Abstract
Background
In sub-Saharan Africa, continued clinical follow-up, after cardiac surgery, is only available at urban referral centres. We implemented a decentralised, integrated care model to provide longitudinal care for patients with advanced rheumatic heart disease (RHD) at district hospitals in rural Rwanda before and after heart surgery.
Methods
We collected data from charts at non-communicable disease (NCD) clinics at three rural district hospitals in Rwanda to describe the outcomes of 54 patients with RHD who received cardiac valve surgery during 2007–2015.
Results
The majority of patients were adults (46/54; 85%), and 74% were females. The median age at the time of surgery was 22 years in adults and 11 years in children. Advanced symptoms—New York Heart Association class III or IV—were present in 83% before surgery and only 4% afterwards. The mitral valve was the most common valve requiring surgery. Valvular surgery consisted mostly of a single valve (56%) and double valve (41%). Patients were followed for a median of 3 years (range 0.2–7.9) during which 7.4% of them died; all deaths were patients who had undergone bioprosthetic valve replacement. For patients with mechanical valves, anticoagulation was checked at 96% of visits. There were no known bleeding or thrombotic events requiring hospitalisation.
Conclusion
Outcomes of postoperative patients with RHD tracked in rural Rwanda health facilities were generally good. With appropriate training and supervision, it is feasible to safely decentralise follow-up of patients with RHD to nurse-led specialised NCD clinics after cardiac surgery.
Keywords: cardiac surgery, health care delivery, global health care delivery
Background
Rheumatic heart disease (RHD) is a major cause of cardiovascular morbidity and death among children and young adults in low-income and middle-income countries.1 RHD is largely preventable, yet >300 000 people die from RHD each year, and >1.2 million suffer from long-term complications including heart failure, atrial fibrillation, stroke and infective endocarditis.2
Sub-Saharan Africa has the second highest prevalence of RHD in the world, and RHD in this region is often diagnosed at an advanced stage.3 More than half of the patients with RHD in a registry involving 14 sub-Saharan Africa countries had severe valvular disease.4 Questions remain about the best strategies for prevention and early diagnosis of RHD.5 As a consequence, cardiac surgery is a key intervention in preventing early mortality among patients with RHD in this region.6 Cardiac surgery for RHD consists of valve repair or replacement using either bioprosthetic or mechanical valves.7 However, despite the need, access to cardiac surgery is severely limited in sub-Saharan Africa,8 with an estimated 18 cardiac surgical procedures per million people compared with 1222 per million in the USA.9 10
One of the main barriers to expansion of cardiac surgery for RHD in low and lower-middle income countries (LLMICs) in sub-Saharan Africa is concern regarding the quality of follow-up.11 12 After cardiac surgery, patients with RHD require penicillin prophylaxis for prevention of rheumatic fever recurrence, and most patients will also require anticoagulation to prevent thromboembolic events in the setting of a mechanical prosthesis and/or atrial fibrillation.13 In the few LLMICs in the region that have established cardiac surgery programmes, follow-up has been done by cardiologists at major referral centres.14 The outcomes at these centres have been poor, with inadequate anticoagulation and high rates of loss to follow-up.4 10 11 14 15 Additionally, centralised cardiac services are less likely to either find or retain impoverished patients from rural areas.16
In November 2006, the Rwandan Ministry of Health (MOH) initiated a partnership with the Rwanda Heart Foundation and four international humanitarian organisations to increase access to cardiac surgery for children and young adults with rheumatic and congenital heart disease.17 18
Rwanda, like most sub-Saharan African countries, has high rates of RHD (>123 000 cases estimated by the Global Burden of Disease Study) and extremely few cardiologists (only four working in the public sector).3 19 All of the cardiologists in the country are based in the capital city. In order to increase case finding for structural heart disease and decrease barriers to follow-up before and after cardiac surgery, the MOH partnered with the non-governmental organisation (NGO) Partners In Health/Inshuti Mu Buzima (PIH/IMB) to decentralise advanced non-communicable disease (NCD) care, including cardiovascular care, to three rural district hospitals, each serving a catchment area of around 250 000 people. Beginning in 2007, these three rural district hospital outpatient clinics were equipped with a portable echocardiographic machine and point-of-care International Normalized Ratio (INR) testing, and supplied with warfarin and basic heart failure medications. Nurses at these clinics underwent 3 months of training, involving didactics and precepted clinical experiences with cardiologists and other specialists to prepare them to care for patients with severe chronic NCDs such as rheumatic and congenital heart disease, cardiomyopathies, severe hypertension, type 1 diabetes, severe chronic respiratory disease and advanced malignancies in need of palliative care.20 Core competencies included preliminary echocardiographic evaluation of heart failure as well as anticoagulation and heart failure management. Nurses were supervised through regular outreach by cardiologists based in the capital, as well as by generalist physicians based at the local district hospital. Ultimately, the three initial outpatient clinics became training facilities that enabled the rest of the district hospitals in the country to establish similar services. In this paper, we describe the postoperative clinical management and outcomes for patients with RHD receiving care at the three original district-level clinics between 2007 and 2015.
Methods
Study setting
Rwanda has a population of approximately 11 million people and is among the most densely populated countries in sub-Saharan Africa.21 Over 80% of the Rwandan population works in agriculture; the poverty rate in 2013–2014 was 39% (at the national poverty line), and life expectancy was 64 years.22 In 2015, Rwanda had 35 district hospitals, and there were 742 physicians and 8751 nurses working in the public sector.22
PIH/IMB is a Rwanda-based NGO that works in coordination with its counterpart, Boston-based organisation, PIH. In 2005, PIH was invited by the Rwandan MOH to help strengthen healthcare services in rural districts of Rwanda. PIH/IMB support focuses on infrastructure, training, monitoring and evaluation, and quality improvement. PIH/IMB currently supports two districts in Eastern Rwanda (Kirehe and Southern Kayonza), as well as one district in Northern Rwanda (Burera).
Patients presenting with symptoms of heart failure or rheumatic fever to health centres in these PIH/IMB-supported districts are referred to district hospitals where they are initially evaluated by specialised NCD nurses trained in simplified echocardiographic diagnostic strategies that have been described elsewhere.23 Patients thought to have cardiac disease that is potentially amenable to surgery are started on penicillin prophylaxis and referred to cardiologists at referral centres in Kigali (the capital). Those patients deemed to be good candidates for cardiac surgery based on the absence of significant comorbidities such as advanced kidney disease and significantly depressed left ventricular systolic function are placed on a waiting list.
Most cardiac surgery for Rwandans is performed by visiting teams at King Faisal Hospital in Kigali, which has been selected by the MOH as the national cardiac surgical referral centre. Although the number of surgeries performed is limited, patients covered by the community health insurance scheme (called ‘Mutuelle de Santé’ in French) benefit from a full subsidy by the MOH with support from different humanitarian organisations.24 Additionally, the MOH supports some out-of-country referral (primarily to centres in India), and there are occasional opportunities for cardiac surgery at a regional humanitarian cardiac surgical centre in Sudan.
After cardiac surgery, patients from PIH/IMB-supported districts are followed by specialised NCD nurses at district hospitals, who monitor their clinical status and support adherence to penicillin prophylaxis.20 Those patients in need of anticoagulation are seen at least monthly for INR testing and adjustment of warfarin dosing based on standardised algorithms. In cases where the INR falls below 2, patients are sometimes hospitalised for subcutaneous heparin until they return to therapeutic range. Nurses are supervised at least monthly by a visiting Rwandan MOH cardiologist based in Kigali (author ER) and are also regularly in contact by mobile phone with Kigali. Patients see a cardiologist at least twice a year. Patients in the lowest income categories do not have any co-payment and also receive transport assistance and food packages if needed.
Data collection and analysis
This study is a retrospective review of patients with RHD who received cardiac surgery and were subsequently followed in the integrated NCD clinics at one of three PIH/IMB-supported rural district hospitals at Rwinkwavu (Southern Kayonza District), Kirehe (Kirehe District) and Butaro (Butaro District). All patients who underwent surgery for RHD from March 2007 to May 2015 and were followed up in one of these three districts were included in this study. Clinical data were manually extracted from patient records during chart reviews conducted between March through May 2015 by two cardiologists (ER, GB). The data extracted included patient demographics, socioeconomic status, clinical status before and after surgery using the New York Heart Association (NYHA) Classification, preoperative and postoperative echocardiography findings, and anticoagulation status using INR results.
We described patient characteristics, preoperative diagnoses and postoperative status, using Fisher’s exact test to assess difference by gender. We produced a heat map indicating the distribution of surgical cases per 100 000 population using ArcGIS.25 We reported the median number of postoperative follow-up visits per year, the median percentage of a patient’s visits where anticoagulation was assessed using the INR, the median percentage of a patient’s visits when INR was <2, the median warfarin dose provided and the median per cent of a patient’s visits when the warfarin dose changed. We described patient outcomes at the time of chart review, namely death or lost-to-follow-up defined as missing clinical visits or drug refill appointments for >2 months past the last scheduled date. For patients that died, we provided details on the patients’ characteristics and surgery type. We used Stata V.12.1 for analyses.26
Results
Patient characteristics and preoperative diagnosis
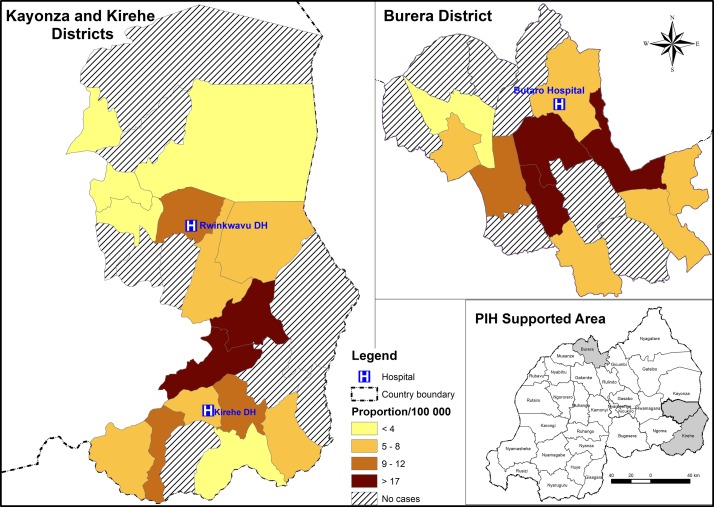
A total of 54 postoperative patients with RHD were included in this study, representing 6.5, 4.5 and 2.9 per 100 000 population for Kirehe, Burera and Southern Kayonza Districts, respectively (see figure 1). The median time from the initial NCD clinic visit to cardiac surgery for these patients was 1.3 years (range 0.2–5.5 years). The majority of patients were women (n=40/54; 74%) (table 1). Most patients were adults (age>15 years; n=46/54; 85%); the median age of adults was 22 years (IQR 18–27) and the median age of children was 11 years (IQR 9–13). About two-thirds of patients were residents of the Eastern Province, primarily in the districts of Kirehe (n=20/54; 37%) or Rwinkwavu (n=14/54; 26%). The remaining patients were residents of Burera District in the Northern Province (n=20/54; 37%). The majority of patients (34/43; 79%) had a dirt floor. There were no statistical differences in patient demographics by gender (P>0.05 for all factors).
Figure 1.
Heat map for the geographical location of patients and proportion of patients per 100 000 per sector.
Table 1.
Description of the patients’ population
| Characteristics | All | Males | Females | P values |
| Age | ||||
| Children (<15 years)* | 8 (15%) | 3 (21%) | 5 (12%) | |
| Adults | 46 (85%) | 11 (79%) | 35 (88%) | 0.41* |
| Location of the healthcare facility | ||||
| Butaro | 20 (37%) | 4 (29%) | 16 (40%) | |
| Kirehe | 20 (37%) | 5 (36%) | 15 (37%) | |
| Rwinkwavu | 14 (26%) | 5 (36%) | 9 (23%) | 0.67* |
| Income level—floor is made of dirt at home | ||||
| No | 9 (21%) | 0 | 9 (26%) | |
| Yes | 34 (79%) | 8 (100%) | 26 (74%) | 0.17* |
| Marital status | ||||
| Single | 20 (59%) | 6 (60%) | 14 (58%) | |
| Married | 12 (35%) | 4 (40%) | 8 (33%) | |
| Divorced | 2 (6%) | 0 | 2 (8%) | 1.00* |
| Occupation | ||||
| Farmer | 20 (41%) | 5 (42%) | 15 (40%) | |
| In school | 14 (29%) | 2 (17%) | 12 (32%) | |
| Professional | 3 (6%) | 0 | 3 (8%) | |
| Unemployed/out of school | 12 (24%) | 5 (43%) | 7 (19%) | 0.40* |
| Preoperative diagnosis and operation | ||||
| Symptoms (NYHA) preoperation | n=48 | n=12 | n=36 | |
| Stage II | 8 (17%) | 5 (42%) | 3 (8%) | |
| Stage III | 25 (52%) | 3 (25%) | 22 (61%) | |
| Stage IV | 15 (31%) | 4 (33%) | 11 (31%) | 0.02* |
| Single valve | n=28 | n=6 | n=23 | |
| Pure MR | 20 (69%) | 4 (67%) | 16 (69.6%) | |
| Pure MS | 4 (13.8%) | 1 (17%) | 3 (13%) | |
| Mixed MR+MS | 2 (10.3%) | 0 | 3 (13%) | |
| Pure AR | 2 (6.9%) | 1 (17%) | 1 (4.4%) | 0.65* |
| ≥2 valves | n=25 | n=8 | n=17 | |
| Mixed MR+TR | 12 (48%) | 1 (13%) | 11 (64.7%) | |
| Mixed MR+AR | 4 (16%) | 4 (50%) | 0 | |
| Mixed MS+TR | 4 (16%) | 0 | 4 (23.5%) | |
| Mixed MR+MS+TR | 3 (12%) | 1 (13%) | 2 (11.8%) | |
| Mixed MS+TR+AR | 2 (8%) | 2 (25%) | 0 | <0.01* |
| Location of the surgical site | ||||
| Rwanda | 40 (82%) | 12 (92%) | 28 (78%) | |
| Sudan | 6 (12%) | 1 (8%) | 5 (14%) | |
| South Africa | 1 (2%) | 0 | 1 (3%) | |
| India | 2 (4%) | 0 | 2 (6%) | 0.87* |
| Number of valves replacement | n=54 | n=14 | n=40 | |
| 1 | 30 (56%) | 7 (50%) | 23 (58%) | |
| 2 | 22 (41%) | 5 (36%) | 17 (42%) | |
| 3 | 2 (4%) | 2 (14%) | 0 | 1.0* |
| Postoperation | ||||
| Symptoms (NYHA) postoperation | n=53 | n=13 | n=40 | |
| Stage I | 41 (77%) | 11 (85%) | 30 (75%) | |
| Stage II | 10 (19%) | 2 (15%) | 8 (20%) | |
| Stage IV | 2 (4%) | 0 | 2 (5%) | 1.00* |
*Fisher’s exact test.
AR, aortic regurgitation; MR, mitral regurgitation; MS, mitral stenosis; NYHA, New York Heart Association; TR, tricuspid regurgitation.
Prior to surgery, 83% of patients had NYHA class III and IV symptoms. The mitral valve was predominantly affected by rheumatic lesions where pure mitral regurgitation (MR) was found in the majority of men (4/6; 67%) or in combination with tricuspid or aortic regurgitation for the remaining (5/8; 62.5%). Similarly, a majority of women (19/23; 82.6%) presented with isolated MR and/or mitral stenosis (MS) or in combination of tricuspid and aortic regurgitation in all the 17 cases. Disorders involving three valves were less common (2/25; 8%), all appearing in males with mitral valve disease, tricuspid and aortic regurgitation.
Type of surgery and outcomes
describes the distribution of patients by sex, type of surgery performed and clinical outcomes. Of the 40 females who had cardiac surgery, 19 (47.5%) had mechanical replacement, 15 (37.5%) had bioprosthetic replacement and 6 (15%) had valve repair (figure 2). For the 14 males receiving surgery, all underwent valve replacement: 11 (79%) mechanical and 3 (21%) bioprosthetic. The majority of the patients received their surgery in Rwanda (40/49; 82%), with a relatively equal distribution in between the three district hospitals (P=0.67) (table 1).
Figure 2.

Distribution of patients by age, surgical procedures and outcomes.
Among the 54 patients, there were 80 total valves replaced or repaired. The majority of patients required surgery for only one valve (n=30/54; 56%) while 41% (22/54) required surgery on two valves, and only 4% (2/54) underwent surgery on three valves. Half of the male participants (7/14; 50%) and over half of the female participants (23/40; 58%) underwent one valve replacement (P>0.99).
After surgery, most of the patients (41/53; 77%) were asymptomatic (NYHA class I), and there was no statistical difference between men and women. During the period of follow-up (median of 3 years, with a range of 0.2–7.9 years), only four patients died (7.4%) and all four had a bioprosthetic valve replacement (table 2). This included 2/3 (67%) of males (P=0.01) and 2/15 (13%) of females (P=0.19) with the bioprosthetic valve replacement. One female has received bioprosthetic valve replacement at age 9 and underwent mechanical valve replacement at age 15 due to severe calcification of bioprosthetic valve. The causes of death included heart failure secondary to postoperative left ventricle dysfunction, calcification of bioprosthesis and infective endocarditis.
Table 2.
Patients with postoperative death
| Patient number | Sex | Age at surgery (years) | Preoperative diagnosis | Valve position | Surgery type | Period of postsurgery follow-up | Comment |
| 29 | M | 8 | Pure MR | Mitral | Bioprosthetic valve replacement | 5 years | Died of severe calcification of biological valve |
| 33 | F | 14 | Mixed MR and TR | Mitral tricuspid |
Bioprosthetic valve replacement | 4.1 years | Died due to serve LV dysfunction and AF |
| 34 | F | 16 | Pure MR | Mitral | Bioprosthetic valve replacement | 2.4 years | Died of severe calcification of biological valve |
| 35 | M | 15 | MR and TR | Mitral tricuspid |
Mitral Bioprosthetic valve replacement and TV repair | 4 months | Died of infective endocarditis 3 months post surgery |
AF, atrial fibrillation; F, female; LV, left ventricle; M, male; MR, mitral regurgitation; TR, tricuspid regurgitation; TV, tricuspid valve.
Patients who were alive (50/54; 92.6%) received penicillin at their most recent clinical follow-up for secondary prevention of rheumatic fever. Penicillin was administered to 40/50 (80%) orally and 10/50 (20%) by intramuscular injection. Of the women, 6/40 (15%) became pregnant—all with bioprosthetic valves—and no death events were reported among them.
Anticoagulation
The INR was checked during 96% of all visits (table 3). The median number of visits per year during which the INR was checked was 14 for all patients since surgery (Supplementary file 1). The warfarin dose was changed during 35% of clinic visits with a median dose of 5.75 mg.
Table 3.
Anticoagulation
| Median | Min | Max | |
| Visits per patient per year since surgery | 14.3 | 3.8 | 26.2 |
| Number of time, per patient, INR was checked or documented during visited | 24 | 2 | 77 |
| Percentage of visits, per patient, when International Normalized Ratio was <2.0 | 16% | 0% | 58% |
| Median warfarin dose | 5.75 | 2.5 | 10 |
| Percentage of visits, per patient, when warfarin dose changed | 35% | 3% | 83% |
heartjnl-2017-312644supp001.pdf (76.4KB, pdf)
Discussion
Our population of patients with RHD who underwent cardiac surgery was composed of children and young adults and predominantly females. Our patients were mostly the rural poor, as indicated by their district of residence and the fact that they were living in homes with dirt floors and were occupied in subsistence farming. Cardiac surgical registries in sub-Saharan Africa have not previously reported much socioeconomic information about their patients. However, it is well known that there is a dramatic urban bias in access to cardiac surgery in this region. For example, a recent review of 30 years of experience at a cardiac surgical centre in Ethiopia found that >90% of the patients who received cardiac surgery came from a big city or town, whereas >80% of Ethiopia is rural (even in 2016).27 In contrast, our decentralised, integrated strategy was able to identify, diagnose and follow an impoverished rural population.
During an 8-year period, 54 patients with advanced RHD underwent cardiac surgery and were followed by nurses at clinical sites in rural Rwanda operated by the Rwandan MOH in partnership with PIH/IMB. Patients’ symptoms greatly improved as 77% were asymptomatic after surgery. For those who remained in care, INR was regularly monitored with warfarin dosages adjusted as needed. These favourable outcomes demonstrate that patient follow-up after cardiac surgery can be conducted safely and effectively by nurses at rural district hospitals, and integrated with management of other severe NCDs. The success of this decentralised management depended on the monthly in-person supervision by the MOH cardiologist.
Over half of the patients (55%) underwent mechanical valve replacement, while bioprosthetic replacement was performed in 33% of cases and repair in 11%. Bioprostheses were mainly placed in young females with the intent of avoiding complications of anticoagulation during pregnancy.
Anticoagulation monitoring was robust and there were no known bleeding or thrombotic complications. Only 4 (7%) of the patients died during a median follow-up of 3 years. There were no deaths among patients with mechanical valves.
Though the number of patients receiving cardiac surgery was small in our study, there were two concerning trends that warrant future research. First, our study suggests that bioprosthetic valves in young patients with RHD may be prone to rapid degeneration and calcification. Of the 18 patients with bioprosthetic valves, 2 patients (11%) died of severe valve calcification. Mechanical valve replacement for patients in rural settings may be preferred. Over the life span of the Rwandan cardiac surgery experience, there was increasing confidence in the quality of decentralised anticoagulation for mechanical valves. Beginning in 2013, most of the surgeries performed among our cohort used a mechanical valve.
Second, the geographical distribution of the patients that received surgery within the PIH/IMB-supported districts shows a concentration in certain geographic sectors (figure 1). This suggests that there is likely underdiagnosis of advanced RHD—particularly in areas that are far from district hospitals. Since the time that the programme was initiated, the specialised nurses at the district hospital NCD clinics have facilitated further decentralisation of services to health centres serving populations of around 20 000 people. We hope that this decentralisation of chronic care for common NCDs such as hypertension and asthma will also increase recognition of more severe conditions such as heart failure and lead to more RHD diagnosis.
Nearly 93% of the patients in our study had received penicillin for secondary prophylaxis against group A streptococcal infections at the time of their most recent clinic visit. Strategies to prevent RHD should be pursued in parallel to increasing capacity for cardiac surgery and surgical follow-up. Consistent with the Addis Ababa communiqué on eradication of RHD in Africa, these strategies include (i) ensuring the supply of benzathine and oral penicillin to primary health centres to facilitate primary prophylaxis of streptococcal pharyngitis and (ii) making early diagnosis of RHD possible by decentralising echocardiography to first-level hospitals.23 28 The role of echocardiographic screening in the control of RHD is not yet clear due to questions regarding the natural history of mild disease identified in asymptomatic individuals.29
Scaling up access to both cardiac surgery and medical interventions to address RHD will require increased financing for health services. Additionally, there is need to support research on strategies for prevention and early detection.30
Limitations
The major limitation of this study is that it is a retrospective review of outcomes in a relatively small number of patients followed for between 2 months and 8 years. It will be important in the future to closely monitor the outcomes of this approach at a larger scale and over a longer period of time, particularly relative to challenges in supply chain management, information systems and supervision.
Conclusions
To our knowledge, this is first published report from an LLMIC in sub-Saharan Africa of nurse-led, decentralised follow-up of advanced RHD after cardiac surgery. Patients were followed in integrated NCD clinics at rural district hospitals. This programme was able to reach an extremely impoverished population with good results.
Since the time of this initial experience at three district hospitals, this service delivery model has now been implemented at most district hospitals in Rwanda and is facilitating expansion of access to cardiac surgery for the poor both by increasing case finding and assuring good outcomes. We believe that this model (of specialised, nurse-led clinics for severe chronic NCDs) has great potential for other LLMICS in sub-Saharan African with cardiac surgical programmes facing similar challenges.
Key questions.
What is already known on this subject?
In sub-Saharan Africa, there is no evidence yet on the clinical outcomes for nurse-led and decentralised follow-up of advanced rheumatic heart disease (RHD) after cardiac surgery.
What might this study add?
This study adds evidence to the possibility of good outcomes in rural and resource-limited settings of patients with RHD after heart surgery, such as in rural Rwanda, by using a decentralised care model.
How might this impact on clinical practice?
With appropriate training and supervision, it is feasible to safely decentralise follow-up of patients with RHD to nurse-led specialised non-communicable disease clinics after cardiac surgery.
Footnotes
EKR and ZE-K contributed equally.
Contributors: EKR, BH-G, GN, SD, NT, CM, FM, EH, JM, GFK and GB: study conception, and data collection. EKR and ZE-K: data analysis and drafting the first draft of the manuscript. EKR, ZE-K, BH-G, GFK and GB: revising the work critically and final approval of the version published.
Funding: ZE-K is supported by the Harvard Medical School Global Health Equity Research Fellowship, funded by Jonathan M. Goldstein and Kaia Miller Goldstein. GFK is supported by the American Heart Association (17MCPRP33460298).
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The study was approved by the Rwanda National Ethics Committee (673/RNEC/2013) and Brigham and Women’s Hospital Institution Review Board (2013P000047/BWH).
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This article has been corrected since it was published Online First. The equal contributorship footnotes have been added in. The affiliation of author Bethany Hedt-Gauthier has been changed from affiliation number 3 to 5.
References
- 1. Kwan GF, Mayosi BM, Mocumbi AO, et al. Endemic cardiovascular diseases of the poorest billion. Circulation 2016;133:2561–75. 10.1161/CIRCULATIONAHA.116.008731 [DOI] [PubMed] [Google Scholar]
- 2. Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med 2017;377:713–22. 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 3. Institute for Health Metrics and Evaluation (IHME). Global Burden of Disease (GBD) study. 2016. http://viz.healthmetricsandevaluation.org/gbd-compare/ (accessed 6 Oct 2017).
- 4. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countriesclinical perspective. Circulation 2016;134:1456–66. 10.1161/CIRCULATIONAHA.116.024769 [DOI] [PubMed] [Google Scholar]
- 5. Celermajer DS, Chow CK, Marijon E, et al. Cardiovascular Disease in the Developing World. J Am Coll Cardiol 2012;60:1207–16. 10.1016/j.jacc.2012.03.074 [DOI] [PubMed] [Google Scholar]
- 6. Mirabel M, Grimaldi A, Freers J, et al. Access to cardiac surgery in sub-Saharan Africa. Lancet 2015;385:606 10.1016/S0140-6736(15)60235-5 [DOI] [PubMed] [Google Scholar]
- 7. Finucane K, Wilson N. Priorities in cardiac surgery for rheumatic heart disease. Glob Heart 2013;8:213–20. 10.1016/j.gheart.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 8. Zhang W, Okello E, Nyakoojo W, et al. Proportion of patients in the Uganda rheumatic heart disease registry with advanced disease requiring urgent surgical interventions. Afr Health Sci 2015;15:1182–8. 10.4314/ahs.v15i4.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pezzella AT. Global aspects of cardiothoracic surgery with focus on developing countries. Asian Cardiovasc Thorac Ann 2010;18:299–310. 10.1177/0218492310370060 [DOI] [PubMed] [Google Scholar]
- 10. Zheleva B, Atwood JB. The invisible child: childhood heart disease in global health. Lancet 2017;389:16–18. 10.1016/S0140-6736(16)32185-7 [DOI] [PubMed] [Google Scholar]
- 11. Longenecker CT, Morris SR, Aliku TO, et al. Rheumatic heart disease treatment cascade in uganda. Circ Cardiovasc Qual Outcomes 2017;10:e004037 10.1161/CIRCOUTCOMES.117.004037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mirabel M, Lachaud M, Offredo L, et al. Cardiac surgery in low-income settings: 10 years of experience from two countries. Arch Cardiovasc Dis 2017;110:82–90. 10.1016/j.acvd.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 13. Butchart EG, Gollke-Barwolf C, Antunes M, et al. Heart valve disease: a guide to patient management after surgery. Abingdon, UK: Informa Healthcare, 2006. [Google Scholar]
- 14. Anakwue R, Ocheni S, Madu A. Utilization of oral anticoagulation in a teaching hospital in Nigeria. Ann Med Health Sci Res 2014;4:S286–90. 10.4103/2141-9248.141973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ogendo SW. Pattern of anticoagulation control after heart valve surgery at the Kenyatta national hospital, nairobi. East Afr Med J 2000;77:354–8. [DOI] [PubMed] [Google Scholar]
- 16. Tudor Hart J. The inverse care law. The Lancet 1971;297:405–12. 10.1016/S0140-6736(71)92410-X [DOI] [PubMed] [Google Scholar]
- 17. Binagwaho A, Rusingiza E, Mucumbitsi J, et al. Uniting to address pediatric heart disease in Africa: advocacy from Rwanda. SA Hear 2013;10:440–6. [Google Scholar]
- 18. Swain JD, Mucumbisti J, Rusingiza E, et al. Cardiac surgery for advanced rheumatic heart disease in Rwanda. Lancet Glob Health 2014;2:e141–e142. 10.1016/S2214-109X(14)70022-1 [DOI] [PubMed] [Google Scholar]
- 19. Mucumbitsi J, Bulwer B, Mutesa L, et al. Prevalence of rheumatic valvular heart disease in Rwandan school children: echocardiographic evaluation using the World Heart Federation criteria. Cardiovasc J Afr 2017;28:285–92. 10.5830/CVJA-2017-007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bukhman G, Kidder A. The PIH guide to chronic care integration for endemic non- communicable diseases. Boston, Massachusetts: Partners In Health, 2011. [Google Scholar]
- 21. World Bank. World development indicators. 2017. https://data.worldbank.org/data-catalog/world-development-indicators (accessed 6 Oct 2017).
- 22. National Institute of Statistics - The Republic of Rwanda. Statistical yearbook, 2016. [Google Scholar]
- 23. Kwan GF, Bukhman AK, Miller AC, et al. A simplified echocardiographic strategy for heart failure diagnosis and management within an integrated noncommunicable disease clinic at district hospital level for sub-Saharan Africa. JACC Heart Fail 2013;1:230–6. 10.1016/j.jchf.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 24. Lu C, Chin B, Lewandowski JL, et al. Towards universal health coverage: an evaluation of Rwanda Mutuelles in its first eight years. PLoS One 2012;7:7 10.1371/journal.pone.0039282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ESRI. ArcGIS desktop: release 10, 2011. [Google Scholar]
- 26. Stata Corp. Stata 12 base reference manual, 2011. [Google Scholar]
- 27. Guteta S, Yadeta D, Azazh A, et al. Cardiac surgery for valvular heart disease at a referral hospital in ethiopia: a review of cases operated in the last 30 years. Ethiop Med J 2016;54:49–55. [PubMed] [Google Scholar]
- 28. Watkins D, Zuhlke L, Engel M, et al. Seven key actions to eradicate rheumatic heart disease in Africa: the addis ababa communiqué. Cardiovasc J Afr 2016;27:184–47. 10.5830/CVJA-2015-090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zühlke L, Mayosi BM. Echocardiographic screening for subclinical rheumatic heart disease remains a research tool pending studies of impact on prognosis. Curr Cardiol Rep 2013;15:343 10.1007/s11886-012-0343-1 [DOI] [PubMed] [Google Scholar]
- 30. Marijon E, Celermajer DS, Jouven X. Rheumatic heart disease - an iceberg in tropical waters. N Engl J Med 2017;377:780–1. 10.1056/NEJMe1705840 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2017-312644supp001.pdf (76.4KB, pdf)