 2009;284:25065–25076. doi: 10.1074/jbc.M109.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian P., Boomsma W., Wang Y., Otzen D.E., Jensen M.H., Lindorff-Larsen K. Structure of a functional amyloid protein subunit computed using sequence variation. J. Am. Chem. Soc. 2015;137:22–25. doi: 10.1021/ja5093634. [DOI] [PubMed] [Google Scholar]
- 14.Tayeb-Fligelman E., Tabachnikov O., Moshe A., Goldshmidt-Tran O., Sawaya M.R., Coquelle N., Colletier J.-P., Landau M. The cytotoxic Staphylococcus aureus PSMα3 reveals a cross-α amyloid-like fibril. Science (80-. ) 2017;355:831–833. doi: 10.1126/science.aaf4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn S.E., Andrikopoulos S., Verchere C.B. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 16.Sipe J.D., Benson M.D., Buxbaum J.N., Ikeda S., Merlini G., Saraiva M.J.M., Westermark P. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23:209–213. doi: 10.1080/13506129.2016.1257986. [DOI] [PubMed] [Google Scholar]
- 17.Kyle R.A. Amyloidosis: a convoluted story. Br. J. Haematol. 2001;114:529–538. doi: 10.1046/j.1365-2141.2001.02999.x. [DOI] [PubMed] [Google Scholar]
- 18.Sipe J.D., Cohen A.S. Review: history of the amyloid fibril. J. Struct. Biol. 2000;130:88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 19.Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E.C. The characterization of soluble amyloid prepared in water. J. Clin. Invest. 1968;47:924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Channon K.J., Devlin G.L., Magennis S.W., Finlayson C.E., Tickler A.K., Silva C., MacPhee C.E. Modification of fluorophore photophysics through peptide-driven self-assembly. J. Am. Chem. Soc. 2008;130:5487–5491. doi: 10.1021/ja710310c. [DOI] [PubMed] [Google Scholar]
- 21.Goyal P., Krasteva P.V., Van Gerven N., Gubellini F., Van den Broeck I., Troupiotis-Tsaïlaki A., Jonckheere W., Péhau-Arnaudet G., Pinkner J.S., Chapman M.R., Hultgren S.J., Howorka S., Fronzes R., Remaut H. Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature. 2014;516:250–253. doi: 10.1038/nature13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt A., Annamalai K., Schmidt M., Grigorieff N., Fändrich M. Cryo-EM reveals the steric zipper structure of a light chain-derived amyloid fibril. Proc. Natl. Acad. Sci. U. S. A. 2016;113:6200–6205. doi: 10.1073/pnas.1522282113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howie A.J., Brewer D.B., Howell D., Jones A.P. Physical basis of colors seen in Congo red-stained amyloid in polarized light. Lab. Investig. 2008;88:232–242. doi: 10.1038/labinvest.3700714. [DOI] [PubMed] [Google Scholar]
- 24.Howie A.J., Brewer D.B. Optical properties of amyloid stained by Congo red: history and mechanisms. Micron. 2009;40:285–301. doi: 10.1016/j.micron.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Vassar P.S., Culling C.F. Fluorescent stains, with special reference to amyloid and connective tissues. Arch. Pathol. 1959;68:487–498. [PubMed] [Google Scholar]
- 26.Levine H. [18] Quantification of β-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 27.Hudson S.A., Ecroyd H., Kee T.W., Carver J.A. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J. 2009;276:5960–5972. doi: 10.1111/j.1742-4658.2009.07307.x. [DOI] [PubMed] [Google Scholar]
- 28.De Ferrari G.V., Mallender W.D., Inestrosa N.C., Rosenberry T.L. Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites. J. Biol. Chem. 2001;276:23282–23287. doi: 10.1074/jbc.M009596200. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto S., Arita-Morioka K.I., Mizunoe Y., Yamanaka K., Ogura T. Thioflavin T as a fluorescence probe for monitoring RNA metabolism at molecular and cellular levels. Nucleic Acids Res. 2015;43:1–12. doi: 10.1093/nar/gkv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amdursky N., Erez Y., Huppert D. Molecular rotors: what lies behind the high sensitivity of the thioflavin-T fluorescent marker. Acc. Chem. Res. 2012;45:1548–1557. doi: 10.1021/ar300053p. [DOI] [PubMed] [Google Scholar]
- 31.Stsiapura V.I., Maskevich A.A., Kuzmitsky V.A., Uversky V.N., Kuznetsova I.M., Turoverov K.K. Thioflavin T as a molecular rotor: fluorescent properties of thioflavin T in solvents with different viscosity. J. Phys. Chem. B. 2008;112:15893–15902. doi: 10.1021/jp805822c. [DOI] [PubMed] [Google Scholar]
- 32.O'Nuallain B., Wetzel R. Conformational Abs recognizing a generic amyloid fibril epitope. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1485–1490. doi: 10.1073/pnas.022662599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kayed R., Head E., Sarsoza F., Saing T., Cotman C.W., Necula M., Margol L., Wu J., Breydo L., Thompson J.L., Rasool S., Gurlo T., Butler P., Glabe C.G. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol. Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly S.M., Jess T.J., Price N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta Protein Proteomics. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Micsonai A., Wien F., Kernya L., Lee Y.-H., Goto Y., Réfrégiers M., Kardos J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E3095–E3103. doi: 10.1073/pnas.1500851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makin O.S., Serpell L.C. Amyloid Proteins. Humana Press; New Jersey: 2005. X-ray diffraction studies of amyloid structure; pp. 067–080. [Google Scholar]
- 37.Greenwald J., Riek R. Biology of amyloid: structure, function, and regulation. Structure. 2010;18:1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Fitzpatrick A.W.P., Debelouchina G.T., Bayro M.J., Clare D.K., Caporini M.A., Bajaj V.S., Jaroniec C.P., Wang L., Ladizhansky V., Müller S.A., MacPhee C.E., Waudby C.A., Mott H.R., De Simone A., Knowles T.P.J., Saibil H.R., Vendruscolo M., Orlova E.V., Griffin R.G., Dobson C.M. Atomic structure and hierarchical assembly of a cross-β amyloid fibril. Proc. Natl. Acad. Sci. U. S. A. 2013;110:5468–5473. doi: 10.1073/pnas.1219476110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzpatrick A.W.P., Falcon B., He S., Murzin A.G., Murshudov G., Garringer H.J., Crowther R.A., Ghetti B., Goedert M., Scheres S.H.W. Cryo-EM structures of tau filaments from Alzheimer's disease. Nature. 2017;547:185–190. doi: 10.1038/nature23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasmer C., Lange A., Van Melckebeke H., Siemer A.B., Riek R., Meier B.H. Amyloid fibrils of the HET-s(218–289) prion form a solenoid with a triangular hydrophobic core. Science (80-. ) 2008;319:1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 41.van der Wel P.C.A., Lewandowski J.R., Griffin R.G. Structural characterization of GNNQQNY amyloid fibrils by magic angle spinning NMR. Biochemistry. 2010;49:9457–9469. doi: 10.1021/bi100077x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langkilde A.E., Morris K.L., Serpell L.C., Svergun D.I., Vestergaard B. The architecture of amyloid-like peptide fibrils revealed by X-ray scattering, diffraction and electron microscopy. Acta Crystallogr. D Biol. Crystallogr. 2015;71:882–895. doi: 10.1107/S1399004715001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frederick K.K., Michaelis V.K., Caporini M.A., Andreas L.B., Debelouchina G.T., Griffin R.G., Lindquist S. Combining DNP NMR with segmental and specific labeling to study a yeast prion protein strain that is not parallel in-register. Proc. Natl. Acad. Sci. U. S. A. 2017;114:3642–3647. doi: 10.1073/pnas.1619051114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.C.E. MacPhee, C.M. Dobson, Formation of mixed fibrils demonstrates the generic nature and potential utility of amyloid nanostructures, (2000).
- 45.Baldwin A.J., Knowles T.P.J., Tartaglia G.G., Fitzpatrick A.W., Devlin G.L., Shammas S.L., Waudby C.A., Mossuto M.F., Meehan S., Gras S.L., Christodoulou J., Anthony-Cahill S.J., Barker P.D., Vendruscolo M., Dobson C.M. Metastability of native proteins and the phenomenon of amyloid formation. J. Am. Chem. Soc. 2011;133:14160–14163. doi: 10.1021/ja2017703. [DOI] [PubMed] [Google Scholar]
- 46.Larsen P., Nielsen J.L., Otzen D., Nielsen P.H. Amyloid-like adhesins produced by floc-forming and filamentous bacteria in activated sludge. Appl. Environ. Microbiol. 2008;74:1517–1526. doi: 10.1128/AEM.02274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsén A., Jonsson A., Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989;338:652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- 48.Oli M.W., Otoo H.N., Crowley P.J., Heim K.P., Nascimento M.M., Ramsook C.B., Lipke P.N., Brady L.J. Functional amyloid formation by Streptococcus mutans. Microbiology. 2012;158:2903–2916. doi: 10.1099/mic.0.060855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chapman M.R., Robinson L.S., Pinkner J.S., Roth R., Heuser J., Hammar M., Normark S., Hultgren S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dueholm M.S., Nielsen S.B., Hein K.L., Nissen P., Chapman M., Christiansen G., Nielsen P.H., Otzen D.E. Fibrillation of the major curli subunit CsgA under a wide range of conditions implies a robust design of aggregation. Biochemistry. 2011;50:8281–8290. doi: 10.1021/bi200967c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collinson S.K., Emödy L., Müller K.H., Trust T.J., Kay W.W. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J. Bacteriol. 1991;173:4773–4781. doi: 10.1128/jb.173.15.4773-4781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X., Hammer N.D., Chapman M.R. The molecular basis of functional bacterial amyloid polymerization and nucleation. J. Biol. Chem. 2008;283:21530–21539. doi: 10.1074/jbc.M800466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X., Smith D.R., Jones J.W., Chapman M.R. In vitro polymerization of a functional Escherichia coli amyloid protein. J. Biol. Chem. 2007;282:3713–3719. doi: 10.1074/jbc.M609228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammer N.D., Schmidt J.C., Chapman M.R. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12494–12499. doi: 10.1073/pnas.0703310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dueholm M.S., Petersen S.V., Sønderkaer M., Larsen P., Christiansen G., Hein K.L., Enghild J.J., Nielsen J.L., Nielsen K.L., Nielsen P.H., Otzen D.E. Functional amyloid in Pseudomonas. Mol. Microbiol. 2010;77 doi: 10.1111/j.1365-2958.2010.07269.x. (no-no) [DOI] [PubMed] [Google Scholar]
- 56.Larsen P., Nielsen J.L., Dueholm M.S., Wetzel R., Otzen D., Nielsen P.H. Amyloid adhesins are abundant in natural biofilms. Environ. Microbiol. 2007;9:3077–3090. doi: 10.1111/j.1462-2920.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- 57.Dueholm M.S., Søndergaard M.T., Nilsson M., Christiansen G., Stensballe A., Overgaard M.T., Givskov M., Tolker-Nielsen T., Otzen D.E., Nielsen P.H. Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. MicrobiologyOpen. 2013;2:365–382. doi: 10.1002/mbo3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stöver A.G., Driks A. Secretion, localization, and antibacterial activity of TasA, a Bacillus subtilis spore-associated protein. J. Bacteriol. 1999;181:1664–1672. doi: 10.1128/jb.181.5.1664-1672.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero D., Aguilar C., Losick R., Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diehl A., Roske Y., Ball L., Chowdhury A., Hiller M., Molière N., Kramer R., Stöppler D., Worth C.L., Schlegel B., Leidert M., Cremer N., Erdmann N., Lopez D., Stephanowitz H., Krause E., van Rossum B.-J., Schmieder P., Heinemann U., Turgay K., Akbey Ü., Oschkinat H. Structural changes of TasA in biofilm formation of Bacillus subtilis. Proc. Natl. Acad. Sci. March 27, 2018;115(13):3237–3242. doi: 10.1073/pnas.1718102115. (201718102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alteri C.J., Xicohténcatl-Cortes J., Hess S., Caballero-Olín G., Girón J.A., Friedman R.L. Mycobacterium tuberculosis produces pili during human infection. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5145–5150. doi: 10.1073/pnas.0602304104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mann K.M., Pride A.C., Flentie K., Kimmey J.M., Weiss L.A., Stallings C.L. Analysis of the contribution of MTP and the predicted Flp pilus genes to Mycobacterium tuberculosis pathogenesis. Microbiology. 2016;162:1784–1796. doi: 10.1099/mic.0.000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramsugit S., Guma S., Pillay B., Jain P., Larsen M.H., Danaviah S., Pillay M. Pili contribute to biofilm formation in vitro in Mycobacterium tuberculosis. Antonie Van Leeuwenhoek. 2013;104:725–735. doi: 10.1007/s10482-013-9981-6. [DOI] [PubMed] [Google Scholar]
- 64.Ayakawa G.Y., Boushell L.W., Crowley P.J., Erdos G.W., McArthur W.P., Bleiweis A.S. Isolation and characterization of monoclonal antibodies specific for antigen P1, a major surface protein of mutans streptococci. Infect. Immun. 1987;55:2759–2767. doi: 10.1128/iai.55.11.2759-2767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Besingi R.N., Wenderska I.B., Senadheera D.B., Cvitkovitch D.G., Long J.R., Wen Z.T., Brady L.J. Functional amyloids in Streptococcus mutans, their use as targets of biofilm inhibition and initial characterization of SMU_63c. Microbiology (U. K.) 2017;163:488–501. doi: 10.1099/mic.0.000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehlin C., Headley C.M., Klebanoff S.J. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J. Exp. Med. 1999;189 doi: 10.1084/jem.189.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwartz K., Syed A.K., Stephenson R.E., Rickard A.H., Boles B.R. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng Y., Joo H.-S., Nair V., Le K.Y., Otto M. Do amyloid structures formed by Staphylococcus aureus phenol-soluble modulins have a biological function? Int. J. Med. Microbiol. 2018 Aug;308(6):675–682. doi: 10.1016/j.ijmm.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boles B.R., Thoendel M., Roth A.J., Horswill A.R. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dutta A., Bhattacharyya S., Kundu A., Dutta D., Das A.K. Macroscopic amyloid fiber formation by staphylococcal biofilm associated SuhB protein. Biophys. Chem. 2016;217:32–41. doi: 10.1016/j.bpc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 71.Taglialegna A., Navarro S., Ventura S., Garnett J.A., Matthews S., Penades J.R., Lasa I., Valle J. Staphylococcal Bap proteins build amyloid scaffold biofilm matrices in response to environmental signals. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.López-Ochoa J., Montes-García J.F., Vázquez C., Sánchez-Alonso P., Pérez-Márquez V.M., Blackall P.J., Vaca S., Negrete-Abascal E. Gallibacterium elongation factor-Tu possesses amyloid-like protein characteristics, participates in cell adhesion, and is present in biofilms. J. Microbiol. 2017;55:745–752. doi: 10.1007/s12275-017-7077-0. [DOI] [PubMed] [Google Scholar]
- 73.Kim J.-G., Park B.K., Yoo C.-H., Jeon E., Oh J., Hwang I. Characterization of the Xanthomonas axonopodis pv. glycines Hrp pathogenicity island. J. Bacteriol. 2003;185:3155–3166. doi: 10.1128/JB.185.10.3155-3166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oh J., Kim J.-G., Jeon E., Yoo C.-H., Moon J.S., Rhee S., Hwang I. Amyloidogenesis of type III-dependent harpins from plant pathogenic bacteria. J. Biol. Chem. 2007;282:13601–13609. doi: 10.1074/jbc.M602576200. [DOI] [PubMed] [Google Scholar]
- 75.Peterson C.P., Sauer C., Chatfield C.H. The extracellular polymeric substances of Legionella pneumophila biofilms contain amyloid structures. Curr. Microbiol. 2018 Jun;75(6):736–744. doi: 10.1007/s00284-018-1440-1. [DOI] [PubMed] [Google Scholar]
- 76.Markande A.R., Nerurkar A.S. Bioemulsifier (BE-AM1) produced by Solibacillus silvestris AM1 is a functional amyloid that modulates bacterial cell-surface properties. Biofouling. 2016;32:1153–1162. doi: 10.1080/08927014.2016.1232716. [DOI] [PubMed] [Google Scholar]
- 77.de Lorenzo V. Isolation and characterization of microcin E492 from Klebsiella pneumoniae. Arch. Microbiol. 1984;139:72–75. doi: 10.1007/BF00692715. [DOI] [PubMed] [Google Scholar]
- 78.Bieler S., Estrada L., Lagos R., Baeza M., Castilla J., Soto C. Amyloid formation modulates the biological activity of a bacterial protein. J. Biol. Chem. 2005;280:26880–26885. doi: 10.1074/jbc.M502031200. [DOI] [PubMed] [Google Scholar]
- 79.Shahnawaz M., Park K.-W., Mukherjee A., Diaz-Espinoza R., Soto C. Prion-like characteristics of the bacterial protein Microcin E492. Sci. Rep. 2017;7:45720. doi: 10.1038/srep45720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arranz R., Mercado G., Martín-Benito J., Giraldo R., Monasterio O., Lagos R., Valpuesta J.M. Structural characterization of microcin E492 amyloid formation: identification of the precursors. J. Struct. Biol. 2012;178:54–60. doi: 10.1016/j.jsb.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 81.Claessen D., Wösten H.A.B., van Keulen G., Faber O.G., Alves A.M.C.R., Meijer W.G., Dijkhuizen L. Two novel homologous proteins of Streptomyces coelicolor and Streptomyces lividans are involved in the formation of the rodlet layer and mediate attachment to a hydrophobic surface. Mol. Microbiol. 2002;44:1483–1492. doi: 10.1046/j.1365-2958.2002.02980.x. [DOI] [PubMed] [Google Scholar]
- 82.Elliot M.A., Karoonuthaisiri N., Huang J., Bibb M.J., Cohen S.N., Kao C.M., Buttner M.J. The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev. 2003;17:1727–1740. doi: 10.1101/gad.264403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wildermuth H., Wehrli E., Horne R.W. The surface structure of spores and aerial mycelium in Streptomyces coelicolor. J. Ultrastruct. Res. 1971;35:168–180. doi: 10.1016/s0022-5320(71)80149-1. [DOI] [PubMed] [Google Scholar]
- 84.Claessen D., Rink R., de Jong W., Siebring J., de Vreugd P., Boersma F.G.H., Dijkhuizen L., Wosten H.A.B. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev. 2003;17:1714–1726. doi: 10.1101/gad.264303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sawyer E.B., Claessen D., Haas M., Hurgobin B., Gras S.L. The assembly of individual chaplin peptides from Streptomyces coelicolor into functional amyloid fibrils. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang W., Willemse J., Sawyer E.B., Lou F., Gong W., Zhang H., Gras S.L., Claessen D., Perrett S. The propensity of the bacterial rodlin protein RdlB to form amyloid fibrils determines its function in Streptomyces coelicolor. Sci. Rep. 2017;7:42867. doi: 10.1038/srep42867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Römling U. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell. Mol. Life Sci. 2005;6205:1234–1246. doi: 10.1007/s00018-005-4557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith D.R., Price J.E., Burby P.E., Blanco L.P., Chamberlain J., Chapman M.R. The production of curli amyloid fibers is deeply integrated into the biology of Escherichia coli. Biomolecules. 2017;7 doi: 10.3390/biom7040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Serra D.O., Richter A.M., Klauck G., Mika F., Hengge R. Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. MBio. 2013;4:e00103–e00113. doi: 10.1128/mBio.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vidal O., Longin R., Prigent-Combaret C., Dorel C., Hooreman M., Lejeune P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 1998;180:2442–2449. doi: 10.1128/jb.180.9.2442-2449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gophna U., Barlev M., Seijffers R., Oelschlager T.A., Hacker J., Ron E.Z. Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect. Immun. 2001;69:2659–2665. doi: 10.1128/IAI.69.4.2659-2665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ryu J.-H., Kim H., Frank J.F., Beuchat L.R. Attachment and biofilm formation on stainless steel by Escherichia coli O157:H7 as affected by curli production. Lett. Appl. Microbiol. 2004;39:359–362. doi: 10.1111/j.1472-765X.2004.01591.x. [DOI] [PubMed] [Google Scholar]
- 93.Uhlich G.A., Cooke P.H., Solomon E.B. Analyses of the red-dry-rough phenotype of an Escherichia coli O157:H7 strain and its role in biofilm formation and resistance to antibacterial agents. Appl. Environ. Microbiol. 2006;72:2564–2572. doi: 10.1128/AEM.72.4.2564-2572.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thongsomboon W., Serra D.O., Possling A., Hadjineophytou C., Hengge R., Cegelski L. Phosphoethanolamine cellulose: a naturally produced chemically modified cellulose. Science. 2018;359:334–338. doi: 10.1126/science.aao4096. [DOI] [PubMed] [Google Scholar]
- 95.Serra D.O., Richter A.M., Hengge R. Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J. Bacteriol. 2013;195:5540–5554. doi: 10.1128/JB.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Evans M.L., Chapman M.R. Curli biogenesis: order out of disorder. Biochim. Biophys. Acta. 2014;1843:1551–1558. doi: 10.1016/j.bbamcr.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hobley L., Harkins C., MacPhee C.E., Stanley-Wall N.R. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015 Sep;39(5):649–669. doi: 10.1093/femsre/fuv015. fuv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nenninger A.A., Robinson L.S., Hultgren S.J. Localized and efficient curli nucleation requires the chaperone-like amyloid assembly protein CsgF. Proc. Natl. Acad. Sci. U. S. A. 2009;106:900–905. doi: 10.1073/pnas.0812143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Gerven N., Klein R.D., Hultgren S.J., Remaut H. Bacterial amyloid formation: structural insights into curli biogenesis. Trends Microbiol. 2015;23:693–706. doi: 10.1016/j.tim.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barnhart M.M., Chapman M.R. Curli biogenesis and function. Annu. Rev. Microbiol. 2006;60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moradali M.F., Ghods S., Rehm B.H.A. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017;7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Skariyachan S., Sridhar V.S., Packirisamy S., Kumargowda S.T., Challapilli S.B. Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal. Folia Microbiol. (Praha) 2018:1–20. doi: 10.1007/s12223-018-0585-4. [DOI] [PubMed] [Google Scholar]
- 103.Zeng G., Vad B.S., Dueholm M.S., Christiansen G., Nilsson M., Tolker-Nielsen T., Nielsen P.H., Meyer R.L., Otzen D.E. Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front. Microbiol. 2015;6:1099. doi: 10.3389/fmicb.2015.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otto M. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013;64:175–188. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- 105.Rohde H., Burandt E.C., Siemssen N., Frommelt L., Burdelski C., Wurster S., Scherpe S., Davies A.P., Harris L.G., Horstkotte M.A., Knobloch J.K.-M., Ragunath C., Kaplan J.B., Mack D. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials. 2007;28:1711–1720. doi: 10.1016/j.biomaterials.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 106.Schwartz K., Ganesan M., Payne D.E., Solomon M.J., Boles B.R. Extracellular DNA facilitates the formation of functional amyloids in Staphylococcus aureus biofilms. Mol. Microbiol. 2016;99:123–134. doi: 10.1111/mmi.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lei M.G., Gupta R.K., Lee C.Y. Proteomics of Staphylococcus aureus biofilm matrix in a rat model of orthopedic implant-associated infection. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lopez D., Vlamakis H., Kolter R. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol. Rev. 2009;33:152–163. doi: 10.1111/j.1574-6976.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 109.Vlamakis H., Chai Y., Beauregard P., Losick R., Kolter R. Sticking together: building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013;11:157–168. doi: 10.1038/nrmicro2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Branda S.S., González-Pastor J.E., Ben-Yehuda S., Losick R., Kolter R. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Branda S.S., Chu F., Kearns D.B., Losick R., Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 112.Romero D., Vlamakis H., Losick R., Kolter R. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol. Microbiol. 2011;80:1155–1168. doi: 10.1111/j.1365-2958.2011.07653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chai L., Romero D., Kayatekin C., Akabayov B., Vlamakis H., Losick R., Kolter R. Isolation, characterization, and aggregation of a structured bacterial matrix precursor. J. Biol. Chem. 2013;288:17559–17568. doi: 10.1074/jbc.M113.453605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Erskine E., Morris R.J., Schor M., Earl C., Gillespie R.M.C., Bromley K., Sukhodub T., Clark L., Fyfe P.K., Serpell L.C., Stanley-Wall N.R., MacPhee C.E. Formation of functional, non-amyloidogenic fibres by recombinant Bacillus subtilis TasA. Mol. Microbiol. 2018 doi: 10.1111/mmi.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Romero D., Sanabria-Valentín E., Vlamakis H., Kolter R. Biofilm inhibitors that target amyloid proteins. Chem. Biol. 2013;20:102–110. doi: 10.1016/j.chembiol.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jain N., Ådén J., Nagamatsu K., Evans M.L., Li X., McMichael B., Ivanova M.I., Almqvist F., Buxbaum J.N., Chapman M.R. Inhibition of curli assembly and Escherichia coli biofilm formation by the human systemic amyloid precursor transthyretin. Proc. Natl. Acad. Sci. 2017;114:12184–12189. doi: 10.1073/pnas.1708805114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Esteban J., García-Coca M. Mycobacterium biofilms. Front. Microbiol. 2017;8:2651. doi: 10.3389/fmicb.2017.02651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Basaraba R.J., Ojha A.K. Tuberc. Tuber. Bacillus. Second ed. American Society of Microbiology; 2017. Mycobacterial biofilms: revisiting tuberculosis bacilli in extracellular necrotizing lesions; pp. 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ojha A.K., Baughn A.D., Sambandan D., Hsu T., Trivelli X., Guerardel Y., Alahari A., Kremer L., Jacobs W.R., Hatfull G.F., Hatfull G.F. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 2008;69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Crick D.C., Jackson M., Daffé M. Genetics of capsular polysaccharides and cell envelope (glyco)lipids. Microbiol. Spectr. 2014;2 doi: 10.1128/microbiolspec.MGM2-0021-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bayer M.E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J. Bacteriol. 1977;130:911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rigano L.A., Siciliano F., Enrique R., Sendín L., Filippone P., Torres P.S., Qüesta J., Dow J.M., Castagnaro A.P., Vojnov A.A., Marano M.R. Biofilm formation, epiphytic fitness, and canker development in Xanthomonas axonopodis pv. citri. Mol. Plant Microbe Interact. MPMI. 2007;201094:1222–1230. doi: 10.1094/MPMI-20-10-1222. [DOI] [PubMed] [Google Scholar]
- 123.Hirano S.S., Upper C.D. Ecology and epidemiology of foliar bacterial plant pathogens. Annu. Rev. Phytopathol. 1983;21:243–270. [Google Scholar]
- 124.Zimaro T., Thomas L., Marondedze C., Sgro G.G., Garofalo C.G., Ficarra F.A., Gehring C., Ottado J., Gottig N. The type III protein secretion system contributes to Xanthomonas citri subsp. citri biofilm formation. BMC Microbiol. 2014;14:96. doi: 10.1186/1471-2180-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cucarella C., Solano C., Valle J., Amorena B., Lasa I., Penadés J.R. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 2001;183:2888–2896. doi: 10.1128/JB.183.9.2888-2896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Costalonga M., Herzberg M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014;162:22–38. doi: 10.1016/j.imlet.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xiao J., Klein M.I., Falsetta M.L., Lu B., Delahunty C.M., Yates J.R., Heydorn A., Koo H. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bowen W.H., Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Larson M.R., Rajashankar K.R., Patel M.H., Robinette R.A., Crowley P.J., Michalek S., Brady L.J., Deivanayagam C. Elongated fibrillar structure of a streptococcal adhesin assembled by the high-affinity association of alpha- and PPII-helices. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5983–5988. doi: 10.1073/pnas.0912293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Larson M.R., Rajashankar K.R., Crowley P.J., Kelly C., Mitchell T.J., Brady L.J., Deivanayagam C. Crystal structure of the C-terminal region of Streptococcus mutans antigen I/II and characterization of salivary agglutinin adherence domains. J. Biol. Chem. 2011;286:21657–21666. doi: 10.1074/jbc.M111.231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bély M. Sensitivity and specificity of Congo red staining according to Romhányi. Comparison with Puchtler's or Bennhold's methods. Acta Histochem. 2006;108:175–180. doi: 10.1016/j.acthis.2006.03.017. [DOI] [PubMed] [Google Scholar]