Abstract
Background
The Malaria Atlas Project (MAP) has worked to assemble and maintain a global open-access database of spatial malariometric data for over a decade. This data spans various formats and topics, including: geo-located surveys of malaria parasite rate; global administrative boundary shapefiles; and global and regional rasters representing the distribution of malaria and associated illnesses, blood disorders, and intervention coverage. MAP has recently released malariaAtlas, an R package providing a direct interface to MAP’s routinely-updated malariometric databases and research outputs.
Methods and results
The current paper reviews the functionality available in malariaAtlas and highlights its utility for spatial epidemiological analysis of malaria. malariaAtlas enables users to freely download, visualise and analyse global malariometric data within R. Currently available data types include: malaria parasite rate and vector occurrence point data; subnational administrative boundary shapefiles; and a large suite of rasters covering a diverse range of metrics related to malaria research. malariaAtlas is here used in two mock analyses to illustrate how this data may be incorporated into a standard R workflow for spatial analysis.
Conclusions
malariaAtlas is the first open-access R-interface to malariometric data, providing a new and reproducible means of accessing such data within a freely available and commonly used statistical software environment. In this way, the malariaAtlas package aims to contribute to the environment of data-sharing within the malaria research community.
Electronic supplementary material
The online version of this article (10.1186/s12936-018-2500-5) contains supplementary material, which is available to authorized users.
Keywords: Malaria, Open-access, Malariometric data, Parasite rate, R package
Background
Since 2005, the Malaria Atlas Project (MAP) has worked to assemble and maintain a global open-access database of spatially explicit malariometric data. This work has been motived by dual aims to both enhance open-access malaria data availability and to provide operationally relevant information for national and international policymakers [1–4]. The availability of this repository of global malariometric data has underpinned numerous studies in the field [5–15]; and continues to support prominent international research such as the Global Burden of Disease study [16, 17] and the World Malaria Report [18–22]. The fundamental need for accurate local information on malaria burden is evident now more than ever, as more countries approach malaria elimination and the challenges of limited funding, insecticide resistance and antimalarial resistance continue to grow [18]. To this end, MAP maintains a routinely updated assembly of national and subnational malariometric data, while developing tools to enable open access to this data for researchers and policymakers worldwide.
The data estate hosted at MAP is one of the largest open-access collections of global malariometric data, both in terms of number of records and geographic coverage. This data spans various formats, topic areas and spatial resolutions, including survey data for precise point locations, administrative-unit level routine surveillance data, and raster grids of spatially continuous modelled predictions (see Table 1). The geo-located survey data specifically encompass: malaria parasite rate (cross-sectional point prevalence), malaria-relevant blood disorder prevalence, intervention coverage, and Anopheles vector occurrence. The subnational routine surveillance data covers metrics such as API (annual parasite incidence) and malaria mortality. Finally, the predicted global and regional rasters represent estimates of the distribution of malaria infection and associated disease (e.g. clinical incidence; malaria-attributable fever), malaria-relevant blood disorders, vector occurrence and relative abundance, intervention coverage, and accessibility to cities. This database comprises published data from scientific publications, national surveys (e.g. DHS and MIS [23, 24]), and grey literature produced by national ministries of health and international organizations; as well as unpublished data from researchers and malaria control programmes worldwide. Altogether this represents decades of collaborative work and countless person-hours of on-the-ground data collection.
Table 1.
Outline of the Malaria Atlas Project open-access data estate and current availability
| Data type and format | Open-access availability | |
|---|---|---|
| malariaAtlas | Web-toolsa | |
| Geo-located point data | ||
| Malaria parasite rate (PR; for P. falciparum and P. vivax) | Available now | Available now |
| Dominant mosquito vectors | Available now | Available now |
| Malaria-relevant blood disorders | Coming soon | Available now |
| Administrative-unit (polygon) level data | ||
| Administrative boundary shapefiles | Available now | Not currently available |
| Annual Parasite Incidence (API; for P. falciparum and P. vivax) | Coming soon | Coming soon |
| Malaria reproductive number (P. falciparum) | Coming soon | Available now |
| Global/regional raster grids | ||
| Predicted malaria infection risk, prevalence, and associated illness | Available now | Available now |
| Predicted prevalence of malaria-relevant blood disorders | Available now | Available now |
| Predicted mosquito vector distribution and relative abundance | Available now | Available now |
| Intervention Coverage (ITNs; IRS; ACT) | Available now | Available now |
| Global travel time to cities | Available now | Available now |
aAvailable at map.ox.ac.uk
Along with a newly released suite of online tools that enable open-access availability to MAP’s databases and associated research outputs (available at http://www.map.ox.ac.uk), MAP has recently released malariaAtlas, an R package providing a direct interface to MAP’s open-access databases and research outputs [25–27]. This interface offers three main advantages to traditional data repositories, including: user-defined queries to enable efficient downloading of subsets of large datasets; automatic access to the most up-to-date version of the database including new data and/or database amendments; and transparent and reproducible data access in the form of a few lines of shareable R code. This paper introduces malariaAtlas, outlining the available data and functions in the package and illustrating its utility in two reproducible mock analyses.
Results and discussion
Data available through malariaAtlas
malariaAtlas currently enables users to download, visualize and manipulate three types of data: parasite rate (PR) survey data; administrative boundary shapefiles; and a large suite of rasters covering a range of modelled outputs related to malaria research (see Table 1). Georeferenced PR survey data is a core component of MAP’s data estate and a common measure of malaria endemicity [1, 28]. The PR survey points entered into MAP’s database are screened for robust sampling methods and geographic specificity to ensure they provide representative parasite species-specific information on the local prevalence of malaria infection. This database includes 73,326 survey points as of July 2018 (64,685 Plasmodium falciparum; 14,412 Plasmodium vivax), covering the period 1975–2017. In addition to georeferenced data on malaria endemicity, up-to-date and topologically correct shapefiles of a region’s administrative boundaries are fundamental to visualizing, interpreting and analysing spatial epidemiological data. As such, MAP maintains a collated set of subnational administrative boundary shapefiles assembled from various publicly available sources (see [29]). MAP also makes a large number of raster grids publicly available, representing the major outputs of MAP’s spatiotemporal epidemiological research. At the time of writing, 86 raster surfaces were available to download using malariaAtlas. These cover a variety of relevant metrics, such as predicted malaria parasite prevalence, clinical incidence and malaria-attributable fever [8, 30–32]; prevalence of malaria-related human blood disorders [33–35]; predicted risk of zoonotic Plasmodium knowlesi infection [36]; predicted mosquito vector distribution and relative abundance [37–40]; coverage of insecticide-treated bed nets (ITNs), indoor residual spraying (IRS) and artemisinin-based combination therapy (ACT) [8]; and travel time to cities [41]. By providing an R-interface to MAP’s hosted survey data, shapefiles and rasters, malariaAtlas enhances direct and reproducible access to this data source.
Downloading and visualizing data with malariaAtlas
Using malariaAtlas to download and visualize data from MAP in R is achieved through four main classes of functions as outlined in Table 2. These include: ‘list’ functions that allow the user to see how much data is available for a given data type; ‘get’ functions for data downloads; ‘autoplot’ methods that enable quick visualisation of downloaded data using functions from the ggplot2 package [42]; and a number of utility functions that enable common manipulations of downloaded data (see Table 2).
Table 2.
Outline of malariaAtlas functions
| Category | Function name | Purpose | Data type | R object class |
|---|---|---|---|---|
| ‘List’ available data | listData | Wrapper for below functions, returning a data.frame outlining data availability | – | data.frame |
| listPoints | Return a data.frame listing countries with parasite rate survey points | Point data | data.frame | |
| listShp | Return a data.frame listing administrative units with shapefiles available to download | Shapefile | data.frame | |
| listRaster | Return a data.frame listing rasters available to download | Raster | data.frame | |
| ‘Get’ available data | getPR | Download parasite rate survey data for specified location(s) and species | Point data | data.frame; pr.points a |
| getShp | Download shapefiles for specified location(s) and administrative level(s) | Shapefile | SpatialPolygon(s); data.frame; mapShp a | |
| getRaster | Download specified rasters for specified location(s) and year(s) | Raster | RasterLayer; RasterBrick; RasterStack; data.frame; mapRaster a | |
| ‘Autoplot’ downloaded data | autoplot.pr.points | Quickly visualise parasite rate survey locations and raw PR values for data downloaded using malariaAtlas | Point data | gg |
| autoplot.mapShp | Quickly visualise shapefiles downloaded using malariaAtlas | Shapefile | gg | |
| autoplot.mapRaster | Quickly visualise rasters downloaded using malariaAtlas | Raster | gg/list | |
| Utility functions | extractRaster | Extract values from specified rasters at specified point locations (lat/long) | Point data | data.frame |
| convertPrevalence b | Convert parasite rate from a given age-range to another | Prevalence | numeric | |
| as.mapShp | Convert SpatialPolygon or SpatialPolygons objects to mapShpa objects | Shapefile | mapShpa; data.frame | |
| as.mapRaster | Convert objects of RasterLayer; RasterBrick; RasterStack classes or a list of RasterBrick/RasterStacks to mapRastera objects | Raster | mapRastera; data.frame |
a malariaAtlas specific object class defined for purposes of quick visualisation using autoplot (pr.points; mapShp; and mapRaster) or in-built optional conversion of Spatial* classes to data.frame formats (mapShp; mapRaster)
b See the ageStand R package on GitHub [43] or malariaAtlas help files for additional information on convertPrevalence
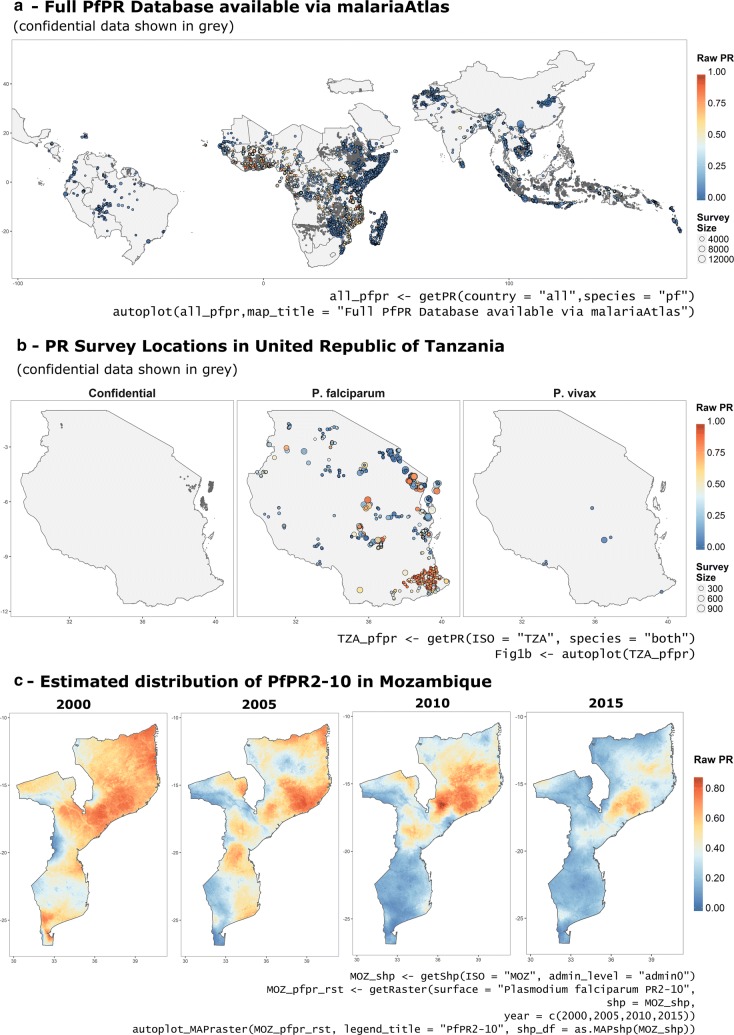
Within malariaAtlas, the functions listPoints, getPR and autoplot.pr.points provide a quick and simple way of downloading and visualising publicly available PR survey data hosted by MAP. listPoints returns a data.frame outlining the countries for which parasite rate survey data is available in MAPs database. getPR returns a data.frame of geo-located PR point data including: number of individuals examined; number of positive diagnoses by species; age range of the sample population; sampling date and location information; diagnostic method(s) used; and source citation. Arguments are included to enable queries based on location (Continent; Country Name; 3 letter ISO code; or spatial extent) and species (either P. falciparum or P. vivax). The returned data has the additional class ‘pr.points’ which enables quick visualization of downloaded points using autoplot. A subset of the PR survey points in MAP’s database remain confidential, in accordance with the respective data-use agreements under which they have been shared. For these confidential data points, MAP has either limited or no permission to share measured PR values and/or geo-location data, however citations to the original data source are provided for all downloaded points. Accordingly, data-sharing restrictions for any given point are provided in the ‘permissions_info’ column of a downloaded pr.points data.frame. Figure 1 illustrates the use of malariaAtlas to download and visualise PR survey points, including maps of (a) the full database of available P. falciparum PR points at the time of publication (Fig. 1a, b) all PR survey points hosted by MAP from Tanzania (Fig. 1b).
Fig. 1.
Using malariaAtlas to download and visualise geolocated parasite rate data and modelled raster data. a malariaAtlas-derived map of the full PfPR database available to download using getPR. Points are coloured according to PR value and sized according to sample size. Grey points illustrate confidential data. b Map of all PR points from The United Republic of Tanzania hosted by MAP for both Plasmodium falciparum and Plasmodium vivax. c Rasters of estimated spatial distribution of PfPR in Mozambique in 2000, 2005, 2010 and 2015 from Bhatt et al. [8]. For all panels, the malariaAtlas R code used to download and visualise the relevant data is included below the map
Analogous to the functions described above, listShp, getShp and autoplot.mapShp allow users to download and visualise the set of shapefiles collated by MAP (see Table 2). listShp returns a data.frame indicating all administrative regions covered by these shapefiles along with their administrative level and corresponding parent administrative unit. getShp returns either a SpatialPolygons object or mapShp object (as chosen by the user) containing polygons at either ADMIN0 (national) or ADMIN1 (state; province) levels for any given country; and down to ADMIN3 level for some malaria-endemic countries. Quick visualisation of mapShp objects is possible through an autoplot method.
Rasters are the final datatype available to download and visualise using malariaAtlas via the functions: listRaster, getRaster and autoplot.mapRaster. listRaster returns a data.frame that serves as a catalogue of rasters available to download using getRaster, mirroring the catalogue of rasters available on MAP’s online interactive explorer tool (map.ox.ac.uk/explorer). This data.frame includes columns that provide descriptive metadata including an abstract outlining raster content, a citation to the original publication associated with a given raster, and the time period covered for time-varying raster datasets. getRaster provides the means to download one or more raster layers at a time, queried by location (using either an input SpatialPolygon shapefile or a user-defined extent (xmin, xmax, ymin, ymax)), and year (for temporally dynamic raster datasets). The data is returned as a Raster* object: a RasterLayer for a single raster; a RasterBrick for two or more rasters of the same extent/resolution; or a list of Raster* objects for two or more rasters of differing extents/resolutions. Downloaded rasters represent the mean predicted value from various geostatistical models. For further information on specific modelling approaches and/or associated uncertainty of predicted values users are encouraged to consult the associated publication (citation information available via listRaster) or to contact MAP directly. The utility function as.mapRaster converts any object downloaded using getRaster into a mapRaster object (long-format data.frame with columns x, y, z (longitude, latitude, value) and raster_name) enabling tabular manipulation and ggplot-friendly visualisation. Quick visualization of mapRaster objects is provided via included autoplot methods. Figure 1c illustrates example code used to download and quickly visualise a raster for a given shapefile extent via malariaAtlas.
Data manipulation and utility functions
Three additional utility functions have been added to provide an easy means to perform common data manipulations. extractRaster allows users to download values from MAP rasters at specific point locations supplied in a user-specified set of coordinates (see malariaAtlas Vignette; [27]). This enables users to input a list of locations (latitude, longitude) and get back the associated raster value (e.g. malaria prevalence) for each location. as.mapShp and as.mapRaster provide a means of converting between Spatial* class objects (for polygon data) or Raster* class objects (for raster data) to the malariaAtlas data.frame-based object classes mapShp and mapRaster respectively. This permits tabular manipulation and ggplot-friendly plotting through provided autoplot methods. convertPrevalence is an additional utility function that provides a principled approach to age-standardization of malaria prevalence data [43], based on models defined by Smith et al. [28] for P. falciparum and Gething et al. [30] for P. vivax. Altogether, the above functions provide a simple means of downloading, visualising and manipulating spatial malariometric data. The flexibility of R as a statistical software platform and the wealth of existing R packages enable users to easily extend their analysis beyond these functions and integrate malariaAtlas into more complex analytical workflows.
Zoon modules
To further aid the dissemination and use of these data, malariaAtlas modules were developed for the species distribution modelling software zoon [44]. Zoon provides a modular framework for species distribution modelling, allowing users to collect and model data in a simple pipeline. Species distribution modelling is a subfield of ecology in which the spatial distribution of an organism is estimated from known presence and absence (if available) locations. There are strong parallels between species distribution modelling and parasite rate mapping as both use binomial data to estimate a spatial probability surface; in species distribution modelling this surface is the probability of species occurrence while in parasite rate mapping the surface is probability of infection. Two zoon modules have been added (‘malariaAtlas_PR’ and ‘malariaAtlas_covariates’) allowing parasite rate surveys to be used as response data and raster data to be used as covariates within a zoon workflow. The parasite rate data offers a useful benchmark dataset for testing new methods. However, state-of-the-art models of malaria prevalence (e.g. [8]) are currently beyond the scope of zoon, and as such zoon is not expected to be directly used for risk mapping and/or policymaking.
Mock analysis 1: predicting the spatial distribution of Plasmodium vivax using malariaAtlas-derived response and covariate data
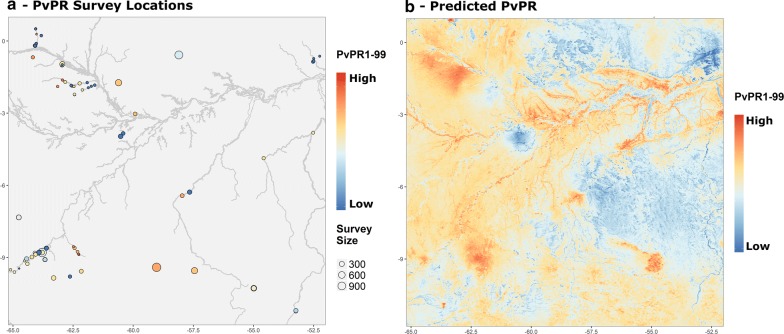
The first mock analysis illustrates the use of malariaAtlas to download response and covariate data for use in spatial epidemiological analysis. P. vivax parasite rate (PvPR) survey points and covariate raster data were downloaded using malariaAtlas (see Box 1) and used to fit a Bayesian geostatistical model of malaria risk (see full example code in Additional file 1). For illustrative purposes, an arbitrary spatial extent was chosen for this analysis. All PvPR points in the study area were downloaded using getPR, and then subsetted to only publicly available data for analysis. convertPrevalence was used to standardize values to all-ages PvPR (see Fig. 2a; Box 1). The R-INLA package [45, 46] was used to fit a Bayesian geostatistical model with a binomial likelihood to these data. Covariate data included rasters of environmental factors (night-time land surface temperature [47]; log elevation [48]; rainfall [49]) and log travel time to the nearest city (downloaded using getRaster as in Box 1, hereafter referred to as ‘human accessibility’; [41]). These fixed effects were given minimally informative (INLA default) priors.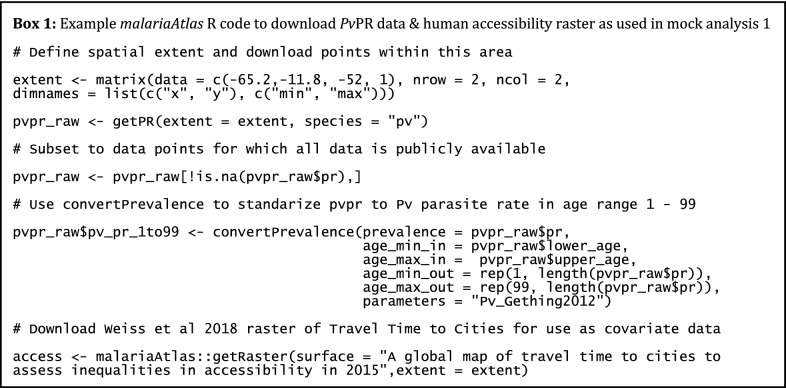
Fig. 2.
Predicting the spatial distribution of Plasmodium vivax using malariaAtlas-derived response and covariate data. a Map illustrating locations of age-standardised PvPR survey points within the study area as used for response data in mock analysis 1. River locations were downloaded from the Global Lakes and Wetlands Database [52]. b Predicted Plasmodium vivax parasite rate within the study area. Predictions are derived from a Bayesian geostatistical model using data in panel a and environmental covariates including night-time temperature, elevation and rainfall. Both maps were produced using malariaAtlas’ autoplot methods and ggplot2 [42]. Absolute values were removed from the colour scales to reflect the purely illustrative nature of this analysis
The spatial autocorrelation in the data was modelled using a continuous, spatial Gaussian random field with a Matern covariance function [45]. The hyperparameters of the random field were given Penalised Complexity (PC) priors, which by design prefer a simpler model with a smoother random field [50]. The hyperparameters of the random field are the range (the distance within which the correlation of the field is essentially zero) and the standard deviation (the amount the field can vary). For the current model, the priors on these values were parameterised by setting the probability that the range of the field was smaller than an extreme minimum value (2 decimal degrees) as 0.01 and the probability that the standard deviation of the field was greater than an extreme maximum value (2.7) as 0.01. A random field with a standard deviation of 2.7 would be able to explain all the residual variance from a previously fitted logistic regression. The above prior was thus defined such that this undesirable level of overfitting was unlikely.
The fitted model was used to predict PvPR across the spatial extent of the study area (see Fig. 2b). Within this model, night-time temperature and elevation were significant predictors of PvPR (estimated coefficients (95% CI) of − 0.98 (− 1.70 to − 0.30) and − 1.43 (− 2.69 to − 0.38) respectively), while human accessibility did not significantly predict PvPR (− 0.16 (− 0.44 to 0.16)). Overall interpretation of these results is limited due to its small sample size and arbitrary spatial extent. Nevertheless, this mock analysis illustrates the use of malariaAtlas to download spatial malariometric response and covariate data for incorporation into further analysis.
Mock analysis 2: testing a new modelling approach using in-built malariaAtlaszoon modules
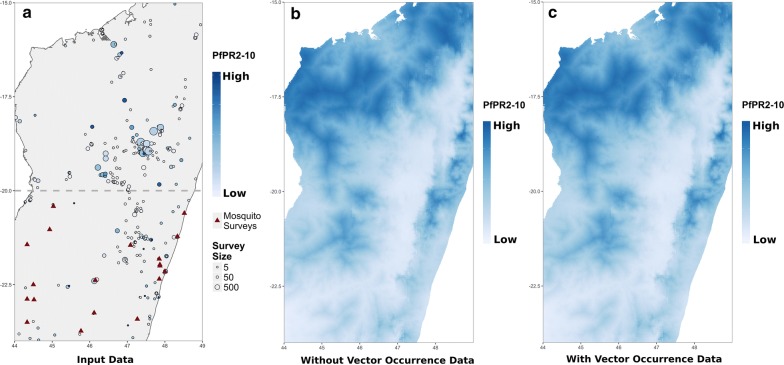
The second mock analysis demonstrates how malariaAtlas can be used to access malariometric data within a zoon workflow [44]. As an illustrative example, this analysis investigates whether including mosquito occurrence data can improve predictive models of PfPR, using data from a second arbitrary study area (bounded by latitudes of − 24 and − 15 and longitudes of 44 and 49). A simple spatial validation scheme was implemented, using PfPR data from north of latitude − 20 (28,921 individuals from 208 locations) as a hold-out validation data set. Logistic regression models were fitted to two datasets and their predictive performance was compared. The first data set was simply the PfPR data from 116 locations and 8546 individuals south of latitude − 20. The second dataset was comprised of the same PfPR data with the addition of known occurrence locations of Anopheles arabiensis and Anopheles gambiae collected from the Global Biodiversity Information Facility [51], treating each vector occurrence location as equivalent to a single positive case of P. falciparum (total 147 locations and 8592 individuals/mosquitoes; see Fig. 3a). For covariates, WorldClim layers 1, 4, 12 and 15 (mean and within-year variation of temperature and precipitation [49]) as well as human accessibility [41] were used. PfPR data and human accessibility rasters were downloaded using malariaAtlas zoon modules (see Box 2). Model performance was compared using the AUC (Area Under the Curve) model evaluation criterion which assessed the ability of each model to correctly assign an infected/non-infected status to individuals in the hold-out set.

Fig. 3.
Including mosquito occurrence data alongside PR survey data in models of Plasmodium falciparum parasite rate. a Map of geolocated input data, PR points (coloured circles) were obtained from MAP using the malariaAtlas_PR zoon module; mosquito presence data (red triangles) were obtained from GBIF using the SpOcc zoon module [44, 51, 53]. b, c Predicted Plasmodium falciparum parasite rate from logistic regression models using either PR data only (b) or PR data and mosquito occurrence data (c). Maps were produced using malariaAtlas’ autoplot methods and ggplot2 [42]. Absolute values were removed from the colour scales to reflect the purely illustrative nature of this analysis
Including mosquito occurrence data very marginally improved predictive performance. AUC was 0.577 without mosquito occurrence data and 0.578 with the addition of mosquito data. Maps created using both models are shown in Fig. 3b, c showing almost identical outcomes. It is worth noting that the difference in model performance has no practical relevance. However, this serves as an illustrative example of how malariaAtlas data can be used within zoon to test new methods. Larger scale comparisons, and a less naive approach to incorporating mosquito data, would be needed to truly examine whether this method has analytical merit.
Conclusions
malariaAtlas is the first open-access R-interface to malariometric data, providing a new and reproducible means of accessing this data within a freely available and commonly used statistical software environment. As such, by using malariaAtlas, any individual with internet access can directly download, visualise and analyse data from the Malaria Atlas Project. Furthermore, this package is designed to fit into existing research workflows, enabling importation of multiple data-types in a few simple lines of code, as illustrated in the mock analyses above. As the MAP data estate continues to grow, malariaAtlas will offer an up-to-date interface to the most recent malariometric data. Future updates will seek to provide access to additional data-types (e.g. publicly reported routine surveillance data; site-level geolocated survey data of other types such as prevalence data for glucose-6-phosphate dehydrogenase deficiency and the Duffy negative blood group; and new raster datasets such as modelled resistance to the insecticides used in malaria control). Future updates will also include the option for date-specific data queries (e.g. ‘download data as at 01/04/2018’), enabling truly reproducible data download irrespective of potential amendments to the source database. malariaAtlas rests upon decades of valuable collaboration and data-sharing within the malaria research community. By providing a new means of open-access to malariometric data it is hoped that this package both contributes to this environment of open data-sharing and also provides a valuable tool to malaria researchers worldwide.
Additional file
Additional file 1. Illustrative R code used to conduct the Mock Analyses in this paper.
Authors’ contributions
DP and TL conceived of the project, and created and maintain the R package. DM, JH and HG created and maintain the database systems underlying the R package and contributed technical advice to interfacing with these systems. JR, KT, UD, CG, CM, MT, MN, SB, EC, DW, RH, KB, HG and PG gathered and processed malariometric data and/or produced modelled rasters available via malariaAtlas. PG and MT obtained funding for this project and offered advice on the R package design. DP wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are thankful to the many people who have generously contributed their unpublished data to MAP’s research and/or open-access database. These individuals are identified on the MAP website at http://www.map.ox.ac.uk/acknowledgements. We also thank Scott Chamberlain for fixing bugs in the spocc package that were encountered during our analyses, as well as Nick Golding and David Smith for providing the code and permissions to include the convertPrevalence function in malariaAtlas.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The malariometric datasets generated and/or analysed during the current study are available from the Malaria Atlas Project database, http://www.map.ox.ac.uk. Environmental covariate data that support the analyses in this study are available from NASA LP DAAC [47, 48] and WorldClim [49]. Mosquito occurrence data used in this study are available from GBIF [51].
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
The development of malariaAtlas was funded by the Bill and Melinda Gates Foundation’s grant to the ROAD-MAP (Repository of Open Access Data—Malaria Atlas Project) group within MAP (Gates Foundation opportunity code: OPP1106023).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guerra CA, Hay SI, Lucioparedes LS, Gikandi PW, Tatem AJ, Noor AM, et al. Assembling a global database of malaria parasite prevalence for the Malaria Atlas Project. Malar J. 2007;6:17. doi: 10.1186/1475-2875-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay SI, Snow RW. The Malaria Atlas Project: developing global maps of malaria risk. PLoS Med. 2006;3:e473. doi: 10.1371/journal.pmed.0030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyes CL, Temperley WH, Henry AJ, Burgert CR, Hay SI. Providing open access data online to advance malaria research and control. Malar J. 2013;12:161. doi: 10.1186/1475-2875-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piel FB, Howes RE, Nyangiri OA, Moyes CL, Williams TN, Weatherall DJ, et al. Online biomedical resources for malaria-related red cell disorders. Hum Mutat. 2013;34:937–944. doi: 10.1002/humu.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker PG, Griffin JT, Ferguson NM, Ghani AC. Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: a modelling study. Lancet Glob Health. 2016;4:e474–e484. doi: 10.1016/S2214-109X(16)30073-0. [DOI] [PubMed] [Google Scholar]
- 6.Amoah B, Giorgi E, Heyes DJ, Burren S, Diggle PJ. Geostatistical modelling of the association between malaria and child growth in Africa. Int J Health Geogr. 2018;17:7. doi: 10.1186/s12942-018-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrenho E, Miraldo M, Shaikh M, Atun R. Vertical and horizontal equity of funding for malaria control: a global multisource funding analysis for 2006–2010. BMJ Glob Health. 2017;2:e000496. doi: 10.1136/bmjgh-2017-000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korenromp E, Hamilton M, Sanders R, Mahiané G, Briët OJ, Smith T, et al. Impact of malaria interventions on child mortality in endemic African settings: comparison and alignment between LiST and Spectrum-Malaria model. BMC Public Health. 2017;17:781. doi: 10.1186/s12889-017-4739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatem AJ, Guerra CA, Kabaria CW, Noor AM, Hay SI. Human population, urban settlement patterns and their impact on Plasmodium falciparum malaria endemicity. Malar J. 2008;7:218. doi: 10.1186/1475-2875-7-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuadros DF, Branscum AJ, García-Ramos G. No evidence of association between HIV-1 and malaria in populations with low HIV-1 prevalence. PLoS ONE. 2011;6:e23458. doi: 10.1371/journal.pone.0023458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golding N, Burstein R, Longbottom J, Browne AJ, Fullman N, Osgood-Zimmerman A, et al. Mapping under-5 and neonatal mortality in Africa, 2000–15: a baseline analysis for the Sustainable Development Goals. Lancet. 2017;390:2171–2182. doi: 10.1016/S0140-6736(17)31758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. UNICEF . Achieving the malaria MDG target: reversing the incidence of malaria 2000–2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 14.Briët OJ, Gething PW, Maire N, Tarantino M, Hay SI. Estimated malaria epidemiologically effective lifetime of mass LLIN distributions depending on transmission in African countries. Report for African Leaders Malaria Alliance, 2012.
- 15.Gething PW, Casey DC, Weiss DJ, Bisanzio D, Bhatt S, Cameron E, et al. Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N Engl J Med. 2016;375:2435–2445. doi: 10.1056/NEJMoa1606701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GBD 2016 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . World malaria report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 19.WHO . World malaria report 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 20.WHO . World malaria report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 21.WHO . World malaria report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 22.WHO . World malaria report 2013. Geneva: World Health Organization; 2013. [Google Scholar]
- 23.The DHS Program. Demographic and health surveys 2018. https://dhsprogram.com. Accessed 12 Apr 2018.
- 24.Malariasurveys.org. Malaria indicator surveys 2018. http://www.malariasurveys.org. Accessed 12 Apr 2018.
- 25.Malaria Atlas Project. MAP data R package 2018. http://www.map.ox.ac.uk/application-project/malariaatlas_package. Accessed 13 Apr 2018.
- 26.Malaria Atlas Project. malariaAtlas 2018. https://github.com/malaria-atlas-project/malariaAtlas. Accessed 10 Apr 2018.
- 27.Pfeffer D, Lucas T, May D, Rozier J, Gibson H, Golding N, et al. malariaAtlas: An R interface to open-access malaria data, hosted by the ‘Malaria Atlas Project’ 2018. https://cran.r-project.org/web/packages/malariaAtlas/index.html. Accessed 10 Apr 2018.
- 28.Smith DL, Guerra CA, Snow RW, Hay SI. Standardizing estimates of the Plasmodium falciparum parasite rate. Malar J. 2007;6:131. doi: 10.1186/1475-2875-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malaria Atlas Project. Administrative boundaries 2018. https://map.ox.ac.uk/data-project/administrative-boundaries. Accessed 13 Apr 2018.
- 30.Gething PW, Elyazar IRF, Moyes CL, Smith DL, Battle KE, Guerra CA, et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012;6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gething PW, Patil AP, Smith DL, Guerra CA, Elyazar IR, Johnston GL, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10:378. doi: 10.1186/1475-2875-10-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalrymple U, Cameron E, Bhatt S, Weiss DJ, Gupta S, Gething PW. Quantifying the contribution of Plasmodium falciparum malaria to febrile illness amongst African children. eLife. 2017;6:e29198. doi: 10.7554/eLife.29198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, et al. The global distribution of the Duffy blood group. Nat Commun. 2011;2:266. https://www.nature.com/articles/ncomms1265#supplementary-information. [DOI] [PMC free article] [PubMed]
- 34.Howes RE, Piel FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9:e1001339. doi: 10.1371/journal.pmed.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shearer FM, Huang Z, Weiss DJ, Wiebe A, Gibson HS, Battle KE, et al. Estimating geographical variation in the risk of zoonotic Plasmodium knowlesi infection in countries eliminating malaria. PLoS Negl Trop Dis. 2016;10:e0004915. doi: 10.1371/journal.pntd.0004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinka ME, Golding N, Massey NC, Wiebe A, Huang Z, Hay SI, et al. Modelling the relative abundance of the primary African vectors of malaria before and after the implementation of indoor, insecticide-based vector control. Malar J. 2016;15:142. doi: 10.1186/s12936-016-1187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiebe A, Longbottom J, Gleave K, Shearer FM, Sinka ME, Massey NC, et al. Geographical distributions of African malaria vector sibling species and evidence for insecticide resistance. Malar J. 2017;16:85. doi: 10.1186/s12936-017-1734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moyes CL, Shearer FM, Huang Z, Wiebe A, Gibson HS, Nijman V, et al. Predicting the geographical distributions of the macaque hosts and mosquito vectors of Plasmodium knowlesi malaria in forested and non-forested areas. Parasit Vectors. 2016;9:242. doi: 10.1186/s13071-016-1527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss D. J., Nelson A., Gibson H. S., Temperley W., Peedell S., Lieber A., Hancher M., Poyart E., Belchior S., Fullman N., Mappin B., Dalrymple U., Rozier J., Lucas T. C. D., Howes R. E., Tusting L. S., Kang S. Y., Cameron E., Bisanzio D., Battle K. E., Bhatt S., Gething P. W. A global map of travel time to cities to assess inequalities in accessibility in 2015. Nature. 2018;553(7688):333–336. doi: 10.1038/nature25181. [DOI] [PubMed] [Google Scholar]
- 42.Wickham H. ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- 43.Golding N. ageStand: age standardisation of malaria prevalence 2014. https://github.com/SEEG-Oxford/ageStand. Accessed 12 Apr 2018.
- 44.Golding N, August TA, Lucas TC, Gavaghan DJ, Loon EE, McInerny G. The zoon R package for reproducible and shareable species distribution modelling. Methods Ecol Evol. 2018;9:260–268. doi: 10.1111/2041-210X.12858. [DOI] [Google Scholar]
- 45.Lindgren F, Rue H, Lindström J. An explicit link between Gaussian fields and Gaussian Markov random fields: the stochastic partial differential equation approach. J R Stat Soc Series B Stat Methodol. 2011;73:423–498. doi: 10.1111/j.1467-9868.2011.00777.x. [DOI] [Google Scholar]
- 46.Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc Series B Stat Methodol. 2009;71:319–392. doi: 10.1111/j.1467-9868.2008.00700.x. [DOI] [Google Scholar]
- 47.NASA LP DAAC. Land surface temperature and emissivity 8-day L3 global 1 km. version 005; 2015. https://lpdaac.usgs.gov. Accessed Feb 2017.
- 48.NASA LP DAAC. SRTMGL3S: NASA Shuttle Radar Topography Mission Global 3 arc second sub-sampled. Version 003; 2013. https://lpdaac.usgs.gov. Accessed Mar 2016.
- 49.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 50.Simpson D, Rue H, Riebler A, Martins TG, Sørbye SH. Penalising model component complexity: a principled, practical approach to constructing priors. Stat Sci. 2017;32:1–28. doi: 10.1214/16-STS576. [DOI] [Google Scholar]
- 51.GBIF.org. GBIF home page 2018. https://www.gbif.org. Accessed 12 Apr 2018.
- 52.WWF, Center for Environmental Systems Research UoK, Germany. Global lakes and wetlands database 2004. https://www.worldwildlife.org/pages/global-lakes-and-wetlands-database. Accessed 12 Apr 2018.
- 53.Chamberlain S. spocc: Interface to species occurrence data sources 2018. R package version 0.7.3.9318. https://github.com/ropensci/spocc. Accessed 20 June 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Illustrative R code used to conduct the Mock Analyses in this paper.
Data Availability Statement
The malariometric datasets generated and/or analysed during the current study are available from the Malaria Atlas Project database, http://www.map.ox.ac.uk. Environmental covariate data that support the analyses in this study are available from NASA LP DAAC [47, 48] and WorldClim [49]. Mosquito occurrence data used in this study are available from GBIF [51].