Abstract
A number of recent studies have documented rapid changes in behavioral sensory acuity induced by aversive learning in the olfactory and auditory modalities. The effect of aversive learning on the discrimination of low-level features in the visual system of humans remains unclear. Here, we used a psychophysical staircase procedure to estimate discrimination thresholds for oriented grating stimuli, before and after differential aversive learning. We discovered that when a target grating orientation was conditioned with an aversive loud noise, it subsequently led to an improvement of discrimination acuity in nearly all subjects. However, no such change was observed in a control group conditioned to an orientation shifted by ± 90° from the target. Our findings cannot be explained by contextual learning or sensitization factors. The results converge with those reported in the olfactory modality and provide further evidence that early sensory systems can be rapidly modified by recently experienced reinforcement histories.
Adaptive behavior in rapidly changing environments entails making fine discriminations between sensory patterns associated with danger against other, potentially similar patterns, associated with safety. Accumulating evidence suggests that reinforcement with motivationally salient outcomes produces lasting changes in perceptual representations, even at the earliest stages of sensory processing, across multiple modalities and animal species (McGann, 2015; Weinberger, 2007). These findings challenge traditional notions that the early sensory systems encode stimuli in a relatively fixed, feedforward manner as determined by a static neural wiring diagram. Instead, sensory systems are increasingly understood as being subject to rapid modification by factors including learned contingency rules that are contained in prior reinforcement histories (Aton, 2013; Fontanini & Katz, 2008).
Several reports have documented experience-dependent modification of perceptual acuity, sensitivity and/or discrimination performance following aversive learning in human subjects. Perhaps the most intriguing evidence for these perceptual learning effects comes from the conditioning of mirror-image odor molecules (enantiomers) that are initially perceptually indiscriminable. Li and colleagues (2008) demonstrated that pairing one of the enantiomers (but not its counterpart) with electric shock produced within-session improvement of psychophysical discrimination such that the smells became perceptually distinct after conditioning. Subsequently, the finding was independently replicated with aversive conditioning being accompanied by rapid augmentation of odor detection sensitivity (Åhs et al., 2013). Specifically, the aversively conditioned odorant (CS+) was capable of being detected at ~67% reduced concentration levels compared to the non-paired (CS-) stimulus. When learning regimens include repeated odorant-shock pairings, the changes in sensory sensitivity persisted for days (Parma et al., 2015).
In a parallel line of studies, perceptual discrimination was found to be impaired for aversively conditioned auditory tones (Resnik et al., 2011). Tones associated with monetary loss led to a deterioration of perceptual discrimination such that tone frequencies surrounding the loss predictive tone were judged to be more similar (Schechtman et al., 2008). Taken together, these findings suggest distinct patterns of results (either increased or decreased perceptual discriminability) for aversively conditioned cues across two different sensory modalities.
Our goal was to address two questions: does recent reinforcement history modify discrimination in the visual system and, if so, does this follow a pattern similar to olfaction (improved performance) or audition (deteriorated performance)? A recent study reported increased discriminability for an aversively conditioned human face when the learning was followed by a sleep interval (Sterpenich et al., 2014). However, faces are visually complex and do not lend themselves to clear manipulations of sensory characteristics unlike simpler stimuli such as sinewave gratings. Low-level vision has been conceptualized as consisting of a set of spatial filters or channels that are selective for canonical features (e.g., orientation) (Nassi & Callaway, 2009). Here, we isolated grating orientation as the critical feature and paired it with aversive reinforcement to evaluate experience-dependent changes in discrimination acuity of human visual channels. We used a psychophysical staircase procedure to test for the rapidly occurring effects of aversive learning on low-level visual discrimination (just noticeable difference [JND]) thresholds for oriented grating stimuli. If aversive learning sharpens perceptual representations for a visual CS+, then one would expect to see decreased JND thresholds following learning, as in olfaction. On the other hand, conditioning-induced increases in JND thresholds would be the expected outcome if, as in the auditory modality, aversively conditioned cues produce broader generalization curves.
Method
Participants
Participants were recruited from the undergraduate and graduate student campus community at SUNY Binghamton. Since anxiety is known to influence conditioned response generalization (Duits et al., 2015), all potential participants were screened for neuropsychiatric illness using the Mini International Neuropsychiatric Illness (MINI version 6.0, Lecrubier et al., 1997) questionnaire administered via telephone interview. Initial screening resulted in the elimination of 8 potential participants. The remaining participants were screened at the time of testing for self-reported drug or alcohol use 12 hours prior to participation, history of photic seizure, and for natural or corrected Snellen acuity of 20/20 or better (one exclusion). In total, 32 participants (18 female, M age = 20.16 years, S.D. = 2.13) met all inclusion criteria. Participants were randomly assigned to either the experimental (conditioned at target orientation tested in the psychophysical procedure) or control groups (conditioned at target orientation ± 90°) using a between-subjects1 design (n = 16 each). Subjects were compensated at a rate of 20 USD for their participation. All experimental procedures were approved by the local Institutional Review Board.
Stimuli
The visual stimuli consisted of circular Gabor patches (1.36 c/deg, 52% Michelson contrast) covering a 4.5° viewing angle set against a uniform gray background (luminance of 32 cd/m2). The CS+ and CS- cues had an orientation of either 45° or 135°, counterbalanced across participants. The unconditioned stimulus (UCS) consisted of an aversive sound (metal scraping on slate for 3.69 secs, played at 90 dB sound pressure level) used in previous human conditioning studies (Resnik et al., 2011) and delivered using free-field stereo speakers positioned directly behind participants.
Procedure and Design
Upon arrival to the laboratory, all participants first provided written informed consent followed by Snellen acuity tests. Participants then completed the State-Trait Anxiety Inventory for Adults (Spielberger, 1977). The general study design is illustrated in Figure 1A. All participants completed three experimental phases: (i) a baseline (pre-) psychophysical assessment of their discrimination threshold, followed by (ii) an aversive conditioning procedure and finally, (iii) a post-conditioning discrimination threshold measurement that was identical to the first one (but without practice trials). The aversive conditioning component of the experiment was conducted in an adjoining experimental room that was physically separate from the room in which visual thresholds were estimated. Distinct rooms were used to remove any putative effects of contextual conditioning on subsequent threshold estimates (Weinberger, 2007).
Figure 1:
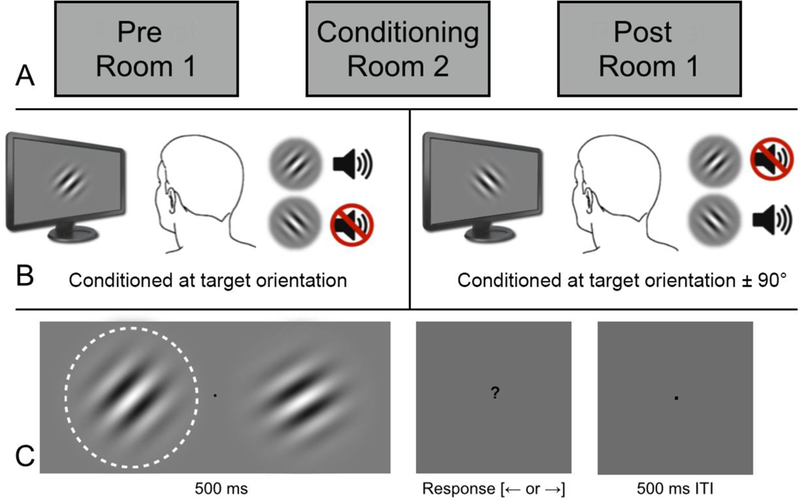
Panel A depicts the three experimental phases, involving physical contextual shifts between the threshold estimation and aversive conditioning procedures. As indicated in Panel B, differential aversive conditioning was identical in the experimental (left) and control (right) groups, except that the former was conditioned to the target orientation being measured during the psychophysical task (here 45°) while the latter was conditioned to an orientation shifted by ± 90° from the target. Panel C illustrates the trial sequence in the 2AFC task, with the target (outlined in white for illustrative purposes only) and foil on opposite sides of the screen. Participants indicated whether the target was presented on the ‘left’ or ‘right’ response. Foil angular offset was controlled by an adaptive staircase method. (Note: Gabor grating spatial frequency and contrast do not match ones used in the experiment, but are presented for illustrative purposes.)
Aversive Conditioning.
Participants were informed immediately prior to conditioning that something about grating orientation (rather than another potential sensory feature, such as spatial frequency) may have a relationship with whether or not they would hear the aversive noise. A differential cue conditioning procedure was used for all participants. There were 10 each of CS+ (100% reinforcement schedule) and CS- trials. Trial duration was 5 s with a 1 s ITI. The CS+ and the UCS temporally co-terminated (i.e., a delay conditioning procedure). Trial order was pseudo randomized so that no more than two CS+ or CS- trials were presented consecutively. Critically, participants in the experimental group were conditioned at the target orientation measured during pre- and post- testing while participants in the control group were conditioned at an orientation that was shifted by 90° from the target (see Figure 1B).
Immediately after conditioning, participants rated their dislike of the UCS (6-point Likert scale, 0 = “Neutral”, 5 = “Very unpleasant”, M = 4.31, S.D. = 1.09). Participants next completed a contingency awareness questionnaire in which they rated the perceived strength of the relationship between the grating orientation and UCS occurrence using a simple 6-point Likert scale, (0 = “No relationship”, 5 = “Strong relationship”). Participants also briefly described CS+/UCS contingencies using a free written response. Two naïve experimenters independently rated these descriptions with high inter-rater agreement (Spearman’s rho = 0.96, Cohen’s kappa = 0.75). The descriptions were scored from 0 to 5, with 0 being very low contingency awareness (reporting sounds as occurring entirely at random) and 5 being very high (reporting sounds co-occurring only with the correct orientation). Overall, the majority of participants seemed to learn that grating orientation was a strong predictor of the aversive noise (Likert rating of contingency awareness, M = 4.06, S.D. = 1.37; written description scores, M = 3.25, S.D. = 1.96).
Following the contingency awareness evaluation, participants were returned to the original room to perform the post-conditioning threshold assessment.
JND Threshold Estimation.
Participants were seated at a desk with heads stabilized using a chin and forehead rest. A two-alternative forced choice (2AFC) psychophysical task was used to estimate JND orientation thresholds. As indicated in Figure 1C, a target orientation (45° or 135°, counterbalanced) was displayed alongside a foil grating. Participants indicated which side (left or right) contained the target grating. The foil gratings differed from the target only in the relative orientation offset, between ± 0.5° and ± 20° (over 16 linear steps), with trial-by-trial offsets controlled by three interleaved one-up three-down staircases. One-up three-down staircases are designed to converge on the subject’s point of 75% accuracy (Leek, 2001). Initial staircases offsets were ±10, ±15, and ±20°. On each trial, orientation offset was increased (to a maximum of ± 20°) by one step after each incorrect response within that staircase, or decreased (to a minimum of ± 0.5°) by one step after three consecutive correct responses. The foil orientation offset was recorded after each incorrect response (a staircase ‘reversal’). Participants’ JND thresholds were quantified as the average of all staircase reversals within a session (minimum of 34 reversals). Feedback (visual) was provided following incorrect responses. It was expected that this feedback would encourage participant engagement, especially as the task was described as difficult. The pre- and post- assessments each had 150 trials. The pre-assessment run was preceded by 15 practice trials during which the experimenter remained in the room with the subject to ensure task compliance.
Statistical Analyses
Non-parametric, randomization statistics were used to test our main hypotheses, since these tests permit exact inferences, are robust to violations of assumptions underlying parametric tests and are less sensitive to outliers in study designs with relatively small sample sizes (Edgington & Onghena, 2007). To test for reliable differences in discrimination thresholds as a function of aversive learning, we used a Monte Carlo method with 5000 random within-subject data permutations to construct an empirical null hypothesis distribution (no difference from pre- to post-assessment points). This sampling distribution was then used to derive pperm values by dividing the number of t-scores more extreme than the observed t (tobs) by the total number of permutations. A similar approach was adopted for exploratory analyses of between-group effects, except using 5000 random between-subject data permutations. All statistical analyses were performed using MATLAB (Mathworks, 2010) and R (R Development Core Team, 2008).
Results
We first ensured that the groups did not differ on STAI State or Trait anxiety scores (all ps > 0.30). Both groups found the UCS to be equally aversive (experimental group M = 4.38, S.D. = 0.89; control group M = 4.25, S.D. = 1.29, p = 0.66). Participants across the two groups also did not differ with respect to either the Likert ratings of CS+/UCS contingency strength or in the quality of their written descriptions (all ps > 0.30). Any subsequent differences, therefore, could not be attributed to differential learning or perceived UCS salience.
As revealed by inspecting Figure 2A, when the aversively conditioned grating orientation matched the target orientation being measured in the psychophysical threshold estimation procedure, nearly all participants exhibited a pre to post-conditioning improvement in orientation discrimination accuracy (median shift of 2.12° or approximately a 21% improvement). It is interesting to note that one participant in the experimental group who exhibited a strong post-conditioning discrimination decrement (increased JND threshold) also failed to acquire the CS+/UCS contingency as indicated in their written description which mentioned that the aversive loud sounds were delivered randomly2.
Figure 2:
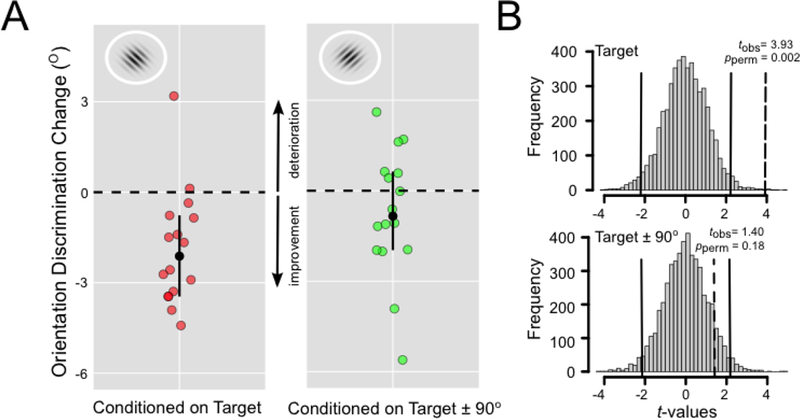
Panel A depicts the post minus pre individual difference scores in JND thresholds (°), separately for the experimental (conditioned at the target orientation) and control groups (conditioned at target orientation ±90°). The black circle indicates the median difference score bounded by 99% confidence intervals estimated using a basic bootstrap (5,000 replicates). Dashed horizontal line indicates the no change reference point. Panel B depicts the Monte Carlo sampling distribution of paired t-test scores (5,000 random within-subject data permutations). Solid vertical lines denote the critical t values for a pperm = 0.05. Dashed vertical lines denote the tobs scores (df = 15).
By comparison to participants in the experimental group, a more even distribution of pre- to post discrimination accuracy was observed in the control group, conditioned to an orientation that was shifted from the psychophysical target grating by ± 90°. This visual impression is further confirmed by inspecting the Monte Carlo sampling distribution of paired t-test scores across the two groups (see Figure 2B). These findings suggest that a reliable shift in post-conditioning orientation discrimination thresholds occurred only in the experimental group that was psychophysically tested at the orientation that was aversively conditioned.
Although it was not the primary goal of our study, in a final, exploratory set of analyses, we wished to examine between-group differences in JND thresholds. There were no reliable differences in the initial, baseline assessment of discrimination acuity, tobs(15) = 1.66, pperm = 0.13. However, the post-conditioning JND threshold was lower in the experimental compared to the con trol group, tobs(15) = -2.406, pperm = 0.03.
Discussion
We examined the rapidly occurring (within-session) effects of differential aversive learning on visual discrimination acuity. Our findings revealed that aversive conditioning of a target grating orientation subsequently improved discrimination accuracy (lowered the JND threshold) by ~21%, suggesting that visual cues predictive of threat produce sharpened perceptual representations. By contrast, there was no reliable effect of aversive conditioning when the CS+ was shifted by ± 90° relative to the psychophysical target. The aversive learning effects on visual discrimination thresholds could presumably not be attributed to simple sensitization or contextual conditioning effects, since both groups experienced identical learning preparations and the aversive conditioning took place in a room that was physically separate from the one used for psychophysical testing.
The perceptual discrimination findings reported here can be interpreted to generally complement an existing literature in human electrophysiology, which documents amplification of sensory-evoked brain potentials for aversively conditioned cues (see Miskovic & Keil, 2012 for a review). Perhaps the most relevant set of findings comes from a recent study that measured changes in neuronal orientation tuning following aversive conditioning by probing the visual system with a graded continuum of sine-wave orientations surrounding the reinforced target (McTeague et al., 2015). Subsequent changes in population-level visuocortical responses were best fit by a so-called ‘Mexican hat’ pattern in which amplification for the aversively conditioned orientation was accompanied by response suppression for nearby orientations. Their results pointed to increased lateral inhibition of orientations surrounding the CS+ cue, which would presumably lead to sharpened perceptual representations of the CS+, though this was not directly tested. Converging evidence from the murine somatosensory system suggests that the refinement of sensory discrimination following aversive conditioning could be mediated by a sparse coding strategy that enhances the efficiency of the conditioned cue (Gdalyahu et al., 2012). It is likely that the effects observed here do not only reflect local changes in early sensory areas, but also the contribution of additional structures. For instance, hippocampal pattern separation might be more finely resolved for the CS+ cue, which would subsequently provide a more accurate template for comparing orientation offsets (Lissek, 2012).
Our findings agree with those in the olfactory domain, where enhanced discrimination of enantiomers is often observed after aversive conditioning (Åhs et al., 2013; Li et al., 2008; Parma et al., 2015) but are opposed to the pattern of results obtained with conditioned auditory cues (Resnik et al., 2011; Schechtman et al., 2008). Although some of the reported differences might be explained by modality-specific constraints (perhaps owing to distinct resolutions offered by the various sensory systems), another possibility is that the apparent discrepancy is partially a function of learning parameters. In the olfactory experiments, the CS+ and CS- cues are perceptually isomorphic to begin with – the specificity of aversive learning is therefore very high and produces improved discrimination as a result. By contrast, the distinct tone frequencies (Δ1KHz) used in a previous study of auditory conditioning (Resnik et al., 2011) are perceptually quite dissimilar at the beginning of aversive learning. In rodents, it has been demonstrated that aversive conditioning can exert bidirectional effects on sensory acuity, depending on the specificity of initial learning (Aizenberg & Geffen, 2013; Chapuis & Wilson, 2011). For instance, single cue conditioning leads to broader generalization while differential conditioning produces narrower tuning profiles (Chen et al., 2011). This suggests that improved sensory discrimination is an advantage when the CS+ and CS- share many sensory features in common, but that broader generalization gradients may be beneficial when this is not the case (McGann, 2015). Manipulating the relative coarseness (or fineness) in the specificity of aversive learning – for instance by varying the angular offset between the CS+ and CS- cues in our case or comparing single versus differential cue conditioning – would then be expected to lead to either worse or improved discrimination acuity. Ongoing work in our laboratory is currently testing these predictions in the human visual system.
In summary, we found that aversive conditioning of a low-level visual feature (orientation) improved subsequent discrimination accuracy in a relatively rapid manner. This suggests that perceptual representations are refined by prior reinforcement history and adds to a growing body of evidence that early sensory systems can be modified by motivationally salient statistical contingencies. Although our study was focused on aversive learning, there is evidence that instrumental reward histories may also refine visual discrimination (Baldassi & Simoncini, 2011).
Acknowledgments
This research was supported by a research grant from the NIMH (1R03MH105716–01A1) to V. Miskovic. Appreciation is expressed to Dr. Brandon Gibb for his help with the mental health screening portion of the study. The authors would also like to thank Chris Caracciolo, Molly O’Hagan, Ryan O’Rourke, and Kendra Deschamps for their assistance with data collection.
Footnotes
1We opted for a between-subjects design in an effort to minimize participant fatigue and potential practice effects that would accrue from doubling the number of experimental trials.
2This is consistent with evidence from non-human animal studies that non-learners evidence worse discrimination acuity while learners evidence increased discrimination acuity in an aversive conditioning preparation (Aizenberg & Geffen, 2013).
References
- Åhs F, Miller SS, Gordon AR, & Lundström JN (2013). Aversive learning increasessensory detection sensitivity. Biological Psychology, 92, 135–141. doi.org/ 10.1016/j.biopsycho.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Aton SJ (2013). Set and setting: how behavioral state regulates sensory function and plasticity. Neurobiology of Learning and Memory, 106, 1–10. doi: 10.1016/j.nlm.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassi S, & Simoncini C (2011). Reward sharpens orientation coding independently of attention. Frontiers in Neuroscience, 5, 13. doi: 10.3389/fnins.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenberg M, & Geffen MN (2013). Bidirectional effects of aversive learning on perceptual acuity are mediated by the sensory cortex. Nature Neuroscience, 16, 994–996. doi: 10.1038/nn.3443 [DOI] [PubMed] [Google Scholar]
- Chapuis J, & Wilson DA (2011). Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nature Neuroscience, 15, 155–161. doi: 10.1038/nn.2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Barnes DC, & Wilson DA (2011). Generalized vs. stimulus-specific learned fear differentially modifies stimulus encoding in primary sensory cortex of awake rats. Journal of Neurophysiology, 106, 3136–3144. doi: 10.1152/jn.00721.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington ES, & Onghena P (2007). Randomization Tests (4th Edition). Boca Raton, FL: CRC Press. [Google Scholar]
- Fontanini A, & Katz DB (2008). Behavioral states, network states, and sensory response variability. Journal of Neurophysiology, 100, 1160–1168. doi: 10.1152/jn.90592.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdalyahu A, Tring E, Polack PO, Gruver R, Golshani P, Fanselow MS et al. (2012). Associative fear learning enhances sparse network coding in primary sensory cortex. Neuron, 75, 121–132. doi: 10.1016/j.neuron.2012.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan D, Weiller E, Amorim P, Bonora I, Sheehan K, Janavs J, & Dunbar G (1997). The M.I.N.I. International Neuropsychiatric Interview (M.I.N.I.) A short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry, 12, 224–231. [Google Scholar]
- Leek MR (2001). Adaptive procedures in psychophysical research. Perception & Psychophysics, 63, 1279–1292. doi: 10.3758/BF03194543 [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, & Gottfried JA (2008). Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science, 319, 1842–1845. doi: 10.1126/science.1152837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S (2012). Toward an account of clinical anxiety predicated on basic, neurally-mapped mechanisms of Pavlovian fear-learning: The case for conditioned overgeneralization. Depression and Anxiety, 29, 257–263. doi: 10.1002/da.21922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann JP (2015). Associative learning and sensory neuroplasticity: how does it happen and what is it good for? Learning & Memory, 22, 567–576. doi.org/ 10.1101/lm.039636.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Gruss LF, & Keil A (2015). Aversive learning shapes neuronal orientation tuning in human visual cortex. Nature Communications, 6, 7823. doi: 10.1038/ncomms8823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, & Keil A (2012). Acquired fears reflected in cortical sensory processing: a review of electrophysiological studies of human classical conditioning. Psychophysiology, 49, 1230–1241. doi: 10.1111/j.1469-8986.2012.01398.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi JJ, & Callaway EM (2009). Parallel processing strategies of the primate visual system. Nature Reviews Neuroscience, 10, 360–372. doi: 10.1038/nrn2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Ferraro S, Miller SS, Åhs F, & Lundström JN (2015). Enhancement of odor sensitivity following repeated odor and visual fear conditioning. Chemical Senses, 40, 497–506. doi.org/ 10.1093/chemse/bjv033 [DOI] [PubMed] [Google Scholar]
- R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- Resnik J, Sobel N, & Paz R (2011). Auditory aversive learning increases discrimination thresholds. Nature Neuroscience, 14, 791–796. doi.org/ 10.1038/nn.2802 [DOI] [PubMed] [Google Scholar]
- Schechtman E, Laufer O, & Paz R (2010). Negative valence widens generalization of learning. The Journal of Neuroscience, 30, 10460–10464. doi: 10.1523/jneurosci.2377-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C (1983). Manual for the State-Trait Anxiety Inventory. Rev. ed. Consulting Psychologists Press; Palo Alto (CA). [Google Scholar]
- Sterpenich V, Piguet C, Desseilles M, Ceravolo L, Gschwind M, Van De Ville D, et al. (2014). Sleep sharpens sensory stimulus coding in human visual cortex after fear conditioning. NeuroImage, 100, 608–618. doi.org/ 10.1016/j.neuroimage.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Weinberger NM (2007). Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning & Memory, 14, 1–16. doi.org/ 10.1101/lm.421807 [DOI] [PMC free article] [PubMed] [Google Scholar]


