Abstract
Background
Studies investigating salivary cortisol level as susceptibility marker for post-traumatic stress disorder (PTSD) produced inconsistent results. The aim of this study was to compare salivary cortisol concentration levels in PTSD patients with those in controls by synthesizing published data.
Methods
We did a systematic review, meta-analysis and meta-regression of studies comparing concentrations of salivary cortisol between patients with PTSD and controls. The electronic databases of PubMed, Embase, Web of Science and Psyc-ARTICLES were searched for relevant articles. A random-effects model with restricted maximum-likelihood estimator is used to synthesize the effect size (assessed by standardized mean difference).
Results
A total of 784 articles were identified of which 22 were included in the final analysis. A trend of lower salivary cortisol levels was found in PTSD patients when compared with the controls (SMD = − 0.28, 95% CI-0.53;-0.04, p = 0.022). Subgroup analysis showed that the salivary cortisol levels were lower in patients with PTSD than in controls in studies conducted after 2007 or in studies using saliva samples collected in the morning.
Conclusions
The evidence from this meta-analysis supports that salivary samples collected in the morning consistently showed a lower salivary cortisol level in patients with PTSD than in controls, although whether salivary cortisol could be used as a diagnostic tool requires further research.
Electronic supplementary material
The online version of this article (10.1186/s12888-018-1910-9) contains supplementary material, which is available to authorized users.
Keywords: Post-traumatic stress disorder, Salivary cortisol, Systematic review, Meta-analysis
Background
Post-traumatic stress disorder (PTSD) is a common psychiatric and anxiety disorder caused by traumatic events [1]. It has a negative impact on the physical and mental health of the affected patients [2], as well as their professional and social life [3, 4], which may impose a large burden on patients’ families and society. Indeed, PTSD is a kind of complex disease which may also be related to genetic factors (internal factors) and environmental factors (external factors) [5, 6]. Its etiology and pathogenesis have not been fully understood [6]. However, there is plenty of evidence that PTSD might be attributed to the disorder of the Hypothalamic-Pituitary-Adrenal axis (HPA axis) [7, 8]. Cortisol is an adrenal glucocorticoid hormones secreted by the zona fasciculata in the adrenal cortex [9, 10]. It is the end product of the HPA axis in humans. When faced with stressors the body may produce a corresponding stress response [11]. At the same time the body may secrete large amounts of cortisol to inhibit stress response by metabolic action, which in turn restores the body back to its normal functionality [12, 13]. However, if the body has been in a state of high pressure which stimulates its stress response too often, then this may lead to passivation of the HPA axis [10, 14]. Moreover, if the HPA axis is not restored to normal, then abnormal cortisol levels may arise in patients with PTSD [15]. Therefore, cortisol could be used as a biomarker for patients with PTSD [16].
Various biological specimens, including plasma, serum, saliva, cerebrospinal fluid and urine are used to measure cortisol [12, 17]. In addition, acquisition process of salivary measurement has been proposed as a noninvasive method [18]. We focus on the salivary cortisol mainly for the consideration of practice applications. Salivary cortisol is more readily available than urine specimens, and is more convenient to collect at home for large scale epidemiological studies [19].
A much debated question is whether salivary cortisol could be used as a susceptibility marker for PTSD patients [20]. Studies investigating salivary cortisol as a susceptibility marker for PTSD patients have produced different results [12], which may be attributed to the differences in investigation time, sampling time, type of trauma, assessment tools for PTSD symptoms, and collection and analysis of salivary cortisol [21, 22].
There has been no meta-analysis specifically targeted on salivary cortisol as a susceptibility marker for PTSD patients, although some previous studies have included salivary cortisol in subgroup analyses [12].
The purpose of this study is to compare salivary cortisol levels between PTSD patients and controls using a meta-analysis of existing studies. We also used regression and subgroup analyses to explore the sources of heterogeneity among studies [23].
Methods
Identification and selection of studies
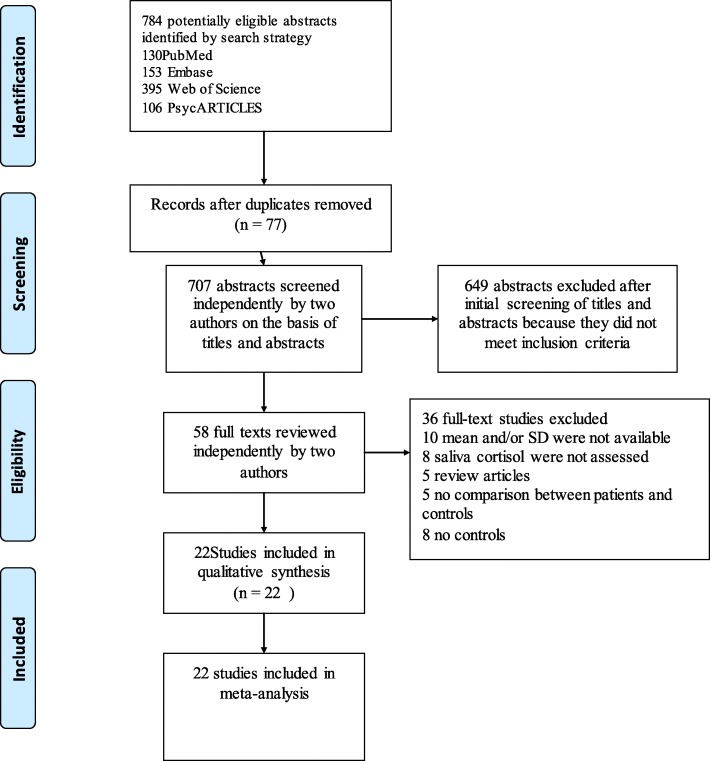
PRISMA guidelines were used for this systematic review and meta-analysis [24]. Online electronic databases were searched from September 1987 until September 2017 for articles published in English. These included PubMed, Embase, Web of Science and Psyc-ARTICLES. Experienced librarians designed these searches, which used the following keywords:(((((((((((cortisol in saliva [Title/Abstract]) OR saliva cortisol [Title/Abstract]) OR glucocorticoids in saliva[Title/Abstract]) OR saliva glucocorticoid[Title/Abstract])OR steroid hormones in saliva [Title/Abstract]) OR saliva steroid [Title/Abstract]) OR corticosteroids in saliva[Title/Abstract]) OR saliva corticosteroid[Title/Abstract])) OR Salivary Cortisol[Title/Abstract]))AND((PTSD[Title/Abstract]) OR Posttraumatic Stress Disorder[Title/Abstract]). These terms were adapted for the other databases and the detailed search strategies are shown in the Additional file 1. The detailed search strategies are shown in the Fig. 1.
Fig. 1.
Study selection
Eligibility criteria
Two researchers independently screened and selected the relevant articles. In case of disagreement the final decision was made after consultation with a third party [25]. Moreover, the grey literatures were not included in our study. Primary inclusion criteria for articles for this study were: the study included PTSD cases and control groups, reported PTSD diagnostic criteria, and reported the mean and standard deviation (SD) of salivary cortisol. Exclusion criteria were: studies investigating PTSD with other comorbid disorders, or HPA axis disorder and review articles [26].
Data extraction
This study used Note Express of Central South University in order to gain insights into analyses, and manage articles. Trauma-exposed controls (TC) and non-trauma-exposed controls (NTC) were all eligible as control groups. Since most studies reported TC for 81.8% (18/22), we chose TC as control groups when both TC and NTC were included. In addition, we analyzed the relation between PTSD and all NTC as control groups as a supplement [27]. The following characteristics were extracted from each eligible article: first author, publication year, sex, ages of participants, participant number, salivary cortisol concentration level mean ± standard deviation, country where the study was conducted (study country), trauma type [28], PTSD assessment method, saliva collection time [28], salivary cortisol collection and assay methods, inter-assay variation, intra-assay variation, sensitivity and T-frozen (temperature of frozen). Salivary cortisol unit conversion which we used was 1 g/dl = 27.59 nmol/L [29].
Statistical analyses
All analyses were carried out using R software (version R i386 3.4.2). Meta-analysis and meta-regression analysis were performed using R software with package metafor [30]. The random-effects model with restricted maximum-likelihood estimator was used to synthesize the effect size in the studies. Standardised mean difference (SMD) was used to assess the effect size, calculated by using Cohen’s d. Significance level was set at 0.05 for all statistical tests. If the SMD level was ≤0.2 then it was considered low effect; if it was 0.2–0.7 then moderate effect; if it was ≥0.8 then large effect [31, 32]. Begg’s rank correlation test was used to check publication bias. The Q statistic was used to test the presence of heterogeneity and the I2 statistic was used to quantify the percentage of variability. Maximal heterogeneity is indicated by an I2 = 100% whereas no heterogeneity is indicated by an I2 = 0 [33]. In addition, subgroup analyses were conducted with regard to saliva collection time and study year. We also performed meta-regression analysis to explore other sources of heterogeneity. The following 8 variables were included in this analysis: Country (USA = 1,other = 0), PTSD assessment (DSM-IV CAPS = 1, other = 0), collection time (am = 1,pm = 0), publication year (study year≥2007 = 1, < 2007 = 0), assayed methods (report = 1,Unreported = 0), inter-assay variation (report = 1,Unreported = 0), intra-assay variation (report = 1,Unreported = 0), sensitivity (report = 1,Unreported = 0) and frozen (report = 1,Unreported = 0).
Results
Literature search
Literature search produced an aggregate of 784 relevant articles of which 130 were from PubMed, 153 from Embase, 395 from Web of Science and 106 from PsycARTICLES. Of these, 77 were excluded because they were duplicates. Further assessment of abstracts of the 707 remaining articles resulted in 649 exclusions for failing to meet the inclusion criteria. The 58 full articles left were reviewed by two authors independently. Then 10 articles were excluded for not reporting means (SDs), 8 articles were excluded for not assessing saliva cortisol, 5 articles were excluded because they were review articles, 5 articles were excluded for not reporting results comparing patients and controls, and 8 articles were excluded for not reporting results in controls. In the end, 22 eligible articles were included for this study (Fig. 1).
Characteristics of eligible articles
Table 1 presents characteristics of the 22 eligible studies. Most articles reported saliva collection time, salivary cortisol collection and assay methods, inter-assay variation, intra-assay variation, sensitivity and T-frozen.
Table 1.
Characteristics of studies included in the meta-analysis
| Study | N | Country | Trauma type | Controls | Female | Mean Age | PTSD Assessment | Collection time | Assayed Methods | Interassay variation | Intra-assay variation | Sensitivity | T-frozen |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carrion 2001 [43] | 51 | USA | Mixed trauma | NTC | 21 | 10.7 | DSM-IV Reaction Index | AM,PM | RIA | 12% | NR | NR | -20 °C |
| Coupland 2003 [44] | 66 | Canada | Abuse | NTC | 66 | 38 ± 11 | DSM-IV CAPS-1 | AM,PM | ELISA | 2% | 8% | 0.05 mg/dl. | −80 °C |
| Feldman 2013 [45] | 232 | Israeli | Combat | TC,NTC | 110 | 1.5–5.0 | Clinicians diagnose | AM | ELISA | 10.5% | 13.4% | NR | −20 °C |
| Gill 2008 [46] | 71 | USA | Civilian trauma | TC,NTC | 71 | 42.9 ± 7.82 | DSM-IV CAPS | PM | ELISA | 9% | 9% | NR | −80 °C |
| Kloet 2006 [47] | 83 | Netherland | Combat | TC,NTC | 0 | 34.1 ± 5.8 | DSM-IV CAPS | AM,PM | RIA | 4% | 5.5–9% | NR | −80 °C |
| Kobayashi 2014 [48] | 39 | USA | Injury | TC | 7 | 40.3 ± 10.7 | DSM-IV CAPS | AM,PM | RIA | NR | NR | NR | −20 °C |
| Lindauer 2006 [49] | 24 | Netherland | Mixed trauma | TC | 10 | 35.1 ± 11.4 | DSM-IV CAPS | AM,PM | RIA | 10% | 10% | NR | −20 °C |
| Lipschitz, DS 2003 [50] | 48 | USA | Mixed trauma | TC,NTC | 37 | 16.4 ± 2.6 | DSM-IV CTQ | AM | RIA | 8.00% | 9.00% | NR | −80 °C |
| Mcfarlane 2011 [51] | 48 | Australia | Traumatic accident | TC | 12 | 34 ± 12.7 | DSM-IV CAPS | AM,PM | RIA | NR | NR | NR | − 20 °C |
| Neylan 2009 [52] | 22 | USA | Combat | TC | 0 | 51.1 ± 2.5 | DSM-IV SCID | AM | NR | NR | NR | NR | NR |
| Tucker 2010 [53] | 100 | USA | Bombing registry | TC,NTC | 54 | 47.0 ± 10.0 | DSM-IV DIS | AM | RIA | NR | NR | NR | −20 °C |
| Roth 2007 [54] | 218 | Sweden | Combat | TC | 122 | NR | DSM-IV HTQ | AM | RIA | 10% | 10% | 0.8 nmol/l | −70 °C |
| Shalev 2007 [55] | 155 | Israel | Road traffic accidents | TC | 64 | 31.2 ± 11.6 | DSM-IV CAPS | AM | NR | NR | NR | NR | −40 °C |
| Su, T 2009 [56] | 27 | China | Mixed trauma | NTC | 2 | 43.15 ± 12.8 | DSM-IV CAPS | AM | RIA | 3% | 6% | 10 pg/tube | −80 °C |
| Wahbeh 2013 [57] | 71 | USA | Combat | TC | 0 | 55.5 ± 8.9 | DSM-IV CAPS | AM,PM | ELISA | 4.74% | 3.03% | NR | NR |
| Witteveen, AB 2010 [58] | 1880 | Netherlands | Mixed trauma | TC | 141 | 47.0 ± 8.0 | DSM-IV CAPS | AM,PM | RIA | NR | NR | NR | −20 °C |
| Yehuda 2005 [59] | 63 | USA | Holocaust | TC,NTC | 36 | 69.7 ± 5.0 | DSM-IV CAPS | AM,PM | RIA | 3.90% | 12.00% | 10 ng/dl | NR |
| Yehuda, R 2005 [60] | 67 | USA | Holocaust | TC,NTC | 34 | 68.5 ± 5.9 | DSM-IV CAPS | AM,PM | RIA | 3.90% | 12.00% | 10 ng/dl | NR |
| Young, EA 2004 [61] | 516 | USA | Mixed trauma | TC,NTC | 457 | 36.8 ± 2.2 | DSM-III | AM,PM | NR | 6.50% | 5.00% | 1 ng/mL | −20 °C |
| Young 2004 [62] | 171 | USA | Mixed trauma | TC,NTC | 171 | 18–54 | DSM-III | AM,PM | NR | 10% | NR | 2 μg/dL | −20 °C |
| Steven 2004 [63] | 34 | USA | Childhood trauma | NTC | 30 | 40.3 ± 3.3 | DSM-IV CAPS | AM | ELISA | 5.70% | 6.90% | 7 μg/dL | NR |
| Neylan 2003 [64] | 32 | USA | Combat | TC | 0 | 49.4 ± 5.7 | DSM-IV CAPS | AM | NR | NR | NR | NR | NR |
TC Trauma-exposed controls, NTC Non-trauma-exposed controls, RIA Radioimmunoassay, ELISA Enzyme linked immunosorbent assay, CAPS Clinician-administered PTSD scale, NR Not report, USA United States of America, T-frozen Temperature of frozen
Salivary cortisol levels in PTSD as compared with controls
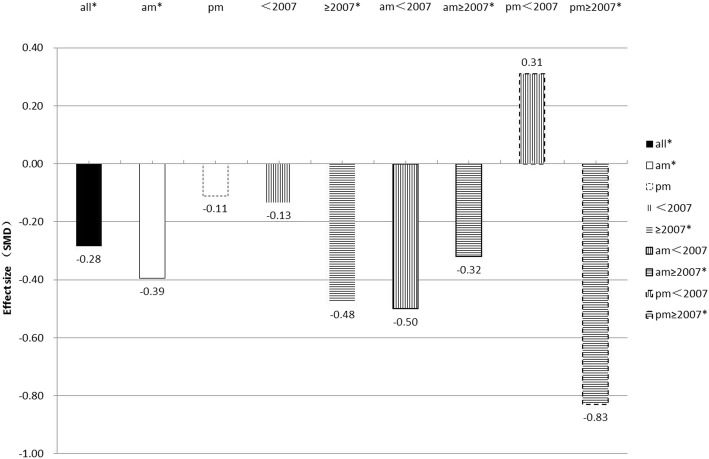
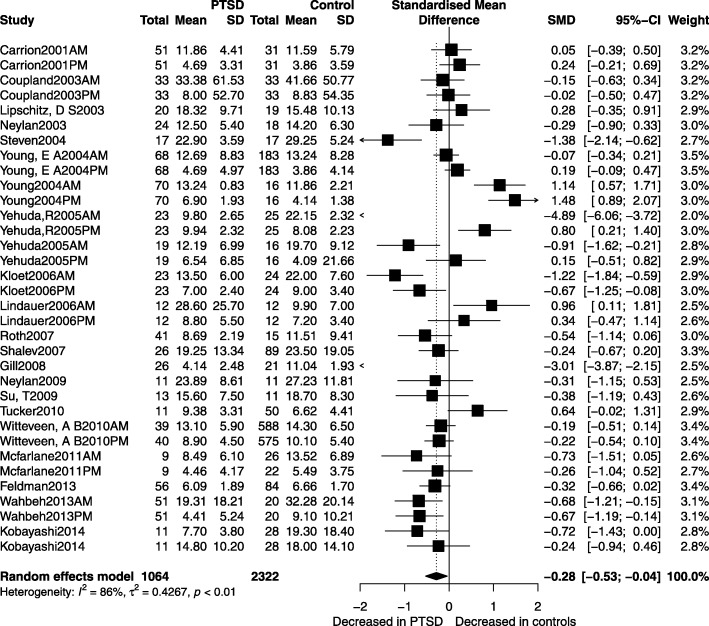
Table 2 displays the summarized salivary cortisol concentration levels between PTSD patients and the controls. A trend showing lower salivary cortisol concentration levels was observed in PTSD patients as compared to the controls (SMD = − 0.28, 95% CI -0.53; − 0.04, p = 0.022). There was also an overall difference in the pooled effect size between people with PTSD and NTC (SMD = − 0.33, 95% CI -0.63; − 0.03, p = 0.032). Figures 2 and 3 compare the salivary cortisol effect sizes (SMD) of studies using samples collected in the morning (am) with those of studies using samples collected in the afternoon (pm). The same was also compared between studies conducted before 2007 and those conducted after 2007. It should be noted that a recent similar systematic review and meta-analysis on adults was published in 2007 and it performed subgroup analysis of salivary cortisol in PTSD [12]. Since then, there has been a rapid development of various salivary cortisol analyzers. Therefore, we wanted to know whether the situation had changed 10 years later after the publication of that systematic review and meta-analysis in 2007. Hence, we performed subgroup analysis related to the eligible studies published before 2007, and those published after 2007. The results of subgroup analyses showed that the differences in salivary cortisol concentration levels between PTSD patients and the controls was bigger in studies that used samples collected in the morning than in those studies that used samples collected in the afternoon. Also, higher differences were observed in studies conducted after 2007 than in those conducted before 2007. Moreover, the biggest difference in salivary cortisol concentration level was observed in studies that used morning samples and those conducted after 2007. No differences were found for afternoon samples (p = 0.598), whereas in the morning people with PTSD had lower levels of cortisol than controls (SMD = − 0.39, 95% Cl − 0.70; − 0.09, p = 0.012). Whereas the studies conducted before 2007 did not reveal significant differences (p = 0.479), PTSD in studies conducted after 2007 had highly significant lower salivary cortisol levels than their comparison groups (SMD = − 0.48, 95% Cl − 0.75; − 0.20, p = 0.001).
Table 2.
Meta-analysis of salivary cortisol markers in PTSD
| Participants with PTSD, n | controls n | SMD (95% CI) | p value | Heterogeneity | Begg’S test Kendall’s tau statistic (p value) | |||
|---|---|---|---|---|---|---|---|---|
| Q statistic (DF; p value) | τ2 | I2 | ||||||
| all | 1064 | 2322 | −0.28 (−0.53; −0.04) | 0.022 | 236.00(33 < 0.0001) | 0.427 | 86.00% | −0.1907, p = 0.117 |
| am | 628 | 1316 | −0.39 (− 0.70; − 0.09) | 0.012 | 132.68(20 < 0.0001) | 0.412 | 84.90% | −0.2000, p = 0.219 |
| pm | 436 | 1006 | −0.11 (− 0.52; 0.30) | 0.598 | 96.40(12 < 0.0001) | 0.476 | 87.60% | −0.1795, p = 0.435 |
| <2007 | 659 | 734 | −0.13 (− 0.50; 0.23) | 0.479 | 163.59(18 < 0.0001) | 0.568 | 89.00% | −0.2281, p = 0.186 |
| ≥2007 | 405 | 1588 | −0.48 (− 0.75; − 0.20) | 0.001 | 52.71(14 < 0.0001) | 0.199 | 73.40% | −0.2952, p = 0.140 |
PTSD post-traumatic stress disorder, SMD standardised mean difference, DF degrees of freedom
Fig. 2.
Salivary cortisol effect size. Salivary cortisol effect size (SMD) for studies examining in the morning (am), afternoon (pm), before 2007, and after 2007 levels in PTSD and control groups. PTSD,posttraumatic stress disorder; am, morning (before 12 pm); pm, afternoon (after 12 pm);<2007,before 2007;≥2007, after 2007;*p < 0.05
Fig. 3.
Meta-analyses of salivary cortisol
Meta-regression analyses
Table 3 presents the results of meta-regression analysis. It shows that country of study, sample collection time, study year, saliva cortisol assayed instrument reporting method, inter-assay variation reporting, intra-assay variation reporting, sensitivity reporting and frozen sample reporting were not significantly different. Recall that the meta-regression analysis was used to explore sources of heterogeneity among the eligible studies. If results indicate not significantly different, it means the variables in question cannot explain the overall heterogeneity. However, in our case, after introducing PTSD assessment methods into the meta-regression analysis model, results showed that sources of heterogeneity can be explained by PTSD assessment methods as the difference was significant. (b = − 0.812, 95%CI -1.540;-0.084, p = 0.0288).
Table 3.
Separate univariate meta-regression model of salivary cortisol in PTSD, PTSD = post-traumatic stress disorder
| Estimate | se | zval | pval | ci.lb | ci.ub | |
|---|---|---|---|---|---|---|
| Collection time | − 0.306 | 0.362 | − 0.844 | 0.399 | −1.015 | 0.404 |
| Country | −0.085 | 0.362 | −0.236 | 0.814 | −0.795 | 0.624 |
| Publication year | −0.352 | 0.351 | −1.002 | 0.316 | −1.041 | 0.337 |
| PTSD assessment | −0.812 | 0.371 | −2.187 | 0.029 | −1.540 | −0.084 |
| Assayed methods | −0.344 | 0.384 | −0.896 | 0.370 | −1.097 | 0.409 |
| Inter-assay variation | −0.090 | 0.391 | −0.229 | 0.819 | −0.857 | 0.677 |
| Frozen | 0.686 | 0.390 | 1.762 | 0.078 | −0.077 | 1.450 |
Heterogeneity and bias analysis
Heterogeneity was reported to be high among the eligible studies (I2 > 75%). However, for the subgroup analysis stratified by study year, the combined heterogeneity of studies declined from 92 to 33%. This change in heterogeneity indicated that studies conducted after 2007 had more consistent and homogeneous results.
Begg’s rank correlation test revealed no potential publication bias (p = 0.117), implying that there was low probability of publication bias.
Discussion
Generally, concentrations of salivary cortisol were lower in patients with PTSD than in the controls (SMD = − 0.28, 95% CI -0.53; − 0.04, p = 0.022). There was also an overall difference in the pooled effect size between people with PTSD and NTC. Specifically, our findings suggest that PTSD status affects basal salivary cortisol levels; some people do not develop PTSD despite experiencing a trauma similar to that of PTSD patients [34]. The exact biological mechanisms underlying the altered long-term salivary cortisol output as a result of trauma remain largely unknown [35]. Speculatively, decreased output of salivary cortisol after developing PTSD may evolve as a compensatory anti-glucocorticoid mechanism [16, 36], to inhibit negative effects of long-term increased negative glucocorticoid feedback sensitivity of glucocorticoid receptors that have been observed in PTSD patients irrespective of trauma-exposed status. However, this remains to be further investigated [16].
As far as we know, this is the first study to perform systematic review, meta-analysis and meta-regression on the salivary cortisol concentration levels in PTSD, particularly by considering studies reporting salivary cortisol as a susceptibility marker for PTSD. Nonetheless, there are three previous systematic reviews and meta-analyses which investigated the relationship between Cortisol and post-traumatic stress disorder. One of which included only 7 salivary cortisol studies from two databases [12], another one only used 9 PTSD salivary cortisol subgroup studies [16], and the next one used only 3 salivary cortisol studies [20]. These studies found no relationship between cortisol concentration levels and PTSD. In addition, these studies did not take into account the sample source from which cortisol concentration levels were measured. For example, they included plasma/serum, saliva or urine samples, even if it is well known that the cortisol concentration levels varied from sample to sample between sufficient samples from different sources. The use of small sample sizes might have contributed to the results of no significant relationship between cortisol concentration levels and PTSD. Noteworthy, the methods for collecting salivary cortisol, testing and analyzing cortisol concentration levels have improved in more recent years. These include immunoassay or liquid chromatography-tandem mass spectrometry (LC-MS/MS) for the measurement of salivary cortisol [37, 38]. Therefore, there is need to update literature to include these new methods when investigating the relationship between cortisol concentration levels and PTSD. Accordingly, in this study, we enlarged the scope of article searching in the online electronic databases, which eventually yielded 22 eligible studies which collectively had 1064 participants with PTSD and 2322 controls. Thus, compared with the preceding previous studies, this study used relatively larger sample size which would make conclusions more comparable and convincing. Also, unlike in the preceding studies, this study focused only on salivary cortisol concentration levels when investigating its association with PTSD because results of the same investigation, using different sample sources for cortisol concentration levels, may be problematic to interpret since cortisol concentration levels differ with different sample sources. Thus, using the 22 eligible studies, some interesting significant results were found. In the analysis according to whether saliva samples were taken in the morning or in the afternoon, it was found that PTSD patients had lower levels of salivary cortisol than controls in studies which used saliva samples taken in the morning but not in those studies which used saliva samples taken in the afternoon. With regard to methodological aspects, and due to the pulsatile nature of adrenal steroid release, there are inherent limitations when using single-point measurements of basal salivary cortisol concentration levels [39]. More specifically, it is known that the cortisol release follows a circadian rhythm such as cortisol awakening response [40]. Additionally, specific basal morning salivary cortisol concentration levels seem to be lower in PTSD patients, while basal cortisol concentration level assessments at other times during the day do not seem to be associated with PTSD. Therefore, it could be speculated that PTSD patients’ cortisol awakening response was insensitive [40], whereas that of the control groups was more nimble in the morning. Considering the preceding cortisol awakening response hypothesis, it could be suggested that the ideal time for morning saliva sampling is after awakening. We suggest that researchers control for waking when saliva samples are to be collected in the morning in practical investigation (by getting people to wake at the same time, e.g. 8.00 am).
Moreover, the indicator of publication year is selected through the group discussion and the literature review, we want to evaluate that whether the previous mixed findings on cortisol levels in PTSD could be due to time of sample collection and older assays (prior to 2007). Since the recent meta-analysis article was appear in 2007 which included subgroup analysis to distribution and function of salivary cortisol in PTSD [12]. Furthermore, in the analysis according to whether studies were conducted before 2007 or after 2007, it was observed that the difference in salivary cortisol concentration levels between PTSD patients and controls was observed only in studies which were conducted after 2007. Results of Begg’s rank correlation test revealed low probability of publication bias.
In summary, this systematic review and meta-analysis found that concentrations of salivary cortisol were consistently and homogeneously lower in patients with PTSD than in the controls when the analysis included only studies conducted after 2007 and studies which used saliva samples collected in the morning. Future studies on salivary cortisol concentration levels in PTSD patients should take the following aspects into consideration:
① Saliva collection time should be unified. Considering the preceding cortisol awakening response hypothesis, it could be suggested that the ideal time for morning saliva sampling is after awakening. We suggest that researchers control for waking when saliva samples are to be collected in the morning in practical investigation (by getting people to wake at the same time, e.g. 8.00 am). Besides, the guidelines for assessment of the salivary cortisol must be strictly followed and these include: objective control of sampling adherence, participant instructions, sampling protocols and quantification strategies, as well as reporting and interpreting of salivary cortisol data [40].
② Storage methods of saliva must be unified. That is, the collected saliva sample should be stored at − 20 °C~ − 80 °C until assayed. Assay methods of saliva must be unified. We recommend using enzyme-linked immunoassay and radioimmunoassay; and inter-assay and intra-assay coefficients of variation of lower than 10%.
③ The meta-regression analysis had 8 variables, of which only one was significant: PTSD assessment method (Table 3). It means this variable can explain the overall heterogeneity. Thus, we recommend using DSM-IVdiagnostic criteria with the clinician-administered PTSD scale (CAPS). This scale has proved to show high degree of reliability and validity, and can provide a better reference for further study of PTSD and salivary cortisol [41]. It is expected that meeting these consensus guidelines in future research could create more powerful research designs that would yield reliable and reproducible data and results. Although no biomarkers have yet demonstrated clinical applicability for PTSD [23, 41], in future, we believe that salivary cortisol could be a quick biomarker assay to assist in screening patients for PTSD hence promising a possibility for screening a lot of people within a short time for PTSD. After screening, clinicians can then assess symptom severity by conducting clinical interviews with those suspected of having PTSD. On the one hand, as we know from natural disasters (like earthquake, hurricane, tsunami and flood) [42]; the affected population is generally large. Especially for developing countries such as China and India, with an enormous population density and limited psychiatrists, millions of people would be at high risk for PTSD after natural disasters. It can save a lot of clinicians and psychological guidance resources to enable the large-scale PTSD screening to become a reality.
Saliva is a specimen that is safe and easy to obtain in a large-scale epidemiological study. Therefore, development of rapid salivary cortisol test methods for PTSD screening could provide fast and cost-efficient means in large epidemiological studies, and in clinical practice, which could facilitate PTSD diagnosis, treatment, control and prevention.
Conclusions
The evidence from this meta-analysis supports that salivary samples collected in the morning consistently showed a lower salivary cortisol level in patients with PTSD than in controls, although whether salivary cortisol could be used as a diagnostic tool requires further research.
Additional file
Search strategies: details of search strategy. (DOC 27 kb)
Acknowledgements
We are grateful to Central South University Library for his assistance during the literature search. Besides, we are also thankful to Atipatsa C Kaminga for providing professional editing. Last but not least, we thank all the authors whose articles contributed an indispensable data for this systematic review and meta-analysis.
Funding
This study has no funding. The corresponding author is ultimately responsible for the decision to submit it for publication and make full use of all the data in the study by Central South University Library.
Availability of data and materials
Available upon request to the corresponding author Aizhong Liu: lazroy@live.cn.
Abbreviations
- <2007
Before 2007
- ≥2007
After 2007
- 95% CI
95% confidence interval
- Am
Morning (before 12 pm)
- CAPS
Clinician-administered PTSD scale
- DF
Degrees of freedom
- DSM-IV
Diagnostic and statistical manual of mental disorders, 4th edition
- ELISA
Enzyme linked immunosorbent assay
- NR
Not report
- NTC
Non-trauma-exposed Controls
- pm
Afternoon (after 12 pm)
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- PTSD
Post-traumatic stress disorder
- RIA
Radioimmunoassay
- SMD
Standardised mean difference
- TC
Trauma-exposed controls
Authors’ contributions
XP and AL contributed to the study design, while XP, ZW and XW contributed to the data collection. Statistical analyses and interpretation of results were performed by XP, ZW and XW. XP, AL and SW drafted the manuscript and edited the language. All the authors participated in the critical revision, and approved the final version of the manuscript.
Ethics approval and consent to participate
Ethical approval and participant consent were not applicable for this systematic review and meta-analysis, since this study involved data and materials from published articles.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kornor H, Winje D, Ekeberg O, Weisaeth L, Kirkehei I, Johansen K, et al. Early trauma-focused cognitive-behavioural therapy to prevent chronic post-traumatic stress disorder and related symptoms: a systematic review and meta-analysis. BMC Psychiatry. 2008;8:81. doi: 10.1186/1471-244X-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Prog Neuro-Psychoph. 2018;84(A):221–228. doi: 10.1016/j.pnpbp.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Heeke C, Stammel N, Heinrich M, Knaevelsrud C. Conflict-related trauma and bereavement: exploring differential symptom profiles of prolonged grief and posttraumatic stress disorder. BMC Psychiatry. 2017;17(1):118. doi: 10.1186/s12888-017-1286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knezevic M, Krupic D, Sucurovic S. Emotional competence and combat-related PTSD symptoms in Croatian Howeland war veterans. Drustvena Istrazivanja. 2017;26(1):1–18. doi: 10.5559/di.26.1.01. [DOI] [Google Scholar]
- 5.Rona RJ, Burdett H, Bull S, Jones M, Jones N, Greenberg N, et al. Prevalence of PTSD and other mental disorders in UK service personnel by time since end of deployment: a meta-analysis. BMC Psychiatry. 2016;16(1):333. doi: 10.1186/s12888-016-1038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lolk M, Byberg S, Carlsson J, Norredam M. Somatic comorbidity among migrants with posttraumatic stress disorder and depression - a prospective cohort study. BMC Psychiatry. 2016;16(1):447. doi: 10.1186/s12888-016-1149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: a meta-analysis. Psychoneuroendocrinology. 2012;37(3):317–331. doi: 10.1016/j.psyneuen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Savic D, Knezevic G, Damjanovic S, Spiric Z, Matic G. The role of personality and traumatic events in cortisol levels - where does PTSD fit in? Psychoneuroendocrinology. 2012;37(7):937–947. doi: 10.1016/j.psyneuen.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Lokhmatkina NV, Feder G, Blake S, Morris R, Powers V, Lightman S. Longitudinal measurement of cortisol in association with mental health and experience of domestic violence and abuse: study protocol. BMC Psychiatry. 2013;13:188. doi: 10.1186/1471-244X-13-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suliman S, Ericksen T, Labuschgne P, de Wit R, Stein DJ, Seedat S. Comparison of pain, cortisol levels, and psychological distress in women undergoing surgical termination of pregnancy under local anaesthesia versus intravenous sedation. BMC Psychiatry. 2007;7:24. doi: 10.1186/1471-244X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventura-Junca R, Symon A, Lopez P, Fiedler JL, Rojas G, Heskia C, et al. Relationship of cortisol levels and genetic polymorphisms to antidepressant response to placebo and fluoxetine in patients with major depressive disorder: a prospective study. BMC Psychiatry. 2014;14:220. doi: 10.1186/s12888-014-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 13.Savic D, Knezevic G, Damjanovic S, Spiric Z, Matic G. Is there a biological difference between trauma-related depression and PTSD? DST says ‘NO’. Psychoneuroendocrinology. 2012;37(9):1516–1520. doi: 10.1016/j.psyneuen.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Melin EO, Thunander M, Landin-Olsson M, Hillman M, Thulesius HO. Depression differed by midnight cortisol secretion, alexithymia and anxiety between diabetes types: a cross sectional comparison. BMC Psychiatry. 2017;17(1):335. doi: 10.1186/s12888-017-1495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fragkaki I, Thomaes K, Sijbrandij M. Posttraumatic stress disorder under ongoing threat: a review of neurobiological and neuroendocrine findings. Eur J Psychotraumatol. 2016;7:30915. doi: 10.3402/ejpt.v7.30915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology. 2017;77:261–274. doi: 10.1016/j.psyneuen.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Skoluda N, Linnemann A, Nater UM. The role of week(end)-day and awakening time on cortisol and alpha-amylase awakening responses. Stress. 2016;19(3):333–338. doi: 10.1080/10253890.2016.1174850. [DOI] [PubMed] [Google Scholar]
- 19.Olivera-Figueroa LA, Juster RP, Morin-Major JK, Marin MF, Lupien SJ. A time to be stressed? Time perspectives and cortisol dynamics among healthy adults. Biol Psychol. 2015;111:90–99. doi: 10.1016/j.biopsycho.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris MC, Hellman N, Abelson JL, Rao U. Cortisol, heart rate, and blood pressure as early markers of PTSD risk: a systematic review and meta-analysis. Clin Psychol Rev. 2016;49:79–91. doi: 10.1016/j.cpr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wild B, Stadnitski T, Wesche D, Stroe-Kunold E, Schultz JH, Rudofsky G, et al. Temporal relationships between awakening cortisol and psychosocial variables in inpatients with anorexia nervosa - a time series approach. Int J Psychophysiol. 2016;102:25–32. doi: 10.1016/j.ijpsycho.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Hankin BL, Badanes LS, Smolen A, Young JF. Cortisol reactivity to stress among youth: stability over time and genetic variants for stress sensitivity. J Abnorm Psychol. 2015;124(1):54–67. doi: 10.1037/abn0000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2(11):1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Samara MT, Goldberg Y, Levine SZ, Furukawa TA, Geddes JR, Cipriani A, et al. Initial symptom severity of bipolar I disorder and the efficacy of olanzapine: a meta-analysis of individual participant data from five placebo-controlled studies. Lancet Psychiatry. 2017;4(11):859–867. doi: 10.1016/S2215-0366(17)30331-0. [DOI] [PubMed] [Google Scholar]
- 26.Steudte S, Kirschbaum C, Gao W, Alexander N, Schonfeld S, Hoyer J, et al. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry. 2013;74(9):639–646. doi: 10.1016/j.biopsych.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Nygaard M, Sonne C, Carlsson J. Secondary psychotic features in refugees diagnosed with post-traumatic stress disorder: a retrospective cohort study. BMC Psychiatry. 2017;17(1):5. doi: 10.1186/s12888-016-1166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeSantis AS, Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Racial and ethnic differences in diurnal cortisol rhythms: are they consistent over time? Psychosom Med. 2015;77(1):6–15. doi: 10.1097/PSY.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 29.Kolassa IT, Eckart C, Ruf M, Neuner F, de Quervain DJ, Elbert T. Lack of cortisol response in patients with posttraumatic stress disorder (PTSD) undergoing a diagnostic interview. BMC Psychiatry. 2007;7:54. doi: 10.1186/1471-244X-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbui C, Purgato M, Ostuzzi G. Regulatory meta-analyses for the evaluation of psychotropic medicines. Lancet Psychiatry. 2017;4(9):660–661. doi: 10.1016/S2215-0366(17)30161-X. [DOI] [PubMed] [Google Scholar]
- 31.Bouras N, Ikkos G, Craig T. Meta-community mental health care: towards a new concept. Lancet Psychiatry. 2017;4(8):581–582. doi: 10.1016/S2215-0366(17)30108-6. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression-correlation analysis. J R Stat Soc. 2010;52(4):691.
- 33.Tran US, Gregor B. The relative efficacy of bona fide psychotherapies for post-traumatic stress disorder: a meta-analytical evaluation of randomized controlled trials. BMC Psychiatry. 2016;16:266. doi: 10.1186/s12888-016-0979-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norrholm SD, Jovanovic T. Fear processing, psychophysiology, and PTSD. Harvard Rev Psychiat. 2018;26(3):129–141. doi: 10.1097/HRP.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 35.Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: focus on the Trier social stress test. Neurosci Biobehav Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Allen AP, Curran EA, Duggan A, Cryan JF, Chorcorain AN, Dinan TG, et al. A systematic review of the psychobiological burden of informal caregiving for patients with dementia: focus on cognitive and biological markers of chronic stress. Neurosci Biobehav Rev. 2017;73:123–164. doi: 10.1016/j.neubiorev.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: a focus on health and disease in children. Stress. 2008;11(1):1–14. doi: 10.1080/10253890701365527. [DOI] [PubMed] [Google Scholar]
- 38.Bae YJ, Gaudl A, Jaeger S, Stadelmann S, Hiemisch A, Kiess W, et al. Immunoassay or LC-MS/MS for the measurement of salivary cortisol in children? Clin Chem Lab Med. 2016;54(5):811–822. doi: 10.1515/cclm-2015-0412. [DOI] [PubMed] [Google Scholar]
- 39.Norrholm SD, Jovanovic T, Gerardi M, Breazeale KG, Price M, Davis M, et al. Baseline psychophysiological and cortisol reactivity as a predictor of PTSD treatment outcome in virtual reality exposure therapy. Behav Res Ther. 2016;82:28–37. doi: 10.1016/j.brat.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wuest S, et al. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Kruger-Gottschalk A, Knaevelsrud C, Rau H, Dyer A, Schafer I, Schellong J, et al. The German version of the posttraumatic stress disorder checklist for DSM-5 (PCL-5): psychometric properties and diagnostic utility. BMC Psychiatry. 2017;17(1):379. doi: 10.1186/s12888-017-1541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai W, Kaminga AC, Tan H, Wang J, Lai Z, Wu X, et al. Long-term psychological outcomes of flood survivors of hard-hit areas of the 1998 Dongting Lake flood in China: prevalence and risk factors. PLoS One. 2017;12(2):e171557. doi: 10.1371/journal.pone.0171557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol Psychiatry. 2002;51(7):575–582. doi: 10.1016/S0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- 44.Coupland NJ, Hegadoren KM, Myrholm J. Increased beclomethasone-induced vasoconstriction in women with posttraumatic stress disorder. J Psychiatr Res. 2003;37(3):221–228. doi: 10.1016/S0022-3956(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 45.Feldman R, Vengrober A, Eidelman-Rothman M, Zagoory-Sharon O. Stress reactivity in war-exposed young children with and without posttraumatic stress disorder: relations to maternal stress hormones, parenting, and child emotionality and regulation. Dev Psychopathol. 2013;25(4pt1):943–955. doi: 10.1017/S0954579413000291. [DOI] [PubMed] [Google Scholar]
- 46.Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-α, and IL-6 in women with PTSD. J Trauma Stress. 2008;21(6):530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EGWM, Westenberg HGM. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2007;32(3):215–226. doi: 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi I, Delahanty DL. Awake/sleep cortisol levels and the development of posttraumatic stress disorder in injury patients with peritraumatic dissociation. Psychol Trauma Theory Res Pract Policy. 2014;6(5):449–456. doi: 10.1037/a0033013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindauer RJL, Olff M, van Meijel EPM, Carlier IVE, Gersons BPR. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biol Psychiatry. 2006;59(2):171–177. doi: 10.1016/j.biopsych.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 50.Lipschitz DS, Rasmusson AM, Yehuda R, Wang S, Anyan W, Gueoguieva R, et al. Salivary cortisol responses to dexamethasone in adolescents with posttraumatic stress disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(11):1310–1317. doi: 10.1097/01.chi.0000084832.67701.0d. [DOI] [PubMed] [Google Scholar]
- 51.McFarlane AC, Barton CA, Yehuda R, Wittert G. Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(5):720–727. doi: 10.1016/j.psyneuen.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Neylan TC, Schuff N, Lenoci M, Yehuda R, Weiner MW. Cortisol levels are positively correlated with hippocampal N-Acetylaspartate. Biol Psychiatry. 2003;54(10):1118–1121. doi: 10.1016/S0006-3223(03)01974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tucker P, Pfefferbaum B, North CS, Kent A, Jeon-Slaughter H, Parker DE. Biological correlates of direct exposure to terrorism several years postdisaster. Ann Clin Psychiatry. 2010;22(3):186–195. [PubMed] [Google Scholar]
- 54.Roth G, Ekblad S, Ågren H. A longitudinal study of PTSD in a sample of adult mass-evacuated Kosovars, some of whom returned to their home country. Eur Psychiat. 2006;21(3):152–159. doi: 10.1016/j.eurpsy.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Shalev AY, Videlock EJ, Peleg T, Segman R, Pitman RK, Yehuda R. Stress hormones and post-traumatic stress disorder in civilian trauma victims: a longitudinal study. Part I: HPA axis responses. Int J Neuropsychopharmacol. 2008;11(03). 10.1017/S1461145707008127. [DOI] [PubMed]
- 56.Su T, Zhang L, Chung M, Chen Y, Bi Y, Chou Y, et al. Levels of the potential biomarker p11 in peripheral blood cells distinguish patients with PTSD from those with other major psychiatric disorders. J Psychiatr Res. 2009;43(13):1078–1085. doi: 10.1016/j.jpsychires.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Wahbeh H, Oken BS. Salivary cortisol lower in posttraumatic stress disorder. J Trauma Stress. 2013;26(2):241–248. doi: 10.1002/jts.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witteveen AB, Huizink AC, Slottje P, Bramsen I, Smid T, van der Ploeg HM. Associations of cortisol with posttraumatic stress symptoms and negative life events: a study of police officers and firefighters. Psychoneuroendocrinology. 2010;35(7):1113–1118. doi: 10.1016/j.psyneuen.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Yehuda R, Golier JA, Kaufman S. Circadian rhythm of salivary cortisol in holocaust survivors with and without PTSD. Am J Psychiatry. 2005;162(5):998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- 60.Yehuda R, Golier JA, Harvey PD, Stavitsky K, Kaufman S, Grossman RA, et al. Relationship between cortisol and age-related memory impairments in holocaust survivors with PTSD. Psychoneuroendocrinology. 2005;30(7):678–687. doi: 10.1016/j.psyneuen.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Young EA, Tolman R, Witkowski K, Kaplan G. Salivary cortisol and posttraumatic stress disorder in a low-income community sample of women. Biol Psychiatry. 2004;55(6):621–626. doi: 10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder: a community epidemiologic study. Biol Psychiatry. 2004;56(3):205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 63.Lindley SE, Carlson EB, Benoit M. Basal and dexamethasone suppressed salivary cortisol concentrations in a community sample of patients with posttraumatic stress disorder. Biol Psychiatry. 2004;55(9):940–945. doi: 10.1016/j.biopsych.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 64.Neylan TC, Lenoci M, Maglione ML, Rosenlicht NZ, Metzler TJ, Otte C, et al. Delta sleep response to metyrapone in post-traumatic stress disorder. Neuropsychopharmacology. 2003;28(9):1666–1676. doi: 10.1038/sj.npp.1300215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategies: details of search strategy. (DOC 27 kb)
Data Availability Statement
Available upon request to the corresponding author Aizhong Liu: lazroy@live.cn.