Abstract
Multidrug-resistant gram-negative pathogens are a significant health threat. Burkholderia spp. encompass a complex subset of gram-negative bacteria with a wide range of biological functions that include human, animal, and plant pathogens. The treatment of infections caused by Burkholderia spp. is problematic due to their inherent resistance to multiple antibiotics. The major β-lactam resistance determinant expressed in Burkholderia spp. is a class A β-lactamase of the PenA family. In this study, significant amino acid sequence heterogeneity was discovered in PenA (37 novel variants) within a panel of 48 different strains of Burkholderia multivorans isolated from individuals with cystic fibrosis. Phylogenetic analysis distributed the 37 variants into 5 groups based on their primary amino acid sequences. Amino acid substitutions were present throughout the entire β-lactamase and did not congregate to specific regions of the protein. The PenA variants possessed 5 to 17 single amino acid changes. The N189S and S286I substitutions were most prevalent and found in all variants. Due to the sequence heterogeneity in PenA, a highly conserved peptide (18 amino acids) within PenA was chosen as the antigen for polyclonal antibody production in order to measure expression of PenA within the 48 clinical isolates of B. multivorans. Characterization of the anti-PenA peptide antibody, using immunoblotting approaches, exposed several unique features of this antibody (i.e., detected <500 pg of purified PenA, all 37 PenA variants in B. multivorans, and Pen-like β-lactamases from other species within the Burkholderia cepacia complex). The significant sequence heterogeneity found in PenA may have occurred due to selective pressure (e.g., exposure to antimicrobial therapy) within the host. The contribution of these changes warrants further investigation.
Keywords: Burkholderia, β-Lactamase, Sequencing
1. Introduction
The genus Burkholderia encompasses ~114 to 117 species that include human, animal, and/or plant pathogens as well as species that possess environmental benefits (e.g., endophytic B. phytofirmans can prevent onion rot) (Eberl and Vandamme, 2016). Based on advances in whole genomic sequencing and phylogenetic analysis, researchers proposed reorganizing the plant-beneficial-environmental species into new genera, Paraburkholderia and Caballeronia, while the human pathogens would remain within the Burkholderia genus (Dobritsa et al., 2017; Dobritsa and Samadpour, 2016; Eberl and Vandamme, 2016; Sawana et al., 2014). Discrepancies remain, however, as some species possess dual beneficial and pathogenic potential (Paraburkholderia ginsengisoli and Paraburkholderia tropica) (Deris et al., 2010; Marks et al., 2016). Thus, the taxonomic organization of these species remains controversial.
Focusing on the Burkholderia pathogens, 2 major groups exist, the Burkholderia cepacia complex (Bcc) and the Burkholderia pseudomallei complex (Bpc). Bcc species can cause infections (e.g., pneumonia) in immunocompromised persons or in individuals with cystic fibrosis (Abbott and Peleg, 2015; Chiappini et al., 2014; Gautam et al., 2011; Hanulik et al., 2013; Marson et al., 2015). Among the Bcc, Burkholderia multivorans and Burkholderia cenocepacia are the most prevalent species recovered from persons with cystic fibrosis in the United States. Within the Bpc, B. pseudomallei is capable of causing a necrotizing pneumonia known as melioidosis and is considered a potential bioweapon (Perumal Samy et al., 2017). Bcc and Bpc species are typically antibiotic resistant, rendering effective antimicrobial therapy of infections a challenge (Abbott and Peleg, 2015; Chiappini et al., 2014; Lipuma, 2010; Rhodes and Schweizer, 2016).
A major antibiotic resistance determinant present in all species of Burkholderia is an inducible class A β-lactamase of the Pen family (e.g., PenA). In 2009, Poirel et al. (2009) characterized several Pen β-lactamases present in Bcc and published the initial nomenclature for the Pen-like β-lactamase family. Since that time, the number of Burkholderia species has increased as has the number of Pen-type enzymes (Table 1). Expression of blapen genes is regulated by a LysR-type transcriptional regulator, PenR through a system analogous to AmpC/AmpR regulatory pathways present in members of the Enterobacteriaceae and in Pseudomonas aeruginosa (Dhar et al., 2018; Trepanier et al., 1997). In addition, each Pen-like β-lactamase possesses a different substrate profile (Papp-Wallace et al., 2013a; Poirel et al., 2009). PenA of B. multivorans possesses a very broad substrate profile that includes carbapenems and is capable of hydrolyzing β-lactamase inhibitors (i.e., clavulanic acid, sulbactam, and tazobactam). Here, we describe the identification of 37 novel PenA variants from B. multivorans. Moreover, using sequence analysis, we generated a polyclonal antibody using on an antigen that comprised an 18-amino-acid peptide from PenA that is able to detect all of the different PenA variants via immunoblotting.
Table 1.
The Pen family of Burkholderial class A β-lactamases.
| Bacterial species (complex) | Pen-like β-lactamase | Reference |
|---|---|---|
| Burkholderia multivorans (Bcc) | PenA | (Trepanier et al., 1997) |
| Burkholderia cenocepacia (Bcc) | PenB | (Poirel et al., 2009) |
| Burkholderia stabilis (Bcc) | PenC | (Poirel et al., 2009) |
| Burkholderia pyrrocina (Bcc) | PenD | (Poirel et al., 2009) |
| Burkholderia vietnamiensis (Bcc) | PenE | (Poirel et al., 2009) |
| Burkholderia ambifaria (Bcc) | PenF | (Poirel et al., 2009) |
| Burkholderia dolosa (Bcc) | PenG | (Poirel et al., 2009) |
| Burkholderia ubonensis (Bcc) | PenH | (Poirel et al., 2009) |
| Burkholderia pseudomallei (Bpc) | PenI | (Poirel et al., 2009) |
| Burkholderia oklahomensis (Bpc) | PenJ | (Poirel et al., 2009) |
| Burkholderia mallei (Bpc) | PenK | (Poirel et al., 2009) |
| Burkholderia thailandensis (Bpc) | PenL | (Poirel et al., 2009) |
| Burkholderia humptydooensis (Bpc) | PenM | This study |
| Burkholderia gladioli | PenN | This study |
2. Materials and methods
2.1. Strains
The Burkholderia spp. clinical isolates were from the B. cepacia Research Laboratory and Repository strain collection as previously described (Papp-Wallace et al., 2017b). The construction of Escherichia coli DH10B pBC SK(+)blapenA and E. coli DH10B pBC SK(+)blapenI was as described (Papp-Wallace et al., 2013b).
2.2. Whole-genome sequencing of B. multivorans
Genomic DNA was purified from the clinical B. multivorans isolates using the MasterPure™ gram-positive DNA purification kit (Epicentre Inc, Madison, WI) as recommended by the manufacturer. The genomes of 48 B. multivorans isolates were sequenced at JCVI by Illumina NextSeq (2 × 150 bp). Paired-end libraries were constructed using Illumina NexteraXT kits. Sequence reads were generated with a target average read depth of ~100-fold coverage. Sequence reads for each isolate were assembled individually using SPAdes (Bankevich et al., 2012) and annotated using National Center for Biotechnology Information’s (NCBI’s) Prokaryotic Genome Annotation Pipeline (Tatusova et al., 2016). Raw DNA sequence reads were submitted to the NCBI Sequence Read Archive, and annotated genomes were deposited in the GenBank whole-genome sequencing repository, which can be obtained within BioProject PRJNA434393. Clustal Ω from the European Bioinformatics Institute (EMBL-EBI) was used to create a multiple sequence alignment using the primary amino acid sequences of the 37 PenA variants and a phylogenetic tree (Li et al., 2015; McWilliam et al., 2013; Sievers et al., 2011).
2.3. PCR and DNA sequencing of blapen from Burkholderia spp
Overnight cultures of B. cenocepacia AU0583, Burkholderia pyrrocinia AU1114, Burkholderia vietnamiensis AU3578, Burkholderia ambifaria AU5203, Burkholderia stabilis AU9035, Burkholderia dolosa AU9336, Burkholderia gladioli AU1009, and Burkholderia ubonensis AU7314 carrying different blaPen genes were boiled for 10 minutes at 99 °C. Polymerase chain reaction (PCR) was conducted using 1 μL of boiled cells (containing the extracted DNA) with the PCR Master Mix (Promega) and primers generated to detect blapenB, blapenC, blapenD, blapenE, blapenF, blapenG, blapenH, and blapenN (Supplementary Table 1). The PCR products were cleaned using QIAquick PCR Purification Kit (Qiagen) and sent to Molecular Cloning Laboratories for DNA sequencing. The resulting DNA sequence files were analyzed using DNAstar software, and the final nucleotide sequences were submitted to NCBI and assigned the following Genbank accession numbers: MG839177–MG839184. Peptide sequences from the different Pen-like β-lactamases were aligned using Clustal Ω as described above.
2.4. Immunoblotting
All strains were grown in lysogeny broth to log phase at an OD600nm between 0.6 and 0.7. In addition, the Burkholderia spp. were treated with 1 mg/L imipenem for 2 hours to induce expression of blapen. Subsequently, the cells were pelleted and lysed using stringent periplasmic fractionation to prepare crude extracts, as previously described (Papp-Wallace et al., 2012). These crude extracts and purified full-length PenA protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% nonfat dry milk in 20 mM Tris–Cl with 150 mM NaCl at pH 7.4 (TBS) for 1 hour and probed in 5% nonfat dry milk in TBS with 1 μg/mL of polyclonal anti–PenA-peptide antibody (which was raised in rabbits by New England Peptide (NEP) using a selected PenA 18 amino acid peptide as the antigen). Membranes were washed 5 times for 10 minutes with TBS with 0.05% Tween 20 (TBST), and for protein detection, blots were incubated for 1 hour in 1:5000 dilutions of antirabbit and antimouse secondary horseradish peroxidase–conjugated antibodies in 5% nonfat dry milk in TBS. Blots were washed 5 times for 10 minutes with TBST and developed using the ECL-Plus™ developing kit (GE Healthcare Life Sciences) or the SuperSignal West Femto Chemiluminescent Substrate (Thermoscientific) according to the manufacturers’ instructions. The Fotodyne Luminary/FX was used to capture images.
3. Results and discussion
3.1. The PenA carbapenemase of B. multivorans exhibits considerable sequence heterogeneity
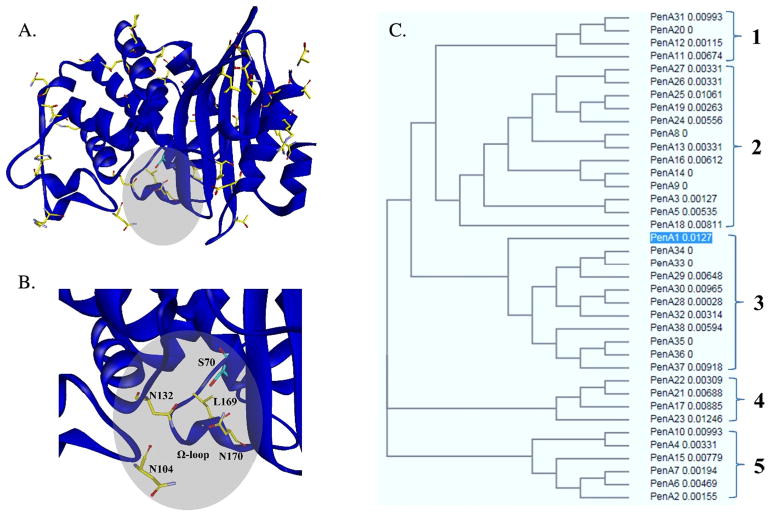
To determine the primary amino acid sequence diversity between PenA’s in B. multivorans, we compared the PenA amino acid sequences from 48 different clinical isolates; 37 novel PenA variants were identified (Table 2). PenA variants possessed 5 to 17 different amino acid substitutions (amino acid numbering is based on the Ambler system; Ambler, 1980) compared with PenA from B. multivorans ATCC 17616, the strain in which PenA was first described (Prince et al., 1988). The locations of these amino acid substitutions were mapped to the PenA crystal structure (Fig. 1A). Several amino acid substitutions (N104S, N132S, L169P, and N170K) were found within the motifs associated with the active site (Fig. 1B). These amino acid substitutions are likely to alter the activity of PenA. Previously, amino acid substitutions at position L169 in PenA were shown to possess enhanced ceftazidime resistance (Papp-Wallace et al., 2017a; Yi et al., 2012). Moreover, substitutions at residues N104, N132, and N170 in other class A β-lactamases were shown to influence either the binding of β-lactams and/or β-lactamase inhibitors as well as the turnover or inhibition (Frase et al., 2011; Ourghanlian et al., 2017; Ramdani-Bouguessa et al., 2011).
Table 2.
Amino acid comparison of the PenA variants sequenced in clinical isolates of B. multivorans to B. multivorans ATCC 17616; (L), correspond to amino acid substitutions in the PenA leader peptide; and amino acid numbering is based on the Ambler system (Ambler, 1980).
| Strain | Amino acid substitutions, insertions, or deletions present | No. of Δs | PenA allele | PenA RefSeq accession | GenBank genome accession |
|---|---|---|---|---|---|
| ATCC 17616, AU21747 | 0 | PenA1 | WP_012216561.1 | NC_010086, PVGM00000000 | |
| AU17545, AU19729 | S(L5)P, N189S, V247A, T267A, S286I | 5 | PenA2 | WP_105796499.1 | PVGB00000000, PVGH00000000 |
| AU11233 | T19A, N189S, V247A, T267A, S286I | 5 | PenA3 | WP_105769622.1 | PVFL0000000 |
| AU26250, AU15954, AU22892 | H60Y, N189S, V247A, T267A, S286I, A290G | 6 | PenA4 | WP_105781374.1 | PVGY00000000, PVFW00000000, PVGO00000000 |
| AU10398 | S(L5)P, T19A, N170K, N189S, V247A, T267A, S286I | 7 | PenA5 | WP_105822028.1 | PVFH00000000 |
| AU19518, AU28069 | S(L5)P, N189S, A205T, G228A, V247A, T267A, S286I | 7 | PenA6 | WP_105803562.1 | PVGD00000000, PVHB00000000 |
| AU14371, AU10897 | S(L5)P, N189S, A205T, V247A, T267A, S286I, A290G | 7 | PenA7 | WP_105758546.1 | PVFT00000000, PVFI00000000 |
| AU14786 | S(L5)P, T19A, G77A, N189S, V247A, T267A, S286I | 7 | PenA8 | WP_048804470.1 | PVFU00000000 |
| AU25543 | T19A, G77A, A86E, N189S, P201A, V247A, T267A, S286I | 8 | PenA9 | WP_105772526.1 | PVGX00000000 |
| AU19564 | A30V, N189S, V192M, V247A, T267A, S286I, A287S, A290G | 8 | PenA10 | WP_105846710.1 | PVGE00000000 |
| AU13919, AU14328 | T19A, F34L, N189S, A205T, V247A, T267A, S286I, A290G | 8 | PenA11 | WP_105777201.1 | PVFQ00000000, PVFR00000000 |
| AU23995 | T19A, S25L, T52A, N189S, V247A, T267A, S286I, A290G | 8 | PenA12 | WP_105809721.1 | PVGT00000000 |
| AU11772, AU23919 | S(L5)P, T19A, P67R, G77A, N189S, V247A, T267A, S286I | 8 | PenA13 | WP_105766562.1 | PVFN00000000, PVGS00000000 |
| AU18096 | T19A, G77A, A86E, N189S, P201A, V247A, T267A, S286I | 8 | PenA14 | WP_105772526.1 | PVGC00000000 |
| AU21015 | S(L5)P, P26R, N189S, D239A, V247A, T267A, S286I, A290G | 8 | PenA15 | WP_107999608.1 | PZZC00000000 |
| AU30760 | T19A, H60Y, G77A, N189S, P201A, V247A, T267A, S286I, A290G | 9 | PenA16 | WP_105825204.1 | PVHJ00000000 |
| AU4507 | A24P, A30S, N189S, P201H, G228A, V247A, T267A, S286I, A290G | 9 | PenA17 | WP_105951298.1 | PVHL00000000 |
| AU24277 | S(L5)P, A15T, T19A, N189S, P201L, V247A, T267A, S286I, A290G | 9 | PenA18 | WP_105835300.1 | PVGU00000000 |
| AU12481 | T19A, A30S, H60Y, G77A, N189S, V247A, T267A, S286I, A290P | 9 | PenA19 | WP_105791036.1 | PVFO00000000 |
| AU30441 | T19A, S25L, T52A, G77A, N189S, V247A, T267A, S286I, A290G | 9 | PenA20 | WP_088926609.1 | PVHH00000000 |
| AU20929 | S(L5)P, A23T, N189S, P201A, I208S, G228A, V247A, T267A, S286I, A290G | 10 | PenA21 | WP_105841035.1 | PVGJ00000000 |
| AU29198 | S(L5)P, ΔA29, A30L, N189S, P201A, G228A, V247A, T267A, S286I, A290G | 10 | PenA22 | WP_105842598.1 | PVHD00000000 |
| AU17534 | T19A, A30S, Q92R, N189S, Q206R, G228A, V247A, T267A, S286I | 10 | PenA23 | WP_105782476.1 | PVGA00000000 |
| AU23668 | T(L2)P, S(L5)P, T19A, A30S, H60Y, G77A, N189S, V247A, T267A, S286I | 10 | PenA24 | WP_105813799.1 | PVGQ00000000 |
| AU21596 | T19A, A30S, T52A, H60Y, G77A, N189S, V247A, T267A, D276E, S286I | 10 | PenA25 | WP_105854385.1 | PVGL00000000 |
| AU14364 | S(L5)P, T19A, A23T, H60Y, D63G, G77A, L169P, A184E, N189S, V247A, T267A, S286I | 12 | PenA26 | WP_105765688.1 | PVFS00000000 |
| AU15814 | S(L5)P, T19A, A23T, H60Y, D63G, G77A, N132S, A184E, N189S, V247A, T267A, S286I | 12 | PenA27 | WP_105765076.1 | PVFV00000000 |
| AU17135, AU10047 | T3A, L10V, T19A, A58T, N104S, R141L, N189S, P201A, V247A, A280T, S286I, A290G | 12 | PenA28 | WP_088924033.1 | PVFZ00000000, PVFE00000000 |
| AU16734 | T3A, L10V, T19A, R99Q, N104S, R141L, N189S, P201A, T227A, A280T, S286I, A290G | 12 | PenA29 | WP_105792310.1 | PVFY00000000 |
| AU19659 | T3A, L10V, P26A, A58T, N104S, R141L, N189S, P201A, K219R, A280T, S286I, A290G | 12 | PenA30 | WP_105807557.1 | PVGF00000000 |
| AU10086 | T19A, S25L, T52A, G77A, H112Y, T118A, N189S, V247A, T267A, R283Q, S286I, A290G | 12 | PenA31 | WP_039217008.1 | PVFF00000000 |
| AU23690, AU11358, AU11204 | T3A, L10V, T19A, A58T, N104S, R141L, N189S, P201A, T227A, V247A, A280T, S286I, A290G | 13 | PenA32 | WP_105762373.1 | PVGR00000000, PVFM00000000, PVFK00000000 |
| AU23365 | T3A, L10V, T19A, N104S, R141L, N189S, P201A, T227A, V247A, L250M, A280T, S286I, A290G | 13 | PenA33 | WP_069220914.1 | PVGP00000000 |
| AU27706 | T3A, L10V, T19A, N104S, R141L, N189S, insertion VL 191–192, P201A, T227A, V247A, L250M, A280T, S286I, A290G | 15 | PenA34 | WP_105856053.1 | PVHA00000000 |
| AU30050 | T3A, L10V, T19P, V20T, S21D, D22N, V27G, N104S, R141L, N189S, P201A, T227A, V247A, A280T, S286I, A290G | 16 | PenA35 | WP_105795180.1 | PVHF00000000 |
| AU30438 | T3A, L10V, T19P, V20T, S21D, D22N, V27G, N104S, R141L, N189S, P201A, T227A, V247A, T267A, S286I, A290G | 16 | PenA36 | WP_105795180.1 | PVHG00000000 |
| AU22436 | T3A, L10V, T19P, V20T, S21D, D22N, V27G, A58T, N104S, R141L, N189S, P201A, T227A, L250M, A280T, S286I, A290G | 17 | PenA37 | WP_088929369.1 | PVGN00000000 |
| AU25057 | T3A, L10V, G11C, T19P, V20T, S21D, D22N, V27G, N104S, R141L, N189S, P201A, T227A, V247A, A280T, S286I, A290G | 17 | PenA38 | WP_105837729.1 | PVGW00000000 |
Fig. 1.
A, PenA crystal structure representing residues (yellow sticks) that are variable in the different clinical isolates. A gray circle highlights the location of the active site, catalytic S70 (cyan sticks). Bg, Enlarge view of the PenA active site revealing the active site residues (N104, N132, L169, and N170) that possess amino acid substitutions in the PenA variants. C, Phylogenetic tree constructed using Clustal Ω reveals the presence of 5 clades; PenA1 is highlighted in blue (Li et al., 2015; McWilliam et al., 2013; Sievers et al., 2011)
The 37 variants clustered into ~5 clades based on phylogenetic analysis (Fig. 1C). The N189S and S286I amino acid substitutions were found in all PenA variants. Other prevalent amino acid substitutions included V247A and T267A present in 92% and 73% of the variants, respectively. The largest cluster (2) consisted of the 13 variants with a high occurrence of T19A, H60Y, and G77A substitutions (Fig. 1C and Table 2). The second largest group (3) possessed the most (i.e., 12–17) amino acid substitutions in PenA. The majority of these variants carried the T3A, L10V, N104S, and R141L amino acid substitutions, as well as T227A and A280T, which were not present in the other 4 subgroups. The remaining 3 clades contained 4–6 variants; however, the differentiating factor between them included the occurrence of T19A, T52A, and/or S25L in group 1 and P201X and/or G228A in group 4. Clade 5 mostly possessed the prevalent amino acid substitutions mentioned above with a few others interspersed.
3.2. Identification of a PenA peptide for antibody production
Due to the significant heterogeneity in amino acid sequence, the polyclonal anti-PenA antibody that we had generated previously (Papp-Wallace et al., 2017a) using the full-length purified PenA β-lactamase as the antigen was unable to recognize the PenA variants in the clinical isolates via immunoblotting. Thus, we set out to generate a better anti-PenA antibody. Based on the 37 different PenA amino acid sequences, we identified conserved regions within the protein (Fig. 2). Four regions of PenA were found to not possess any amino acid substitutions. With additional guidance provided by NEP, a single PenA-peptide (CARSIGDDTFRLDRWETE; the first amino acid of this peptide, tyrosine [Y] was replaced by cysteine [C]) was chosen for polyclonal antibody production in rabbits (Fig. 2).
Fig. 2.
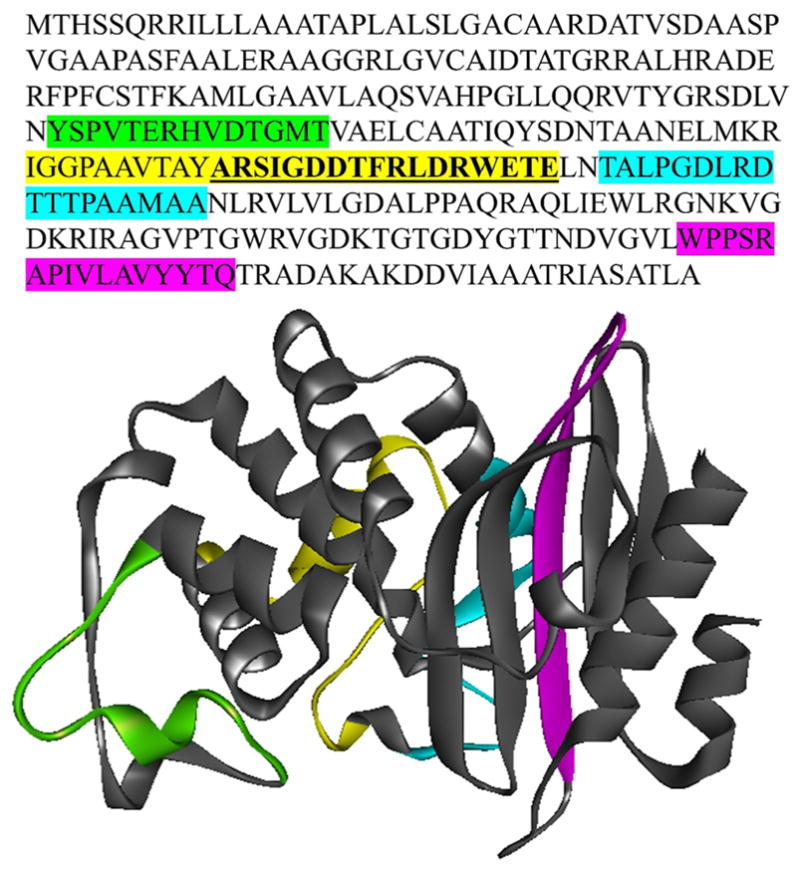
Based on the amino acid sequence variability observed with PenA in the 50 clinical isolates (Table 2), 4 regions (highlighted in green, yellow, cyan, and magenta) were identified within the PenA amino acid sequence that did not possess any substitutions (top); regions are mapped onto the PenA proteins structure (below). NEP conducted further analysis and found that the yellow region (bold and underlined) would make the most favorable antigen.
3.3. The polyclonal anti–PenA-peptide antibody possesses a low limit of detection
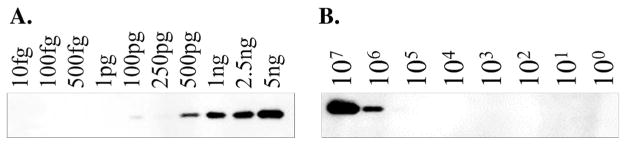
To determine the limits of detection for the anti–PenA-peptide antibody, decreasing concentrations (10 fg–5 ng) of purified PenA protein were used for an immunoblot. The antibody could distinguish small amounts of protein, and a band for 500 pg of purified PenA was easily detected; moreover, a very weak band was observed at 100 pg (Fig. 3A). Our previous polyclonal anti-PenA antibody could only recognize 250 ng of purified PenA protein (data not shown). Thus, the anti–PenA-peptide antibody discriminates 200–500× better than the former anti-PenA antibody. The detection limit of the antibody was further assessed using B. multivorans ATCC 17616 after induction of blaPenA using imipenem. The anti–PenA-peptide antibody could detect PenA protein using ~106 CFUs (Fig. 3B).
Fig. 3.
Determining the sensitivity of the anti–PenA-peptide antibody. A, Immunoblot using decreasing concentrations of purified PenA β-lactamase. B, Immunoblot using decreasing CFUs of B. multivorans ATTC 17616 after induction with 1 μg/mL of imipenem.
3.4. The polyclonal anti–PenA-peptide antibody detects PenA in 50 different B. multivorans clinical isolates
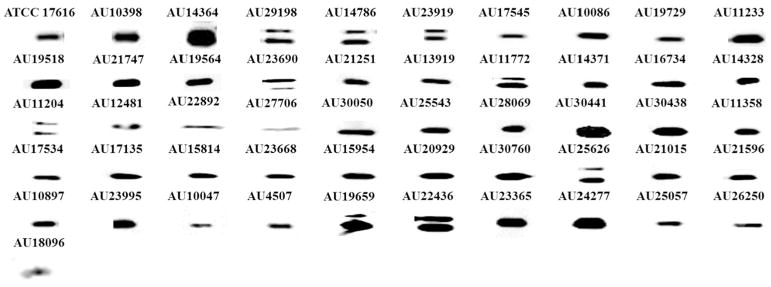
To test the ability of the anti–PenA-peptide antibody to detect different variants of PenA, 50 B. multivorans clinical isolates were grown to log phase, induced with imipenem, and prepared for immunoblotting. The anti–PenA-peptide antibody was able to detect all 37 PenA variants within the 50 isolates (Fig. 4). A nonspecific higher-molecular-weight band was observed in some of the isolates (e.g., AU29198, AU14786, and AU22436); studies are underway to identify this protein. We speculate that the higher-molecular-weight band represents unprocessed PenA protein prior to cleavage of the signal peptide; PenA’s signal peptide is 27 amino acids and 2.7 kDa.
Fig. 4.
Immunoblot on crude extracts of 50 different clinical isolates of B. multivorans after induction with 1 μg/mL of imipenem. Strains AU25626 and AU21251 were not sequenced, but are presented in the immunoblot.
3.5. The polyclonal anti–PenA-peptide antibody detects other Pen-like β-lactamases within Bcc
The cross-reactivity of the PenA-peptide antibody was assessed against other Pen-like β-lactamases (PenB, PenC, PenD, PenE, PenF, PenG, PenH, and PenN) produced by Burkholderia species. Interestingly, among the clinical isolates tested using ~108 CFUs, the antibody detected PenB, PenD, PenE, PenF, PenC, PenG, and PenH, which are produced by strains within the Bcc (Fig. 5A). The PenE and PenG β-lactamases of B. vietnamiensis and B. dolosa, respectively, demonstrated the most intense bands. The PenN β-lactamase of B. gladioli, which is not a member of the Bcc, was not within the limit of detection. Comparison of the analogous peptide sequences from the different Pens revealed that the PenN peptide possessed the most sequence diversity with 4-amino-acid substitutions compared with the peptide used for antibody production (Fig. 5B).
Fig. 5.
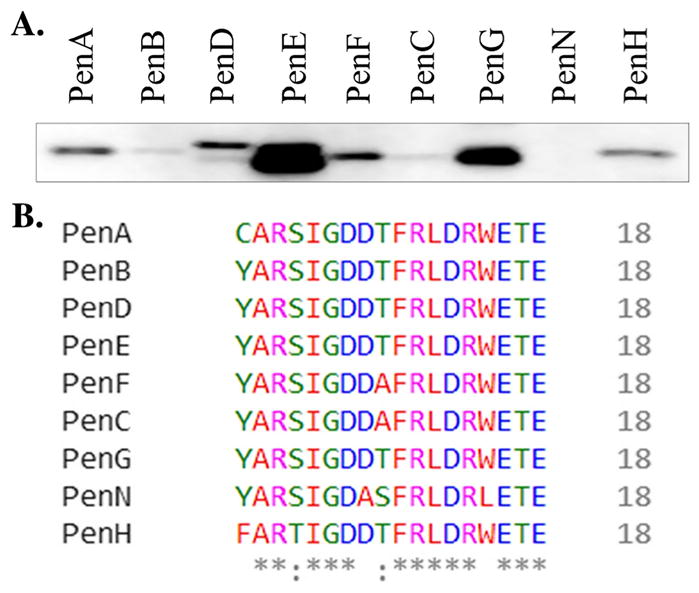
Assessing the cross-reactivity of anti–PenA-peptide antibody against Burkholderia spp. A, Immunoblot on B. multivorans ATTC 17616 blaPenA, B. cenocepacia AU0583 blaPenB, B. pyrrocinia AU1114 blaPenD, B. vietnamiensis AU3578 blaPenE, B. ambifaria AU5203 blaPenF, B. stabilis AU9035 blaPenC, B. dolosa AU9336 blaPenG, B. gladioli AU1009 blaPenN and B. ubonensis AU7314 blaPenH grown to log phase, induced with 1 μg/mL of imipenem, and prepared as crude extracts. B, Clustal Ω sequence alignment of the peptides from the different Pen-like β-lactamases compared with the PenA peptide used for antibody production.
4. Conclusions
Here, we document the significant sequence heterogeneity in the PenA carbapenemase based on the analysis of 48 different clinical strains of B. multivorans. Thirty-seven novel PenA variants were identified with 5–17 different single amino acid substitutions. Most of the amino acid changes are not to active site residues; thus, we hypothesize that they may increase the stability of the β-lactamase. The impact is currently under investigation.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program BX002872 to KMP-W from the US Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The contents do not represent the views of the US Department of Veterans Affairs or the US Government. This project has been funded in whole or part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services under Award Number U19AI110819 to JCVI and U19-AI109713-SRP to KMP-W. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by funding (to JJL) from the Cystic Fibrosis Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diagmicrobio.2018.06.005.
Competing interests
The authors have no competing interests to declare.
References
- Abbott IJ, Peleg AY. Stenotrophomonas, Achromobacter, and nonmelioid Burkholderia species: antimicrobial resistance and therapeutic strategies. Semin Respir Crit Care Med. 2015;36:99–110. doi: 10.1055/s-0034-1396929. [DOI] [PubMed] [Google Scholar]
- Ambler RP. The structure of β-lactamases. Philos Trans R Soc Lond Ser B Biol Sci. 1980;289:321–31. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–77. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini E, Taccetti G, de Martino M. Bacterial lung infections in cystic fibrosis patients: an update. Pediatr Infect Dis J. 2014;33:653–4. doi: 10.1097/INF.0000000000000347. [DOI] [PubMed] [Google Scholar]
- Deris ZZ, Van Rostenberghe H, Habsah H, Noraida R, Tan GC, Chan YY, et al. First isolation of Burkholderia tropica from a neonatal patient successfully treated with imipenem. Int J Infect Dis. 2010;14:e73–4. doi: 10.1016/j.ijid.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Dhar S, Kumari H, Balasubramanian D, Mathee K. Cell-wall recycling and synthesis in Escherichia coli and Pseudomonas aeruginosa—their role in the development of resistance. J Med Microbiol. 2018;67:1–21. doi: 10.1099/jmm.0.000636. [DOI] [PubMed] [Google Scholar]
- Dobritsa AP, Samadpour M. Transfer of eleven species of the genus Burkholderia to the genus Paraburkholderia and proposal of Caballeronia gen. nov. to accommodate twelve species of the genera Burkholderia and Paraburkholderia. Int J Syst Evol Microbiol. 2016;66:2836–46. doi: 10.1099/ijsem.0.001065. [DOI] [PubMed] [Google Scholar]
- Dobritsa AP, Linardopoulou EV, Samadpour M. Transfer of 13 species of the genus Burkholderia to the genus Caballeronia and reclassification of Burkholderia jirisanensis as Paraburkholderia jirisanensis comb. nov Int J Syst Evol Microbiol. 2017;67:3846–53. doi: 10.1099/ijsem.0.002202. [DOI] [PubMed] [Google Scholar]
- Eberl L, Vandamme P. Members of the genus Burkholderia: good and bad guys. F1000Res. 2016:5. doi: 10.12688/f1000research.8221.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frase H, Toth M, Champion MM, Antunes NT, Vakulenko SB. Importance of position 170 in the inhibition of GES-type β-lactamases by clavulanic acid. Antimicrob Agents Chemother. 2011;55:1556–62. doi: 10.1128/AAC.01292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam V, Singhal L, Ray P. Burkholderia cepacia complex: beyond Pseudomonas and Acinetobacter. Indian J Med Microbiol. 2011;29:4–12. doi: 10.4103/0255-0857.76516. [DOI] [PubMed] [Google Scholar]
- Hanulik V, Webber MA, Chroma M, Uvizl R, Holy O, Whitehead RN, et al. An outbreak of Burkholderia multivorans beyond cystic fibrosis patients. J Hosp Infect. 2013;84:248–51. doi: 10.1016/j.jhin.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Li W, Cowley A, Uludag M, Gur T, McWilliam H, Squizzato S, et al. The EMBL-EBI bioinfor-matics web and programmatic tools framework. Nucleic Acids Res. 2015;43:W580–4. doi: 10.1093/nar/gkv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks LR, Dodd H, Russo TA, Berenson CS. Burkholderia ginsengisoli bacteraemia: emergence of a novel pathogen. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-213584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson FA, Hortencio TD, Aguiar KC, Ribeiro JD. Demographic, clinical, and laboratory parameters of cystic fibrosis during the last two decades: a comparative analysis. BMC Pulm Med. 2015;15:3. doi: 10.1186/1471-2466-15-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, et al. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013;41:W597–600. doi: 10.1093/nar/gkt376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourghanlian C, Soroka D, Arthur M. Inhibition by avibactam and clavulanate of the β-lactamases KPC-2 and CTX-M-15 harboring the substitution N(132)G in the conserved SDN motif. Antimicrob Agents Chemother. 2017:61. doi: 10.1128/AAC.02510-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Taracila MA, Smith KM, Xu Y, Bonomo RA. Understanding the molecular determinants of substrate and inhibitor specificities in the carbapenemase KPC-2: exploring the roles of Arg220 and Glu276. Antimicrob Agents Chemother. 2012;56:4428–38. doi: 10.1128/AAC.05769-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Taracila MA, Gatta JA, Ohuchi N, Bonomo RA, Nukaga M. Insights into β-lactamases from Burkholderia species, two phylogenetically related yet distinct resistance determinants. J Biol Chem. 2013a;288:19090–102. doi: 10.1074/jbc.M113.458315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Taracila MA, Gatta JA, Ohuchi N, Bonomo RA, Nukaga M. Insights into beta-lactamases from Burkholderia species, two phylogenetically related yet distinct resistance determinants. J Biol Chem. 2013b;288:19090–102. doi: 10.1074/jbc.M113.458315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Becka SA, Taracila MA, Zeiser ET, Gatta JA, LiPuma JJ, et al. Exploring the role of the Ω-Loop in the evolution of ceftazidime resistance in the PenA β-lactamase from Burkholderia multivorans, an important cystic fibrosis pathogen. Antimicrob Agents Chemother. 2017a:61. doi: 10.1128/AAC.01941-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Becka SA, Zeiser ET, Ohuchi N, Mojica MF, Gatta JA, et al. Overcoming an extremely drug resistant (XDR) pathogen: avibactam restores susceptibility to ceftazidime for Burkholderia cepacia complex isolates from cystic fibrosis patients. ACS Infect Dis. 2017b;3:502–11. doi: 10.1021/acsinfecdis.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal Samy R, Stiles BG, Sethi G, Lim LHK. Melioidosis: clinical impact and public health threat in the tropics. PLoS Negl Trop Dis. 2017;11:e0004738. doi: 10.1371/journal.pntd.0004738. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Poirel L, Rodriguez-Martinez JM, Plesiat P, Nordmann P. Naturally occurring class A β-lactamases from the Burkholderia cepacia complex. Antimicrob Agents Chemother. 2009;53:876–82. doi: 10.1128/AAC.00946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A, Wood MS, Cacalano GS, Chin NX. Isolation and characterization of a penicillinase from Pseudomonas cepacia 249. Antimicrob Agents Chemother. 1988;32:838–43. doi: 10.1128/aac.32.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdani-Bouguessa N, Manageiro V, Jones-Dias D, Ferreira E, Tazir M, Canica M. Role of SHV β-lactamase variants in resistance of clinical Klebsiella pneumoniae strains to β-lactams in an Algerian hospital. J Med Microbiol. 2011;60:983–7. doi: 10.1099/jmm.0.030577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KA, Schweizer HP. Antibiotic resistance in Burkholderia species. Drug Resist Updat. 2016;28:82–90. doi: 10.1016/j.drup.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawana A, Adeolu M, Gupta RS. Molecular signatures and phylogenomic analysis of the genus Burkholderia: proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov harboring environmental species. Front Genet. 2014;5:429. doi: 10.3389/fgene.2014.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Ω. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–24. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanier S, Prince A, Huletsky A. Characterization of the penA and penR genes of Burkholderia cepacia 249 which encode the chromosomal class A penicillinase and its LysR-type transcriptional regulator. Antimicrob Agents Chemother. 1997;41:2399–405. doi: 10.1128/aac.41.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Cho KH, Cho YS, Kim K, Nierman WC, Kim HS. Twelve positions in a β-lactamase that can expand its substrate spectrum with a single amino acid substitution. PLoS One. 2012;7:e37585. doi: 10.1371/journal.pone.0037585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.