Abstract
Background
Although we know that quality of life generally improves after left ventricular assist device (LVAD) implantation, we know little about how symptoms change in response to LVAD.
Methods
The purpose of this paper was to compare changes in symptoms between bridge and destination therapy patients as part of a prospective cohort study. Physical (dyspnea and wake disturbances) and affective symptoms (depression and anxiety) were measured prior to, and at 1, 3 and 6 months after LVAD. Multiphase growth modeling was used to capture the two major phases of change; initial improvements between pre-implant and 1 month after LVAD; and subsequent improvements between 1 and 6 months after LVAD.
Results
The sample included 64 bridge and 22 destination therapy patients as the pre-implant strategy. Destination patients had worse pre-implant dyspnea and wake disturbances, and they experienced greater initial improvements in these symptoms compared with bridge patients (all p<0.05); subsequent change in both symptoms were similar between groups (both p>0.05). Destination patients had worse pre-implant depression (p=0.042) but experienced similar initial and subsequent improvements in depression in response to LVAD compared with bridge patients (both p>0.05). Destination patients had similar pre-implant anxiety (p=0.279), but experienced less initial and greater subsequent improvements in anxiety after LVAD compared with bridge patients (both p<0.05).
Conclusions
There are many differences in the magnitude and timing of change in symptom responses to LVAD between bridge and destination therapy patients. Detailed information on changes in specific symptoms may better inform shared decision making regarding LVAD.
Introduction
Heart failure (HF) is the fastest growing cardiovascular disorder in the U.S.1 Similarly, the number of adults living with advanced HF2 will invariably increase with the aging of the population.3 There is a limited supply of donor hearts,4 and for a myriad of reasons many patients with advanced HF are ineligible for the gold standard treatment of heart transplantation.5 As such, the use of left ventricular assist devices (LVAD) as a bridge to transplantation/decision or destination therapy is an increasingly utilized therapeutic option in the management of advanced HF.6 In the current era of continuous-flow LVADs, the average 1-year survival is 80%.7
Health-related quality of life (HRQOL) is also known to improve with LVAD therapy irrespective of age8 or HF severity.9 Because there is no LVAD-specific measure of HRQOL,10 and because HRQOL is poorly defined in advanced HF,11 it is often used as a broad and catch-all term that can be difficult for providers and patients to appreciate.12 In addition to poor HRQOL, HF is also known to be associated with burdensome physical symptoms like dyspnea13 and wake disturbances,14 as well as affective symptoms like depression and anxiety.15,16 Symptoms are important in HF because they are the main progenitor of healthcare utilization17 and independent predictors of event-risk.18,19 Compared with our extensive knowledge about HRQOL, however, we know little about how specific symptoms change in response to LVAD in general,10,20 or comparing LVAD therapy as a bridge or destination therapy in particular. A more detailed understanding of symptom responses to LVAD therapy will allow us to better support shared decision-making, provide sufficient education to patients and families about what to expect, and tailor monitoring strategies to identify and ameliorate barriers to optimal patient-reported outcomes. The purpose of this paper was to characterize and compare changes in physical and affective symptoms among adults undergoing LAVD implantation as bridge to heart transplantation/decision or as destination therapy from pre-implantation through the first six months after LVAD implantation.
Methods
Design
Profiling Biobehavioral Responses to Mechanical Support in Advanced Heart Failure (PREMISE) is a U.S. National Institutes of Health-sponsored prospective cohort study designed, in part, to better characterize patient-reported outcomes in responses to LVAD. The full background and design of the PREMISE study have been reported in detail elsewhere.21 In brief, adults (≥21 years of age) who were undergoing LVAD with a commercially-available and U.S. Food and Drug Administration-approved continuous-flow LVAD as a bridge to transplantation/decision or as destination therapy were approached for participation and enrolled prior to the initiation of LVAD and followed for six months after LVAD implantation. All participants met criteria for Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile 1–4.7 Patients were not eligible if they had a heart transplantation or previous LVAD prior to enrollment, major psychiatric illness or documented major cognitive impairment such as Alzheimer’s disease, or if they had concomitant terminal illness that impeded participation in a six month study. For the purposes of this study, patients were identified as requiring an LVAD as bridge (i.e. bridge to transplantation or decision) or destination therapy as the pre-implantation strategy designated by a multidisciplinary advanced HF section committee. Participants were recruited through a single center between April, 2012 and December, 2015. Written informed consent was obtained from all study participants and the study was reviewed and approved by our institutional review board.
Data Collection
Our perspectives on patient-reported outcomes in response to LVAD were informed by the patient-reported outcomes (PROMIS) Adult Health Domain Framework.22 Thus, in addition to our use of HRQOL as a broad general health outcome, we chose valid and reliable measures of common physical health (i.e. symptoms of dyspnea and wake disturbances) and mental health (i.e. affective symptom of depression and anxiety) as more perceptible patient-reported outcomes. The following measures were used prior to LVAD (median of 5 days pre-implant), and at 1, 3 and 6 months after LVAD.
Heart failure HRQOL was measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ).12 The KCCQ HRQOL score is derived from 3 items focused on how HF has limited enjoyment of life, how the patient would feel if they had to spend the rest of their life with HF the way it is right now, and how often the patient felt discouraged or down in the dumps because of HF; scores on the KCCQ HRQOL range from 0–100 with higher scores reflecting better HRQOL.12 Cronbach’s alpha on the 3-item HRQOL scale was 0.63 in this study. There is no LVAD-specific measure of HRQOL; hence, we included the KCCQ HRQOL score for comparison to other studies because it is used extensively in LVAD studies, and often as the only patient-reported outcome. In general, the KCCQ is more sensitive to change in clinical status compared with both other HF-specific23 and general measures of HRQOL.24 Overall, however, the KCCQ has sufficient predictive validity only when the risk of clinical events is low, not when patients have a high risk of death or hospitalization like in advanced HF.25
Dyspnea was measured using the Heart Failure Somatic Perception Scale (HFSPS).26 The HFSPS asks about how much the participant was bothered by common HF symptoms during the last week and provides six response options ranging from 0 (not at all) to 5 (extremely bothersome). The HFSPS has a 6-item subscale for dyspnea that was used in this analysis (HFSPS Dyspnea; range 0–30; higher scores indicate worse dyspnea).27 Cronbach’s alpha on the HFSPS Dyspnea scale was 0.89 in this study.
Wake disturbances were measured using the 8-item Epworth Sleepiness Scale (ESS).28 The ESS asks respondents to rate how likely they would be to fall asleep in 8 situations by choosing response options that range from 0 (would never fall asleep) to 3 (high chance). The ESS correlates significantly with sleep latency measures, and scores distinguish normal sleep patterns, idiopathic hypersomnia and insomnia.28 Cronbach’s alpha was 0.89 in this study.
Depression was measured using the 9-Item Patient Health Questionnaire (PHQ9).29 The PHQ9 scores each of the 9 related DSM-IV criteria providing four response options ranging from 0 (not at all) to 3 (nearly every day). The PHQ9 scores of 5, 10, 15, and 20 are indicative of mild, moderate, moderately severe, and severe depression, respectively.29 Cronbach’s alpha on the PHQ9 was 0.82 in this study.
Anxiety was measured using the Brief Symptom Inventory (BSI).30 The BSI asks about feelings during the past seven days and provides five response options ranging from 0 (no) to 4 (extreme). Subscale scores (ranging from 0 to 4) are calculated by adding the ratings and dividing the total by the number of items in the subscale, with higher scores indicating higher distress. Cronbach’s alpha on the BSI anxiety items was 0.85 in this study.
Statistical Analysis
Means and standard deviations or counts and proportions were used to describe the sample, and t- and χ2-tests were used to compare characteristics between bridge and destination therapy patients. Latent growth curve modeling31 was performed to estimate change in symptoms across four time points from pre-implant to 6 months post-implant. We performed multiphase growth modeling32,33 to capture the two major phases of change in patient-reported outcomes observed previously in response to LVAD implantation;34 initial improvements observed between pre-implant and 1 month post implant (Δ1), and subsequent improvements between 1 and 6 months post implant (Δ2). Based on multiphase growth models, pre-implant values are presented in means and standard errors; phases of change are presented as mean slope, standard error of the slope, and the significance of change as well as Cohen’s d (i.e. standardized mean difference) to quantify the magnitude of change (0.2–0.3 is a small effect, ≈ 0.5 is a moderate effect, and ≥ 0.8 is a large effect).35 To test for differences in symptoms between bridge and destination patients, we then compared pre-implant values and each phase of change by therapy type (results are reported in t-tests (i.e. mean/standard error of difference between implant strategy) and p-values); this approach takes into consideration multiphase growth modeling estimates and employs full information maximum likelihood estimation to mitigate bias due to missing data.36 Trajectory graphs were prepared to compare changes in symptoms by implant strategy. There is no standard approach for sample size considerations in growth modeling; with four symptom measurements, however, our n-to-items ratio exceeded sample size recommendations for related approaches (10:1).37 Since the formal tests of difference were independence means tests, we were powered to detect moderate differences (i.e. Cohen’s d ≥0.5) with an allocation ratio of ≈ 3:1, assuming α = 0.05 and power = 0.80. All statistical analyses were performed using Stata v14 (College Station, Texas) or Mplus v7.31 (Los Angeles, California).
Results
Characteristics of the sample (n=86) prior to LVAD are presented in Table 1. Patients requiring an LVAD as bridge therapy were 15 years younger on average and fewer had HF of ischemic etiology compared with destination therapy patients. Destination therapy patients had more complicated comorbid conditions including diabetes, kidney disease and sleep disordered breathing, and more were former smokers compared with bridge therapy patients. Although not statistically significant, more destination therapy patients had a history of depressive or anxiety disorders and were on routine psychotropic medications compared with bridge therapy patients. Otherwise, the two groups had similar clinical hallmarks of advanced HF including dilated left ventricles with poor contractility, elevated right- and left-sided filling pressures and reduced cardiac output. During 6 months of follow-up, 2 destination therapy patients died and another 2 required device exchanges; 3 bridge therapy patients died, 1 required a device exchange, and 8 were transplanted.
Table 1.
Pre-implant characteristics of the sample
| Characteristic (mean±SD or n (%)) | Full Sample (n=86) | Bridge (n=64) | Destination (n=22) | p-value |
|---|---|---|---|---|
| Patient age (in years) | 53.0 ± 14.4 | 49.2 ± 14.1 | 64.1 ± 8.4 | <0.001 |
| Female | 18 (20.9%) | 14 (21.9%) | 4 (18.2%) | 0.713 |
| Caucasian | 72 (83.7%) | 51 (79.7%) | 21 (95.5%) | 0.628 |
| Body Mass Index (kg/m2) | 29.0 ± 5.5 | 28.8 ± 5.3 | 29.6 ± 6.1 | 0.590 |
| Former vs never smokers | 54 (62.8%) | 36 (56.3%) | 18 (81.8%) | 0.032 |
| Diabetes with end-organ damage | 9 (10.5%) | 3 (4.7%) | 6 (27.3%) | 0.026 |
| Stage 3 chronic kidney disease | 34 (39.5%) | 21 (32.8%) | 13 (59.1%) | 0.030 |
| Sleep disordered breathing | 41 (47.7%) | 26 (40.6%) | 15 (68.2%) | 0.026 |
| Depressive/anxiety disorder | 13 (15.1%) | 8 (12.5%) | 5 (22.7%) | 0.248 |
| Antidepressant/anxiolytic therapy | 24 (27.9%) | 16 (25.0%) | 6 (27.3%) | 0.833 |
| Ischemic Etiology | 29 (33.7%) | 17 (26.6%) | 12 (54.6%) | 0.022 |
| New York Heart Association Class III/IV | 83 (96.5%) | 61 (95.3%) | 22 (100%) | 0.142 |
| Left ventricular ejection fraction (%) | 20.4 ± 3.0 | 20.4 ± 3.3 | 20.5 ± 2.0 | 0.838 |
| Left ventricular internal diastolic diameter (cm) | 7.4 ± 1.2 | 7.4 ± 1.2 | 7.3 ± 1.1 | 0.847 |
| PCWP (mm Hg) | 23.4 ± 8.6 | 23.9 ± 8.7 | 22.0 ± 8.3 | 0.363 |
| RAP (mm Hg) | 9.3 ± 4.8 | 9.4 ± 9 | 8.9 ± 4.7 | 0.674 |
| Cardiac Index (by Fick equation) (L/min/m2) | 1.9 ± 0.5 | 1.9 ± 0.5 | 1.9 ± 0.5 | 0.859 |
| V02 max (L/min) | 12.4 ± 4.8 | 13.2 ± 5.1 | 10.1 ± 2.5 | 0.062 |
| Serum Sodium (mEq/L) | 134.5 ± 4.3 | 134.8 ± 3.9 | 133.5 ± 5.4 | 0.285 |
| Serum Hemoglobin (%) | 11.9 ± 1.9 | 11.7 ± 1.8 | 12.6 ± 2.2 | 0.098 |
| Continuous Inotropic Support | 52 (60.5%) | 41 (64.1%) | 11 (50.0%) | 0.403 |
| Intra-aortic Balloon Pump | 33 (38.4%) | 27 (42.2%) | 6 (27.3%) | 0.215 |
Abbreviations: PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure.
Over the course of 6 months, there were large and significant improvements in HRQOL in response to LVAD in both patient groups (Figure 1). Compared with bridge therapy patients, destination therapy patients had similar HRQOL prior to implantation (p=0.111) but they experienced significantly greater initial improvement in HRQOL in response to LVAD (p=0.048). Subsequent improvements in HRQOL beyond 1 month after LVAD were significant, small-to-moderate in effect size, but were not different between bridge and destination therapy patients (p=0.590).
Figure 1. Changes in quality of life in response to LVAD by implant strategy.
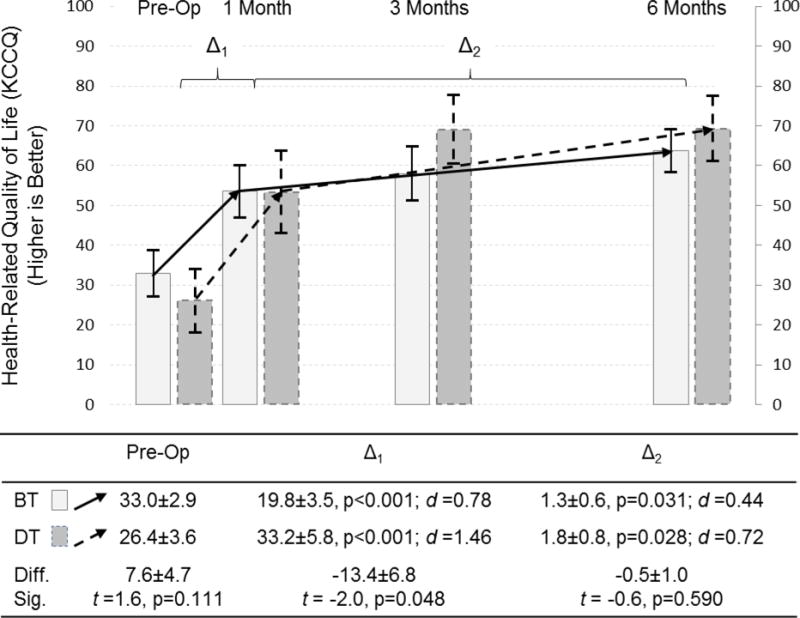
Changes in quality of life, as measured by 3-items of the Kansas City Cardiomyopathy Questionnaire, are depicted comparing pre-operative assessment with measure taken at 1, 3 and 6 months after LVAD; the mean and 95% confidence interval is represented by column height and the high and low whisker bars, respectively. Solid lines and lighter bar graphs represent bridge therapy patients, and dashed lines and darker bars represent destination therapy patients. Two phases of change in quality of life are depicted; the initial response to LVAD between pre-implant and 1 month post-implant (Δ1), and the subsequent change between 1 and 6 months post-implant (Δ2). The effect size of each phase of change is presented as Cohen’s d (d) (0.2–0.3 = small, ≈ 0.5 = moderate, and ≥ 0.8 = large effect). The difference (diff) and significance thereof (sig) of HRQOL pre-implant and during each phase of change between destination and bridge therapy patients are presented below the figure.
Abbreviations: BT = bridge therapy; Diff. = difference; DT = destination therapy; KCCQ = Kansas City Cardiomyopathy Questionnaire; LVAD = left ventricular assist device; Sig. = significance; t = t-test.
There were large and significant improvements in dyspnea, and moderate and significant improvements in wake disturbances in response to 6 months of LVAD in both groups (Figure 2). Destination therapy patients had worse dyspnea prior to implantation (p=0.034) and experienced greater initial improvements in dyspnea in response to LVAD compared with bridge therapy patients (p=0.012). Subsequent improvements in dyspnea beyond 1 month of LVAD were small in effect size, similar by therapy type (p=0.588), and significant only for bridge therapy patients. Destination therapy patients had worse wake disturbances prior to implantation (p=0.001) and experienced greater initial improvements in wake disturbances in response to LVAD compared with bridge therapy patients (p=0.009). In fact, wake disturbances did not change at 1 month compared with pre-implant for bridge therapy patients. Additional improvements in wake disturbances beyond 1 month of LVAD were significant, moderate in effect size, and similar between bridge and destination therapy patients (p=0.523).
Figure 2. Changes in dyspnea and wake disturbances in response to LVAD by implant strategy.
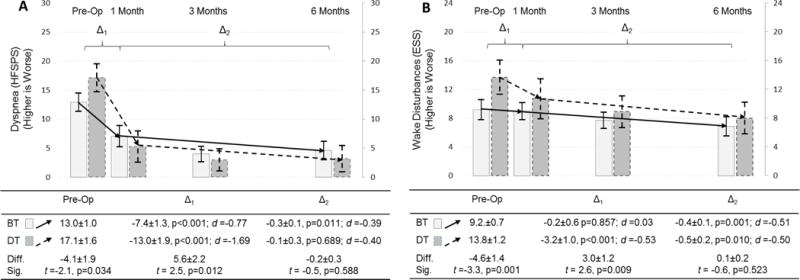
Changes in dyspnea (A; as measured by the Heart Failure Somatic Perception Scale dyspnea score) and wake disturbances (B; as measured by the Epworth Sleepiness Scale) are depicted comparing pre-operative assessment with measure taken at 1, 3 and 6 months after LVAD; the mean and 95% confidence interval is represented by column height and the high and low whisker bars, respectively. Solid lines and lighter bar graphs represent bridge therapy patients, and dashed lines and darker bars represent destination therapy patients. Two phases of change in symptoms are depicted; the initial response to LVAD between pre-implant and 1 month post-implant (Δ1), and the subsequent change between 1 and 6 months post-implant (Δ2). The effect size of each phase of change is also presented as Cohen’s d (d) (0.2–0.3 = small, ≈ 0.5 = moderate, and ≥ 0.8 = large effect). The difference (diff) and significance thereof (sig) of symptoms pre-implant and during each phase of change between destination and bridge therapy patients are presented below the figure.
Abbreviations: BT = bridge therapy; Diff. = difference; DT = destination therapy; ESS = Epworth Sleepiness Scale; HFSPS = Heart Failure Somatic Perception Scale; LVAD = left ventricular assist device; Sig. = significance; t = t-test.
Depression and anxiety improved significantly in response to LVAD in both groups over the course of 6 months (Figure 3). Destination therapy patients had worse depression prior to implant (p=0.042), but experienced similar initial (p=0.420) and subsequent improvements (p-0.188) in response to LVAD compared with bridge therapy patients. Improvements in depression beyond 1 month of LVAD were only significant only for destination therapy patients. Destination therapy patients had similar anxiety prior to LVAD compared with bridge patients (p=0.279); but, they experienced less initial (p=0.025) improvement, and greater subsequent improvements (p=0.003) in anxiety in response to LVAD compared with bridge therapy patients. Anxiety was not better at 1 month compared with pre-implant for destination therapy patients, and anxiety did not improve beyond 1 month of LVAD for bridge therapy patients.
Figure 3. Changes in depression and anxiety in response to LVAD by implant strategy.
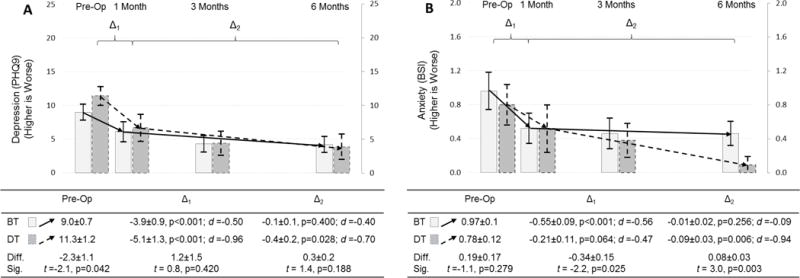
Changes in depression (A; as measured by the patient health questionnaire) and anxiety (B; as measured by the brief symptom inventory) are depicted comparing pre-operative assessment with measure taken at 1, 3 and 6 months after LVAD; the mean and 95% confidence interval is represented by column height and the high and low whisker bars, respectively. Solid lines and lighter bar graphs represent bridge therapy patients, and dashed lines and darker bars represent destination therapy patients. Two phases of change in symptoms are depicted; the initial response to LVAD between pre-implant and 1 month post-implant (Δ1), and the subsequent change between 1 and 6 months post-implant (Δ2). The effect size of each phase of change is also presented as Cohen’s d (d) (0.2–0.3 = small, ≈ 0.5 = moderate, and ≥ 0.8 = large effect). The difference (diff) and significance thereof (sig) of symptoms pre-implant and during each phase of change between destination and bridge therapy patients are presented below the figure.
Abbreviations: BSI = Brief Symptom Inventory; BT = bridge therapy; Diff. = difference; DT = destination therapy; PHQ9 = patient health questionnaire; LVAD = left ventricular assist device; Sig. = significance; t = t-test.
Discussion
We sought to characterize and compare changes in common symptoms in response to LVAD as bridge or destination therapy. In this sample of 86 adults undergoing LVAD, we observed large and significant improvements in dyspnea, wake disturbances, depression and anxiety that in many instances were different comparing bridge versus destination therapy patients in both magnitude and in the timing of change. Destination therapy patients had similar pre-implant and anxiety, but worse pre-implant dyspnea, wake disturbances and depression compared with bridge therapy patients. Destination therapy patients had greater initial (1-month) improvements in dyspnea and wake disturbance, similar initial improvements in depression, and smaller initial improvements in anxiety compared with bridge therapy patients. Finally, destination therapy patients had similar subsequent (1 to 6-month) improvements in dyspnea, wake disturbances and depression, and greater subsequent improvements in anxiety compared with bridge therapy patients. We are hopeful that our insights on specific symptom responses to LVAD that are concrete and tangible can facilitate shared decision making and be complementary to the large body of literature on HRQOL in LVAD.
Our findings of significant improvement in HRQOL in response to LVAD mirror the early and sustained changes reported previously34 with a few notable exceptions. First, we have provided evidence that pre-implant HRQOL is similar comparing destination and bridge therapy patients. This is important because at the same time we have provided evidence that destination therapy patients have worse pre-implant dyspnea, wake disturbances and depression compared with bridge therapy patients. Hence, HRQOL cannot serve well as a catch-all patient-reported outcome if it doesn’t capture significant differences in hallmark physical and affective symptoms associated with HF. Second, we provide evidence that the greatest gains in HRQOL occur within 1 month and during this same time destination therapy patients have much greater initial improvements in HRQOL compared with bridge therapy patients. Hence, the largest gains in HRQOL should be expected between pre-implant and 1 month of LVAD, particularly among destination therapy patients, followed by significant but small to moderate improvements through 6 months of LVAD therapy. This also means that there may be room for improvement in optimizing HRQOL after 1 month.
Our novel insights into one of the hallmark symptoms of HF, dyspnea, are important for a few reasons. First, communicating directly with patients and their families about what to expect regarding dyspnea may be more concrete than talking only about HRQOL in response to LVAD. For example, one talking point might be that on average patients have nearly half (bridge therapy) or less than one third (destination therapy) the amount of shortness of breath at 1 month of LVAD compared with what they are experiencing now pre-implantation. Second, it is also important to note that whatever destination therapy patients gain in dyspnea reduction at 1 month will plateau compared with bridge therapy patients who will continue to have significant reductions in dyspnea after 1 month. This difference by implant strategy is important because destination therapy patients are living longer than ever with durable devices yet may live with a similar level of dyspnea they have just 1 month after LVAD. Indeed, this information derived from our novel findings may aid shared decision-making more effectively than a similar discussion about improvements in HRQOL, particularly for destination therapy patients.
Wake disturbances in HF are fascinating because they are common, closely related to fatigue, and are often observable (e.g. when the patient falls asleep during an exam). Prior work has provided evidence that wake disturbances are prevalent before and up to 6 months after LVAD.38 To the best of our knowledge, however, our findings of worse wake disturbances pre-implant and greater initial improvements in wake disturbances with destination therapy compared with bridge therapy patients are novel. After 1 month, subsequent reductions in wake disturbances were similar between bridge and destination therapy patients. Here again, changes in wake disturbances may be a more concrete way of describing the potential value of LVAD regarding patient-reported outcomes compared with HRQOL. Specifically, insight that destination therapy patients in particular are much less likely to fall asleep while reading, watching television or talking with someone after LVAD compared with what they are experiencing pre-implant may help them make better shared decisions. Moreover, there is room for improvement in minimizing wake disturbances after 1 month in both groups.
Depression and anxiety have been shown to improve in an early and sustained fashion in response to LVAD in prior research.39 We observed several new insights into depression and anxiety in response to LVAD. First, although depression was significantly worse pre-implant among destination therapy compared with bridge patients, initial and subsequent improvements in depression in response to LVAD was similar between therapy types. Information that on average patients experience less than half the level of depression by 3 months compared with what they experience pre-implant may help guide shared decision-making prior to LVAD. Said another way, by three months the level of depression in both groups would be considered mild (PHQ9 of 5) compared with pre-implant depression also that was in the moderate range (PHQ9 of 10). Second, depression and anxiety continued to improve significantly after 1 month in destination therapy patients whereas there was a plateau in both depression and anxiety after 1 month for bridge therapy patients. It is unclear if therapy-related differences in how depression and anxiety change in response to LVAD reflect inherent variances in state (e.g. the contribution of advanced HF) or trait (e.g. the contribution of non-HF related factors) symptoms, or simply differences between destination therapy patients who know that LVAD is their enduring therapy as opposed to bridge therapy patients who are hopeful for transplantation. Interestingly, recent work has provided evidence that LVAD-related changes in anxiety and depression are not dependent on the use of psychotropic medications.39 Knowing that bridge therapy patients do not have significant improvements in depression and anxiety after 1-month, but have significant concomitant improvements in HRQOL and physical symptoms, may point to the need for strategy-specific cognitive therapy that has been shown to be helpful in other phases of HF.40
There are both strengths and limitation to this research. This study is one of few studies that obtained detailed, valid and reliable patient-reported measures of physical and affective symptoms before and after LVAD; hence, these results contribute to the emerging area of symptom science. An additional strength of this research is the use of a type of growth modeling that matches the two distinct phases of change in symptoms in response to LVAD. In addition to inherent limitations of observational research, this was a single center study that included a sample mainly comprised of men and a relatively short follow-up period of 6 months of LVAD. Hence, more work will need to be done to codify or further refine these insights in future research. It is also our intention to identify the influence of demographic, clinical, and other treatment factors on the magnitude of change in symptoms in future reports. Finally, although the bridge vs. destination therapy designation was helpful in characterizing differences and similarities of change in symptoms in this report, there are limitations to viewing this designation as mutually exclusive41 that need to be considered when interpreting our findings.
Conclusions
Although LVAD is generally associated with significant improvements in physical and affective symptoms over time, there are many difference in the magnitude and timing of change in symptom responses to LVAD between bridge and destination therapy patients. Detailed information on changes in specific physical and affective symptoms may better inform shared LVAD decision making compared with discussion about the generic patient-reported outcome of HRQOL.
Contributor Information
Christopher S. Lee, Oregon Health & Science University School of Nursing and Knight Cardiovascular Institute, Portland, OR.
Jill M. Gelow, Oregon Health & Science University Knight Cardiovascular Institute, Portland, OR.
Christopher V. Chien, University of North Carolina REX Healthcare, Raleigh, NC.
Shirin O. Hiatt, Oregon Health & Science University School of Nursing, Portland, OR.
Julie T. Bidwell, Emory University Nell Hodgson Woodruff School of Nursing, Atlanta, GA.
Quin E. Denfeld, Oregon Health & Science University Knight Cardiovascular Institute, Portland, OR.
Kathleen L. Grady, Feinberg School of Medicine, Northwestern University, Chicago, IL.
James O. Mudd, Oregon Health & Science University Knight Cardiovascular Institute, Portland, OR.
References
- 1.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metra M, Ponikowski P, Dickstein K, et al. Advanced chronic heart failure: A position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9(6–7):684–694. doi: 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report–2012. J Heart Lung Transplant. 2012;31(10):1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287(5):628–640. doi: 10.1001/jama.287.5.628. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 7.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015 doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Grady KL, Naftel DC, Myers S, et al. Change in health-related quality of life from before to after destination therapy mechanical circulatory support is similar for older and younger patients: analyses from INTERMACS. J Heart Lung Transplant. 2015;34(2):213–221. doi: 10.1016/j.healun.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grady KL, Naftel D, Stevenson L, et al. Overall quality of life improves to similar levels after mechanical circulatory support regardless of severity of heart failure before implantation. J Heart Lung Transplant. 2014;33(4):412–421. doi: 10.1016/j.healun.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grady KL, Magasi S, Hahn EA, Buono S, McGee EC, Jr, Yancy C. Health-related quality of life in mechanical circulatory support: Development of a new conceptual model and items for self-administration. J Heart Lung Transplant. 2015;34(10):1292–1304. doi: 10.1016/j.healun.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Nieminen MS, Dickstein K, Fonseca C, et al. The patient perspective: Quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol. 2015;191:256–264. doi: 10.1016/j.ijcard.2015.04.235. [DOI] [PubMed] [Google Scholar]
- 12.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 13.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 14.Riegel B, Ratcliffe SJ, Weintraub WS, et al. Double jeopardy: the influence of excessive daytime sleepiness and impaired cognition on health-related quality of life in adults with heart failure. Eur J Heart Fail. 2012;14(7):730–736. doi: 10.1093/eurjhf/hfs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 16.Yohannes AM, Willgoss TG, Baldwin RC, Connolly MJ. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25(12):1209–1221. doi: 10.1002/gps.2463. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg RJ, Spencer FA, Szklo-Coxe M, et al. Symptom presentation in patients hospitalized with acute heart failure. Clin Cardiol. 2010;33(6):E73–80. doi: 10.1002/clc.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KS, Song EK, Lennie TA, et al. Symptom clusters in men and women with heart failure and their impact on cardiac event-free survival. J Cardiovasc Nurs. 2010;25(4):263–272. doi: 10.1097/JCN.0b013e3181cfbb88. [DOI] [PubMed] [Google Scholar]
- 19.Song EK, Moser DK, Rayens MK, Lennie TA. Symptom clusters predict event-free survival in patients with heart failure. J Cardiovasc Nurs. 2010;25(4):284–291. doi: 10.1097/JCN.0b013e3181cfbcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brouwers C, Denollet J, de Jonge N, Caliskan K, Kealy J, Pedersen SS. Patient-reported outcomes in left ventricular assist device therapy: a systematic review and recommendations for clinical research and practice. Circ Heart Fail. 2011;4(6):714–723. doi: 10.1161/CIRCHEARTFAILURE.111.962472. [DOI] [PubMed] [Google Scholar]
- 21.Lee CS, Mudd JO, Gelow JM, et al. Background and Design of the Profiling Biobehavioral Responses to Mechanical Support in Advanced Heart Failure Study. J Cardiovasc Nurs. 2014;29(5):405–415. doi: 10.1097/JCN.0b013e318299fa09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eurich DT, Johnson JA, Reid KJ, Spertus JA. Assessing responsiveness of generic and specific health related quality of life measures in heart failure. Health Qual Life Outcomes. 2006;4:89. doi: 10.1186/1477-7525-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Sawadogo K, Ambroise J, Vercauteren S, et al. Interaction between the Kansas City Cardiomyopathy Questionnaire and the Pocock’s clinical score in predicting heart failure outcomes. Qual Life Res. 2016;25(5):1245–1255. doi: 10.1007/s11136-015-1154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurgens CY. Somatic awareness, uncertainty, and delay in care-seeking in acute heart failure. Res Nurs Health. 2006;29(2):74–86. doi: 10.1002/nur.20118. [DOI] [PubMed] [Google Scholar]
- 27.Jurgens CY, Lee CS, Riegel B. Psychometric analysis of the heart failure somatic perception scale as a measure of patient symptom perception. J Cardiovasc Nurs. doi: 10.1097/JCN.0000000000000320. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 31.Burant CJ. Latent Growth Curve Models: Tracking Changes Over Time. Int J Aging Hum Dev. 2016;82(4):336–350. doi: 10.1177/0091415016641692. [DOI] [PubMed] [Google Scholar]
- 32.Grimm KJ, Ram N, Hamagami F. Nonlinear growth curves in developmental research. Child Dev. 2011;82(5):1357–1371. doi: 10.1111/j.1467-8624.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ram N, Grimm KJ. Using simple and complex growth models to articulate developmental change: Matching theory to method. International Journal of Behavioral Development. 2007;31(4):303–316. [Google Scholar]
- 34.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16(6):e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Mahwah, New Jersey: Lawrence Elbaum Associates; 1988. [Google Scholar]
- 36.Muthé LK, Muthén BO. Mplus: Statistical Analysis with Latent Variables User’s Guide. 6th. Los Angeles: Muthén & Muthén; 2010. [Google Scholar]
- 37.MacCallum RC, Widaman KF, Zhang S, Hong S. Sample size in factor analysis. Psychological Methods. 1999;4:84–99. [Google Scholar]
- 38.Casida JM, Davis JE, Brewer RJ, Smith C, Yarandi H. Sleep and daytime sleepiness of patients with left ventricular assist devices: a longitudinal pilot study. Prog Transplant. 2011;21(2):131–136. doi: 10.1177/152692481102100208. [DOI] [PubMed] [Google Scholar]
- 39.Reynard AK, Butler RS, McKee MG, Starling RC, Gorodeski EZ. Frequency of depression and anxiety before and after insertion of a continuous flow left ventricular assist device. Am J Cardiol. 2014;114(3):433–440. doi: 10.1016/j.amjcard.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Dekker RL. Cognitive therapy for depression in patients with heart failure: a critical review. Heart Fail Clin. 2011;7(1):127–141. doi: 10.1016/j.hfc.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang JC, Stehlik J. Moving beyond “bridges”. JACC Heart Fail. 2013;1(5):379–381. doi: 10.1016/j.jchf.2013.08.003. [DOI] [PubMed] [Google Scholar]


