Abstract
Background
Cecropin A (CeA), a natural cationic antimicrobial peptide, exerts potent antimicrobial activity against a broad spectrum of Gram-positive and Gram-negative bacteria, making it an attractive candidate substitute for antimicrobials. However, the low production rate and cumbersome, expensive processes required for both its recombinant and chemical synthesis have seriously hindered the exploitation and application of CeA. Here, we utilized a short β-structured self-aggregating protein, ELK16, as a fusion partner of CeA, which allowed the efficient production of high-purity CeA antibacterial peptide with a simple inexpensive process.
Results
In this study, three different approaches to the production of CeA peptide were investigated: an affinity tag (His-tag)-fused protein expression system (AT-HIS system), a cell-free protein expression system (CF system), and a self-assembling peptide (ELK16)-fused protein expression system (SA-ELK16 system). In the AT-HIS and CF systems, the CeA peptide was obtained with purities of 92.1% and 90.4%, respectively, using one or more affinity-chromatographic purification steps. The procedures were tedious and costly, with CeA yields of only 0.41 and 0.93 μg/mg wet cell weight, respectively. Surprisingly, in the SA-ELK16 system, about 6.2 μg/mg wet cell weight of high-purity (approximately 99.8%) CeA peptide was obtained with a simple low-cost process including steps such as centrifugation and acetic acid treatment. An antimicrobial test showed that the high-purity CeA produced in this study had the same antimicrobial activity as synthetic CeA peptide.
Conclusions
In this study, we designed a suitable expression system (SA-ELK16 system) for the production of the antibacterial peptide CeA and compared it with two other protein expression systems. A high yield of high-purity CeA peptide was obtained with the SA-ELK16 system, which greatly reduced the cost and time required for downstream processing. This system may provide a platform for the laboratory scale production of the CeA antibacterial peptide.
Electronic supplementary material
The online version of this article (10.1186/s12896-018-0473-7) contains supplementary material, which is available to authorized users.
Keywords: Self-aggregating protein, Cecropin A, Antimicrobial peptide, ELK16, Escherichia coli
Background
Antimicrobial peptides (AMPs) are small peptides of 10–50 amino acids that are effective in the innate immune systems of a wide variety of organisms, including bacteria, plants, insects, and mammals [1–3]. In general, they are cationic, amphipathic, β-folded or α-helix peptides that display rapid, strong, and broad-spectrum activities against a wide range of pathogens, including bacteria, fungi, plants, and even insects [4–6].
In recent decades, the widespread use of antimicrobials has resulted in a rapid increase in antimicrobial resistance, which has increased the worldwide morbidity [7] and economic losses [8, 9]. This has led to enormous efforts to explore and develop new antimicrobial agents, such as AMPs. Moreover, because of their broad spectrum of antibacterial properties but no hemolytic or cytotoxic activities, AMPs are considered attractive targets for the development of new antimicrobial compounds [10, 11]. Cecropin A (CeA) is a natural linear cationic α-helical AMP isolated from insects [12–14]. It contains a strongly cationic region at its N-terminus and a large hydrophobic tail at its C-terminus, which allow its interaction with the microbial membrane, and then induces cell lysis by pore formation [1]. It has a wide spectrum of antimicrobial activities against Gram-negative bacteria, Gram-positive bacteria, and fungal phytopathogens [15]. More importantly, this peptide does not induce the lysis of erythrocytes or lymphocytes, even at high concentrations [16]. Therefore, the CeA peptide has become a potentially useful antimicrobial substance in biochemical and pharmaceutical research [17].
Because the chemical synthesis of the CeA peptide or extraction from natural sources are extremely expensive, with low yields [6, 18–20], the development of a simple way to efficiently produce high-purity CeA peptide is urgently required. In recent years, there has been much research into the production of CeA and its derivatives [21–24]. To reduce the production costs, Pichia pastoris and Bacillus subtilis have been used as the expression hosts for the secretion of the CeA peptide [23, 25, 26]. To avoid the formation of inclusion bodies, some researchers have fused the peptide to glutathione S-transferase (GST) or thioredoxin (TRX), which significantly increased the soluble fraction of the peptide [16, 21, 27]. To obtain high-purity CeA peptide, affinity tags such as the hexahistidine (His6) tag, cysteine protease domain tag, and strep tag, have been used, and high-purity peptide produced [24, 28, 29]. However, all these methods still require expensive purification with affinity chromatography or high-performance liquid chromatography and the yields are relatively low. More importantly, the peptide toxicity for host cells and the proteolysis of the product are still two obstacles to the high production of the CeA peptide.
Recently, a small β-strand self-assembling peptide, ELK16, which induces the formation of “active inclusion bodies” in vivo, has attracted the attention of many researchers [30, 31]. Based on the self-assembling property of ELK16, a CeA fusion protein containing Mxe GyrA intein and ELK16 was constructed and expressed in E. coli BL21(DE3) cells (SA-ELK16 system). Besides, two other approaches were also investigated: an affinity tag (His tag) fusion protein expression system (AT-HIS system) and a cell-free protein expression system (CF system). We compared the costs and yields of each process, and thus established a simple, inexpensive, single-step purification method for the efficient production of high-purity CeA peptide. This method provides a novel platform for the laboratory large-scale production of CeA peptide.
Results
Design and construction of three different CeA fusions
Because AMPs are toxic to host cells and are readily degraded by proteases [32], various proteins such as small ubiquitin-related modifier (SUMO) [33, 34], GST [35], and TRX [31, 36] were selected as fusion partners for the efficient expression of AMPs. Here, we incorporated a self-cleaving peptide Mxe GyrA intein [37] as the fusion carrier in the AT-HIS system to avoid the degradation of CeA by proteases to improve its expression. High-purity CeA peptide was released by the self-cleavage of Mxe GyrA intein and then purified with nickel ion affinity chromatography (fusion mode “CeA–Mxe–His”, shown in Fig. 1a). In the CF expression system, a His6 tag was fused to the CeA peptide by RF cloning to allow its downstream purification. RF cloning is a simple and restriction-free method for insertion of foreign DNA into a plasmid at any desired location [38]. To achieve the high-level expression of the CeA peptide, two different fusions were constructed: N-terminal fusion (His6–CeA) or C-terminal fusion (CeA–His6) (fusion mode “His6–CeA” or “CeA–His6”, respectively; shown in Fig. 1a). Recent studies have reported that some peptides like ELK16 can assemble into aggregates when expressed in E. coli. Based on the results of this study, ELK16 was selected and attached to the C-terminus of Mxe GyrA intein (CeA–Mxe–ELK16) in the SA-ELK16 system to induce and increase the formation of active fusion protein aggregates in E. coli (fusion mode “CeA–Mxe–ELK16”, shown in Fig. 1a). These aggregates were then separated by simple centrifugation and high-purity CeA peptide was successfully released into soluble fractions after dithiothreitol (DTT)-induced Mxe GyrA intein cleavage and acetic acid treatment (Fig. 1b).
Fig. 1.
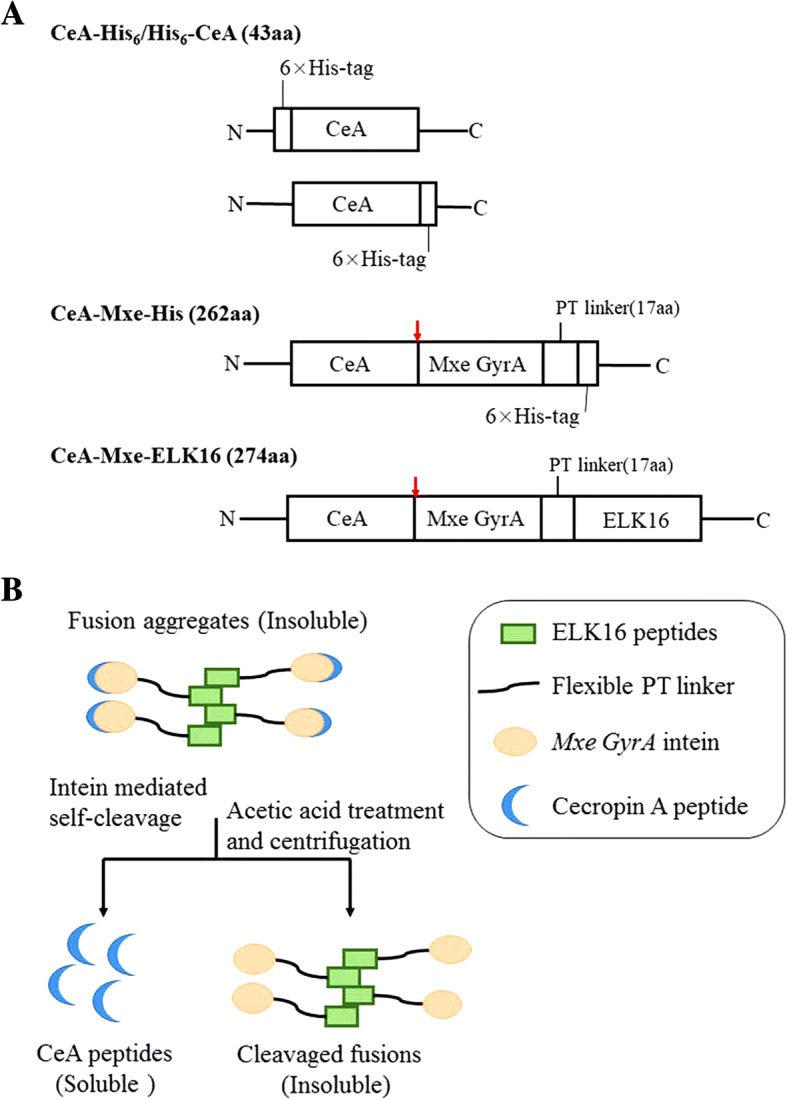
Schematic diagram of three different fusions and quick purification of CeA–Mxe–ELK16 fusion. a Compositions of the three different fusions (CeA–Mxe–His, CeA–His6 or His6–CeA, and CeA–Mxe–ELK16) used in the three different expression systems (AT-HIS system, CF system, and SA-ELK16 system, respectively). “N” and “C” represent the amino and carboxy terminals of the polypeptide; Red arrow indicates the cleavage site of Mxe GyrA intein. b Easy simple purification process for CeA–Mxe–ELK16 fusion
Expression, optimization and purification of CeA peptide with the AT-HIS system
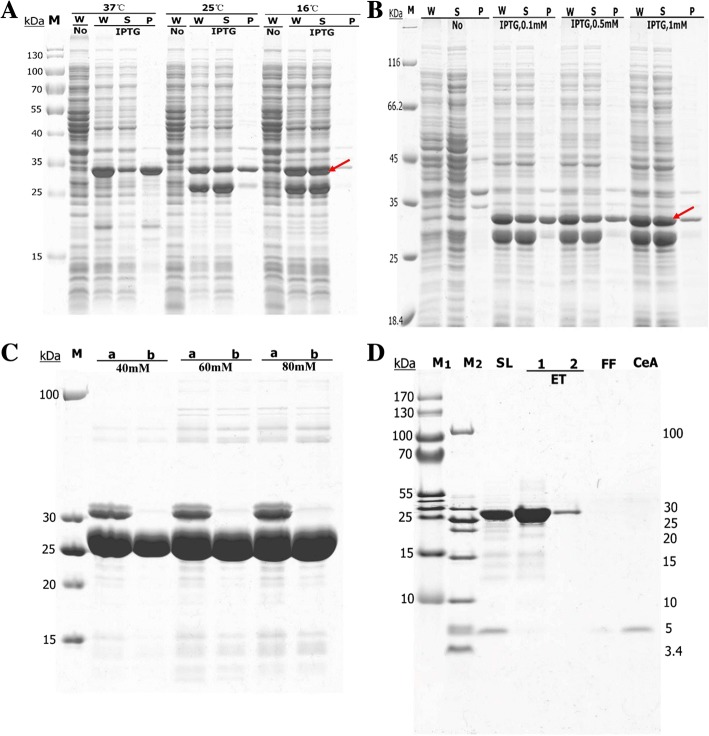
E. coli BL21(DE3) cells harboring plasmid pET21a–AM–His were cultured at 37 °C and isopropyl β-D-1-thiogalactopyranoside (IPTG) was added when the optical density of the cells at a wavelength of 600 nm (OD600) reached 0.6–0.8 to initiate the expression of CeA–Mxe–His fusion. To achieve high-level expression of the soluble CeA–Mxe–His fusion, different incubation temperatures (37 °C, 25 °C, and 16 °C) were investigated. As shown in Fig. 2a, large amounts of the CeA–Mxe–His fusion were successfully expressed in E. coli BL21(DE3) cells at all temperatures (bands marked with red arrow). However, the fusion polypeptide was expressed and deposited primarily in inclusion bodies when the cells were incubated at 37 °C for 5 h. When the temperature was reduced to 25 °C or 16 °C, large amounts of soluble CeA–Mxe–His fusion were produced (Fig. 2a). Different concentrations of IPTG (0.1, 0.5, or 1 mM) were also tested. As shown in Fig. 2b, a sufficient amount of soluble fusion was produced after induction with 0.1 mM IPTG and there was no obvious increase in the amount of CeA–Mxe–His fusion when 0.5 or 1 mM IPTG was used. In summary, we selected 25 °C, 0.1 mM IPTG and 12 h as the optimal conditions for the expression of CeA–Mxe–His fusion.
Fig. 2.
Expression, optimization, and purification of CeA peptide with AT-HIS system in E. coli. Competent E. coli BL21(DE3) cells were transformed with plasmid pET21–CeA–Mxe–His, which produced the CeA–Mxe–His fusion. Cells carrying plasmid pET21–CeA–Mxe–His were cultured in LB medium to express the fusion. To induce high expression, different temperatures (16 °C, 25 °C, or 37 °C) (A) and a series of IPTG concentrations (B) were used. Different concentrations of DTT (40–80 mM) were tested to optimize the efficiency of Mxe GyrA intein cleavage (C). High-purity CeA peptide was obtained after nickel ion affinity chromatography (D). Red arrow indicates CeA–Mxe–His fusion; NO, the cells were not induced with IPTG; W, whole-cell lysate; S, supernatant fractions; and P, insoluble pellets of induced cells after sonication. “a” and “b” in C indicate the sample before and after cleavage by DTT, respectively. In D: SL, supernatant before it was loaded onto the column; ET, elution fraction; FF, fast-flow fraction; CeA, final concentrated CeA peptide. M, M1 and M2, protein markers of different molecular weights
The CeA–Mxe–His fusion was purified with nickel ion affinity chromatography and high-purity CeA–Mxe–His fusion was collected (Additional file 1). To obtain more CeA peptide, the efficiency of Mxe GyrA intein cleavage was optimized. Figure 2c shows that nearly 100% of the CeA–Mxe–His fusion was successfully cleaved by the addition of 40 mM DTT, which is consistent with other reports [34, 37], and the CeA peptide was successfully released from the fusion (the band at 5 kDa in line SL in Fig. 2d). Approximately 92.1% of the CeA peptide was obtained after a second round of nickel ion affinity chromatography, with a final yield estimated to be 0.41 μg/mg wet cell weight (Fig. 2d and Table 1). Unfortunately, severe intracellular self-cleavage of the CeA–Mxe–His fusion in the AT-HIS system could not be prevented, even when the low temperature (16 °C) and low concentration of IPTG (0.1 mM) were used, which greatly reduced the production of CeA peptide (two major bands in Fig. 2a and b).
Table 1.
Summary of the expression and purification schemes in the three different systems
| Expression systems | AT-HIS expression system | CF expression system | SA-ELK16 expression system |
|---|---|---|---|
| Required steps | Step 1: Expression of fusion protein CeA-Mxe-PT-His6 | Step 1: Expression of antibacterial peptide His6-CeA | Step 1: Expression of fusion protein CeA-Mxe-ELK16 |
| Step 2: Cell disruption and supernatant collection | Step 2: Ni2+ affinity chromatography | Step 2: Cell disruption and acquisition of protein aggregates | |
| Step 3: Ni2+ affinity chromatography | Step 3: Intein-mediated cleavage | ||
| Step 4: Intein-mediated cleavage | Step 4: acetic acid treatment and collection of CeA peptide. | ||
| Step 5: The second Ni2+ affinity chromatography | |||
| Time consuming | 5 days | 2 days | 2 days |
| Final yield of peptide production (μg/mg wet cell pellet) a | 0.41 | 0.93 b | 6.20 |
| Purity of bio-produced Cecropin A peptide (%) | 92.1 | 90.4 | 99.8 |
aYield of cecropin A peptide after intein-mediated cleavage in LB culture: 2.87 ± 0.62 mg/ml wet cell weight in the SA-ELK16 system and 1.48 ± 0.48 mg/ml wet cell weight in the AT-HIS system, estimated with the densitometric analysis software Gel-Pro Analyzer or BCA Protein Assay Kit
bYield of CeA peptide in CF system, 0.93 μg/ml of reaction mixture
Expression and purification of CeA peptide with the CF system
In the CF system, two different plasmids (pET21–His6–CeA and pET21–CeA–His6) were constructed to express the CeA peptide. The purified plasmid DNA was added as the template to the cell free reaction mixtures (RMs) and expressed at 30 °C for 16–18 h and then analyzed by tricine SDS-PAGE. However, the CeA peptide was not expressed when plasmid pET21–CeA–His6 was added to RM as the template (data not show). Fortunately, as shown in Fig. 3a, His6–CeA was successfully expressed as the soluble form, which suggests that the N-terminal fusion of the His tag improved the expression of the CeA peptide. Different concentrations (12–22 mM) of Mg2+ were also evaluated for enhanced expression of CeA peptide. Results in Fig. 3a shows that the expression of the CeA peptide increased with increments in the Mg2+ concentration and the highest production of soluble CeA peptide was achieved at an Mg2+ concentration of 16 mM. A further increase in the Mg2+ concentration did not improve the expression of the soluble CeA peptide, so an optimum Mg2+ concentration of 16 mM was selected.
Fig. 3.
Expression and purification of CeA peptide in CF system. a Different Mg2+ ion concentrations (12–22 mM) were tested to optimize His6–CeA production in the CF system. The supernatant and pellet fractions of RM were separated by centrifugation at 12,000 g for 5 min and analyzed with tricine SDS–PAGE stained with Coomassie Blue. S, supernatant samples; P, pellet samples. Red frame contains the band of CeA peptide, processed with high gray-scale resolution. b Purification of His6–CeA with Ni2+-NTA affinity chromatography. SL, samples containing His6–CeA before purification; FF, flow-through fraction; ET (1–4), elution fractions with imidazole; M1, M2, different protein markers, in kDa
The CeA peptide was purified with nickel ion affinity chromatography, as reported previously [39]. As shown in Fig. 3b, the amount of CeA peptide successfully purified was about 0.93 μg/ml of reaction mixture, with a purity of approximately 90.4% (Table 1).
High-purity CeA peptide released in the SA-ELK16 system
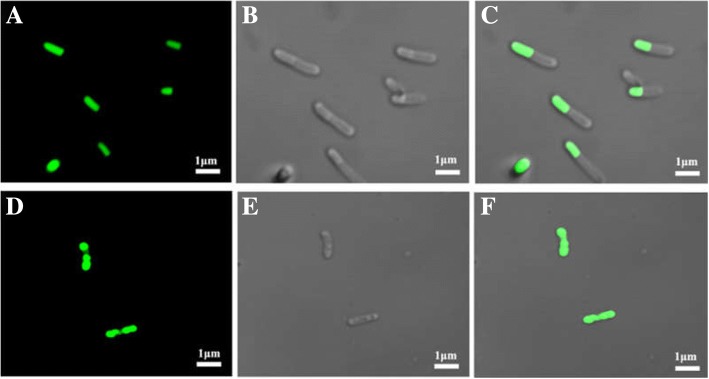
Previous studies have reported that some polypeptides like ELP, 18A, and ELK16 can induce the formation of aggregates in vivo [30, 40–42]. To verify the self-assembly property of ELK16, two different fusions, GFP–Mxe–ELK16 and GFP–Mxe-His, were constructed, expressed in E. coli BL21(DE3) cells, and then analyzed with fluorescence confocal microscopy. As shown in Fig. 4, the fluorescent signal clearly localized the cellular distribution of GFP–Mxe–ELK16 (Fig. 4a), whereas the GFP–Mxe–His expressing cells displayed uniform green fluorescence throughout the cytoplasm (Fig. 4d). These data indicate that the ELK16 fusion could induce the formation of active protein aggregates in E. coli BL21(DE3) cells.
Fig. 4.
Differences in localization and form of the ELK16 fusion and HIS fusion in E. coli. a–c Confocal fluorescence micrographs of GFP–Mxe–ELK16 fusion protein; d–f, confocal fluorescence micrographs of GFP–Mxe–HIS fusion protein. a and d Green fluorescent images; b and e, bright-field images; c and f, merged images. Scale bars represent 1 μm
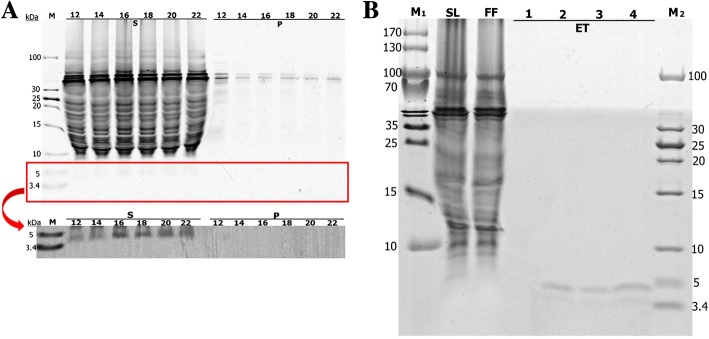
Based on the above results, ELK16 was chosen as the carrier protein fused to the CeA peptide (CeA–Mxe–ELK16) to induce the formation of active inclusion bodies in E. coli, achieving the highly efficient production of the CeA peptide. To prevent the formation of inclusion bodies, a lower temperature (16 °C) was selected as the induction temperature for 24 h, and different concentrations of IPTG (0.2, 0.5, and 1 mM) were tested. As shown in Fig. 5, a major band at approximately 30 kDa was observed, indicating that the CeA–Mxe–ELK16 fusion was successfully expressed. Approximately 50.23–78.16 μg/mg wet cell weight accumulated as insoluble aggregate and there was no intracellular self-cleavage of the fusion which reduced the toxicity to the host cells (Table 2). There was a slight increase in the expression of the CeA–Mxe–ELK16 fusion at a higher IPTG concentration (0.5 or 1 mM), and ultimately 1 mM IPTG was selected as the optimal concentration.
Fig. 5.
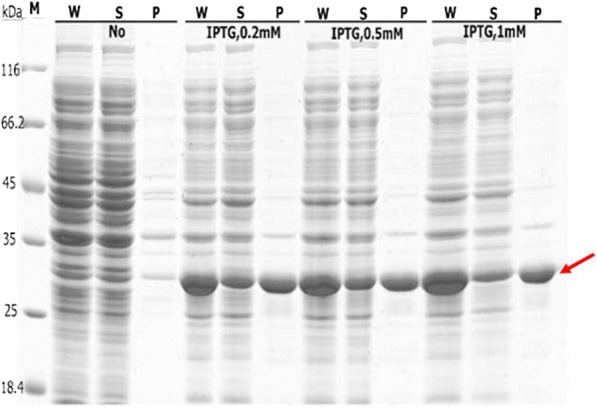
Expression of CeA–Mxe–ELK16 fusion in the SA-ELK16 system and its optimization. Cells producing the CeA–Mxe–ELK16 fusion were cultured at 16 °C for 24 h. Different concentrations of IPTG (0.2–1.0 mM) were tested to maximize expression. Positions corresponding to the CeA–Mxe–ELK16 fusion are marked with red arrows. NO, the cells were not induced with IPTG; W, whole-cell lysate; S, supernatant fractions; and P, insoluble pellets of induced cells
Table 2.
Quantification of cecropin A peptides cleaved from the fusions in the three different schemes
| Expression systems | MW (kDa) | Fusions yielda (μg/mg wet cell pellet) | Peptide yieldb (μg/mg wet cell pellet) | Cleavage effciencyc (%) | Percent recoveryd (%) | Peptide puritye (%) |
|---|---|---|---|---|---|---|
| CF system | 4.6 | ─ | 0.93f | ─ | ─ | 90.4 |
| AT-HIS system | 4.4 | 4.85 | 0.41 | 100 | 52.14 | 92.1 |
| SA-ELK16 system | 4.4 | 51.16 | 6.20 | 79.50 | 78.38 | 99.9 |
aYield of fusion proteins in the form of aggregates or soluble fractions
bYield of cecropin A peptides after intein-mediated cleavage in LB culture: 2.87 ± 0.62 mg/ml wet cell weight in the SA-ELK16 system and 1.48 ± 0.48 mg/ml wet cell weight in the AT-HIS system, estimated with the densitometric analysis software Gel-Pro Analyzer or BCA Protein Assay Kit
cCleavage efficiency was calculated by dividing the amount of cleaved protein aggregate by the amount of total aggregate before cleavage
dPercentage recovery of cecropin A peptide was calculated by dividing the mass of cecropin A peptide after cleavage by the mass that could be theoretically obtained from the fusion protein
ePurity of CeA peptide was calculated with the BandScan software
fYield of CeA peptide in the CF system was 0.93 μg/ml of reaction mixture
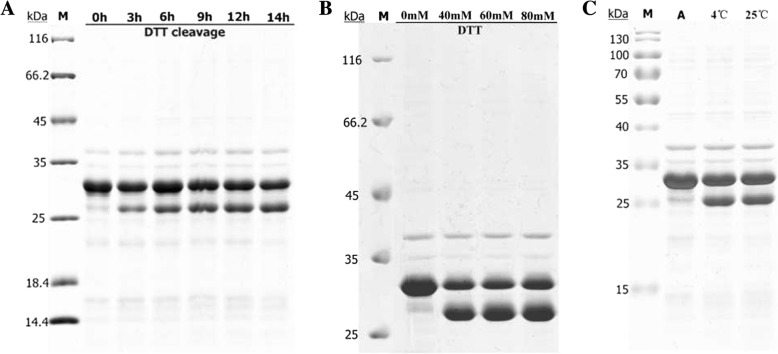
To obtain more CeA peptide, the efficiency of Mxe GyrA intein cleavage was investigated and optimized. The CeA–Mxe–ELK16 aggregates were first subjected to cleavage with 40 mM DTT at 25 °C for 0–14 h. As shown in Fig. 6a, about 22.7% of the aggregates were cleaved after 3 h, and when the incubation time was extended to 12 h, nearly 46.7% of the fusion was cleaved, and this proportion remained constant with longer incubation time. DTT concentrations of 40–80 mM were also tested at 25 °C for 12 h, and 55–76.2% of the fusion aggregate was cleaved, indicating that 40 mM DTT was sufficient to induce the cleavage of the majority of CeA–Mxe–ELK16 aggregates (Fig. 6b and Table 2). Considering high temperature (25 °C) can reduce the stability and activity of the CeA peptide, different incubation temperatures (including 25 °C and 4 °C) were tested. As shown in Fig. 6c, incubation of the samples at 4 °C and 25 °C generated the same amounts of cleaved fusion aggregates. Based on these results, we performed all subsequent cleavage reactions with 40 mM DTT at 4 °C for 12 h. Then the cleaved samples were centrifuged at 12,000 g for 30 min at 4 °C. Unfortunately, the released CeA peptide remained in the insoluble fraction after centrifugation (band marked with a red star in Fig. 7-P1). According to recent studies, acetic acid treatment can denature and precipitate the majority of E. coli proteins [30, 43]. Moreover, the structure of CeA peptide is simple (only two α helices with no disulphide bond) and has a higher flexibility (it can fit its conformation to different circumstances) [44, 45]. Therefore, CeA peptide can resume a suitable structure in PBS buffer after acetic acid treatment. Based on this principle, different concentrations of acetic acid (0.5–3% [v/v]) were added to the cleaved samples to solubilize the CeA peptide. As can be seen in Fig. 7, CeA peptide of high purity (approximately 99.8%, Table 2) was successfully dissolved in the soluble fraction when the cleaved samples were incubated with acetic acid. Interestingly, increasing amounts of CeA peptide were dissolved in the soluble fraction as the increased amount addition of acetic acid added. Ultimately, the addition of 2.5% acetic acid was selected, because higher concentrations of acetic acid may affect the stability of CeA peptide. The dissolved CeA peptide was then concentrated and dialized in phosphate-buffered saline (PBS) for further study.
Fig. 6.
Combinatorial optimization analysis of Mxe GyrA intein cleavage activity in CeA–Mxe–ELK16 fusion. Different incubation times (a) and concentrations of DTT (40–80 mM) (b) were used to optimize cleavage activity. Different incubation temperatures (4 °C or 25 °C) were also tested (c). P, samples before cleavage with DTT; M, protein marker
Fig. 7.
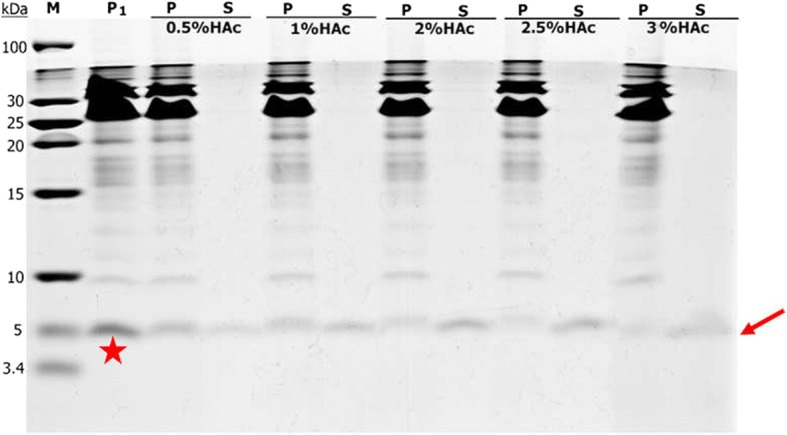
High-purity CeA peptide released with acetic acid treatment. Different concentrations of acetic acid (0.5–3% [v/v]) were used to release high-purity CeA peptide. Position corresponding to high-purity CeA peptide is marked with a red arrow. Red star indicates CeA peptide released from fusion protein. P1, insoluble fraction of the cleaved fusion protein; P, insoluble fraction after acetic acid treatment; S, soluble fraction after acetic acid treatment
Antibacterial properties of the bioproduced CeA peptide
The in vitro antimicrobial activity against E. coli ATCC 25922 of the CeA peptide bio-produced with the three different expression systems was determined as the minimal inhibitory concentrations (MICs) with the broth microdilution method [45]. The results are shown in Fig. 8. The MICs of the CeA peptides generated in the three systems against E. coli ATCC25922 were the same (9.75 ng/μl), and consistent with the MIC of the chemically synthesized CeA peptide against E. coli ATCC 25922. These results indicate that the CeA peptides produced by these three protein expression systems had the same antimicrobial activity as synthetic CeA, and that the N-terminal additional of histidine to the CeA peptide in the CF system had no negative effect on its antibacterial activity.
Fig. 8.
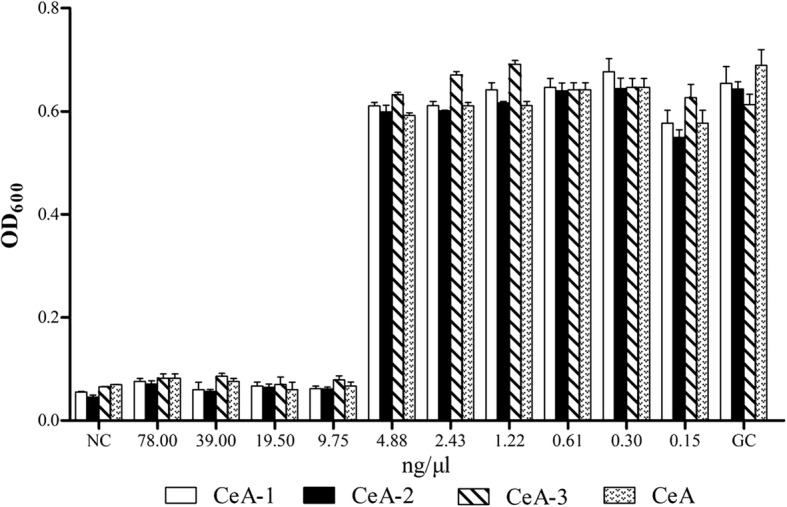
MIC assay against E. coli of CeA peptides produced in three different expression systems. Growth inhibition of E. coli ATCC 25922 is shown in the presence of various concentrations of CeA peptide produced in the CF system (white rectangle: CeA-1), AT-HIS system (black rectangle: CeA-2), and SA-ELK16 system (rectangle filled with oblique lines: CeA-3). Chemically synthesized CeA peptide was also tested (rectangle filled with point: CeA). GC, growth control for E. coli ATCC 25922 in the absence of CeA. NC, sterilized Müller–Hinton broth used as the normal control in this experiment. Experiments were performed in triplicate, and standard deviations (error bars) are shown
Discussion
In this study, we investigated three different protein expression systems for the production of the CeA peptide: the AT-HIS system, CF system, and SA-ELK16 system. By comparing the results, we developed a cost-effective and efficient protein expression system for high-purity CeA peptide.
In the AT-HIS system, two rounds of nickel ion affinity chromatography were required which was both expensive and time-consuming (approximately 5 days, Table 1). More importantly, a large amount of CeA peptide was lost in the second round of Ni2+ affinity chromatography, which significantly limited the large-scale production of CeA peptide. Finally, CeA peptide with 92.1% purity of was obtained at a yield of 0.41 μg/mg wet cell pellet, indicating that AT-HIS system is not an ideal system for the production of the CeA peptide. Due to its open nature and membrane-free, cell free protein expression system was considered the most suitable platform for expression of toxic proteins in recent years [46]. We tested the efficacy of the CF expression system for producing the CeA peptide. Table 1 shows that about 0.93 μg/ml reaction mixture of the CeA peptide was eventually collected, with 90.4% purity. When the CF and AT-HIS systems were compared, the CF system performed better than the AT-HIS system in that the final yield of CeA peptide was 2-fold higher than with the AT-HIS system. However, this yield is relatively low compared with the production of other AMPs, and falls far short of the yields required for large scale production [47, 48]. Moreover, the purity of the CeA peptide produced by these two expression systems was less than 95% and is inappropriate for further research. Most importantly, several cumbersome and expensive procedures like nickel affinity chromatography are required, which are not practicable for the large-scale production of the CeA peptide. Therefore, there is an urgent need to investigate and explore a high and efficient system for the production of CeA peptide.
To achieve a highly efficient low-cost production process for high-purity (> 98%) CeA peptide, the small self-assembling protein ELK16 was attempted in this study. In SA-ELK16 system, the recovery of CeA peptide was estimated to be 78.38%, higher than that in AT-HIS system (52.14%, Table 2). Finally, highly pure (~ 99.8%) CeA peptide was obtained after the cleavage of Mxe GyrA intein (Table 1), with a yield of 6.20 μg/mg wet cell pellet, which was 15-fold higher than the yield of the AT-HIS system and 6.7-fold higher than that of the CF protein expression system (Table 2). These results indicated that SA-ELK16 system was more efficient than AT-HIS system and CF system for production of CeA peptide. Furthermore, the CeA peptide was produced rapidly (2 days) with simple centrifugation and acetic acid treatment, eliminating expensive and tedious procedures like nickel affinity chromatography or high-performance liquid chromatography, dramatically reducing the production cost (Table 1). These factors make the SA-ELK16 system an ideal candidate for the laboratory scale production of the CeA peptide.
However, in SA-ELK16 system, much effort is still needed for the industrial production of CeA peptide. Such as the cleavage of CeA–Mxe–ELK16 fusion was induced by DTT in this study which was expensive at industry scale. Some other self-cleavage inteins like Ssp dnaB and sortase, which cleavage reactions were activated by pH and calcium ion, can be used to reduce the costs at industry production of CeA peptide [49–51]. Besides, the downstream process like dialysis also need to be further optimized to meet the needs of industrial production of CeA peptide.
Conclusions
Cecropin A is a natural linear cationic AMP, with rapid and potent activity against a broad spectrum of pathogens, including bacteria, fungi, viruses, and neoplastic cells. Therefore, it has great potential utility in various biotechnological applications [2, 19, 22]. However, the exploitation of the CeA peptide is seriously limited by the low yields and expensive purification costs of its production. Here, we report a fast, economical and efficient expression system, the SA-ELK16 system, for the laboratory scale production of high-purity CeA peptide, which circumvents the difficulties encountered by ordinary recombinant methods or expensive chemical syntheses. It completely avoids the use of affinity resins and greatly reduces the cost and time required, providing a novel platform for laboratory scale production of the CeA peptide. This study lays a solid foundation for further research into the CeA peptide.
Methods
Strains and materials
Competent E. coli DH5α and BL21(DE3) cells were purchased from Tiangen Biotech (Beijing, China). Oligonucleotides for gene manipulation were synthesized by Invitrogen (Shanghai, China) or Tianyi Huiyuan Gene Technology (Guangzhou, China). The restriction endonuclease DpnI used for restriction-free cloning (RF cloning) was obtained from Fermentas Thermo Scientific (Glen Burnie, MD, USA). The Hi Trap 5 mL pre-packed column used for protein purification was purchased from Qiagen (Dusseldorf, Germany). All chemical reagents used in this study were of analytical grade.
Construction of recombinant plasmids
All three recombinant expression vectors used in the study were constructed with RF cloning. Like fusion PCR cloning, RF cloning uses the appropriate DNA fragment as a megaprimer for the linear amplification of the vector to introduce a foreign DNA into the plasmid at a predetermined position [38]. CeA peptide, Mxe GyrA intein, and ELK16 were synthesized with the appropriate DNA sequences by codon-optimized expression in E. coli BL21(DE3) by Sangon Biotech (Shanghai, China). The DNA fragments encoding the CeA peptide, Mxe GyrA intein, and ELK16 were cloned into the vector pET30a with RF cloning to construct pET30a–AM–ELK16. To construct pET21a–AM–His, primers AMH-F and AMH-R (see Additional file 2) were used to amplify the AM sequence encoding both the CeA peptide and the Mxe GyrA intein from plasmid pET30a-AM–ELK16. The amplified DNA fragment was then integrated into the pET21a vector with RF cloning. Plasmid pET21–His6–AMP was obtained by amplifying the CeA gene from pET30a–AM–ELK16 and inserting it into pET21a. Plasmid pET21–AMP–His6 was constructed in the same way as pET21–His6–AMP. All the primers used in this work are listed in Additional file 2.
Expression of fusion proteins in three different systems
In the SA-ELK16 and AT-HIS systems, Luria–Bertani (LB) medium containing 50 μg/ml kanamycin or 100 μg/ml ampicillin was inoculated with E. coli BL21(DE3) cells carrying pET30a–AM–ELK16 or pET21a–AM–His, respectively, and incubated at 37 °C. Different concentrations of IPTG (0.1–1 mM) were added to initiate protein expression when the cell density (OD600) reached 0.6–08. The cultures were then incubated under different conditions (5 h/37 °C, 12 h/25 °C, or 24 h/16 °C) to optimize protein expression. The cells were harvested by centrifugation at 7500×g for 15 min and the pellets were stored at − 80 °C for further analysis. In the CF system, pET21–His6–AMP and its derivative were expressed with a previously described method [52]. The reaction solution, containing 60 μl of reaction mixture (RM) and 900 μl of feeding mixture (FM), was added to the wells of standard 24-well microplates. To optimize the Mg2+ concentration, regenerated cellulose membranes with a molecular-weight cut-off of 14 kDa were used. The reactions were performed in the continuous-exchange cell-free configuration and incubated for 12–16 h at 30 °C with shaking (180 rpm).
Intein-mediated cleavage and peptide purification
In the CF system, the soluble fractions were harvested by centrifuging the reaction mixture at 12,000 g for 10 min. Ten-fold volumes of cold acetone were added to the supernatants to precipitate the protein [47]. The pellets were air dried, dissolved in 1 × loading buffer (20 mM Tris–HCl, 3% [w/v] glycerol, 1.5% [w/v] SDS, 5% [w/v] β-mercaptoethanol, 0.02% [w/v] bromophenol blue, pH 8.5), and denatured by heating at 95 °C for 10 min. The samples were separated and analyzed with 16% tricine SDS-PAGE. The CeA fusion produced with the CF system was purified with nickel ion affinity chromatography, as previously reported [39].
The harvested cell pellets containing the CeA–Mxe–ELK16 or CeA–Mxe–His fusions were resuspended to 10 OD600 units/ml of culture with lysis buffer (20 mM Tris-HCl, 500 mM NaCl, 1 mM EDTA, pH 8.5) and the suspended cells were lysed with a high-pressure homogenizer (Constant Systems Limited, United Kingdom). The CeA–Mxe–ELK16 aggregates were separated by centrifugation at 12,000 g for 30 min at 4 °C. The soluble fractions containing the CeA–Mxe–His fusions were isolated from the aggregates by centrifugation at 12,000 g for 30 min at 4 °C, and then purified with nickel ion affinity chromatography, as described above. For Mxe GyrA intein mediate cleavage, the CeA–Mxe–ELK16 aggregates or purified CeA–Mxe–His fusions were resuspended in cleavage buffer (20 mM Tris-HCl, 500 mM NaCl, 1 mM EDTA, pH 8.5) containing different concentrations of DTT (40–80 mM). The cleavage reaction was assessed by incubating the samples under different conditions (4 °C or 25 °C for 0–14 h) to optimize the efficiency of cleavage. The quantity of protein was determined with a BCA Protein Assay Kit (Sangon) or the Gel-Pro Analyzer software (Media Cybernetics, Houston, USA), using aprotinin as the standard and adjusting for the loading volume.
Acetic acid treatment for high-purity CeA peptide in the SA-ELK16 system
To obtain high-purity (≥ 98%) CeA peptide, different proportions of acetic acid (0.5–3%) were added to the suspensions of the cleaved CeA–Mxe–ELK16 fusion and incubated at room temperature for 10 min. The supernatant containing high-purity CeA peptide was separated by centrifugation at 12,000 g for 30 min at 4 °C. The soluble and insoluble fractions were analyzed with tricine SDS-PAGE.
Fluorescence confocal microscopy
Escherichia coli BL21(DE3) cells carrying the pET30–GFP–Mxe–ELK16 or pET21–GFP–Mxe–His plasmid were cultured and the expression of the fusion was induced by the addition of 0.2 mM IPTG. After expression, 200 μl of cells were harvested by centrifugation at 7000 g at 4 °C for 5 min, resuspended in sterile PBS (1% w/v), spotted onto a slide, air dried, and visualized with a 63× phase-contrast objective on a Zeiss LSM 710 microscope (Germany).
MIC assay
The MICs of the CeA peptides produced in the three different expression systems against E. coli ATCC 25922 were assessed with the broth microdilution method, as previously reported [45], and compared with that of a synthetic CeA peptide. For the antimicrobial assays, overnight cultures of E. coli ATCC 25922 cells were diluted with fresh Müller–Hinton broth (MHB) to an OD625 of 0.08–0.13 (0.5 McFarland standards), and further diluted with fresh MHB (1:100) to a final concentration of approximately 107 colony-forming units (CFU) per ml. The bio-produced CeA peptides were serially diluted in MHB medium and 50 μl aliquots of the diluted CeA peptides were added to 96-well plates. The standardized bacterial suspension (50 μl) was then added to each well. Growth controls contained no peptide antimicrobial, and sterility controls contained only broth. The plates were incubated at 37 °C for 16–18 h. The OD600 values were monitored with a microplate reader and the lowest concentration of peptide at which the growth of E. coli ATCC 25922 was inhibited was determined as the MIC.
Additional files
Purification of CeA-Mxe-His fusions in AT-HIS system. (DOCX 181 kb)
Primers used in this study. (DOCX 145 kb)
Acknowledgements
We thank Janine Miller, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This work was supported by the financial support of Natural Science Foundation of Guangdong Province, China (grant number: 2015A030310322); the Innovative Program of Department of Education of Guangdong Province, China (grant number: 2013KJCX0013). We are also grateful the important role of the funding in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All data for this study are included in this published article and its Additional files.
Abbreviations
- CeA
Cecropin A
- E.coli
Escherichia coli
- FM
feeding mixture
- IPTG
Isopropyl β-D-Thiogalactoside
- LB
Luria-Bertani
- MHB
Müller–Hinton broth
- Mxe
Mxe GyrA intein
- RF cloning
Restriction Free cloning
- RM
reaction mixture
Authors’ contributions
JW and MW designed the experiments. MW and KZ performed the experiments. JL, MH and XL carried out the data analysis. MW wrote the manuscript. SL, YM and JW supervised the whole work attended the discussions and revised the manuscript. All authors have read and approved the manuscript, and ensure that this is the case.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Meng Wang, Email: skymeng612@163.com.
Kaiwen Zheng, Email: 821908046@qq.com.
Jinglian Lin, Email: 1040305338@qq.com.
Minhua Huang, Email: 2314416316@qq.com.
Yi Ma, Email: bimayikobe@scut.edu.cn.
Shan Li, Email: lishan@scut.edu.cn.
Xiaochun Luo, Email: xcluo@scut.edu.cn.
Jufang Wang, Phone: +86-20-39380626, Email: jufwang@scut.edu.cn.
References
- 1.Schmitt P, Mercado L, Díaz M, Guzmán F, Arenas G, Marshall SH. Characterization and functional recovery of a novel antimicrobial peptide (CEC dir–CEC ret) from inclusion bodies after expression in Escherichia coli. Peptides. 2008;29(4):512–519. doi: 10.1016/j.peptides.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 2.DeLucca AJ, Bland JM, Jacks TJ, Grimm C, Cleveland TE, Walsh TJ. Fungicidal activity of cecropin A. Antimicrob Agents Chemother. 1997;41(2):481–483. doi: 10.1128/aac.41.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavallarin L, Andreu D, San Segundo B. Cecropin A—derived peptides are potent inhibitors of fungal plant pathogens. Mol Plant-Microbe Interact. 1998;11(3):218–227. doi: 10.1094/MPMI.1998.11.3.218. [DOI] [PubMed] [Google Scholar]
- 4.Andreu D, Merrifield R, Steiner H, Boman H. Solid-phase synthesis of cecropin A and related peptides. Proc Natl Acad Sci. 1983;80(21):6475–6479. doi: 10.1073/pnas.80.21.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silvestro L, Gupta K, Weiser JN, Axelsen PH. The concentration-dependent membrane activity of cecropin A. Biochemistry. 1997;36(38):11452–11460. doi: 10.1021/bi9630826. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Movahedi A, Wang X, Wu X, Yin T, Zhuge Q. Molecular structure, chemical synthesis, and antibacterial activity of ABP-dHC-cecropin A from drury (Hyphantria cunea) Peptides. 2015;68:197–204. doi: 10.1016/j.peptides.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P. Prophylactic antibiotic administration in pregnancy to prevent infectious morbidity and mortality. Cochrane Database Syst Rev. 2002;4(4):CD002250. doi: 10.1002/14651858.CD002250. [DOI] [PubMed] [Google Scholar]
- 8.Gauger PC, Loving CL, Khurana S, Lorusso A, Perez DR, Kehrli ME, Roth JA, Golding H, Vincent AL. Live attenuated influenza a virus vaccine protects against a (H1N1) pdm09 heterologous challenge without vaccine associated enhanced respiratory disease. Virology. 2014;471:93–104. doi: 10.1016/j.virol.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Paudel S, Park J, Jang H, Hyun B, Yang D, Shin H. Evaluation of antibody response of killed and live vaccines against porcine epidemic diarrhea virus in a field study. Vet Q. 2014;34(4):194–200. doi: 10.1080/01652176.2014.973999. [DOI] [PubMed] [Google Scholar]
- 10.Wu R, Wang Q, Zheng Z, Zhao L, Shang Y, Wei X, Liao X, Zhang R. Design, characterization and expression of a novel hybrid peptides melittin (1–13)-LL37 (17–30) Mol Biol Rep. 2014;41(7):4163–4169. doi: 10.1007/s11033-013-2900-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Li J, Movahedi A, Sang M, Xu C, Xu J, Wei Z, Yin T, Zhuge Q. A novel inclusion complex (β-CD/ABP-dHC-cecropin A) with antibiotic propertiess for use as an anti-Agrobacterium additive in transgenic poplar rooting medium. Enzym Microb Technol. 2015;81:72–79. doi: 10.1016/j.enzmictec.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Efimova SS, Schagina LV, Ostroumova OS. Channel-forming activity of cecropins in lipid bilayers: effect of agents modifying the membrane dipole potential. Langmuir. 2014;30(26):7884–7892. doi: 10.1021/la501549v. [DOI] [PubMed] [Google Scholar]
- 13.Yi H-Y, Chowdhury M, Huang Y-D, Yu X-Q. Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol. 2014;98(13):5807–5822. doi: 10.1007/s00253-014-5792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kylsten P, Samakovlis C, Hultmark D. The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J. 1990;9(1):217. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.X-m L, X-b J, J-y Z, H-f M, Ma Y, F-j C, Wang Y, Li X-b. Expression of the antimicrobial peptide cecropin fused with human lysozyme in Escherichia coli. Appl Microbiol Biotechnol. 2010;87(6):2169–2176. doi: 10.1007/s00253-010-2606-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Li X, Wang X, Jin X, Shi D, Wang J, Bi D. Cecropin B represses CYP3A29 expression through activation of the TLR2/4-NF-κB/PXR signaling pathway. Sci Rep. 2016;6. 10.1038/srep27876. [DOI] [PMC free article] [PubMed]
- 17.Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membr Biol. 1997;156(3):197. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]
- 18.Boman H, Boman IA, Andreu D, Li Z-Q, Merrifield R, Schlenstedt G, Zimmermann R. Chemical synthesis and enzymic processing of precursor forms of cecropins A and B. J Biol Chem. 1989;264(10):5852–5860. [PubMed] [Google Scholar]
- 19.Hancock RE, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16(2):82–88. doi: 10.1016/S0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 20.Gordon YJ, Romanowski EG, McDermott AM. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res. 2005;30(7):505–515. doi: 10.1080/02713680590968637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Jin F, Yu X, Ji S, Wang J, Cheng H, Wang C, Zhang W. Expression and purification of a recombinant antibacterial peptide, cecropin, from Escherichia coli. Protein Expr Purif. 2007;53(2):293–301. doi: 10.1016/j.pep.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Andersons D, Engström Å, Josephson S, Hansson L, Steiner H. Biologically active and amidated cecropin produced in a baculovirus expression system from a fusion construct containing the antibody-binding part of protein a. Biochem J. 1991;280(1):219–224. doi: 10.1042/bj2800219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang K, Su Y, Li J, Sun J, Yang Y. Expression and purification of the antimicrobial peptide cecropin AD by fusion with cationic elastin-like polypeptides. Protein Expr Purif. 2012;85(2):200–203. doi: 10.1016/j.pep.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Téllez GA, Castaño-Osorio JC. Expression and purification of an active cecropin-like recombinant protein against multidrug resistance Escherichia coli. Protein Expr Purif. 2014;100:48–53. doi: 10.1016/j.pep.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Ji S, Li W, Baloch AR, Wang M, Li H, Cao B, Zhang H. Efficient biosynthesis of a Cecropin A-melittin mutant in Bacillus subtilis WB700. Sci Rep. 2017;7. 10.1038/srep40587. [DOI] [PMC free article] [PubMed]
- 26.F-l J, X-x X, X-q Y, S-x R. Expression and characterization of antimicrobial peptide CecropinAD in the methylotrophic yeast Pichia pastoris. Process Biochem. 2009;44(1):11–16. doi: 10.1016/j.procbio.2008.08.012. [DOI] [Google Scholar]
- 27.Shen A, Lupardus PJ, Morell M, Ponder EL, Sadaghiani AM, Garcia KC, Bogyo M. Simplified, enhanced protein purification using an inducible, autoprocessing enzyme tag. PLoS One. 2009;4(12):e8119. doi: 10.1371/journal.pone.0008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang Y, Wang J-X, X-f Z, Du X-J, Xue J-F. Molecular cloning and characterization of cecropin from the housefly (Musca domestica), and its expression in Escherichia coli. Dev Compar Immunol. 2006;30(3):249–257. doi: 10.1016/j.dci.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Xia L, Liu Z, Ma J, Sun S, Yang J, Zhang F. Expression, purification and characterization of cecropin antibacterial peptide from Bombyx mori in Saccharomyces cerevisiae. Protein Expr Purif. 2013;90(1):47–54. doi: 10.1016/j.pep.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Zhou B, Xing L, Wu W, Zhang X-E, Lin Z. Small surfactant-like peptides can drive soluble proteins into active aggregates. Microb Cell Factories. 2012;11(1):10. doi: 10.1186/1475-2859-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu W, Zhao Q, Xing L, Lin Z. Recombinant production of influenza hemagglutinin and HIV-1 GP120 antigenic peptides using a cleavable self-aggregating tag. Sci Rep. 2016;6. 10.1038/srep35430. [DOI] [PMC free article] [PubMed]
- 32.Da Costa JP, Cova M, Ferreira R, Vitorino R. Antimicrobial peptides: an alternative for innovative medicines? Appl Microbiol Biotechnol. 2015;99(5):2023–2040. doi: 10.1007/s00253-015-6375-x. [DOI] [PubMed] [Google Scholar]
- 33.Satakarni M, Curtis R. Production of recombinant peptides as fusions with SUMO. Protein Expr Purif. 2011;78(2):113–119. doi: 10.1016/j.pep.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y, Yu J, Lin J, Wu S, Li S, Wang J. High efficient expression, purification, and functional characterization of native human epidermal growth factor in Escherichia coli. Biomed Res Int. 2016;2016:3758941. doi: 10.1155/2016/3758941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia C, Wang S, Chen Y, Xiao X, Su Z, Zheng Q. Improving Soluble Expression of Curcin-Transferrin Receptor-binding Peptide Fusion Protein with Glutathione Transferase-Small Ubiquitin-related Modifier System. J Pharm Biomed Sci. 2017;7:2. [Google Scholar]
- 36.Chen Y, Tan S, Yang F, Chen Z, Wu Z, Huang J. Soluble expression and purification of a functional harpin protein in Escherichia coli. Process Biochem. 2017;57:200–206. doi: 10.1016/j.procbio.2017.03.010. [DOI] [Google Scholar]
- 37.Marshall CJ, Grosskopf VA, Moehling TJ, Tillotson BJ, Wiepz GJ, Abbott NL, Raines RT, Shusta EV. An evolved Mxe GyrA intein for enhanced production of fusion proteins. ACS Chem Biol. 2014;10(2):527–538. doi: 10.1021/cb500689g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ent FVD, Löwe J. RF cloning: a restriction-free method for inserting target genes into plasmids. J Biochem Biophys Methods. 2006;67(1):67–74. doi: 10.1016/j.jbbm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Huang M, Gu H, Li S, Ma Y, Wang J. Mutational analysis to identify the residues essential for the acetyltransferase activity of GlmU in Bacillus subtilis. RSC Adv. 2017;7(23):13858–13867. doi: 10.1039/C7RA00086C. [DOI] [Google Scholar]
- 40.Ge X, Yang DS, Trabbic-Carlson K, Kim B, Chilkoti A, Filipe CD. Self-cleavable stimulus responsive tags for protein purification without chromatography. J Am Chem Soc. 2005;127(32):11228–11229. doi: 10.1021/ja0531125. [DOI] [PubMed] [Google Scholar]
- 41.Vázquez E, Villaverde A. Engineering building blocks for self-assembling protein nanoparticles. Microb Cell Factories,9,1(2010-12-30) 2010;9(1):1–4. doi: 10.1186/1475-2859-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin Zhanglin, Zhou Bihong, Wu Wei, Xing Lei, Zhao Qing. Self-assembling amphipathic alpha-helical peptides induce the formation of active protein aggregates in vivo. Faraday Discussions. 2013;166:243. doi: 10.1039/c3fd00068k. [DOI] [PubMed] [Google Scholar]
- 43.Pane Katia, Durante Lorenzo, Pizzo Elio, Varcamonti Mario, Zanfardino Anna, Sgambati Valeria, Di Maro Antimo, Carpentieri Andrea, Izzo Viviana, Di Donato Alberto, Cafaro Valeria, Notomista Eugenio. Rational Design of a Carrier Protein for the Production of Recombinant Toxic Peptides in Escherichia coli. PLOS ONE. 2016;11(1):e0146552. doi: 10.1371/journal.pone.0146552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holak TA, Engström A, Kraulis PJ, Lindeberg G, Bennich H, Jones TA, Gronenborn AM, Clore GM. The solution conformation of the antibacterial peptide cecropin A: a nuclear magnetic resonance and dynamical simulated annealing study. Biochemistry. 1988;27(20):7620–7629. doi: 10.1021/bi00420a008. [DOI] [PubMed] [Google Scholar]
- 45.Lee E, Shin A, Kim Y. Anti-inflammatory activities of cecropin A and its mechanism of action. Arch Insect Biochem Physiol. 2015;88(1):31–44. doi: 10.1002/arch.21193. [DOI] [PubMed] [Google Scholar]
- 46.Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y, Ghoshdastider U, Wang J, Ye W, Dötsch V, Filipek S, Bernhard F, Wang X. Cell-free expression of human glucosamine 6-phosphate N-acetyltransferase (HsGNA1) for inhibitor screening. Protein Expr Purif. 2012;86(2):120–126. doi: 10.1016/j.pep.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Rao X, Li S, Jc JX, Hu X, Huang J, Chen Z, Zhu J, Hu F. A novel carrier molecule for high-level expression of peptide antibiotics in Escherichia coli. Protein Exp Purification. 2004;36(1):11–18. doi: 10.1016/j.pep.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Feng X, Liu C, Guo J, Song X, Li J, Xu W, Li Z. Recombinant expression, purification, and antimicrobial activity of a novel hybrid antimicrobial peptide LFT33. Appl Microbiol Biotechnol. 2012;95(5):1191–1198. doi: 10.1007/s00253-011-3816-z. [DOI] [PubMed] [Google Scholar]
- 50.Vieweg S, Ansaloni A, Wang ZM, Warner JB, Lashuel HA. An Intein-based strategy for the production of tag-free huntingtin exon 1 proteins enables new insights into the Polyglutamine dependence of Httex1 aggregation and fibril formation. J Biol Chem. 2016;291(23):12074. doi: 10.1074/jbc.M116.713982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hay ID, Du J, Reyes PR, Rehm BHA. In vivo polyester immobilized sortase for tagless protein purification. Microb Cell Factories. 2015;14(1):190. doi: 10.1186/s12934-015-0385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Y, Muench D, Schneider T, Sahl H-G, Bouhss A, Ghoshdastider U, Wang J, Doetsch V, Wang X, Bernhard F. Preparative scale cell-free production and quality optimization of MraY homologues in different expression modes. J Biol Chem. 2011;286(45):38844–38853. doi: 10.1074/jbc.M111.301085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Purification of CeA-Mxe-His fusions in AT-HIS system. (DOCX 181 kb)
Primers used in this study. (DOCX 145 kb)
Data Availability Statement
All data for this study are included in this published article and its Additional files.