Abstract
Objective:
To examine the BRCA1-associated protein-1 (BAP1) expression of primary uveal melanomas without and with metastasis, and to analyze the correlation between the BAP1 immunoreactivity of primary uveal melanoma and other clinico-pathologic features.
Design:
Retrospective case series.
Subjects:
Forty uveal melanoma patients (mean age: 57.98±14.75 years) were included in this analysis, of which twenty patients had no metastatic disease and 20 patients had metastasis.
Methods:
Medical records and histology slides of patients with primary uveal melanoma treated by enucleation were reviewed. BAP1 expression was evaluated by immunohistochemical staining of formalin fixed paraffin embedded sections. Immunoreactivity in the nucleus and cytoplasm were graded by estimating the percentage of primary tumor cells showing a positive staining of their nucleus or cytoplasm per 1 high power field 200× (grade 0 to 3).
Main outcome measures:
Tumor size, histologic features, nuclear and cytoplasmic BAP1 immunoreactivity grade and patient outcome including development of metastasis.
Results:
Significantly lower nuclear (P=0.025) BAP1 immunoreactivity was observed in the metastatic melanoma group. Greater tumor thickness, basal diameter and more advanced TNM stage were associated with an increased odds ratio of developing metastasis (P<0.05). Additionally, tumors with a higher proportion of cells expressing nuclear BAP1 had decreased odds of developing metastatic disease in a multivariate model (P=0.042). Metastasis-free survival was significantly longer in uveal melanoma patients with high nuclear BAP1 stain (P=0.004).
Conclusions:
There is a difference in time to metastasis in uveal melanoma patients with different grades of nuclear BAP1 immunoreactivity measured in primary uveal melanoma. Nuclear BAP1 stain is the only significant independent predictor of metastatic disease in this study. Our data support the role of BAP1 immunohistochemical staining of primary uveal melanoma to evaluate metastatic risk.
Introduction
Uveal melanoma is the most common primary intraocular malignancy in adults.1 At the time of diagnosis, fewer than 5% of patients with uveal melanoma have detectable metastatic disease. In further course, however, about 40% of patients will develop metastases, and there is a high rate of mortality in patients with metastatic uveal melanoma.1,2 Five genes have been identified to be frequently mutated in uveal melanoma, including BRCA1-associated protein (BAP1), Eukaryotic translation initiation factor 1A X-chromosomal (EIF1AX), Guanine nucleotide-binding protein subunit alpha-11 (GNA11), Guanine nucleotide-binding protein (Gq) subunit alpha (GNAQ) and Splicing factor 3B subunit 1 (SF3B1).1–8 The BAP1 gene is located on chromosome 3p21.1 and encodes a nuclear ubiquitinase involved in epigenetic modulation of chromatin.9 Inactivating mutations in the tumor suppressor BAP1 are strongly associated with metastasis, suggesting that BAP1 may function as a metastasis suppressor in uveal melanoma.10 Koopmans et al. demonstrated a strong association between BAP1 immunostaining and BAP1 mutation status (sensitivity: 88% and specificity: 97%) in 74 patients with histologically proven uveal melanoma.11
The purpose of this study is to examine the BAP1 expression of primary uveal melanomas without and with metastasis as well as to analyze the correlation between the BAP1 immunoreactivity of primary uveal melanoma and the clinico-pathologic features of patients with this tumor.
Patients and methods
Enucleated eyes with uveal melanoma that were accessioned into the L. F. Montgomery Ophthalmic Pathology Laboratory, Emory Eye Center, between January 2002 and October 2016 were collected, and the medical charts were reviewed. Inclusion criteria for the study patients were: (1) histologically proven uveal melanoma and (2) available clinical follow-up data. Exclusion criteria were: (1) tumor location confined only to the iris and (2) non-melanoma histopathologic diagnosis. The clinico-pathological features evaluated included: patient age, sex, pretreatment modality, largest basal diameter (LBD), tumor thickness, histopathologic tumor cell type, scleral invasion, extrascleral extension at the time of surgery, rupture of Bruch’s membrane, vortex vein invasion, gene expression profile (Decision Dx-UM; Castle Biosciences Inc., Phoenix, AZ), TNM stage12, presence or absence of metastasis, and time to metastasis or metastasis-free follow up time. The study was HIPAA-compliant, followed the tenets of the Declaration of Helsinki and was approved by the Emory University Institutional Review Board.
Histology
Enucleation specimens were immediately fixed in formalin (10%) and embedded in paraffin. 5 μm thick pupil-optic nerve sections that included the center of the melanoma were mounted on glass slides, then sections were deparaffinized with xylene and rehydrated through a graded series of ethanol and distilled water. The sections were stained with hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) as well as bleached sections were stained with H&E according to routine protocols. The slides were evaluated qualitatively and quantitatively with a light microscope (Olympus BHTU, Tokyo, Japan). The histologic cell type (spindle, mixed, epithelioid), largest basal diameter (LBD) and thickness of the primary tumor, scleral invasion, extrascleral extension, rupture of Bruch’s membrane and vortex vein invasion were recorded.
Immunohistochemical staining
BAP1 immunohistochemistry was performed on paraffin tissue sections with the Leica Bond-III automated system (Leica Microsystems, Chicago, IL) using a red chromogen according to the manufacturer protocol. After deparaffinizing sections, heat-induced antigen retrieval was performed, and the sections were incubated for 20 minutes with BAP1 antibody (1:40 dilution; Santa Cruz Biotechnology, USA). This was followed by incubation with hematoxylin counterstain. The tissue sections were screened under low magnification (40×), and the three areas exhibiting the most intense BAP1 staining were selected for grading. Immunoreactivity was semiquantitatively evaluated in the nucleus and cytoplasm separately under 200× magnification using a 4-point scoring system (0 = positive staining in less than 10% of cells per high-power field, 1 = positive staining in between 10% and 33% of cells per high-power field, 2 = positive staining in between 33% and 66% of cells per high-power field, 3 = positive staining in 66% or greater number of cells per high-power field) (Figure 1, 2). BAP1 immunoreactivity grade was calculated as the mean score in the three most intensely stained areas separately for the nuclear and cytoplasmic BAP1 stain. The slides were graded twice in a masked fashion.
Figure 1.
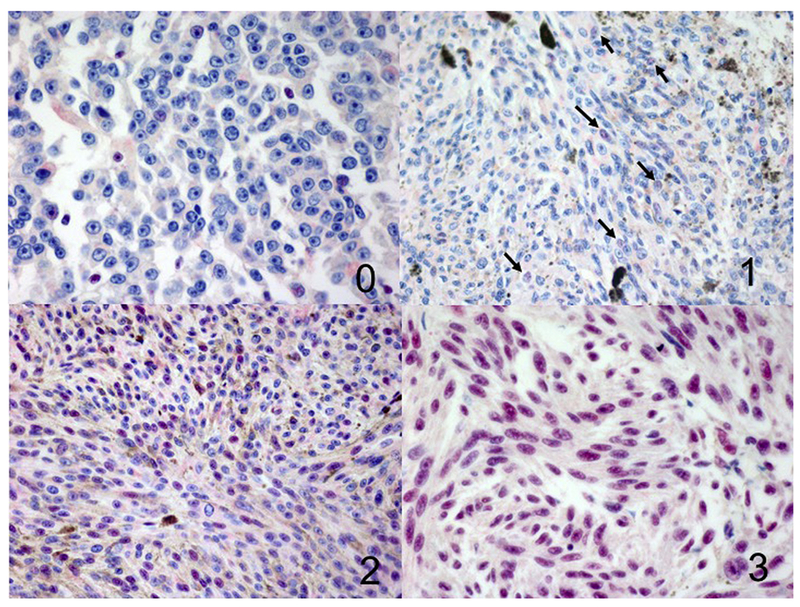
Different grades of BAP1 immunoreactivity in the nucleus (100×), 0 = positive staining in less than 10% of cells per high-power field, 1 = positive staining (arrows) in more than 11% but in less than 33% of cells per high-power field, 2 = positive staining in more than 34% but less than 66% of cells per high-power field, 3 = positive staining in more than 67% of cells per high-power field.
Figure 2.
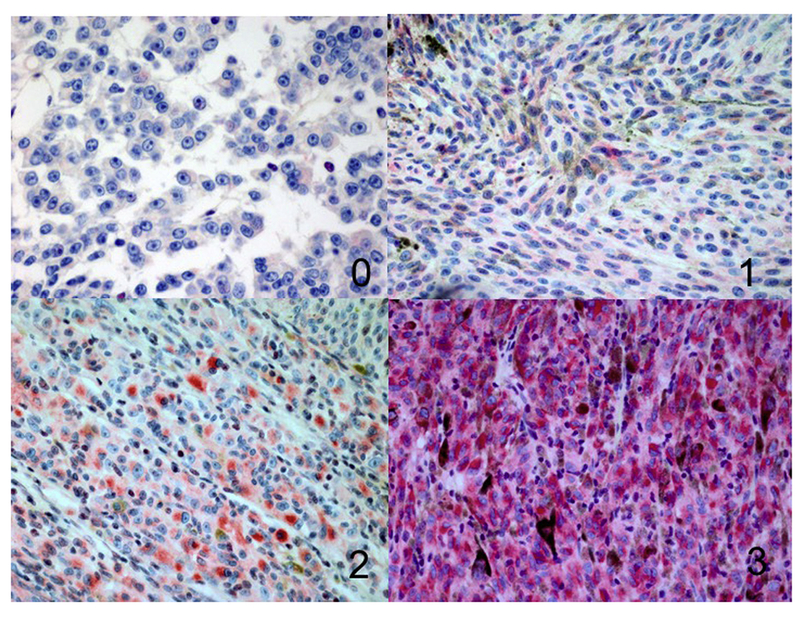
Different grades of BAP1 immunoreactivity in the cytoplasm (100×), 0 = positive staining in less than 10% of cells per high-power field, 1 = positive staining in more than 11% but in less than 33% of cells per high-power field, 2 = positive staining in more than 34% but less than 66% of cells per high-power field, 3 = positive staining in more than 67% of cells per high-power field.
Data analysis
Descriptive statistics were calculated on demographic and clinical factors for those without metastasis and those with metastasis. Descriptive statistics are expressed as mean ± standard deviation (SD) for continuous variables and frequency and percent for categorical variables. For comparison of clinical variables of interest between those with and without melanoma, a t-test or Chi-square test was performed, as appropriate. Nuclear and cytoplasmic BAP1 stain was analyzed as a binary variable (low/high) using grade 1 as a cut-off (> grade 1 was considered a high BAP1 stain group and ≤ grade 1 was considered a low BAP1 stain group).
The relationship between the presence of metastasis and clinical variables of interest (LBD, tumor thickness, nuclear BAP1 stain) were also examined with bivariate and multivariate logistic regression models. Corresponding odds ratios and 95% confidence intervals were also calculated. An additional outcome, metastasis-free survival, defined as the time to the development of metastatic disease, was analyzed using the Kaplan-Meier method. If a subject did not develop metastic disease, they were censored at the time of last study visit. All statistical tests were completed with SAS 9.4 with an alpha of 0.05.
Results
Forty patients (22 males, 18 females) with primary uveal melanoma treated with enucleation were included in this analysis. The mean age of the patients was 57.98 ± 14.75 years (ranging from 24 to 86 years). Twenty patients had no metastatic disease during the 77.45 ± 42.26 months mean follow-up (Group A). Twenty patients developed metastasis in an average of 30.7 ± 23.13 months from the enucleation surgery (Group B). In Group A, ten patients had prior plaque brachytherapy and one patient was previously treated with transpupillary thermotherapy and proton beam radiation. In Group B, 4 patients were treated with plaque brachytherapy, 2 patients had proton beam radiation and 1 patient underwent ophthalmic artery embolization. In Group B, every patient had liver metastasis, six patients had lung, three patients had bone, 3 patients had local, 2 patients had subcutaneous and one patient had lymph node metastasis. Two patients (5%) had metastasis at presentation. All patients are alive in Group A, whereas five patients (25%) are alive with 15 patients (75%) deceased in Group B. Gene expression profiling was performed in 17 patients. In Group A, two patients had class 2, four patients had class 1 (not specified) and two patients had class 1A and 1B melanoma. Five patients had gene expression profile class 2, two patients had class 1B tumor in Group B.
Clinico-pathologic features of uveal melanoma patients without and with metastasis are summarized in Table 1. In Group A patients the histologic cell type was mixed in 95% (19/20) and epitheloid in 5% (1/20), in Group B mixed cell type was found in 80% (16/20) and epitheloid in 20% (4/20) (P = 0.191). Significantly higher mean LBD (P = 0.006) and mean tumor thickness (P = 0.005) was observed in Group B. Optic nerve invasion was not found in either group. Statistical analysis did not disclose significant difference between the two groups in Bruch’s membrane rupture (P = 0.204), scleral invasion (P = 0.127), extrascleral extension (P = 0.231) and vortex vein invasion (P = 1.000).
Table 1.
Clinico-pathologic features of uveal melanoma patients without (Group A) and with metastasis (Group B).
| Group A (n=20) | Group B (n=20) | P-value | ||
|---|---|---|---|---|
| Age# (years) | 58.5 (15.5) | 57.4 (14.3) | 0.809 | |
| Largest basal diameter# (mm) | 14.28 (4.37) | 18.14 (3.93) | 0.006 | |
| Tumor thickness# (mm) | 6.41 (2.71) | 10.65 (8.02) | 0.005 | |
| TNM Stage | 1 | 5 (25%) | 0 (0%) | 0.001 |
| 2 | 2 (10%) | 5 (25%) | ||
| 3 | 10 (50%) | 3 (15%) | ||
| 4 | 3 (15%) | 12 (60%) | ||
| Nuclear BAP1 stain | Low | 5 (25%) | 12 (60%) | 0.025* |
| High | 15 (75%) | 8 (40%) | ||
| Cytoplasmic BAP1 stain | Low | 1 (5%) | 6 (30%) | 0.092 |
| High | 19 (95%) | 14 (70%) | ||
| BM rupture | 7 (35%) | 11 (55%) | 0.204* | |
| Vortex vein invasion | 0 (0%) | 1 (5%) | 1.000 | |
| Sclera invasion | 13 (65%) | 18 (90%) | 0.127 | |
| Extrascleral extension | 0 (0%) | 3 (15%) | 0.231 | |
| GEP class | 1 | 8/10 (80%) | 2/7 (29%) | 0.058 |
| 2 | 2/10 (20%) | 5/7 (71%) |
Mean (standard deviation)
Chi-square test
Immunohistochemistry
BAP1 expression was analyzed immunohistochemically on paraffin sections from primary uveal melanoma patients without (Group A) and with metastasis (Group B). In Group A, nuclear BAP1 stain was low (grade 0–1) in 10% (2/20) and high (grade 2–3) in 90% (18/20). In Group B, low nuclear BAP1 immunoreactivity was found in 70% (14/20) and high nuclear BAP1 stain was observed in 30% (6/20). No detectable nuclear BAP1 expression was observed in none of the patients without metastasis and 45% (9/20) of patients with metastasis. Cytoplasmic BAP1 stain was high in 100% (20/20) in patients without metastasis. In patients with metastatic disease, low cytoplasmic BAP1 immunoreactivity was observed 35% (7/20) and high cytoplasmic BAP1 stain was found in 65% (13/20). A significantly lower nuclear BAP1 immunoreactivity score was found in Group B (P = 0.025). There was no statistically significant difference in cytoplasmic BAP1 stain between both groups (P = 0.092).
There was a significant difference in nuclear BAP1 immunoreactivity between patients with and without prior treatment (P = 0.006). No statistically significant difference in nuclear BAP1 stain was observed between patients with liver metastasis and those with multiple metastasis (P = 0.337). BAP1 reactivity in the nucleus did not differ significantly between patients with metastatic uveal melanoma who are alive and deceased (P = 0.433). Significantly lower nuclear BAP1 expression was found in patients with class 2 gene expression profile when compared to those with class 1 tumor (P = 0.001).
Bivariate logistic regression analysis confirmed that higher tumor thickness, basal diameter and more advanced TNM stage significantly increased the odds of developing metastatic disease (Table 2). Additionally, it revealed that having higher nuclear BAP1 expression decreased the odds of developing metastasis. No other clinico-pathologic parameters contributed significantly to the development of metastatic uveal melanoma (P > 0.05). Multivariate logistic regression model showed that nuclear BAP1 stain was the only significant independent predictor of metastatic disease (P = 0.042) (Table 3). Patients with high nuclear BAP1 stain had longer metastasis-free survival than those with low BAP1 stain (P = 0.004, Figure 3). The estimated mean time to metastasis was 39.06 ± 7.41 months for low nuclear BAP1 stain and 138.12 ± 16.22 months for high nuclear BAP1 stain.
Table 2.
Logistic regression analysis of predictors of metastasis risk in uveal melanoma
| OR (95% Confidence Interval) | P-value | ||
|---|---|---|---|
| Age | 0.947 (0.618, 1.451)* | 0.8032 | |
| LBD | 1.268 (1.048, 1.534) | 0.0146 | |
| Tumor thickness | 1.302 (1.063, 1.595) | 0.0107 | |
| TNM Stage | 1 | 0.053 (0, 0.348) | 0.0008 |
| 2 | 0.639 (0.053, 9.877) | ||
| 3 | 0.085 (0.008, 0.585) | ||
| 4 | Reference group | ||
| Nuclear BAP1 stain | Low | Reference Group | 0.0291 |
| High | 0.222 (0.058, 0.858) | ||
| Cytoplasmic BAP1 stain | Low | Reference Group | 0.0649 |
| High | 0.123 (0.013, 1.138) | ||
| BM rupture | 2.270 (0636, 8.106) | 0.2069 | |
| Scleral invasion | 4.4846 (0.863, 27.221) | 0.0731 |
For every 10-year increase in age
Table 3.
Multivariate logistic regression model evaluating the metastatic risk
| OR (95% CI) | P-value | ||
|---|---|---|---|
| LBD | 1.090 (0.833, 1.426) | 0.531 | |
| Tumor thickness | 1.222 (0.932, 1.603) | 0.147 | |
| Nuclear BAP1 stain | Low | Reference Group | 0.042 |
| High | 0.194 (0.040, 0.942) |
Figure 3.
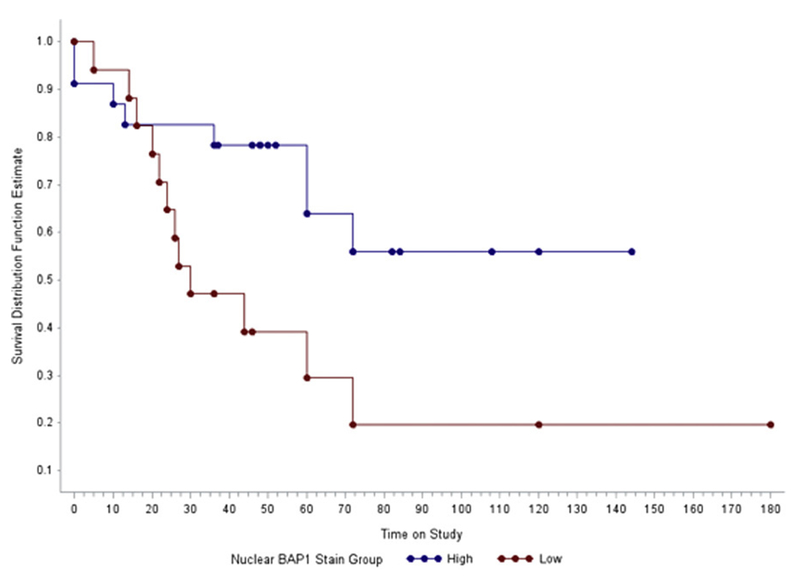
Kaplan-Meier metastasis-free survival curve for uveal melanoma patients with low and high nuclear BAP1 stain.
Discussion
It has been reported that germline BAP1 mutations measured in peripheral blood are present in approximately 22% (range 8% to 50%) of familial uveal melanomas compared with 2–4% in spontaneously occurring uveal melanoma cases.13–15 However, a higher rate (approximately 47.4%) of somatic BAP1 mutations has been reported in primary uveal melanoma.10–11 Another investigation found a mutation frequency of 45% (29/64) for BAP1 in unselected cases using exome and Sanger sequencing.8 Whole-exome sequencing of metastatic uveal melanoma identified inactivating somatic mutations in BAP1 in 81-84% of metastatic tumors.10,16 Ewens et al. reported that 77% of uveal melanomas carrying BAP1 mutations developed metastasis.17 Harbour et al. strongly implicated that progression to metastatic competence of uveal melanomat requires inactivation of BAP1 as a crucial event.10
Patients with tumor predisposition syndrome (TPDS) associated with BAP1 mutation have been reported to have an increased risk for different malignancies, including uveal melanoma. The affected patients can have multiple primary tumors which can be more aggressive than those in the average population.18 TPDS associated with BAP1 mutation is inherited in an autosomal dominant pattern with a high penetrance.18 Cebulla et al. identified a BAP1 germline mutation in young patients with uveal melanoma.19 Early age of cancer onset is thought to be an important feature of high-risk for hereditary predisposition to cancer even for TPDS in which uveal melanoma has been the earliest reported cancer.20,21 This finding supports that BAP1 screening should be considered in young patients with uveal melanoma for prognostic stratification.
In our study, significantly lower nuclear BAP1 immunoreactivity was observed in patients with metastatic uveal melanoma when compared to patients without metastasis. We found no statistically significant difference between both patient groups regarding cytoplasmic BAP1 staining. The nuclear BAP1 stain was low (Grade 1) in 10% of patients without metastasis and 70% of patients with metastatic melanoma. None of the patients without metastasis had an absence of nuclear BAP1 staining (Grade 0) whereas 45% of patients with metastasis had Grade 0 nuclear BAP1 staining. Van de Nes et al. observed no detectable BAP1 mutations in melanomas with positive nuclear BAP1 staining.22 They found a loss of BAP1 immunoreactivity in all of their specimens with a BAP1 mutation regardless of the type of mutation measured with Sanger sequencing, gene dosage and methylation analysis.22
Previous studies reported that BAP1 is a chromatin associated protein and located in the nucleus assessed by immunohistochemistry.23,24 Wild type BAP1 was shown to be preferentially found in the nucleus, whereas mutant BAP1 proteins showed impaired nuclear localization with increased cytoplasmic appearance.25 Nuclear localization of BAP1 protein has been demonstrated to be required for tumor suppressor activity.26 This corresponds to our study of uveal melanoma patients with low nuclear BAP1 immunoreactivity which showed an increased incidence of metastasis. The estimated mean time to metastasis was 39.06 ± 7.41 months for low nuclear BAP1 stain and 138.12 ± 16.22 months for high nuclear BAP1 stain. We confirmed that tumor thickness, largest tumor basal diameter, more advanced TNM stage and nuclear BAP1 stain have a statistically significant relationship with the presence of metastasis. However, when we looked these parameters with multi-variate analysis, nuclear BAP1 staining was the only significant variable. Similar to our results, others have found a significant association between BAP1 immunoreactivity and metastatic progression of uveal melanoma.22,27
Matatall et al. demonstrate that BAP1 is necessary for maintenance of melanocyte identity in uveal melanoma cells, and that loss of BAP1 leads to a damaged cell identity and a gain in stem cell-like behavior.28 Decatur et al. assumed that BAP1 mutation likely develops later in tumor progression and is of prognostic significance.8 They found BAP1 mutations to be associated with class 2 gene expression profile (P < 0.001), older patient age (P = 0.007) and high metastatic risk in their study population. Gene expression profiling (GEP) is a prognostic test to predict the risk of metastasis in uveal melanoma.29 It is an RNA-based classification test which includes 12 discriminating genes and three control genes.29 Uveal melanomas with the class 1A and 1B GEP have a very low and low metastatic risk, whereas those with the class 2 GEP have a higher risk of metastatic disease. However, Decatur et al. highlighted that the gene expression profiling is prognostically superior to the mutation status of BAP1 gene, thus the role of mutational analysis for prognostic stratification may serve as a supplement to gene expression profiling.8
Previous studies investigated the BAP1 mutational status of metastatic uveal melanoma cells in the liver.30,31 Kalirai et al. reported that the grade of BAP1 protein expression observed in primary uveal melanoma cells were retained in the liver metastasis.30 Variable BAP1 expression was demonstrated in liver metastases of uveal melanomas, supporting the concept that although BAP1 mutations are important for promoting uveal melanoma metastasis, the BAP1 expression may or may not be lost in the metastases in the liver.31 However, the present study confirms the importance of BAP1 mutation of the primary tumor cells in the development of metastatic disease.
Metastasis-free survival was significantly longer in uveal melanoma patients with strong nuclear BAP1 stain of the primary tumor cells. BAP1 immunoreactivity in the nucleus was detected in all patients without metastasis, nuclear BAP1 expression indicating properly functioning BAP1 protein was completely lost in 45% and low in 70% of patients with metastatic uveal melanoma. There is substantial evidence for a difference in time to metastasis in uveal melanoma patients with different grades of nuclear BAP1 immunoreactivity. Patients with low nuclear BAP1 stain showed an increased chance of metastasis. Besides lower nuclear BAP1 immunoreactivity, higher tumor thickness, basal diameter and more advanced TNM stage also contributed significantly to the development of metastatic uveal melanoma. However, nuclear BAP1 stain was the only significant independent predictor of metastatic disease in a multivariate analysis. Our data support the role of BAP1 immunohistochemical stain of primary uveal melanoma cells in evaluating the metastatic risk.
Acknowledgments
This study was supported in part by: NIH R01CA176001, NEI P300630 and Research to Prevent Blindness, Inc.
The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
No conflicting relationship exists for any author.
References
- 1.Bakalian S, Marshall JC, Logan P, et al. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin Cancer Res 2008;14:951–956. [DOI] [PubMed] [Google Scholar]
- 2.Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 2003;44:4651–4659. [DOI] [PubMed] [Google Scholar]
- 3.Onken MD, Worley LA, Long MD, et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci 2008;49:5230–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010;363:2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbour JW, Roberson ED, Anbunathan H, et al. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nat Genet 2013;45:133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin M, Maßhöfer L, Temming P, et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat Genet 2013;45:933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol 2016;134:728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheuermann JC, de Ayala Alonso AG, Oktaba K, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koopmans AE, Verdijk RM, Brouwer RW, et al. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod Pathol 2014;27:1321–1330. [DOI] [PubMed] [Google Scholar]
- 12.Uveal melanoma in: Amin MB, editor-in-chief AJCC Cancer Staging Manual, 8th edition Springer: New York; 2017, p. 805–817. [Google Scholar]
- 13.Gupta MP, Lane AM, DeAngelis MM, et al. Clinical characteristics of uveal melanoma in patients with germline BAP1 mutations. JAMA Ophthalmol 2015;133:881–887. [DOI] [PubMed] [Google Scholar]
- 14.Field MG, Harbour JW. Recent developments in prognostic and predictive testing in uveal melanoma. Curr Opin Ophthalmol 2014;25:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rai K, Pilarski R, Boru G, et al. Germline BAP1 alterations in familial uveal melanoma. Genes Chromosomes Cancer. 2017;56:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griewank KG, van de Nes J, Schilling B, et al. Genetic and clinico-pathologic analysis of metastatic uveal melanoma. Mod Pathol 2014;27:175–183. [DOI] [PubMed] [Google Scholar]
- 17.Ewens KG, Kanetsky PA, Richards-Yutz J, et al. Chromosome 3 status combined with BAP1 and EIF1AX mutation profiles are associated with metastasis in uveal melanoma. Invest Ophthalmol Vis Sci 2014;55:5160–5167. [DOI] [PubMed] [Google Scholar]
- 18.Pilarski R, Rai K, Cebulla C, et al. BAP1 Tumor predisposition syndrome 2016. October 13 In: Pagon RA, Adam MP, Ardinger HH, et al. , editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK390611/?report=classic;2016 Accessed 15.04.17. [PubMed] [Google Scholar]
- 19.Cebulla CM, Binkley EM, Pilarski R, et al. Analysis of BAP1 germline gene mutation in young uveal melanoma patients. Ophthalmic Genet 2015;36:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hampel H, Sweet K, Westman JA, et al. Referral for cancer genetics consultation: a review and compilation of risk assessment criteria. J Med Genet 2004;41:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoiom V, Edsgard D, Helgadottir H, et al. Hereditary uveal melanoma: a report of a germline mutation in BAP1. Genes Chromosomes Cancer. 2013;52:378–384. [DOI] [PubMed] [Google Scholar]
- 22.van de Nes JA, Nelles J, Kreis S, et al. Comparing the prognostic value of BAP1 mutation pattern, chromosome 3 status, and BAP1 immunohistochemistry in uveal melanoma. Am J Surg Pathol 2016;40:796–805. [DOI] [PubMed] [Google Scholar]
- 23.Joseph RW, Kapur P, Serie DJ, et al. Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low-risk clear cell renal cell carcinoma. Cancer. 2014;120:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arzt L, Quehenberger F, Halbwedl I, et al. BAP1 protein is a progression factor in malignant pleural mesothelioma. Pathol Oncol Res 2014;20:145–151. [DOI] [PubMed] [Google Scholar]
- 25.Hakiri S, Osada H, Ishiguro F, et al. Functional differences between wild-type and mutant-type BRCA1-associated protein 1 tumor suppressor against malignant mesothelioma cells. Cancer Sci 2015;106:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ventii KH, Devi NS, Friedrich KL, et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res 2008;68;6953–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue H, Qian J, Yuan Y, et al. Clinicopathological characteristics and prognosis for survival after enucleation of uveal melanoma in Chinese patients: long-term follow-up. Curr Eye Res 2016. December 2:1–7. [In Press] [DOI] [PubMed] [Google Scholar]
- 28.Matatall KA, Agapova OA, Onken MD, et al. BAP1 deficiency causes loss of melanocytic cell identity in uveal melanoma. BMC Cancer. 2013;13:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119:1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br J Cancer. 2014;111:1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossniklaus HE, Zhang Q, You S, et al. Metastatic ocular melanoma to the liver exhibits infiltrative and nodular growth patterns. Hum Pathol 2016;57:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]


