Summary
Symptoms of the central disorders of hypersomnolence extend beyond excessive daytime sleepiness to include non-restorative sleep, fatigue, and cognitive dysfunction. They share much in common with myalgic encephalomyelitis/chronic fatigue syndrome, recently renamed systemic exertion intolerance disease, whose additional features include post-exertional malaise and orthostatic intolerance. We sought to determine the frequency and correlates of systemic exertion intolerance disease in a hypersomnolent population. One-hundred eighty-seven hypersomnolent patients completed questionnaires regarding sleepiness and fatigue; questionnaires and clinical records were used to assess for systemic exertion intolerance disease. Sleep studies, hypocretin, and cataplexy were additionally used to assign diagnoses of hypersomnolence disorders or sleep apnea. Included diagnoses were idiopathic hypersomnia (n=63), narcolepsy type 2 (n=25), persistent sleepiness after obstructive sleep apnea treatment (n=25), short habitual sleep duration (n=41), and sleepiness with normal sleep study (n=33). Twenty-one percent met systemic exertion intolerance disease criteria, and frequency of systemic exertion intolerance disease was not different across sleep diagnoses (p=0.37). Systemic exertion intolerance disease patients were no different from those without this diagnosis by gender, age, Epworth, depressive symptoms, or sleep study parameters. The whole cohort reported substantial fatigue on questionnaires, but the systemic exertion intolerance disease group exhibited more profound fatigue and was less likely to respond to traditional wake-promoting agents (88.6% vs. 67.7%, p=0.01). Systemic exertion intolerance disease appears to be a common comorbidity in patients with hypersomnolence, which is not specific to hypersomnolence subtype but may portend a poorer prognosis for treatment response.
Keywords: chronic fatigue syndrome, systemic exertion intolerance disease, narcolepsy, idiopathic hypersomnia, sleepiness, fatigue
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome affects between 836,000 and 2.5 million Americans (Jason et al., 2006). Despite its potentially debilitating effects, many patients struggle with symptoms for years before receiving a diagnosis due to the non-specific symptom profile and difficulties in operationalizing the construct of fatigue. Fatigue, as opposed to sleepiness, is characterized as the “lack of energy without inadvertent or excessive sleep” (Khan and Trotti, 2015). However, patients and clinicians often have trouble distinguishing between fatigue and sleepiness, not least because the two words share the synonym of “tired”. Furthering diagnostic challenge, patients with diseases in which the cardinal symptom is fatigue, and those with diseases centered on excessive daytime sleepiness (EDS), often report both fatigue and sleepiness as part of their symptom profile. For example, patients with obstructive sleep apnea (OSA) who display an increased sleep propensity on multiple sleep latency testing (MSLT) often prefer the terms “lack of energy” or “fatigue” (Chervin, 2000). Furthermore, patients with chronic fatigue syndrome have MSLT sleep latencies that are significantly shorter (i.e., sleepier) than controls (Neu et al., 2008).
Recently, the Institute of Medicine (IOM) convened an expert panel to evaluate the evidence on chronic fatigue syndrome and develop new diagnostic criteria to decrease time to diagnosis and improve providers’ understanding of the disease. The IOM recommended the name of the disease be changed to Systemic Exertion Intolerance Disease (SEID) to more accurately reflect its systemic nature (IOM, 2015). Under the SEID criteria, patients must have profound, new-onset fatigue accompanied by unrefreshing sleep, post-exertional malaise, and either cognitive dysfunction or orthostatic intolerance. Each criterion can be fulfilled by one or more symptoms in that domain. For example, “unrefreshing sleep” can be met any of these patient-reported symptoms: difficulty falling and staying asleep, feeling unrefreshed despite long sleep hours, or increased need for naps. Post-exertional malaise can be met by a “crash” after exercise, feeling physically sick or drained after mild activity, or feeling mentally tired after mild exertion. The cognitive dysfunction and orthostatic intolerance criteria can similarly be met by a variety of symptoms, such as “brain fog” or difficulty concentrating for the former, and syncope or feeling unwell while standing for extended periods for the latter (IOM, 2015). A similar domain-based approach for diagnosis was used in the development of the International Consensus Criteria for Myalgic Encephalomyelitis (Carruthers et al., 2011). This domain-based method captures a more homogenous group of individuals who exhibit the same cardinal features of disease compared to cohorts identified by a polythetic approach (requiring a certain number of a list of symptoms) (Fukuda et al., 1994; Jason et al., 2015a). Unlike prior criteria, which excluded patients who had sleep apnea or a central disorder of hypersomnolence (CDH) or required their satisfactory treatment before the diagnosis could be made, the IOM criteria do not require the use of polysomnography (PSG) or MSLT to rule out other disorders (IOM, 2015).
Given the tenuous semiologic distinction between sleepiness and fatigue and the overlap in symptoms between hypersomnolence and SEID, we applied the new Institute of Medicine criteria for SEID to a group of patients with hypersomnolence to determine: 1) the frequency of SEID among hypersomnolent patients; 2) the frequency of SEID among patients being evaluated for hypersomnolence, but not meeting International Classification of Sleep Disorders, Third Edition (ICSD-3) criteria for any hypersomnolence disorder (ICSD-3, 2014); and 3) the associations between symptoms, treatment-responsiveness, quality of fatigue, and the presence or absence of SEID. We hypothesized that if the new SEID criteria capture a homogenous disease centered on fatigue as opposed to sleepiness, the rate of SEID positivity in our hypersomnolent group would be low. We also hypothesized that the group of patients reporting sleepiness but not meeting ICSD-3 criteria for a hypersomnolence disorder would have higher rates of SEID, reflecting either patient or physician misperception of fatigue as sleepiness. Finally, because commonly used wake-promoting agents target sleepiness more than fatigue (Sheng et al., 2013), we anticipated that patients meeting SEID criteria would be more likely to report failure of traditional wake-promoting medications.
Methods
Subjects
This study was a retrospective chart review of 341 patients who presented to the Emory Sleep Center for hypersomnolence evaluation between October 2011 and June 2016. As part of a standard clinical work-up, patients complete questionnaires on sleepiness, fatigue, and related symptoms. Overnight, in-laboratory PSG was performed to assess for sleep apnea and other sleep disorders, and followed by MSLT. The MSLT provides 4 or 5 opportunities, spaced two hours apart, to try to fall asleep. This yields two measures: average time to fall asleep (i.e., the mean sleep latency, MSLT-SOL) and number of nap opportunities containing REM sleep (i.e., SOREMPs). Patients who had sufficient questionnaire, clinical, and PSG/MSLT data to assign diagnosis were included. Data were collected on additional features of sleepiness, ancillary symptoms, and cataplexy from medical records. The most recent PSG/MSLT performed at our center, or at a referring facility, conforming to guidelines for nocturnal sleep time of at least 360 minutes (Littner et al., 2005), was used in the case of multiple testing. Patients were excluded for: small sample size (Kleine-Levin syndrome, narcolepsy type 1, psychiatric hypersomnolence), OSA with resolution of sleepiness with OSA treatment or absence of follow up, fewer than 360 minutes of sleep on nocturnal PSG, or missing diagnostic data. Final analyses included 187 patients. Clinical data, PSG/MSLT results, CSF hypocretin level (when available), and cataplexy were used to assign each patient an ICSD-3 diagnosis of a central disorder of hypersomnolence (CDH) or sleep apnea (Figure 1). Patients reporting problematic hypersomnolence but not meeting any ICSD-3 criteria (i.e., with MSLT-SOL > 8 min) were labeled subjective EDS (sEDS). Patients were asked to summate their typical weekly sleep duration, considering both weekdays and weekends. If patients reported excessive daytime sleepiness and weekly sleep <49 hours, they were categorized as having short sleep time. This was chosen to align with the AASM’s consensus statement on optimal sleep duration of 7-9 hours/night (Watson et al, 2015). It differs from the ICSD-3 diagnosis of insufficient sleep syndrome in that patients did not necessarily report resolution of symptoms with extension of sleep time (ICSD-3, 2014). Number of medications attempted was extracted from the medical record. Each medication was considered individually, whether prescribed as mono- or poly-therapy. Treatment-failure was defined as insufficient symptom control or side effect requiring medication discontinuation. This study was approved by the Emory University Institutional Review Board.
Figure 1. Diagnostic algorithm.
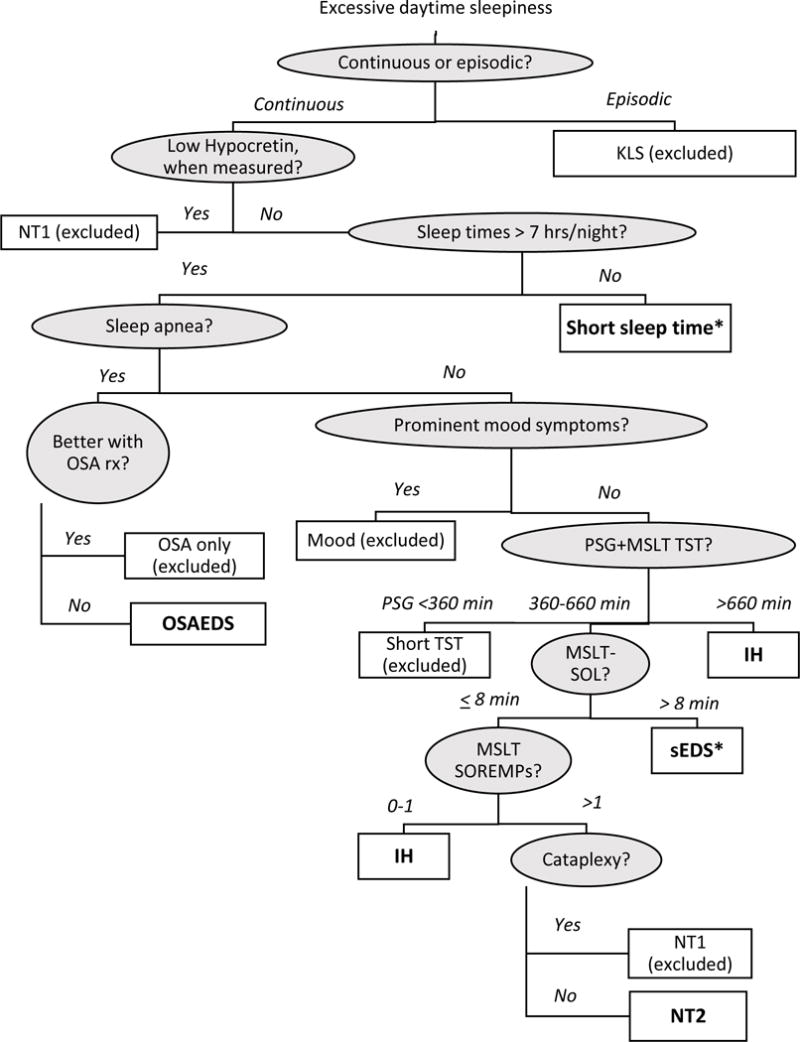
Patients were assigned diagnoses based on clinical features as specified in the algorithm. Shaded circles indicate clinical features used to differentiate among diagnoses, while clear squares indicate assigned diagnosis. Diagnoses included in SEID analyses are in bold; patients were excluded for KLS, NT1, OSA only, Mood, and Short PSG TST. * indicates a diagnosis not included in ICSD-3 (see text for details). Abbreviations: KLS: Kleine-Levin Syndrome; TST: Total sleep time; NT1: narcolepsy type 1; Short sleep time: short habitual sleep duration; OSA: Obstructive sleep apnea; OSA rx: treatment for OSA; OSAEDS: hypersomnia due to a medical condition, i.e., EDS despite adequate treatment for OSA; Mood: hypersomnolence associated with a psychiatric disorder; PSG: polysomnography; MSLT-SOL: MSLT mean sleep latency; IH: Idiopathic hypersomnia; sEDS: Patients reporting problematic hypersomnolence but not meeting ICSD-3 criteria; SOREMP: Sleep onset REM period; NT2: narcolepsy type 2
Measures
Questionnaire battery included questions about onset and quality of sleepiness, the Beck Depression Inventory-Short Form (BDI-SF) (Furlanetto et al., 2005), Epworth Sleepiness Scale (ESS) (Johns, 1991), Multidimensional Fatigue Inventory (MFI) (Smets et al., 1995), Fatigue Severity Scale (FSS) (Krupp et al., 1989), the Horne-Ostberg Test (Horne and Ostberg, 1976), and the Functional Outcomes of Sleep Questionnaire (FOSQ) (Weaver et al., 1997). The BDI-SF is a 13-question scale in which higher scores indicate more severe depressive symptoms, with scores greater than 10 associated with moderate or severe depression. The Epworth Sleepiness Scale is a 24-point scale that quantifies the likelihood of dozing under various conditions, with scores > 10 suggesting substantial sleepiness (Johns, 1991).
The MFI is a 20-item self-assessment comprised of five subscales: general fatigue, physical fatigue, mental fatigue, reduced activity, and reduced motivation. Each sub-score ranges from 4-20, where higher values indicate greater fatigue. In chronic fatigue syndrome patients, mean MFI total score is 67.1 (Lin et al., 2009). The FSS is a 9-item tool that assesses the physical aspects of fatigue and the effect of fatigue on ability to function. Patients rate their agreement with statements on a scale of 1 to 7, where 1 indicates strongly disagreeing and 7 strongly agreeing. Items are summed and divided by 9 to yield a final score between 1 and 7. A score of greater than 5 has been used to define fatigue (Bakshi et al., 1999;Flachenecker et al., 2002).
The Horne-Ostberg (Lark Owl Self-Test) is a 19-item morningness-eveningness self-assessment that indicates whether a patient prefers daytime or nighttime activity. Scores below 41 indicate an “evening” chronotype, scores 42-58 indicate an “intermediate” chronotype, and scores above 59 indicate a “morning” chronotype. The FOSQ is a 30-item questionnaire that assesses the impact of sleepiness on domains of functioning, explicitly asking about “sleepiness as opposed to fatigue” (Weaver et al., 1997). Lower scores indicate greater impairment from sleepiness.
Operationalizing the SEID Criteria
In order to meet SEID criteria, a patient must have “a substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities (criterion 1), that persists for more than 6 months (criterion 2) and is accompanied by fatigue (criterion 3), which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion (criterion 4), and is not substantially alleviated by rest (criterion 5)” and is accompanied by post-exertional malaise (criterion 6), unrefreshing sleep (criterion 7), and either cognitive impairment (criterion 8) or orthostatic intolerance (criterion 9) (Syndrome., 2015). The IOM guidelines acknowledge that there are multiple questions and scales that are useful for capturing these components, rather than specifying a single tool or set of tools that must be used. We operationalized these IOM criteria using components of our clinical battery.
Criterion 1 was assessed based on the FSS. If a patient rated their agreement with the statements “Fatigue interferes with carrying out certain duties and responsibilities” or “Fatigue interferes with my work, family or social life” as 5 or greater, they were considered positive for criterion 1.
As part of their initial evaluation, patients self-reported information regarding symptom onset and duration, which was used to assess whether they fulfilled the criterion for duration of 6 months or more, but not lifelong (criterion 2).
A final average FSS scores of 5 or greater was used to fulfill the SEID requirement of experiencing profound fatigue (criterion 3), which is consistent with prior authors’ cutoff for fatigued versus non-fatigued (Flachenecker et al., 2002).
The FOSQ asks for a general rating of activity level from “very low” (1) to “high” (4). Any answer other than “high” was used to indicate that the patient’s fatigue was not the result of excessive exertion (criterion 4).
When queried about amount of sleep needed to feel rested, patients were considered to fulfill criterion 5 “…not substantially alleviated by rest” if they reported never feeling rested after a night’s sleep or required more than 12 hours of sleep to feel rested.
The FSS queries post-exertional malaise (criterion 6) in question item 2. Patients were considered positive for post-exertional malaise if they scored 5 or greater on this question.
Items 5 and 7 on the Horne-Ostberg assess a patient’s level of alertness and refreshment in the first half hour after awaking from sleep. Patients who indicated they were “slightly alert” or “not at all alert,” or who reported feeling “fairly tired” or “very tired” as opposed to refreshed in the first half hour of waking were said to fulfill criterion 7.
Cognitive impairment (criterion 8) was based on the mental fatigue sub score on the MFI. Any patient whose mental fatigue sub score was ≥ 9.0 (the 75th percentile in healthy controls (Lin et al., 2009)), was recorded as having cognitive impairment.
Information on orthostatic intolerance (criterion 9) was assessed from patient charts. Orthostatic intolerance was defined as any of: history of orthostatic hypotension, history of postural orthostatic tachycardia syndrome, light-headedness, near-syncope or syncope, impaired concentration, headaches, dimming or blurry vision, palpitations, tremulousness, chest pain, a feeling of weakness, facial pallor, nervousness, shortness of breath, low tolerance for upright posture, and heat intolerance (Syndrome., 2015). Subjects who met all of criteria 1-7 and either criterion 8 or 9 were categorized as meeting SEID criteria.
Statistics
We tested for differences between those meeting and not meeting the operationalized SEID criteria with respect to demographic factors, diagnosis, questionnaire scores, sleep study data, and treatment response, using t-tests (corrected for unequal variances as necessary) for continuous variables and Chi-square (or Fisher exact test) for categorical variables. Similar comparisons were calculated across hypersomnia diagnostic groups using one-way, mixed-model ANOVA (to control for unequal sample sizes and variances) (Littell et al, 2002) for continuous variables and Chi-square for categorical variables. In the case of significant ANOVA results, post-hoc, pairwise comparisons were performed via Tukey’s multiple comparison test. Individual pairs of SEID criteria (or the question(s) used for their assessment) were made using Pearson’s correlation, t-tests, or Chi-square, as indicated by each variable type. Cohen’s d was calculated in the case of significant t-test results to generate effect size estimates. Study data were managed using REDCap electronic data capture hosted at Emory University (Harris et al., 2009) and analyzed in SAS (version 9.4). P-values < 0.05 were considered to be significant. Values are presented as means (+/− standard deviation) or count (percent) unless otherwise specified. Degrees of freedom are shown in parentheses following each statistical test.
Results
Final sample size was 187, with mean age 36.1 years (+/−15.1; 63% women). Sixty-three patients had idiopathic hypersomnia (IH), 25 narcolepsy type 2, 25 persistent sleepiness after OSA treatment, 41 habitually short sleep times, and 33 subjective sleepiness not meeting criteria for any of these disorders (sEDS; see Table 1 for clinical features by diagnosis).
Table 1.
Clinical characteristics by diagnosis
| sEDS (n=33) | IH (n=63) | SST (n=41) | NT2 (n=25) | OSA (n=25) | DF | F or Chi-square | p-value | Pairwise tests* | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years | 30.8 (12.2) | 34.0 (13.7) | 43.7 (17.7) | 26.4 (8.2) | 45.4 (12.8) | 4, 53 | 13.9 | <0.0001 | NT2<IH< SST=OSA; sEDS<SST; sEDS<OSA |
| Body mass index | 25.3 (4.7) | 24.3 (4.5) | 28.2 (6.5) | 24.8 (4.6) | 32.0 (8.0) | 4, 48 | 6.7 | 0.0002 | IH<SST= OSA; NT2=sEDS<OSA; NT2=sEDS= IH; EDS=SST= NT2 |
| Women, % | 66.7 | 69.8 | 61.0 | 64.0 | 44.0 | 4 | 5.2 | 0.27 | – |
| Age of EDS Onset, years | 20.3 (8.1) | 20.4 (13.7) | 24.4 (16.3) | 16.3 (7.6) | 25.6 (12.5) | 4, 5 | 3.2 | 0.02 | NT2<OSA |
| Sleep time/week, hours** | 76.1 (19.4) | 71.4 (15.8) | 39.7 (6.5) | 69.7 (16.7) | 66.2 (10.5) | 4, 77 | 91.4 | <0.0001 | SST<all others |
| TST, minutes | 442.5 (68.7) | 460.1 (135.7) | 391.5 (50.5) | 409.7 (34.4) | 430.3 (97.1) | 4, 48 | 4.8 | 0.003 | IH=sEDS> SST |
| Sleep efficiency | 83.9 (9.7) | 89.5 (6.7) | 88.3 (11.6) | 90.0 (6.2) | 83.1 (9.2) | 4, 46 | 4.2 | 0.006 | IH=NT2> OSA; sEDS<IH |
| PSG N1% | 6.6 (3.0) | 5.8 (3.9) | 5.3 (3.3) | 4.7 (4.1) | 8.0 (5.5) | 4, 32 | 1.8 | 0.16 | – |
| PSG N2% | 61.2 (9.3) | 59.1 (14.1) | 62.5 (13.8) | 54.7 (9.9) | 60.4 (11.5) | 4, 38 | 1.5 | 0.23 | – |
| PSG N3% | 16.2 (7.9) | 15.8 (10.9) | 13.2 (12.2) | 18.9 (7.2) | 13.6 (11.7) | 4, 33 | 1.1 | 0.35 | – |
| PSG REM% | 16.0 (6.2) | 19.3 (8.7) | 18.7 (10.1) | 23.6 (10.3) | 18.0 (8.9) | 4, 43 | 2.4 | 0.07 | – |
| AHI** | 0.9 (1.1) | 1.1 (1.2) | 3.9 (4.5) | 1.3 (1.1) | 11.4 (8.7) | 4, 31 | 9.87 | <0.0001 | IH=sEDS< SST<OSA; NT2<OSA |
| PLMI | 4.9 (9.3) | 10.8 (18.0) | 10.2 (17.0) | 1.5 (2.7) | 13.7 (22.3) | 4, 32 | 6.1 | 0.001 | IH>NT2 |
| MSLT-SOL** | 12.5 (3.3) | 5.2 (2.8) | 4.6 (3.3) | 4.2 (2.0) | 7.0 (4.2) | 4, 35 | 41.0 | <0.0001 | sEDS>all others |
| Number of SOREMPs** | 0.6 (1.1) | 0.3 (0.5) | 1.6 (1.5) | 3.2 (1.2) | 0.8 (1.2) | 4, 38 | 40.0 | <0.0001 | NT2>all others; IH<SST |
Values are reported as mean (SD) or percentage.
pairwise group differences calculated using post-hoc Tukey test.
this variable was used to assign patients to diagnostic group and therefore differs perforce between some groups. sEDS: subjective sleepiness with normal multiple sleep latency test; IH: idiopathic hypersomnia; SST: habitually short sleep durations; NT2: narcolepsy type 2; OSA: obstructive sleep apnea with residual sleepiness after apnea treatment; DF: degrees of freedom; Sleeptime/week: patient reported summed hours of sleep during a typical week, including weekdays and weekends; TST: total sleep time on PSG; PSG: polysomnogram; AHI: apnea-hypopnea index; PLMI: periodic limb movement index; MSLT-SOL: MSLT mean sleep latency; SOREMP: sleep onset rapid eye movement periods on polysomnography/multiple sleep latency test.
Frequency of SEID
Thirty-nine (20.9%) patients met operationalized criteria for SEID. The percentage of patients meeting SEID criteria did not vary across the included diagnoses (chi-square(4)=4.3, p=0.37) (Table 2). Those meeting and not meeting SEID criteria were similar in terms of demographic and polysomnographic findings (Table 3), including mean sleep latency on MSLT (6.58 +/− 4.5 minutes in the SEID group vs. 6.7 +/− 4.3 minutes in the no SEID group, t(156)=0.2, p=0.87), with both groups demonstrating substantial objective sleepiness.
Table 2.
Frequency of systemic exertion intolerance disease (SEID) in different disorders of excessive daytime sleepiness
| Diagnosis | All patients (n=187) | SEID (n=39) | No SEID (n=148) |
|---|---|---|---|
| Subjective sleepiness | 33 (17.7%) | 5 (12.8%) | 28 (18.9%) |
| Idiopathic hypersomnia | 63 (33.7%) | 13 (33.3%) | 50 (33.8%) |
| Short sleep times | 41 (21.9%) | 6 (15.4%) | 35 (23.7%) |
| Narcolepsy type 2 | 25 (13.4%) | 7 (18.0%) | 18 (12.2%) |
| Sleep apnea with residual sleepiness after treatment | 25 (13.4%) | 8 (20.5%) | 17 (11.5%) |
There were no significant differences in the frequency of SEID across ICSD-3 diagnoses (Chi-square(4)=4.3, p=0.37).
Table 3.
Comparison of demographic and clinical data between those with and without systemic exertion intolerance disease (SEID)
| Characteristic | All Patients (n=187) | SEID (n=39) | No SEID (n=148) | DF | t-value or chi-square | P value |
|---|---|---|---|---|---|---|
| Age, years | 36.1 (15.1) | 35.9 (14.1) | 36.1 (15.4) | 185 | 0.1 | 0.94 |
| Body mass index | 26.4 (6.2) | 26.2 (5.6) | 26.5 (6.3) | 167 | 0.3 | 0.77 |
| Women, % | 63.1 | 66.7 | 62.2 | 1 | 0.3 | 0.60 |
| Age of EDS Onset, years | 21.4 (13.0) | 21.6 (10.6) | 21.4 (13.6) | 169 | -0.1 | 0.89 |
| Sleep time/week, hours | 64.4 (19.7) | 68.3 (20.4) | 63.3 (19.4) | 185 | −1.4 | 0.16 |
| TST, minutes | 434.9 (100.5) | 438.0 (77.1) | 434.1 (106.0) | 71 | −0.2 | 0.81 |
| Sleep efficiency | 87.5 (8.9) | 86.4 (9.5) | 87.8 (8.7) | 162 | 0.8 | 0.42 |
| PSG N1% | 6.1 (4.0) | 6.5 (4.7) | 5.9 (3.8) | 134 | −0.7 | 0.47 |
| PSG N2% | 59.7 (12.4) | 63.4 (12.3) | 58.7 (12.2) | 128 | −1.8 | 0.07 |
| PSG N3% | 15.5 (10.3) | 13.1 (11.1) | 16.1 (10.1) | 128 | 1.4 | 0.17 |
| PSG REM% | 19.0 (9.0) | 17.3 (8.9) | 19.4 (9.0) | 138 | 1.1 | 0.27 |
| AHI | 2.9 (5.0) | 4.3 (6.9) | 2.4 (4.2) | 40 | −1.5 | 0.13 |
| PLMI | 8.5 (16.0) | 8.1 (15.6) | 8.6 (16.2) | 134 | 0.1 | 0.90 |
| MSLT-SOL | 6.7 (4.3) | 6.6 (4.5) | 6.7 (4.3) | 156 | 0.2 | 0.87 |
| Number of SOREMPs | 1.1 (1.4) | 1.1 (1.3) | 1.0 (1.5) | 155 | −0.2 | 0.87 |
| GABA Percent Potentiation | 98.6 (21.6) | 100.7 (23.6) | 97.7 (21.0) | 43 | −0.4 | 0.68 |
Values are reported as mean (SD) or percentage. DF: degrees of freedom; Sleeptime/week: patient reported summed hours of sleep during a typical week, including weekdays and weekends; TST: total sleep time on PSG; PSG: polysomnogram; AHI: apnea-hypopnea index; PLMI: periodic limb movement index; MSLT-SOL: MSLT mean sleep latency; SOREMP: sleep onset REM periods on polysomnography/multiple sleep latency test. GABA Percent Potentiation: percent potentiation by cerebrospinal fluid on GABA-A receptors in vitro (Rye et al, 2012).
Subjective Measures of Sleepiness and Fatigue in Patients with and without SEID
As anticipated, the group as a whole also demonstrated substantial subjective sleepiness, with average ESS of 15.0 (+/− 5.1). There was no difference in ESS between the two groups (SEID 14.7 +/− 4.8 vs. no SEID 15.1 +/− 5.2, t(185)=0.4, p=0.71). Because individual questions were used for SEID determination from the Fatigue Severity Scale, the Multidimensional Fatigue Inventory, and the Functional Outcomes of Sleep scale, sum scores for these scales may have differed between groups by definition (see Supplementary Table 1). Questions from the Horne-Ostberg were also used for SEID determination, but this scale did not differ between groups. Pairwise comparisons of individual criteria were typically only modestly correlated or non-significant, with the exception of moderate-to-strong and statistically significant relationships between: criteria 1 and 3 (r=0.80, p<0.0001), 3 and 6 (r=0.50, p<0.0001), 5 and 7 (Cohen’s d=0.68, p<0.0001), and 7 and 9 (Cohen’s d=0.52, p=0.0003).
Multidimensional Fatigue Inventory
The mean MFI total score for all patients in our cohort was 71.7 +/− 14.4. When the mental fatigue component (criterion 8 in assessment of SEID status) was removed from the score, the SEID group remained significantly more fatigued than the group not meeting SEID criteria (62.4 +/− 10.2 vs. 55.9 +/− 12.4, t(185)= −3.0, p=0.003). On MFI domains, those with SEID reported higher levels of general fatigue (18.0 +/− 1.8 vs. 16.8 +/− 2.7, t(87)= −3.2, p=0.002) and physical fatigue (15.6 +/− 3.6 vs. 13.5 +/− 4.1, t(185)= −3.0, p=0.003), with more reduction in activity (15.5 +/− 3.9 vs. 13.5 +/− 4.5, t(185)= −2.5, p=0.01). There were no differences between those with and without SEID on motivation (13.3 +/− 3.7 vs. 12.1 +/− 4.2, t(185)= −1.6, p=0.11) or mental fatigue (15.5 +/− 3.4 vs. 14.2 +/− 4.3, t(185)= −1.7, p=0.09), despite the latter being used in SEID determination.
Fatigue Severity Scale
The mean FSS score was 5.8 (+/− 1.1). Excluding questions used for classification, average FSS scores were significantly higher in those meeting SEID criteria (6.5 +/− 0.60 vs. 5.8 +/− 1.3, t(132)= −4.8, p < 0.001).
Other scales
Seventy-six patients (40.6%) were evening-type, 16 (8.6%) were morning-type, and 95 (50.8%) were neither. Excluding questions 5 and 7 (used for SEID assessment), average scores were no different between the groups that met and did not meet SEID criteria (39.6 +/− 10.4 vs 41.6 +/− 10.2, t(184)=1.1, p=0.29). Mean FOSQ score was 12.0 (+/− 3.3). Excluding question 26, patients meeting SEID criteria reported more functional impairment from sleepiness than did those not meeting SEID criteria (11.0 +/− 2.9 vs. 12.2 +/− 3.4, t(185)=2.0, p=0.048). Mean BDI-SF score was 9.4 (+/− 6.8). There was no difference in BDI-SF scores between those who met and did not meet SEID criteria (10.0 +/− 6.7 vs. 9.3 +/− 6.8, t(185)= −0.6, p=0.55).
Fatigue in patients with subjective sleepiness (sEDS) versus idiopathic hypersomnia
There was no difference in the percentage of patients meeting SEID criteria in the sEDS group compared to other diagnoses, even though PSG/MSLT were normal in the sEDS group by definition. To further evaluate the possibility that patients with sEDS may have fatigue that they misperceive as sleepiness, we compared sEDS patients to those with idiopathic hypersomnia, who are clinically similar to sEDS patients other than in MSLT results (Pizza et al., 2013). Rates of SEID positivity were no different between the sEDS and IH groups (15.2% vs 20.6%, chi-square(1)=0.43, p = 0.52). The sEDS and IH groups were similar on MFI scores (72.3 +/− 13.8 for sEDS vs. 73.2 +/− 14.2 for IH, t(94)= −0.3, p=0.78) and FSS scores (5.9 +/− 1.0 for sEDS vs. 5.9 +/− 0.9 for IH, t(94)= −0.4, p=0.69).
Treatment response in Patients with SEID vs. those without SEID
The average number of medications unsuccessfully used for hypersomnolence treatment was 3.6 (+/− 3.2). Of the 165 patients (88.2%) who had tried traditional wake-promoting agents (e.g. modafinil/armodafinil, amphetamines, methylphenidate), 46 (27.9%) experienced an adequate response and 119 (72.1%) did not. Patients meeting SEID criteria were more likely to have failed treatment with these agents (88.6% vs. 67.7%, chi-square(1)=6.0, p=0.01). One-hundred eighteen patients were prescribed clarithromycin for hypersomnolence (Trotti et al., 2015). Patients with and without SEID exhibited similar response rates to clarithromycin (37.5% vs. 46.5%, chi-square(1)=0.7, p=0.38). Ninety-one patients were prescribed flumazenil (Trotti et al., 2016) and response rates were similar for those meeting (64.0%) and not meeting (60.6%) SEID criteria (chi-square(1)=0.1, p=0.77).
Discussion
One-fifth of hypersomnolent patients met operationalized SEID criteria, in contrast to the U.S. case rate of 338/100,000 (Reyes et al., 2003). Several explanations for this discrepancy are possible. First, SEID criteria may be overly broad. Comparing cohorts captured by SEID versus other criteria, SEID criteria identify a larger, less functionally-impaired group (Jason et al., 2015b). However, fatigue was abundant in our hypersomnolent patients, even those not meeting SEID criteria, suggesting that the symptom overlap with fatigue is important regardless of specific diagnostic criteria. Second, unlike prior criteria that excluded patients with inadequately-treated sleep disorders (Carruthers et al., 2011), SEID criteria might erroneously capture patients with partially-treated hypersomnolence who misperceive sleepiness as fatigue. However, our subjects with and without SEID were equally sleepy by subjective and objective measures, suggesting that the SEID criteria are capturing a construct distinct from sleepiness. Third, the high rate of SEID-positivity in hypersomnolent patients may point to a shared biology. The pathophysiology of chronic fatigue and most hypersomnolence disorders remains poorly understood. Given that the similarities are many and the differences are subtle, it is possible that these disorders exist on a temporal or phenotypic spectrum of disease. This latter hypothesis is deserving of further research.
Even among patients not meeting criteria for SEID, our hypersomnolent cohort demonstrated substantial fatigue, suggesting that hypersomnia disorders themselves may cause fatigue. Hypersomnia patients use hyperactivity and multi-tasking to resist sleepiness more than controls, such that fatigue may be the result of expending physical and mental energy to maintain alertness (Vernet et al., 2010). Our patients with and without SEID demonstrated some differences in quality of fatigue that may suggest two different etiologies for fatigue in these patients. Patients with SEID and hypersomnolence reported more global fatigue, more physical fatigue, and reduced activity, and the key difference between those with and without SEID appears to be an increase in baseline physical fatigue. On the contrary, there was no difference in reported mental fatigue between the two groups, which was high for both. Speculatively, mental fatigue in this cohort could be accounted for by an increased expenditure of mental energy to combat sleepiness.
We could not confirm our second hypothesis, that patients not meeting ICSD-3 criteria for a hypersomnolence disorder would have higher rates of SEID. Rates of SEID positivity did not differ across diagnoses. In particular, patients with sEDS were no more likely to meet SEID criteria than patients with the other disorders, and fatigue scores were similar in those with sEDS and those with IH, the most closely related CDH. This speaks against the common clinical supposition that patients who report sleepiness but have normal multiple sleep latency testing are simply misperceiving fatigue as sleepiness. Rather, it may be another indication that the MSLT does not optimally classify hypersomnolent patients. The MSLT is normal in a substantial group of patients with symptoms of idiopathic hypersomnia (Vernet and Arnulf, 2009) or problematic sleepiness (Pizza et al., 2013) and has poor test-retest reliability in several hypersomnolence disorders (Ruoff et al., 2017;Trotti et al., 2013).
Finally, the SEID group was significantly less likely to have an adequate response to traditional wake-promoting agents. However, both groups found comparable benefit to the gamma-aminobutyric acid (GABA)-A receptor modulators clarithromycin and flumazenil. Speculatively, this might reflect involvement of the GABA system in the pathogenesis of chronic fatigue, as previously proposed (Corrigan et al., 1994). Alternately, it might reflect non-GABAergic mechanisms of clarithromycin or flumazenil. Clarithromycin may treat hypersomnolence partially because of effects on inflammation or the gut microbiome (Trotti et al., 2015). In a pilot study, a 6-day course of erythromycin reduced gut gram-positive bacteria in chronic fatigue syndrome patients and significantly improved subjective sleep quality (Jackson et al., 2015). Alternatively, it is possible that a portion of the benefit from these and traditional wake-promoting medications could be placebo response, as observed previously in SEID treatment (Pigeon et al., 2003). One implication of this work is that there may be a role for cognitive-behavioral or exercise therapies in hypersomnolent patients with comorbid SEID, as these treatments improve physical functioning, sleep, and perception of health in chronic fatigue (Kroenke and Swindle, 2000; Larun et al., 2016;Pigeon et al., 2003). Treatment studies targeting patients with both hypersomnolence and SEID are needed.
Our study has several limitations. Our clinic is referred patients who are treatment-refractory, so the SEID rate might be lower in other sleep clinic populations. Conversely, because patients often have extensive evaluation for other causes of symptoms, including SEID, before referral, our observed rate of comorbidity may be low. There is no single, validated questionnaire recommended for SEID diagnosis. As a result, the combination of questions and scales used here may have resulted in different findings than would a different method of assessing each criterion. In particular, rather than directly assessing the effect of rest, we used as a surrogate the amount of sleep needed to feel rested, which may have imprecisely captured this criterion. Our use of a retrospective design limited our ability to compare the cohort derived from our operationalized SEID criteria to that generated from other validated assessments of chronic fatigue such as the DePaul questionnaire (Jason et al., 2010). Despite these limitations, our pilot study suggests that SEID symptoms are commonly comorbid with hypersomnolence, occur equally across included ICSD-3 diagnoses, and do not merely represent fatigued patients who have been misdiagnosed as sleepy. Future work is needed to prospectively validate these findings, elucidate the biological bases for this clinical overlap and to determine optimal treatments for hypersomnolent patients with comorbid SEID.
Supplementary Material
Acknowledgments
This project was supported by: K23-NS083748 (LMT), R01-NS089719 (DBR), Mind Science Foundation (DBR), and UL1-TR000424.
Footnotes
DR. LYNN MARIE TROTTI (Orcid ID : 0000-0003-2329-6847)
Conflict of Interest Disclosure: Dr. Bliwise reports personal fees from Ferring Pharmaceuticals, Merck and Respicardia, outside the submitted work. Dr. Rye reports personal fees from Jazz Pharmaceuticals, UCB Pharma, Xenoport Inc., Flamel Technologies, and Balance Therapeutics, outside the submitted work. Dr. Rye has U.S. patent 20110028418A1. Dr. Trotti reports institutional funds from Balance Therapeutics and Jazz Pharmaceuticals, outside the submitted work. Authors Maness, Saini, and Olvera report no disclosures.
Author Contributions: Conception/design (CM, LMT); Data acquisition, analysis, interpretation (all); Drafting the work (CM, LMT); Revision for intellectual content (PS, DLB, VO, DBR); approval of final version (all)
References
- Bakshi R, Miletich RS, Henschel K, et al. Fatigue in multiple sclerosis. Neurology. 1999;53:1151–3. doi: 10.1212/wnl.53.5.1151. [DOI] [PubMed] [Google Scholar]
- Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis. Journal of Internal Medicine. 2011;270:327–38. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin RD. Sleepiness fatigue tiredness and lack of energy in obstructive sleep apnea. Chest. 2000;118:372–9. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, MacDonald S, Brown A, Armstrong K, Armstrong EM. Neurasthenic fatigue, chemical sensitivity and GABAa receptor toxins. Med Hypotheses. 1994;43:195–200. doi: 10.1016/0306-9877(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Flachenecker P, Kumpfel T, Kallmann B, et al. Fatigue in multiple sclerosis. Mult Scler. 2002;8:523–6. doi: 10.1191/1352458502ms839oa. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome. International Chronic Fatigue Syndrome Study Group. Annals of internal medicine. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Furlanetto LM, Mendlowicz MV, Romildo Bueno J. The validity of the Beck Depression Inventory-Short Form as a screening diagnostic instrument for moderate severe depression in medical inpatients. Journal of affective disorders. 2005;86:87–91. doi: 10.1016/j.jad.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington DC: 2015. http://www.ncbi.nlm.nih.gov/books/NBK274235/ [Google Scholar]
- International classification of sleep disorders. 3rd. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- Jackson ML, Butt H, Ball M, Lewis DP, Bruck D. Sleep quality the treatment of intestinal microbiota imbalance in Chronic Fatigue Syndro me: A pilot study. Sleep Sci. 2015;8:124–33. doi: 10.1016/j.slsci.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason LA, Torres-Harding S, Njoku MGC. The Face of CFS in the US CIFIDS Chron. 2006:16–21. [Google Scholar]
- Jason LA, Evans M, Porter N, et al. The development of a revised Canadian myalgic encephalomyelitis chronic fatigue syndrome case definition. American Journal of Biochemistry and Biotechnology. 2010;6:120–35. [Google Scholar]
- Jason LA, Kot B, Sunnquist M, et al. Chronic Fatigue Syndrome and Myalgic Encephalomyelitis. Health Psychol Behav Med. 2015a;3:82–93. doi: 10.1080/21642850.2015.1014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason LA, Sunnquist M, Brown A, Newton JL, Strand EB, Vernon SD. Chronic Fatigue Syndrome versus Systemic Exertion Intolerance Disease. Fatigue. 2015b;3:127–41. doi: 10.1080/21641846.2015.1051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Khan Z, Trotti LM. Central Disorders of Hypersomnolence. Chest. 2015;148:262–73. doi: 10.1378/chest.14-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Swindle R. Cognitive-behavioral therapy for somatization symptom syndromes. Psychother Psychosom. 2000;69:205–15. doi: 10.1159/000012395. [DOI] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2016;12:CD003200. doi: 10.1002/14651858.CD003200.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JM, Brimmer DJ, Maloney EM, Nyarko E, Belue R, Reeves WC. Further validation of the Multidimensional Fatigue Inventory in a US adult population sample. Popul Health Metr. 2009;7:18. doi: 10.1186/1478-7954-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Stroup WW, Freund RJ. SAS for linear models. fourth SAS Institute Inc; Cary, NC: 2002. [Google Scholar]
- Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- Neu D, Hoffmann G, Moutrier R, Verbanck P, Linkowski P, Le Bon O. Are patients with chronic fatigue syndrome just ‘tired’ or also ‘sleepy’? J Sleep Res. 2008;17:427–31. doi: 10.1111/j.1365-2869.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness fatigue. Journal of psychosomatic research. 2003;54:61–9. doi: 10.1016/s0022-3999(02)00542-1. [DOI] [PubMed] [Google Scholar]
- Pizza F, Moghadam KK, Vandi S, et al. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res. 2013;22:32–40. doi: 10.1111/j.1365-2869.2012.01032.x. [DOI] [PubMed] [Google Scholar]
- Reyes M, Nisenbaum R, Hoaglin DC, et al. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch Intern Med. 2003;163:1530–6. doi: 10.1001/archinte.163.13.1530. [DOI] [PubMed] [Google Scholar]
- Ruoff C, Pizza F, Trotti LM, et al. The MSLT is Repeatable in Narcolepsy Type 1 But Not Narcolepsy Type 2. J Clin Sleep Med. 2017 doi: 10.5664/jcsm.6882. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng P, Hou L, Wang X, et al. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders. PLoS One. 2013;8:e81802. doi: 10.1371/journal.pone.0081802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of psychosomatic research. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy idiopathic hypersomnia. J Clin Sleep Med. 2013;9:789–95. doi: 10.5664/jcsm.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti LM, Saini P, Bliwise DL, Freeman AA, Jenkins A, Rye DB. Clarithromycin in gamma-aminobutyric acid-Related hypersomnolence. 2015;78:454–65. doi: 10.1002/ana.24459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti LM, Saini P, Koola C, LaBarbera V, Bliwise DL, Rye DB. Flumazenil for the Treatment of Refractory Hypersomnolence. J Clin Sleep Med. 2016;12:1389–94. doi: 10.5664/jcsm.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet C, Arnulf I. Idiopathic hypersomnia with without long sleep time. Sleep. 2009;32:753–9. doi: 10.1093/sleep/32.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet C, Leu-Semenescu S, Buzare MA, Arnulf I. Subjective symptoms in idiopathic hypersomnia. J Sleep Res. 2010;19:525–34. doi: 10.1111/j.1365-2869.2010.00824.x. [DOI] [PubMed] [Google Scholar]
- Watson NF, Badr MS. Recommended Amount of Sleep for a Healthy Adult. J Clin Sleep Med. 2015;11:591–2. doi: 10.5664/jcsm.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


