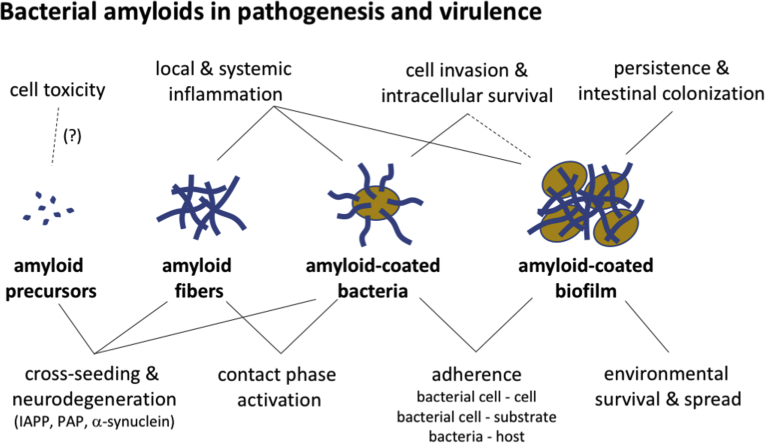
Abstract
Amyloid fibrils are best known as a product of human and animal protein misfolding disorders, where amyloid formation is associated with cytotoxicity and disease. It is now evident that for some proteins, the amyloid state constitutes the native structure and serves a functional role. These functional amyloids are proving widespread in bacteria and fungi, fulfilling diverse functions as structural components in biofilms or spore coats, as toxins and surface-active fibers, as epigenetic material, peptide reservoirs or adhesins mediating binding to and internalization into host cells. In this review, we will focus on the role of functional amyloids in bacterial pathogenesis. The role of functional amyloids as virulence factor is diverse but mostly indirect. Nevertheless, functional amyloid pathways deserve consideration for the acute and long-term effects of the infectious disease process and may form valid antimicrobial targets.
Keywords: biofilm, bacterial adhesion, bacterial pathogenesis, curli, amyloid
Abbreviations: MTP, Mycobacterium tuberculosis curli-like pili; FTIR, Fourier-transform infrared spectroscopy; ThT, Thioflavin T; ECM, extracellular matrix; QS, quorum-sensing; UPEC, uropathogenic E. coli; TLR, Toll-like receptor; PMN, polymorphonuclear neutrophil; WapA, wall-associated protein A; PSMs, phenol-specific modulins; TSEs, transmissible spongiform encephalopathies; EGCG, epigallocatechin-3-gallate; Aβ, amyloid beta; CR, Congo red; CD, circular dichroism; AFM, atomic force microscopy; XRD, X-ray diffraction; EM, electron microscopy
Graphical abstract
Highlights
-
•
Functional amyloids are widespread in bacteria, pathogenic and non-pathogenic.
-
•
Bacterial biofilms most commonly function as structural support in the extracellular matrix of biofilms or spore coats, and in cell–cell and cell-surface adherence.
-
•
The amyloid state can be the sole structured and functional state, or can be facultative, as a secondary state to folded monomeric subunits.
-
•
Bacterial amyloids can enhance virulence by increasing persistence, cell adherence and invasion, intracellular survival, and pathogen spread by increased environmental survival.
-
•
Bacterial amyloids may indirectly inflict disease by triggering inflammation, contact phase activation and possibly induce or aggravate human pathological aggregation disorders.
Introduction
Several human and animal degenerative disorders are associated with the accumulation of fibrous deposits known as amyloids. The term amyloid was originally coined by Rudolph Virchow in the mid 19th century to denote iodine-staining tissue abnormalities seen in histology, initially mistaken as starch (amylum) deposits [1]. A vast body of research has since established that amyloid deposits consist of non-covalent protein and peptide polymers and that the amyloid conformation can be regarded as a separate folding class based on a number of common structural and biophysical properties [2]. In these disease-associated deposits, the amyloid state represents an aberrant, non-native fold of the composing proteins or peptides. Importantly, for reasons that are often not understood at the molecular level, the accumulation, or the process leading to the formation of the amyloid deposits is associated with gain-of-function cell toxicity and tissue damage [3]. In the last two decades, however, it has become apparent that the amyloid fold is not restricted to protein aggregation disorders, but can also be found as a functional state in a number of fibrous structures produced by bacteria and fungi, as well as higher eukaryotes [4]. In these “functional amyloids,” the amyloid conformation can comprise the primary native state of the proteins, or the implicated proteins can adopt a globular folded as well as amyloid state depending on environmental conditions or post-translational processing, with both states thought to have biological function. In bacteria, amyloids of different origin have been found in major phyla such as Proteobacteria, Actinobacteria and Firmicutes, and several more are likely undiscovered [5], [6], [7]. The first and best-described bacterial functional amyloid is curli, discovered in S. Typhimurium and described from an Escherichia coli cow mastitis infection [8], [9]. Several more distinct bacterial amyloids have since been described, often discovered in the context of human and animal pathogens such as pathogenic gammaproteobacteria (Escherichia, Shigella, Salmonella, etc.), Pseudomonads, Mycobacterium, Streptococcus, Staphylococcus and Bacillus [8], [10], [11], [12], [13], [14], [15], [16]. It should be noted, however, that the discovery rate of new bacterial functional amyloids in pathogenic versus environmental genera is likely skewed by the biased research efforts on the former. For example, phylogenetic analysis of curli has indeed shown this bacterial amyloid is widespread in environmental Proteobacteria and Bacteriodetes [7]. Others, including Mycobacterium tuberculosis curli-like pili (MTP), are more restricted to pathogenic species and strains [11]. In any case, the occurrence of functional amyloids in pathogenic bacteria and the potential of the amyloid fold or formation process to inflict cell and tissue damage bring the question of their possible contribution to bacterial virulence and in infectious disease. Here we review known bacterial amyloids, their biosynthetic pathways and their roles in bacterial physiology and pathogenesis in particular. Known bacterial amyloids are found predominantly in the context of bacterial biofilm formation, and for most, the contribution to pathogenesis appears indirect. Nevertheless, the presence of bacterial amyloids can be advantageous to the colonization, persistence and spread of bacterial pathogens, and as such can represent a virulence factor. Equally important are the observations that the presence of bacterial amyloids can trigger acute and longer term host responses or may set off protein aggregation disorders that lead to tissue damage and disease secondary to the bacterial infection. We conclude our review with a summary of the ongoing efforts and approaches for the inhibition of bacterial amyloid formation as a component in fighting infectious disease.
Functional Amyloids, What's in a Name?
Amyloid is a laden term and the field tends to adopt a rather fluid definition. Not all “functional amyloids” proposed in the literature and covered in this review are necessarily experimentally confirmed to be amyloid or to adhere to the structural and self-propagating characteristics of amyloids sensu strictu, so that some systems may better be dubbed amyloid-like. For clarity, we briefly try to define functional amyloids in a biophysical and structural way, highlighting the techniques one can use to identify protein deposits as amyloid. Amyloids are highly ordered protein aggregates with an unbranched filamentous morphology, typically featuring a helical array of β-strands packed orthogonal to the fiber long axis [2]. This cross-β architecture gives rise to characteristic fiber X-ray diffraction patterns with reflection maxima at ~ 4.8 and ~ 10 Å corresponding to inter strand and inter sheet distances, respectively. This characteristic cross-β pattern can also be identified through solid-state NMR, Fourier-transform infrared spectroscopy (FTIR) and electron paramagnetic resonance profiles [17]. The amyloid fibrils usually feature a “steric zipper” of stacked and interdigitating side chains that stabilize and drive oligomerization [18], [19], [20]. Because of this highly ordered and tightly packed form, amyloid fibers show high resistance to proteolysis and chemical and thermal denaturation [21], [22]. The amyloid cross-β architecture also results in indicative tinctorial properties based on the binding and ordering of the dyes Congo red and thioflavin T (ThT) on the cross-β backbone, resulting in a red shift and birefringence, or increased fluorescence efficiency, respectively [23]. However, care should be taken not to over interpret these indicators [24] as Congo red also binds biopolymers such as cellulose [25] and partially folded proteins [26], while ThT can recognize non-amyloid ordered protein folds and protein gels. Conversely, lack of CR or ThT binding does not rule out a possible amyloid nature of an aggregate [23], [24]. Another characteristic feature of amyloids is that fiber growth is a nucleation-dependent self-assembly and structure propagation process, where a seed, defined as a fiber fragment or a small amyloid-like nucleus, provides a conformational scaffold that facilitates rapid quasi-irreversible conformational transition of the composing protein subunits or peptides into the amyloid state as they are built into the fiber [27]. This nucleation-dependent self-assembly gives rise to characteristic sigmoidal growth curves when a protein or peptide's amyloid formation is followed spectroscopically over time (e.g., by ThT fluorescence, circular dichroism or dynamic light scattering). The initial or so-called “lag phase” in these growth curves corresponds to the build-up of nuclei in size and number to an aggregate concentration that results in the exponential consumption of free subunits [28]. The molecular pathways and species adopted during the amyloid formation process are of interest because these are the stages most strongly associated with cell toxicity seen in pathological amyloids [3]. For functional amyloids, where the amyloid state and the amyloid formation processes are physiologically functional traits optimized by natural selection, it is often still unclear whether similarly toxic species are formed during the amyloidogenesis process. In the following paragraphs, we review the role of bacterial functional amyloids as a potential virulence factor in host–pathogen interaction, whether this comes from off- or on-pathway toxic properties of the amyloid formation process, or as a direct or indirect trait from the amyloid fibers.
Bacterial Amyloids and Biofilm
Probably the most prevalent occurrence of bacterial functional amyloids is in the context of community behavior and biofilm formation. Biofilms are single- or multiple species microbial communities held together by an extracellular matrix (ECM) produced by the included microorganism. These biofilms or “microbial cities” are the dominant mode of microbial life in many natural, medical and industrial settings [29]. Apart from constituting a unique ecological niche, biofilms offer bacteria protection against their surrounding environment, providing increased resistance to antimicrobials, protozoal grazers, UV radiation, desiccation, mechanical stress and various host defenses [30], [31], [32], [33]. In a clinical setting, biofilms increase treatment failure and account for up to 60% of the nosocomial infections through fouling of various indwelling medical devices [34], [35]. The list of biofilm-associated infectious diseases includes chronic ear, urinary tract and wound infections; cystic fibrosis; periodontitis; recurrent tonsillitis; and chronic rhinosinusitis [31], [34].
Bacteria inside these self-assembled, well-organized, multicellular communities display very dissimilar characteristics to their planktonic counterparts, and can show physiological differentiation that is often controlled through the excretion of quorum-sensing (QS) signal molecules [36]. They show remarkably increased resistance to antibiotics and other environmental stresses and are able to survive and evade host immunity due to the physical protection afforded by an ECM [37]. This biofilm ECM consists of exopolysaccharides, extracellular DNA, proteins and lipids [33]. Proteins in the ECM include enzymes, which act as the biofilms' digestive system, as well as structural proteins [33]. Among the structural proteins are the functional amyloids, which can constitute the bulk of the matrix [38], [39]. Amyloid staining has been observed in many natural biofilms, and the production of extracellular functional amyloid has been demonstrated in environmental isolates within the phyla Bacteriodetes, Firmicutes, Actinobacteria and Gammaproteobacteria [5], [6]. Proposed functions for ECM-associated amyloids include adherence to biological and non-biological substrates, and provision of mechanical robustness to the biofilm. Biofilm-associated amyloids are most often pictured as structural scaffolds [14], [15], [38], [40], [41], and differential expression can guide spatial patterning of the biofilm [40] (Fig. 1). Together with polysaccharides, they are often the prime determinants of biofilm morphology [38], [45]. Other reported roles include a protective function against phage attack by entrapment of phage particles [46].
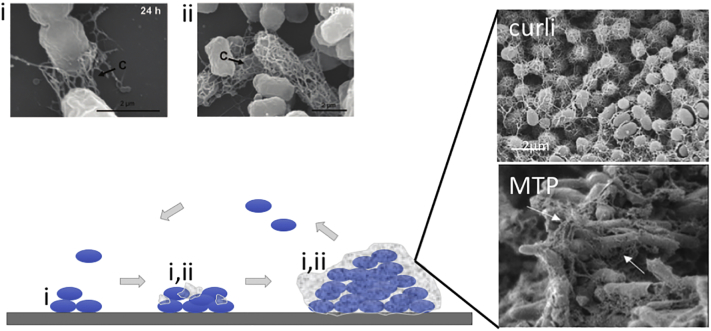
Fig. 1.
Role of bacterial amyloids in biofilm formation. Representative scanning electron microscopy (SEM) images of curli-expressing E. coli (strain MG1655) show two contributions of curli to biofilm formation: (i) adherence of cells to the underlying substrate, and (ii) formation of an ECM that encapsulates cells and mediates cell–cell contacts in microcolonies and mature biofilms (images reproduced from Ref. [42]). The schematic drawing shows the different stages of biofilm formation, from left to right: initial adhesion to a biotic or abiotic surface, microcolony formation that starts production of a protective ECM, full encapsulation of the colony into a mature 3D biofilm. The contribution of functional amyloids such as curli for adherence and ECM formation is labeled (i) and (ii), respectively. Upper and lower SEM images on the right show the abundant presence of curli or MTP in E. coli or M. tuberculosis biofilms, respectively (images reproduced from Refs. [43] and [44], respectively). Arrows indicate curli (labeled c) or MTP.
A diverse set of functional amyloids have been shown or proposed to be associated with bacterial biofilms, including curli (Proteobacteria and Bacteroidetes), Fap (Pseudomonas), TasA (Bacillus), Bap and PSM (Staphylococcus), MTP (M. tuberculosis) and Streptococcus mutans P1 (Fig. 2). In following paragraphs, known bacterial functional amyloids, their biosynthetic pathways and implication in bacterial virulence are described on an individual basis.
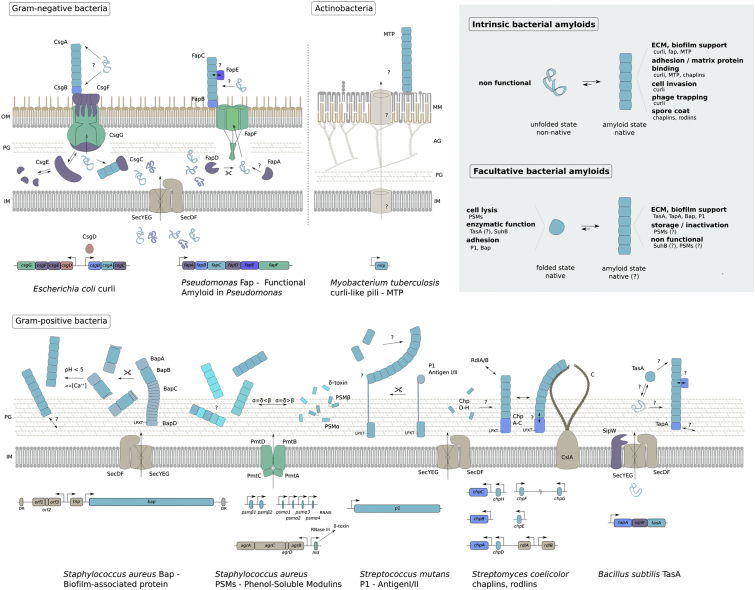
Fig. 2.
Bacterial amyloid assembly pathways. Schematic representation of known bacterial amyloid secretion and assembly pathways, showing the model systems for each of the amyloids. Most systems utilize the generic SEC machinery (SecYEG–SecDF; beige) to transport unfolded units across the cytoplasmic membrane. Some systems encompass pathway-specific transporters (shown in green). In Staphylococci, PSMs are exported via a specialized tetrameric ABC-transporter (PmtA–D), and in Gram-negatives, a dedicated outer membrane channel is required for export to the cell surface, that is, CsgG or FapF in curli and fap, respectively. Several amyloid assembly pathways require accessory proteins (shown in purple), for proteolytic processing (SipW, FapD), export (CsgE, CsgF, FapA) or as a protection against premature amyloid nucleation (CsgC, CsgE). In some systems, minor subunits take a role as nucleator (shown in blue; CsgB, FapB, ChpA–C, TapA), accelerating and localizing the amyloid transition of the major subunit forming the bacterial amyloid fiber (light blue). In Gram-positives, the amyloid fiber can be covalently attached to the peptidoglycan by sortase via the C-terminal LPTX motif. Some proproteins (Bap, P1) are only forming amyloids upon proteolytic processing, and the lighter blue parts indicate protein domains with different folds and functionalities. Where there are clear unfolded intermediates in the amyloid assembly process, these are pictured as random coils in the corresponding colours. Alpha-helical peptides are represented as rods (PSMs, ChpD–H). Genetic organization of the various pathways is drawn with corresponding colors and adjacent genes are indicated were relevant. The curli assembly system is the only system with a known dedicated transcription factor (CsgD; shown in orange). Labels and abbreviations: (DR) Direct Repeat, indicating the bap gene is part of a transposon, encoding its own transposase (Tnp) and putative ABC transporter (ORF1-3). (?) Outstanding questions, (↔) interactions/parallel pathways, (→) unidirectional transport/reactions, (⇄) equilibria, (CslA) cellulose synthase, (IM) cytoplasmic membrane, (OM) outer membrane, (MM) mycomembrane, (PG) peptidoglycan, (AG) arabinogalactan, (C) cellulose. (Inset, upper right) We propose that known bacterial amyloids can be broadly divided into two classes: (I) Intrinsic bacterial amyloids, where the amyloid state is the primary functional form of the proteins involved. These systems appear to have dedicated secretion/assembly pathways. With the exception of chaplins, which form helical precursors, the fiber subunits do not appear to have a folded conformation prior to reaching the amyloid state. (II) Facultative bacterial amyloids are systems where the subunits adopt a (functional) globular folded structure, and can give rise to amyloid fiber formation depending on proteolytic processing and/or environmental triggers such as pH. In these systems, amyloid formation appears to have evolved as a secondary functionality or may be non-functional. In some of these systems, the amyloid state is in equilibrium with free monomers and may act as storage or inactivating state.
Curli
Curli were first identified as a novel class of extracellular filaments in S. Typhimurium, originally referred to as thin curled fimbriae [47]. Soon thereafter, similar surface structures were detected in an E. coli isolate associated with bovine mastitis [8] and in S. Enteritidis [12], where they were termed curli and thin, aggregative fimbriae (tafi in short), respectively. The name curli refers to the coiled appearance of the filaments in electron microscopy [8]. Morphologically, curli are thin (2–5 nm diameter) aggregative fibers of varying length that protrude from the bacterial surface as a thick ECM [8], [48], [49] (Fig. 1, Fig. 3). The amyloid-like nature of curli was first demonstrated by Chapman et al. [9], based on the fibers' ability to bind the dyes Congo red and ThT, extreme chemical stability and β-sheet structure. Purified curli fibers indeed remain intact after boiling in the presence of sodium dodecyl sulfate (SDS), exposure to sodium hydroxide or digestion with proteinase K [12]. Dissociation of curli fibers into their structural subunits requires resuspension in formic acid (> 70%) [12], [54]. Furthermore, purified curli subunits self-assemble into fibrils similar to those produced in vivo [9], a process that is expedited by incubation with pre-existing curli fragments or “seeds” [55]. Curli fibers are the product of a dedicated and highly regulated assembly system encoded by curli-specific genes (csg), which are clustered on two divergently described operons csgDEFG and csgBAC [56] (Fig. 2). The major curli subunit, CsgA, is transported to the cell surface as an unfolded protein, where it assembles to form extracellular amyloid polymers upon interaction with the minor curli protein CsgB. The safe guidance through the periplasm and export of the curli subunits to the outside of the cell are an interplay between accessory proteins CsgC, CsgE, and CsgF and a transport pore CsgG in the outer membrane (reviewed in Ref. [57]).
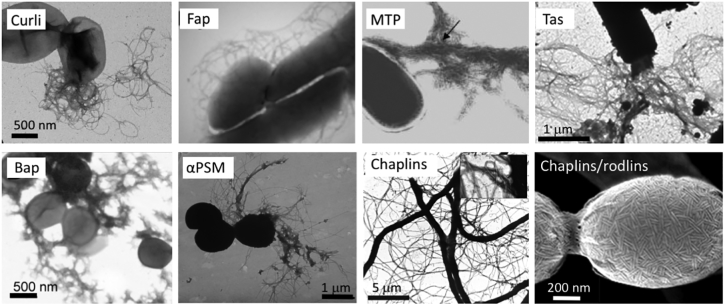
Fig. 3.
Transmission electron microscopy images of negatively stained bacteria displaying different bacterial amyloids discussed in this review. From left to right, E. coli cell expressing curli (N. Van Gerven), P. aeruginosa cell expressing fap fibers [50]. M. tuberculosis cell expressing MTP [11]. B. cereus cell expressing TasA fibers [51], S. aureus cell expressing Bap [16], or αPSMs in their fibrillar form [15]. Last two images show S. coelicolor expressing chaplins in their adhesive fibrillary form [52] or chaplin/rodlin fibers as part of spore coats ([53]; SEM image).
Although curli were first characterized in clinical isolates [8], [49] and have several virulence-associated features assigned to them [8], [58], [59], [60], [61], their importance in pathogenesis is still elusive. Looking at the phylogenetic occurrence of curli operons, it is apparent that curli do not find their origin in bacterial virulence. Indeed curli are widespread in environmental, non-pathogenic species of the genera Proteobacteria and Bacteroidetes [7]. Nevertheless, curli have primarily been studied in the context of Salmonella and E. coli biofilms [60], [62], [63], [64], [65], where they serve as an adhesive and structuring support of the biofilm ECM, together with cellulose and extracellular DNA [66] (Fig. 1). It is noteworthy that the transcriptional regulator CsgD not only activates the biosynthesis of curli, but also of cellulose, the other major biofilm polymer [67], [68]. Both curli and cellulose confer physical protection to cells and generate strong cohesion, elasticity and the striking macroscopic morphology of mature biofilms [38], [69]. Curli also facilitate surface attachment of individual cells and cell–cell adherence in developing biofilms [42] (Fig. 1). The ability of strains to form biofilms is predicted to be a conserved strategy for increased persistence and survival in different environments [70]. For example, curli production by the foodborne pathogen E. coli O157:H7 enhances its ability to form biofilm on stainless steel, glass and spinach leaves, thereby resulting in increased difficulty in removing or killing cells by routine cleaning and sanitizing procedures used in food-processing plants [70], [71]. In this way, curli contribute to the increasing number of infections caused by ingestion of biofilm contaminated fruits, vegetables or meat, not only by E. coli and Salmonella, but also by Citrobacter, Enterobacter, and potentially Klebsiella species [25], [72].
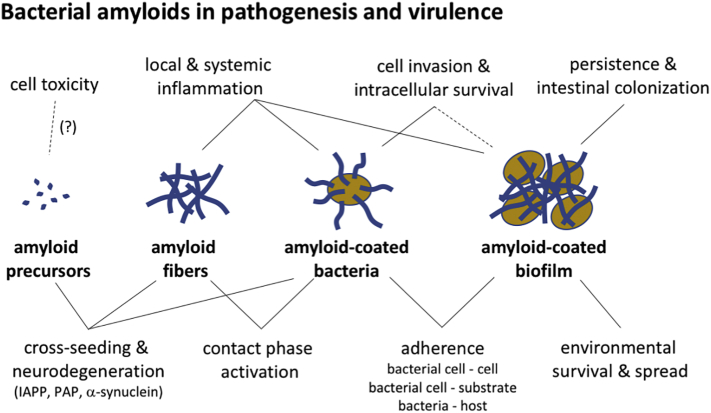
The curli genes occur in most commensal as well as human and animal pathogenic E. coli isolates and Salmonella enterica serovars [73], [74]. However, although the csg genes are present, their expression pattern can vary considerably depending on the lifestyle of the bacterium or the infection site, even within a species. While curli were originally thought to be expressed mainly at ambient temperatures [8], [75], it now seems that depending on the growth medium, commensal E. coli as well as enteropathogenic E. coli, enterotoxigenic E. coli, uropathogenic E. coli (UPEC), enteroaggregative E. coli, avian pathogenic E. coli and E. coli isolates causing sepsis can express curli at 37 °C [74], [76], [77], [78], [79], [80]. Strikingly, in enteroinvasive E. coli, Shigella spp. and enterohaemorrhagic E. coli, curli expression is often abolished through mutations or deletions, suggesting that there is a negative selective pressure on curli expression in these pathogens [61], [81], [82], [83], [84]. Also in Salmonella, curli expression is found to be mosaic, where curli-associated biofilm formation is highly conserved in broad host range Salmonella serovars associated with gastroenteritis like S. Typhimurium, but is often lost in Salmonella strains that are responsible for invasive disease, like S. Typhi [85]. For enteric pathogens, biofilm formation is likely advantageous for transmission, increasing pathogen survival in diverse environmental niches. However, it appears that a loss of the curli-associated biofilm phenotype has emerged as a pathoadaptive trait in invasive strains or species, presumably to improve the fitness of the pathogen in the strong selective settings experienced in host tissues [86]. Although many chronic infections involve biofilm formation, this appears an anti-virulence factor in acute S. Typhimurium infection (a systemic typhus-like infection in rodents), resulting in a significant barrier to colonization of internal organs such as the spleen and mesenteric lymph nodes of mice [87]. Curli were also found to promote colonization in the initial stages of urinary tract infections, but curliated mutants are more efficiently eliminated after 48 h post-infection [77]. In contrast to invasive isolates, commensal inhabitants of the gastrointestinal tract appear to retain the ability to express curli [80]. These fibers contribute to colonization and biofilm formation in the gut, but also seem to play a critical role in regulating epithelial translocation during infection [88], [89]. Recognition of amyloid fibers in enteric biofilms by the Toll-like receptor (TLR) 2/1 complex has been seen to reinforce barrier function by promoting intestinal epithelial integrity and overall gut health [88], [89]. These studies also found that treatment of mice with a single dose of curli ameliorated pathology in 2,4,6-trinitrobenzenesulfonic acid-induced colitis, leading them to propose curli fibers as candidate therapeutic to treat intestinal inflammatory disorders [89].
There are various reports on the direct interactions of microbial amyloids and host systems, which form the basis for assigning virulence properties to curli [8], [60]. Several studies have linked curli to adherence and invasion of eukaryotic host cells [63], [76], [77], [90], [91], [92], [93], which is at least partly attributed to curli's fibronectin-binding capacity [8], [94]. In avian pathogenic E. coli isolates, curli promote adherence to avian intestinal cells, internalization of HeLa cells [90] and persistence in the cecum of chickens [95]. However, the role of curli in pathogenicity seems more complicated. Expression of curli by a typical commensal E. coli isolate enhanced adherence and internalization of the bacteria in intestinal or bladder cells and triggered IL-8 production [93]. However, the same study showed co-expression of cellulose counteracted these curli-mediated interactions [93]. In general, the internalization efficiency in most studies is low (0.013% of inoculum number) [77], [93] to moderate (0.19% to 0.35%) [90], [94], and curli fibers are not the only virulence factor involved in the internalization of avian E. coli strains [90] or Salmonella, as deletion of csgBA, encoding the main curli subunit proteins, caused no noticeable impairment of Salmonella virulence [96]. Comparable results were observed for enteroaggregative E. coli curli mutants, which were still able to adhere to epithelial cells [97]. These observations can be explained considering the repertoire of redundant adhesins produced by these organisms. Apart from fibronectin, curli bind a variety of other host ECM and serum proteins, such as laminin [98], plasminogen [61] and human contact phase proteins [99], a feature hypothesized to be caused by the conserved quaternary structure of amyloids. Curli have also been shown to trigger innate immune responses. They interact with major histocompatibility complex I (MHC-I) [8], activating innate immune responses in macrophages and vascular smooth muscle cells [79], [100], and curli fibers of E. coli and S. Typhimurium were found to activate the TLR1/2/CD14 complex in HeLa, HEK293 and THP1 cells, inducing the production of pro-inflammatory mediators such as NO, IL-6 and IL-8 [101], [102], [103], [104]. The fibrillar structure of the amyloid fiber appears to be required for CD14 binding [104] and the presence of fibrils contributes to the TLR1/2- mediated response in vivo [101]. On the other hand, curli have also been found to protect E. coli cells from innate responses, such as complement-mediated killing via inhibition of the classical complement pathway [105]. However, the survival advantage demonstrated in vivo at 2 h post-infection disappeared as the infection progressed [105]. Curli have also been found to sequester the antimicrobial peptide LL-37 produced by urothelial cells and neutrophils in the urinary tract, thereby protecting UPEC from lysis [77].
In conclusion, it seems that the primary role for curli is in the environment, where they provide a better fitness advantage in soils, sediments and on plant tissues. The expression of curli does not seem to be a prerequisite for colonization of the gastrointestinal or urinary tract. However, it may be a factor contributing to persistence in non-invasive enteric commensals and pathogens, and facilitating dispersal via temporary environmental stages. While curli can provide a level of protection from complement and antimicrobial peptide-based killing, the advantage is limited and temporary, as they do trigger a strong innate response. As a result curli are often found to be under negative selection in invasive strains or isolates.
Functional Amyloid in Pseudomonas—Fap
The success of Pseudomonas species as a resilient human and plant pathogen derives in great part from their ability to form robust biofilms [106]. In humans, these Gram-negative opportunistic bacilli are responsible for various, often chronic and life-threatening infections, particularly in immunocompromised patients, and patients with cancer, cystic fibrosis or burn wounds [107]. The presence of amyloid fibrils as a component of Pseudomonas biofilms was first proposed based on binding of ThT and amyloid-specific conformational antibodies [5]. Bacteria positive for Congo red staining were later shown to be surrounded by a bulky ECM that contains embedded fibers similar in appearance to curli produced by E. coli [10] (Fig. 3). Synchrotron radiation circular dichroism and FTIR both showed the presence of extensive β-sheet structure in the purified fibers, which like curli are SDS insoluble, but readily self-assemble from subunits isolated after fiber dissolution in formic acid [10]. A distinct operon of six genes, called functional amyloid in Pseudomonas or fap (fapA-F), was found responsible for the biosynthesis of the fibers [10] (Fig. 2). Fap proteins are genetically distinct to curli proteins and make use of alternative strategies for handling and export of the amyloid protein sequences [10], [108], [109]. FapC forms the main component of the Fap fibers, which also contain smaller fractions of FapB and FapE [110], [111]. FapB is proposed to take on a function as nucleator protein, but can take over as the main amyloid component in strains lacking FapA, a periplasmic component that is thought to serve a regulatory or chaperoning role in Fap subunit secretion [110]. FapD is a periplasmic cysteine protease required for secretion of FapC, and FapF forms a trimer of gated β-barrel channels in the outer membrane required for secretion of the Fap subunits [109].
Overexpression of the fap operon leads to a highly aggregative and adherent phenotype with enhanced biofilm forming capacity [10], [110]. Fap fibrils hereby likely function as an adhesin for the attachment to abiotic surfaces, such as plastic and glass [110]. Using atomic force microscopy imaging and force spectroscopy, it was shown that the Fap amyloid makes a major contribution to the build-up of the mechanical robustness of Pseudomonas biofilms, increasing the hydrophobicity of the biofilm surface and enhancing biofilm stiffness and resistance to drying [50]. However, Fap overexpression markedly altered the overall proteome of the bacterium, so that the altered biofilm phenotype may not be fully ascribed to the mechanical properties of the amyloid fibrils. Proteome changes included a lowered abundance in classical virulence factors such as elastase B and the secretion system of alkaline protease A, the two main proteases that lead to tissue damage, and an increase in biofilm-associated proteins and alginate biosynthesis, leading to a mucoid phenotype similar to that seen in chronic cystic fibrosis infections [111]. The combined secretion of Fap and the polysaccharide alginate confer physical protection to cells in this phenotype [38], [69], similar to what is seen for curli and cellulose secretion in E. coli and Salmonella [38], [64]. Inclusion of amyloidogenic CsgA or FapC fragments into methylcellulose or alginate gels was found to alter their viscoelastic properties of alginate gels, leading to the suggestion that mechanical properties of the biofilm matrix may be adapt to environmental conditions.
In addition to giving mechanical support to the ECM, Fap fibrils were found to modulate the retention and release of small extracellular metabolites such as QS molecules [112]. QS molecules are instrumental in bacterial intercellular communication and biofilm build-up [113], and binding to Fap may constitute a very useful feature especially in turbulent and competitive environments, such as rivers, wastewater treatment plants or acute respiratory infections [112]. This involvement of the Fap amyloids in interbacterial information transfer makes it harder to distinguish a direct causative or secondary effect on pathogenicity.
The fap operon is found predominantly in Pseudomonas species, but although not as widespread as the curli system, it also occurs in other Gamma-proteobacteria including some Aeromonas, Vibrio, and Shewanella; in Beta-proteobacteria such as Bulkholderia and Ralstonia; and in the Delta-proteobacterial genus Desulfohalobium [108]. It is interesting to notice, that unlike curli, there is a comparatively high frequency of known pathogenic species within the identified bacteria carrying the fap operon (39%). These include pathogens associated with not only opportunistic infections in the airway and lungs, gastroenteritis, and diarrhea, but also sepsis [108]. Even so, fap occurrence is sporadic and not conserved among all pathogenic strains of these genera, showing Fap is not indispensable for pathogenicity in these bacteria [108]. Nevertheless, the role of Fap as potential virulence factor in Pseudomonas is supported by the identification of a fapC deletion mutant of P. aeruginosa which is highly attenuated in a Caenorhabditis elegans infection model and in a polymorphonuclear neutrophil (PMN) phagocytosis assay [114]. Fap was also shown to be expressed in vivo in murine infection models using a novel amyloid-targeting fluorescent probe [115]. This observation is further supported by the fact that transcription of the fap operon was highly up-regulated in murine models of acute burn and chronic surgical wound infections when compared to traditional laboratory conditions [116].
Many functional amyloids have the ability to bind to ECM proteins such as laminin. Although not shown for Fap, Pseudomonas infection in cystic fibrosis patients causes PMNs to release large amounts of elastase, collagenase and oxygen radicals that cause degradation of the matrix and elastic framework of the bronchi and bronchioles [117], [118], providing a potential niche for Fap.
M. tuberculosis Curli-Like Pili—MTP
M. tuberculosis is capable of assembling two distinct pilus types: type IV pili and M. tuberculosis curli-like pili [11]. The latter are 2–3 nm in width and appear microscopically similar in structure to curli of E. coli and Salmonella spp. (Fig. 3), forming a dense fibrillar meshwork that extends many microns away from the bacterial surface [8], [12] (Fig. 1). Although the amyloid nature of M. tuberculosis pili (MTP) is not yet conclusively proven, the aggregative fibrillar nature of the pili and biochemical properties such as the ability to bind to Congo red dye and resistance to SDS support the classification as bacterial amyloids [11]. Despite being dubbed “curli-like pili,” there is no obvious primary amino acid sequence homology between MTP and curli, or for the other functional amyloids described [11].
Purified MTP are composed of 4-kDa protein subunits, encoded by the Rv3312A (mtp) gene [11] (Fig. 2). Gene knockout and complementation confirmed its necessity for pili formation. The mtp gene is not organized in an operon or cluster with curli- or pili-associated biogenesis genes, but is located between genes involved in intermediary metabolism [11]. For that reason, the secretion, assembly and association of MTP with the complex mycobacterial cell wall remains enigmatic [119]. Possibly, as yet unidentified additional MTP biogenesis genes are located distantly on the chromosome.
Amplicon sequencing of clinical isolates shows that the mtp gene is present only in M. tuberculosis complex strains and not in non-tuberculous mycobacteria or in other bacteria [11], [120], [121]. This suggests that MTP production may be limited to pathogenic mycobacteria species. Sera from TB patients contain IgG antibodies that specifically react to the native MTP antigen, providing evidence that the pili are produced in vivo during human TB infection and that they play a role in stimulating the humoral immune response [11]. The host inflammatory response associated with invasive pathogens like M. tuberculosis causes tissue damage and possible subsequent exposure of ECM proteins, such as laminin. Studies in an organ culture system have shown M. tuberculosis preferentially attaches and invades these damaged sites [122]. Alteri et al. [11] reported binding of MTP to laminin in vitro and showed that MTP are produced during adherence to epithelial cells, indirectly implicating a role as an adherence factor. As mtp deletion mutants are unable to bind to laminin [11], MTP may be critical for interaction and colonization of host cells during the establishment of TB infection in the lung or other tissue sites. M. tuberculosis ability to attach to, enter, and survive in host cells including macrophages and dendritic cells is critical to its survival, replication and dissemination in the host. MTP-deficient mutants displayed a significant decrease in the adhesion and invasion of human cultured THP-1-derived macrophages or A549 alveolar epithelial cells [119], [123]. In M. tuberculosis mouse infection models, MTP were not required for survival of the pathogen, although differences in mtp expression did affect lesion architecture in infected lungs [124]. M. tuberculosis most likely brings into play additional strategies to adhere to and invade its host to compensate for the loss of MTP. The role of MTP as adhesive structures is further supported by their involvement in bacterial aggregation and in vitro biofilm formation. An MTP-deficient strain displayed a 68 % reduction in biofilm mass compared to the parental strain, although no significant difference between mtp expression in cells of the biofilm to those growing planktonically was observed [125]. Whether M. tuberculosis also forms biofilms in the host is a controversial topic [126]. Non-tuberculous mycobacterial species colonize the host as biofilms [127], [128] and M. tuberculosis isolated throughout the world do form biofilms that persist despite exposure to high levels of antibiotics in vitro [129], [130]. Although the long-term persistence of M. tuberculosis indeed bears similarities to the chronic infections of biofilm-forming pathogens [131], biofilm development in vivo and the implication of MTP remain to be conclusively shown.
Streptococcus mutans Biofilm-Associated Amyloids
S. mutans is an acidogenic Gram-positive oral bacterium that is well adapted to a biofilm lifestyle for survival and persistence in dental plaques. This organism is the main causative agent associated with human dental caries, although non-lethal, one of the most ubiquitous infectious diseases that affect humans (reviewed in Ref. [132]). S. mutans biofilms show birefringence when stained with Congo red, pointing to the presence of functional amyloids in the biofilm matrix [13]. Three candidate amyloid proteins have been associated with the CR staining of the matrix and were shown to form fibers in vitro: adhesin P1, the wall-associated protein A (WapA) and the secreted protein SMU_63c [133]. The P1 adhesin is a large (185-kDa), extended protein comprising multiple folded domains spaced by coiled-coil repeat regions [134]. It is covalently anchored to the cell peptidoglycan via a C-terminal LPXTG motif, characteristic for Gram-positive cell wall-anchored proteins (Fig. 2) [135]. The adhesive carbohydrate-binding properties are ascribed to a β-rich “globular head” domain that intervenes the A- and P-repeats at the tip of the extended stalk. The C-terminal region is also globular and comprised of three structurally related β-sandwich domains. Intact P1 covalently attached to the cell wall, but interacts with more loosely associated proteolytic P1 fragments, particularly the C-terminal fragment C123 originally identified as AgII, to form the functional adhesive layer [136], [137], [138]. The C123 fragment of P1 readily forms fiber-like structures in vitro [138] (Fig. 3), suggesting that it may contribute to the formation of functional amyloid during biofilm development. Although P1 fragments form fibers in vitro, these have not yet been visualized in the biofilm context. C123 is also readily extracted from the cell surface with SDS or mechanical shaking in phosphate buffer, and is also readily found in the culture medium [13]. This is in contrast to biofilm-associated functional amyloids like curli or Fap fibers, which form mechanically and chemically robust fibrils. P1 deletion mutants of S. mutans were less virulent in a rat dental caries model [139], but this may reflect the protein's adhesive properties more than its ability to form amyloid-like fibers. P1 is a multifunctional adhesin that is implicated in the interaction of S. mutans with ECM proteins such as fibronectin, fibrinogen and collagen (reviewed in Ref. [140]). It also induces the production of pro-inflammatory mediators, such as NO, PGE2, TNF-α, IL-6 and IL-8 [141], [142], [143] in monocytes, epithelial and endothelial cells, by binding directly to α5β1 integrins and TLR4 [141], [144]. Seeing that P1 can be released from the bacterial cell surface, these molecules may act at a distance to cause more systemic inflammation. P1 is indeed hypothesized to participate in the initiation and/or perpetuation of rheumatic diseases, by stimulating pro-inflammatory cytokine release from various synovial cells [145]. Whether these P1 attributes involve its ability to form fibers is unknown, however.
P1 is not essential for S. mutans biofilm formation [146] and bacterial colonies lacking P1 demonstrate residual green birefringence [13]. More recently, WapA and SMU_63c were also found to form amyloid-like fibers in vitro [133]. Individual mutants lacking spaP (encoding P1), wapA or 63c did not drastically reduce S. mutans biofilm formation, but spaP/wapA or spaP/wapA/63c double and triple mutants were severely attenuated in biofilm formation in glucose medium, though had no effect on sucrose-dependent biofilm formation [133]. To what extent these effects on S. mutans biofilm formation depend on these proteins' ability to form amyloid-like fibrils is still unclear, although glucose-dependent biofilm formation was also significantly inhibited in the presence of known polyphenolic amyloid inhibitors such as tannic acid or epigallocatechin-3-gallate (EGCG) [13], [133]. These compounds also inhibited P1 and WapA fibril formation in vitro [133].
TasA as Functional Amyloid In Bacillus subtilis Biofilms
In the genus Bacillus, biofilm formation has been extensively studied for the saprophyte B. subtilis. Bacilli can form different types of biofilms but the model system is its formation of biofilms at the air–liquid interface, so-called pellicle biofilms. In B. subtilis, the main components of these biofilms are polysaccharides encoded by the eps operon [147], and amyloid-like proteinaceous filaments encoded by the tapA-sipW-tasA operon [148], [149] (Fig. 2). Notably, both of these operons are under regulation of the sinR regulon, a master switch in biofilm formation [148]. In addition, the self-assembling BslA protein forms a thin hydrophobic layer on top of the biofilm. Together, BslA and the biofilm structuring eps and TasA components give the biofilm matrix its typical impenetrable characteristics, with non-wetting properties superior even to teflon [150]. The tapA–sipW–tasA operon was originally associated with the assembly and structure of the spores [151], [152], although the function of the different proteins in this process was never really clear. Later, SipW, TapA and TasA have been linked to biofilm formation [149] with TasA posited as a possible functional amyloid. TasA was found to assemble in fibers in vitro and in vivo, which stain with amyloid-indicating dyes such as ThT and Congo red (Fig. 3) [14]. Interestingly, TasA can be purified in physiological buffer as a soluble protomer and only aggregates under acidic conditions or on hydrophobic surfaces [153]. TasA and TasA-like proteins are part of the zinc-dependent M73 metallo-proteinase family. A recent report of the B. subtilis TasA shows the protein to form a stable folded monomer with a M73 family β-sandwich topology, though lacking measurable proteinase activity, and readily forming fibers at low pH by means of localized conformational rearrangements [154]. TapA is the main accessory protein coupling TasA to the peptidoglycan in the cell wall and decorating the TasA fibers in about 1:100 TapA:TasA ratio (Fig. 2) [155]. It also acts as a potentiator for TasA assembly [155], [156] reminiscent to the nucleator function of CsgB or FabB in curli and Fap, respectively. Both TasA and TapA are proteolytically processed by the membrane-bound protease SipW, which also has a signaling function to allow biofilm formation on solid surfaces [157]. Interestingly, on solid surfaces, neither TasA nor TapA is required for biofilm formation, in contrast to SipW which is essential in the early stages of biofilm formation for attachment to surfaces, suggesting that the latter has a more universal function than merely activating the TasA/TapA system [157], [158]. For B. subtilis, it was shown that TasA is important for colonization of plant roots [159]. Notably, the TasA protein has been associated with potent antimicrobial activity by an unknown mechanism [152] and is found upregulated in response to pathogen presence [160], suggesting that it is used by Bacillus for competitive colonization purposes.
Can TasA/TapA be regarded a virulence factor? Both proteins are present in the Bacillus cereus group. In B. cereus, a notable food-borne pathogen where biofilm formation is regarded important in bacterial persistence [161], the sipW operon contains two tasA homologues calY1 and calY2, which play a role in pellicle biofilms but not in submerged biofilms [162]. In B. cereus and Bacillus thuringiensis, biofilms are naturally found in the environment [163], [164] and possibly aid virulence by secreting toxins in situ [165]. In contrast, Bacillus anthracis biofilm formation is more controversial. Although B. anthracis can form biofilms in culture [166], their contribution to the infective life cycle is more obscure and not needed sensu strictu [167]. This is consistent with the report that the sinR regulon in this pathogen is more tailored toward the secretion of metalloproteases, different from that in other Bacilli, indicating that the TasA might have a different function in B. anthracis [168]. Whether in the B. cereus group the TasA protein has amyloid-like fiber forming properties similar to B. subtilis, and whether the folded monomeric state of the protein has retained M73 metallo-proteinase activity are yet unclear.
Chaplins as polyvalent amyloids in Streptomyces coelicolor
Chaplins are functional amyloids found in the genus of filamentous soil bacteria Streptomyces, where they are essential for the formation of aerial hyphae [169], [170]. Streptomyces are rarely associated with pathogenesis, though S. somaliensis, S. sudanensis, S. scabies and related species can cause human and plant infections. Functionally, chaplins resemble the hydrophobins found in filamentous Fungi [171]. In concert with SapB, they act as surfactants, needed for the hyphae to breach the air–liquid interface [172]. Together with the rodlins, chaplins form the rod-like hydrophobic surface on top of the hyphae [173] (Fig. 3). In the model species S. coelicolor, eight chaplin proteins have been identified, each characterized by the hydrophobic 40-residue chaplin domain. These proteins cluster together in two families with five short chaplins (ChpD,E,F,G,H) having one chaplin domain, while the three long chaplins (ChpA,B,C) harbor two chaplin domains in addition to a sortase signal (Fig. 2). Short chaplins have been shown to harbor amyloidogenic sequences as verified by microscopy, X-ray diffraction, binding to amyloid-indicating dyes and FTIR [174], [175]. The inclusion of the sortase signal possibly implies that long chaplins are anchored to the peptidoglycan, and this has been shown to be true for ChpC [176]. It is therefore speculated that long chaplins serve as attachment sites for the shorter amyloidogenic chaplins, although they are not absolutely required in later stages of aerial hyphae development where sortases SrtE1 and SrtE2 play a more crucial role (Fig. 2) [176]. Finally, ChpE is likely involved in regulating coordination of assembly/polymerization of the other subunits, but deviates from its other short chaplin counterparts in that it lacks the otherwise conserved cysteine residues [53]. It also shows a pH-dependent fibrillation, staying soluble at low pH, which might contribute to its special function [177].
Apart from their role in aerial hyphae formation, chaplins have also been identified to form adhesive fimbriae, facilitating surface attachment. In these specialized fimbrae, chaplins are thought to be attached to the cells through cellulose fibrils [52], with no eminent role for the long chaplins. These chaplins might function in attachment or contribute to biofilm formation. Interestingly, amyloid fibrils engaging in fimbrae have different superstructural properties than those making up the hydrophobic surface of the aerial hyphae [178] with the fimbriae resembling stacked β-fibrils, while the surface coating amyloids assemble in pairs and adopt a special amphipathic orientation.
Functional Amyloids in Staphylococcus Species
Staphylococcal biofilms present a major challenge in medicine as they adhere to abiotic surfaces such as indwelling medical devices, as well as biotic surfaces such as mammalian host tissues [35]. Clinical isolates of Staphylococcus aureus display both polysaccharide-dependent and -independent biofilm formation [179] and S. aureus and Staphylococcus epidermidis rely on different matrix components for structural stability [180]. The main components of polysaccharide-dependent staphylococcal biofilms are PIA/PNAG polymers [181], [182]. However, the involvement of biofilm-associated proteins is increasingly recognized. In 2001, Cucarella et al. [183] discovered Bap (biofilm-associated protein) as a virulence factor in S. aureus subclinical bovine mastitis infections. Bap is a large secreted protein composed of 2276 amino acids, with an N-terminal signal peptide and a carboxy-terminal segment containing the LPXTG cell wall anchor motif. Bap is a multidomain proprotein that becomes proteolytically processed on the cell surface. The B domain (BapB) features two EF-hand domains which bind calcium at millimolar concentrations, inhibiting biofilm formation and surface adherence [184]. Moreover, BapB shows pH-dependent bacterial clumping and biofilm formation, when bacteria are grown in the presence of glucose and a pH below 5 [16]. Interestingly, at this acidic pH, purified BapB forms amyloid oligomers as verified by ATR-FTIR, electron microscopy and their binding to dyes such as Congo red, ThT and Proteostat (a protein aggregation dye with increased fluorescence efficiency compared to ThT) [16]. The authors also identify two possible BapB-derived peptides with amyloidogenic properties forming fibers as seen by electron microscopy (Fig. 3). Also, mass spectrometry analysis on S. aureus aggregates showed that these fibers primarily contained peptides consisting of BapB, adding to the belief that the main component of Bap contributing to bacterial clumping and biofilm formation is the B-domain. Therefore, the authors put forward a model in which bap is proteolytically processed under acidic conditions yielding BapB fragments which at low concentrations of calcium form into amyloid fibers and scaffold biofilm formation. Two other proteolytic fragments, BapC and BapD, have been proposed to help in anchoring biofilm to the bacterial membrane, although exogenously added BapB protein could also polymerize on top of bap-negative strains [16]. In another report, peptides with the consensus sequence STVTVTF derived from the BapC repeat region from S. epidermidis were found to be amyloidogenic in vitro [185]. The functional relevance of this amyloid-prone region and its implication in Bap fiber formation are unknown, however. The primary function of BapC likely lies in cell adhesion, as it comprises anywhere from 3 to 50 repeats of so-called HYR modules, extracellular modules with an Ig-like fold, involved in adhesion [186], [187].
How widespread is bap in pathogenic Staphylococci and how important it is in virulence? In S. aureus, bap has only been found in cases of bovine mastitis and not in human isolates [183], [188], [189]. In this respect, the protein appears to be tailored for cattle udder infections, where the naturally fluctuating calcium levels allow the bacteria to switch between the persistent biofilm state during the dry periods and the motile infectious state during lactation [184]. Nevertheless, bap orthologs have been identified in other so-called coagulase-negative Staphylococcus species [190], and in five species at least, Bap has been shown to be amyloid competent [16]. Thus, Bap could contribute to biofilm formation, persistence and virulence as some species colonize regions with low pH beneficial for biofilm formation, among them S. epidermidis, S. warneri and S. saprophyticus. Nevertheless, in human clinical isolates bap seems not to be a major determinant for virulence as it is absent from MRSA isolates [191] and S. chromogenes isolates [192]. There are reported cases where a bap-homologue, Bhp, identified in human S. epidermidis isolates, could have a similar role to Bap [190]. This indicates that, though being involved in biofilm formation, Staphylococci have a plethora of other factors contributing to biofilm formation and bap is definitely not the essential determinant for virulence. However, bap-positive isolates are shown to be more adherent to mammalian cells than other genetic factors associated with biofilm formation [193], indicating that they can be important in long-lived persistence. Finally, mouse models challenged with bap-positive staphylococci take longer to clear the infection compared to bap-negative strains [183].
Recently, a second amyloidogenic protein has been identified in S. aureus, SuhB [194]. The amyloidogenic nature was confirmed by X-ray diffraction, amyloid-indicating dyes and electron microscopy imaging of fibers. However, SuhB functions as a folded phosphatase, and the physiological role of the protein's amyloid formation, if any, is yet unclear and may be a secondary function.
Finally, though controversial, also phenol-specific modulins (PSMs) have been proposed to form amyloid-like structures in staphylococci. PSMs were first discovered in S. epidermidis to induce cytokine release and activate inflammatory pathways in macrophages [195]. PSMs are exclusive to staphylococci and are particularly associated with pathogens [196], [197], although the sequence determinants differ significantly among the pathogens [198]. Research has focused on S. aureus and the discussion below will follow S. aureus nomenclature. PSMs are amphipathic alpha helices, and this fold has been confirmed by their solution structures in NMR [199]. They can be divided into two main groups, the smaller (20–25 aa) PSMα, including the similar δ-toxin, and larger PSMβ (about 45 aa) [196]. Lacking a typical secretion signal, PSMs are exported by a dedicated ABC protein secretion system which also protects the bacteria from self and non-self PSMs (Fig. 2) [200]. Off all discussed proteins/peptides in this review, the link between PSMs and virulence/pathogenesis in Staphylococcus is undoubtedly the most outspoken (PSM mode of actions reviewed in Ref. [198]). PSMs lyse blood cells [196], [201] and trigger macrophage escape during pathogen replication [202]. They also widely elicit immune responses [195], [196], [198] and modulate biofilm structuring and dispersal, essential to coordinate the pathogens' life cycle with infectivity [196], [203], [204]. Nevertheless, there is ample controversy about the possible amyloidogenic properties of these peptides and whether or not the resulting aggregated forms contribute to the typical toxic effects associated with virulence. The first amyloid-like characteristics of these PSMs were described by the laboratory of Blaise Boles [15] identifying them as the main component of fiber-like extensions from the cells through mass spectrometry. Furthermore, when purifying PSMα1 in vitro, the peptides form fibers that bind Congo red and ThT, and show high β-sheet propensity by ATR-FTIR and CD [15], [205]. PSMα peptides are notably less amyloidogenic compared to the longer PSMβ [206] and their amyloidogenic properties are inversely correlated with their cytolytic activities [207]. This is also true within the PSMα with PSMα2,3 being more cytolytic than PSMα1,4 which are in turn more amyloidogenic [205], [207]. A striking incongruity regarding PSMs is their superstructure. Whereas the initial papers [15], [205], [208] described PSMs as of amyloid nature with the distinct cross β-sheet fold, the recently solved crystal structure of PSMα3 unexpectedly shows a “cross-alpha”-amyloid fold [209]. This alpha-amyloid form of PSMα3 was speculated to trigger the cytolytic activity [209], although this claim is questioned by a study disentangling the amyloid properties from the cytotoxicity by virtue of an alanine mutagenesis scan [206]. Based on current observations, the physiological relevance and functional contribution of the aggregating properties seen for PSMs remain unclear.
Bacterial Amyloids as Toxins and Immunogens
Pathological amyloids associated with human amyloidosis and degenerative diseases are well known for their cytotoxic effects. The molecular mechanisms of toxicity in these various amyloid systems are diverse and include tissue damage and inflammation caused by deposited mature fibers, as well as cell toxicity of prefibrillar intermediates in the amyloid assembly process [3]. Similar to pathological amyloids, bacterial amyloids might be associated with cell and tissue damage as a collateral consequence of the amyloid formation process at the infection site, but could also be used by pathogenic bacteria as an evolved trait during virulence. We found no known examples of bacterial amyloid systems where amyloid toxicity is directly used or exacerbated as a virulence mechanism. However, bacterial amyloid depositions do trigger host responses that are important to consider in the acute and long-term effects of the disease process.
Interaction with the contact phase system
The contact system is a proteolytic cascade that provides an interface between coagulation and innate immunity driving both pro-inflammatory and pro-coagulant pathways. Pre-fibrillar oligomers and aggregates of pathological amyloids like Aβ42, but not mature fibrils, have been found to activate the contact phase system [210]. Also curliated bacteria have been found to sequester various contact-phase proteins onto their surface, including the serine protease factor XII (FXII), the non-enzymatic co-factor high molecular weight kininogen (HK) and fibrinogen, thereby triggering an activation of the contact system leading to release of pro-inflammatory peptide bradykinin (BK) and fibrinopeptides at the site of infection [58], [99], [211]. The role of curli in contact system activation was clearly demonstrated since E. coli and S. Typhimurium strains mutant strains lacking curli expression also lack the ability to trigger the system [58]. However, whether the curli fibers, or soluble curli monomers or oligomers directly trigger the system is currently unknown. Although the contact system is part of the innate immune system and originally a defense mechanism, a controlled induction of inflammation can be beneficial for bacteria by increasing vascular permeability, and thereby improving the influx of nutrients and promoting bacterial invasion into surrounding tissue [2], [211], [212], [213]. However, genomic and proteomic observations of invasive E. coli and Salmonella infections show a negative selection toward curli expression, suggesting that curli-mediated activation of the contact system is not used as a virulence-enhancing trait by these pathogens, and may reflect a response of the host seeking to encapsulate the bacteria in a fibrin network to prevent their further spread. Either way, Herwald and co-workers [58] showed that curliated bacteria prolonged blood clotting times and suggested that during severe sepsis, the sequestration of host contact factors onto the surface of the bacteria can cause a systemic fibrinogen depletion and a hypocoagulatory state, possibly explaining the bleeding disorders commonly seen in septicemia. It is unknown at present whether other bacterial functional amyloids activate the contact system.
Bacterial amyloids in neurodegeneration
Although amyloids are highly diverse in sequence, composition and ultrastructure, common structural features such as the underlying cross-β spine give rise to a phenomenon known as cross-seeding, where amyloid deposits of one protein or peptide act as heterologous aggregation nuclei or facilitate secondary amyloid nucleation of another [2]. A notorious example of intra- or cross-species contagious amyloid deposition is that of the prion protein PrP, giving rise to transmissible spongiform encephalopathies (TSEs) in cattle and human [2]. TSEs involve related prion proteins; however, and in other amyloid systems, lack of sequence similarity in amyloid regions has been seen to provide a selectivity barrier to cross-seeding efficiency [214]. Nevertheless, an important question to take into consideration is the extent by which bacterial amyloids have prion-like cross-seeding properties toward human pathological amyloids. Curli cross-seeding has been demonstrated to enable inter-species biofilm formation and in vitro, curli fibers were able to seed fibrillation in distant CsgA homologs, with sequence identities as low as 30% [215]. However, despite this low-sequence similarity, the consensus motifs in the amyloid regions are conserved and it can reasonably be expected that CsgA homologs form structurally similar fibrils. A systematic study of curli cross-seeding is lacking, but different observations indicate that the presence of curli can influence aggregation processes of dissimilar, human amyloids. In vitro, 1 mol% CsgA has been found to reduce the lag times for human islet amyloid polypeptide (IAPP) fibrillation and increased elongation rates in human prostatic acid phosphatase fragment PAP248–286, [216]. In vivo, purified curli or curliated bacteria enhanced silver nitrate-induced amyloid protein A (AA) deposition in mouse liver [217], and Chen and coworkers [218] used a rat model to show that the presence of curli producing bacteria in the gut enhanced inflammation and the deposition of α-synuclein in the brain. Intriguingly, previous studies have pointed to an enteric link or even origin of α-synuclein aggregation and Parkinson's disease [219], and also for Alzheimer's disease, an involvement of the gut and gut microbiota has been suggested [220], [221], [222]. The gut–brain axis may provide contact of gut-localized bacterial amyloid deposits and host amyloid-prone proteins in the central nervous system, similar to the infection route of ingested prions in TSEs [223], [224]. Of note, CsgA and α-synuclein both share a D-Q-Φ-X0,1-G-K-N-ζ-E motif (where Φ = W/L, ζ = S/E), and the natural curli assembly inhibitor CsgC also has inhibitory activity toward α-synuclein, suggesting that localized common structural epitopes may be present in curli and α-synuclein fibrils and or nuclei [225]. However, it is unclear at this moment whether the presence of functional amyloids like curli in the gut triggers α-synuclein or AA aggregation by cross-seeding, or more indirectly, by priming local or systemic inflammation. In this context, it is of interest that curli as well as Aβ and αS fibrils induce pro-inflammatory responses by engaging with TLR1 and TLR2, leading to the translocation of nuclear factor κβ and the increased production of pro-inflammatory cytokines [101], [226], [227]. For a more comprehensive perspective on the interplay between functional amyloids and human amyloid disorders, the interested reader is referred to the recent review by Friedland and Chapman [228].
Countering Bacterial Amyloid Assembly
In the above paragraphs, we reviewed the occurrence of functional amyloids in human and animal pathogenic bacteria. Since the discovery of curli in E. coli and S. enterica, distinct functional amyloid pathways have been identified in M. tuberculosis, Pseudomonads, S. mutans, Staphylococci, Streptomyces and the Bacillus group, and several more may remain to be discovered. With the exception of PSMs found in Staphylococci, these functional amyloids appear to serve a primary role as a structural and possibly adhesive component of the ECM, supporting bacterial organization into biofilms and colonization of biotic and abiotic surfaces. The contribution to virulence of these various bacterial amyloids is mostly indirect, in the context of their biofilm-supporting and regulating roles. Biofilm formation can be a key part of the pathogenic cascade, by direct involvement in bacterial persistence and increased tolerance to antibiotics and host responses, as seen for example in chronic lung or urinary tract infections or dental carries, or more indirectly by increasing persistence and dispersion in diverse environments, for example, in catheter-related or food-borne infections. To efficiently control and manage bacterial infectious diseases, much attention has been directed toward searching for novel therapeutics that prevent biofilm development or break up existing biofilms, as alternatives or supplements to conventional antibiotics [229], [230], [231]. Biofilm formation is multifactorial process, and different bacteria employ a diverse array of often redundant cell-surface adhesins and ECM components, such that a multipronged approach will likely be required [232]. Encouraging examples indicate that inhibition of functional amyloid formation may be a component in this search.
Several natural compounds that inhibit biofilm formation have been found to alter expression levels of the biosynthetic pathways responsible for functional amyloid production. One such molecule that has been found able to inhibit curli production and prevent biofilm in E. coli is the antibiotic rifapentine [233]. Rifapentine was identified in a cell-based high-throughput curli-specific screen of 446 compounds, and it seems that at low micromolar and submicromolar concentrations, it did not inhibit cell viability but instead prevented curli gene transcription [233]. Comparisons with a cephalosporin antibiotic further revealed that curli production is not affected by standard antibiotic treatment and cell killing pressure [233]. Indeed, in contrast to the majority of conventional antimicrobial treatments, rifampin has a reasonable activity against biofilm-forming cells [229]. In higher concentrations, rifapentine targets RNA polymerase and is often used to treat tuberculosis [234]. Although it is still debated whether M. tuberculosis forms biofilms in vivo [126], [131], it is interesting to consider the possibility that an additional activity of rifapentine may be the targeting and blocking of MTP formation [125]. Other natural products found to affect E. coli biofilm formation are Indole-3-acetaldehyde isolated from the plant pathogen Rhodococcus sp. strain BF 332 [235] or Ginkgo biloba plant extracts and ginkgolic acids [236]. In both cases, inhibition of biofilm formation was accompanied with a down-regulation of the curli operons csgDEFG and csgBAC. Remarkably, both compounds also significantly reduced the biofilm formation of S. aureus and S. epidermidis [235], [236], although the mechanism was not further investigated in these pathogens. Transcriptome analyses showed that also the apple flavonoid phloretin and low (0.5% v/v) concentrations of honey significantly repressed the curli operon csgBAC in E. coli O157:H7 [237], [238]. In a rat colitis model, phloretin significantly ameliorated colon inflammation [238]. Curli and biofilm production have complex and multifactorial regulons, and with few exceptions, the direct molecular targets of inhibitory compounds are unknown. One notable exception is the molecular chaperone DnaK, which plays an important role in biofilm formation by regulating curli biosynthesis [239]. Chemical inhibition of DnaK cellular functions by myricetin (Myr), a plant-derived flavonoid, prevented the production of curli and consequent biofilm formation in E. coli [240]. Myr also decreased the production of S. aureus virulence, including adhesion, biofilm formation, hemolysis and staphyloxanthin production, without interfering with growth [241]. In this case, Myr most likely exerts its effect by down-regulating the saeR global regulator and interacting with sortase A and α-hemolysin. Myr conferred a significant degree of protection against staphylococcal infection in the in vivo Galleria mellonella model [241].
Functional amyloid assembly can also be inhibited by targeting the amyloid formation process directly at the level of the aggregating protein subunits. Tremendous efforts have gone to the developments of small molecules that inhibit aggregation of amyloid proteins associated with human diseases (reviewed in Refs. [242], [243]). Taken into account some of the common structural properties observed in various amyloids and the shared binding capacity of amyloid-specific dyes as Congo red and ThT, compounds developed for the inhibition of aberrant human amyloid assembly could also be active against bacterial functional amyloids. One such molecule is EGCG, the principal polyphenol present in green tea. EGCG has been postulated to have multiple effects on human pathological and physiological processes, including anti-cancer, antioxidant, anti-inflammatory and anti-fibrosis effects (reviewed in Ref. [244]). Its anti-amyloidogenic activity was demonstrated with amyloid beta (Aβ) and α-synuclein peptides associated with Alzheimer's and Parkinson's neurodegenerative diseases, respectively [245], [246]. EGCG was seen to redirect natively unfolded aggregation-prone polypeptides into a new type of off-pathway, SDS-stable, nontoxic oligomer protein assemblies [245]. The compound also has the ability to convert large, mature α-synuclein and Aβ fibrils into these smaller, amorphous protein aggregates that are nontoxic to mammalian cells [246]. Mechanistic studies indicate that EGCG directly binds β-sheet-rich aggregates and mediates a conformational change without a disassembly into monomers or small diffusible oligomers [246]. While EGCG's antimicrobial potential has been intensively studied for many years (reviewed in Ref. [247]), a detailed molecular understanding of how green tea exerts its broad protective effects against both Gram-positive and Gram-negative pathogens has been lacking. Although the beneficial effects of EGCG are likely attributable to diverse modes of action, it has recently been observed that EGCG may be affecting naturally occurring functional amyloids in a similar way to human disease-associated amyloids. In S. aureus, EGCG was seen to prevent the assembly of amyloidogenic PSMs and progressively disentangles preformed PSM amyloid fibrils, converting them into amorphous aggregates [205]. In P. aeruginosa, EGCG inhibited fibrillation of the major fiber subunit FapC and structurally remodeled existing fibrils in vitro, thereby stabilizing non-amyloid aggregates [248]. This leads to a reduced amyloid content in biofilms in situ and an increased susceptibility of Pseudomonas biofilms to the antibiotic tobramycin [248]. EGCG also showed activity toward P1-induced biofilm formation of S. mutans [13] and completely eliminated curli production by the 2011 German outbreak E. coli strain O104:H4 and related enteroaggregative E. coli [249]. EGCG may act against other bacterial amyloid fibers and have a more general antibiofilm activity. In E. coli, the pronounced anti-biofilm activity of EGCG was found to depend on two complementary modes of action, that is, (i) direct interference in the fiber assembly of curli subunits, and (ii) triggering of the σE-mediated cell envelope stress response which downregulates the biofilm regulator CsgD, required for the biosynthesis of both curli and cellulose, by targeting csgD mRNA via the small RNA RybB [249]. It should be noted, however, that not all effects of EGCG against bacteria are beneficial, and that the triggering of bacterial stress responses could have adverse outcomes. Exposure of Staphylococcus strains to sublethal doses of EGCG, for example, leads to its reduced susceptibility to antibiotics targeting bacterial cell wall synthesis (e.g., vancomycin, oxacillin, ampicillin) [250]. Thus, informed care has to be taken in choosing appropriate combinations of EGCG and antibiotics [241].
Thiazolo ring-fused 2-pyridones form a group of peptidomimetics that can target essential protein-protein interactions in macromolecular assembly including bacterial pilus biogenesis [251]. Substitution of the various R groups on the 2-pyridone ring scaffold provided compounds that inhibited the in vitro polymerization of the major curli subunit CsgA [252], [253]. One of the more potent “curlicides,” FN075, inhibited E. coli biofilm formation, and the pretreatment of UPEC with FN075 resulted in decreased bladder colonization and intracellular biofilm formation in a mouse cystitis model [252]. FN075 also inhibit type 1 pilus assembly, so that it is difficult to compare the relative contribution of the compounds' curlicide versus “pilicide” activity during virulence attenuation. Nevertheless, genetic knockout of the curli biosynthesis pathway showed reduced colonization efficiency in this same mouse model, indicating that curli formation can form an anti-virulence target in itself. FN057's inhibitory activity on CsgA aggregation extends to other amyloids including the Aβ peptide, and functions in a similar manner to EGCG, by stabilizing an off-pathway non-amyloidogenic oligomeric form of the CsgA protein that does not proceed to fibers [252], [254]. Although FN075 inhibits in vitro fibril formation of the human Aβ peptide, the oligomeric species formed by α-synuclein unexpectedly worked as a template that increased the rate of fibrillation [254]. This example demonstrates that small molecules that directly inhibit bacterial amyloid formation could potentially exacerbate or trigger human aggregation diseases, highlighting the importance of assessing the level and kind of cross-reactivity of amyloid inhibitory compounds.
Conversely, let us consider the possibility that the cytotoxicity often associated with the amyloid formation process might be turned against the bacterial pathogens. When assembling functional amyloids, bacteria are likely to have evolved different ways to circumvent or deal with the build-up of toxic intermediates that would harm the producing cell. For example, curli assembly employs a direct nucleation route that is thought to circumvents toxic intermediates [255], and includes a chaperone, CsgC, that inhibits toxicity of premature fiber assembly in the periplasm [225]. When functional amyloid assembly is inhibited, can there be a toxic gain-of-function activity by the off-pathway build-up of toxic species? From this perspective, transporters of the amyloid subunits may form promising therapeutic targets because their inactivation would prevent the translocation aggregation prone peptides to the extracellular space, thereby causing their intracellular accumulation and aggregation. Targeting S. aureus PSMs for therapeutic intervention is hampered by their multitude and diversity. However, as one distinct α-PSM transporter (pmt) is responsible for the export of all PSM classes, pmt represents a potential target to interfere with the production of all PSMs. Indeed, pmt affects key virulence-associated phenotypes to a similarly strong extent as the sum of all PSMs [200]. The pmt transporter is essential for bacterial survival when PSM peptides are expressed. Expression of PSMs in the absence of pmt led to abnormal cell division and severe damage to the cytoplasmic membrane caused by cytosolic accumulation of PSMs, resulting in a loss of bacterial fitness [200]. A later report showed that the deleterious impact of intracellular accumulation of PSMαs in S. aureus upon blockage of their secretion would be essentially exerted by the more soluble PSMα3, and not by PSMα1 and PSMα4, that would likely aggregate into inert inclusions [205]. Interestingly, the induction of protein aggregation may extend beyond functional amyloid pathways. Peptides encoding target-specific aggregation-prone sequence segments were recently demonstrated to have antimicrobial activity by entering and accumulating in the bacterial cytosol where they initiate toxic aggregation of bacterial proteins [256]. Such peptides displayed a strong bactericidal effect against important human pathogens such as S. aureus and Enterococcus faecalis, as well as their drug resistant derivatives. Administration of the peptides cured mice from sepsis caused by S. aureus, without apparent toxic side effects, as judged from histological and hematological evaluation of mice tissues [256].
Finally, functional amyloids may serve as target for vaccination and other immunomodulatory strategies. Active and passive immunization with P1 antigen or with P1 specific antibodies (e.g., expressed in lactobacilli) can prevent S. mutans colonization and protect rodents and non-human primates from experimental dental caries (reviewed in Refs. [257], [258], [259]). Antibodies against S. epidermidis PSM peptides inhibited bacterial spread from indwelling medical devices suggesting that interference with biofilm detachment mechanisms may prevent dissemination of biofilm-associated infections [260]. A vaccination approach was also suggested for M. tuberculosis MTP pili, as the presence of MTP-specific antibodies represents a powerful indication that the antigen is produced during natural infection [26]. The mtp gene is further conserved in M. tuberculosis complex strains, but not in non-tuberculous mycobacteria or other respiratory bacteria [120], making it a prime vaccine candidate [261].
Perspectives
Since the discovery of curli and the realization that these bacterial surface fibers represent an amyloid structure that is the product of a dedicated biosynthetic pathway [8], [9], at least 10 distinct bacterial amyloids have been recognized [8], [10], [11], [12], [13], [14], [15], [16]. These systems differ in the secretion and assembly pathways they employ, as well as in the amyloidogenic subunit(s), which are non-homologous and expected to differ in fiber ultrastructure. They are found in diverse bacterial phyla and are likely to represent just a fraction of the amyloid repertoire in bacteria. Here we reviewed those bacterial amyloids that are found in pathogenic species and which may have a direct or indirect role in virulence and disease (Fig. 4, Table 1). Based on available genetic, biochemical and structural information, one can make a distinction between intrinsic and facultative bacterial amyloids (Fig. 2). In the former, the amyloid state represents the primary structural and functional state of the composing proteins, which are part of a biosynthetic pathway dedicated to the assembly of the functional amyloid structure. Among the known bacterial amyloids, curli, fap, MTP, chaplins and rodlins can be recognized as intrinsic bacterial amyloids. Their primary function appears to be as a structuring and supporting component of the ECM in biofilms or spore coats, and as an adhesive structure. In facultative amyloids on the other hand, the composing protein subunits can attain a globular folded state, as well as a fibrous amyloid state (Fig. 2). In these systems, the amyloid state appears a secondary feature and does not necessarily comprise a functional form of the protein. In the known examples, the folded state of the individual subunits can serve as adhesin (P1 and Bap) [134], [184], belong to a protein family with enzymatic function (TasA, SuhB) [154], [194], or function as a toxin (PSMs) [198]. These proteins can form amyloid fibers as a result of proteolytic processing or environmental triggers such as pH and divalent ion concentrations. For TasA, TapA, Bap and P1, the resulting fibers have been shown or suggested to serve as ECM component during biofilm formation [138], [154], [183]. For others, it is not clear whether the amyloid conformation represents a functional state. An interesting possibility is that the amyloid state is used as a means to inactivate or store the composing protomers. Such reservoir mechanism has been proposed for microcin E492 (mccE492), an 84-residue pore-forming bacteriotoxin, produced by Klebsiella pneumoniae RYC492 to kill a variety of competing Enterobacteria [262]. Apart from the active monomeric form, secreted mccE492 can be found in a second β-sheet-rich conformer that forms amyloid-like fibers [263], which can dissociate to the active monomers by exposure to high-pH or low-ionic-strength conditions as well as upon dilution [264]. The reversible formation of inactive β-rich fibrils is thought to provide a post-translation control of bacteriocin activity depending on environmental and quorum-related conditions. Also for TasA, P1 and PSMs, the amyloid-like fibrils have been found able to dissociate into the monomeric subunits under physiological conditions [13], [50], [154], [183]. This is in contrast with curli, where the adoption of the amyloid state is quasi-irreversible, with little to no dissociation of protomers from the fibrils even with high concentrations of chaotropes such as urea or guanidinium hydrochloride, or when monomers are depleted from the surrounding solution [12], [255]. In curli and other known intrinsic bacterial amyloids, the monomeric subunits appear to have no discernible function other than to serve as building blocks for the fibrils. Reversible association–dissociation of these amyloid fibrils would not likely have any functional advantage, unless to provide a means to alter the concentration and spatiotemporal occurrence of the amyloid fibers. Whether bacteria can induce the dissociation or degradation of curli, fap or MTP as a control mechanism for ECM integrity or adherence properties is unknown, however.
Fig. 4.
Summarizing scheme of direct and indirect implication of bacterial amyloids in infectious disease and bacterial virulence.
Table 1.
Summary of known bacterial amyloids, their taxonomic distribrution (“pathogens” lists most important pathogenic species, non-exhaustively; “model organism” lists primary organism used in amyloid studies), their genetic organization [amyloidogenic subunits are in bold, (putative) cell anchoring subunits are underscored] and the structural characteristics of the amyloidogenic subunits and the fiber, as well as their function and potential role as virulence determinant
| Functional amyloids | Occurrence | Genetic organisation | Structural and physical properties | Attributed functionalities and role in virulence | Evidence for amyloid nature |
|---|---|---|---|---|---|
| Intrinsic bacterial amyloids | |||||
| Curli |
Taxa: Proteobacteria, Bacteroidetes Pathogens: pathogenic E. coli, Salmonella Model:E. coli |
csgDEFG; csgBAC |
Fiber: 2-5 nm in width, SDS and protease resistant Subunits: CsgA, CsgB 150–1300 aa; SP followed by an amyloidogenic core of concatenated pseudorepeats of ~ 22 aa |
Biofilm matrix scaffolding; adhesion to ECM and serum proteins including fibronectin, laminin, plasminogen, and human contact phase proteins; adherence and invasion of eukaryotic host cells; interacts with MHC-I and TLR1 and TLR2; sequesters the antimicrobial peptide LL-37; sequesters bacteriophages |
CR, ThT, CD, FTIR, AFM, XRD, EM |
| Fap |
Taxa: γ-, β-, δ-Proteobacteria Pathogens:P. aeruginosa Model:P. aeruginosa |
fapABCDEF |
Fiber: ~ 10 nm in width, SDS and protease resistant Subunits: FapB/A: 180–340 aa, SP followed by N-terminal domain (~ 37 aa), an amyloid core with 3 repeats (R1–3) of 17/37 aa, separated by 2 variable linker regions (L1–2) of 30 to 275 aa |
Biofilm formation; attachment to abiotic surfaces; virulence factor in C. elegans infection model and PMN leukocyte phagocytosis assay; retention of QS molecules | CR, ThT, CD, FTIR, AFM, EM, amyloid-specific conformational antibodies |
| MTP |
Taxa: Mycobacterium Pathogens:M. tuberculosis Model:M. tuberculosis |
Single gene, mtp |
Fiber: 2–3 nm in width, SDS resistant Subunit: MTP: ~ 100 aa; SP followed by single amyloidogenic domain of ~ 70 aa |
In vitro biofilm formation and bacterial aggregation; adhesion to ECM protein laminin, pulmonary epithelial cells; adhesion to and invasion of THP-1 macrophages |
CR, EM |
| Chaplins/Rodlins |
Taxa: Streptomyces Pathogens:S. scabies, S. sudanensis, S. somaliensis Model:S. coelicolor |
All monocistronic: chpC–chpH; chpF–chpG; chpB; chpE; chpA–chpD–rdlA–rdlB |
Fiber: Variable width between 3.6 and 17.6 nm, protease resistance, pH-dependent fibrillation for ChpE. Subunits: ChpD–H: 50-60 aa; SP followed by hydrophobic chaplin domain (~ 40 aa)/RdlB ~ 133 aa; SP followed by amyloidogenic domain |
Aerial hyphae development, adherence to abiotic surfaces |
[Chaplins] EM, XRD, CD, FTIR, CR, ThT, AFM [Rodlins] EM, ThT, XRD |
| Facultative bacterial amyloids | |||||
| P1 |
Taxa: Streptococcus Pathogens:S. mutans, S. pyogenes Model:S. mutans |
Single gene, p1 |
Fiber: 7–12 nm in width, SDS resistant Subunits: spaP (AgI/II): ~ 1500 aa; SP followed by multidomain region; proteolytic processing of preprotein into C-terminal ~ 500 residues (C123 or AgII) forms amyloidogenic fragment |
Native form: functions as adhesin Fibrous form: implicated in biofilm matrix scaffolding; adhesion (?); induction of pro-inflammatory mediators |
CR, ThT, EM, CD |
| TasA |
Taxa: Bacillus Pathogens:B. cereus group Model:B. subtilis |
tapA–sipW–tasA |
Fiber: 10–15 nm in width, SDS and protease resistant, pH-dependent fibrillation Subunits: TasA ~ 260 aa; SP followed by M73 proteinase family jelly-roll fold that is amyloidogenic at low pH and on hydrophobic surfaces |
Native form: function unknown, enzymatic activity (?), antimicrobial activity (?) Fibrous form: biofilm matrix scaffolding, antimicrobial activity (?) |
CR, ThT, EM, CD, AFM, FTIR, XRD |
| Bap |
Taxa: Staphylococcus Pathogens:S. aureus; coagulase-negative Staphylococci Model:S. aureus |
Single gene, bap |
Fiber: ~ 10 nm in width Subunits: Bap ~ 2300 aa; SP followed by multidomain region; the proteolytic BapB (residue ~ 360–820) and BapC (residues ~ 820–2210) fragments show calcium and pH-dependent fibrillation, and contain aggregation-prone sequences resp. |
Virulence factor in mouse model of S. aureus bacteremia. Native form: Adhesion; binding to GP96 Fibrous form: Biofilm matrix scaffolding |
ATR-FTIR, CD, EM, CR, ThT, Proteostat |
| PSMs |
Taxa: Staphylococcus Pathogens: S. aureus; coagulase-negative Staphylococci Model:S. aureus |
All monocistronic: psmα1–4; psmβ1–2; hld region in RNAIII encodes δ-toxin |
Fiber: ~ 12 nm in width, SDS and protease resistance Subunits:PSMα/δ-toxin: 20–25 aa α-helical peptides. PSMβ: 45 aa helix-turn-helix peptides |
Native form: cytolytic toxin, induces macrophage inflammation and cytokine release Fibrous form: biofilm matrix scaffolding, reversible storage form of cytolytic peptides (?) |
ATR-FTIR, CD, CR, ThT |
The table also summarizes the experimental evidence for the proposed amyloid nature of the respective systems (CR, congo red binding; ThT, thioflavin T fluorescence; CD, circular dichroism; (ATR-)FTIR, (attenuated total reflection-) Fourier-transform infrared spectroscopy; AFM, atomic force microscopy; XRD, X-ray diffraction; EM, electron microscopy). See main text for references.
The role of bacterial amyloids in virulence is diverse (Fig. 4). As a component of the ECM in biofilm, they can increase the producing bacteria's ability to withstand changing and challenging environmental conditions. Biofilm formation can promote persistence in the host, as well as facilitate spread by enhancing a pathogens' survival outside the host, including on medical devices and clinical facilities. Fouling of devices and surfaces can further be facilitated by adhesive properties of the fibers [42] (Fig. 1). When acting as adhesive structures, this is likely through a high multivalency of non-specific interactions with biotic and abiotic surfaces and polymers, rather than via specific high- or medium-affinity interactions as seen in most canonical bacterial adhesins [265]. Several bacterial amyloids have also been found to bind with host matrix proteins such as fibronectin, fibrinogen, laminin and collagen [8], [11], [98], [140], although the contribution of these interactions to the infectious disease development is often difficult to discern. While these properties have the potential to increase pathogen virulence and persistence, this can be local and temporary. Indeed the presence of bacterial amyloids can trigger strong host responses and may then expedite pathogen clearance. As an example, while in enteric E. coli infections, curli can enhance colonization and persistence, they appear to be under negative selection in invasive Enterobacteriaceae [61], [81], [82], [83], [84], [85], [86]. However, when not acting as a bacterial virulence factor per se, exposure to bacterial amyloids or amyloid-producing bacteria can bring important consequences for disease development. Bacterial amyloid fibers can induce local and systemic inflammation [79], [100], and at least curli have been found to activate the contact phase system, thought to result in life-threatening blood coagulation disorders during sepsis with curli-expressing bacteria [8], [58], [59], [60], [61], [99]. These are indirect effects, coming from host-responses toward bacterial amyloids fibers. To date, there is no evidence that toxicity of bacterial amyloid fibers or of any intermediates en route to fiber formation is used as a virulence mechanism by pathogens. Rather, it appears that at least for intrinsic bacterial amyloids such a curli, the pathway has evolved to avoid or neutralize the formation of any toxic species in the amyloid formation process [225], [255]. A point garnering attention is the possibility that bacterial amyloids instigate or exaggerate the amyloidosis of host amyloid-prone proteins and can thereby have a link to the development of neurodegenerative disorders [228]. The ability of bacterial amyloids to cross-seed human amyloid deposits or the possibility that bacterial amyloid-induced inflammation sets off human amyloidosis will be an important point of future investigation to evaluate the longer-term effects of exposure to amyloid-producing bacteria or bacterial amyloid products. The level of inertness of bacterial amyloids and derivative fibers and materials will important also in the context of their possible use as biomaterial in different biotech applications [266].
Finally, because of their direct or indirect implications in bacterial virulence, attempts have been made to target the formation of different bacterial amyloids as a contribution in antibacterial or anti-virulence therapy. The two main approaches emerging are the inhibition of the biosynthetic pathways at the transcriptional level, and the development of inhibitors that block the amyloid formation process itself. Several compounds known to inhibit fibrillation of pathological amyloids have also shown a level of activity toward different bacterial amyloids, and vice versa. However, caution should be taken to carefully evaluate the level and type of cross-reactivity of such compounds, since some inhibitors of bacterial amyloids were found to facilitate the amyloid transition of host amyloids and may thus aggravate human aggregation diseases [254].
The discovery of bacterial and other functional amyloids has led to the realization that the amyloid fold, long thought to be exclusively associated with disease, can be used as a functional fold in biological systems. This allows us to address important questions related to the mechanisms of toxicity or toxicity avoidance in pathological and functional amyloids, can inform on inhibitory strategies, and may open routes to exploit interesting properties of the fold such as self-assembly and mechanical strength for biotechnological use. Functional amyloids have proven widespread in bacteria and many more are likely to be discovered. It will be interesting to see the new discovery, application and possibly inhibition of bacterial amyloids develop over the coming years.
Acknowledgments
We acknowledge financial support from VIB and ERC through consolidator grant BAS-SBBT (No. 649082). S.V.d.V. is supported by FWO via a PhD fellowship.
Declarations of Interest: N.V.G., S.V.d.V. and H.R. hold IP related to the use of curli pathway components in biotechnological applications.
Edited by Sven J Saupe
References
- 1.Sipe J.D., Cohen A.S. Review: history of the amyloid fibril. J. Struct. Biol. 2000;130:88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg D.S., Sawaya M.R. Structural studies of amyloid proteins at the molecular level. Annu. Rev. Biochem. 2017;86:69–95. doi: 10.1146/annurev-biochem-061516-045104. [DOI] [PubMed] [Google Scholar]
- 3.Chiti F., Dobson C.M. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu. Rev. Biochem. 2017;86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- 4.Fowler D.M., Koulov A.V., Balch W.E., Kelly J.W. Functional amyloid—from bacteria to humans. Trends Biochem. Sci. 2007;32:217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Larsen P., Nielsen J.L., Dueholm M.S., Wetzel R., Otzen D., Nielsen P.H. Amyloid adhesins are abundant in natural biofilms. Environ. Microbiol. 2007;9:3077–3090. doi: 10.1111/j.1462-2920.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- 6.Larsen P., Nielsen J.L., Otzen D., Nielsen P.H. Amyloid-like adhesins produced by floc-forming and filamentous bacteria in activated sludge. Appl. Environ. Microbiol. 2008;74:1517–1526. doi: 10.1128/AEM.02274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dueholm M.S., Albertsen M., Otzen D., Nielsen P.H. Curli functional amyloid systems are phylogenetically widespread and display large diversity in operon and protein structure. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen A., Jonsson A., Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989;338:652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- 9.Chapman M.R., Robinson L.S., Pinkner J.S., Roth R., Heuser J., Hammar M., Normark S., Hultgren S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dueholm M.S., Petersen S.V., Sonderkaer M., Larsen P., Christiansen G., Hein K.L., Enghild J.J., Nielsen J.L., Nielsen K.L., Nielsen P.H., Otzen D.E. Functional amyloid in Pseudomonas. Mol. Microbiol. 2010;77:1009–1020. doi: 10.1111/j.1365-2958.2010.07269.x. [DOI] [PubMed] [Google Scholar]
- 11.Alteri C.J., Xicohténcatl-Cortes J., Hess S., Caballero-Olín G., Girón J.A., Friedman R.L. Mycobacterium tuberculosis produces pili during human infection. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5145–5150. doi: 10.1073/pnas.0602304104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collinson S.K., Emody L., Muller K.H., Trust T.J., Kay W.W. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J. Bacteriol. 1991;173:4773–4781. doi: 10.1128/jb.173.15.4773-4781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oli M.W., Otoo H.N., Crowley P.J., Heim K.P., Nascimento M.M., Ramsook C.B., Lipke P.N., Brady L.J. Functional amyloid formation by Streptococcus mutans. Microbiol. Read. Engl. 2012;158:2903–2916. doi: 10.1099/mic.0.060855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero D., Aguilar C., Losick R., Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz K., Syed A.K., Stephenson R.E., Rickard A.H., Boles B.R. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taglialegna A., Navarro S., Ventura S., Garnett J.A., Matthews S., Penades J.R., Lasa I., Valle J. Staphylococcal Bap proteins build amyloid scaffold biofilm matrices in response to environmental signals. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shewmaker F., McGlinchey R.P., Wickner R.B. Structural insights into functional and pathological amyloid. J. Biol. Chem. 2011;286:16533–16540. doi: 10.1074/jbc.R111.227108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawaya M.R., Sambashivan S., Nelson R., Ivanova M.I., Sievers S.A., Apostol M.I., Thompson M.J., Balbirnie M., Wiltzius J.J.W., McFarlane H.T., Madsen A.Ø., Riekel C., Eisenberg D. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447:453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 19.Riek R. The three-dimensional structures of amyloids. Cold Spring Harb. Perspect. Biol. 2017;9 doi: 10.1101/cshperspect.a023572. (pii:a023572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenwald J., Riek R. Biology of amyloid: structure, function, and regulation. Struct. Lond. Engl. 2010;1993(18):1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Nordstedt C., Näslund J., Tjernberg L.O., Karlström A.R., Thyberg J., Terenius L. The Alzheimer A beta peptide develops protease resistance in association with its polymerization into fibrils. J. Biol. Chem. 1994;269:30773–30776. [PubMed] [Google Scholar]
- 22.Sitaras C., Naghavi M., Herrington M.B. Sodium dodecyl sulfate-agarose gel electrophoresis for the detection and isolation of amyloid curli fibers. Anal. Biochem. 2011;408:328–331. doi: 10.1016/j.ab.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Groenning M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J. Chem. Biol. 2010;3:1–18. doi: 10.1007/s12154-009-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisert R., Felau L., Brown L.R. Methods for enhancing the accuracy and reproducibility of Congo red and thioflavin T assays. Anal. Biochem. 2006;353:144–146. doi: 10.1016/j.ab.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Zogaj X., Bokranz W., Nimtz M., Römling U. Production of cellulose and curli fimbriae by members of the family Enterobacteriaceae isolated from the human gastrointestinal tract. Infect. Immun. 2003;71:4151–4158. doi: 10.1128/IAI.71.7.4151-4158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khurana R., Uversky V.N., Nielsen L., Fink A.L. Is Congo red an amyloid-specific dye? J. Biol. Chem. 2001;276:22715–22721. doi: 10.1074/jbc.M011499200. [DOI] [PubMed] [Google Scholar]
- 27.Jarrett J.T., Lansburry P.T. Seeding “one dimensional cristallization” of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 28.Arosio P., Knowles T.P.J., Linse S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015;17:7606–7618. doi: 10.1039/c4cp05563b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Toole G., Kaplan H.B., Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 30.Davies D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 31.Hall-Stoodley L., Stoodley P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009;11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 32.Flemming H.-C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 33.Flemming H.-C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 34.Davey M.E., O’toole G.A. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000;64:847–867. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Götz F. Staphylococcus and biofilms. Mol. Microbiol. 2002;43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 36.McDougald D., Rice S.A., Barraud N., Steinberg P.D., Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2011;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- 37.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 38.Serra D.O., Richter A.M., Hengge R. Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J. Bacteriol. 2013;195:5540–5554. doi: 10.1128/JB.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCrate O.A., Zhou X., Reichhardt C., Cegelski L. Sum of the parts: composition and architecture of the bacterial extracellular matrix. J. Mol. Biol. 2013;425:4286–4294. doi: 10.1016/j.jmb.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serra D.O., Richter A.M., Klauck G., Mika F., Hengge R. Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. MBio. 2013;4:e00103–e00113. doi: 10.1128/mBio.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taglialegna A., Lasa I., Valle J. Amyloid structures as biofilm matrix scaffolds. J. Bacteriol. 2016;198:2579–2588. doi: 10.1128/JB.00122-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May T., Okabe S. Escherichia coli harboring a natural IncF conjugative F plasmid develops complex mature biofilms by stimulating synthesis of colanic acid and curli. J. Bacteriol. 2008;190:7479–7490. doi: 10.1128/JB.00823-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serra D.O., Klauck G., Hengge R. Vertical stratification of matrix production is essential for physical integrity and architecture of macrocolony biofilms of Escherichia coli. Environ. Microbiol. 2015;17:5073–5088. doi: 10.1111/1462-2920.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alteri C. Graduate College University of Arizona; 2005. Novel pili of Mycobacterium tuberculosis. (Ph.D. thesis) [Google Scholar]
- 45.Kobayashi K., Iwano M. BslA(YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol. Microbiol. 2012;85:51–66. doi: 10.1111/j.1365-2958.2012.08094.x. [DOI] [PubMed] [Google Scholar]
- 46.Vidakovic L., Singh P.K., Hartmann R., Nadell C.D., Drescher K. Dynamic biofilm architecture confers individual and collective mechanisms of viral protection. Nat. Microbiol. 2018;3:26–31. doi: 10.1038/s41564-017-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grund S., Weber A. A new type of fimbriae on Salmonella typhimurium. Zentralbl. Veterinarmed. B. 1988;35:779–782. doi: 10.1111/j.1439-0450.1988.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 48.Bian Z., Normark S. Nucleator function of CsgB for the assembly of adhesive surface organelles in Escherichia coli. EMBO J. 1997;16:5827–5836. doi: 10.1093/emboj/16.19.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collinson S.K., Clouthier S.C., Doran J.L., Banser P.A., Kay W.W. Characterization of the agfBA fimbrial operon encoding thin aggregative fimbriae of Salmonella enteritidis. Adv. Exp. Med. Biol. 1997;412:247–248. doi: 10.1007/978-1-4899-1828-4_37. [DOI] [PubMed] [Google Scholar]
- 50.Zeng G., Vad B.S., Dueholm M.S., Christiansen G., Nilsson M., Tolker-Nielsen T., Nielsen P.H., Meyer R.L., Otzen D.E. Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front. Microbiol. 2015;6:1099. doi: 10.3389/fmicb.2015.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caro-Astorga J., Pérez-García A., de Vicente A., Romero D. A genomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms. Front. Microbiol. 2015;5 doi: 10.3389/fmicb.2014.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Jong W., Wösten H.A.B., Dijkhuizen L., Claessen D. Attachment of Streptomyces coelicolor is mediated by amyloidal fimbriae that are anchored to the cell surface via cellulose. Mol. Microbiol. 2009;73:1128–1140. doi: 10.1111/j.1365-2958.2009.06838.x. [DOI] [PubMed] [Google Scholar]
- 53.Di Berardo C., Capstick D.S., Bibb M.J., Findlay K.C., Buttner M.J., Elliot M.A. Function and redundancy of the chaplin cell surface proteins in aerial hypha formation, rodlet assembly, and viability in Streptomyces coelicolor. J. Bacteriol. 2008;190:5879–5889. doi: 10.1128/JB.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collinson S.K., Parker J.M., Hodges R.S., Kay W.W. Structural predictions of AgfA, the insoluble fimbrial subunit of Salmonella thin aggregative fimbriae. J. Mol. Biol. 1999;290:741–756. doi: 10.1006/jmbi.1999.2882. [DOI] [PubMed] [Google Scholar]
- 55.Wang X., Smith D.R., Jones J.W., Chapman M.R. In vitro polymerization of a functional Escherichia coli amyloid protein. J. Biol. Chem. 2007;282:3713–3719. doi: 10.1074/jbc.M609228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hammar M., Arnqvist A., Bian Z., Olsen A., Normark S. Expression of two csg operons is required for production of fibronectin- and congo red-binding curli polymers in Escherichia coli K-12. Mol. Microbiol. 1995;18:661–670. doi: 10.1111/j.1365-2958.1995.mmi_18040661.x.. [DOI] [PubMed] [Google Scholar]
- 57.Van Gerven N., Klein R.D., Hultgren S.J., Remaut H. Bacterial amyloid formation: structural insights into curli biogensis. Trends Microbiol. 2015;23:693–706. doi: 10.1016/j.tim.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herwald H., Morgelin M., Olsen A., Rhen M., Dahlback B., Muller-Esterl W., Bjorck L. Activation of the contact-phase system on bacterial surfaces—a clue to serious complications in infectious diseases. Nat. Med. 1998;4:298–302. doi: 10.1038/nm0398-298. [DOI] [PubMed] [Google Scholar]
- 59.Olsen A., Herwald H., Wikstrom M., Persson K., Mattsson E., Bjorck L. Identification of two protein-binding and functional regions of curli, a surface organelle and virulence determinant of Escherichia coli. J. Biol. Chem. 2002;277:34568–34572. doi: 10.1074/jbc.M206353200. [DOI] [PubMed] [Google Scholar]
- 60.Barnhart M.M., Chapman M.R. Curli biogenesis and function. Annu. Rev. Microbiol. 2006;60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sjöbring U., Pohl G., Olsén A. Plasminogen, absorbed by Escherichia coli expressing curli or by Salmonella enteritidis expressing thin aggregative fimbriae, can be activated by simultaneously captured tissue-type plasminogen activator (t-PA) Mol. Microbiol. 1994;14:443–452. doi: 10.1111/j.1365-2958.1994.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 62.Vidal O., Longin R., Prigent-Combaret C., Dorel C., Hooreman M., Lejeune P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 1998;180:2442–2449. doi: 10.1128/jb.180.9.2442-2449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prigent-Combaret C., Prensier G., Le Thi T.T., Vidal O., Lejeune P., Dorel C. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colanic acid. Environ. Microbiol. 2000;2:450–464. doi: 10.1046/j.1462-2920.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 64.Jonas K., Tomenius H., Kader A., Normark S., Römling U., Belova L.M., Melefors O. Roles of curli, cellulose and BapA in Salmonella biofilm morphology studied by atomic force microscopy. BMC Microbiol. 2007;7:70. doi: 10.1186/1471-2180-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Austin J.W., Sanders G., Kay W.W., Collinson S.K. Thin aggregative fimbriae enhance Salmonella enteritidis biofilm formation. FEMS Microbiol. Lett. 1998;162:295–301. doi: 10.1111/j.1574-6968.1998.tb13012.x. [DOI] [PubMed] [Google Scholar]
- 66.Hufnagel D.A., Depas W.H., Chapman M.R. The biology of the Escherichia coli extracellular matrix. Microbiol. Spectr. 2015;3 doi: 10.1128/microbiolspec.MB-0014-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Römling U., Rohde M., Olsén A., Normark S., Reinköster J. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 2000;36:10–23. doi: 10.1046/j.1365-2958.2000.01822.x. [DOI] [PubMed] [Google Scholar]
- 68.Zogaj X., Nimtz M., Rohde M., Bokranz W., Römling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 2001;39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]
- 69.Lembré P., Di Martino P., Vendrely C. Amyloid peptides derived from CsgA and FapC modify the viscoelastic properties of biofilm model matrices. Biofouling. 2014;30:415–426. doi: 10.1080/08927014.2014.880112. [DOI] [PubMed] [Google Scholar]
- 70.Chauret C. Survival and control of Escherichia coli O157:H7 in foods, beverages, soil and water. Virulence. 2011;2:593–601. doi: 10.4161/viru.2.6.18423. [DOI] [PubMed] [Google Scholar]
- 71.Ryu J.H., Kim H., Frank J.F., Beuchat L.R. Attachment and biofilm formation on stainless steel by Escherichia coli O157:H7 as affected by curli production. Lett. Appl. Microbiol. 2004;39:359–362. doi: 10.1111/j.1472-765X.2004.01591.x. [DOI] [PubMed] [Google Scholar]
- 72.Chekabab S.M., Daigle F., Charette S.J., Dozois C.M., Harel J. Survival of enterohemorrhagic Escherichia coli in the presence of Acanthamoeba castellanii and its dependence on Pho regulon. Microbiology Open. 2012;1:427–437. doi: 10.1002/mbo3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frömmel U., Lehmann W., Rödiger S., Böhm A., Nitschke J., Weinreich J., Groß J., Roggenbuck D., Zinke O., Ansorge H., Vogel S., Klemm P., Wex T., Schröder C., Wieler L.H., Schierack P. Adhesion of human and animal Escherichia coli strains in association with their virulence-associated genes and phylogenetic origins. Appl. Environ. Microbiol. 2013;79:5814–5829. doi: 10.1128/AEM.01384-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schiebel J., Böhm A., Nitschke J., Burdukiewicz M., Weinreich J., Ali A., Roggenbuck D., Rödiger S., Schierack P. Genotypic and phenotypic characteristics associated with biofilm formation by human clinical Escherichia coli isolates of different pathotypes. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.01660-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Römling U., Sierralta W.D., Eriksson K., Normark S. Multicellular and aggregative behaviour of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 1998;28:249–264. doi: 10.1046/j.1365-2958.1998.00791.x. [DOI] [PubMed] [Google Scholar]
- 76.Kikuchi T., Mizunoe Y., Takade A., Naito S., Yoshida S. Curli fibers are required for development of biofilm architecture in Escherichia coli K-12 and enhance bacterial adherence to human uroepithelial cells. Microbiol. Immunol. 2005;49:875–884. doi: 10.1111/j.1348-0421.2005.tb03678.x. [DOI] [PubMed] [Google Scholar]
- 77.Kai-Larsen Y., Lüthje P., Chromek M., Peters V., Wang X., Holm Å., Kádas L., Hedlund K.-O., Johansson J., Chapman M.R., Jacobson S.H., Römling U., Agerberth B., Brauner A. Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maurer J.J., Brown T.P., Steffens W.L., Thayer S.G. The occurrence of ambient temperature-regulated adhesins, curli, and the temperature-sensitive hemagglutinin tsh among avian Escherichia coli. Avian Dis. 1998;42:106–118. [PubMed] [Google Scholar]
- 79.Bian Z., Brauner A., Li Y., Normark S. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J. Infect. Dis. 2000;181:602–612. doi: 10.1086/315233. [DOI] [PubMed] [Google Scholar]
- 80.Bokranz W., Wang X., Tschäpe H., Römling U. Expression of cellulose and curli fimbriae by Escherichia coli isolated from the gastrointestinal tract. J. Med. Microbiol. 2005;54:1171–1182. doi: 10.1099/jmm.0.46064-0. [DOI] [PubMed] [Google Scholar]
- 81.Cookson A.L., Cooley W.A., Woodward M.J. The role of type 1 and curli fimbriae of Shiga toxin-producing Escherichia coli in adherence to abiotic surfaces. Int. J. Med. Microbiol. 2002;292:195–205. doi: 10.1078/1438-4221-00203. [DOI] [PubMed] [Google Scholar]
- 82.Sakellaris H., Hannink N.K., Rajakumar K., Bulach D., Hunt M., Sasakawa C., Adler B. Curli loci of Shigella spp. Infect. Immun. 2000;68:3780–3783. doi: 10.1128/iai.68.6.3780-3783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uhlich G.A., Chen C.-Y., Cottrell B.J., Hofmann C.S., Dudley E.G., Strobaugh T.P., Nguyen L.-H. Phage insertion in mlrA and variations in rpoS limit curli expression and biofilm formation in Escherichia coli serotype O157: H7. Microbiol. Read. Engl. 2013;159:1586–1596. doi: 10.1099/mic.0.066118-0. [DOI] [PubMed] [Google Scholar]
- 84.Uhlich G.A., Keen J.E., Elder R.O. Mutations in the csgD promoter associated with variations in curli expression in certain strains of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2001;67:2367–2370. doi: 10.1128/AEM.67.5.2367-2370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gerstel U., Römling U. The csgD promoter, a control unit for biofilm formation in Salmonella typhimurium. Res. Microbiol. 2003;154:659–667. doi: 10.1016/j.resmic.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 86.MacKenzie K.D., Palmer M.B., Köster W.L., White A.P. Examining the link between biofilm formation and the ability of pathogenic Salmonella strains to colonize multiple host species. Front. Vet. Sci. 2017;4:138. doi: 10.3389/fvets.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.White A.P., Gibson D.L., Grassl G.A., Kay W.W., Finlay B.B., Vallance B.A., Surette M.G. Aggregation via the red, dry, and rough morphotype is not a virulence adaptation in Salmonella enterica serovar Typhimurium. Infect. Immun. 2008;76:1048–1058. doi: 10.1128/IAI.01383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oppong G.O., Rapsinski G.J., Newman T.N., Nishimori J.H., Biesecker S.G., Tükel Ç. Epithelial cells augment barrier function via activation of the Toll-like receptor 2/phosphatidylinositol 3-kinase pathway upon recognition of Salmonella enterica serovar Typhimurium curli fibrils in the gut. Infect. Immun. 2013;81:478–486. doi: 10.1128/IAI.00453-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oppong G.O., Rapsinski G.J., Tursi S.A., Biesecker S.G., Klein-Szanto A.J., Goulian M., McCauley C., Healy C., Wilson R.P., Tükel C. Biofilm-associated bacterial amyloids dampen inflammation in the gut: oral treatment with curli fibres reduces the severity of hapten-induced colitis in mice. NPJ Biofilms Microbiomes. 2015;1 doi: 10.1038/npjbiofilms.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gophna U., Barlev M., Seijffers R., Oelschlager T.A., Hacker J., Ron E.Z. Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect. Immun. 2001;69:2659–2665. doi: 10.1128/IAI.69.4.2659-2665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johansson C., Nilsson T., Olsen A., Wick M.J. The influence of curli, a MHC-I-binding bacterial surface structure, on macrophage-T cell interactions. FEMS Immunol. Med. Microbiol. 2001;30:21–29. doi: 10.1111/j.1574-695X.2001.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 92.Uhlich G.A., Keen J.E., Elder R.O. Variations in the csgD promoter of Escherichia coli O157:H7 associated with increased virulence in mice and increased invasion of HEp-2 cells. Infect. Immun. 2002;70:395–399. doi: 10.1128/IAI.70.1.395-399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X., Rochon M., Lamprokostopoulou A., Lünsdorf H., Nimtz M., Römling U. Impact of biofilm matrix components on interaction of commensal Escherichia coli with the gastrointestinal cell line HT-29. Cell. Mol. Life Sci. 2006;63:2352–2363. doi: 10.1007/s00018-006-6222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gophna U., Oelschlaeger T.A., Hacker J., Ron E.Z. Role of fibronectin in curli-mediated internalization. FEMS Microbiol. Lett. 2002;212:55–58. doi: 10.1111/j.1574-6968.2002.tb11244.x. [DOI] [PubMed] [Google Scholar]
- 95.La Ragione R.M., Collighan R.J., Woodward M.J. Non-curliation of Escherichia coli O78:K80 isolates associated with IS1 insertion in csgB and reduced persistence in poultry infection. FEMS Microbiol. Lett. 1999;175:247–253. doi: 10.1111/j.1574-6968.1999.tb13627.x. [DOI] [PubMed] [Google Scholar]
- 96.Weening E.H., Barker J.D., Laarakker M.C., Humphries A.D., Tsolis R.M., Bäumler A.J. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect. Immun. 2005;73:3358–3366. doi: 10.1128/IAI.73.6.3358-3366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saldaña Z., Xicohtencatl-Cortes J., Avelino F., Phillips A.D., Kaper J.B., Puente J.L., Girón J.A. Synergistic role of curli and cellulose in cell adherence and biofilm formation of attaching and effacing Escherichia coli and identification of Fis as a negative regulator of curli. Environ. Microbiol. 2009;11:992–1006. doi: 10.1111/j.1462-2920.2008.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olsen A., Arnqvist A., Hammar M., Normark S. Environmental regulation of curli production in Escherichia coli. Infect. Agents Dis. 1993;2:272–274. [PubMed] [Google Scholar]
- 99.Ben Nasr A., Olsén A., Sjöbring U., Müller-Esterl W., Björck L. Assembly of human contact phase proteins and release of bradykinin at the surface of curli-expressing Escherichia coli. Mol. Microbiol. 1996;20:927–935. doi: 10.1111/j.1365-2958.1996.tb02534.x. [DOI] [PubMed] [Google Scholar]
- 100.Bian Z., Yan Z.Q., Hansson G.K., Thorén P., Normark S. Activation of inducible nitric oxide synthase/nitric oxide by curli fibers leads to a fall in blood pressure during systemic Escherichia coli infection in mice. J. Infect. Dis. 2001;183:612–619. doi: 10.1086/318528. [DOI] [PubMed] [Google Scholar]
- 101.Tükel C., Nishimori J.H., Wilson R.P., Winter M.G., Keestra A.M., van Putten J.P.M., Bäumler A.J. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell. Microbiol. 2010;12:1495–1505. doi: 10.1111/j.1462-5822.2010.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tükel C., Raffatellu M., Humphries A.D., Wilson R.P., Andrews-Polymenis H.L., Gull T., Figueiredo J.F., Wong M.H., Michelsen K.S., Akçelik M., Adams L.G., Bäumler A.J. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol. Microbiol. 2005;58:289–304. doi: 10.1111/j.1365-2958.2005.04825.x. [DOI] [PubMed] [Google Scholar]
- 103.Tükel C., Wilson R.P., Nishimori J.H., Pezeshki M., Chromy B.A., Bäumler A.J. Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell Host Microbe. 2009;6:45–53. doi: 10.1016/j.chom.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rapsinski G.J., Newman T.N., Oppong G.O., van Putten J.P.M., Tükel Ç. CD14 protein acts as an adaptor molecule for the immune recognition of Salmonella curli fibers. J. Biol. Chem. 2013;288:14178–14188. doi: 10.1074/jbc.M112.447060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Biesecker S.G., Nicastro L.K., Wilson R.P., Tükel Ç. The functional amyloid curli protects Escherichia coli against complement-mediated bactericidal activity. Biomolecules. 2018;8:5. doi: 10.3390/biom8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Donlan R.M., Costerton J.W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moser C., Pedersen H.T., Lerche C.J., Kolpen M., Line L., Thomsen K., Høiby N., Jensen P.Ø. Biofilms and host response—helpful or harmful. Acta Pathol. Microbiol. Immunol. Scand. 2017;125:320–338. doi: 10.1111/apm.12674. [DOI] [PubMed] [Google Scholar]
- 108.Dueholm M.S., Otzen D., Nielsen P.H. Evolutionary insight into the functional amyloids of the pseudomonads. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rouse S.L., Hawthorne W.J., Lambert S., Morgan M.L., Hare S.A., Matthews S. Purification, crystallization and characterization of the Pseudomonas outer membrane protein FapF, a functional amyloid transporter. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016;72:892–896. doi: 10.1107/S2053230X16017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dueholm M.S., Søndergaard M.T., Nilsson M., Christiansen G., Stensballe A., Overgaard M.T., Givskov M., Tolker-Nielsen T., Otzen D.E., Nielsen P.H. Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. Microbiology Open. 2013;2:365–382. doi: 10.1002/mbo3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Herbst F.-A., Søndergaard M.T., Kjeldal H., Stensballe A., Nielsen P.H., Dueholm M.S. Major proteomic changes associated with amyloid-induced biofilm formation in Pseudomonas aeruginosa PAO1. J. Proteome Res. 2015;14:72–81. doi: 10.1021/pr500938x. [DOI] [PubMed] [Google Scholar]
- 112.Seviour T., Hansen S.H., Yang L., Yau Y.H., Wang V.B., Stenvang M.R., Christiansen G., Marsili E., Givskov M., Chen Y., Otzen D.E., Nielsen P.H., Geifman-Shochat S., Kjelleberg S., Dueholm M.S. Functional amyloids keep quorum-sensing molecules in check. J. Biol. Chem. 2015;290:6457–6469. doi: 10.1074/jbc.M114.613810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y.-H., Tian X. Quorum sensing and bacterial social interactions in biofilms. Sensors. 2012;12:2519–2538. doi: 10.3390/s120302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wiehlmann L., Munder A., Adams T., Juhas M., Kolmar H., Salunkhe P., Tümmler B. Functional genomics of Pseudomonas aeruginosa to identify habitat-specific determinants of pathogenicity. Int. J. Med. Microbiol. 2007;297:615–623. doi: 10.1016/j.ijmm.2007.03.014. [d] [DOI] [PubMed] [Google Scholar]
- 115.Kim J.-Y., Sahu S., Yau Y.-H., Wang X., Shochat S.G., Nielsen P.H., Dueholm M.S., Otzen D.E., Lee J., Delos Santos M.M.S., Yam J.K.H., Kang N.-Y., Park S.-J., Kwon H., Seviour T., Yang L., Givskov M., Chang Y.-T. Detection of pathogenic biofilms with bacterial amyloid targeting fluorescent probe, CDy11. J. Am. Chem. Soc. 2016;138:402–407. doi: 10.1021/jacs.5b11357. [DOI] [PubMed] [Google Scholar]
- 116.Turner K.H., Everett J., Trivedi U., Rumbaugh K.P., Whiteley M. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gaggar A., Hector A., Bratcher P.E., Mall M.A., Griese M., Hartl D. The role of matrix metalloproteinases in cystic fibrosis lung disease. Eur. Respir. J. 2011;38:721–727. doi: 10.1183/09031936.00173210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bruce M.C., Poncz L., Klinger J.D., Stern R.C., Tomashefski J.F., Dearborn D.G. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am. Rev. Respir. Dis. 1985;132:529–535. doi: 10.1164/arrd.1985.132.3.529. [DOI] [PubMed] [Google Scholar]
- 119.Ramsugit S., Pillay M. Mycobacterium tuberculosis Pili promote adhesion to and invasion of THP-1 macrophages. Jpn. J. Infect. Dis. 2014;67:476–478. doi: 10.7883/yoken.67.476. [DOI] [PubMed] [Google Scholar]
- 120.Naidoo N., Ramsugit S., Pillay M. Mycobacterium tuberculosis pili (MTP), a putative biomarker for a tuberculosis diagnostic test. Tuberc. Edinb. Scotl. 2014;94:338–345. doi: 10.1016/j.tube.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hosseini H., Fooladi A.A.I., Arjomandzadegan M., Emami N., Bornasi H. Genetics study and transmission electron microscopy of pili in susceptible and resistant clinical isolates of Mycobacterium tuberculosis. Asian Pac J Trop Med. 2014;7S1:S199–S203. doi: 10.1016/S1995-7645(14)60232-7. [DOI] [PubMed] [Google Scholar]
- 122.Middleton A.M., Chadwick M.V., Nicholson A.G., Dewar A., Groger R.K., Brown E.J., Ratliff T.L., Wilson R. Interaction of Mycobacterium tuberculosis with human respiratory mucosa. Tuberc. Edinb. Scotl. 2002;82:69–78. doi: 10.1054/tube.2002.0324. [DOI] [PubMed] [Google Scholar]
- 123.Ramsugit S., Pillay B., Pillay M. Evaluation of the role of Mycobacterium tuberculosis pili (MTP) as an adhesin, invasin, and cytokine inducer of epithelial cells. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 2016;20:160–165. doi: 10.1016/j.bjid.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mann K.M., Pride A.C., Flentie K., Kimmey J.M., Weiss L.A., Stallings C.L. Analysis of the contribution of MTP and the predicted Flp pilus genes to Mycobacterium tuberculosis pathogenesis. Microbiol. Read. Engl. 2016;162:1784–1796. doi: 10.1099/mic.0.000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramsugit S., Guma S., Pillay B., Jain P., Larsen M.H., Danaviah S., Pillay M. Pili contribute to biofilm formation in vitro in Mycobacterium tuberculosis. Antonie Van Leeuwenhoek. 2013;104:725–735. doi: 10.1007/s10482-013-9981-6. [DOI] [PubMed] [Google Scholar]
- 126.Nayak N. Mycobacterium Tuberculosis Biofilm—a new perspective. Indian J. Tuberc. 2015;62:4–6. doi: 10.1016/j.ijtb.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 127.Carter G., Wu M., Drummond D.C., Bermudez L.E. Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J. Med. Microbiol. 2003;52:747–752. doi: 10.1099/jmm.0.05224-0. [DOI] [PubMed] [Google Scholar]
- 128.Marsollier L., Aubry J., Coutanceau E., André J.-P.S., Small P.L., Milon G., Legras P., Guadagnini S., Carbonnelle B., Cole S.T. Colonization of the salivary glands of Naucoris cimicoides by Mycobacterium ulcerans requires host plasmatocytes and a macrolide toxin, mycolactone. Cell. Microbiol. 2005;7:935–943. doi: 10.1111/j.1462-5822.2005.00521.x. [DOI] [PubMed] [Google Scholar]
- 129.Ojha A.K., Baughn A.D., Sambandan D., Hsu T., Trivelli X., Guerardel Y., Alahari A., Kremer L., Jacobs W.R., Hatfull G.F. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 2008;69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pang J.M., Layre E., Sweet L., Sherrid A., Moody D.B., Ojha A., Sherman D.R. The polyketide Pks1 contributes to biofilm formation in Mycobacterium tuberculosis. J. Bacteriol. 2012;194:715–721. doi: 10.1128/JB.06304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Basaraba R.J., Ojha A.K. Mycobacterial biofilms: revisiting tuberculosis bacilli in extracellular necrotizing lesions. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.TBTB2-0024-2016. (TBTB2-0024-2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nobbs A.H., Lamont R.J., Jenkinson H.F. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 2009;73:407–450. doi: 10.1128/MMBR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Besingi R.N., Wenderska I.B., Senadheera D.B., Cvitkovitch D.G., Long J.R., Wen Z.T., Brady L.J. Functional amyloids in Streptococcus mutans, their use as targets of biofilm inhibition and initial characterization of SMU_63c. Microbiol. Read. Engl. 2017;163:488–501. doi: 10.1099/mic.0.000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heim K.P., Crowley P.J., Long J.R., Kailasan S., McKenna R., Brady L.J. An intramolecular lock facilitates folding and stabilizes the tertiary structure of Streptococcus mutans adhesin P1. Proc. Natl. Acad. Sci. U. S. A. 2014;111:15746–15751. doi: 10.1073/pnas.1413018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Navarre W.W., Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heim K.P., Sullan R.M.A., Crowley P.J., El-Kirat-Chatel S., Beaussart A., Tang W., Besingi R., Dufrene Y.F., Brady L.J. Identification of a supramolecular functional architecture of Streptococcus mutans adhesin P1 on the bacterial cell surface. J. Biol. Chem. 2015;290:9002–9019. doi: 10.1074/jbc.M114.626663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sullan R.M.A., Li J.K., Crowley P.J., Brady L.J., Dufrêne Y.F. Binding forces of Streptococcus mutans P1 adhesin. ACS Nano. 2015;9:1448–1460. doi: 10.1021/nn5058886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tang W., Bhatt A., Smith A.N., Crowley P.J., Brady L.J., Long J.R. Specific binding of a naturally occurring amyloidogenic fragment of Streptococcus mutans adhesin P1 to intact P1 on the cell surface characterized by solid state NMR spectroscopy. J. Biomol. NMR. 2016;64:153–164. doi: 10.1007/s10858-016-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Crowley P.J., Brady L.J., Michalek S.M., Bleiweis A.S. Virulence of a spaP mutant of Streptococcus mutans in a gnotobiotic rat model. Infect. Immun. 1999;67:1201–1206. doi: 10.1128/iai.67.3.1201-1206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brady L.J., Maddocks S.E., Larson M.R., Forsgren N., Persson K., Deivanayagam C.C., Jenkinson H.F. The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol. Microbiol. 2010;77:276–286. doi: 10.1111/j.1365-2958.2010.07212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cha S.-M., Cha J.-D., Jang E.-J., Kim G.-U., Lee K.-Y. Sophoraflavanone G prevents Streptococcus mutans surface antigen I/II-induced production of NO and PGE2 by inhibiting MAPK-mediated pathways in RAW 264.7 macrophages. Arch. Oral Biol. 2016;68:97–104. doi: 10.1016/j.archoralbio.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 142.Neff L., Zeisel M., Sibilia J., Schöller-Guinard M., Klein J.P., Wachsmann D. NF-kappaB and the MAP kinases/AP-1 pathways are both involved in interleukin-6 and interleukin-8 expression in fibroblast-like synoviocytes stimulated by protein I/II, a modulin from oral streptococci. Cell. Microbiol. 2001;3:703–712. doi: 10.1046/j.1462-5822.2001.00148.x. [DOI] [PubMed] [Google Scholar]
- 143.Neff L., Zeisel M., Druet V., Takeda K., Klein J.-P., Sibilia J., Wachsmann D. ERK 1/2- and JNKs-dependent synthesis of interleukins 6 and 8 by fibroblast-like synoviocytes stimulated with protein I/II, a modulin from oral streptococci, requires focal adhesion kinase. J. Biol. Chem. 2003;278:27721–27728. doi: 10.1074/jbc.M212065200. [DOI] [PubMed] [Google Scholar]
- 144.Al-Okla S., Chatenay-Rivauday C., Klein J.P., Wachsmann D. Involvement of alpha5beta1 integrins in interleukin 8 production induced by oral viridans streptococcal protein I/IIf in cultured endothelial cells. Cell. Microbiol. 1999;1:157–168. doi: 10.1046/j.1462-5822.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- 145.Gourieux B., Al-Okla S., Schöller-Guinard M., Klein J., Sibilia J., Wachsmann D. Pro-inflammatory cytokine production by synoviocytes following exposure to protein I/II, a modulin from oral streptococci. FEMS Immunol. Med. Microbiol. 2001;30:13–19. doi: 10.1111/j.1574-695X.2001.tb01544.x. [DOI] [PubMed] [Google Scholar]
- 146.Ahn S.-J., Ahn S.-J., Wen Z.T., Brady L.J., Burne R.A. Characteristics of biofilm formation by Streptococcus mutans in the presence of saliva. Infect. Immun. 2008;76:4259–4268. doi: 10.1128/IAI.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kearns D.B., Chu F., Branda S.S., Kolter R., Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 2005;55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- 148.Chu F., Kearns D.B., Branda S.S., Kolter R., Losick R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 2006;59:1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- 149.Branda S.S., Chu F., Kearns D.B., Losick R., Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 150.Epstein A.K., Pokroy B., Seminara A., Aizenberg J. Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration. Proc. Natl. Acad. Sci. U. S. A. 2011;108:995–1000. doi: 10.1073/pnas.1011033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Serrano M., Zilhão R., Ricca E., Ozin A.J., Moran C.P., Henriques A.O. A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J. Bacteriol. 1999;181:3632–3643. doi: 10.1128/jb.181.12.3632-3643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stöver A.G., Driks A. Secretion, localization, and antibacterial activity of TasA, a Bacillus subtilis spore-associated protein. J. Bacteriol. 1999;181:1664–1672. doi: 10.1128/jb.181.5.1664-1672.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chai L., Romero D., Kayatekin C., Akabayov B., Vlamakis H., Losick R., Kolter R. Isolation, characterization, and aggregation of a structured bacterial matrix precursor. J. Biol. Chem. 2013;288:17559–17568. doi: 10.1074/jbc.M113.453605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Diehl A., Roske Y., Ball L., Chowdhury A., Hiller M., Molière N., Kramer R., Stöppler D., Worth C.L., Schlegel B., Leidert M., Cremer N., Erdmann N., Lopez D., Stephanowitz H., Krause E., van Rossum B.-J., Schmieder P., Heinemann U., Turgay K., Akbey Ü., Oschkinat H. Structural changes of TasA in biofilm formation of Bacillus subtilis. Proc. Natl. Acad. Sci. 2018;115:3237–3242. doi: 10.1073/pnas.1718102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Romero D., Vlamakis H., Losick R., Kolter R. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol. Microbiol. 2011;80:1155–1168. doi: 10.1111/j.1365-2958.2011.07653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Romero D., Kolter R. Functional amyloids in bacteria. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2014;17:65–73. doi: 10.2436/20.1501.01.208. [DOI] [PubMed] [Google Scholar]
- 157.Terra R., Stanley-Wall N.R., Cao G., Lazazzera B.A. Identification of Bacillus subtilis SipW as a bifunctional signal peptidase that controls surface-adhered biofilm formation. J. Bacteriol. 2012;194:2781–2790. doi: 10.1128/JB.06780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hamon M.A., Stanley N.R., Britton R.A., Grossman A.D., Lazazzera B.A. Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis. Mol. Microbiol. 2004;52:847–860. doi: 10.1111/j.1365-2958.2004.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beauregard P.B., Chai Y., Vlamakis H., Losick R., Kolter R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E1621–E1630. doi: 10.1073/pnas.1218984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khezri M., Jouzani G.S., Ahmadzadeh M. Fusarium culmorum affects expression of biofilm formation key genes in Bacillus subtilis. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2016;47:47–54. doi: 10.1016/j.bjm.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stenfors Arnesen L.P., O’sullivan K., Granum P.E. Food poisoning potential of Bacillus cereus strains from Norwegian dairies. Int. J. Food Microbiol. 2007;116:292–296. doi: 10.1016/j.ijfoodmicro.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 162.Gao T., Foulston L., Chai Y., Wang Q., Losick R. Alternative modes of biofilm formation by plant-associated Bacillus cereus. Microbiol. Open. 2015;4:452–464. doi: 10.1002/mbo3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Verplaetse E., Slamti L., Gohar M., Lereclus D. Cell differentiation in a Bacillus thuringiensis population during planktonic growth, biofilm formation, and host infection. MBio. 2015;6 doi: 10.1128/mBio.00138-15. (e00138-00115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hayrapetyan H., Muller L., Tempelaars M., Abee T., Nierop Groot M. Comparative analysis of biofilm formation by Bacillus cereus reference strains and undomesticated food isolates and the effect of free iron. Int. J. Food Microbiol. 2015;200:72–79. doi: 10.1016/j.ijfoodmicro.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 165.Fagerlund A., Dubois T., Økstad O.-A., Verplaetse E., Gilois N., Bennaceur I., Perchat S., Gominet M., Aymerich S., Kolstø A.-B., Lereclus D., Gohar M. SinR controls enterotoxin expression in Bacillus thuringiensis biofilms. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee K., Costerton J.W., Ravel J., Auerbach R.K., Wagner D.M., Keim P., Leid J.G. Phenotypic and functional characterization of Bacillus anthracis biofilms. Microbiol. Read. Engl. 2007;153:1693–1701. doi: 10.1099/mic.0.2006/003376-0. [DOI] [PubMed] [Google Scholar]
- 167.Majed R., Faille C., Kallassy M., Gohar M. Bacillus cereus biofilms-same, only different. Front. Microbiol. 2016;7:1054. doi: 10.3389/fmicb.2016.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pflughoeft K.J., Sumby P., Koehler T.M. Bacillus anthracis sin locus and regulation of secreted proteases. J. Bacteriol. 2011;193:631–639. doi: 10.1128/JB.01083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Elliot M.A., Karoonuthaisiri N., Huang J., Bibb M.J., Cohen S.N., Kao C.M., Buttner M.J. The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev. 2003;17:1727–1740. doi: 10.1101/gad.264403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Claessen D., Rink R., de Jong W., Siebring J., de Vreugd P., Boersma F.G.H., Dijkhuizen L., Wosten H.A.B. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev. 2003;17:1714–1726. doi: 10.1101/gad.264303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wösten H.A., van Wetter M.A., Lugones L.G., van der Mei H.C., Busscher H.J., Wessels J.G. How a fungus escapes the water to grow into the air. Curr. Biol. 1999;9:85–88. doi: 10.1016/s0960-9822(99)80019-0. [DOI] [PubMed] [Google Scholar]
- 172.Dragoš A., Kovács Á.T., Claessen D. The role of functional amyloids in multicellular growth and development of Gram-positive bacteria. Biomolecules. 2017;7 doi: 10.3390/biom7030060. (pii:E60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Claessen D., Stokroos I., Deelstra H.J., Penninga N.A., Bormann C., Salas J.A., Dijkhuizen L., Wösten H.A.B. The formation of the rodlet layer of streptomycetes is the result of the interplay between rodlins and chaplins. Mol. Microbiol. 2004;53:433–443. doi: 10.1111/j.1365-2958.2004.04143.x. [DOI] [PubMed] [Google Scholar]
- 174.Sawyer E.B., Claessen D., Haas M., Hurgobin B., Gras S.L. The assembly of individual chaplin peptides from Streptomyces coelicolor into functional amyloid fibrils. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Capstick D.S., Jomaa A., Hanke C., Ortega J., Elliot M.A. Dual amyloid domains promote differential functioning of the chaplin proteins during Streptomyces aerial morphogenesis. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9821–9826. doi: 10.1073/pnas.1018715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Duong A., Capstick D.S., Di Berardo C., Findlay K.C., Hesketh A., Hong H.-J., Elliot M.A. Aerial development in Streptomyces coelicolor requires sortase activity. Mol. Microbiol. 2012;83:992–1005. doi: 10.1111/j.1365-2958.2012.07983.x. [DOI] [PubMed] [Google Scholar]
- 177.Dokouhaki M., Hung A., Day L., Gras S.L. The pH-dependent assembly of Chaplin E from Streptomyces coelicolor. J. Struct. Biol. 2017;198:82–91. doi: 10.1016/j.jsb.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 178.Bokhove M., Claessen D., de Jong W., Dijkhuizen L., Boekema E.J., Oostergetel G.T. Chaplins of Streptomyces coelicolor self-assemble into two distinct functional amyloids. J. Struct. Biol. 2013;184:301–309. doi: 10.1016/j.jsb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 179.Chaignon P., Sadovskaya I., Ragunah C., Ramasubbu N., Kaplan J.B., Jabbouri S. Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl. Microbiol. Biotechnol. 2007;75:125–132. doi: 10.1007/s00253-006-0790-y. [DOI] [PubMed] [Google Scholar]
- 180.Izano E.A., Amarante M.A., Kher W.B., Kaplan J.B. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 2008;74:470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cramton S.E., Gerke C., Schnell N.F., Nichols W.W., Götz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Speziale P., Pietrocola G., Foster T.J., Geoghegan J.A. Protein-based biofilm matrices in Staphylococci. Front. Cell. Infect. Microbiol. 2014;4:171. doi: 10.3389/fcimb.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cucarella C., Solano C., Valle J., Amorena B., Lasa I., Penadés J.R. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 2001;183:2888–2896. doi: 10.1128/JB.183.9.2888-2896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Arrizubieta M.J., Toledo-Arana A., Amorena B., Penadés J.R., Lasa I. Calcium inhibits bap-dependent multicellular behavior in Staphylococcus aureus. J. Bacteriol. 2004;186:7490–7498. doi: 10.1128/JB.186.22.7490-7498.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lembré P., Vendrely C., Martino P.D. Identification of an amyloidogenic peptide from the Bap protein of Staphylococcus epidermidis. Protein Pept. Lett. 2014;21:75–79. doi: 10.2174/09298665113209990072. [DOI] [PubMed] [Google Scholar]
- 186.Callebaut I., Gilgès D., Vigon I., Mornon J.P. HYR, an extracellular module involved in cellular adhesion and related to the immunoglobulin-like fold. Protein Sci. Publ. Protein Soc. 2000;9:1382–1390. doi: 10.1110/ps.9.7.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lasa I., Penadés J.R. Bap: a family of surface proteins involved in biofilm formation. Res. Microbiol. 2006;157:99–107. doi: 10.1016/j.resmic.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 188.Cucarella C., Tormo M.A., Ubeda C., Trotonda M.P., Monzón M., Peris C., Amorena B., Lasa I., Penadés J.R. Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect. Immun. 2004;72:2177–2185. doi: 10.1128/IAI.72.4.2177-2185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vautor E., Abadie G., Pont A., Thiery R. Evaluation of the presence of the bap gene in Staphylococcus aureus isolates recovered from human and animals species. Vet. Microbiol. 2008;127:407–411. doi: 10.1016/j.vetmic.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 190.Tormo M.A., Knecht E., Götz F., Lasa I., Penadés J.R. Bap-dependent biofilm formation by pathogenic species of Staphylococcus: evidence of horizontal gene transfer? Microbiol. Read. Engl. 2005;151:2465–2475. doi: 10.1099/mic.0.27865-0. [DOI] [PubMed] [Google Scholar]
- 191.Serray B., Oufrid S., Hannaoui I., Bourjilate F., Soraa N., Mliji M., Sobh M., Hammoumi A., Timinouni M., El Azhari M. Genes encoding adhesion factors and biofilm formation in methicillin-resistant Staphylococcus aureus in Morocco. J. Infect. Dev. Ctries. 2016;10:863–869. doi: 10.3855/jidc.8361. [DOI] [PubMed] [Google Scholar]
- 192.Bochniarz M., Adaszek Ł., Dzięgiel B., Nowaczek A., Wawron W., Dąbrowski R., Szczubiał M., Winiarczyk S. Factors responsible for subclinical mastitis in cows caused by Staphylococcus chromogenes and its susceptibility to antibiotics based on bap, fnbA, eno, mecA, tetK, and ermA genes. J. Dairy Sci. 2016;99:9514–9520. doi: 10.3168/jds.2016-11723. [DOI] [PubMed] [Google Scholar]
- 193.Castilho I.G., Dantas S.T.A., Langoni H., Araújo J.P., Fernandes A., Alvarenga F.C.L., Maia L., Cagnini D.Q., Rall V.L.M. Host–pathogen interactions in bovine mammary epithelial cells and HeLa cells by Staphylococcus aureus isolated from subclinical bovine mastitis. J. Dairy Sci. 2017;100:6414–6421. doi: 10.3168/jds.2017-12700. [DOI] [PubMed] [Google Scholar]
- 194.Dutta A., Bhattacharyya S., Kundu A., Dutta D., Das A.K. Macroscopic amyloid fiber formation by staphylococcal biofilm associated SuhB protein. Biophys. Chem. 2016;217:32–41. doi: 10.1016/j.bpc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 195.Mehlin C., Headley C.M., Klebanoff S.J. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J. Exp. Med. 1999;189:907–918. doi: 10.1084/jem.189.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang R., Braughton K.R., Kretschmer D., Bach T.-H.L., Queck S.Y., Li M., Kennedy A.D., Dorward D.W., Klebanoff S.J., Peschel A., DeLeo F.R., Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 197.Berlon N.R., Qi R., Sharma-Kuinkel B.K., Joo H.-S., Park L.P., George D., Thaden J.T., Messina J.A., Maskarinec S.A., Mueller-Premru M., Athan E., Tattevin P., Pericas J.M., Woods C.W., Otto M., Fowler V.G. Clinical MRSA isolates from skin and soft tissue infections show increased in vitro production of phenol soluble modulins. J. Infect. 2015;71:447–457. doi: 10.1016/j.jinf.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Peschel A., Otto M. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 2013;11:667–673. doi: 10.1038/nrmicro3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Towle K.M., Lohans C.T., Miskolzie M., Acedo J.Z., van Belkum M.J., Vederas J.C. Solution structures of phenol-soluble modulins α1, α3, and β2, virulence factors from Staphylococcus aureus. Biochemistry (Mosc) 2016;55:4798–4806. doi: 10.1021/acs.biochem.6b00615. [DOI] [PubMed] [Google Scholar]
- 200.Chatterjee S.S., Joo H.-S., Duong A.C., Dieringer T.D., Tan V.Y., Song Y., Fischer E.R., Cheung G.Y.C., Li M., Otto M. Essential Staphylococcus aureus toxin export system. Nat. Med. 2013;19:364–367. doi: 10.1038/nm.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cheung G.Y.C., Duong A.C., Otto M. Direct and synergistic hemolysis caused by Staphylococcus phenol-soluble modulins: implications for diagnosis and pathogenesis. Microbes Infect. 2012;14:380–386. doi: 10.1016/j.micinf.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Grosz M., Kolter J., Paprotka K., Winkler A.-C., Schäfer D., Chatterjee S.S., Geiger T., Wolz C., Ohlsen K., Otto M., Rudel T., Sinha B., Fraunholz M. Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin α. Cell. Microbiol. 2014;16:451–465. doi: 10.1111/cmi.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Periasamy S., Joo H.-S., Duong A.C., Bach T.-H.L., Tan V.Y., Chatterjee S.S., Cheung G.Y.C., Otto M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dastgheyb S.S., Villaruz A.E., Le K.Y., Tan V.Y., Duong A.C., Chatterjee S.S., Cheung G.Y.C., Joo H.-S., Hickok N.J., Otto M. Role of phenol-soluble modulins in formation of Staphylococcus aureus biofilms in synovial fluid. Infect. Immun. 2015;83:2966–2975. doi: 10.1128/IAI.00394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Marinelli P., Pallares I., Navarro S., Ventura S. Dissecting the contribution of Staphylococcus aureus α-phenol-soluble modulins to biofilm amyloid structure. Sci. Rep. 2016;6 doi: 10.1038/srep34552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zheng Y., Joo H.-S., Nair V., Le K.Y., Otto M. Do amyloid structures formed by Staphylococcus aureus phenol-soluble modulins have a biological function? Int. J. Med. Microbiol. 2017 doi: 10.1016/j.ijmm.2017.08.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Laabei M., Jamieson W.D., Yang Y., van den Elsen J., Jenkins A.T.A. Investigating the lytic activity and structural properties of Staphylococcus aureus phenol soluble modulin (PSM) peptide toxins. Biochim. Biophys. Acta. 2014;1838:3153–3161. doi: 10.1016/j.bbamem.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 208.Schwartz K., Sekedat M.D., Syed A.K., O’Hara B., Payne D.E., Lamb A., Boles B.R. The AgrD N-terminal leader peptide of Staphylococcus aureus has cytolytic and amyloidogenic properties. Infect. Immun. 2014;82:3837–3844. doi: 10.1128/IAI.02111-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tayeb-Fligelman E., Tabachnikov O., Moshe A., Goldshmidt-Tran O., Sawaya M.R., Coquelle N., Colletier J.-P., Landau M. The cytotoxic Staphylococcus aureus PSMα3 reveals a cross-α amyloid-like fibril. Science. 2017;355:831–833. doi: 10.1126/science.aaf4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zamolodchikov D., Renn T. 2016. The Alzheimer's disease peptide b-amyloid promotes thrombin generation through activation of coagulation factor XII; pp. 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Persson K., Russell W., Mörgelin M., Herwald H. The conversion of fibrinogen to fibrin at the surface of curliated Escherichia coli bacteria leads to the generation of proinflammatory fibrinopeptides. J. Biol. Chem. 2003;278:31884–31890. doi: 10.1074/jbc.M302522200. [DOI] [PubMed] [Google Scholar]
- 212.Nickel K.F., Renné T. Crosstalk of the plasma contact system with bacteria. Thromb. Res. 2012;130(Suppl. 1):S78–S83. doi: 10.1016/j.thromres.2012.08.284. [DOI] [PubMed] [Google Scholar]
- 213.Hofman Z., de Maat S., Hack C.E., Maas C. Bradykinin: Inflammatory product of the coagulation system. Clin. Rev. Allergy Immunol. 2016;51:152–161. doi: 10.1007/s12016-016-8540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.O’Nuallain B., Williams A.D., Westermark P., Wetzel R. Seeding specificity in amyloid growth induced by heterologous fibrils. J. Biol. Chem. 2004;279:17490–17499. doi: 10.1074/jbc.M311300200. [DOI] [PubMed] [Google Scholar]
- 215.Zhou Y., Smith D., Leong B.J., Brännström K., Almqvist F., Chapman M.R. Promiscuous cross-seeding between bacterial amyloids promotes interspecies biofilms. J. Biol. Chem. 2012;287:35092–35103. doi: 10.1074/jbc.M112.383737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hartman K., Brender J.R., Monde K., Ono A., Evans M.L., Popovych N., Chapman M.R., Ramamoorthy A. Bacterial curli protein promotes the conversion of PAP248-286 into the amyloid SEVI: cross-seeding of dissimilar amyloid sequences. PeerJ. 2013;1 doi: 10.7717/peerj.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lundmark K., Westermark G.T., Olsén A., Westermark P. Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: cross-seeding as a disease mechanism. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6098–6102. doi: 10.1073/pnas.0501814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen S.G., Stribinskis V., Rane M.J., Demuth D.R., Gozal E., Roberts A.M., Jagadapillai R., Liu R., Choe K., Shivakumar B., Son F., Jin S., Kerber R., Adame A., Masliah E., Friedland R.P. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged fischer 344 rats and Caenorhabditis elegans. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Del Tredici K., Braak H. Sporadic Parkinson's disease: development and distribution of α-synuclein pathology. Neuropathol. Appl. Neurobiol. 2016;42:33–50. doi: 10.1111/nan.12298. [DOI] [PubMed] [Google Scholar]
- 220.Minter M.R., Zhang C., Leone V., Ringus D.L., Zhang X., Oyler-Castrillo P., Musch M.W., Liao F., Ward J.F., Holtzman D.M., Chang E.B., Tanzi R.E., Sisodia S.S. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci. Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Marizzoni M., Provasi S., Cattaneo A., Frisoni G.B. Microbiota and neurodegenerative diseases. Curr. Opin. Neurol. 2017;30:630–638. doi: 10.1097/WCO.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 222.Vuong H.E., Yano J.M., Fung T.C., Hsiao E.Y. The microbiome and host behavior. Annu. Rev. Neurosci. 2017;40:21–49. doi: 10.1146/annurev-neuro-072116-031347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shmakov A.N., Ghosh S. Prion proteins and the gut: une liaison dangereuse? Gut. 2001;48:443–447. doi: 10.1136/gut.48.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hoffmann C., Ziegler U., Buschmann A., Weber A., Kupfer L., Oelschlegel A., Hammerschmidt B., Groschup M.H. Prions spread via the autonomic nervous system from the gut to the central nervous system in cattle incubating bovine spongiform encephalopathy. J. Gen. Virol. 2007;88:1048–1055. doi: 10.1099/vir.0.82186-0. [DOI] [PubMed] [Google Scholar]
- 225.Evans M.L., Chorell E., Taylor J.D., Åden J., Götheson A., Li F., Koch M., Sefer L., Matthews S.J., Wittung-Stafshede P., Almqvist F., Chapman M.R. The bacterial curli system possesses a potent and selective inhibitor of amyloid formation. Mol. Cell. 2015:445–455. doi: 10.1016/j.molcel.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu S., Liu Y., Hao W., Wolf L., Kiliaan A.J., Penke B., Rübe C.E., Walter J., Heneka M.T., Hartmann T., Menger M.D., Fassbender K. TLR2 is a primary receptor for Alzheimer's amyloid β peptide to trigger neuroinflammatory activation. J. Immunol. Baltim. Md. 2012;1950(188):1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 227.Daniele S.G., Béraud D., Davenport C., Cheng K., Yin H., Maguire-Zeiss K.A. Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders. Sci. Signal. 2015;8:ra45. doi: 10.1126/scisignal.2005965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Friedland R.P., Chapman M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017;13:1–12. doi: 10.1371/journal.ppat.1006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Römling U., Kjelleberg S., Normark S., Nyman L., Uhlin B.E., Åkerlund B. Microbial biofilm formation: a need to act. J. Intern. Med. 2014;276:98–110. doi: 10.1111/joim.12242. [DOI] [PubMed] [Google Scholar]
- 230.Elchinger P.-H., Delattre C., Faure S., Roy O., Badel S., Bernardi T., Taillefumier C., Michaud P. Effect of proteases against biofilms of Staphylococcus aureus and Staphylococcus epidermidis. Lett. Appl. Microbiol. 2014;59:507–513. doi: 10.1111/lam.12305. [DOI] [PubMed] [Google Scholar]
- 231.Goncalves M.D.S., Delattre C., Balestrino D., Charbonnel N., Elboutachfaiti R., Wadouachi A., Badel S., Bernardi T., Michaud P., Forestier C. Anti-biofilm activity: a function of Klebsiella pneumoniae capsular polysaccharide. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cegelski L., Marshall G.R., Eldridge G.R., Hultgren S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Maher M.C., Lim J.Y., Gunawan C., Cegelski L. Cell-based high-throughput screening identifies rifapentine as an inhibitor of amyloid and biofilm formation in Escherichia coli. ACS Infect. Dis. 2015;1:460–468. doi: 10.1021/acsinfecdis.5b00055. [DOI] [PubMed] [Google Scholar]
- 234.Rothstein D.M. Rifamycins, alone and in combination. Cold Spring Harb. Perspect. Med. 2016;6 doi: 10.1101/cshperspect.a027011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lee J.-H., Kim Y.-G., Kim C.-J., Lee J.-C., Cho M.H., Lee J. Indole-3-acetaldehyde from Rhodococcus sp. BFI 332 inhibits Escherichia coli O157:H7 biofilm formation. Appl. Microbiol. Biotechnol. 2012;96:1071–1078. doi: 10.1007/s00253-012-3881-y. [DOI] [PubMed] [Google Scholar]
- 236.Lee J.-H., Kim Y.-G., Ryu S.Y., Cho M.H., Lee J. Ginkgolic acids and Ginkgo biloba extract inhibit Escherichia coli O157:H7 and Staphylococcus aureus biofilm formation. Int. J. Food Microbiol. 2014;174:47–55. doi: 10.1016/j.ijfoodmicro.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 237.Lee J.-H., Park J.-H., Kim J.-A., Neupane G.P., Cho M.H., Lee C.-S., Lee J. Low concentrations of honey reduce biofilm formation, quorum sensing, and virulence in Escherichia coli O157:H7. Biofouling. 2011;27:1095–1104. doi: 10.1080/08927014.2011.633704. [DOI] [PubMed] [Google Scholar]
- 238.Lee J.-H., Regmi S.C., Kim J.-A., Cho M.H., Yun H., Lee C.-S., Lee J. Apple flavonoid phloretin inhibits Escherichia coli O157:H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun. 2011;79:4819–4827. doi: 10.1128/IAI.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Arita-Morioka K.-I., Yamanaka K., Mizunoe Y., Tanaka Y., Ogura T., Sugimoto S. Inhibitory effects of Myricetin derivatives on curli-dependent biofilm formation in Escherichia coli. Sci. Rep. 2018;8:8452. doi: 10.1038/s41598-018-26748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Arita-Morioka K., Yamanaka K., Mizunoe Y., Ogura T., Sugimoto S. Novel strategy for biofilm inhibition by using small molecules targeting molecular chaperone DnaK. Antimicrob. Agents Chemother. 2015;59:633–641. doi: 10.1128/AAC.04465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Silva L.N., Da Hora G.C.A., Soares T.A., Bojer M.S., Ingmer H., Macedo A.J., Trentin D.S. Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Sci. Rep. 2017;7:2823. doi: 10.1038/s41598-017-02712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Porat Y., Abramowitz A., Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 243.Nie Q., Du X., Geng M. Small molecule inhibitors of amyloid β peptide aggregation as a potential therapeutic strategy for Alzheimer's disease. Acta Pharmacol. Sin. 2011;32:545–551. doi: 10.1038/aps.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chu C., Deng J., Man Y., Qu Y. Green tea extracts epigallocatechin-3-gallate for different treatments. Biomed. Res. Int. 2017:5615647. doi: 10.1155/2017/5615647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ehrnhoefer D.E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 246.Bieschke J., Russ J., Friedrich R.P., Ehrnhoefer D.E., Wobst H., Neugebauer K., Wanker E.E. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Steinmann J., Buer J., Pietschmann T., Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013;168:1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Stenvang M., Dueholm M.S., Vad B.S., Seviour T., Zeng G., Geifman-Shochat S., Søndergaard M.T., Christiansen G., Meyer R.L., Kjelleberg S., Nielsen P.H., Otzen D.E. Epigallocatechin gallate remodels overexpressed functional amyloids in Pseudomonas aeruginosa and increases biofilm susceptibility to antibiotic treatment. J. Biol. Chem. 2016;291:26540–26553. doi: 10.1074/jbc.M116.739953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Serra D.O., Mika F., Richter A.M., Hengge R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the σ(E)-dependent sRNA RybB. Mol. Microbiol. 2016;101:136–151. doi: 10.1111/mmi.13379. [DOI] [PubMed] [Google Scholar]
- 250.Bikels-Goshen T., Landau E., Saguy S., Shapira R. Staphylococcal strains adapted to epigallocathechin gallate (EGCG) show reduced susceptibility to vancomycin, oxacillin and ampicillin, increased heat tolerance, and altered cell morphology. Int. J. Food Microbiol. 2010;138:26–31. doi: 10.1016/j.ijfoodmicro.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 251.Pinkner J.S., Remaut H., Buelens F., Miller E., Aberg V., Pemberton N., Hedenstrom M., Larsson A., Seed P., Waksman G., Hultgren S.J., Almqvist F. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17897–17902. doi: 10.1073/pnas.0606795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cegelski L., Pinkner J.S., Hammer N.D., Cusumano C.K., Hung C.S., Chorell E., Aberg V., Walker J.N., Seed P.C., Almqvist F., Chapman M.R., Hultgren S.J. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 2009;5:913–919. doi: 10.1038/nchembio.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Andersson E.K., Bengtsson C., Evans M.L., Chorell E., Sellstedt M., Lindgren A.E.G., Hufnagel D.A., Bhattacharya M., Tessier P.M., Wittung-Stafshede P., Almqvist F., Chapman M.R. Modulation of curli assembly and pellicle biofilm formation by chemical and protein chaperones. Chem. Biol. 2013;20:1245–1254. doi: 10.1016/j.chembiol.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Horvath I., Weise C.F., Andersson E.K., Chorell E., Sellstedt M., Bengtsson C., Olofsson A., Hultgren S.J., Chapman M., Wolf-Watz M., Almqvist F., Wittung-Stafshede P. Mechanisms of protein oligomerization: inhibitor of functional amyloids templates α-synuclein fibrillation. J. Am. Chem. Soc. 2012;134:3439–3444. doi: 10.1021/ja209829m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sleutel M., Van den Broeck I., Van Gerven N., Feuillie C., Jonckheere W., Valotteau C., Dufrêne Y.F., Remaut H. Nucleation and growth of a bacterial functional amyloid at single-fiber resolution. Nat. Chem. Biol. 2017;13:902–908. doi: 10.1038/nchembio.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bednarska N.G., van Eldere J., Gallardo R., Ganesan A., Ramakers M., Vogel I., Baatsen P., Staes A., Goethals M., Hammarström P., Nilsson K.P.R., Gevaert K., Schymkowitz J., Rousseau F. Protein aggregation as an antibiotic design strategy. Mol. Microbiol. 2016;99:849–865. doi: 10.1111/mmi.13269. [DOI] [PubMed] [Google Scholar]
- 257.Taubman M.A., Nash D.A. The scientific and public-health imperative for a vaccine against dental caries. Nat. Rev. Immunol. 2006;6:555–563. doi: 10.1038/nri1857. [DOI] [PubMed] [Google Scholar]
- 258.Russell M.W., Childers N.K., Michalek S.M., Smith D.J., Taubman M.A. A caries vaccine? The state of the science of immunization against dental caries. Caries Res. 2004;38:230–235. doi: 10.1159/000077759. [DOI] [PubMed] [Google Scholar]
- 259.Yan H. Salivary IgA enhancement strategy for development of a nasal-spray anti-caries mucosal vaccine. Sci. China Life Sci. 2013;56:406–413. doi: 10.1007/s11427-013-4473-5. [DOI] [PubMed] [Google Scholar]
- 260.Wang R., Khan B.A., Cheung G.Y.C., Bach T.-H.L., Jameson-Lee M., Kong K.-F., Queck S.Y., Otto M. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J. Clin. Invest. 2011;121:238–248. doi: 10.1172/JCI42520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Naidoo N., Pillay M. Bacterial pili, with emphasis on Mycobacterium tuberculosis curli pili: potential biomarkers for point-of care tests and therapeutics. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2017;22:93–105. doi: 10.1080/1354750X.2016.1252960. [DOI] [PubMed] [Google Scholar]
- 262.Duquesne S., Destoumieux-Garzón D., Peduzzi J., Rebuffat S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat. Prod. Rep. 2007;24:708–734. doi: 10.1039/b516237h. [DOI] [PubMed] [Google Scholar]
- 263.Bieler S., Estrada L., Lagos R., Baeza M., Castilla J., Soto C. Amyloid formation modulates the biological activity of a bacterial protein. J. Biol. Chem. 2005;280:26880–26885. doi: 10.1074/jbc.M502031200. [DOI] [PubMed] [Google Scholar]
- 264.Shahnawaz M., Soto C. Microcin amyloid fibrils A are reservoir of toxic oligomeric species. J. Biol. Chem. 2012;287:11665–11676. doi: 10.1074/jbc.M111.282533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Moonens K., Remaut H. Evolution and structural dynamics of bacterial glycan binding adhesins. Curr. Opin. Struct. Biol. 2017;44:48–58. doi: 10.1016/j.sbi.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 266.Li C., Qin R., Liu R., Miao S., Yang P. Functional amyloid materials at surfaces/interfaces. Biomater. Sci. 2018;6:462–472. doi: 10.1039/c7bm01124e. [DOI] [PubMed] [Google Scholar]