Abstract
Production of virus-free plants is necessary to control viral diseases, import novel cultivars from other countries, exchange breeding materials between countries or regions and preserve plant germplasm. In vitro techniques represent the most successful approaches for virus eradication. In vitro thermotherapy-based methods, including combining thermotherapy with shoot tip culture, chemotherapy, micrografting or shoot tip cryotherapy, have been successfully established for efficient eradication of various viruses from almost all of the most economically important crops. The present study reviewed recent advances in in vitro thermotherapy-based methods for virus eradication since the twenty-first century. Mechanisms as to why thermotherapy-based methods could efficiently eradicate viruses were discussed. Finally, future prospects were proposed to direct further studies.
Keywords: Cryotherapy, Chemotherapy, Micrografting, Shoot tip culture, Thermotherapy, Virus eradication
Necessity to produce virus-free plants
Virus diseases cause great losses of crop yield and have long been a constraint for sustainable developments of agricultural production [1, 2]. For example, potato leafroll virus (PLRV), potato virus S (PVS), potato virus X and potato virus Y (PVY) are among the most serious viruses attacking potato [3]. Single infection caused yield losses of 40–60% by PLRV, 10–20% by PVS, 10–50% by PVX and 20–50% by PVY [4]. Mixed infection with two viruses resulted in a much larger loss of yield than the single infection [4]. Plum pox virus (PPV), one of the most serious viral diseases attacking Prunus fruit trees, widely occurred in almost all stone fruit producing countries [5]. Annual yield losses caused by PPV infection were 1.5 million for plum and 0.6 million metric tons for apricot, approximately valuing at €5400 million and €3600 million for the former and latter in Europe [5]. By 2013, over €33 million had been invested in research projects on PPV control in Europe [5].
Plant viruses are obligate intracellular parasites that colonize only inside the living cells of the host and can be transmitted by vegetative propagation from generation to generation and insect vectors from the virus-infected plants to the healthy ones [6]. Although the use of chemicals had potential applications to control viral diseases [7, 8], cultivation of virus-free plants has been/is an agricultural strategy for efficient control of them [2, 9]. As early as 1968, the European Union (EU) issued an EU Council directive [10], which required that propagative materials of fruit crops must meet the phytosanitary requirements. Virus-free plants are currently widely grown throughout the world to control viral diseases in many of economically important crops like tuber crops [4, 11], fruit trees [2, 12], herbaceous ornamentals [13, 14]. Virus-free materials are required in importing novel cultivars from other countries and exchanging breeding materials between countries or regions [2, 15]. In addition, preservation of plant germplasm also emphasizes use of virus-free plants [16, 17].
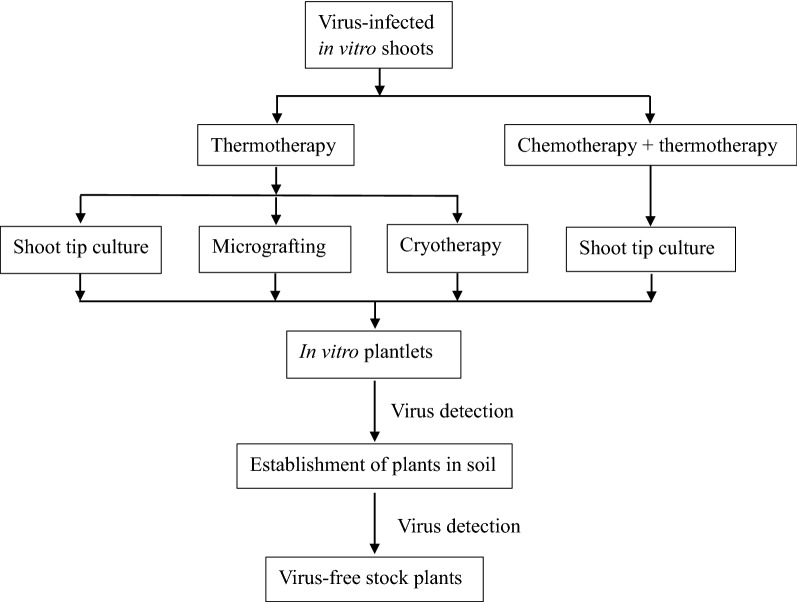
In vitro culture techniques represent the most successful strategies for production of virus-free plants [18–20]. So far, various methods have been established for eradication of plant viruses, including shoot tip culture (also called meristem culture) [2, 4, 11, 12, 19, 21, 23], micrografting [12, 21], chemotherapy [12, 17, 21], thermotherapy [2, 11, 19, 22, 23] and shoot tip cryotherapy [24, 25]. Accumulative data have proven combining thermotherapy with each of them (herein called thermotherapy-based methods, Fig. 1) are much more efficient for virus eradication than single use of them. For example, shoot tip cryotherapy completely failed to eradicate raspberry bushy dwarf virus (RBDV) [26] and apple stem grooving virus (ASGV) [27], while combining thermotherapy with shoot tip cryotherapy produced 33% and 100% of plants free of RBDV [22] and ASGV [26].
Fig. 1.
In vitro thermotherapy-based methods for production of virus-free plants
Detailed information on the said subject in the last century can be found in a number of excellent reviews [2, 12, 19, 21–23]. The present study reviewed recent advances in thermotherapy-based methods for virus eradication since the 21st century. Mechanisms as to why thermotherapy-based methods could efficiently eradicate virus eradication were discussed. Finally, future prospects were also proposed to direct further studies.
Thermotherapy-based methods for virus eradication
In thermotherapy-based methods, infected in vitro cultures are first heat-treated and then subjected to a given procedure (Fig. 1), as described below.
In general, the higher the temperature and the longer the exposure duration are, the higher the virus-eradication frequency is. Often, thermotherapy of 35–42 °C for 4–6 weeks is applied to the target plants, mainly depending on virus type and plant species, as well as the virus-host combination [2, 12, 19, 21–23]. Choice of a thermotherapy regime should allow the treated plant to survive and at the same time inactivate the virus, thus resulting in production of virus-free plants.
Combining thermotherapy with shoot tip culture
This technique includes thermotherapy of the diseased in vitro shoots, followed by shoot tip culture.
Skiada et al. [28] reported combining thermotherapy with shoot tip culture for eradication of grapevine leafroll-associated virus 1 (GLRaV-1), an easy-to-eradicate virus, and grapevine rupestris stem pitting-associated virus (GRSPaV), a difficult-to-eradicate virus, from grapevine ‘Agiorgitiko’. The in vitro shoots mix-infected with GLRaV-1 and GRSPaV were heat-treated for 6 weeks by an alternating temperature of 40 °C/37 °C (day/night), followed by shoot tip culture. This procedure resulted in about 53% survival of the heat-treated shoots, 56% regeneration of shoot tips, and 91% and 74% of GLRaV-1- and GRSPaV-1-free plants. Thermotherapy followed by shoot tip culture was reported to eradicate PVY from infected potato [29]. In vitro virus-infected potato shoots were thermo-treated for 40 days at a consistent temperature of 37 °C, followed by shoot tip culture (0.1–0.3 mm). Culture of 0.1 mm shoot tips resulted in about 88% survival levels of the treated shoots and 75–81% virus-free plants in two potato cultivars, and larger shoot tips decreased the virus eradication frequencies [29].
Tobacco mosaic virus (TMV) represented a type of viruses that were still difficult to eradicate by thermotherapy followed by shoot tip culture [30]. Greenhouse-grown plants of soybean (Glycine max) were mechanically inoculated with tobacco mosaic virus and then thermo-treated at 40 °C/6 °C (day/night) for 15 days. Heat-treated plants grew as well as the control (25 °C/6 °C). Virus infectivity increased in the leaves of the treated plants heat-treated in these two thermotherapy regimes. TMV was detected in all the newly developed news. Shoot tips that developed during the alternating temperature treatments were isolated from the treated TMV-infected tobacco and cultured in vitro for plantlet regeneration. As results, all plantlets regenerated were still TMV-infected [30].
Thermotherapy followed by shoot tip culture was the most frequently used method for virus eradication from plants including herbaceous crops and woody species. Some examples of successful virus eradication by thermotherapy followed by shoot tip culture are listed in Table 1.
Table 1.
Some examples of in vitro thermotherapy followed by shoot tip culture for virus eradication
| Plant species and cultivars or genotypes | Viruses | Temperature and duration | Shoot survival after thermotherapy (%) | Size of shoot tips (mm) | Shoot tip survival (%) | Virus-free frequency (%) | References |
|---|---|---|---|---|---|---|---|
| Herbaceous crops | |||||||
| Potato ‘Diamond’ | Potato virus Y (PVY) | 37 °C, 40 days | 43 | 1.0 cm | NT | 33 | [64] |
| Potato ‘Burren’ and ‘Binella’ | PVY | 37 °C, 40 days | NT | 0.1–0.3 | 88–100 | 56–81 | [25] |
| Potato genotype ‘040138’ | Potato virus X (PVX) | 32 °C/42 °C, (day/night), 35 days | 80–100 | NS | NT | 80 | [66] |
| Cassava ‘Olho Junto’ | Cassava common mosaic virus (CsCMV) | 39 °C/28 °C, (day/night), 30 days | NT | NS | NT | 72 | [115] |
| Cassava ‘Kibandameno’ | East African cassava mosaic virus (EACMV) |
35 °C, 42 days | 81 | NS | NT | 78 | [55] |
| Garlic ‘Morado’, ‘Union’, ‘Fuego’, ‘Lican’, ‘Nieve’, ‘Castaño’, ‘Violeta’, ‘Gostoso’, ‘Norteño’, ‘Perla’, ‘Sureño’ | OYDV, LYSV, GCLV, garlic mite-borne filamentous virus (GarMbFV), garlic virus D (GarVD) | 36 °C, 30–40 days | 55–57 | 0.3 | NT | 63–100 (OYDV), 80–100 (LYSV), 93–100 (GCLV), 67–100 (GarMbFV), 18–97 (GarVD) | [116] |
| Garlic (cultivars not specified) | Onion yellow dwarf virus (OYDV) and leek yellow stripe virus (LYSV) | 50 °C, 2 h | NT | 0.3–0.5 | NT | 19 (OYDV) 33 (LYSV) |
[117] |
| Garlic ‘Hamedan’ | OYDV and garlic virus A, B, C, D (GarVs) | 36 °C, 5 weeks | NT | 1.0–2.0 | 71 | 0 (OYDV) 90 (GarVs) |
[118] |
| Garlic ‘Jonas’ | OYDV, LYSV and garlic common latent virus (GCLV) | 38 °C, 30 days | NT | 0.3–0.5 | 72 | 83 (OYDV) 100 (LYSV) 39 (GCLV) |
[112] |
| Herbaceous crops | |||||||
| Chrysanthemum ‘Regol Time’ | Chrysanthemum B virus (CVB) | 38 °C, 30 days | NT | 0.3–1.0 | NT | 12 | [28] |
| Lilium × elegans genotypes 409 and 599 | Lilium symptomless virus (LSV) | 35 °C, 42 days | NT | 0.3 | NT | 100 | [83] |
| Lilium Asiatic hybrid ‘Visconti’, and LA hybrids ‘Fangio’ and ‘Lacorno’ | LSV, lily mottle virus (LMoV) and cucumber mosaic virus (CMV) | 35 °C, 5 weeks | NT | NS | NT | 100 (LSV) 100 (CMV) 100 (LMoV) |
[51] |
| Begonia | Prunus necrotic ringspot virus (PNRSV) | 38 °C/22 °C, (day/night), 35 days | NT | 2–3 cm | NT | 37.5 | [63] |
| Horseradish | Turnip Mosaic Virus (TuMV) | 37 °C, 23 days | 100 | 0.5 | NT | 100 | [53] |
| Artichoke | Artichoke Italian latent virus (AILV), artichoke latent virus (ArLV) | 38 °C, 15 days | NT | 0.3–0.8 | 75 | 100 | [84] |
| Woody species | |||||||
| Apple ‘Idared’ and ‘Sampion’ | Apple chlorotic leaf spot virus (ACLSV) and (ASPV) (Idared) ACLSV, ASPV and apple stem grooving virus (ASGV) (Sampion) |
39 °C, 6 days | NT | 1.0–2.0 | 63 (Idared) 44 (Sampion) |
60 (Idared) 0 (Sampion) |
[71] |
| Apple ‘Yanfu9’, ‘Xinyanfu3’, ‘Xin2001’, ‘Gala’, Huafu, ‘Apple 123’ and ‘Zhengzhou No. 5’ | ACLSV, ASGV, ASPV and apple mosaic virus (ApMV) | 38 °C, 30 days | NT | 1.0 | 26 (Total) | 43 (Total) | [40] |
| Woody species | |||||||
| Apple (Malus × domestica cvs. Gala, Ruiyang, Nongguo 25, Fuji, M. paradisiaca M9) | ASGV | 36 °C/32 °C, (day/night), 4 weeks | 100 | 1.5 | 63–90 | 7–38 | [23] |
| Apple ‘Oregon Spur-II’ | ACLSV, ApMV, ASGV, ASPV | 37–40 °C, 4 weeks | NT | 0.3–0.6 | 26–33 | 75 (ACLSV) 100 (ApMV, ASGV and ASPV) |
[77] |
| Pear ‘Huang-hua’ | ASGV and ACLSV | 37 °C, 35 days | 64 | 1.0 | NT | 66.7 (ACLSV) 33.3 (ASGV) |
[60] |
| Pear ‘Fengshui’, ‘Jingshui No. 1’, ‘4’ and ‘14’ | ASGV, ACLSV and ASPV | 42 °C/34 °C, (day/night), 55 days | 20–57 | 0.5–1.0 | NT | 100 | [58] |
| Pear ‘Jinshui no. 2’ | ASGV ACLSV | 35 °C, 40 days | 100 | 0.5 | 66.7 | 33.3 | [61] |
| Apricot ‘Bebecou’ | Plum pox virus (PPV) | 35–37 °C, 20 days | NT | 1.0–2.0 (meristem), 5.0 cm (Shoot tip) | 35 (Meristem), 28 (Shoot tip) | 74 (Meristem) 82 (Shoot tip) |
[69] |
| Nectarine ‘Arm King’ | PPV and prunus necrotic ringspot virus (PNRSV) | 35 °C, 2 weeks | NT | 1.3–2.0 | 38 | 86 (PPV) 81 (PNRSV) |
[56] |
| Woody species | |||||||
| Plum ‘Earliblue’ | PNRSV | 35–38 °C, 10–12 days | 14–71 | 5.0 | NT | 100 | [57] |
| Grapevine ‘Napoleon’ | Grapevine leafroll-asso ciated virus-3 (GLRaV-3), grapevine fan leaf virus (GFLV) | 37 °C/34 °C, (day/night) 1.5 months |
NT | 0.5–3.0 | 53–80 | 72–100 (GLRaV-3) 100 (GFLV) |
[79] |
| Grapevine ‘Sagrantino’ | Grape virus A (GVA) | 36 °C, 57 days | NT | 1.0 cm | NT | 60 | [72] |
| Grapevine ‘Mantilaria’ and ‘Prevezaniko’ | Grapevine leafroll associated virus (GLRaV) and GRSPaV | 40 °C/37 °C, (day/night), 1 weeks | 59.37 (Mantilaria) 41.86 (Prevezaniko) |
5.0 | 96 (Mantilaria) 78 (Prevezaniko) |
38 (Mantilaria) 89 (Prevezaniko) |
[81] |
| Grapevine ‘Bidaneh Sefid’ and ‘Shahroodi’ | GFLV | 40 °C/30 °C, (day/night), 7 weeks | NT | NT | NT | 100 | [119] |
| Grapevine ‘Agiorgitiko’ | GLRaV-1 and GRSPaV | 40 °C/37 °C, (day/night), 1 weeks | 53 | 0.1–0.2 (Meristem) 5.0 (Shoot tip) |
56 (Meristem) 88 (Shoot tip) |
62 (Meristem) 38 (Shoot tip) |
[24] |
| Grapevine ‘Kober 5BB’ | GVA, GFLV, grapevine fleck virus (GFkV), GLRaV-1 and GLRaV-3 | 37 °C, 48 days | 100 | NS | NT | 100 (GFLV), 70 (GVA), 25 (GFLaV-1), 25 (GLRaV-3) and 0 (GFKV) | [86] |
| Fig ‘Biadi’ and ‘Aswad’ | Fig leaf mottle-associated virus-1 and 2 (FLMaV-1 and FLMaV-2), and fig mosaic virus (FMV) | 35 °C, 30 days | NT | 6.0 | 39 (Biadi) 42 (Aswad) |
0–81 | [87] |
| Raspberry ‘Gatineau’ | Raspberry bushy dwarf virus (RBDV) | 37 °C, 21 days | NT | 0.2–0.3 | NT | 39 | [120] |
| Black raspberry (cultivars not specified) | Black raspberry necrosis virus (BRNV) | 29 °C and 38 °C (in 4-h interval), 5 weeks | NT | NS | 90 | 100 | [62] |
| Caper (cultivars not specified) | Caper latent virus (CapLV) | 38 °C, 45–60 days | 97 | 0.3–0.4 | 60–93 | 89–93 | [80] |
NS not specified, NT not tested
Combining chemotherapy with thermotherapy and shoot tip culture
In this technique, diseased in vitro shoots were cultured on an antivirus chemical-containing medium and then subjected to heat treatments, followed by shoot tip culture. In a few cases, diseased in vitro shoots were first heat-treated and then cultured on antivirus chemicals-containing medium, followed by shoot tip culture [31, 32]. Although several antivirus chemicals were available against plant viruses, ribavirin was the most frequently used, and sometimes 2-thiouracil was also used, for virus eradication [12, 19, 23]. Concentrations of antivirus agents used for virus eradication ranged between 20–50 mg L−1 for ribavirin [12, 19, 23] and 25–40 mg L−1 for 2-thiouracil [33, 34], depending on types of virus and hosts, as well as the virus-host combinations.
Fletcher and Fletcher [35] reported combining chemotherapy with thermotherapy for virus eradication from three Andean root crops including oca (Oxalis tuberosa), ulluco (Ullucus tuberosus) and arracacha (Arracacia xanthorrhiz). Diseased in vitro shoots were grown on a growth medium composed of MS supplemented with 50 mg L−1 ribavirin, which was added to the medium before autoclaving, and the cultures were then thermo-treated by an alternating temperature of 35 °C/31 °C (day/night). After about 10 days of thermotherapy, new shoots (1 cm in length) developed from the lateral shoot apices of oca and ulluco, and apical shoot apices of arracacha were excised and cultured for shoot regeneration on the same medium without ribavirin. This protocol produced about 80% of explant survivals in all three crops. Plants regenerated were free of arracacha virus B (AVB), papaya mosaic virus (PapMV) and ullucus mild mottle virus (UMMV) in 7 out of 8 accessions, but still infected with UMMV in one accession in oca. PapMV, ullucus virus C (UVC), UMMV and ullucus mosaic virus (UMV) were successfully eradicated in 5 accessions of ulluco, and arracacha virus A (AVA) was eradicated in arracacha ‘Racacha Blanca’.
Combining chemotherapy with thermotherapy was reported to eradicate apple viruses from diseased in vitro shoots [36]. Virus-infected in vitro apple shoots were cultured on a shoot maintenance medium composed of MS medium supplemented with 25 µg mL−1 ribavirin, which was filter-sterilized using a Millipore filter (0.22 µm) and added into the medium after autoclaving. The cultures were then thermo-treated at a constant temperature of 36 °C. After 20 days of thermotherapy, shoot tips (1.0 mm in size) were excised from the treated axillary shoots and cultured for shoot regeneration. About 90% of the treated shoots survived. All shoot tips regenerated into shoots, and all shoots regenerated were free of apple chlorotic leaf spot virus (ACLSV), apple stem pitting virus (ASPV) and ASGV.
Combining chemotherapy with thermotherapy was frequently used for virus eradication in herbaceous crops and sometimes also in woody plants. Some examples of virus eradication by combining chemotherapy with thermotherapy are listed in Table 2.
Table 2.
Some examples of in vitro chemotherapy followed by thermotherapy for virus eradication
| Plant species and cultivars or genotypes | Viruses | Antiviral chemicals (mg/L) | Survival of the treated shoots (%) | Survival of shoot tips (%) | Virus-free frequency (%) | References |
|---|---|---|---|---|---|---|
| Herbaceous crops | ||||||
| Garlic (10 accessions) | OYDV, LYSV, GCLV, shallot latent virus (SLV) and MbFV | Ribavirin (50) | NT | NT | 100 (OYDV, SLV and LYSV), 90 (GLCV) and 88 (MbFV) | [12] |
| Oca (8 accessions), ulluco (5 accessions), arracacha ‘Racacha Blanca’ | Papaya mosaic virus (PapMV), arracacha virus B (AVB) and ullucus mild mottle virus (UMMV) in Oca; PapMV, ullucus virus C (UVC), UMMV, ullucus mosaic virus (UMV) in ulluco; arracacha A virus (AVA) in arracacha | Ribavirin (50) | ≥ 80% | NT | (% not specified) free of viruses in 7 accessions, and still infected with UMMV in one accession in oca, free of viruses in 5 accessions in ulluco, and free of virus in arracacha | [30] |
| Potato ‘Baraka’ | PVY | Ribavirin (20) | NT | NT | 83 | [74] |
| Potato ‘F9-99’ | PLRV and PVY | Ribavirin (20) | 45–85 | NT | 26–60 (PLRV) 98–100 (PVY) |
[49] |
| Potato ‘Diamond’ | PVY | Ribavirin (20) | 26 | NT | 43 | [64] |
| Begonia × semperflorens | PNRSV | Ribavirin (20) | NT | NT | 58 | [63] |
| Chrysanthemum ‘Regol Time’ | Chrysanthemum B virus (CVB) | 2-Thiouracil (40) | NT | NT | 27 | [28] |
| Chrysanthemum ‘Regol Time’ | Tomato aspermy virus (TAV) | Ribavirin (10) | NT | NT | 52 | [91] |
| Herbaceous crops | ||||||
| Cassava ‘Cidade Rica’, ‘Riqueza II’, ‘Mandioca Lagoa’, ‘CM-425/7’, ‘Sabará’, ‘Sauma’ | Cassava frogskin disease (CFSD) and cassava vein mosaic virus (CsVMV) | Tetracycline (5–15) | 100 | NT | 100 (CFSD) 98 (CsVMV) |
[54] |
| Woody plants | ||||||
| Apple ‘JonagoId’, pear ‘Pierre Corneille’, raspberry ‘Norna’ | ACLSV and ASGV in apple, ACLSV in pear and raspberry vein chlorosis virus (RVCV) in raspberry | Ribavirin (50) | 42 (apple), 80 (pear) and 34 (raspberry) | NT | 100 (ACLSV and ASGV in apple), 83 (ACLSV in pear), 89 (RVCV in raspberry) | [71] |
| Apple ‘Xinhongjiangjun’ | ASPV, ASGV and ACLSV | Ribavirin (25) | 90 | 59 (apical shoots) and 100 (axillary shoots) | 94 (apical shoots) 100 (axillary shoots) |
[31] |
| Pear ‘Pierre Corneille’ | ACLSV | Ribavirin (50) | 71 | NT | 90 | [26] |
| Pear ‘Jinshui no. 2’ | ASGV, ACLSV | Ribavirin (25) | 100 | 71 | 100 | [61] |
NT not tested
Combining thermotherapy with micrografting
Micrografting is referred as the placement of a small meristem or a section of microshoot onto the top of a rootstock cultured in vitro [37]. Murashige et al. [38] and Navarro et al. [39] were the first to use micrografting for virus eradication from diseased citrus in vitro cultures. Since then, a number of studies on micrografting for virus eradication have been reported [12, 21, 37].
There have been only a few studies using thermotherapy followed by micrografting for virus eradication [40–42]. Several studies that used in vivo system were not included in the present study [43–46]. Sharma et al. [41] reported combining thermotherapy with micrografting for efficient eradication of Indian citrus ringspot virus (ICRSV) from the diseased plants of Kinnow (Citrus nobilis × · Citrus deliciosa). One-year-old pot-grown plants were exposed to 38–40 °C by gradually increasing temperatures (1 °C/day) from 30 °C to 38–40 °C within 9–11 days and then maintained at 38–40 °C until new shoots elongated. Shoot tips (0.7 mm) were excised from the new shoots, surface-disinfected and micrografted upon in vitro 2-weeks old seedling rootstocks of rough lemon (C. jambhiri). Micrografts developed into plantlets after 5–6 weeks of micrografting. This procedure resulted in 28–40% of micrografting success rates and 59–60% of ICRSV-free frequencies.
Applying thermotherapy (40 °C, 1 week) followed by micrografting, Chae et al. [42] completely eradicated citrus tristeza virus (CTV), satsuma dwarf virus (SDV) and citrus tatter leaf virus (CTLV) for six citrus cultivars.
Combining thermotherapy with micrografting was mainly applied to woody plants like citrus [38–43], apple [44–46] and pear [46], because shoot regeneration from shoot tip culture is difficult in these plants [12]. Some examples of successful eradication by combining thermotherapy with micrografting are listed in Table 3.
Table 3.
Some examples of thermotherapy followed by in vitro micrografting or shoot tip cryotherapy for virus eradication
| Methods | Plant species and cultivars or genotypes | Viruses | Size of shoot tips (mm) | Survival of the treated shoots (%) | Success (%) of micrografting or shoot regrowth (%) in cryotherapy | Virus-free frequency (%) | References |
|---|---|---|---|---|---|---|---|
| Thermotherapy + micrografting | Citrus ‘Early satsuma mandarin’, ‘Yuzu’, ‘Shiranuhi’ | Citrus tristeza virus (CTV), satsuma dwarf virus (SDV), citrus tatter leaf virus (CTLV) | 0.3 | NT | 13–75 | 76 (CTV), 100 (SDV) and 83 (CTLV) | [35] |
| Citrus hybrid ‘Kinnow’ | Indian citrus ringspot virus (ICRSV) | 0.7 | 42 (treatment of nodal segments at 50 °C for 120 min in water bath), 28 (exposure of nodal segments to 50 °C for 120 min of hot air) and NT (exposure of whole plants to 38 °C for 9 days in growth chamber) | 38 (water bath), 26 (hot air) and 40 (growth chamber) | 37 (water bath), 23 (hot air) and 59 (growth chamber) | [36] | |
| Citrus ‘Ehimekashi dai28go’, ‘Setoka’, ‘Shiranuhi’, ‘Haraejosaeng’, ‘Pungkwang’, ‘Samdajosaeng’ | CTV | NS | NT | NT | 100 | [37] | |
| Thermotherapy + cryotherapy | Raspberry genotype ‘Z13’ | Raspberry bushy dwarf virus (RBDV) | 1.0 | NT | 30 | 35 | [22] |
| Garlic ‘Jonas’ | OYDV, SYSV and GCLV | NS | NT | 40 | 90 (OYDV) 100 (LYSV) 80 (GCLV) |
[112] | |
| Apple ‘Gala’, ‘Ruixue’, ‘Ruiyang’, ‘Nongguo 25’, ‘Fuji’and ‘M9’ | ASGV | 1.5 | 100 | 33–76 | 30–100 | [23] |
NT not tested, NS not specified
Combining thermotherapy with shoot tip cryotherapy
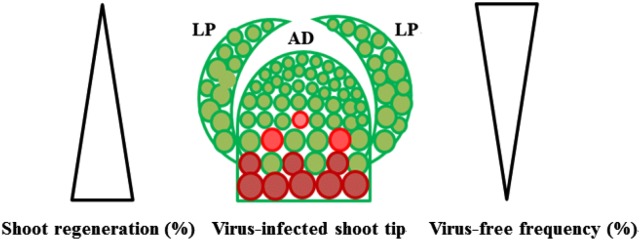
Shoot tip cryotherapy refers to treatment of infected materials for a short time in liquid nitrogen (LN) using cryopreservation protocols to cure infected plants [24, 25]. When shoot tips are frozen in LN, only cells in the upper parts of the apical dome (AD) are able to survive, while those in the lower parts are killed [26, 27, 47–49]. Virus is unevenly distributed inside plants [50]: virus concentration increases with increased distance from the AD; the AD contains low virus and even free of virus infection (Fig. 2). Thus, plants regenerated from shoot tip cryotherapy may be free of virus infection [24, 25]. Shoot tip cryotherapy has proven to be much more efficient, than the traditional methods like shoot tip culture, for eradication of viruses that do not infect the meristematic cells of the shoot tips [24, 25]. However, shoot tip cryotherapy cannot eradicate the viruses that can infect the meristematic cells of shoot tips, like RBDV [26], ASGV [27, 49], pelargonium flower break virus (PFBV) [51] and pelargonium line pattern virus (PLPV) [51].
Fig. 2.
Size of shoot tip in relation with shoot regeneration and virus-free frequency in thermotherapy-based methods. Cells in green color are healthy cells and in red color are virus-infected cells. Increased virus tirer is indicated in increased intensity of red color. AD apical dome, LP leaf primordium
Wang et al. [24] reported combining thermotherapy with shoot tip cryotherapy for RBDV eradication. In vitro RBDV-infected shoots of raspberry (Rubus idaeus) were thermo-treated using an alternating temperature of 38 °C/26 °C (day/night) under a 16-h photoperiod. After 28–35 days of thermotherapy, shoot tips (0.2 mm in size) containing two leaf primordia (PLs) were excised from the treated shoots and used for cryotherapy, as described by Wang et al. [47]. This procedure produced 20–36% and 30–40% of survival and shoot regrowth levels in cryo-treated shoot tips. About 30–35% plants recovered from combining thermotherapy with shoot cryotherapy were free of RBDV [24].
Recently, combining thermotherapy with shoot tip cryotherapy was shown to efficiently eradicate ASGV, a difficult-to-eradicate virus [27]. In vitro shoots infected with ASGV were thermo-treated using an alternating temperature of 36 °C/32 °C (day/night). After 4 weeks of thermotherapy, shoot tips (1.5 mm in size) containing 4–5 LPs were excised from the treated shoots and subjected to cryotherapy, as described by Li et al. [49]. This protocol yielded 33–76% and 30–100% of shoot regrowth rates and virus eradication frequencies across the four apple cultivars and one rootstock tested. Zhao et al. [27] believed thermotherapy followed by shoot tip cryotherapy might be considered to be the most efficient method so far reported for virus eradication. Some examples of virus eradication by combining thermotherapy with shoot tip cryotherapy are listed in Table 3.
Key factors affecting success of virus eradication
Thermotherapy temperatures and durations
High temperatures induce stress to plants, and such stress is intensified as their durations increase [52, 53]. High temperatures and their prolonged durations were shown to reduce survival levels of the treated shoots and shoot tips excised from the treated shoots, the regenerative ability and micrografting success of shoot tips excised from the treated shoots. However, increased temperatures and thermotherapy durations enhanced virus eradication [26, 27, 36, 53].
Applying combining chemotherapy with thermotherapy for virus eradication from apple, Hu et al. [41] found survival levels of the treated in vitro shoots and shoot tips excised from the treated shoots decreased from 100 to 40% and 70 to 8%, respectively, as thermotherapy temperatures increased from 34 to 38 °C. Chemotherapy followed by thermotherapy at 36 °C produced higher frequencies of plants free of ACLSV, ASPV and ASGV than 34 °C [36]. Similar results were repeatedly found in combining chemotherapy with thermotherapy in potato [54], garlic [55] and lily [56], in thermotherapy followed by shoot tip culture in garlic [57], horseradish [53], cassava [54, 55], nectarine [56], plum [57] and pear [58], in combining thermotherapy with shoot tip cryotherapy in raspberry [26] and apple [27], and in thermotherapy followed by micrografting in Citrus [41, 42].
Working on combining thermotherapy with shoot tip culture for virus eradication from pear, Tan et al. [63] found that survival levels, shoot length and proliferation efficiency of in vitro shoots decreased, while virus-free frequencies of ACLSV and ASPV increased, as thermotherapy durations increased from 10 to 50 days. Negative effects of prolonged thermotherapy durations on in vitro shoots and shoot tips excised from the treated shoots were frequently found in woody plants such as apricot [64], peach [64], cherry [64], pear [65, 66], plum [62] and black raspberry [67], and herbaceous species such as horseradish [58], chrysanthemum [33], begonia [63] and potato [64]. Increased virus eradication frequencies by increasing thermotherapy durations were also frequently reported in various virus-host combinations. Examples of herbaceous crops included horseradish infected with horseradish mosaic virus (HMV) [53], begonia infected with prunus necrotic ringspot virus (PNRSV) [63], chrysanthemum infected with chrysanthemum virus B (CVB) [28] and potato infected with PVY [64]. Examples of woody plants were apricot infected with ACLSV [59], peach infected with PNRSV and ACLSV [59], sour cherry infected with prune dwarf virus (PDV) and ACLSV [59], raspberry infected with RBDV [22], pear mix-infected with ACLSV, ASPV and ASGV [58] and ACLSV and ASGV [60, 61], and apple infected with ASGV [23].
Preculture of the virus-infected in vitro shoots on a medium containing 10−5 M salicylic acid (SA) for 4 weeks increased survival levels of the heat-treated shoots (42 °C, 30 days) from 58 to 64% and PVX-free frequencies from 75 to 98% among the seven potato genotypes [65]. Similar results were also reported by Fang et al. [49] and Aguilar-Camacho et al. [66], who applied thermotherapy-based methods for virus eradication from in vitro potato shoots. SA treatments decreased catalase activity and increased hydrogen peroxide (H2O2) levels of the in vitro potato shoots, thus enhancing their tolerance to thermotherapy [65, 66]. SA induced plant defense to virus infection and was beneficial for virus eradication [67]. Therefore, SA had double positive effects in thermotherapy for virus eradication: enhancing plant tolerance to thermotherapy and increasing virus eradication frequency. Detailed information of SA-mediated biotic and abiotic stress signalling in plants can be found in a recent review [67].
Step-wise increasing temperatures and alternating temperatures
Many temperate plant species like Prunus, Vaccinium and Vitis are sensitive to sudden increased temperatures, and step-wise increasing temperature treatments (preconditioning) helped them adapt themselves to thermotherapy [68]. In the study of Bruna [52] for eradication of onion yellow dwarf virus (OYDV), in vitro shoots of garlic were preconditioned at 30 °C for 7 days and then heat-treated at 38 °C for 38 days. Step-wise increasing temperatures were also used in Prunus fruits such as peach [68], nectarine [56] and apricot [69].
Thermotherapy using an alternating day/night temperature was found to alleviate negative effects of high constant temperature on the in vitro shoots during thermotherapy and increased survival and growth of the heat-treated shoots, thus improving virus eradication efficiency. For example, Knapp et al. [14] reported that shoot survival levels were much higher in in vitro apple shoots that had been heat-treated for 30 days by an alternating temperature of 38 °C/36 °C (day/night) than by a constant temperature of 38 °C. Higher survival levels, and greater shoot length and proliferation index were obtained in in vitro pear shoots that had been heat-treated for 50 days by an alternating temperature of 42 °C/34 °C (day/night) than by a consistent temperature of 37 °C [58]. Beneficial effects of the alternating temperature for thermotherapy on the treated shoots were also observed in other woody plants like peach [68], pear [70], apple [23, 71], plum [57] and grapevine [24].
Types and concentrations of antivirus agents
Types and concentrations of antivirus agents used in thermotherapy-based chemotherapy influenced survival of the treated in vitro shoots, shoot regeneration of shoot tips excised from the treated shoots and virus eradication frequencies. Verma et al. [68] tested the effects of three antivirus agents (6-azauracil, 2-thiouracil and ribavirin) on PNRSV eradication from the infected in vitro Begonia shoots, and found chemotherapy of 20–35 mg L−1 ribavirin yielded 20–45% of virus-free plants, which were higher than 0–15% and 0–20% produced by the same concentrations of 2-thiouracil and 6-azauracil, respectively. Chemotherapy (20 mg L−1 ribavirin) followed by thermotherapy (38 °C, 25 days) yielded almost 100% of shoot survival levels and 57.5% of PNRSV-free plants. Rribavirin treatment (30 mg L−1) followed by thermotherapy (38 °C, 25 days) produced less than 30% shoot survival and 75% of PNRSV-free plants. Reduced survival levels of the treated shoots by increased ribavirin concentrations were reported in garlic [16], pear [31, 76], apple [44, 75], raspberry [76] and grapevine [77] for grapevine. Optimal ribavirin concentration for production of virus-free plants was 12.21 mg L−1 (50 μM used) for lily infected with lily symptomless virus (LSV), tulip breaking virus-lily (TBV-L) and cucumber mosaic virus (CMV) [7], 20 mg L−1 for potato infected with PLRV and PVY [49, 74], Begonia infected with PNRSV [68], artichoke infected with artichoke latent virus (ALV) [32] and cassava infected with east African cassava mosaic virus (EACMV), [60], 25 mg L−1 for apple infected with ACLSV, ASPV and ASGV [36] and pear infected with ACLSV and ASGV [65], 50 mg L−1 for pear infected with ACLSV [31], garlic infected with OYDV, leek yellow stripe virus (LYSV), shallot latent virus (SLV), mite borne filamentous virus (MbFV) and garlic common latent virus (GCLV) [16].
Ram et al. [33] found chemotherapy (30–40 mg L−1 2-thiouracil) followed by thermotherapy (38 °C, 30 days) was most effective in CVB eradication among the five antiviral chemicals tested. 2-thiouracil was also shown to be effective in eradicating LSV, TBV-L and CMV from lily [73], and PLRV from potato [34].
Filter-sterilized antivirus agents caused much severer toxic to plants but produced higher frequencies of PVY-free plants than the autoclaved agents, indicating autoclaving may reduce effects of antivirus agents on virus eradication [79]. In many cases, filter-sterilized antivirus agents were added to the medium after autoclaving [34, 55, 60, 69, 77, 80, 81], and in some cases, antivirus agents were added to the medium before autoclaving [35].
Size of shoot tips
As addressed above, virus is unevenly distributed inside plants [50] (Fig. 2). Therefore, size of shoot tips is critical for virus eradication. In general, size of shoot tips is positively related to survival and shoot regeneration, while it is negatively proportional to virus eradication frequency [21], (Fig. 2). With grapevine, Skiada et al. [28] reported although shoot regeneration levels were much higher (80%) in shoot tip culture (0.5 cm) than that (56%) in meristem culture (0.1–0.2 mm), the latter produced 91.2% and 73.8% plants free of GLRaV-1 and GRSPaV-1, which were much higher than 68% and 51% by the former. Similar results were obtained in a great number of studies using thermotherapy-based methods for eradication of different types of viruses from various plant species ranging from woody to herbaceous plants and originating from temperate to tropical regions [2, 12, 19, 21, 23]. Shoot tip size is critical for virus eradication and combining thermotherapy with shoot tip culture allows use of larger shoot tips than those used for shoot tip culture without thermotherapy [12, 19, 21, 23].
Source of explants and position of shoot tips
Source of explants and shoot tip position influenced success of virus eradication in thermotherapy-based methods. Survival and growth levels of apple shoot tips following thermotherapy were higher in the buds harvested from the actively growing shoots than from the dormant ones, and in the apical buds than in axillary ones [82]. These effects were related to higher endogenous contents of auxins and cytokinins in the actively growing and apical buds than the dormant and axillary ones [83]. Survival levels of the heat-treated grapevine shoots were higher when in vitro cultures were established from the middle and basal buds than from the terminal buds [84]. GLRaV-3-free frequencies were similar among the terminal, the first and second axillary shoot tips, but were lower in the third axillary shoot tips [84]. Virus-free frequencies were higher plants when the diseased in vitro shoots were established in summer than in spring [84]. The authors attributed this effect to “natural thermotherapy’’, since when the samples were collected in summer, the day temperatures in the vineyards (Murcia, Spain) reached 38–40 °C [84]. Hu et al. [36] found virus elimination frequencies were higher in the plants regenerated from axillary shoots than from apical shoots in apple, using combining chemotherapy with thermotherapy. The authors believed the axillary shoot tips elongated faster than apical ones in their experiments, thus helping new growth of the axillar buds to escape virus infection [36].
Genotype-specific responses
Survival levels of the heat-treated shoots, and survival and shoot regrowth levels of the shoots tips excised from the heat-treated shoots of Capparis spinosa were significantly higher in ‘Pantelleria’ than in ‘Salina’ [85]. Similar virus-free frequencies were obtained between these two cultivars by combining thermotherapy with shoot tip culture. Survival levels of the heat-treated shoots and virus free frequencies differed from two apple cultivars by thermotherapy followed by shoot tip culture [75]. Genotype-specific responses were also found in a number of plants including woody species such as Prunus [64] and Vitis [86], and herbaceous species such as Allium sativum [55, 87], artichoke infected with ALV [32], Solanum tuberosum [29], Lilium [56, 88], Cynara cardunculus var. scolymus [89] and Manihot esculenta [59].
It has been known that tolerance/resistance to high temperatures varies with plant genotypes [53]. Ability of a given virus to infect meristematic cells of the shoot tips and its distribution pattern in shoot tips following heat treatment also vary with plant genotypes [6, 27, 90]. These variations lead to genotype-specific responses to thermotherapy-based methods for success of virus eradication. For example, using in situ hybridization for localization of chrysanthemum stunt viroid (CSVd) in shoot tips of the four infected Argyranthemum genotypes, Zhang et al. [90] reported CSVd was present in all tissues including the uppermost cell layers in the apical dome (AD) and the youngest leaf primordia (LPs) 1 and 2 in ‘Yellow Empire’ and ‘Butterfly’, but it was detected only in the lower part of the AD, while not in the upper part of the AD, and LPs 1–2 in ‘Border Dark Red’ and ‘Border’. Virus distribution patterns analyzed by immunohistologial localization were similar in shoot tips of the ASGV-infected apple cultivars ‘Gala’ and ‘Ruixue’ before thermotherapy [27]. However, 4 weeks of thermotherapy resulted in different virus distribution patterns between them [27]. In ‘Gala’, the virus was not detected in the AD and LPs 1–5 but was detected in LP 6 and older LPs of the shoot tips. In ‘Ruixue’, the virus was not detected in the AD and LPs 1–3 but was found in LP 4 and older LPs [27].
Types of virus and infection status
In general, phloem-limited viruses are easy to eradicate, while those that can infect the meristematic cells of shoot tips are difficult to eradicate.
Artichoke latent virus (ArLV) could be easily eliminated by shoot tip culture alone, while artichoke Italian latent virus (AILV) could be removed only when thermotherapy followed by shoot tip culture was used [89]. Virus-free frequencies were much higher for GLRaV-1 (67–91%) than for GRSPaV-1 (51–74%), regardless of size of shoot tips used in combining thermotherapy with shoot tip culture [28]. Thermotherapy followed by shoot tip culture resulted in virus-free frequencies of 100% for grapevine fanleaf virus (GFLV), 70% for grapevine virus A (GVA), 25 for GLRaV-1, 25 for GLRaV-3 and 0% for grapevine fleck virus (GFKV) [91]. Varying virus-free frequencies with types of the virus were repeatedly reported in various virus-host combinations by thermotherapy-based methods, for example, garlic infected with LYSV, OYDV, GarMbFV and GCLV [87], potato infected with PVY and PLRV [49]; apple infected with ACLSV, ApMV, ASPV and ASGV [36, 75, 82], pear infected with ACLSV and ASGV [65, 66]; figs infected with fig leaf mottle-associated virus 1 (FLMaV-1), FLMaV-2 and fig mosaic virus (FMV) [92].
Viruses differ in their abilities to infect shoot tips of the same plant [6], thus leading to differences in virus eradication frequencies in different viruses from the same plant. Wang and Valkonen [48] found sweetpotato chlorotic stunt virus (SPCSV) and sweetpotato feathery mottle virus (SPFMV) were not present in the AD and LPs 1–3 of the shoot tips of sweetpotato. SPCSV was detected in LP5 and the older tissue, but not in LP 1–4, and SPFMV in LP4 and the older tissue. Li et al. [49] found that ASPV was present in lower part of AD, and LP 4 and older tissues of the shoot tips, but not present in upper part of AD and LPs 1–3, leaving a ASPV-free area in AD (approximately 0.8 mm in length). ASGV was detected across the AD and LPs 1–6 of the shoot tips, leaving only the very top layers of cells in AD (approximately 0.5 mm in length) free of ASGV infection. Similar distribution patterns of ASPV and ASGV were observed in shoot tips of apple ‘Gala’ [93]. These data provided explanations to why virus-free frequencies varied with the types of virus in thermotherapy-based methods.
Virus eradication was easier from single-infected plants than from mix-infected ones [12]. Following combining thermotherapy with shoot tip culture, Knapp et al. [18] found ACLSV-free frequency was much higher in the in vitro single-infected shoots than in the shoots mix-infected with ASGV and ACLSV. Fang et al. [54] also reported PLRV was much easier to remove from single-infected shoots than co-infected ones with PVY and PLVR. However, little has been known about mechanism of the synergistic effects of viruses in terms of virus eradication.
Genetic stability and field behaviors in virus-free plants
High temperatures cause stress to plants [52, 53] and antivirus chemicals are toxic to plants [19, 31, 34, 68, 80, 81]. These create ricks of genetic variations in plants regenerated following thermotherapy-based methods. In addition, in vitro culture procedure may also induce genetic variations, due to the use of high concentrations of plant growth regulators, repeated subculture and shoot regeneration through callus formation [94, 95]. The purpose of cultivation of virus-free plants is to improve quality and yield of agricultural production, while maintaining the genetic stability and unique straits of the original cultivars [1, 6]. Therefore, it is necessary to assess genetic stability and observe field behaviors in virus-free plants derived from thermotherapy-based methods. Unfortunately, information on the said subject has been quite limited.
Acquadro et al. [89] applied simple sequence repeat (SSR) and amplified fragment length polymorphism (AFLP) markers to assess genetic stability in virus-free Globe artichoke plants obtained by thermotherapy followed by shoot tip culture. SSR did not detect any polymorphic bands. Although some polymorphic bands were detected by AFLP, no obvious variations were found between the control and treated samples, and polymorphic bands detected were genotype-specific. Nevertheless, these results indicate thermotherapy may induce genetic variations.
Nesi et al. [88] reported LSV-free lily plants produced by combining thermotherapy with shoot tip culture grew more vigorously and produced more leaves than the virus-infected plants after the first year of growth in the field. Ram et al. [96] observed field behaviors of CMV, CVB and TAV-free chrysanthemum plants produced by thermotherapy followed by shoot tip culture, and found the virus-free plants produced significantly higher vegetative growth (plant height and number of stems) and flower production (number and diameter of flowers) than the infected plants. Ramírez-Malagón et al. [55] reported Potyvirus-free garlic plants derived from thermotherapy or chemotherapy followed by shoot tip culture had better vegetative growth and higher yield and quality of cloves than the virus-infected plants. Compared field behaviors between virus-infected potato plants and virus-free plants derived from virus-free minitubers resulted from thermotherapy followed by shoot tip culture, Mahmound et al. [69] found plant height, and tuber weight and yield were significantly greater in virus-free plants than those single-infected with PVY, and mix-infected with PLRV, PVX and PVY. These data indicated virus-free plants produced by thermotherapy-based methods significantly increased their potential of vegetative growth and yield, compared with virus-infected ones.
Mechanism
Thermotherapy
Possible mechanisms involved in thermotherapy-based methods for virus eradication were well discussed in a number of publications [12, 19, 22, 23]. Recent studies added valuable information on the said subject:
High temperature treatments were found to prevent virus movement toward the meristematic cells of the treated shoots of raspberry infected with RBDV [26], pear infected with ACLSV and ASGV [63], and apple infected with ACLSV and ASGV [27]. This effect resulted in production of larger virus-free areas of the infected shoot tips, thus helping virus eradication [26, 27].
Thermotherapy was found to inhibit viral replication [92, 97] or caused virus RNA degradation [26, 27], thus decreasing virus titer in the infected shoot tips. Liu et al. [93] found reduced ASGV titers in thermo-treated pear shoot tips were associated with a number of miRNA-mediated genes related to disease defense and hormone signal transduction pathways in the apical meristem of pear shoots. These results suggested that miRNAs may have important functions in the thermotherapy-induced decreases of virus titer in the heat-treated shoots [98].
High temperatures promoted virus-induced RNA silencing in tobacco infected with cymbidium ringspot virus (CRSV) [99] and PVX [100], cassava and tobacco infected with cassava Geminivirus [101] and raspberry infected with RBDV [26]. Recently, Liu et al. [97] found thermotherapy drastically decreased viral genome accumulation in shoot tips of the treated pear shoots infected with ASGV, which was accompanied with the elevated levels of virus-derived small interfering RNA (vsiRNA). Thermotherapy induced the biogenesis of vsiRNAs and inhibited viral RNA accumulation, by up-regulating the expression of key genes in the RNA silencing pathway [102]. miRNAs have been known to regulate key genes in resistance to virus infections [103–106]. Increased virus-induced silencing by thermotherapy is so far the most convinced mechanism involved in improved virus eradication by thermotherapy-based methods [26, 98, 102]. Nevertheless, specific mechanism as to why thermotherapy improves virus eradication has not been well understood yet and needs further studies.
Chemotherapy
Studies that elucidate mechanism involved in antivirus agents for plant virus have been quite limited. Existing studies demonstrated exogenous applications of antivirus agents inhibited synthesis of virus RNA [107–113]. Inhibition of viral RNA synthesis decreased the number of virus particles released from the infected cells into the newly divided cells [107, 108], thus resulting in production of a larger virus-free area or reduction of virus titers in the treated shoot tips and helping virus eradication [34]. Applying ribavirin to treat human cells infected with hepatitis C virus, a RNA virus, Crotty et al. [114, 115] found the antiviral activity of ribavirin was through lethal mutagenic activity by forcing the virus into ‘error catastrophe’, thus destroying the infectivity of the virus genomic RNA. Nevertheless, working model of chemotherapy on plant virus eradication has not been well understood yet and extra studies are needed.
Cryotherapy
As addressed above, distribution of virus is uneven inside plants [50]. When shoot tips are frozen in LN, only cells that are less differentiated, have large nucleo-cytoplasmic ratio and contain less free water are able to survive [24, 25]. Therefore, only cells in top layers of AD and the youngest leaf primordia (LPs) are able to survive, while cells in lower parts of AD and the older PLs are killed, following cryopreservation [24, 25]. Thus, freezing in LN kills virus-infected cells and allows healthy (virus-free) cells to survive and then regenerate into pathogen-free plants [20–22]. In combining thermotherapy with cryotherapy, thermotherapy had dual effects: production of larger virus-free areas in the treated shoots, as addressed above, and reduction in the number of survival cells, eventually improving virus eradication frequency, compared with cryotherapy alone [26, 27].
Further prospects
So far, various thermotherapy-based methods have successfully been developed for eradication of viruses from almost all economically important crops that are vegetatively propagated [2, 12, 19, 22, 23], Tables 1, 2, and 3 in present study. However, simple and efficient methods are still needed for eradication of the viruses, especially those that infect meristematic cells of the shoot tips. RBDV and ASGV are the two representatives of this type of virus and can be effectively eradicated only by combining thermotherapy with shoot tip cryotherapy [25, 26]. Compared with other techniques, cryotherapy is a relatively new technique, and may not be available in many laboratories, which is still limiting wider applications of this method. Therefore, continuous developments of simple and efficient method for eradication of such viruses are necessary.
Methods of virus detection and durations after virus eradication treatments determine virus eradication frequencies in a given method. In many of the previous studies, ELISA-based methods were used to screen the virus status, followed by PCR-based methods to confirm the virus status in plants following virus eradication treatments [17, 34, 42, 116]. In some cases, only ELISA-based methods were used to confirm the virus status in the plants following virus eradication [29, 30, 55, 61, 65, 71, 78]. It is well-known that ELISA-based methods are much less sensitive to detect viruses than PCR-based methods [2, 117]. Often, virus-free frequencies analyzed by ELISA-based methods in plants following virus eradication treatments were much higher than by PCR-based ones [28, 36, 56, 63, 72]. Reduction of virus titer, but not virus eradication, was frequently found in the plants following virus eradication treatments [51, 119]. In such cases, ELISA-based methods or too short time durations after virus eradication treatments failed to detect the ‘real’ virus status in the plants. Using combining thermotherapy with chemotherapy, Xu et al. [78] reported virus was detected by ELISA only in 30% and 6% of in vitro regenerants of lily ‘Georgia’ and ‘Casablanca’, respectively. However, the virus infection analyzed by the same method increased to 100% and 44% in these two cultivars after 6 months of growth in soil under greenhouse condition. Based on these data, we support use of two unrelated methods, for example, combining PCR-based methods with ELISA or biological indexing, or even more sensitive methods like next generation sequencing [2, 117] for detection of virus status in plants following virus eradication treatments, thus increasing the reliability of the results. In addition, to ensure virus status in the regenerants following virus eradication treatments, double tests for viruses should be done: first in in vitro regenerants following virus eradication treatments and then in plants after at least 6 and 10 months (including a dormant season) of growth in soil for herbaceous and woody plants, respectively [16, 27, 49, 56].
Many of the previous studies used single cultivar or genotype for virus eradication. As addressed above, genotype-specific response is very common in virus eradication studies, and a protocol may work well in a given genotype but completely fails in another. Therefore, development of protocols applicable to a wide range of genotypes within a species would facilitate wider applications of the technique to production of virus-free plants for commercial cultivation and preservation of plant germplasm [16, 17, 27].
Extra studies should also be strengthened in assessments of genetic stability and observations on field performance in the virus-free plants resulted from thermotherapy-based methods. These studies would accelerate extensions of virus-free plants to practical agricultural production for improvements of yield and quality of crops.
Authors’ contributions
M-RW and Z-HC collected and analyzed references, and drafted the manuscript; X-YH and J-WL assisted to collection and analysis of references; LZ and Q-CW proposed the subject, provided financial supports and revised manuscript. All authors read and approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This research was funded by National Natural Science Foundation of China (No. 31701761), Chinese Universities Scientific Fund (No. 2452017061) and Department of Science and Technology of Shaanxi Province, China (2014KTCL02-05).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACLSV
apple chlorotic leaf spot virus
- AD
apical dome
- AILV
artichoke Italian latent virus
- ALV or ArLV
artichoke latent virus
- ASGV
apple stem grooving virus
- ASPV
apple stem pitting virus
- AVA
arracacha virus A
- AVB
arracacha virus B
- CMV
cucumber mosaic virus
- CSVd
chrysanthemum stunt viroid
- CTLV
citrus tatter leaf virus
- CTV
citrus tristeza virus
- CVB
chrysanthemum virus B
- SDV
satsuma dwarf virus
- EACMV
East African cassava mosaic virus
- EU
European Union
- FLMaV
fig leaf mottle-associated virus
- FMV
fig mosaic virus
- GCLV
garlic common latent virus
- GFKV
grapevine fleck maculavirus
- GFLV
grapevine fanleaf nepovirus
- GLRaV-1
grapevine leafroll-associated virus 1
- GRSPaV
grapevine rupestris stem pitting-associated virus
- GVA
grapevine vitivirus A
- HMV
horseradish mosaic virus
- ICRSV
Indian citrus ringspot virus
- MbFV
mite borne filamentous viruses
- LPs
leaf primordia
- LSV
lily symptomless virus
- LN
liquid nitrogen
- LYSV
leek yellow stripe virus
- OYDV
onion yellow dwarf virus
- PapMV
papaya mosaic virus
- PDV
prune dwarf virus
- PFBV
pelargonium flower break virus
- PLRV
potato leafroll virus
- PLPV
pelargonium line pattern virus
- PNRSV
prunus necrotic ringspot virus
- PPV
plum pox virus
- PVS
potato virus S
- PVX
potato virus X
- PVY
potato virus Y
- RBDV
raspberry bushy dwarf virus
- SA
salicylic acid
- SLV
shallot latent virus
- SPCSV
sweetpotato chlorotic stunt virus
- SPFMV
sweetpotato feathery mottle virus
- SSR
simple sequence repeat
- AFLP
amplified fragment length polymorphism
- TAV
tomato aspermy virus
- TBV-L
tulip breaking virus-lily
- UMMV
ullucus mild mottle virus
- UMV
ullucus mosaic virus
- UVC
ullucus virus C
- vsiRNA
virus-derived small interfering RNA
Contributor Information
Lei Zhao, Email: zhaolei5105@126.com.
Qiao-Chun Wang, Email: qiaochunwang@nwsuaf.edu.cn.
References
- 1.Hadidi A, Barba M. Economic impact of pome and stone fruit viruses and viroids. In: Hadidi A, Barba M, Candresse T, Jelkman W, editors. Virus and virus-like diseases of pome and stone fruits. St. Paul: American Phytopathological Society; 2011. pp. 1–7. [Google Scholar]
- 2.Barba M, Ilardi V, Pasquini G. Control of pome and stone fruit virus diseases. In: Loebenstein G, Katis NI, editors. Advances in virus research. Burlington: Academic Press; 2015. pp. 47–83. [DOI] [PubMed] [Google Scholar]
- 3.Brunt AA. The main viruses infecting potato crops. In: Loebenstein G, Berger PH, Brunt A, Lawson RH, editors. Virus and virus-like diseases of potatoes and production of seed-potatoes. Dordrecht: Kluwer; 2011. pp. 65–67. [Google Scholar]
- 4.Wang B, Ma Y-L, Zhang Z-B, Wu Z-M, Wu Y-F, Wang Q-C, Li M-F. Potato viruses in China. Crop Prot. 2011;30:1117–1123. doi: 10.1016/j.cropro.2011.04.001. [DOI] [Google Scholar]
- 5.Scorza R, CallahanA Dardick Ch, Ravelonandro M, Polak J, Malinowski T, Zagrai I, Cambra M, Kamenova I. Genetic engineering of Plum pox virus resistance: ‘HoneySweet’ plum—from concept to product. Plant Cell Tissue Org Cult. 2013;115:1–12. doi: 10.1007/s11240-013-0339-6. [DOI] [Google Scholar]
- 6.Hull R. Mathews’ plant virology. London: Academic Press; 2002. [Google Scholar]
- 7.Perring TM, Gruenhagen NM, Farrar CA. Management of plant viral diseases through chemical control of insect vectors. Ann Rev Entomol. 1999;44:457–481. doi: 10.1146/annurev.ento.44.1.457. [DOI] [PubMed] [Google Scholar]
- 8.Luvisi A, Panattoni A, Materazzi A, De Bellis L. Chemical outbreak for tobacco mosaic virus control. Int J Agric Biol. 2017;19:792–800. doi: 10.17957/IJAB/15.0365. [DOI] [Google Scholar]
- 9.Hadidi A, Pasquini G, Barba M, Spiegel S, Zaidi A, Crescebzi A. Strategies for control of systemic pathogens of fruit trees-an overview. In: Hadidi A, Barba M, Candresse T, Jelkmann M, editors. Virus and virus-like diseases of pome and stone fruits. St. Paul: American Phytopathological Society; 2011. pp. 373–375. [Google Scholar]
- 10.European Community. Council directive 92/34 of 28 April 1992 on the marketing of fruit plant propagation material and fruit plants indented for fruit production. EU bulletin n L 157/10 of June 10. 1992.
- 11.Zhang LM, Wang QM, Liu QC, Wang Q-C. Sweetpotato in China. In: Loebenstein G, editor. Sweetpotato biology. Berlin: Springer; 2008. pp. 321–354. [Google Scholar]
- 12.Laimer M, Barba M. Elimination of systemic pathogens by thermotherapy, tissue culture, or in vitro micrografting. In: Hadidi A, Barba M, Candresse T, Jelkmann M, editors. Virus and virus-like diseases of pome and stone fruits. St. Paul: American Phytopathological Society; 2011. pp. 389–393. [Google Scholar]
- 13.Previati A, Benelli C, Da Re F, Giannini M. In vitro production of virus-free chrysanthemum stock plants for cut flowers. Propag Ornam Plant. 2008;8:167–169. [Google Scholar]
- 14.Krezal G. Virus certification of ornamental plant—the European strategy. In: Hadidi A, Khetarpal RK, Koganezawa H, editors. Plant virus disease control. St Paul: APS Press; 1998. pp. 277–287. [Google Scholar]
- 15.Reed PJ, Foster JA. Exclusion of pome and stone fruit viruses, viroids and phytoplasmas by certification and quarantine. In: Hadidi A, Barba M, Candresse T, Jelkmann W, editors. Virus and virus-like diseases of pome and stone fruits. St. Paul: American Phytopathological Society; 2011. pp. 381–388. [Google Scholar]
- 16.Senula A, Keller ERJ, Leseman DE. Elimination of viruses through meristem culture and thermotherapy for the establishment of the in vitro collection of garlic (Allium sativum) Acta Hortic. 2000;530:121–128. doi: 10.17660/ActaHortic.2000.530.12. [DOI] [Google Scholar]
- 17.Postman JD, Sugar D. Elimination of viruses from the USDA Pyrus germplasm collection. Acta Hortic. 2002;596:529–530. doi: 10.17660/ActaHortic.2002.596.88. [DOI] [Google Scholar]
- 18.Knapp E, Hanzer V, Weiss H, da Câmara Machado A, Weiss B, Wang Q, Katinger H, Laimer M. New aspects of virus elimination in fruit tress. Acta Hortic. 1995;386:409–418. doi: 10.17660/ActaHortic.1995.386.56. [DOI] [Google Scholar]
- 19.Panattoni A, Luvisi A, Triolo E. Elimination of viruses in plants: twenty years of progress. Span J Agri Res. 2013;11:173–188. doi: 10.5424/sjar/2013111-3201. [DOI] [Google Scholar]
- 20.Smith GR, Fletcher JD, Marroni V, Kean JM, Stringer LD, Vereijssen J. Plant pathogen eradication: determinants of successful programs. Australas Plant Pathol. 2017;46:277–284. doi: 10.1007/s13313-017-0489-9. [DOI] [Google Scholar]
- 21.Faccioli G, Marani F. Virus elimination by meristem tip culture and tip micrografting. In: Hadidi A, Khetarpal RK, Koganzawa H, editors. Plant virus disease control. St. Paul: APS Press; 1998. pp. 346–380. [Google Scholar]
- 22.Mink GI, Wample R, Howell WE. Heat treatment of perennial plants to eliminate phytoplasmas, viruses and viroids while maintaining plant survival. In: Hadidi A, Khetarpal RH, Koganezawa H, editors. Plant virus disease control. St Paul: APS Press; 1998. pp. 332–345. [Google Scholar]
- 23.Faccioli G. Control of potato viruses using meristem and stem-cuttings cultures, thermotherapy and chemotherapy. In: Loebenstein G, Berger PH, Brunt AA, Lawson RH, editors. Virus and virus-like diseases of potato and production of seed-potatoes. Dordrecht: Kluwer; 2001. pp. 365–390. [Google Scholar]
- 24.Wang Q-C, Panis B, Engelmann F, Lambardi M, Valkonen JPT. Cryotherapy of shoot tips: a technique for pathogen eradication to produce healthy planting materials and prepare healthy plant genetic resources for cryopreservation. Ann Appl Biol. 2009;154:351–363. doi: 10.1111/j.1744-7348.2008.00308.x. [DOI] [Google Scholar]
- 25.Wang QC, Valkonen JPT. Cryotherapy of shoot tips: novel pathogen eradication method. Trend Plant Sci. 2009;14:119–122. doi: 10.1016/j.tplants.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Wang QC, Cuellar WJ, Rajamaki M-L, Hirata Y, Valkonen JPT. Combined thermotherapy and cryotherapy for efficient virus eradication: relation of virus distribution, subcellular changes, cell survival and viral RNA degradation in shoot tips. Mol Plant Pathol. 2008;9:237–250. doi: 10.1111/j.1364-3703.2007.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Wang M-R, Cui Z-H, Chen L, Wang Q-C. Combining thermotherapy with cryotherapy for efficient eradication of Apple stem grooving virus (ASGV) from infected apple in vitro shoots. Plant Dis. 2018;102:1574–1580. doi: 10.1094/PDIS-11-17-1753-RE. [DOI] [PubMed] [Google Scholar]
- 28.Skiada FG, Grigoriadou K, Maliogka VI, Katis NI, Eleftheriou EP. Elimination of Grapevine leafroll-associated virus 1 and Grapevine Rupestris pitting-associated virus from grapevine cv. Agiorgitiko and a micropropagation of protocol for mass production of virus-free plantlets. J Plant Pathol. 2009;91:177–184. [Google Scholar]
- 29.AlMaarri K, Massa R, AlBiski F. Evaluation of some therapies and meristem culture to eliminate Potato Y potyvirus from infected potato plants. Plant Biotechnol. 2012;29:237–243. doi: 10.5511/plantbiotechnology.12.0215a. [DOI] [Google Scholar]
- 30.Lozoya-Saldana H, Dawson WO. Effect of alternating temperature regimes on reduction or elimination of viruses in plant tissues. Phytopathology. 1982;72:1059–1064. doi: 10.1094/Phyto-72-1059. [DOI] [Google Scholar]
- 31.Cieślińska M. Elimination of Apple chlorotic leafspot virus (ACLSV) from pear by in vitro thermotherapy and chemotherapy. Acta Hortic. 2002;596:481–484. doi: 10.17660/ActaHortic.2002.596.80. [DOI] [Google Scholar]
- 32.Navacchi O, Zuccherelli G, Zuccherelli S. In vitro multiplication of artichoke and virus elimination by thermotherapy and chemotherapy. Acta Hortic. 2005;681:397–401. doi: 10.17660/ActaHortic.2005.681.55. [DOI] [Google Scholar]
- 33.Ram R, Verma N, Singh AK, Singh L, Hallan V, Zaid AA. Indexing and production of virus-free chrysanthemums. Biol Plant. 2005;49:149–152. doi: 10.1007/s10535-005-0152-0. [DOI] [Google Scholar]
- 34.Singh B. Effect of antiviral chemicals on in vitro regeneration response and production of PLRV-free plants of potato. J Crop Sci Biotechnol. 2015;18:341–348. doi: 10.1007/s12892-015-0069-x. [DOI] [Google Scholar]
- 35.Fletcher PJ, Fletcher JD. In vitro virus elimination in three Andean root crops: Oca (Oxalis tuberosa), ulluco (Ullucus tuberosus), and arracacha (Arracacia xanthorrhiza) N Z J Crop Hortic Sci. 2001;29:23–27. doi: 10.1080/01140671.2001.9514156. [DOI] [Google Scholar]
- 36.Hu G, Dong Y, Zhang Z, Fan X, Ren F, Zhou J. Virus elimination from in vitro apple by thermotherapy combined with chemotherapy. Plant Cell, Tissue Organ Cult. 2015;121:435–443. doi: 10.1007/s11240-015-0714-6. [DOI] [Google Scholar]
- 37.Jonard R. Micrografting and its applications to tree improvement. In: Bajaj YPS, editor. Biotechnology in agriculture and forestry. Trees I. Heidelberg: Springer; 1986. pp. 31–48. [Google Scholar]
- 38.Murashige T, Bitters WP, Rangan TS, Nauer EM, Roistachek CN, Holliday PB. A technique of shoot apex grafting and its utilization towards recovering virus-free citrus clones. HortScience. 1972;7:118–119. [Google Scholar]
- 39.Navarro L, Roistacher CN, Murashige T. Improvement of shoot-tip grafting in vitro for virus-free citrus. J Am Soc Hortic Sci. 1975;100:471–479. [Google Scholar]
- 40.Kim D, Shim H, Kwon H, Hyun J, Kim K, Lee S. Production of virus-free stocks from citrus plants by the shoot tip grafting and heat treatment. Kor J Plant Biotechnol. 2005;32:45–50. doi: 10.5010/JPB.2005.32.1.045. [DOI] [Google Scholar]
- 41.Sharma S, Singh B, Rani G, Zaidi AA, Hallan VK, Nagpal AK, Virk GS. In vitro production of Indian citrus ringspot virus (ICRSV) free Kinnow plants employing thermotherapy coupled with shoot tip grafting. Plant Cell, Tissue Organ Cult. 2008;92:85–92. doi: 10.1007/s11240-007-9307-3. [DOI] [Google Scholar]
- 42.Chae CW, Yun SH, Park JH, Hyun JW, Koh SW, Lee DH. Micrografting and heat treatment combination for eliminating virus of CTV-infected Citrus. J Life Sci. 2013;23:267–272. doi: 10.5352/JLS.2013.23.2.267. [DOI] [Google Scholar]
- 43.Carvalho SA, Santos FA, Machado MA. Eliminação de vírus do complexo sorose de citros por microenxertia associada a termoterapia. Fitopa Brasil. 2002;27:306–308. doi: 10.1590/S0100-41582002000300012. [DOI] [Google Scholar]
- 44.Hu G-J, Zhang Z-P, Dong Y-F, Fan X-D, Ren F, Zhu H-J. Efficiency of virus elimination from potted apple plants by thermotherapy coupled with shoot-tip grafting. Australas Plant Pathol. 2015;44:167–173. doi: 10.1007/s13313-014-0334-3. [DOI] [Google Scholar]
- 45.Hu G-J, Dong Y-F, Zhang Z-P, Fan X-D, Ren F, Li Z-N. Efficacy of virus elimination from apple by thermotherapy coupled with in vivo shoot-tip grafting and in vitro meristem culture. J Phytopathol. 2017;165:701–706. doi: 10.1111/jph.12610. [DOI] [Google Scholar]
- 46.Zuļġe N, Kâle A, Gospodaryk A, Vēvere K, Moročko-Bičevska I. Establishment of nuclear stock collections for apple and pear in Latvia. Proc Latv Acad Sci Sect B. 2017;71:156–165. [Google Scholar]
- 47.Wang QC, Laamanen J, Uosukainen M, Valkonen JPT. Cryopreservation of in vitro-grown shoot tips of raspberry (Rubus idaeus L.) by encapsulation-vitrification and encapsulation-dehydration. Plant Cell Rep. 2005;24:280–288. doi: 10.1007/s00299-005-0936-x. [DOI] [PubMed] [Google Scholar]
- 48.Wang QC, Valkonen JPT. Elimination of two synergistically interacting viruses from sweetpotato by shoot tip culture and cryotherapy of shoot tips. J Virol Methods. 2008;154:135–145. doi: 10.1016/j.jviromet.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Li B-Q, Feng C-H, Hu L-Y, Wang M-R, Wang Q-C. Shoot tip culture and cryopreservation for eradication of Apple stem pitting virus (ASPV) and Apple stem grooving virus (ASGV) from apple rootstocks ‘M9’ and ‘M26’. Ann Appl Biol. 2016;168:142–150. doi: 10.1111/aab.12250. [DOI] [Google Scholar]
- 50.White PR. Multiplication of the viruses of tobacco and aucuba mosaic in growing excised tobacco roots. Phytopath. 1934;24:1003–1011. [Google Scholar]
- 51.Gallard A, Mallet R, Chevalier M, Grapin A. Limited elimination of two viruses by cryotherapy of pelargonium apices related to virus distribution. Cryo Lett. 2011;32:111–122. [PubMed] [Google Scholar]
- 52.Sharkey TD, Schrader SM. High temperature stress. In: Rao KVM, Raghavendra AS, Reddy KJ, editors. Physiology and molecular biology of stress tolerance in plants. Dordrecht: Springer; 2006. pp. 101–129. [Google Scholar]
- 53.Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ Exp Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 54.Fang YL, Dhital SP, Li KH, Khu DM, Kim HY, Lim HT. Utilization of single nodal cuttings and therapies for eradication of double-infected potato viruses (PLRV and PVY) from in vitro plantlets of potato (Solanum tuberosum) J Kor Soc Hortic Sci. 2005;46:119–125. [Google Scholar]
- 55.Ramírez-Malagón R, Pérez-Moreno L, Borodanenko A, Salinas-Gonzáez G, Ochoa-Alejo N. Differential organ infection studies, potyvirus elimination, and field performance of virus-free garlic plants produced by tissue culture. Plant Cell, Tissue Organ Cult. 2006;86:103–110. doi: 10.1007/s11240-006-9102-6. [DOI] [Google Scholar]
- 56.Chinestra SC, Curvetto NR, Marinangeli PA. Production of virus-free plants of Lilium spp from bulbs obtained in vitro and ex vitro. Sci Hortic. 2015;194:304–312. doi: 10.1016/j.scienta.2015.08.015. [DOI] [Google Scholar]
- 57.Bruna A. Effect of thermotherapy and meristem-tip culture on production of virus-free garlic in Chile. Acta Hortic. 1997;433:631–634. doi: 10.17660/ActaHortic.1997.433.72. [DOI] [Google Scholar]
- 58.Uchanski M, Skirvin RM, Norton MA. The use of in vitro thermotherapy to obtain turnip mosaic virus-free horseradish plants. Acta Hortic. 2004;631:175–179. doi: 10.17660/ActaHortic.2004.631.22. [DOI] [Google Scholar]
- 59.Carvalho MJS, Oliveira EJ, Souza AS, Pereira JS, Diamantino MSAS, Oliveira SAS. Cleaning cassava genotypes infected with cassava frogskin disease via in vitro shoot tip culture. Genet Mol Res. 2017 doi: 10.4238/gmr16029556. [DOI] [PubMed] [Google Scholar]
- 60.Kidulile CE, Miinda Ateka E, Alakonya AE, Ndunguru JC. Efficacy of chemotherapy and thermotherapy in elimination of East African cassava mosaic virus from Tanzanian cassava landrace. J Phytopathol. 2018 doi: 10.1111/jph.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manganaris GA, Economou AS, Boubourakas IN, Katis NI. Elimination of PPV and PNRSV through thermotherapy and meristem-tip culture in nectarine. Plant Cell Rep. 2003;22:195–200. doi: 10.1007/s00299-003-0681-y. [DOI] [PubMed] [Google Scholar]
- 62.Dziedzic E. Elimination of Prunus necrotic ring spot virus (PNRSV) from plum ‘Earliblue’ shoots through thermotherapy in vitro. J Fruit Ornam Plant Res. 2008;16:101–109. [Google Scholar]
- 63.Tan RR, Wang LP, Hong N, Wang GP. Enhanced efficiency of virus eradication following thermotherapy of shoot-tip cultures of pear. Plant Cell, Tissue Organ Cult. 2010;101:229–235. doi: 10.1007/s11240-010-9681-0. [DOI] [Google Scholar]
- 64.Gella R, Errea P. Application of in vitro therapy for ilarvirus elimination in three Prunus species. J Phytopathol. 1998;146:445–449. doi: 10.1111/j.1439-0434.1998.tb04779.x. [DOI] [Google Scholar]
- 65.Wang LP, Wang GP, Nong N, Tang RR, Deng XY, Zhang H. Effect of thermotherapy on elimination of Apple stem grooving virus and Apple chlorotic spot virus from in vitro-cultured pear shoot tips. HortScience. 2006;41:729–732. [Google Scholar]
- 66.Hu G, Hong N, Wang L, Hu H, Wang G. Efficacy of virus elimination from in vitro-cultured sand pear (Pyrus pyrifolia) by chemotherapy combined with thermotherapy. Crop Prot. 2012;37:20–25. doi: 10.1016/j.cropro.2012.02.017. [DOI] [Google Scholar]
- 67.Cheong EJ, Jeon AR, Kang JW, Mock R, Kinard G, Li R. In vitro elimination of Black raspberry necrosis virus from black raspberry (Rubus occidentalis) Hortic Sci (Prague) 2014;2:95–99. doi: 10.17221/266/2013-HORTSCI. [DOI] [Google Scholar]
- 68.Verma N, Ram R, Zaidi AA. In vitro production of Prunus necrotic ringspot virus-free begonias through chemo- and thermotherapy. Sci Hortic. 2005;103:239–247. doi: 10.1016/j.scienta.2004.05.005. [DOI] [Google Scholar]
- 69.Mahmoud SYM, Hosseny MH, Abdel-Ghaffar MH. Evaluation of some therapies to eliminate Potato Y Potyvirus from potato plants. Int J Virol. 2009;5:64–76. doi: 10.3923/ijv.2009.64.76. [DOI] [Google Scholar]
- 70.López-Delgado H, Mora-Herrera ME, Zavaleta-Mancera HA, Cadena-Hinojosa M, Scott IM. Salicylic acid enhances heat tolerance and potato virus X (PVX) elimination during thermotherapy of potato microplants. Am J Potato Res. 2004;81:171–176. doi: 10.1007/BF02871746. [DOI] [Google Scholar]
- 71.Aguilar-Camacho M, Mora-Herrera ME, López-Delgado HA. Potato virus X (PVX) elimination as short and long term effects of hydrogen peroxide and salicylic acid is differentially mediated by oxidative stress in synergism with thermotherapy. Am J Potato Res. 2016;93:360–367. doi: 10.1007/s12230-016-9509-5. [DOI] [Google Scholar]
- 72.Spiegel A, Stein A, Tam Y. In vitro thermotherapy of Rosaceous fruit trees. Acta Hortic. 1995;386:419–420. doi: 10.17660/ActaHortic.1995.386.57. [DOI] [Google Scholar]
- 73.Koubouris GC, Maliogka VI, Efthimiou K, Katis NI, Vasilakakis MD. Elimination of Plum pox virus through in vitro thermotherapy and shoot tip culture compared to conventional heat treatment in apricot cultivar Bebecou. J Gen Plant Pathol. 2007;73:370–373. doi: 10.1007/s10327-007-0028-6. [DOI] [Google Scholar]
- 74.Zilka S, Faingersh E, Rotbaum A, Tam Y, Spiegel S, Malca N. In vitro production of virus-free pear plants. Acta Hortic. 2002;596:477–479. doi: 10.17660/ActaHortic.2002.596.79. [DOI] [Google Scholar]
- 75.Paprštein F, Sedlák J, Polak J, Svobodova L, Hassan M, Bryxiov M. Results of in vitro thermotherapy of apple cultivars. Plant Cell, Tissue Organ Cult. 2008;94:347–352. doi: 10.1007/s11240-008-9342-8. [DOI] [Google Scholar]
- 76.Cieślińska M, Zawadzka B. Preliminary results of investigation on elimination of viruses from apple, pear and raspberry using thermotherapy and chemotherapy in vitro. Phytopathology. 1999;17:41–48. [Google Scholar]
- 77.Panattoni A, D’Anna F, Cristani C, Triolo E. Grapevine vitivirus A eradication in Vitis vinifera explants by antiviral drugs and thermotherapy. J Virol Methods. 2007;146:129–135. doi: 10.1016/j.jviromet.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Xu PS, Niimi Y, Xu PS. Evaluation of virus-free bulblet production by antiviral and/or heat treatment in in vitro scale cultures of Lilium longiflorum ‘Georgia’ and L. × ‘Casablanca’. J Jpn Soc Hortic Sci. 1999;68:640–647. doi: 10.2503/jjshs.68.640. [DOI] [Google Scholar]
- 79.Nascimento LC, Pio-Ribeiro G, Willadino L, Andrade GP. Stock indexing and potato virus Y elimination from potato plants cultured in vitro. Sci Agricola. 2003;60:525–530. doi: 10.1590/S0103-90162003000300017. [DOI] [Google Scholar]
- 80.Paunovic S, Ruzic D, Vujovic T, Milenkovic S, Jevremovic D. In vitro production of Plum pox virus-free plums by chemotherapy with ribavirin. Biotechnol Biotechnol Equip. 2007;21:417–421. doi: 10.1080/13102818.2007.10817486. [DOI] [Google Scholar]
- 81.Paprštein F, Sedlák J, Svobodová L, Polák J, Gadiou S. Results of in vitro chemotherapy of apple cv. Fragrance. Hortic Sci (Prague) 2013;40:186–190. doi: 10.17221/37/2013-HORTSCI. [DOI] [Google Scholar]
- 82.Vivek M, Modgil M. Elimination of viruses through thermotherapy and meristem culture in apple cultivar ‘Oregon Spur-II’. Virus Dis. 2018;29:75–82. doi: 10.1007/s13337-018-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watanabe M, Suzuki M, Komori S, Bessho H. Seasonal changes of IAA and Cytokinin concentration in shoots of columnar type apple trees. Acta Hortic. 2008;774:75–80. doi: 10.17660/ActaHortic.2008.774.8. [DOI] [Google Scholar]
- 84.Valero M, Ibanez A, Morte A. Effects of high vineyard temperatures on the grapevine leafroll associated virus elimination from Vitis vinifera L. cv. Napoleon tissue cultures. Sci Hortic. 2003;97:289–296. doi: 10.1016/S0304-4238(02)00212-1. [DOI] [Google Scholar]
- 85.Tomassoli L, Lernia GD, Tiberini A, Chiota G, Catenaro E, Barba M. In vitro thermotherapy and shoot-tip culture to eliminate Caper latent virus in Capparis spinosa. Phytopathol Mediterr. 2008;47:115–121. [Google Scholar]
- 86.Maliogka VI, Skiad FG, Eleftheriou EP, Katis NI. Elimination of a new ampelovirus (GLRaV-Pr) and Grapevine rupestris stem pitting associated virus (GRSPaV) from two Vitis vinifera cultivars combining in vitro thermotherapy with shoot tip culture. Sci Hortic. 2009;123:280–282. doi: 10.1016/j.scienta.2009.08.016. [DOI] [Google Scholar]
- 87.Perotto MC, Conci VC, Cafrune EE, Alochis P, Brcamonte R. Difference in the responses of garlic cultivars to the eradication of five viruses. Phyton-Rev Int Bot Exp. 2003; 233–240.
- 88.Nesi B, Trinchello D, Lazzereschi S, Grassotti A. Production of Lily Symptomless virus-free plants by shoot meristem tip culture and in vitro thermotherapy. HortScience. 2009;44:217–219. [Google Scholar]
- 89.Acquadro A, Papanice MA, Lanteri S, Bottalico G, Portis E, Campanale A, Finetti-Sialer MM, Mascia T, Sumerano P, Gallitelli D. Production and fingerprinting of virus-free clones in a reflowering globe artichoke. Plant Cell, Tissue Organ Cult. 2010;100:329–337. doi: 10.1007/s11240-009-9654-3. [DOI] [Google Scholar]
- 90.Zhang ZB, Lee YK, Spetz C, Clarke JL, Wang Q-C, Blystad D-R. Invasion of shoot apical meristems by Chrysanthemum stunt viroid differs among Argyranthemum cultivars. Front Plant Sci. 2016 doi: 10.3389/fpls.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Panattoni A, Triolo E. Susceptibility of grapevine viruses to thermotherapy on in vitro collection of Kober 5BB. Sci Hortic. 2010;125:63–67. doi: 10.1016/j.scienta.2010.03.001. [DOI] [Google Scholar]
- 92.Chalak L, Elbeaino T, Elbitar M, Fattal T, Choueiri E. Removal of viruses from Lebanese fig varieties using tissue culture and thermotherapy. Phytopathol Mediterr. 2015;54:531–535. [Google Scholar]
- 93.Wang M-R, Li B-Q, Feng C-H, Wang Q-C. Culture of shoot tips from adventitious shoots can eradicate Apple stem pitting virus but fails in Apple stem grooving virus. Plant Cell, Tissue Organ Cult. 2016;125:283–291. doi: 10.1007/s11240-016-0948-y. [DOI] [Google Scholar]
- 94.Scowcroft WR. Genetic variability in tissue culture: impact on germplasm conservation and utilization. Rome: IBPGR; 1984. [Google Scholar]
- 95.Dobránszki J, Teixeira da Silva JA. Micropropagation of apple—a review. Biotechnol Adv. 2010;28:462–488. doi: 10.1016/j.biotechadv.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 96.Ram R, Verma N, Singh AK, Zaidi AA. Virus-free chrysanthemums: production and quality management. Arch Phytopathol Plant Prot. 2009;42:940–949. doi: 10.1080/03235400701541396. [DOI] [Google Scholar]
- 97.Cooper VC, Walkey DGA. Thermal inactivation of cherry leaf roll virus in tissue cultures of Nicotiana rustica raised from seeds and meristem tips. Ann Appl Biol. 1978;88:273–278. doi: 10.1111/j.1744-7348.1978.tb00706.x. [DOI] [Google Scholar]
- 98.Liu J, Zhang X, Zhang F, Hong N, Wang G, Wang A, Wang L. Identification and characterization of microRNAs from in vitro-grown pear shoots infected with Apple stem grooving virus in response to high temperature using small RNA sequencing. BMC Genom. 2015;16:945. doi: 10.1186/s12864-015-2126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Szittya G, Silhavy D, Molnár A, Havelda Z, Lovas Á, Lakatos L, Bánfalvi Z, Burgyán J. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 2003;22:633–640. doi: 10.1093/emboj/cdg74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qu F, Ye X, Hou G, Sato G, Clemente TE, Morris TJ. RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in Nicotiana benthamiana. J Virol. 2005;9:15209–15217. doi: 10.1128/JVI.79.24.15209-15217.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chellappan P, Vanitharani R, Ogbe F, Fauquet CM. Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol. 2005;138:1828–1841. doi: 10.1104/pp.105.066563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu J, Zhang X, Yang Y, Hong N, Wang G, Wang A, Wang L. Characterization of virus-derived small interfering RNAs in Apple stem grooving virus-infected in vitro-cultured Pyrus pyrifolia shoot tips in response to high temperature treatment. Virol J. 2016;13:166. doi: 10.1186/s12985-016-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bazzini AA, Hopp HE, Beachy RN, Asurmendi S. Infection and coaccumulation of tobacco mosaic virus proteins alter microRNA levels, correlating with symptoms and plant development. Proc Natl Acad Sci USA. 2007;104:12157–12162. doi: 10.1073/pnas.0705114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–687. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 105.Naqvi AR, Haq QM, Mukherjee SK. MicroRNA profiling of tomato leaf curl New Delhi virus infected tomato leaves indicates that deregulation of mir159/319 and mir172 might be linked with leaf curl disease. Virol J. 2010;7:281–296. doi: 10.1186/1743-422X-7-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abreu PMV, Gaspar CG, Buss DS, Ventura JA, Ferreira PCG, Fernandes PMB. Carica papaya MicroRNAs are responsive to Papaya meleira virus infection. PLoS ONE. 2014;9:7e103401. doi: 10.1371/journal.pone.0103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simpkins I, Walkey DGA, Neely HA. Chemical suppression of virus in cultured plant tissues. Ann Appl Biol. 1981;99:161–169. doi: 10.1111/j.1744-7348.1981.tb05143.x. [DOI] [Google Scholar]
- 108.Fraser RSS, Gerwitz A. Effect of hydroxybenzyl benzimidazole on Tobacco mosaic virus and host RNA synthesis in tobacco leaf disc plants. Plant Sci Lett. 1984;34:111–117. doi: 10.1016/0304-4211(84)90133-0. [DOI] [Google Scholar]
- 109.Schulze S, Kluge S. The mode of inhibition of TMV and PVX-induced RNA-dependent RNA polymerase by some antiphytoviral drugs. J Phytopathol. 1994;141:77–85. doi: 10.1111/j.1439-0434.1994.tb01447.x. [DOI] [Google Scholar]
- 110.Xi Z, Zhang R, Yu Z, Ouyang D. The interaction between tylophorine B and TMV next term RNA. Bioorg Med Chem Lett. 2006;16:4300–4304. doi: 10.1016/j.bmcl.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 111.Xia Y, Fan Z, Yao J, Liao Q, Li W, Qua F, Peng L. Discovery of bitriazolyl compounds as novel antiviral candidates for combating the Tobacco mosaic virus. Bioorg Med Chem Lett. 2006;16:2693–2698. doi: 10.1016/j.bmcl.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 112.Zhao WG, Wang JG, Li ZM, Yang Z. Synthesis and antiviral activity against Tobacco mosaic virus and 3DQSAR of α-substituted-1,2,3-thiadiazoleacetamides. Bioorg Med Chem Lett. 2006;16:6107–6111. doi: 10.1016/j.bmcl.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 113.Dawson WO, Lozoya SH. Examination of the mode of action of ribavirin against tobacco mosaic virus. Intervirology. 1984;22:77–84. doi: 10.1159/000149537. [DOI] [PubMed] [Google Scholar]
- 114.Crotty S, Maag D, Arnold JJ, Zhong W, Lau JY, Hong Z, Andino R, Cameron CE. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 115.Crotty S, Cameron CE, Andino R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci USA. 2001;98:6895–6900. doi: 10.1073/pnas.111085598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vieira RL, da Silva AL, Zaffari GR, Steinmacher DA, de Freitas Fraga HP, Guerra MP. Efficient elimination of virus complex from garlic (Allium sativum L.) by cryotherapy of shoot tips. Acta Physiol Plant. 2015;37:1733. doi: 10.1007/s11738-014-1733-3. [DOI] [Google Scholar]
- 117.Boonham N, Kreuze J, Winter S, van der Vlugt R, Bergervoet J, Tomlinson J, Mumford R. Methods in virus diagnostics: from ELISA to next generation sequencing. Virus Res. 2014;186:20–31. doi: 10.1016/j.virusres.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 118.Balamuralikrishnan M, Doraisamy S, Ganapathy T, Viswanathan R. Impact of serial thermotherapy on sugarcane mosaic virus titre and regeneration in sugarcane. Arch Phytopathol Plant Prot. 2003;36:173–178. doi: 10.1080/03235400310001595630. [DOI] [Google Scholar]
- 119.Silva JM, Carnelossi PR, Bijora T, Facco CU, Picoli MH, Souto ER, Oliveira AJB, Almeida ÁM. Immunocapture-RT-PCR detection of Cassava common mosaic virus in cassava obtained from meristem-tip culture in Paraná state. Trop Plant Pathol. 2011;36:271–275. doi: 10.1590/S1982-56762011000500001. [DOI] [Google Scholar]
- 120.Conci VC, Perotto MC, Cafrune E, Lunello P. Program for intensive production of virus-free garlic plants. Acta Hortic. 2005;688:195–200. doi: 10.17660/ActaHortic.2005.688.25. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.