Abstract
Background
Potential relationship between serum soluble programmed cell death ligand 1 and prognosis of small cell lung cancer is not well explored. The aim of the study was to reveal the prognostic significance of serum soluble programmed cell death ligand 1 in patients with small cell lung cancer.
Methods
A total of 250 small cell lung cancer patients and 250 controls were included. Research information was obtained from their medical records. Blood samples were collected on admission. Serum concentration of programmed cell death ligand 1 was measured using Enzyme-Linked Immunosorbent Assay. The patients underwent cisplatin-etoposide chemotherapy with a maximum of six cycles. Subsequently, they were followed-up for 12 months, and therapeutic response and cancer death were recorded.
Results
Serum concentration of programmed cell death ligand 1 was higher in the patients than in the controls on admission (P < 0.001). After chemotherapy, 112 patients had no response to this therapy. In the 12-month follow up period, 118 patients died due to this cancer. Multivariate Cox regression model revealed that the higher serum concentration of programmed cell death ligand 1 on admission was associated with the higher risk of no response to chemotherapy or cancer caused death (HR: 1.40, 95% CI: 1.05 ~ 1.87; HR: 1.43, 95% CI: 1.08 ~ 1.87).
Conclusion
Elevated serum concentration of soluble programmed cell death ligand 1 might be an independent risk factor for non-response to chemotherapy and cancer caused death in small cell lung cancer patients.
Keywords: Chemotherapy, Prognosis, Programmed cell death ligand 1, Response, Small cell lung carcinoma
Background
Lung cancer is the most common cause of cancer death in men and the second common cause in women around the world [1, 2]. There are two major histological lung cancers: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). SCLC accounts for about 15% of the cases [2]. Patients with SCLC primarily received systemic chemotherapy [3]. Cisplatin, etoposide, carboplatin, gemcitabine, paclitaxel, vinorelbine, topotecan, irinotecan and their combinations are commonly used in these patients [4, 5].
Programmed death 1 receptor (PD-1) and its ligand programmed death ligand 1 (PD-L1) are ectopically up-regulated in tumor tissue [6]. They attenuate the activation of T-cells and inhibit the anti-tumor immune response [7]. Due to these characteristics, PD-1 and PD-L1 are regarded as a novel target for immunotherapy, and their inhibitors have been adopted in NSCLC patients [8–10].
Several studies suggested that tissue expressions of PD-1 and PD-L1 were associated with survival in patients with SCLC or NSCLC [11, 12]. Furthermore, Okuma et al. in their study suggested that high serum level of soluble PD-L1 was prognostic for reduced survival in advanced lung cancer, but this study only included seven SCLC patients [13]. Therefore, prognostic significance of soluble PD-L1 in SCLC patients has not been well investigated.
A potential relationship between PD-L1 expression and chemotherapeutic response in NSCLC patients has been reported. Zhang et al. suggested that high expression of PD-L1 after cisplatin-based neo-adjuvant chemotherapy could be an indication of therapeutic resistance and poor prognosis in patients with NSCLC [14]. On the contrary, Ishii et al. reported that PD-L1 expression was associated with high immunoscore, and the highest immunoscore tended to have a favorable disease-free survival in NSCLC [15]. However, studies focusing on this relationship in SCLC patients are lacking.
This study aimed to evaluate the prognostic significance of soluble PD-L1 in SCLC, and explore the relationship between soluble PD-L1 and chemotherapeutic response in this disease.
Methods
The study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University.
Subjects
A total of 250 patients with SCLC were continuously included from Department of Respiratory and Critical Medicine, the First Affiliated Hospital of Zhengzhou University between January 2010 and December 2016. Adequate histological samples with abundant tumor cells from these patients were available, and all these patients were diagnosed by pathology. Patients who did not have enough histological samples were excluded from this study. And, 250 health volunteers were randomly selected from medical examination center, the First Affiliated Hospital of Zhengzhou University in the same period, and served as controls. The patients did not receive any type of anti-tumor therapy before. The subjects did not have acute myocardial infarction, unstable angina, acute cerebral infarction/hemorrhage, severe infection, autoimmune disease or other kind of cancer. The patients and controls agreed to take part in this study, and signed the written informed consents.
Data collection
Demographic and clinical information were obtained from their medical records. Tumor tissue expressions of epidermal growth factor receptor (EGFR) and kirsten rat sarcoma viral oncogene (KRAS) were obtained from their pathological records.
Enzyme-linked immunosorbent assay
Blood samples were collected from the patients and controls on admission, and were immediately centrifuged at 1000 rpm for 12 min. Serum samples were stored at − 70 °C for following determination.
Serum concentration of PD-L1 was measured using a Human/Cynomolgus Monkey PD-L1/B7-H1 Quantikine Enzyme-Linked Immunosorbent Assay (ELISA) Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instruction. Sensitivity was 4.52 pg/ml, and assay range was 25.0–1600 pg/ml. Each sample was measured twice, and the mean was reported.
Immunohistochemistry
Of the 250 SCLC patients, 98 provided available tumor tissue samples, which were stored at − 196 °C (liquid nitrogen) for further measurement.
Mature and reliable immunohistochemical staining technique was adopted to detect the PD-L1 expression in tumor tissue, and its steps were briefly introduced. First, the tissue samples were fixed in formalin (10%), embedded in paraffin and cut into 4 μm slice. Second, the slices were dewaxed in xylene, rehydrated using graded ethanol and rinsed in phosphate buffer saline. Third, antigen retrieval was performed in citrate buffer (0.01 mol/l, pH 6.0). Fourth, the slices were heated using microwave and then cooled at room temperature for 30 min. Fifth, the slices were infiltrated in hydrogen peroxide (3%) at room temperature for 20 min and incubated in goat serum at 37 °C for 40 min. Sixth, the slices were incubated with primary anti- PD-L1 monoclonal antibodies (Catalog Number: 66248–1-Ig; Proteintech, Wuhan, Hubei, China; 1:500 Dilution) at 4 °C overnight. Seventh, after washing in phosphate buffer saline, the slices were incubated with goat anti-mouse IgG (H + L), biotin conjugate secondary antibodies (Catalog Number: SA00004–1; Proteintech, Wuhan, Hubei, China; 1:500 Dilution) at 37 °C for 30 min. Eighth, the slices were exposed to 3, 3′-diaminobenzidine and counterstained in hematoxylin. Percentage of the positive staining SCLC cells was reported. If more than 5% of the SCLC cells in one tissue slice exhibited positive staining, it was considered as positive result [16].
Definition
Tumor staging was determined by the veterans administration lung cancer group (VALG) staging system [17]. Limited disease (LD) was defined as an area that was tolerably treated by one radiotherapy area, but excluded cancers with pleural or pericardial effusions. All other SCLCs were regarded as extensive disease (ED). Performance status was measured according to the eastern cooperative oncology group (ECOG) score [18].
Smoker was defined as a subject who had at least one cigarette per week for six months or more in his or her life. Other subjects were defined as non-smokers.
Therapy
The patients underwent chemotherapy with a maximum of six cycles. Their chemotherapy regimen was cisplatin and etoposide. Therapeutic response was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) [19]. There were four categories: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). In this study, CR and PR were grouped as response, and SD and PD were combined as non-response.
Follow up
All the patients were followed-up for 12 months by telephone. The process was conducted by a well-trained investigator. Major endpoint was SCLC-caused death.
Statistical analysis
Continuous variable was expressed as mean ± standard deviation (SD), and categorical variable was showed as frequency and constituent ratio. Difference of continuous variables was analyzed using independent sample t test, and difference of categorical variables was detected using chi-square test. A two-sided P < 0.05 was regarded to be statistically significant. A cutoff value for serum concentration of PD-L1 was calculated by receiver operating characteristic curve analysis. Relationship between serum concentration of PD-L1 and prognosis of SCLC patients was analyzed using multivariate Cox regression analysis. Hazard ratio (HR) and 95% confidence interval (CI) were reported. If a 95%CI included value one, it was considered statistically significant. All statistical analyses were conducted using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Results
As shown in Table 1, there were 250 SCLC patients and 250 health controls in the study. There was no difference in age and gender between these two groups (P = 0.381, P = 0.143). Smokers were more common in the SCLC patients than in the health controls (P = 0.012). Serum concentration of PD-L1 was higher in the SCLC patients than in the controls (P < 0.001).
Table 1.
Baseline characteristics of the patients and controls
| SCLC a | Control | P value | |
|---|---|---|---|
| Total (n) | 250 | 250 | – |
| Age (n) | 64.5 ± 5.8 | 65.0 ± 5.8 | 0.381 |
| Gender (n) | |||
| Female | 53 | 67 | 0.143 |
| Male | 197 | 183 | |
| Smoking history (n) | |||
| Non-smoker | 107 | 135 | 0.012 |
| Smoker | 143 | 115 | |
| Performance status | |||
| 0–1 | 218 | – | – |
| 2 | 32 | – | – |
| Tumor grade (n) | |||
| High-middle | 193 | – | – |
| Low | 57 | – | – |
| Tumor stage (n) | |||
| LDa | 143 | – | – |
| EDa | 107 | – | – |
| EGFR (n)a | |||
| Negative | 202 | – | – |
| Positive | 48 | – | – |
| KRAS (n)a | |||
| Negative | 234 | – | – |
| Positive | 16 | – | – |
| Serum PD-L1 (ng/ml)a | 7.0 ± 3.0 | 1.2 ± 0.6 | <0.001 |
aSCLC Small cell lung cancer, LD Limited disease, ED Extensive disease, EGFR Epidermal growth factor receptor, KRAS Kirsten rat sarcoma viral oncogene, PD-L1 Programmed death ligand 1
As shown in Table 2, a total of 138 patients had a response and 112 patients had no response to chemotherapy. Age, gender, smoking history, performance status, tumor grade and tumor stage were equivalent between the patients with a response and the patients without a response to chemotherapy (P = 0.472, P = 0.817, P = 0.127, P = 0.076, P = 0.169, P = 0.312). Serum concentration of PD-L1, tissue expressions of EGFR and KRAS were higher in the patients without a response than in the patients with a response to chemotherapy (P = 0.008, P = 0.016, P = 0.046).
Table 2.
Baseline characteristics of the non-response and response patients
| Non-response | Response | P value | |
|---|---|---|---|
| Total (n) | 112 | 138 | – |
| Age (n) | 64.2 ± 6.2 | 64.7 ± 5.5 | 0.472 |
| Gender (n) | |||
| Female | 23 | 30 | 0.817 |
| Male | 89 | 108 | |
| Smoking history (n) | |||
| Non-smoker | 42 | 65 | 0.127 |
| Smoker | 70 | 73 | |
| Performance status | |||
| 0–1 | 93 | 125 | 0.076 |
| 2 | 19 | 13 | |
| Tumor grade (n) | |||
| High-middle | 91 | 102 | 0.169 |
| Low | 21 | 36 | |
| Tumor stage (n) | |||
| LDa | 68 | 75 | 0.312 |
| EDa | 44 | 63 | |
| EGFR (n)a | |||
| Negative | 83 | 119 | 0.016 |
| Positive | 29 | 19 | |
| KRAS (n)a | |||
| Negative | 101 | 133 | 0.046 |
| Positive | 11 | 5 | |
| Serum PD-L1 (ng/ml)a | 7.6 ± 2.5 | 6.6 ± 3.2 | 0.008 |
aLD Limited disease, ED Extensive disease, EGFR Epidermal growth factor receptor, KRAS Kirsten rat sarcoma viral oncogene, PD-L1 Programmed death ligand 1
As shown in Tables 3, 118 patients died due to SCLC in the follow up period, and the remaining 132 patients were still living at the end of the follow up. There was no difference in age, gender, smoking history, performance status, tissue expressions of EGFR and KRAS between the dead patients and the living patients (P = 0.573, P = 0.532, P = 0.370, P = 0.063, P = 0.086, P = 0.074). More dead patients had low-grade or ED-stage tumors (P = 0.014, P = 0.007). More dead patients had no response to chemotherapy (P < 0.001). Serum concentration of PD-L1 was higher in the dead patients than in the living patients (P = 0.003).
Table 3.
Baseline characteristics of the cancer-dead and survival patients
| Cancer death | Survival | P value | |
|---|---|---|---|
| Total (n) | 118 | 132 | – |
| Age (n) | 64.2 ± 5.5 | 64.7 ± 6.1 | 0.573 |
| Gender (n) | |||
| Female | 23 | 30 | 0.532 |
| Male | 95 | 102 | |
| Smoking history (n) | |||
| Non-smoker | 47 | 60 | 0.370 |
| Smoker | 71 | 72 | |
| Performance status | |||
| 0–1 | 98 | 120 | 0.063 |
| 2 | 20 | 12 | |
| Tumor grade (n) | |||
| High-middle | 83 | 110 | 0.014 |
| Low | 35 | 22 | |
| Tumor stage (n) | |||
| LDa | 57 | 86 | 0.007 |
| EDa | 61 | 46 | |
| EGFR (n)a | |||
| Negative | 90 | 112 | 0.086 |
| Positive | 28 | 20 | |
| KRAS (n)a | |||
| Negative | 107 | 127 | 0.074 |
| Positive | 11 | 5 | |
| Chemotherapeutic response (n) | |||
| Response | 12 | 126 | <0.001 |
| Non-response | 106 | 6 | |
| Serum PD-L1 (ng/ml)a | 7.6 ± 3.1 | 6.5 ± 2.7 | 0.003 |
aLD Limited disease, ED Extensive disease, EGFR Epidermal growth factor receptor, KRAS Kirsten rat sarcoma viral oncogene, PD-L1 Programmed death ligand 1
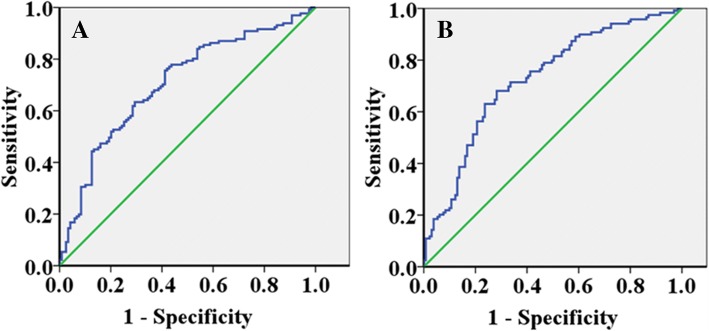
A cutoff point for serum concentration of soluble PD-L1 was 7.0 ng/ml in predicting the risk of chemotherapeutic non-response (Sensitivity = 68.7%, Specificity = 69.8%, Area under the curve = 0.707, P < 0.001) (Fig. 1a). A cutoff point for serum concentration of soluble PD-L1 was 7.1 ng/ml in predicting the risk of cancer death (Sensitivity = 73.9%, Specificity = 71.3%, Area under the curve = 0.726, P < 0.001) (Fig. 1b).
Fig. 1.
Receiver operating characteristic curve analysis of serum soluble programmed cell death ligand 1 in the patients according to the prognosis. a. A cutoff point for serum concentration of soluble programmed cell death ligand 1 was 7.0 ng/ml in predicting the risk of chemotherapeutic non-response (Sensitivity = 68.7%, Specificity = 69.8%, Area under the curve = 0.707, P < 0.001). b: A cutoff point for serum concentration of soluble programmed cell death ligand 1 was 7.1 ng/ml in predicting the risk of cancer death (Sensitivity = 73.9%, Specificity = 71.3%, Area under the curve = 0.726, P < 0.001)
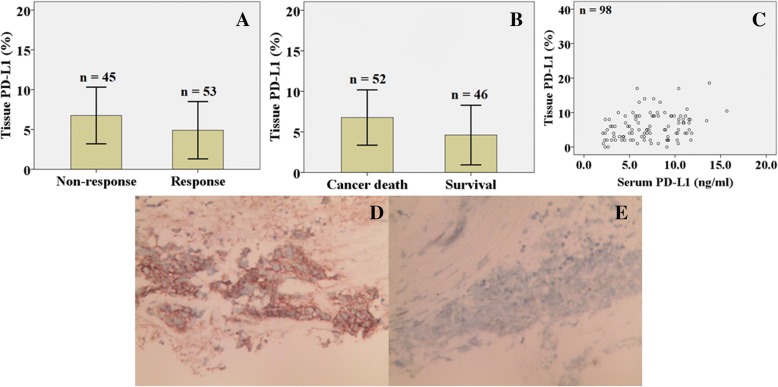
Tissue expression of PD-L1 was higher in the patients without a response than in the patients with a response to chemotherapy (6.8% ± 3.6%, 4.9% ± 3.6%, P = 0.012) (Fig. 2a). Tissue expression of PD-L1 was higher in the dead patients than in the living patients (6.8% ± 3.4%, 4.6% ± 3.7%, P = 0.003) (Fig. 2b). In Fig. 2c, there is a linear relationship between serum level of soluble PD-L1 and tissue expression of PD-L1 (r = 0.214, P = 0.034). Figure 2d and e showed positively and negatively immunohistochemical staining of PD-L1 in SCLC patients, respectively.
Fig. 2.
Tissue expression of soluble programmed cell death ligand 1 in patients with small cell lung cancer patients. a: Tissue expression of programmed cell death ligand 1 was higher in the patients without a response to chemotherapy than in the patients with a response to chemotherapy (6.8% ± 3.6%, 4.9% ± 3.6%, P = 0.012). b: Tissue expression of programmed cell death ligand 1 was higher in the dead patients than in the living patients (6.8% ± 3.4%, 4.6% ± 3.7%, P = 0.003). c: There is a linear relationship between serum level of soluble programmed cell death ligand 1 and tissue expression of soluble programmed cell death ligand 1 (r = 0.214, P = 0.034). d: Positively immunohistochemical staining of programmed death ligand 1 in patients with small cell lung cancer patients (400×). e: Negatively immunohistochemical staining of programmed death ligand 1 in patients with small cell lung cancer patients (400×)
According to the cutoff values of the serum PD-L1 level, the patients were divided into several subgroups for following analysis. As shown in Tables 4 and 5, the higher serum concentration of soluble PD-L1 was associated with the higher risk of no response to chemotherapy or SCLC caused death (HR: 1.40, 95% CI: 1.05 ~ 1.87; HR: 1.43, 95% CI: 1.08 ~ 1.87). The positive expression of PD-L1 in tissue was also associated with the higher risk of no response to chemotherapy or SCLC caused death (HR: 1.76, 95% CI: 1.13 ~ 2.78; HR: 1.86, 95% CI: 1.24 ~ 2.76). In addition, the higher expressions of EGFR and KRAS were related to the higher risk of no response to chemotherapy (HR: 1.45, 95% CI: 1.09 ~ 1.93; HR: 1.57, 95% CI: 1.09 ~ 2.27). Low-grade and ED-stage tumors were associated with the higher risk of cancer death (HR: 1.41, 95% CI: 1.08 ~ 1.85; HR: 1.42, 95% CI: 1.09 ~ 1.84).
Table 4.
Relationship between several markers and chemotherapeutic response
| Non-response (n) |
Total (n) |
Multivariate HRa (95% CI)a,b |
Multivariate HR (95% CI)c |
|
|---|---|---|---|---|
| Total | 112 | 250 | – | – |
| Tumor grade | ||||
| High-middle | 91 | 193 | Reference | Reference |
| Low | 21 | 57 | 0.78 (0.54 ~ 1.13) | 0.76 (0.53 ~ 1.11) |
| Tumor stage | ||||
| LDa | 68 | 143 | Reference | Reference |
| EDa | 44 | 107 | 0.86 (0.65 ~ 1.15) | 0.84 (0.64 ~ 1.13) |
| EGFRa | ||||
| Negative | 83 | 202 | Reference | Reference |
| Positive | 29 | 48 | 1.47 (1.11 ~ 1.95) | 1.45 (1.09 ~ 1.93) |
| KRASa | ||||
| Negative | 101 | 234 | Reference | Reference |
| Positive | 11 | 16 | 1.59 (1.11 ~ 2.29) | 1.57 (1.09 ~ 2.27) |
| Serum PD-L1a | ||||
| <7.0 ng/ml | 45 | 122 | Reference | Reference |
| ≥ 7.0 ng/ml | 67 | 128 | 1.42 (1.07 ~ 1.89) | 1.40 (1.05 ~ 1.87) |
| Tissue PD-L1 | ||||
| Negative | 17 | 50 | Reference | Reference |
| Positive | 28 | 48 | 1.75 (1.12 ~ 2.77) | 1.76 (1.13 ~ 2.78) |
aLD: Limited disease; ED: Extensive disease; EGFR: Epidermal growth factor receptor; KRAS: Kirsten rat sarcoma viral oncogene; PD-L1: Programmed death ligand 1; HR: Hazard ratio; CI: Confidence interval
bThe model was adjusted by age and gender
cThe model was adjusted by age, gender, smoking history, performance status, tumor grade, tumor stage, tissue expressions of epidermal growth factor receptor and kirsten rat sarcoma viral oncogene
Table 5.
Relationship between several markers and cancer caused death
| Cancer death (n) |
Total (n) |
Multivariate HRa (95% CI)a, b |
Multivariate HR (95% CI) c |
|
|---|---|---|---|---|
| Tumor grade | ||||
| High-middle | 83 | 193 | Reference | Reference |
| Low | 35 | 57 | 1.43 (1.10 ~ 1.86) | 1.41 (1.08 ~ 1.85) |
| Tumor stage | ||||
| LDa | 57 | 143 | Reference | Reference |
| EDa | 61 | 107 | 1.43 (1.10 ~ 1.85) | 1.42 (1.09 ~ 1.84) |
| EGFRa | ||||
| Negative | 90 | 202 | Reference | Reference |
| Positive | 28 | 48 | 1.31 (0.99 ~ 1.74) | 1.30 (0.98 ~ 1.73) |
| KRASa | ||||
| Negative | 108 | 234 | Reference | Reference |
| Positive | 10 | 16 | 1.35 (0.90 ~ 2.03) | 1.33 (0.88 ~ 2.01) |
| Serum PD-L1a | ||||
| <7.1 ng/ml | 48 | 124 | Reference | Reference |
| ≥ 7.1 ng/ml | 70 | 126 | 1.44 (1.09 ~ 1.88) | 1.43 (1.08 ~ 1.87) |
| Tissue PD-L1 | ||||
| Negative | 19 | 50 | Reference | Reference |
| Positive | 33 | 48 | 1.85 (1.24 ~ 2.75) | 1.86 (1.24 ~ 2.76) |
aLD Limited disease, ED Extensive disease, EGFR Epidermal growth factor receptor, KRAS Kirsten rat sarcoma viral oncogene, PD-L1 Programmed death ligand 1, HR Hazard ratio, CI Confidence interval
bThe model was adjusted by age and gender
cThe model was adjusted by age, gender, smoking history, performance status, tumor grade, tumor stage, tissue expressions of epidermal growth factor receptor and kirsten rat sarcoma viral oncogene
Discussion
To our knowledge, this is the first published article focusing on the relationship between serum concentration of soluble PD-L1 and prognosis of SCLC patients after chemotherapy. There were 250 patients with confirmed SCLC and 250 health controls in the study. We discovered that serum concentration of soluble PD-L1 significantly elevated in the SCLC patients, which was consistent with a previous study involving several types of advanced lung cancers [13].
A previous study suggested that soluble PD-L1 originated from PD-L1 on the surface of tumor cells by a disengagement mechanism, and serum concentration of soluble PD-L1 should be related to the tumor burden [20]. In the study, we confirmed the relationship between the serum level of soluble PD-L1 and the expression of PD-L1 in tissue. However, this did not mean that the soluble PD-L1 was also associated with the tumor burden, because the expression level of PD-L1 per unit tumor volume was diverse.
Serum PD-L1 was not a specific tumor marker, but an inflammatory and immunoregulatory marker in human [21]. Soluble PD-1 and its receptor PD-1 could be produced by peripheral immunological cells, and represented an unanticipated contributing factor to immune homeostasis [22]. Greisen et al. reported that increased concentration of soluble PD-1 was associated with disease activity and radiographic progression in rheumatoid arthritis [23]. Wu et al. and Shi et al. suggested that soluble PD-1 also contributed to the aberrant activation of T cells and the development of diseases in aplastic anemia and diabetic atherosclerotic complications, respectively [24, 25]. So, an inflammatory lesion in tumor as well as an extra-tumor inflammatory lesion could elevate the concentration of soluble PD-L1. In SCLC patients without other inflammation and immune related diseases, serum concentration of soluble PD-L1 indicated the extents of anti-tumor T cell response and immune suppression induced by PD-1 and PD-L1.
In the study, we reported a 40% increased risk of non-response to chemotherapy in the patients with relatively higher concentration of soluble PD-L1, which was consistent with one previous study by Zhang et al. [14] and was contrary to another study by Ishii et al. [15]. Both these studies included NSCLC patients and measured the tissue expression of PD-L1, but not the serum concentration of PD-L1.
Some studies had revealed the effect of PD-L1 on chemoresistance in other type of cancer. Black et al. suggested that the activation of PD-1/PD-L1 immune checkpoint conferred breast cancer cell chemoresistance associated with increased metastasis [26]. Ishibashi et al. found that the interaction between PD-1 and PD-L1 not only inhibited tumor-specific cytotoxic T lymphocytes, but also induced drug resistance in myeloma cells [27]. Tamura et al. reported that the bone marrow microenvironment up-regulated PD-1 expression on myeloma cells, which linked to aggressive myeloma-cell characteristics and insensitivity to anti-myeloma chemotherapy [28].
Tumor infiltrating lymphocyte (TIL) was regarded to be a protective factor for cancer, and it improved survival in NSCLC patients [29]. Zhang et al. suggested that the higher TIL expression rate was usually found in chemosensitive samples in NSCLC [14]. Potential association between PD-L1 and TILs was also confirmed in lung cancer patients [30]. Furthermore, miR-197/CKS1B/STAT3-mediated network regarded the tumor expression of PD-L1 as a biomarker of this cascade, and the biological interaction between PD-L1 and chemoresistance occurred through this microRNA regulatory cascade [31]. Therefore, the effect of PD-L1 on chemoresistance might be involved in the inhibition of TILs and the dysregulation of miR-197/CKS1B/STAT3-mediated cascade. These findings partly explained the effect of PD-L1 on chemotherapeutic response.
Furthermore, our study revealed a relationship between soluble PD-L1 and prognosis of SCLC patients, and the patients with higher concentration of soluble PD-L1 showed a 40% increased risk of cancer caused death in the 12-month follow up period. This result was consistent with a previous study exploring the prognostic significance of soluble PD-L1 in advanced lung cancer [13]. In the study, most of the dead patients had no response to chemotherapy. Therefore, prognostic significance of soluble PD-L1 might be related to the effect of this ligand on chemotherapeutic response.
Prognostic significance of soluble PD-L1 in other type of cancer had also been explored. Zheng et al. suggested that circulating PD-L1 expression was significantly correlated with differentiation and lymph node metastasis in total advanced gastric cancer patients [32]. Ha et al. reported that advanced biliary tract cancer patients with high soluble PD-L1 showed worse overall survival than patients with low soluble PD-L1, and high soluble PD-L1 was an independent poor prognostic factor in multivariate analysis [33]. Wang et al. revealed that overall response rate to treatment in multiple myeloma patients was higher in low soluble PD-L1 patients than in high soluble PD-L1 patients, and higher soluble PD-L1 level was an independent prognostic factor for shorter 3-year progression free survival [34]. Finkelmeier et al. discovered that soluble PD-L1 level positively correlated with the stage of cirrhosis and with stage of HCC, and patients with high serum PD-L1 concentration had an increased mortality risk, while very low PD-L1 level seemed to come along with better prognosis [35]. Rossille et al. suggested that diffuse large B-cell lymphoma patients with elevated soluble PD-L1 experienced a poorer prognosis with a 3-year overall survival of 76% versus 89% [36]. A meta-analysis from Zhu et al. indicated that high PD-L1 expression was likely to be a negative factor for patients with sarcomas and that it predicted worse survival outcomes [37].
PD-1 and PD-L1 had become a target for anti-tumor therapy. According to this understanding, a novel targeted drug nivolumab had been produced, which was an IgG4 monoclonal antibody against PD-1. It exerted its biological function through inhibiting the PD-1/PD-L1 signal pathway and allowing the immune system to scavenge the tumor cells [38]. Around the world, nivolumab had been used in the treatment of metastatic melanoma, squamous non-small cell lung cancer and renal cell carcinoma [39–41]. Potential therapeutical effect of the drug on SCLC had also been explored. A multicentre, open-label, phase 1/2 trial at 23 hospitals in six countries (CheckMate 032) reported that nivolumab monotherapy and nivolumab plus ipilimumab had an anti-tumor activity with durable responses and manageable safety profiles in previously treated patients with SCLC [42]. Furthermore, Hellmann et al. in their study reported that the efficacy of nivolumab monotherapy and nivolumab plus ipilimumab was enhanced in SCLC patients with high tumor mutational burden [43]. This promising finding required further confirmation in phase 3 randomized controlled trials in SCLC.
Conclusion
In conclusion, elevated serum concentration of soluble PD-L1 might be an independent risk factor for non-response to chemotherapy and cancer caused death in SCLC patients. Further studies should be conducted to explore the mechanisms involved.
Acknowledgments
Availability of data and materials
Not applicable. More than 80% of the subjects did not agree to disclose the data.
Abbreviations
- CI
Confidence interval
- CR
Complete response
- ECOG
Eastern cooperative oncology group
- ED
Extensive disease
- EGFR
Epidermal growth factor receptor
- ELISA
Enzyme Linked Immunosorbent Assay
- HR
Hazard ratio
- KRAS
Kirsten rat sarcoma viral oncogene
- LD
Limited disease
- NSCLC
Non-small cell lung cancer
- PD
Progressive disease
- PD-1
Programmed death 1 receptor
- PD-L1
Programmed death ligand 1
- PR
Partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SCLC
Small cell lung cancer
- SD
Stable disease
- SD
Standard deviation
- TIL
Tumor infiltrating lymphocyte
- VALG
Veterans administration lung cancer group
Authors’ contributions
JJ and SJ contributed to the study concept and design. JJ, SJ, LY, WH, NR and WJ were involved in the acquisition of data. JJ, SJ, LY, WH, NR and WJ conducted the analysis and interpretation of data. JJ, SJ, LY, WH, NR and WJ worked on the preparation of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
A written informed consent was obtained from all the patients and controls. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Consent for publication
Not applicable.
Competing interests
The authors declared that they had no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jianjun Jin, Email: jinjianjun2423@163.com.
Jiming Si, Email: sijiming342423@sina.com.
Yuanhua Liu, Email: liuyuanhua412312@sina.com.
Huanqin Wang, Email: wanghuanqin3242@sina.com.
Ran Ni, Email: niran2131231@tom.com.
Jing Wang, Email: wangjing23441@126.com.
References
- 1.Didkowska J, Wojciechowska U, Mańczuk M, Łobaszewski J. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med. 2016;4:150. doi: 10.21037/atm.2016.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Temraz S, Charafeddine M, Mukherji D, Shamseddine A. Trends in lung cancer incidence in Lebanon by gender and histological type over the period 2005-2008. J Epidemiol Glob Health. 2017;7:161–167. doi: 10.1016/j.jegh.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hann CL, Rudin CM. Management of small-cell lung cancer: incremental changes but hope for the future. Oncology (Williston Park) 2008;22:1486–1492. [PMC free article] [PubMed] [Google Scholar]
- 4.Murray N, Turrisi AT., 3rd A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. doi: 10.1016/S1556-0864(15)31579-3. [DOI] [PubMed] [Google Scholar]
- 5.Azim HA, Jr, Ganti AK. Treatment options for relapsed small-cell lung cancer. Anti-Cancer Drugs. 2007;18:255–261. doi: 10.1097/CAD.0b013e328011a547. [DOI] [PubMed] [Google Scholar]
- 6.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, Overberg P, Rose I, Basu GD, Vranic S, Lynch HT, Von Hoff DD, Hamid O. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomark Prev. 2014;23:2965–2970. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A, POPLAR Study Group Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 10.Rittmeyer Achim, Barlesi Fabrice, Waterkamp Daniel, Park Keunchil, Ciardiello Fortunato, von Pawel Joachim, Gadgeel Shirish M, Hida Toyoaki, Kowalski Dariusz M, Dols Manuel Cobo, Cortinovis Diego L, Leach Joseph, Polikoff Jonathan, Barrios Carlos, Kabbinavar Fairooz, Frontera Osvaldo Arén, De Marinis Filippo, Turna Hande, Lee Jong-Seok, Ballinger Marcus, Kowanetz Marcin, He Pei, Chen Daniel S, Sandler Alan, Gandara David R. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui S, Su X, Dong L Qian J, Ye L, Zhang T, Fu H, Han H, Huang J, Yao Y, Gu Y, Jiang L. Programmed cell death ligand 1 protein levels predicted survival of non-small cell lung cancer. J Cancer. 2017;8:4075–4082. doi: 10.7150/jca.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, Tokito T, Kinoshita T, Kage M, Hoshino T. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10:426–430. doi: 10.1097/JTO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 13.Okuma Y, Hosomi Y, Nakahara Y, Watanabe K, Sagawa Y, Homma S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer. 2017;104:1–6. doi: 10.1016/j.lungcan.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Zhang P, Ma Y, Lv C, Huang M, Li M, Dong B, Liu X, An G, Zhang W, Zhang J, Zhang L, Zhang S, Yang Y. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016;107:1563–1571. doi: 10.1111/cas.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii H, Azuma K, Kawahara A, Matsuo N, Tokito T, Kinoshita T, Yamada K, Sasada T, Akiba J, Hoshino T. Programmed cell death-ligand 1 expression and immunoscore in stage II and III non-small cell lung cancer patients receiving adjuvant chemotherapy. Oncotarget. 2017;8:61618–61625. doi: 10.18632/oncotarget.18651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jotatsu T, Oda K, Yatera K. PD-L1 immunohistochemistry in patients with non-small cell lung cancer. J Thorac Dis. 2018;10:2127–9. doi: 10.21037/jtd.2018.06.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 18.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine. 2011;56:231–238. doi: 10.1016/j.cyto.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 22.Frigola X, Inman BA, Krco CJ, Liu X, Harrington SM, Bulur PA, Dietz AB, Dong H, Kwon ED. Soluble B7-H1: differences in production between dendritic cells and T cells. Immunol Lett. 2012;142:78–82. doi: 10.1016/j.imlet.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greisen SR, Rasmussen TK, Stengaard-Pedersen K, Hetland ML, Hørslev-Petersen K, Hvid M, Deleuran B. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand J Rheumatol. 2014;43:101–108. doi: 10.3109/03009742.2013.823517. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Miao M, Zhang G, Hu Y, Ming Z, Zhang X. Soluble PD-1 is associated with aberrant regulation of T cells activation in aplastic anemia. Immunol Investig. 2009;38:408–421. doi: 10.1080/08820130902912332. [DOI] [PubMed] [Google Scholar]
- 25.Shi B, Du X, Wang Q, Chen Y, Zhang X. Increased PD-1 on CD4(+)CD28(−) T cell and soluble PD-1 ligand-1 in patients with T2DM: association with atherosclerotic macrovascular diseases. Metabolism. 2013;62:778–785. doi: 10.1016/j.metabol.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Black M, Barsoum IB, Truesdell P, Cotechini T, Macdonald-Goodfellow SK, Petroff M, Siemens DR, Koti M, Craig AW, Graham CH. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget. 2016;7:10557–10567. doi: 10.18632/oncotarget.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishibashi M, Tamura H, Sunakawa M, Kondo-Onodera A, Okuyama N, Hamada Y, Moriya K, Choi I, Tamada K, Inokuchi K. Myeloma drug resistance induced by binding of myeloma B7-H1 (PD-L1) to PD-1. Cancer Immunol Res. 2016;4:779–788. doi: 10.1158/2326-6066.CIR-15-0296. [DOI] [PubMed] [Google Scholar]
- 28.Tamura H, Ishibashi M, Yamashita T, Tanosaki S, Okuyama N, Kondo A, Hyodo H, Shinya E, Takahashi H, Dong H, Tamada K, Chen L, Dan K, Ogata K. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27:464–472. doi: 10.1038/leu.2012.213. [DOI] [PubMed] [Google Scholar]
- 29.Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA, Nason KS, Landreneau RJ, Luketich JD, Schuchert MJ. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5. doi: 10.1016/j.jss.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 30.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–755. doi: 10.1177/030089161209800612. [DOI] [PubMed] [Google Scholar]
- 31.Fujita Y, Yagishita S, Hagiwara K, Yoshioka Y, Kosaka N, Takeshita F, Fujiwara T, Tsuta K, Nokihara H, Tamura T, Asamura H, Kawaishi M, Kuwano K, Ochiya T. The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer. Mol Ther. 2015;23:717–727. doi: 10.1038/mt.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Z, Bu Z, Liu X, Zhang L, Li Z, Wu A, Wu X, Cheng X, Xing X, Du H, Wang X, Hu Y, Ji J. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014;26:104–111. doi: 10.3978/j.issn.1000-9604.2014.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha H, Nam AR, Bang JH, Park JE, Kim TY, Lee KH, Han SW, Im SA, Kim TY, Bang YJ, Oh DY. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016;7:76604–76612. doi: 10.18632/oncotarget.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Wang H, Chen H, Wang WD, Chen XQ, Geng QR, Xia ZJ, Lu Y. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget. 2015;6:41228–41236. doi: 10.18632/oncotarget.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finkelmeier F, Canli Ö, Tal A, Pleli T, Trojan J, Schmidt M, Kronenberger B, Zeuzem S, Piiper A, Greten FR, Waidmann O. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer. 2016;59:152–159. doi: 10.1016/j.ejca.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, Le Gouill S, Haioun C, Tarte K, Lamy T, Milpied N, Fest T, Groupe Ouest-Est des Leucémies et Autres maladies du sang; Groupe Ouest-Est des Leucémies et Autres maladies du sang High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28:2367–2375. doi: 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z, Jin Z, Zhang M, Tang Y, Yang G, Yuan X, Yao J, Sun D. Prognostic value of programmed death-ligand 1 in sarcoma: a meta-analysis. Oncotarget. 2017;8:59570–59580. doi: 10.18632/oncotarget.19168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunturi A, McDermott DF. Nivolumab for the treatment of cancer. Expert Opin Investig Drugs. 2015;24:253–260. doi: 10.1517/13543784.2015.991819. [DOI] [PubMed] [Google Scholar]
- 39.Nomura M, Otsuka A, Kondo T, Nagai H, Nonomura Y, Kaku Y, Matsumoto S, Muto M. Efficacy and safety of retreatment with nivolumab in metastatic melanoma patients previously treated with nivolumab. Cancer Chemother Pharmacol. 2017;80:999–1004. doi: 10.1007/s00280-017-3444-0. [DOI] [PubMed] [Google Scholar]
- 40.Grossi F, Crinò L, Logroscino A, Canova S, Delmonte A, Melotti B, Proto C, Gelibter A, Cappuzzo F, Turci D, Gamucci T, Antonelli P, Marchetti P, Santoro A, Giusti S, Di Costanzo F, Giustini L, Del Conte A, Livi L, Giannarelli D, de Marinis F. Use of nivolumab in elderly patients with advanced squamous non-small-cell lung cancer: results from the Italian cohort of an expanded access programme. Eur J Cancer. 2018;100:126–134. doi: 10.1016/j.ejca.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Gunjur A. Nivolumab plus ipilimumab in advanced renal-cell carcinoma. Lancet Oncol. 2018;19:e232. doi: 10.1016/S1470-2045(18)30257-2. [DOI] [PubMed] [Google Scholar]
- 42.Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, Morse MA, Ascierto PA, Horn L, Amin A, Pillai RN, Evans J, Chau I, Bono P, Atmaca A, Sharma P, Harbison CT, Lin CS, Christensen O, Calvo E. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 43.Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G, Szustakowski JD, Sasson A, Golhar R, Vitazka P, Chang H, Geese WJ, Antonia SJ. Tumor mutational burden and efficacy of Nivolumab monotherapy and in combination with Ipilimumab in small-cell lung Cancer. Cancer Cell. 2018;33:853–861. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable. More than 80% of the subjects did not agree to disclose the data.