Abstract
Background.
Diastolic dysfunction is emerging as a leading cause of heart failure in aging population. Induction of hypoxia tolerance and reprogrammed cell metabolism have emerged as novel therapeutic strategies for the treatment of cardiovascular diseases.
Methods and Results.
In the present study, we showed that deletion of sirtuin 3 (SIRT3) resulted in a diastolic dysfunction together with a significant increase in the expression of prolyl hydroxylases (PHD) 1 and 2. We further investigated the involvement of PHD in the development of diastolic dysfunction by treating the 12–14 months old mice with a PHD inhibitor, dimethyloxalylglycine (DMOG) for 2 weeks. DMOG treatment increased the expression of hypoxia-inducible factor (HIF)-1α in the endothelium of coronary arteries. This was accompanied by a significant improvement of coronary flow reserve and diastolic function. Inhibition of PHD altered endothelial metabolism by increasing glycolysis and reducing oxygen consumption. Most importantly, treatment with DMOG completely reversed the pre-existing diastolic dysfunction in the endothelial-specific SIRT3 deficient mice.
Conclusions.
Our findings demonstrate that inhibition of PHD and reprogrammed cell metabolism can reverse the pre-existed diastolic dysfunction in SIRT3 deficient mice. Our study provides a potential therapeutic strategy of induction of hypoxia tolerance for patients with diastolic dysfunction associated with coronary microvascular dysfunction, especially in the aging population with reduced SIRT3.
Keywords: SIRT3, PHD, DMOG, diastolic dysfunction, induction of hypoxia tolerance
1. Introduction
Diastolic dysfunction is one of the major characteristics of heart failure with preserved ejection fraction (HFpEF), as well as in some population of asymptomatic patients and patients with reduced EF (HFrEF) [1]. More than half of the HF patients are diagnosed with diastolic dysfunction [2–5]. Diastolic dysfunction is commonly associated with cardiovascular, metabolic, and inflammatory comorbidities [6]. For instance, age, hypertension, diabetes mellitus, obesity, chronic renal failure, and LV hypertrophy are the major risk factors for diastolic dysfunction [7, 8]. Recent studies demonstrate that persistent or progression of diastolic dysfunction, especially with co-existing comorbidities, promotes the development of heart failure in aging population [9, 10]. However, currently available and effective treatment for HFrEF have failed to show promising results in patients with diastolic dysfunction [11]. Clinical studies reveal that patients with HFpEF have coronary microvascular rarefaction and more cardiac hypertrophy than age-matched patients without clinical diagnosis of coronary artery disease and heart failure [6]. Despite the clinical importance of HFpEF, our understanding of its pathophysiology and molecular mechanism is incomplete.
Sirtuins are a family of Class III histone deacetylases (HDACs) that require NAD+ for their lysine residue deacetylase activity [12, 13]. Sirtuins regulate cellular homeostasis, including energy metabolism and reactive oxygen species (ROS) [14, 15]. Of the Sirtuin family, SIRT3 is primarily localized to the mitochondria in metabolic active organs, including liver, adipose tissue, and heart, where it regulates mitochondrial function and cellular metabolism [15–18]. Increased SIRT3 expression protects cardiomyocytes, pancreatic cells, and neurons from inflammation and apoptosis by reducing oxidative stress [19–22]. SIRT3 levels have been shown to decrease in human cardiac fibroblasts isolated from controls and patients with HF [23]. Hirschey and colleagues report that ablation of SIRT3 in mice impairs glucose tolerance and develops hepatic steatosis and metabolic syndrome [24, 25]. In our previous study, we found that ablation of SIRT3 causes coronary microvascular dysfunction and increases ischemic injury in the heart [26]. Moreover, specific deletion of endothelial Sirt3 impairs glycolysis and causes a diastolic dysfunction in mice [27]. Koentges and colleagues report that SIRT3 deficiency causes mitochondrial and contractile dysfunction in the heart [28]. These studies indicate a critical role of SIRT3 in the development of cardiac dysfunction.
Hypoxia triggers the activation of hypoxia-inducible factors (HIFs) and the expression of many genes involving in glucose uptake, glycolysis, erythropoiesis, and angiogenesis [29–31]. Prolyl hydroxylases (PHDs) play an important role in the regulation of HIFs [32, 33]. Deactivation of PHD1 reduces oxygen consumption and mitochondrial oxidative stress and protects against muscle ischemic necrosis [34]. However, the consequence of administering PHD inhibitor on the diastolic dysfunction is unclear. In the present study, we hypothesized that inhibition of PHDs that mimics induction of hypoxia tolerance is protective against the diastolic dysfunction in the SIRT3 deficient mice. Our study reveals that the expression of PHD1 and PHD2 is significantly upregulated in the SIRT3 deficient mice. Moreover, treatment with PHD inhibitor DMOG reprograms endothelial metabolism, improves coronary microvascular function and diastolic function in global SIRT3 knock-out (KO) mice and endothelial-specific SIRT3 KO mice.
2. Methods
See Online Data Supplement for detailed methods and materials.
2.1. Animals
All animals were fed with laboratory standard chow and water and housed in individually ventilated cages in the Laboratory Animal Facilities at the University of Mississippi Medical Center. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Mississippi Medical Center (Protocol ID: 1280B) and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85–23, Revised 1996).
2.2. DMOG Treatment
Endothelial SIRT3 knockout (SIRT3 ECKO) mice were obtained by crossbreeding SIRT3flox/flox mice with VE-Cadherin-Cre (Cdh5-Cre) transgenic mice [B6.FVB-Tg(Cdh5-cre)7Mlia/J from Jackson Laboratories] expressing Cre recombinase in vascular endothelial cells, as described in previous study [27]. Male SIRT3 global knockout (SIRT3 KO) or SIRT3 ECKO mice at age of 12–14 months were injected with DMOG (25 μg/g/day) intraperitoneally for 14 days as shown in Supplemental Figure S1. The number of mice used for each experiment was indicated in the figure legends. This dosage used in this study was chose based on previous studies with minor change [35–37]. Diastolic function and coronary flow reserve was monitored using echocardiography before the treatment and every 7 days. After 14 days of treatment, the mice were sacrificed, and heart tissue were harvested for Western blot and immunofluorescence staining.
2.3. Statistical Analysis
Data are presented as mean ± S.D. All data were tested for normality and passed the test. Statistical significance was determined using Student’s t-test (two-tailed) between means of two groups, or one-way ANOVA with repeated measures as indicated, followed by Bonferroni’s post-hoc test (Sigmaplot v12.5). P < 0.05 is considered as statistically significant.
3. Results
3.1. SIRT3 KO mice develops diastolic dysfunction
We examined whether SIRT3 KO mice developed a diastolic dysfunction in the presence of impaired CFR. Pulse-wave (PW) Doppler measurements indicated that the isovolumic relaxation time (IVRT) was significantly increased in SIRT3 KO mice (Figure 1A and 1B). In addition, the calculated myocardial performance index was significantly elevated (Figure 1B). The mitral valve inflow velocity during early diastolic (E) phase was similar between WT and SIRT3 KO mice, but it was associated with a significant decrease in late diastolic (A) phase (Supplemental Table 1, Figure 1A and 1B). However, the E/A ratio was not significantly different between the two groups (Supplemental Table 1). Moreover, Tissue Doppler of mitral annulus indicated that the peak velocity of E’ and A’ was significantly decreased, resulting in a significant elevation in the E/E’ ratio (Figure 1C and Supplemental Table 1). These data indicate that loss of SIRT3 is associated with impaired left ventricle (LV) relaxation and filling pressure. SIRT3 KO mice also exhibited a systolic dysfunction compared to the age-matched WT mice as shown in Supplemental Table 2.
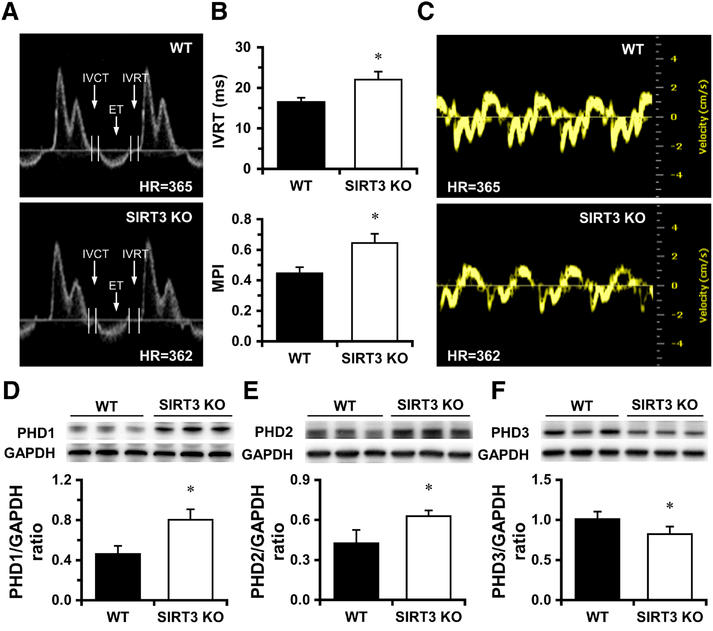
Figure 1. Lack of SIRT3 causes diastolic dysfunction.
A-B, Mitral valve was visualized in apical 4-chamber (A4C) view using Pulsed-wave (PW) Doppler mode. Transmitral inflow Doppler reveals that the isovolumic relaxation time (IVRT) is prolonged in SIRT3 KO mice vs. WT mice. Calculated MPI is significantly increased in SIRT3 KO mice vs. WT mice. C, Representative images of Tissue Doppler of mitral annulus motion of WT and SIRT3 KO mice. *p < 0.05 vs. WT. N=6–9. D-E, The expression of PHD1 and PHD2 is significantly increased in SIRT3 KO mice vs. WT mice. F, The expression of PHD3 is significantly decreased in SIRT3 KO mice. *p < 0.05 vs. WT. N=5–6. IVRT, isovolumic relaxation time; MPI, myocardial performance index.
3.2. Upregulation of PHD 1 and PHD2 in the hearts of SIRT3 KO mice
To further investigate the molecular basis of diastolic dysfunction in SIRT3 deficient mice, we analyzed the expression of PHDs that induces hypoxia tolerance and reprograms cell metabolism. We found that the expression of PHD1 and PHD2 was significantly upregulated whereas the expression of PHD3 was downregulated in SIRT3 KO mouse heart (Figure 1D-F). Intriguingly, the expression of atrial natriuretic peptide (ANP) was diminished in the heart of SIRT3 KO mice (Supplemental Figure S2A). We found that the level of gp91, a subunit of NADPH oxidase, was significantly upregulated in the heart of SIRT3 KO mice (Supplemental Figure S2B). There was a significant decrease in the expression of ERK1/2 in the heart of SIRT3 KO mice (Supplemental Figure S2C). Moreover, the expression of caspase-3 and Wnt7b was markedly increased in the heart of SIRT3 KO mice (Supplemental Figure S2D and S2E), suggesting cardiomyocytes are under stress and vulnerable to apoptosis.
3.3. Inhibition of PHD improves CFR and reverses pre-existed diastolic dysfunction
We further tested if suppression of PHDs could improve diastolic function. SIRT3 KO mice were injected with DMOG (25 μg/g/day) intraperitoneally for 14 days. First, we analyzed the effect of DMOG on the expression of HIF-1α and HIF-2α by Western blots and found only mild increase in HIF-1α in SIRT3 KO heart and no change in HIF-2α (Supplemental Figure S3). Intriguingly, HIF-1α and HIF-2α were enriched in the nuclei of both endothelial cells and cardiomyocytes (Supplemental Figure S4A-S4D), as well as in the endothelium of coronary arteries (Supplemental Figure S5). However, capillary density was not significant different between the two groups (Supplemental Figure S4E). Cardiac diastolic function was measured at day 7 and day 14 by echocardiography after DMOG treatment. As shown in Figure 2A, DMOG treatment significantly increased CFR at day 14 in SIRT3KO mice. Moreover, DMOG treatment significantly improved diastolic function, as evidenced by gradual decline of IVRT and myocardial performance index (MPI) at day 7 (Figure 2B–2C). Transmitral inflow Doppler showed a significantly increase in peak velocity of E and A, along with recovered peak velocity of E’ and A’ at day 7 (Figure 2B, 2D, and Supplemental Table 1). The E/E’ ratio was also significantly decreased (Supplemental Table 1). Systolic function was also improved in SIRT3 KO mice treated with DMOG for 2 weeks (Supplemental Table 2). Although we did not observe any cardiac hypertrophy in SIRT3 KO mice compared to WT mice, DMOG decreased heart weight to tibia length ratio (Supplemental Figure S6A). There was no difference in body weight (Supplemental Figure S6B). Also, DMOG treatment did not alter blood pressure measured by tail-cuff method nor heart rate (Supplemental Figure S7). These data suggest PHD may play an important role in coronary microvascular dysfunction in association with diastolic function during SIRT3 deficiency.
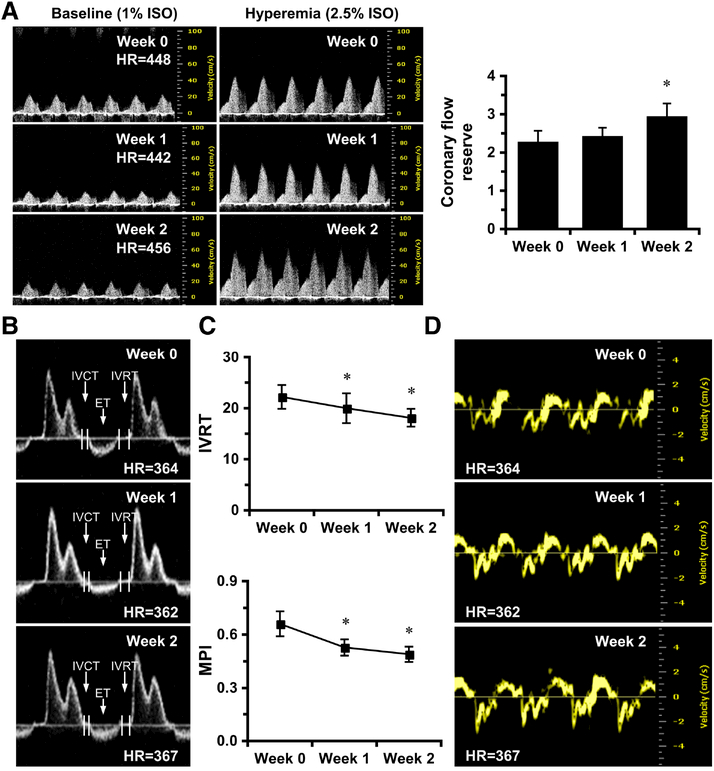
Figure 2. PHD inhibition by DMOG improves coronary microvascular function and diastolic function in SIRT3 KO mice.
A, Representative images of PW Doppler images indicated that CFR is significantly increased in SIRT3 KO mice treated with DMOG at week 2. B-C, Representative images of transmitral PW Doppler and measurements indicates that IVRT and myocardial performance index (MPI) are gradually decreased in SIRT3 KO mice over two weeks of treatment. D, Representative images of Tissue Doppler of mitral annulus motion of SIRT3 KO mice at week 0, week 1 and week 2. *p < 0.05 vs. Week 0, determined by one way ANOVA with repeated measurements. N=6. IVRT, isovolumic relaxation time; MPI, myocardial performance index.
3.4. PHD inhibition alters glycolytic metabolism and oxygen consumption in ECs
Using cultured endothelial cell, we further investigated the effect of PHD inhibition on EC metabolism. In WT-ECs, treatment with DMOG (1 mM) for 24 hours increased the expression of HIF-2α and glycolytic enzyme, PFKFB3 (Supplemental Figure S8A and S9A). Whereas, the expression of HIF-1α did not change (Supplemental Figure S10). This was accompanied by a significant increase in glycolytic metabolism (Figure 3A). Interestingly, basal oxygen consumption and maximum respiratory capacity were suppressed in WT-ECs by DMOG (Figure 3B). Similarly, exposure of SIRT3 KO-ECs to DMOG led to a dose-dependent increase in the expression of HIF-2α and PFKFB3 (Supplemental Figure S8B and S9B) and improvement of glycolysis in SIRT3 KO-ECs (Figure 3A). Furthermore, exposure of SIRT3 KO ECs to DMOG significantly reduced maximum oxygen consumption (Figure 3B). To further test the hypothesis that PHD1 plays an important role in endothelial metabolism, we specifically knockdown PHD1 by using DsiRNA (Supplemental Figure S11A and S11B). Knockdown of PHD1 significantly increased the expression of PFKFB3 (Supplemental Figure S11C). To rule out the potential effect of DMOG on the production of ROS, we measured superoxide level by DHE staining. As shown in Supplemental Figure S12, DMOG treatment did no change the level of ROS in both WT and SIRT3 KO-ECs. These data suggest that PHD1 may contribute to the reprogramming of endothelial metabolism in SIRT3 deficient cells via downregulation of PFKFB3.
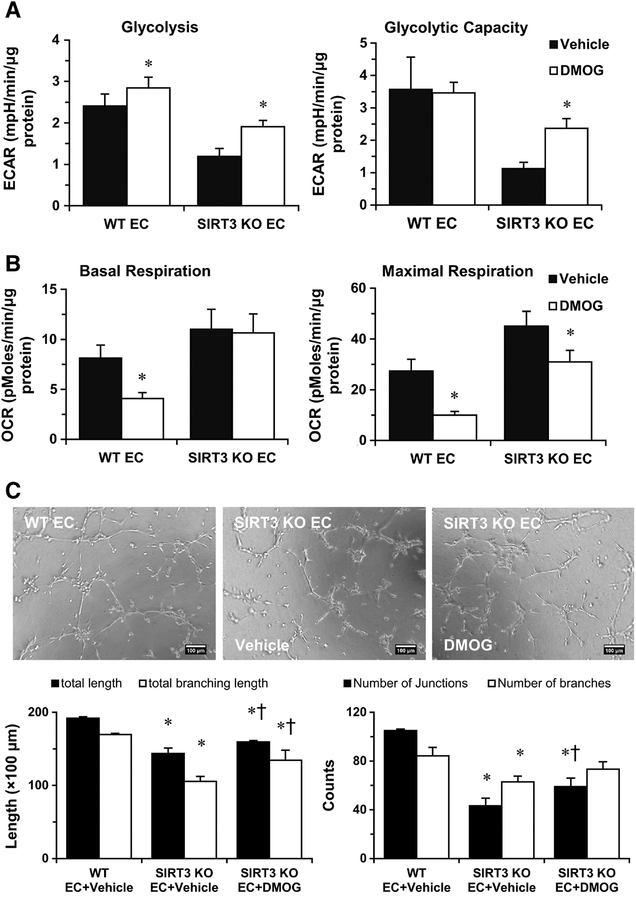
Figure 3. DMOG treatment improves glycolytic function and angiogenesis of SIRT3 KOECs.
A, Glycolysis data are calculated and expressed as extracellular acidification rate (ECAR). Glycolysis and glycolytic capacity in SIRT3 KO-ECs are rescued by the treatment of DMOG (1 mM). B, Treatment with DMOG results in a decrease in maximal respiration in WT-ECs and SIRT3 KO-ECs. However, the basal OCR remains unchanged in SIRT3 KO-ECs. *p<0.05 vs. corresponding vehicle. C, EC tube formation was assessed using a Matrigel assay. Images represented WT and SIRT3 KO-EC network formation at 6 hours of incubation. Quantifications of EC network formation reveals a significant decrease in total length and number of junctions for SIRT3 KO-ECs versus WT-ECs. In the presence of 1 mM DMOG, SIRT3 KO-ECs network formation is improved at 6 hours, as indicated by a significant increase in the number of total branching length in the DMOG treated group. *p<0.05 vs. WT-EC+Vehicle. †p<0.05 vs. SIRT3 KO-EC+Vehicle. Data are representative of 3 independent experiments.
3.5. DMOG treatment increases angiogenic property of EC in SIRT3 KO-ECs
To further test whether DMOG promotes angiogenesis in SIRT3 KO-ECs, we then performed tube formation assay in the presence of 1 mM DMOG. DMOG treatment significantly increased the network formation in the SIRT3 KO-ECs (Figure 3C). To further test the hypothesis that endothelial glycolytic metabolism regulates endothelial angiogenesis, we performed tube formation assay in the presence of 10 μM of PFKFB3 inhibitor-3PO, DMOG, and DMOG/3PO. As expected, DMOG treatment increased network formation in SIRT3 KO-ECs, whereas 3PO abolished the effect of DMOG (Supplemental Figure S13).
3.6. Treatment with DMOG improves CFR and reverses pre-existed diastolic dysfunction in SIRT3 ECKO mice
Our study demonstrated that SIRT3 ECKO mice developed diastolic dysfunction as evidenced by increased E/E’ ratio (Supplemental Table 1). We further investigated the effect of DMOG on diastolic function in SIRT3 ECKO mice. SIRT3 ECKO mice were injected with DMOG (25 μg/g/day) intraperitoneally for 14 days. Treatment with DMOG for 7 days resulted in a significant increase in CFR in SIRT3 ECKO mice (Figure 4A). Moreover, DMOG treatment improved diastolic function, as evidenced by a gradual decline of IVRT and MPI at day 7 (Figure 4B–4C). PW Doppler indicated a significant increase in peak velocity of A wave, along with the recovery of the peak velocity of E’ and A’ waves on day 7 (Figure 4B, 4D and Supplemental Table 1). The E/E’ ratio was also significantly decreased (Supplemental Table 1). Moreover, SIRT3 ECKO mice treated with DMOG resulted in a significant improvement of systolic function (Supplemental Table 2).
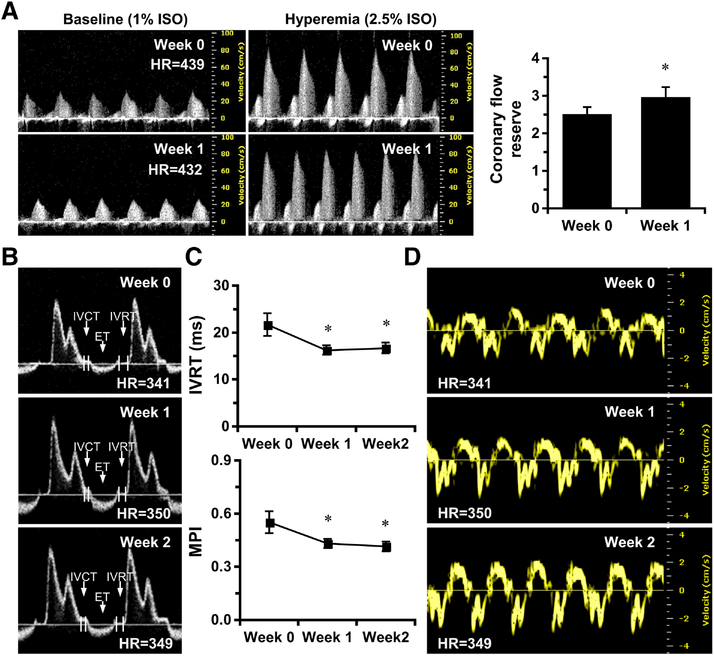
Figure 4. PHD inhibition by DMOG improves coronary microvascular function and diastolic function in SIRT3 ECKO mice.
A, Representative images of PW Doppler images indicates that CFR is significantly increased in SIRT3 ECKO mice treated with DMOG at week 1. B-C, Representative images of transmitral PW Doppler and measurements indicates that IVRT and MPI are gradually decreased in SIRT3 ECKO mice over two weeks of treatment. D, Representative images of Tissue Doppler of mitral annulus motion of SIRT3 ECKO mice at week 0, week 1 and week 2. *p < 0.05 vs. Week 0. N=7. IVRT, isovolumic relaxation time; MPI, myocardial performance index.
4. Discussion
This study demonstrates that SIRT3 deletion results in diastolic dysfunction that is associated with upregulation of PHD1 and PHD2 and alterations in endothelial cell metabolism. These abnormalities lead to coronary microvascular dysfunction manifested as reduced CFR and subsequent diastolic dysfunction in mice. Pharmacological inhibition of PHDs by DMOG improves endothelial glycolytic metabolism and angiogenesis, reverses coronary dysfunction and pre-existed diastolic dysfunction. These results reveal a novel role of SIRT3 on EC metabolism and involvement of PHD in the development of diastolic dysfunction.
Induction of hypoxia tolerance and reprogrammed cell metabolism have emerged as novel therapeutic strategies for the treatment of ischemic diseases [34, 38–40]. Oxygen conformance refers to the ability of cells, upon exposure to insufficient oxygen such as ischemia, to reduce energy expenditure, which allows them to lower oxygen consumption in advance of an energetic crisis. Such energy conservation enhances survival at low oxygen tension and induces a state of hypoxia tolerance. A study demonstrates that PHD1 is the key regulator of oxygen conformance and modulates hypoxia tolerance [34]. Loss of PHD1 reduces oxygen consumption and mitochondrial oxidative stress and protects against muscle ischemic necrosis in a HIF-2α-dependent fashion [34]. This endogenous protection is mediated, at least in part by reprogramming basal metabolism [34]. Intriguingly, this hypoxia tolerance is not attributable to an increase in oxygen supply through enhancing angiogenesis or vasodilatation, but to suppression of reactive oxygen species (ROS) and protecting cells against deleterious effects of oxidative damage in hypoxic conditions [34]. These findings implicate a novel role of PHD1 in the protection of ischemic muscle against oxidative damage and in the induction of hypoxia tolerance by inducing hibernating state and persevering mitochondrial integrity. Consistent with these findings, our data reveals that pharmacological blockade of PHDs with PHD inhibitor DMOG significantly increases EC glycolysis and reduces basal oxygen consumption in WT-ECs. Our previous study shows that SIRT3 deficiency impairs hypoxia tolerance via reduced expression of HIF-2α and PFKFB3 [27]. DMOG treatment also increases the expression of HIF-2α and PFKFB3. In addition, SIRT3 KO mice exhibit increased PHD1 expression and decreased HIF-2α levels and develop cardiac dysfunction at 12 months of age. In contrast, treatment with DMOG reduces maximum respiration in SIRT3 KO-ECs. In vivo study further demonstrates that DMOG treatment improves diastolic function. These data implicate that activation of PHD1, which leads to reduced myocardial tolerance to hypoxia, may contribute to SIRT3 deficiency-induced impairment of endogenous protection and exacerbation of cardiac dysfunction.
One may argue that DMOG-mediated effects may not be specific to PHDs. Therefore, we specifically knockdown PHD1 by using DsiRNA, which significantly increases the expression of PFKFB3 that is similar to the effect of DMOG. We also acknowledge the potential effect of DMOG on ROS production. To verify that, we measured the superoxide level by DHE staining. Our data show that there was no difference in superoxide levels between the control SIRT3 KO ECs and DMOG treated SIRT3 KO ECs (Supplemental Figure S12), suggesting DMOG did not alleviate oxidative stress in SIRT3 KO ECs nor the improvement of microvascular function and cardiac function was attributable to DMOG on ROS. However, overexpression of SIRT3 rescued SIRT3 deletion induced superoxide formation (Supplemental Figure S14). In our recently study, we demonstrated an increase in perivascular fibrosis but not interstitial fibrosis in 12-month-old SIRT3 ECKO mice compared to the age-matched control mice [27]. This suggests that interstitial fibrosis may not play the major role in diastolic dysfunction observed in the SIRT3 ECKO mice that we hypothesized that inhibition of PHDs protects against diastolic dysfunction in the SIRT3 deficient mice via reprograming endothelial metabolism and improving coronary microvascular function. We did not examine the effect of DMOG either on tissue fibrosis or GSK3β–TGFβ–Smad3 signaling pathway that is activated by SIRT3-mediated deacetylation [23]. A recent study demonstrated that DMOG prevented the development of hypoxia-induced myocardial fibrosis via NFκB/HIF-1α pathway [41], suggesting DMOG does play a role in fibrosis. However, whether DMOG can reverse the pre-existing fibrosis needs further investigation.
Our previous studies demonstrated that SIRT3 KO mice exhibited decreased capillary density and CFR, which suggests that coronary microvascular dysfunction could be one of the mechanisms that exacerbates myocardial ischemia (MI) injury and impaired post-MI recovery [26]. Accumulating evidence suggests that impaired CFR is strongly correlated with diastolic dysfunction [42, 43]. Even though reduced CFR may contribute to the development of diastolic dysfunction, the exact mechanism that links SIRT3 and diastolic function has not been studied. In this study we demonstrate for the first time that established diastolic dysfunction in SIRT3 global KO mice can be reversed by DMOG treatment within two weeks. Although the exact mechanism is not clear, we speculate that improvement of endothelial glycolytic metabolism and subsequent coronary microvascular function, in part contributes to the reversed diastolic dysfunction. In addition, endothelial dysfunction and decreased microvascular density has been shown to limit CFR [6]. Our previous study demonstrated that CFR was decreased in SIRT3 KO mice [26] and in SIRT3 ECKO mice [27], indicating EC dysfunction which impaired myocardial perfusion and limited ability to dilate and to increase blood flow in response to increased metabolic demand. Using SIRT3 ECKO mice, we further validate the role of endothelial dysfunction in diastolic dysfunction. Although we cannot completely rule out the contribution of cardiomyocytes, the results are quite similar to that in SIRT3 global KO mice, suggesting endothelial dysfunction does play a major role in diastolic dysfunction. Clinical study reports that reduced microvascular density, endothelial dysfunction, and increased oxidative stress, along with excessive myocardial fibrosis and cardiac hypertrophy, are observed in HFpEF patients compared to age-matched control patients, suggesting endothelium plays an important role in the progression towards HF [6]. Our previous work also demonstrated that apelin gene therapy increases vascular density in the heart and attenuates ischemia-induced HF in diabetic STZ mice, but this effect was not seen in STZ-SIRT3 KO mice [44]. In addition, decreased SIRT3 levels is associated with obesity-induced microvascular deterioration and cardiac dysfunction, suggesting a link among SIRT3, glucose metabolism, and angiogenesis in high fat diet-fed mice [45]. DMOG is an amide analogue of α-ketoglutarate, used as a competitive inhibitor of PHD for HIF stabilization [46, 47]. Recent study reports that DMOG directly inhibits mitochondrial enzymes, which suppresses cellular respiration, ATP production and decreases histone H4 lysine 16 acetylation via HIF-independent pathway before HIF stabilization occurs [48]. Thus, the mechanisms involved in the beneficial effects mediated by DMOG in this study may extend beyond the HIF system. Therefore, future studies are needed to identify the affected targets involved in the regulation of microvascular dysfunction and diastolic dysfunction.
Our data showed that the expression of ANP was significantly reduced in SIRT3 KO heart. The exact mechanism of decreased level of ANP in SIRT3 KO mice was not investigated in the present study. Ruskoaho et al reported that stimulated release of ANP is associated with depletion of endocardial left ventricular stores after excise in both normotensive and hypertensive rats and that the amount of ANP released is correlated with the degree of hypertrophy of the ventricle [49]. This study might partially explain the decreased level of ANP in ventricle tissue found in our study. However, we did not measure the serum level of ANP to support this hypothesis. On the other hand, numerous studies suggest that ANP inhibits renin–angiotensin–aldosterone system and endothelin synthesis and decreases inflammation and has beneficial effects on endothelial function, preserving coronary circulation during ischemia/reperfusion [50–56]. Most importantly, ANP has been shown to stimulate glucose uptake in cardiac myocytes under hypoxia [57]. Therefore, the decreased level of ANP in our SIRT3 KO mice may be one of the contributors of the observed diastolic function.
5. Conclusion
Our study provides the first insight into the potential role of PHD on SIRT3 deficiency-induced diastolic dysfunction. Our results suggest that loss of SIRT3 impairs PHD/HIF signaling pathway and alters cell metabolism. Inhibition of PHD can improve endothelial metabolic homeostasis and reverse pre-existed diastolic dysfunction. This study provides a potential therapeutic strategy that clinically relevant PHD inhibition by DMOG for patients with diastolic dysfunction associated with coronary microvascular dysfunction, especially in the aging population with reduced SIRT3.
Supplementary Material
Highlights:
SIRT3 deletion shifts ECs from glycolytic metabolism to mitochondrial respiration.
Loss of SIRT3 impairs PHD/HIF signaling pathway and glycolytic function.
Treatment with PHD inhibitor DMOG improves endothelial metabolic homeostasis and rescues diastolic dysfunction.
Acknowledgments
The authors thank Dr. Eric Verdin at Gladstone Institute of California for providing the original SIRT3flox/flox mice.
Funding
This study was supported by grants from NIH grant 2R01HL102042–05 and University of Mississippi Medical Center Intramural Research Support Program to J.X. Chen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest associated with this manuscript.
References
- [1].LeWinter MM, Meyer M. Mechanisms of diastolic dysfunction in heart failure with a preserved ejection fraction: If it’s not one thing it’s another. Circ Heart Fail. 6 (2013) 1112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dhingra A, Garg A, Kaur S et al. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 11 (2014) 354–65. [DOI] [PubMed] [Google Scholar]
- [3].Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 43 (2004) 317–27. [DOI] [PubMed] [Google Scholar]
- [4].Owan TE, Hodge DO, Herges RM et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 355 (2006) 251–9. [DOI] [PubMed] [Google Scholar]
- [5].Upadhya B, Taffet GE, Cheng CP et al. Heart failure with preserved ejection fraction in the elderly: scope of the problem. J Mol Cell Cardiol. 83 (2015) 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mohammed SF, Hussain S, Mirzoyev SA et al. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 131 (2015) 550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Redfield MM, Jacobsen SJ, Burnett JC Jr., et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 289 (2003) 194–202. [DOI] [PubMed] [Google Scholar]
- [8].Mandinov L, Eberli FR, Seiler C et al. Diastolic heart failure. Cardiovasc Res. 45 (2000) 813–25. [DOI] [PubMed] [Google Scholar]
- [9].Kane GC, Karon BL, Mahoney DW et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 306 (2011) 856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Banerjee P, Motiwala A, Mustafa HM et al. Does left ventricular diastolic dysfunction progress through stages? Insights from a community heart failure study. Int J Cardiol. 221 (2016) 850–4. [DOI] [PubMed] [Google Scholar]
- [11].Andersen MJ, Borlaug BA. Heart failure with preserved ejection fraction: current understandings and challenges. Curr Cardiol Rep. 16 (2014) 501. [DOI] [PubMed] [Google Scholar]
- [12].Pillai VB, Sundaresan NR, Jeevanandam V et al. Mitochondrial SIRT3 and heart disease. Cardiovasc Res. 88 (2010) 250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Winnik S, Auwerx J, Sinclair DA et al. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 36 (2015) 3404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brouwers FP, de Boer RA, van der Harst P et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 34 (2013) 1424–31. [DOI] [PubMed] [Google Scholar]
- [15].Tanno M, Kuno A, Horio Y et al. Emerging beneficial roles of sirtuins in heart failure. Basic Res Cardiol. 107 (2012) 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guarani V, Potente M. SIRT1 - a metabolic sensor that controls blood vessel growth. Curr Opin Pharmacol. 10 (2010) 139–45. [DOI] [PubMed] [Google Scholar]
- [17].Nogueiras R, Habegger KM, Chaudhary N et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 92 (2012) 1479–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tseng AH, Wu LH, Shieh SS et al. SIRT3 interactions with FOXO3 acetylation, phosphorylation and ubiquitinylation mediate endothelial cell responses to hypoxia. Biochem J. 464 (2014) 157–68. [DOI] [PubMed] [Google Scholar]
- [19].Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol. 309 (2015) H1375–H1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sundaresan NR, Samant SA, Pillai VB et al. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 28 (2008) 6384–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Caton PW, Richardson SJ, Kieswich J et al. Sirtuin 3 regulates mouse pancreatic beta cell function and is suppressed in pancreatic islets isolated from human type 2 diabetic patients. Diabetologia. 56 (2013) 1068–77. [DOI] [PubMed] [Google Scholar]
- [22].Cheng A, Yang Y, Zhou Y et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 23 (2016) 128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sundaresan NR, Bindu S, Pillai VB et al. SIRT3 Blocks Aging-Associated Tissue Fibrosis in Mice by Deacetylating and Activating Glycogen Synthase Kinase 3beta. Mol Cell Biol. 36 (2015) 678–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hirschey MD, Shimazu T, Jing E et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 44 (2011) 177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Osborne B, Bentley NL, Montgomery MK et al. The role of mitochondrial sirtuins in health and disease. Free Radic Biol Med (2016) . [DOI] [PubMed] [Google Scholar]
- [26].He X, Zeng H, Chen JX. Ablation of SIRT3 causes coronary microvascular dysfunction and impairs cardiac recovery post myocardial ischemia. Int J Cardiol. 215 (2016) 349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].He X, Zeng H, Chen ST et al. Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J Mol Cell Cardiol. 112 (2017) 104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Koentges C, Pfeil K, Schnick T et al. SIRT3 deficiency impairs mitochondrial and contractile function in the heart. Basic Res Cardiol. 110 (2015) 36. [DOI] [PubMed] [Google Scholar]
- [29].Metzen E, Stiehl DP, Doege K et al. Regulation of the prolyl hydroxylase domain protein 2 (phd2/egln-1) gene: identification of a functional hypoxia-responsive element. Biochem J. 387 (2005) 711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gonzalez-Flores A, Aguilar-Quesada R, Siles E et al. Interaction between PARP-1 and HIF-2alpha in the hypoxic response. Oncogene. 33 (2014) 891–8. [DOI] [PubMed] [Google Scholar]
- [31].Favier FB, Britto FA, Poncon B et al. Endurance training prevents negative effects of the hypoxia mimetic dimethyloxalylglycine on cardiac and skeletal muscle function. J Appl Physiol (1985 ). 120 (2016) 455–63. [DOI] [PubMed] [Google Scholar]
- [32].Oktay Y, Dioum E, Matsuzaki S et al. Hypoxia-inducible factor 2alpha regulates expression of the mitochondrial aconitase chaperone protein frataxin. J Biol Chem. 282 (2007) 11750–6. [DOI] [PubMed] [Google Scholar]
- [33].Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 40 (2010) 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aragones J, Schneider M, Van GK et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 40 (2008) 170–80. [DOI] [PubMed] [Google Scholar]
- [35].Hegedus P, Li S, Korkmaz-Icoz S et al. Dimethyloxalylglycine treatment of brain-dead donor rats improves both donor and graft left ventricular function after heart transplantation. J Heart Lung Transplant. 35 (2016) 99–107. [DOI] [PubMed] [Google Scholar]
- [36].Ockaili R, Natarajan R, Salloum F et al. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 289 (2005) H542–H548. [DOI] [PubMed] [Google Scholar]
- [37].Yuan Q, Bleiziffer O, Boos AM et al. PHDs inhibitor DMOG promotes the vascularization process in the AV loop by HIF-1a up-regulation and the preliminary discussion on its kinetics in rat. BMC Biotechnol.14 (2014) 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schneider M, Van GK, Fraisl P et al. Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology. 138 (2010) 1143–54. [DOI] [PubMed] [Google Scholar]
- [39].Fraisl P, Aragones J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 8 (2009) 139–52. [DOI] [PubMed] [Google Scholar]
- [40].Aragones J, Fraisl P, Baes M et al. Oxygen sensors at the crossroad of metabolism. Cell Metab. 9 (2009) 11–22. [DOI] [PubMed] [Google Scholar]
- [41].Zhang S, Ma K, Liu Y et al. Stabilization of Hypoxia-inducible Factor by DMOG Inhibits Development of Chronic Hypoxia-Induced Right Ventricular Remodeling. J Cardiovasc Pharmacol. 67 (2016) 68–75. [DOI] [PubMed] [Google Scholar]
- [42].Blomster JI, Svedlund S, Westergren U et al. Coronary flow reserve as a link between exercise capacity, cardiac systolic and diastolic function. Int J Cardiol. 217 (2016) 161–6. [DOI] [PubMed] [Google Scholar]
- [43].Wei J, Mehta PK, Shufelt C et al. Diastolic dysfunction measured by cardiac magnetic resonance imaging in women with signs and symptoms of ischemia but no obstructive coronary artery disease. Int J Cardiol. 220 (2016) 775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hou X, Zeng H, He X et al. Sirt3 is essential for apelin-induced angiogenesis in post-myocardial infarction of diabetes. J Cell Mol Med. 19 (2015) 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zeng H, Vaka VR, He X et al. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med. 19 (2015) 1847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Baader E, Tschank G, Baringhaus KH et al. Inhibition of prolyl 4-hydroxylase by oxalyl amino acid derivatives in vitro, in isolated microsomes and in embryonic chicken tissues. Biochem J. 300 ( Pt 2) (1994) 525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Barrett TD, Palomino HL, Brondstetter TI et al. Pharmacological characterization of 1-(5-chloro-6-(trifluoromethoxy)-1H-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (JNJ-42041935), a potent and selective hypoxia-inducible factor prolyl hydroxylase inhibitor. Mol Pharmacol. 79 (2011) 910–20. [DOI] [PubMed] [Google Scholar]
- [48].Zhdanov AV, Okkelman IA, Collins FW et al. A novel effect of DMOG on cell metabolism: direct inhibition of mitochondrial function precedes HIF target gene expression. Biochim Biophys Acta. 1847 (2015) 1254–66. [DOI] [PubMed] [Google Scholar]
- [49].Ruskoaho H, Kinnunen P, Taskinen T et al. Regulation of ventricular atrial natriuretic peptide release in hypertrophied rat myocardium. Effects of exercise. Circulation. 80 (1989) 390–400. [DOI] [PubMed] [Google Scholar]
- [50].Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 339 (1998) 321–8. [DOI] [PubMed] [Google Scholar]
- [51].Emori T, Hirata Y, Imai T et al. Cellular mechanism of natriuretic peptides-induced inhibition of endothelin-1 biosynthesis in rat endothelial cells. Endocrinology. 133 (1993) 2474–80. [DOI] [PubMed] [Google Scholar]
- [52].Kiemer AK, Vollmar AM. The atrial natriuretic peptide regulates the production of inflammatory mediators in macrophages. Ann Rheum Dis. 60 Suppl 3 (2001) iii68–iii70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kiemer AK, Weber NC, Furst R et al. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ Res. 90 (2002) 874–81. [DOI] [PubMed] [Google Scholar]
- [54].Chu A, Cobb FR. Effects of atrial natriuretic peptide on proximal epicardial coronary arteries and coronary blood flow in conscious dogs. Circ Res. 61 (1987) 485–91. [DOI] [PubMed] [Google Scholar]
- [55].Foreman B, Dai XZ, Homans DC et al. Effect of atrial natriuretic peptide on coronary collateral blood flow. Circ Res. 65 (1989) 1671–8. [DOI] [PubMed] [Google Scholar]
- [56].Egashira K, Inou T, Imaizumi T et al. Effects of synthetic human atrial natriuretic peptide on the human coronary circulation in subjects with normal coronary arteries. Jpn Circ J. 55 (1991) 1050–6. [DOI] [PubMed] [Google Scholar]
- [57].Kudoh A, Katagai H, Takazawa T. Atrial natriuretic peptide increases glucose uptake during hypoxia in cardiomyocytes. J Cardiovasc Pharmacol. 40 (2002) 601–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.