Abstract
Background
Understanding the ecology of exophilic anophelines is a key step toward developing outdoor control strategies to complement existing indoor control tools against malaria vectors. This study was conducted to assess the movement pattern of exophilic Anopheles mosquitoes between blood meal sources and resting habitats, and the landscape factors dictating their resting habitat choice.
Results
Resting clay pots were placed at 5 m, 25 m, 50 m, 75 m and 100 m away from isolated focal houses, radiating from them in four directions. The locations of the clay pots represent heterogeneous land cover types at a relatively fine spatial scale in the landscape. The effect of the landscape characters on the number of both female and male anophelines caught was modelled using zero-inflated negative binomial regression with a log link function. A total of 420 Anopheles mosquitoes (353 females and 67 males) belonging to three species; Anopheles arabiensis, Anopheles pharoensis, and Anopheles tenebrosus were caught in the resting clay pots, with An. arabiensis being the dominant species. Canopy cover, distance from the house, and land cover type were the significant landscape characters influencing the aggregation of resting mosquitoes. Both the count and binary models showed that canopy cover was the strongest predictor variable on the counts and the presence of Anopheles mosquitoes in the clay pots. Female Anopheles were most frequently found resting in the pots placed in banana plantations, and at sampling points that were at the greater distances (75 m and 100 m) from the focal house.
Conclusions
This study showed that exophilic Anopheles mosquitoes tend to rest in shaded areas some distance away from human habitation. These findings are important when targeting mosquitoes outdoors, complementing the existing effort being made to control malaria vectors indoors.
Electronic supplementary material
The online version of this article (10.1186/s12936-018-2499-7) contains supplementary material, which is available to authorized users.
Keywords: Exophilic, Anopheles, Landscape, Canopy, Land cover
Background
Current interventions targeting indoor malaria vectors, particularly the use of long-lasting insecticidal nets (LLINs) and indoor residual sprays (IRS), have been a cornerstone of the recent significant decline in malaria morbidity and mortality [1]. As a result, malaria-related deaths have declined by more than half in sub-Saharan Africa between 2000 and 2015 [1, 2]. The sustainability of these interventions is, however, threatened due to increased vector resistance to available insecticides [3–5], and the change in mosquito biting behaviour to seeking blood meals outdoors [6–8], with some populations shifting the time of biting activity from late night to early evening [8–10]. These behavioural changes favour residual malaria transmission, presenting a major roadblock to further reduce malaria prevalence and enhance the sustainability of malaria vector control [11]. Whilst the current strategy of IRS and ITN control has made great strides against malaria, the global number of malaria cases has not declined in the past few years, but rather has increased by 5 million over the course of a single year in 2016, with no reduction in mortality evident for the first time in a decade [12]. Outdoor interventions directed against adult mosquitoes are lacking [13], and an increased understanding of the ecology and behaviour of exophilic malaria vectors is needed to improve the sustainability of existing control strategies. In addition, this may further act as a guide for the deployment of appropriate outdoor monitoring and control tools [14].
The sustainability of existing integrated vector management (IVM) tools should be actively maintained, and enhanced by the addition of novel interventions, particularly vector control strategies targeting adult anophelines outdoors [13, 15]. Early studies by Gillies [16, 17] revealed that endophilic Anopheles gambiae sensu lato (s.l.), the primary malaria vector at this time, predominantly rested indoors, but with a small proportion of mosquitoes found to be resting in shady zones at some distance from human habitation. In the interim, changes in the biting patterns of several mosquito species have arisen, whereby a far greater proportion of female Anopheles species are found to both feed and rest outdoors [6–10]. Additionally, the habitat has undergone considerable changes, populations of humans are denser, and the agricultural environment is more intensely farmed with greater use of irrigation [18]. In view of the known changes in mosquito feeding behaviour and the habitat, few recent studies describing the outdoor behaviour of mosquitoes have been conducted [19], which may be partly due to the large effort required to catch mosquitoes outdoors as opposed to indoors [20]. Existing knowledge builds extensively on the foundation of the work of Gillies [16] who studied the resting site selection of An. gambiae s.l. and Anopheles funestus in natural and artificial resting sites. More recent studies in Anopheles mosquitoes show that these mosquitoes choose outdoor resting micro-habitats based on several different environmental factors within the landscape at a fine spatial scale [21]. Moreover, a number of studies have associated landscape characters with the distribution or aggregation of exophilic mosquitoes [22–24]. These studies have indicated that different physical and biological components of the environment are important factors affecting mosquito ecology, with habitat type [22], land cover [23], shade [24], microclimate [21] and the availability of blood meal hosts [22] being positively associated with the adult distribution of exophilic mosquito species.
Outdoor monitoring and control tools can be used alone, or to augment other IVM strategies, to alleviate the malaria burden. It is, however, essential to fully understand the behaviour of exophilic populations to make the best use of both existing and novel tools. This study was conducted to explore the resting habitat selection behaviour of Anopheles mosquitoes outdoors and identify landscape characteristics associated with the resting sites which can later be used to optimize the positioning of traps in the landscape around human habitations.
Methods
Study area description
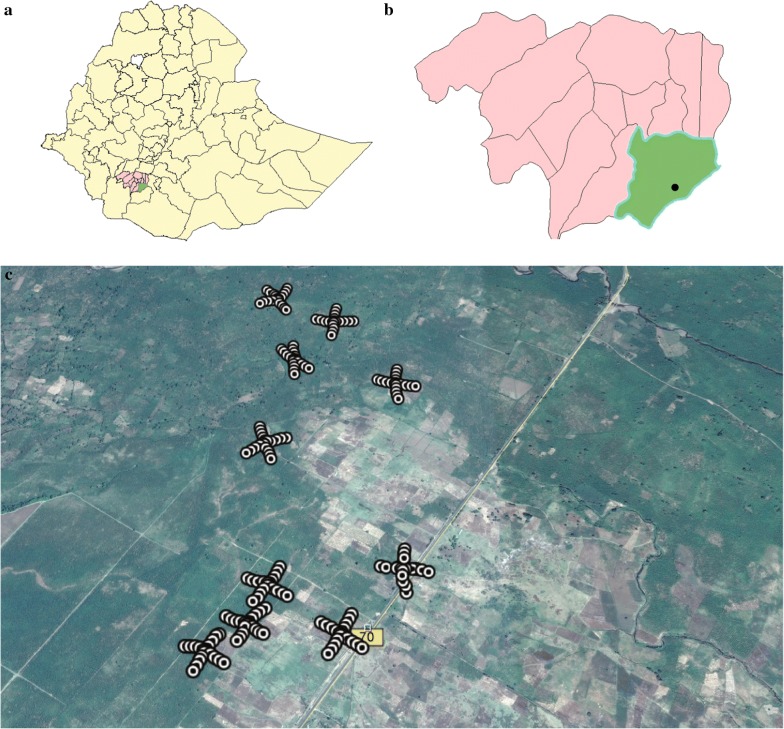
The study was conducted in southern Ethiopia in Arba Minch Zuria district of the Gamo Gofa zone near a village called Sile (5°53′24′′N, 37°29′24′′E) (Additional file 1). The study site is 517 km south of Addis Ababa, the capital city of Ethiopia, and 17 km south of the city of Arba Minch, the capital of Gamo-Gofa zone (Fig. 1). The area is characterized by bimodal rainy seasons with a long rainy period between the months of April and June, and a short rainy season between September and October. This study was conducted between September 2016 and June 2017. The annual rainfall ranges from 900 to 1300 mm, and the average annual temperature is 25 to 36 °C. Banana is the main commercial crop in the area and covers approximately half of the landmass. Maize is cultivated predominantly for subsistence and makes up approximately 20% of the land used. The presence of abundant irrigation canals in the study area, and its proximity to Lake Chamo, creates suitable breeding sites for malaria vectors, making it one of the areas with the highest malaria transmission in the Gamo Gofa zone (based on personal communication with the district health officer). Livestock rearing, including both cattle and small ruminants, is a major activity in the area, and provides potential blood meal sources for mosquitoes.
Fig. 1.
Maps showing a district map of Ethiopia indicating the Gamo-Gofa zone; b the Gamo-Gofa zone indicating Arba Minch Zuria district; and c the study area with the sampling points
Study design and mosquito collection
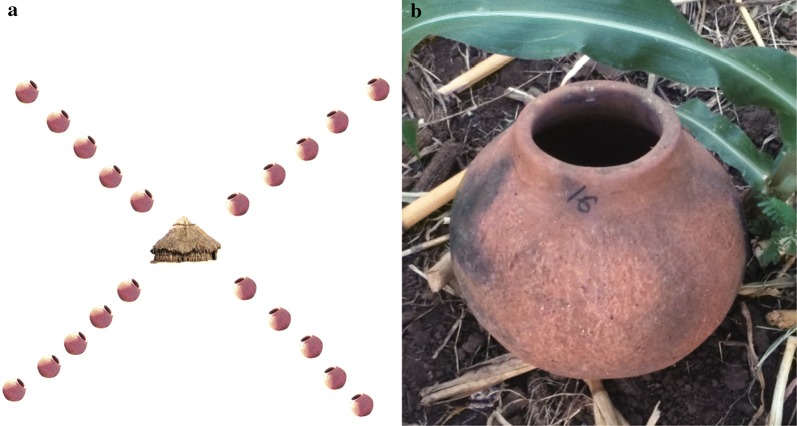
In order to identify the environmental factors affecting outdoor resting site selection by Anopheles mosquitoes, resting clay pots (Fig. 2a, b) were used to collect adult mosquitoes. The clay pots were spherical in shape and made to our specifications by local potters. The pots had an opening of approximately 15 cm, a depth of 40 cm and a capacity to hold ca. 10 l. A 2 cm hole was made at the bottom of the pots in order to avoid rain water accumulation and potential theft.
Fig. 2.
Schematic representation of the clay pot arrangement for collecting outdoor resting Anopheles mosquitoes (a) and a resting clay pot (b)
Ten isolated, inhabited houses, located a minimum of 200 m apart, were selected for the study. The selected houses had mud plastered walls with grass thatched roofs. Twenty clay pots were placed in a criss-cross pattern, with the house at the centre, and single pots being placed at 5 m, 25 m, 50 m, 75 m and 100 m away from the house in each of the four directions (Fig. 2). The hill side of the village was used to orient the position of the pots (Fig. 1).
Environmental variables
Landscape characteristics were determined within a 10 m radius from each sampling points: (1) the distance of the sampling point from the nearest house with potential blood meal sources; (2) the number of potential breeding sites; (3) the land cover type and the percentage canopy cover; and (4) the relative percentage of ground (grasses and other herbs) and tall (shrubs and trees) vegetation. The geographical location of each sampling point and the houses were recorded using a handheld GPS instrument (Additional file 1).
Mosquito sampling and identification
Sampling of mosquitoes was conducted in the morning between 06:00 and 09:00. During collection, a mosquito cage (BugDorm 32.5 cm × 32.5 cm × 32.5 cm) was placed over the opening of the clay pot, and by gently lifting and shaking the pot, as well as blowing air through the small opening at the bottom of the pot, the resting mosquitoes were encouraged into the cage. Then, the mosquitoes were aspirated from the cage, knocked down using ethyl acetate, and transported to the field laboratory.
The collected mosquitoes were counted and sorted according to species group and sex. Female mosquitoes were morphologically identified to species following Verrone [25] and Gillies and Coetzee [26], and subsequently categorized according to their abdominal status as unfed, blood fed, semi-gravid or gravid, following the categories defined by the World Health Organization [27]. Female Anopheles mosquitoes, provisionally identified as An. gambiae s.l., were individually preserved in 1.5 ml Eppendorf tubes containing silica gel and stored at ambient temperature for subsequent molecular identification to sibling species. Molecular identification of female An. gambiae s.l. was conducted using the species-specific polymerase chain reaction (PCR) technique described by Scott et al. [28].
Data analysis
Data analysis was conducted using R statistical software version 3.4.1 [29] and JMP® version 10.0.0. (SAS Institute Inc., Cary, NC, USA). As the response variable was an over-dispersed count data with unequal mean and variance, and due to the excess number of zero captures, a zero-inflated negative binomial regression with log-link function was used to model the effect of environmental factors on the number of outdoor resting Anopheles mosquitoes caught. Before conducting the regression analysis, a multiple correlation analysis was conducted to assess multicollinearity among the continuous predictor variables. Since canopy cover was positively correlated with the percentage of tall vegetation within a 10 m radius of the sampling points, the percentage of tall vegetation was removed from the subsequent model. A pairwise non-parametric Kruskal–Wallis was followed by Wilcoxon pairwise comparison post hoc test to compare the number of mosquitoes between the categories: land cover, shading, and distance from the focal house. A binomial logistic regression was conducted to predict the probability of catching at least a single Anopheles mosquito in the clay pots, followed by a backward selection of non-significant independent variables to model the count and binary outcomes.
Results
Mosquito abundance and physiological state
Surveillance of resting Anopheles mosquitoes was conducted in a rural Ethiopian setting (Fig. 1) using clay pots as artificial resting sites (Fig. 2). A total of 420 Anopheles mosquitoes (353 females and 67 males) were caught in the clay pots. Three Anopheles species/species complexes were collected, of which An. gambiae s.l. was the most abundant species with 370 (88.1%) mosquitoes, followed by Anopheles pharoensis consisting of 49 individuals (11.67%) and Anopheles tenebrosus with a single individual (0.23%). Molecular identification of An. gambiae s.l. using PCR was conducted on 63 individuals (17%) identifying all mosquitoes as Anopheles arabiensis. The physiological state of female anophelines collected from each of the land cover types demonstrated that the highest proportions caught were semi-gravid, followed by unfed (Fig. 3).
Fig. 3.
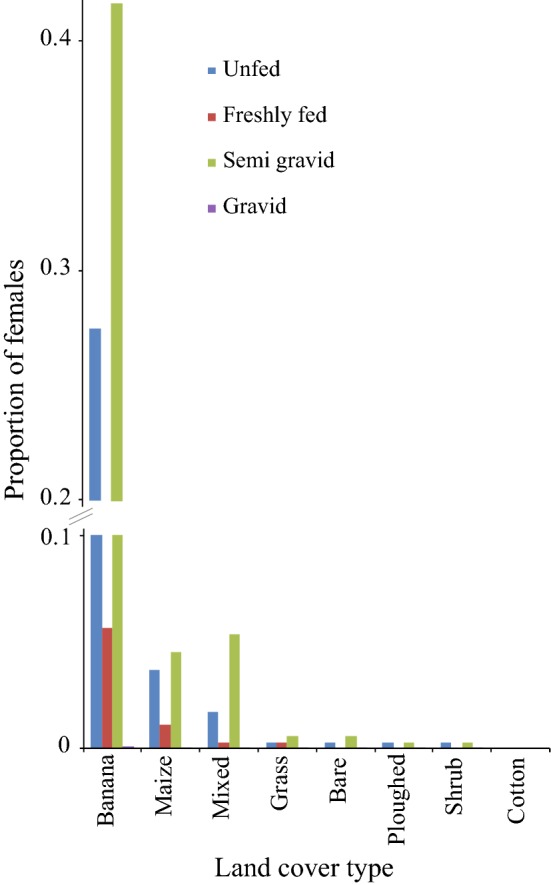
Proportion of different physiological states of Anopheles mosquitoes caught in clay pots distributed amongst different land cover types
Effect of landscape elements on mosquitoes caught
The association between the number of Anopheles mosquitoes caught and the landscape characteristics, within a 10 m radius from each sampling point, was modelled using zero-inflated negative binomial regression (log-likelihood = − 264.8; df = 13; theta = 1.19) for females and (log-likelihood = − 110.8; df = 13; theta = 1.48) for males; (Additional file 2). Backward selection of non-significant independent variables indicated that percent canopy cover (P < 0.001) and distance of sampling points from the nearest dwelling (P < 0.01) significantly affected the number of female Anopheles mosquitoes caught in the resting clay pots, as indicated from count model coefficients in the model (Table 1). Both variables are the dominant characteristics of the banana-dominated land cover, where the highest Anopheles density was recorded. The result from the zero-inflation model also indicated that the odds of having an excess number of zeroes decreased with increasing percent canopy coverage and distance of sampling points from the focal house (Table 1). In contrast, none of the predictor variables from either the count or the zero-inflation models significantly affected the male Anopheles caught (Table 1).
Table 1.
The effect of landscape characteristics within a 10 m radius of the sampling points on the number of Anopheles mosquitoes caught in resting clay pots, as shown by zero-inflated negative binomial regression, followed by backward selection of non-significant independent variables
| Variables | Estimate | Std. error | z value | Pr(> |z|) |
|---|---|---|---|---|
| Females | ||||
| Count model coefficients (negbin with log link) | ||||
| (Intercept) | − 1.2435 | 0.6274 | − 1.982 | 0.04747* |
| Distance to nearest dwelling (m) | 0.0136 | 0.0052 | 2.599 | 0.0094** |
| Percent canopy cover | 0.0238 | 0.0068 | 3.483 | 0.0005*** |
| Zero-inflation model coefficients (binomial with logit link) | ||||
| (Intercept) | 1.3602 | 0.6274 | 1.640 | 0.1010 |
| Distance to nearest dwelling (m) | − 0.0016 | 0.0087 | − 0.185 | 0.8530 |
| Percent canopy cover | − 0.0279 | 0.0094 | − 2.963 | 0.0030** |
| Males | ||||
| Count model coefficients (negbin with log link) | ||||
| (Intercept) | − 1.4289 | 1.2516 | − 1.142 | 0.2536 |
| Percent canopy cover | 0.0215 | 0.0151 | 1.420 | 0.1555 |
| Percent ground vegetation | − 0.0386 | 0.0231 | − 1.672 | 0.0946 |
| Zero-inflation model coefficients (binomial with logit link) | ||||
| (Intercept) | 2.5915 | 1.5563 | 1.665 | 0.0959 |
| Percent canopy cover | − 0.0432 | 0.0290 | − 1.490 | 0.1361 |
| Percent ground vegetation | 0.0075 | 0.0503 | 0.149 | 0.8819 |
negbin negative binomial, log link logarithmic link, logit link logistic link
* P < 0.05, **P < 0.01, ***P < 0.001
Effect of land cover, shade and distance from focal houses on mosquitoes caught
The number of mosquitoes caught in the resting clay pots was compared among land cover types, as well as shading and distance categories from the focal houses. The analysis indicated that land cover type affected the number of both female (P < 0.0001) and male anophelines (P = 0.02) caught. Most of the mosquitoes were recorded in banana-dominated land cover for both sexes (Fig. 4). Shading also had a significant positive effect on the number of both females (P < 0.0001) and males (P < 0.0001). Clay pots placed in fully shaded areas caught a higher number of Anopheles mosquitoes than those positioned in partially shaded or non-shaded areas (Table 2). The number of Anopheles caught at a distance of 5 m, 25 m, 50 m, 75 m or 100 m radius from the focal houses was also compared revealing that the number of female Anopheles mosquitoes was higher at distances farther away from the focal house (P < 0.05). However, the distance of sampling points from the focal house had no significant effect on the number of male Anopheles caught (P > 0.05) (Table 2).
Fig. 4.
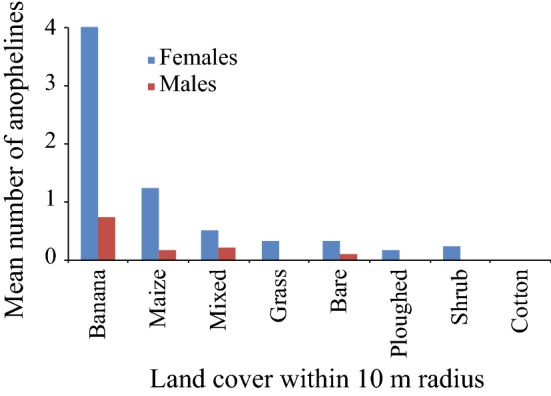
Mean number of Anopheles mosquitoes caught in the resting clay pots in different land cover types
Table 2.
The effect of categorical variables within a 10 m radius of the sampling points on the number of Anopheles mosquitoes caught in resting clay pots, as shown by Kruskal–Wallis test followed by Wilcoxon pair wise comparison method
| Category | Number | Density of mosquitoes | |
|---|---|---|---|
| Males | Females | ||
| Land cover | |||
| Banana | 69 | 0.72a | 4.04a |
| Bare | 12 | 0.08b | 0.33b |
| Cotton | 5 | 0.00b | 0.00b |
| Grass | 12 | 0.00b | 0.33b |
| Maize | 29 | 0.17ab | 1.24b |
| Mixed | 51 | 0.21ab | 0.51b |
| Ploughed | 13 | 0.00b | 0.15b |
| Shrub | 9 | 0.00b | 0.22b |
| P-value | 0.002 | 0.000 | |
| Shading | |||
| Open | 85 | 0.04a | 0.14a |
| Partial | 41 | 0.12a | 1.29b |
| Shaded | 74 | 0.80b | 3.89c |
| P-value | 0.000 | 0.000 | |
| Distance category | |||
| Within 5 m | 40 | 0.3 | 0.32a |
| Within 25 m | 40 | 0.13 | 0.73ac |
| Within 50 m | 40 | 0.25 | 2.15bc |
| Within 75 m | 40 | 0.57 | 2.55b |
| Within 100 m | 40 | 0.43 | 3.08b |
| P-value | 0.429 | 0.0115 | |
abcValues within each category in the same column, followed by the same letter are not significantly different (P > 0.05)
The probability of catching at least a single Anopheles mosquito in the resting clay pots increased with an increasing percentage of canopy cover (P < 0.0001). The rest of the environmental factors had no significant effect on the probability of catching at least one Anopheles mosquito (P > 0.05). The model showing the effect of all predictor variables on the number of mosquito caught is indicated in Additional file 3, and Table 3 shows the model after removing the non-significant predictor variables. The estimated probability of catching at least one single anopheline in relation to canopy coverage is indicated in Fig. 5.
Table 3.
The effect of percentage canopy cover within a 10 m radius of the sampling points on the presence or absence of Anopheles mosquitoes in resting clay pots, as shown by binary logistic regression
| Variables | Estimate | Std. error | z value | Pr(> |z|) |
|---|---|---|---|---|
| (Intercept) | − 2.3495 | 0.3633 | − 6.466 | 0.0011*** |
| Percent canopy cover | 0.0377 | 0.0059 | 6.389 | 0.00003*** |
Fig. 5.
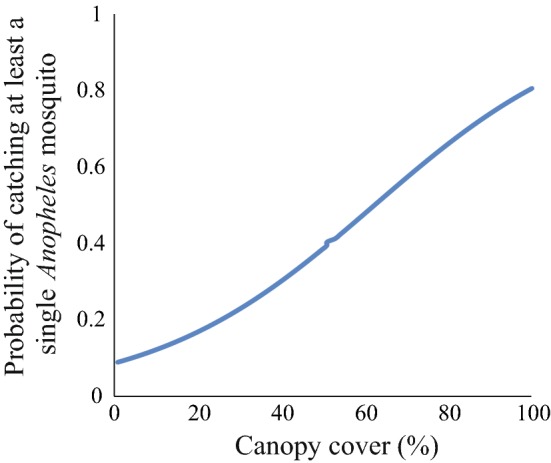
Estimated probability of catching at least one single Anopheles mosquito in relation to percent canopy coverage
Discussion
This study found that the distance of the sampling points from the focal house, the percentage of canopy cover, as well as the land cover characteristics are important landscape predictor variables influencing the resting site selection of exophilic female Anopheles mosquitoes, particularly An. arabiensis. Similarly, canopy and land cover are important factors for male Anopheles. This study reveals that female Anopheles mosquitoes fly 50–100 m away from their blood feeding environment, in contrast to males, to rest in favoured habitats, primarily banana plantations, but also maize fields, which provide optimal shade cover for both males and females. This knowledge is an important step in understanding movement patterns of Anopheles mosquitoes and provides a foundation for further studies on the development of intervention strategies that can complement the IRS and ITNs.
Among the significant explanatory variables in our study, shade is the strongest driver of the distribution of exophilic female Anopheles mosquitoes in the landscape, in line with previous studies on other mosquito species [16, 30, 31]. The two Anopheles species in this study share a preference for shaded resting sites with other Anopheles species in different geographical locations throughout the tropical and subtropical regions of the world [32]. This preference for shaded areas has been linked to the avoidance of excess water loss, as dehydration negatively influences mosquito physiology, survival and fitness [33, 34].
Despite a lack of a statistically significant difference with other land cover types, the maize-dominated areas caught the second highest number of Anopheles. It is noteworthy that maize cultivations have been shown, in this and other regions of eastern Africa, to harbour a large number of resting mosquitoes (personal observation). This is likely due to the fact that maize provides relatively high levels of shade, up to 2 m in height, in comparison with the other land cover classes, where mosquito abundance was found to be low or non-existent. Moreover, it has been shown that there is a direct link between the breeding sites and malaria prevalence during maize and other cereal crop irrigated cultivation [35–38]. The main driver for this is the maize pollen, which provide an important food source for mosquito larvae, increasing the chance of survivorship and higher pupation rate [39]. The adults that emerge from well-nourished larvae are larger in size, less susceptible to chemical insecticides, show increased biting frequency, and have longer blood meal duration and longevity; all of these biological traits are positively contributing to the vectorial capacity of the adult mosquitoes [35, 39–41].
The distance of the sampling points from the nearest house had a positive effect on the number of female Anopheles mosquitoes caught, with catches being higher further away from the house. One likely explanation of this is that sampling clay pots placed near the houses had fewer mosquitoes due to the recurrent disturbance by human and livestock activities. Furthermore, canopy cover, as the strongest predictor variable, is associated with dense banana cultivation, which is located further away from the houses. Thus, female mosquitoes may be motivated to fly a longer distance to reach a shaded refuge. This is in line with previous research, which studied the spatial movement pattern of mosquitoes from the edge of a forest into the interior [42]. Mendez et al. [42] demonstrated that mosquitoes aggregated 100 m and 200 m from the forest edge, leaving the high disturbance, low shade area. One of the pioneer works in understanding the outdoor resting behaviour of Anopheles mosquitoes was conducted by Gillies [16]. The author studied the outdoor resting behaviour of An. gambiae s.l. by using artificially constructed resting boxes placed at different distances from residential houses. The results indicated that resting boxes placed at distant positions caught a higher number of An. gambiae s.l. than resting boxes placed near the houses. However, most of the resting An. gambiae sensu stricto. mosquitoes were caught indoors. The findings of the present study are in partial agreement with the work of Gillies [16], finding that outdoor resting An. arabiensis also prefer heavily shaded resting sites providing optimal microclimate for blood meal digestion.
Conclusion
Previous studies aimed at modelling the effect of landscape characteristics on the distribution of mosquitoes have used a relatively large spatial scale of up to 1000 m to analyse the position of mosquitoes in the landscape [43, 44]. Findings presented in this study show that fine-scale spatial heterogeneity of landscape structures affects the distribution or aggregation of Anopheles mosquitoes, in line with studies on Culex pipiens estuans [45]. Here, the landscape characters are shown to be important drivers of movement patterns and resting site selection of exophilic mosquitoes. In this era of the uncertain sustainability of two major vector control strategies, IRS and ITNs, the search for novel vector control options particularly targeting outdoor populations is of great importance. Knowledge of the mosquito ecology is critical for further studies intended to develop novel monitoring and control tools that work for outdoor feeding and resting Anopheles populations.
Additional files
Additional file 1. Elevation and geographical location of each sampling point.
Additional file 2. Results obtained from zero-inflated negative binomial regression on the association between the number of Anopheles mosquitoes caught and landscape characteristics within a 10 m radius of the sampling points.
Additional file 3. Results obtained from binary logistic regression on the association between the presence or absence of Anopheles mosquitoes and landscape characteristics within a 10 m radius of the sampling points.
Authors’ contributions
The study was designed by RI, RH and SRH. RI, RH, SRH and TH supervised, and YD conducted the collection of data. YD analysed the data and drafted the manuscript together with RH. All authors critically reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the Arba Minch Zuria district health office for facilitating the study. The owners of the houses where the study was conducted are dully acknowledged for letting us use their land. We are grateful to Mr. Yonas Woyza for his indispensable assistance during the field study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data and material that are required to understand the conclusions of this article are provided in this manuscript.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was conducted after obtaining permission from the district administration health office. Verbal consent was also obtained from the house owners to obtain permission to place the resting clay pots on their farms and pasture lands.
Funding
This study was financially supported by the Swedish Research Council (VR/U-forsk) through funding to RI. The funding body had no role in the design of the study, or the collection, analysis and interpretation of the data.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- df
degrees of freedom
- IRS
indoor residual sprays
- ITN
insecticide treated nets
- IVM
integrated vector management
- LLINs
long lasting insecticidal nets
- PCR
polymerase chain reaction
References
- 1.Cibulskis RE, Alonso P, Aponte J, Aregawi M, Barrette A, Bergeron L, et al. Malaria. Global progress 2000–2015 and future challenges. Infect Dis Poverty. 2016;5:61. doi: 10.1186/s40249-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . World malaria report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 3.Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2010;30:1–8. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Global plan for insecticide resistance management in malaria vectors. Geneva: World Health Organization; 2014. [Google Scholar]
- 5.Sougoufara S, Doucouré S, Sembène PM, Harry M, Sokhna C. Challenges for malaria vector control in sub-Saharan Africa: resistance and behavioral adaptations in Anopheles populations. J Vector Borne Dis. 2017;54:4–15. [PubMed] [Google Scholar]
- 6.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moiroux N, Gomez MB, Pennetier C, Elanga E, Djènontin A, Chandre F, et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis. 2012;206:1622–1629. doi: 10.1093/infdis/jis565. [DOI] [PubMed] [Google Scholar]
- 9.Yohannes M, Boelee E. Early biting rhythm in the Afro-tropical vector of malaria, Anopheles arabiensis, and challenges for its control in Ethiopia. Med Vet Entomol. 2011;26:103–105. doi: 10.1111/j.1365-2915.2011.00955.x. [DOI] [PubMed] [Google Scholar]
- 10.Sougoufara S, Diédhiou SM, Doucouré S, Diagne N, Sembène PM, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Anopheles mosquitoes-new insights into malaria vectors; 2013. https://www.intechopen.com/books/residual-transmission-of-malaria-an-old-issue-for-new-approaches, http://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers13-11/010060049.pdf. Accessed 15 Mar 2018.
- 12.WHO . World malaria report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 13.Govella NJ, Ferguson H. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol. 2012;3:199. doi: 10.3389/fphys.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Killeen GF, Marshall JM, Kiware SS, South AB, Tusting LS, Chaki PP, et al. Measuring, manipulating and exploiting behaviours of adult mosquitoes to optimize malaria vector control impact. BMJ Global Health. 2017;2:e000212. doi: 10.1136/bmjgh-2016-000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu L, Muller GC, Marshall JM, Arheart K, Qualls WA, et al. Is outdoor vector control needed for malaria elimination? An individual based modeling study. Malar J. 2017;16:266. doi: 10.1186/s12936-017-1920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillies MT. Studies of house leaving and outside resting of Anopheles gambiae Giles and Anopheles funestus Giles in east Africa. I. The outside resting population. Bull Ent Res. 1954;45:361–374. doi: 10.1017/S0007485300027188. [DOI] [Google Scholar]
- 17.Gillies MT. Studies of house leaving and outside resting of Anopheles gambiae Giles and Anopheles funestus Giles in east Africa. II. The exodus from houses and the house resting population. Bull Ent Res. 1954;45:375–387. doi: 10.1017/S000748530002719X. [DOI] [Google Scholar]
- 18.Güneralp B, Lwasa S, Masundire H, Parnell S, Seto KC. Urbanization in Africa: challenges and opportunities for conservation. Environ Res Lett. 2017;13:015002. doi: 10.1088/1748-9326/aa94fe. [DOI] [Google Scholar]
- 19.Burkett-Cadena ND, Eubanks MD, Unnasch TR. Preference of female mosquitoes for natural and artificial resting sites. J Am Mosq Control Assoc. 2008;24:228–235. doi: 10.2987/5662.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Service MW . Mosquito ecology: field sampling methods. 2. London: Elsevier Applied Science; 1993. [Google Scholar]
- 21.Paaijmans KP, Thomas MB. The influence of mosquito resting behaviour and associated microclimate for malaria risk. Malar J. 2011;10:183. doi: 10.1186/1475-2875-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burkett-Cadena ND, McClure CJW, Estep LK, Eubanks MD. Host or habitats: what drives the spatial distribution of mosquitoes? Ecosphere. 2013;4:30. doi: 10.1890/ES13-00009.1. [DOI] [Google Scholar]
- 23.Overgaard HJ, Ekbom B, Suwonkerd W, Takagi M. Effect of landscape structure on anopheline mosquito density and diversity in northern Thailand: implications for malaria transmission and control. Landscape Ecol. 2003;18:605–619. doi: 10.1023/A:1026074910038. [DOI] [Google Scholar]
- 24.Forattini OP, Kakitani I, Massad E, Marucci D. Studies on mosquitoes (Diptera: Culicidae) and anthropic environment. A—survey of resting adults and synanthropic behaviour in South-Eastern Brazil. Rev Saude Publica. 1993;27:398–411. doi: 10.1590/S0034-89101993000600002. [DOI] [PubMed] [Google Scholar]
- 25.Verrone GA. Outline for the determination of malarial mosquitoes in Ethiopia. Mosq News. 1962;22:37–49. [Google Scholar]
- 26.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Johannesburg: South African Institute of Medical Research; 1987. [Google Scholar]
- 27.World Health Organization . Manual on practical entomology in malaria. Part 2: methods and techniques. Geneva: World Health Organization; 1975. [Google Scholar]
- 28.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 29.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2013. http://www.R-project.org/. Accessed 10 Oct 2017.
- 30.Ibry WS, Apperson CS. Spatial and temporal distribution of resting mosquitoes (Diptera: Culicidae) in the coastal plain of North Carolina. J Med Entomol. 1992;29:150–159. doi: 10.1093/jmedent/29.2.150. [DOI] [PubMed] [Google Scholar]
- 31.Githeko AK, Service MW, Mbogo CM, Atieli FK. Resting behaviour, ecology and genetics of malaria vectors in large scale agricultural areas of western Kenya. Parasitologia. 1996;38:481–489. [PubMed] [Google Scholar]
- 32.Gillies MT. Anopheline mosquitoes: vector behaviour and bionomics. In: Wernsdorfer WH, McGregor I, editors. Malaria: Principles and practice of malariology. Edinburgh: Churchill Livingstone; 1991. pp. 453–486. [Google Scholar]
- 33.Benoit JB, Patrick KR, Desai K, Hardesty JJ, Krause TB, Denlinger DL. Repeated bouts of dehydration deplete nutrient reserves and reduce egg production in the mosquito Culex pipiens. J Exp Biol. 2010;213:2763–2769. doi: 10.1242/jeb.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chown SL, Sørensen JG, Terblanche JS. Water loss in insects: an environmental change perspective. J Insect Physiol. 2011;57:1070–1084. doi: 10.1016/j.jinsphys.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Ye-Ebiyo Y, Pollack RJ, Spielman A. Enhanced development in nature of larval Anopheles arabiensis mosquitoes feeding on maize pollen. Am J Trop Med Hyg. 2000;63:9093. doi: 10.4269/ajtmh.2000.63.90. [DOI] [PubMed] [Google Scholar]
- 36.Ye-Ebiyo Y, Pollack RJ, Kiszewski A, Spielman A. Enhancement of development of larval Anopheles arabiensis by proximity to flowering maize (Zea mays) in turbid water and when crowded. Am J Trop Med Hyg. 2003;68:748–752. doi: 10.4269/ajtmh.2003.68.748. [DOI] [PubMed] [Google Scholar]
- 37.Kebede A, McCann JC, Kiszewski AE, Ye-Ebiyo Y. New evidence of the effects of agro-ecologic change on malaria transmission. Am J Trop Med Hyg. 2005;73:676–680. doi: 10.4269/ajtmh.2005.73.676. [DOI] [PubMed] [Google Scholar]
- 38.Wondwosen B, Hill SR, Birgersson G, Seyoum E, Tekie H, Ignell R. A(maize)ing attraction: gravid Anopheles arabiensis are attracted and oviposit in response to maize pollen odours. Malar J. 2017;16:39. doi: 10.1186/s12936-016-1656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kivuyo HS, Mbazi PH, Kisika DS, Munga S, Rumisha SF, Urasa FM, et al. Performance of five food regimes on Anopheles gambiae senso stricto larval rearing to adult emergence in insectary. PLoS ONE. 2014;9:e110671. doi: 10.1371/journal.pone.0110671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Araujo M, Gil LH, De-Almeida e-Silva A. Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darling under laboratory conditions. Malar J. 2012;11:261. doi: 10.1186/1475-2875-11-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliver SV, Brooke BD. The effect of larval nutritional deprivation on the life history and DDT resistance in laboratory strains of the malaria vector Anopheles arabiensis. Malar J. 2013;12:44. doi: 10.1186/1475-2875-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendez W, Liria J, Navarro J, García CZ, Freier JE, Salas R, et al. Spatial dispersion of adult mosquitoes (Diptera: Culicidae) in a sylvatic focus of Venezuelan equine encephalitis virus. J Med Entomol. 2001;38:813–821. doi: 10.1603/0022-2585-38.6.813. [DOI] [PubMed] [Google Scholar]
- 43.Moncayo AC, Edman JD, Finn JT. Application of geographic information technology in determining risk of eastern equine encephalomyelitis virus transmission. J Am Mosq Control Assoc. 2000;16:28–35. [PubMed] [Google Scholar]
- 44.Diuk-Wasser MA, Brown HE, Andreadis TG, Fish D. Modeling the spatial distribution of mosquito vectors for West Nile virus in Connecticut. USA. Vector Borne Zoonotic Dis. 2006;6:283–295. doi: 10.1089/vbz.2006.6.283. [DOI] [PubMed] [Google Scholar]
- 45.Trawinski PR, Mackay DS. Identification of environmental covariates of West Nile virus vector mosquito population abundance. Vector Borne Zoonotic Dis. 2010;10:515–526. doi: 10.1089/vbz.2008.0063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Elevation and geographical location of each sampling point.
Additional file 2. Results obtained from zero-inflated negative binomial regression on the association between the number of Anopheles mosquitoes caught and landscape characteristics within a 10 m radius of the sampling points.
Additional file 3. Results obtained from binary logistic regression on the association between the presence or absence of Anopheles mosquitoes and landscape characteristics within a 10 m radius of the sampling points.
Data Availability Statement
All data and material that are required to understand the conclusions of this article are provided in this manuscript.