Abstract
Over the past two decades, evidence has accumulated that neurogenesis can occur in both the juvenile and adult mammalian hypothalamus. Levels of hypothalamic neurogenesis can be regulated by dietary, environmental and hormonal signals. Since the hypothalamus has a central role in controlling a broad range of homeostatic physiological processes, these findings may have far ranging behavioral and medical implications. However, many questions in the field remain unresolved, including the cells of origin of newborn hypothalamic neurons and the extent to which these cells actually regulate hypothalamic-controlled behaviors. In this manuscript, we conduct a critical review of the literature on postnatal hypothalamic neurogenesis in mammals, lay out the main outstanding controversies in the field, and discuss how best to advance our knowledge of this fascinating but still poorly understood process.
Introduction
For much of the 20th century, it was regarded as an iron-clad fact that mammalian neurogenesis occurs during embryonic and early postnatal development [1]. Dubbed by some the ‘central dogma of neurobiology’, this is still widely believed by lay audiences, and remains one of the most broadly held public misconceptions about the brain [2]. Over the past few decades, with the development of progressively better tools for labeling and tracking newborn neurons, studies in multiple species has made it clear that that substantial levels of neurogenesis occur in several brain regions in adult mammals.
The two main regions of active neurogenesis in adult rodent brain occur in the subventricular zone (SVZ) of lateral ventricles and the subgranular zone (SGZ) of dentate gyrus in hippocampus. The SVZ produces immature neurons that can migrate along the rostral migratory stream (RMS) connecting to the olfactory bulb, where they then differentiate into mature neurons that process olfactory input [3]. The SGZ gives rise to granule cells of the dentate gyrus, which process information relevant to learning and memory [4]. The behavioral phenotypes that are observed following inhibition of adult neurogenesis in these regions, along with the fact that adult neurogenesis is observed in many different mammalian species, implies that this process is functionally important and evolutionarily conserved [5–8]. These studies have in turn raised the question of whether neurogenesis may occur in other brain regions, either at lower levels or in response to specific physiological states. Over the past decade, evidence has accumulated that low levels of post-developmental neurogenesis occur in multiple mammalian brain regions, including the neocortex, striatum and spinal cord [9]. For several reasons, the brain region that has received the most attention as a possible site of low-level adult neurogenesis is the hypothalamus.
First among these reasons is the presence of a plausible candidate neurogenic niche in the form of the ventricular zone of the basal hypothalamus. Analysis of neurogenic zones in the SVZ and SGZ, as well as the more broadly distributed ventricular neurogenic zones of cold-blooded vertebrates, have identified several common components of a neurogenic niche [10]. These include firstly, a stem/progenitor cell population; secondly, the presence of perivascular basal lamina and other extracellular matrixes harboring soluble factors and cellular molecules that are derived from nearby cells and blood vessels; and finally, persistent expression of developmental morphogens and signaling molecules that contribute to the maintenance or regulation of multipotency and proliferative competence. In the hypothalamus, tanycytes and associated cells of the ventricular zone are strong candidate stem/progenitor cells, while the highly vascularized basal hypothalamic parenchyma and persistent expression of multiple morphogens, cytokines and growth factors all constitute a potentially favorable extracellular environment for neurogenesis.
A second reason is that very low levels of hypothalamic neurogenesis can potentially have outsized effects on physiology and behavior. The hypothalamus is a central homeostatic regulator of many different physiological processes, including sleep, circadian rhythms, core body temperature, blood pressure, thirst, and appetite [11]. It serves as the cockpit of the neuroendocrine system, secreting hormones to the blood that regulate release of pituitary hormones. Located partially outside the blood-brain barrier, the hypothalamus also serves as the main site where changes in levels of circulating metabolites and hormones are sensed, and are used to modulate behavior. The hypothalamus is thus exquisitely positioned to undergo plastic changes in response to long-term changes in environmental conditions. Adding small numbers of specific subtypes of neurons to neural circuits that control these processes could be an effective and parsimonious means of accomplishing this. For this reason, we would expect any levels of hypothalamic neurogenesis to both be low and be highly dependent on changes in diet or hormonal state. These facts add the challenge of detecting and studying hypothalamic neurogenesis.
Third, over the past decade, evidence has accumulated from multiple groups for the existence of postnatal hypothalamic neurogenesis in mammals, although there remains considerable disagreement about its extent, regulation and the source and function of newborn neurons. This review aims to critically review these findings, and build a clearer picture of the precise characteristics of the hypothalamic neural niche, the role of extrinsic factors in controlling hypothalamic neurogenesis, and the function of new neurons generated in juvenile and adult hypothalamus.
The Neurogenic Niche in Postnatal Hypothalamus
The existence of neural stem cells in the adult hypothalamus was first proposed following studies that reported that cells of the hypothalamic ventricular zone were capable of forming multipotent neurospheres in vitro, which gave rise to neurons, astrocytes and oligodendrocytes [12]. Though the neurogenic potential of hypothalamic ventricular cells was small compared to that of lateral ventricles, cells of the hypothalamic parenchyma showed negligible neurogenic potential. Since then, a series of studies have reported direct in vivo evidence for neurogenesis in adult mammalian hypothalamus, which is listed in Table 1. The first of these studies used bromodeoxyuridine (BrdU) labeling to label proliferating cells, and revealed that intracerebral infusion of neurotropic or growth factors robustly induced proliferation of cells in the hypothalamic ventricular zone [13, 14]. Although variable levels of growth factor-induced proliferation in hypothalamic parenchyma were also observed [13, 15–19], the highest levels were reported in the ventricular zone, where both neural progenitors in the embryonic brain and the adult SVZ and SGZ are found. Adult-born neurons have been found in many different regions of the hypothalamus, and these are summarized in Table 2.
Table 1.
Studies reporting neurogenesis in adult mammalian hypothalamus
| Reference | Species | Age | Sex | How progenitors were labeled |
Markers for neurogenesis |
Treatment | Effect of treatment on neurogenesis |
|---|---|---|---|---|---|---|---|
| [13] | Rat | Adult (220–250g) | NS | BrdU (i.c.v. osmotic pump 12d, chased for 28d) | TuJ1+,BrdU+ | BDNF, i.c.v., 12d | Up |
| [14] | Rat | P56 | Both | BrdU (i.p. every 2hrs for 48hrs, chased for 28d) | HuC/D+,BrdU+ | bFGF, single dose i.c.v. | Up |
| [15, 16] | Mouse | P56-63 | Male | BrdU (i.c.v. osmotic pump 7d, chased for 42d) | HuC/D+,BrdU+ | CNTF, i.c.v., 7d | Up |
| [30] | Rat | P60 | Both | BrdU (2x i.p. daily for 3d, chased for 18d) | NeuN+,BrdU+ | IGF-1, i.c.v., 7d | Up |
| [33] | Mouse | P84 | Male | Nestin-CreER.tdTom+ (chased from 30d to 480d) | NeuN+,tdTom+ | Aging/IGF-1R cKO in Nestin+ cells | Down/Up |
| [32] | Mouse | P56-84 | Not specifie d | Glast-CreER.GFP+ (chased for 42 or 270d) | NeuN+,GFP+ and DCX+,GFP+ | Fgf2, i.c.v., 7d | Up |
| [36] | Mouse | P56-84 | Male | NG2-CreER.tdTom+, (chased for 28d), BrdU (DW for 28d) | HuC/D+,tdTom+ ,BrdU+ | NA | NA |
| [19] | Mouse | P90 | Male | Sox2-Cre lentivirus (injected into YFP reporter mice, chased for 80d) | NeuN+,YFP+ | NA | NA |
| [26] | Mouse | P28-32 or P60-70 | Both | Fgf10-CreER.tdTom+ (chased for 30d) | NeuN+,tdTom+ | NA | NA |
| [17] | Mouse | 8 wks | Male | BrdU (i.c.v. osmotic pump 7d, chased 28d) | HuC/D, BrdU+ and NeuN+BrdU+ | HFD 28d, Ob/ob (leptin deficiency) | Down |
| [19] | Mouse | Adult (Not specified) | Male | BrdU (i.c.v. daily for 7d, chased 28d) | NeuN+,BrdU+ | HFD, 4 mths | Down |
| [55] | Mouse | P56 | Male) | BrdU (i.c.v. osmotic pump, 3d) | NeuN+,BrdU+ | Onset of HFD (HFD for 3–7d) | Up for 3d HFD, down after |
| [22] | Mouse | P15 or P45 | Female | BrdU (i.p. 2x daily for 9d, chased 30d) | HuC/D+,BrdU+ | HFD, 30d | Up |
| [57] | Mouse | P42 | Both | BrdU (i.p. 2x daily for 9d, chased 30d) | HuC/D+,BrdU+ | HFD, high protein diet, 30d | Up in ME (females only)/down in ArcN |
| [59, 60] | Mouse | P70-84 | Female | BrdU (i.c.v. osmotic pump 9dys, chased 34d) | HuC/D+,BrdU+ | HFD (38 d) or OVX+E2 implant | Up |
| [66] | Rat | P30 | Both | BrdU (i.p. for 3dys, chased 23d) | NeuN+,BrdU+ | Gonadal hormones | Up |
| [67] | Rat | P28 | Female | BrdU (i.c.v. for 4wks, chased 10d) | NeuN+,BrdU+ | None | NA |
| [79] | Sheep | 2 yrs | Female | BrdU (4x i.v. every 12 hrs, chased 28d) | NeuN+,BrdU+ | Short photoperiod | Up |
| [81] | Sheep | 18–24 mths | Female | NA | DCX+ | Short photoperiod | Up |
| [87] | Rat | P35 | Male | BrdU (i.p. for 5 d, chased up to 53d) | NeuN+,BrdU+ | Long-term heat acclimation | Up |
| [105] | Rat | P35 vs. P70-77 vs.22–25 mths | Male | BrdU (i.p. for 5 d, chased up to 40–50d) | NeuN+,BrdU+ | Aging | Down |
| [88] | Rat | P35 | Male | BrdU (i.p. for 5 ds, chased 6 or 40d) | GAD+,BrdU+ and Glu+,BrdU+ | Long-term heat acclimation | Up |
| [18] | Mouse | P84 | Male | BrdU (i.c.v. osmotic pump for 42d) | Agrp-Cre.LacZ+,PCN A+ and POMC+,PCNA+ | Degenerati on of AgRP+ neurons | Up |
| [97] | Rat | P42 | Male | BrdU (ip for 5d, chased 7d or 21d) | HuC/D+,BrdU and NeuN+,BrdU+ | Voluntary exercise | Up |
Table 2.
Cell of origin and location of newborn neurons
| Reference | Location/Identity of neural progenitors |
Hypothalamic regions where newborn neurons were observed |
|---|---|---|
| [13] | Parenchyma, may originate from near ventricle | Widespread |
| [14] | Ventricular region, including tanycytes | Widespread |
| [15, 16] | Parenchyma | Widespread |
| [30] | Ependymal layer (possibly α1 tanycytes) | Widespread |
| [33] | Nestin+ tanycytes (mainly α-tanycytes) | PH, DMH, VMH, LH ArcN, ME |
| [32] | GLAST+ α-tanycytes | ArcN, VMH, DMH |
| [36] | NG2+ parenchymal cells | Widespread |
| [19] | Sox2+ parenchymal cells | Only ArcN studied |
| [26] | Fgf10+ tanycytes (mainly β-tanycytes) | ArcN, VMH |
| [17] | Parenchyma, may originated from near ventricle | Only ArcN studied |
| [19] | Parenchyma | Only ArcN studied |
| [55] | Parenchyma, may originate from near ventricle | initially ArcN, but later also VMH, and PVN |
| [22] | β-tanycytes (Nestin-CreERT2+) | ME |
| [57] | Not determined | ME |
| [59, 60] | Parenchyma, identity was not verified | ArcN, VMH, DMH |
| [66] | Parenchyma, identity was not verified | AVPV (Female), SDN (Male) |
| [67] | Parenchyma, identity was not verified | AVPV, SCN |
| [79] | Parenchyma | Not specified |
| [81] | Ventricular zone/possibly tanycytes | ArcN, VMH |
| [87] | Ventricular zone/possibly tanycytes | POA/AH, VMH, DMH, PH (n.s in PVH, LH) |
| [105] | Ventricular zone/possibly tanycytes | POA |
| [88] | Parenchyma | POA |
| [18] | Parenchyma | ArcN |
| [97] | Ventricular zone/possibly tanycytes | ArcN, ME |
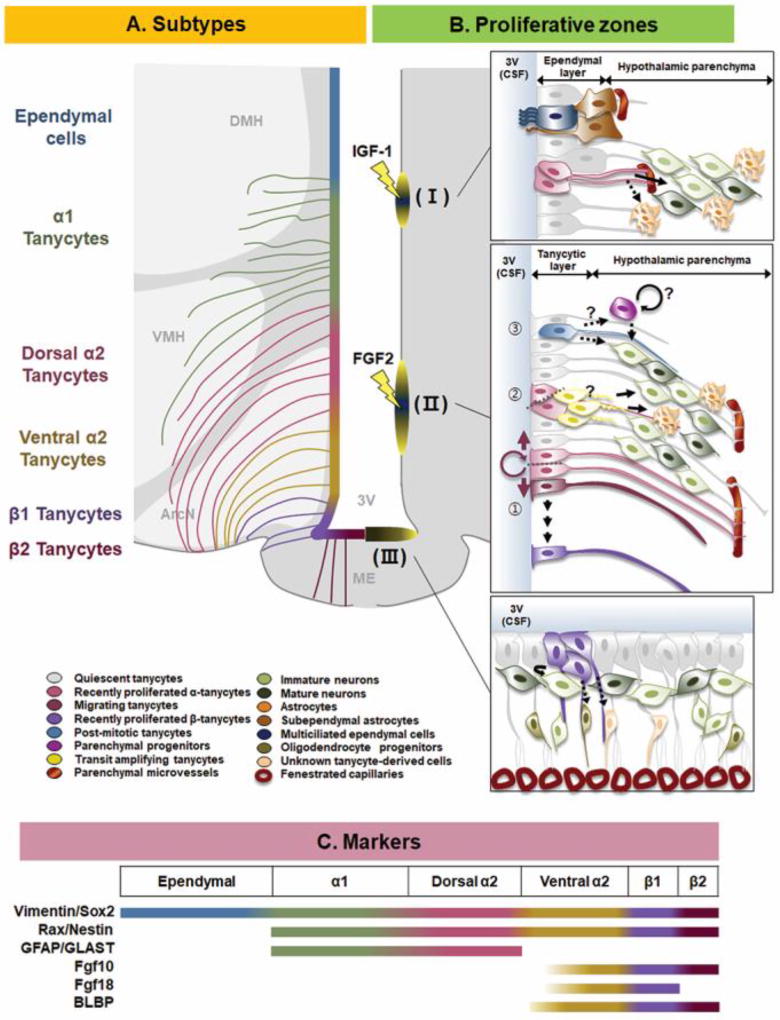
The ventricular zone of the mediobasal hypothalamus is largely comprised of a specialized radial glial-like cell called tanycytes, which line all but the most ventrally located portion of the 3rd ventricular wall in this region [20, 21]. In contrast to the multiciliated ependymal cells that line the ventricles in most of the brain, tanycytes extend only one or two apical cilia into the ventricle and, depending on their location, project a long extended basal process either into the hypothalamic parenchyma or towards the pial surface of the hypothalamus. These radial processes are highly reminiscent of those shown by neural progenitor in the embryonic brain, which also serve as a substrate for radial migration of newly postmitotic neurons. Tanycytes express many genes that are also selectively expressed in embryonic hypothalamic progenitor cells and/or are expressed in neural stem cells of the SVZ and SGZ. These include transcription factors such as Rax, Lhx2, Sox2 and Sox9 [22–25]; intermediate filament proteins such as Nestin, Vimentin and GFAP; growth factors such as Fgf10 and Fgf18 [26]; Notch pathway components such as Notch1, Notch2 and Hes5 [22, 24], and genes of unknown function such as UGS148 [27, 28]. Tanycytes have been traditionally divided into four major subtypes – α1, α2, β1 and β2 – based on their location, morphology and gene expression profile [20]. More recent studies using single-cell RNA-Sequencing (scRNA-Seq) have hinted at the existence of additional functional subtypes of tanycytes [28, 29].
Three different proliferative zones have been identified within the tanycytic layer. These are a subependymal region located in the dorsal α1 region [30, 31], a second region in the dorsal α2 region [14, 20, 32], and a final region that has been termed the “hypothalamic proliferative region”, located adjacent to the median eminence (ME) in the β2 region [22] (Figure 1). Evidence for neurogenesis in all of these regions has been provided using both BrdU incorporation studies and cell lineage analysis using inducible Cre lines.
Figure 1. Tanycytes as neural progenitors in adult mammalian brain.
(A) Schematic illustrating of tanycyte subtypes. From top to bottom: Ependymal cells (Blue), α1 (Green), dorsal (Pink) and ventral α2 (yellow), β1 (Purple), β2 tanycytes (Plum). (B) Three reported proliferative zones in the tanycytic layer. (B–Ⅰ) IGF-1 induces proliferation in the dorsal-most tanycytic zone. These tanycytes give rise to neurons, and possibly astrocytes, in the nearby hypothalamic parenchyma. (B-Ⅱ) FGF-2 induces proliferation in dorsal α2 tanycytes. These α2 tanycytes have the capacity for symmetric self-renewal (①), which may give rise to other subtypes such as β-tanycytes (②). They also divide asymmetrically to give rise to neurons, astrocytes, and more rarely oligodendrocytes (not indicated) directly, or indirectly through tanycyte-derived progenitors located in the hypothalamic parenchyma. These tanycytes may also undergo direct transdifferentiation into neurons without proliferating (③). (B-Ⅲ) β-tanycytes actively proliferate until the early postweaning period, and in puberty in females. These β-tanycytes both divide symmetrically and give rise to neurons, as well as possibly oligodendrocytes progenitors and other uncharacterized cell types in the internal zone of ME. (C) Differential gene expression in different tanycyte subtypes. GLAST shows differential expression between mouse and rat. This schematic shows expression in mouse, while in rat it is expressed mainly in β tanycytes with some expression in α-tanycytes. 3V, third ventricle; ArcN, arcuate nucleus; CSF, cerebrospinal fluid; DMH, dorsomedial hypothalamus; ME, median eminence; VMH, ventromedial hypothalamus.
The first and most dorsally located of these proliferative zones was observed in adult rats following intracerebral infusion of BrdU and IGF-1. Tanycytes, oligodendrocytes and astrocytes in this region all incorporated BrdU, although multiciliated ependymal cells did not. 18 days following the final BrdU infusion, a number of cells double-labeled for BrdU and the neuronal marker NeuN were also observed [30]. More recent studies in mice, which conducted lineage analysis of tanycytes in the aging brain, have also reported IGF-1-dependent regulation of adult neurogenesis in this region in pursuance of aging process [33].
The second proliferative zone is restricted to a subset of dorsally located GFAP and GLAST-positive α2 tanycytes. These cells proliferate in response to intracerebral infusion of EGF and FGF, and in contrast to other tanycyte subtypes, robustly form neurospheres in vitro [14, 20, 32]. Cell lineage analysis using adult Glast-CreER mice indicates that these cells are multipotent, and give rise to neurons and astrocytes, as well as the more ventrally located GFAP-negative α2, β1 and β2 tanycytes [32].
The third proliferative zone is restricted to β2 tanycytes. The β2 tanycytes reside outside the blood-hypothalamus barrier, and make direct contact with the fenestrated capillaries of the ME. They are uniciliated and morphologically distinct from more dosally located tanycytes, and have been designated as E3 cells [34]. High levels of proliferation are observed in the first postnatal month in this region, but this declines dramatically thereafter [22]. Cell lineage analysis using juvenile Nestin-CreER mice shows that these cells give rise to neurons and astrocytes in the ME, and possibly also the arcuate nucleus (ArcN), during the first postnatal month [22, 34]. However, careful anatomical analysis has revealed that the surface area of the ME expands considerably during this period, implying that a substantial fraction of the cell division observed in this zone may represent an increase in the number of β2 tanycytes [22, 34].
In addition to showing dramatically reduced proliferation as animals age [22, 34], adult β2 tanycytes only poorly form neurospheres in vitro [32]. This raises the question of whether cells in this region contribute substantially to adult hypothalamic neurogenesis. Two recent studies have shed light on this question. In the first, β-tanycytes of adult mice were labeled using the Fgf10-CreER knock-in mice, and over the next several months, a progressive accumulation of tanycyte-derived neurons was observed in the VMH and ArcN. Although BrdU labeling of both hypothalamic neurons and tanycytes was observed, no BrdU labeling of Fgf10-derived neurons was reported [26]. In the second, AgRP-positive neurons in the adult ME that underwent excitotoxic destruction were observed to regenerate, but also to not incorporate BrdU when doing so [35]. Taken together, this raises the possibility that dedicated quiescent neural precursors may persist in this region into adulthood, and that these could perhaps arise by direct transdifferentiation of tanycytes.
The observation that α2 tanycytes give rise to cells in this region further suggests that these may function as stem-like cells in vivo, and that β-tanycytes may instead be lineage-restricted neurogenic cells with limited proliferative potential (Figure 1). This is consistent with the observation that with similar chase times following tamoxifen injection, α2 tanycytes labeled with Glast-CreER give rise to a much smaller relative fraction of neurons than do β-tanycytes labeled with Fgf10-CreER [26, 32]. The use of longer chase times could directly address this possibility. Interestingly, lineage analysis conducted using Fgf10-CreER indicates that β tanycyte-derived cells may migrate considerable distances within the hypothalamus, particularly towards more anterior regions that lack tanycytes altogether, suggesting that newly generated neurons may ultimately regulate a broad range of physiological processes [26].
In addition to tanycytes of the hypothalamic ventricular zone, several studies have also reported that neurogenesis also occurs in the hypothalamic parenchyma, although here the evidence is much more controversial [13, 15–17, 19, 36, 37] (Figure 1, Table 1). These studies have reported extensive and widespread neurogenesis, which is stimulated by cytokines such as ciliary-derived neurotropic factor (CNTF) and brain-derived neurotropic factor (BDNF) [13, 15, 16], by degenerative loss of individual subtypes of hypothalamic neurons [18], and inhibited by dietary signals such as high-fat diet (HFD) [19]. Two studies applied cell lineage analysis, and reported that these cells arise from Sox2-expressing cells of unknown identity [19], and NG2-expressing oligodendrocyte progenitors, respectively [36]. Since most oligodendrocyte progenitors coexpress both NG2 and Sox2, these cells may be one and the same. What, if any, relationship exists between these cells and progenitors of the hypothalamic ventricular zone remains unclear. Tanycytes themselves express Sox2, and give rise to both Sox2- and Olig2-positive cells in hypothalamic parenchyma [22, 32, 38]. One possible model comes from the anterior pituitary, where Sox2-positive progenitors of the ventricular-like marginal region give rise to Sox2-positive progenitors that are likewise broadly distributed in the pituitary parenchyma [39]. Addressing this will require fate mapping of ventricular zone cells using selective transgenic lines, such as Rax-CreER [25].
Despite the presence of a potentially permissive neurogenic niche in the hypothalamus, and the fact that other studies have suggested the presence of neural progenitors in other forebrain regions [40–42], it is appropriate to interpret these findings with a dose of healthy skepticism. These studies have typically observed widespread and quite diffuse incorporation of BrdU into neurons, which is difficult to reconcile with our current understanding of how neurogenic niches are organized. This labeling is observed only when BrdU is delivered via continuous intracerebroventricular infusion, rather than by intraperitoneal injection [16], raising the possibility that BrdU incorporation in these cases may reflect low levels of DNA synthesis or repair that are not linked to cell division [43]. Furthermore, more recent studies using the same BrdU delivery method have failed to observe incorporation into parenchymal neurons [35], and likewise failed to observe generation of neurons from NG2-positive cells in hypothalamus or, for that matter, in any other brain region [44–47]. A recent study did observe sporadic labeling of cortical neurons after (though not before) postnatal day 30 (P30) using NG2-CreER mice, and this was ascribed to transient induction of NG2 expression in mature neurons [48]. Likewise, studies that reported that hypothalamic neurons arise from a parenchymal Sox2-positive progenitor used lentiviral delivery of a Sox2 promoter construct driving Cre expression, rather than a knock-in line, which is problematic [19]. The expression pattern of this construct in hypothalamic parenchyma has not been fully characterized, and since the expression level of molecular markers in neurons infected by AAV-based reporters increases steadily over time [49], this may lead to an infection of mature hypothalamic neurons being erroneously interpreted as progenitor cell labeling.
Regulation and Functional Significance of Adult Hypothalamic Neurogenesis
Control of energy homeostasis
For several reasons, most studies of the functional role of postnatal hypothalamic neurogenesis have focused on its role in regulating metabolism and body weight [50]. First among these reasons is the fact that the mediobasal hypothalamus, where the great majority of studies on this topic focused, is a central regulator of food intake and activity. Neurons of the ArcN and ME, in particular, are directly responsive to cues that regulate feeding and satiety. Other nuclei in this region, such as the VMH and DMH, also play central roles in regulating activity and metabolism [51]. Second, these regions directly contact with tanycytes, the best characterized and currently the most plausible cell of origin for newborn hypothalamic neurons, through the long radial processes extended from tanycytic cell bodies. Third, both dietary factors and diet-regulated cytokines have been reported to alter levels of hypothalamic neurogenesis.
A link between adult hypothalamic neurogenesis and energy balance was first suggested in a study that analyzed the effect of intracerebral infusion of CNTF, a treatment that leads to long-lasting reduction in body weight [52]. In this 2005 study, mice subjected to intracerebroventricular (i.c.v.) infusion of both CNTF and BrdU for one week showed a five-fold increase in the number of BrdU-positive cells in hypothalamic parenchyma, along with a two-fold increase in the number of BrdU-positive neurons 42 days after treatment [15]. A subset of these BrdU-positive neurons expressed either pro-opiomelanocortin (POMC) or Neuropeptide Y (NPY), markers of orexinergic and anorexinergic neuronal subtypes, respectively. Simultaneous infusion of the antimitotic drug arabinofuranosyl cytosine (AraC) both blocked BrdU incorporation in the hypothalamus, and counteracted the long-term anorexigenic effect of CNTF [15]. Table 3 lists this and all other studies where a direct link has been made between inhibition of adult hypothalamic neurogenesis using AraC and subsequent effects on physiology and behavior.
Table 3.
Evidence for physiological importance of adult hypothalamic neurogenesis
| Reference | Location/Identity of neural progenitors |
Evidence for functional importance of newborn neurons |
Interval before physiological effect observed |
|---|---|---|---|
| [14] | Ventricular region, including tanycytes | EM analysis (synapse formation) | NA |
| [15, 16] | Parenchyma | AraC (i.c.v. infusion attenuates effects of CNTF on body weight) | 5–10 days* |
| [33] | Nestin+ tanycytes (mainly α-tanycytes) | IGF-1R KO in Nestin+ progenitor cells (increased α-tanycytes density and new born neurons) | NA |
| [36] | NG2+ parenchymal cells | Electrophysiological activity resembling immature neurons | 60 days |
| [22] | β-tanycytes (Nestin-CreERT2+) | ME focal Irradiation (selectively reduced HFD-induced gain in body weight) | 14–21 days* |
| [67] | Parenchyma, identity was not verified | AraC (i.c.v. infusion attenuates estrous-induced surge in LH release) | 28 days |
| [88] | Parenchyma | AraC, (interfered heat tolerance) | 40 days |
| [18] | Parenchyma | AraC (decreased food intake and body fat mass) | 28 days |
indicates time of onset of phenotype determined. All other studies analyzed at listed timepoint only.
Abbreviations used: 3V, third ventricle. AH, anterior hypothalamus. ArcN, arcuate nucleus. AVPV, anterioventral periventricular nucleus. DIO, diet-induced obesity. DMH, dorsomedial hypothalamus. EM, electron microscopy. GDX, Gonadectomy. KO, knockout. LH, lateral hypothalamus. ME, median eminence. n.s., not significant. NS, not specified. OVX, ovariectomy. PH, posterior hypothalamus. POA, preoptic area. PVN, paraventricular nucleus. SCN, suprachiasmatic nucleus. SDN, sexually dimorphic nucleus. VMH, ventromedial hypothalamus.
This study [15] attracted a great deal of attention and stimulated work on this topic, but raised several important concerns, which have yet to be definitively addressed. First, as mentioned above, although several groups have replicated the initial observation of ongoing neurogenesis in parenchymal hypothalamus [36, 53], other more recent studies have not [35]. Second, the fact that i.c.v. infusion of AraC globally blocks cell division throughout the brain [15] raised the question of whether the behavioral effects observed in this study actually resulted from disruption of hypothalamic neurogenesis. Third, although it typically takes at least 3–4 weeks before newborn neurons in the SGZ are synaptically integrated into hippocampal circuitry and modulate behavior [4], this study reported that AraC-dependent inhibition of CNTF-induced weight loss was already detectably with 5 days following treatment, and had maximum effect within 10 days post-treatment [15]. This fact raises the question of whether effects are mediated by neurogenesis at all, rather than proliferation of non-neuronal cells. Finally, the lack of any behavioral phenotype in animals treated with AraC alone [15] raises the question of whether baseline levels of neurogenesis have any role in regulating body weight.
Subsequent studies sought to address this last concern. An inventive study conducted BrdU labeling of embryonically generated hypothalamic neurons, and then measured the appearance of BrdU-negative neurons during postnatal development as an indirect measure of neurogenesis [17]. This study revealed that over half of BrdU-positive ArcN neurons (although only 3% of POMC- and 5% of NPY-positive neurons) lost BrdU label between P28 and P84, but that loss of BrdU labeling was much less pronounced at later ages. Furthermore, in both leptin-deficient mice and animals fed HFD, loss of BrdU labeling was dramatically reduced. This was interpreted to indicate a substantial level of ongoing neurogenesis in ArcN, which was reduced by both increasing age and by obesity induced by long-term feeding with HFD or leptin deficiency. A second study also examined the effects of both HFD and genes that modulate the cellular response to inflammation [19]. This study used i.c.v. BrdU infusion to label proliferating cells and reported that long-term feeding with HFD inhibited BrdU incorporation into neurons in hypothalamic parenchyma, and that this was mediated by an NF-κB-dependent response to inflammatory cytokines. Finally, it was reported that constitutive activation of NF-κB signaling in Sox2-positive hypothalamic cells led to prediabetes and obesity.
A close examination of this data raises questions about exactly how HFD and leptin-dependent changes in cell proliferation in general, and neurogenesis in particular, might regulate body weight and metabolism. Both studies reported that HFD primarily reduced BrdU incorporation in non-neuronal cells of the hypothalamic parenchyma, and that while reduced numbers of BrdU-labeled neurons were observed, both POMC and NPY-positive subtypes were equally affected, which is not consistent with a model where selective generation of anorexigenic neurons modulates neural circuitry controlling feeding. Furthermore, the two studies reported opposite effects of long-term HFD treatment on the formation of hypothalamus-derived neurospheres. In one case, stem-like cells derived from the mice fed HFD for 2 months formed 350% more, and substantially larger, neurospheres from the same number of seeded cells than did mice fed normal chow, as if they had been stimulated to replace lost progeny [17]. In the second study, in contrast, stem-like cells cultured from the mice fed HFD for 4 months generated smaller and fewer neurospheres [19]. This discrepancy could reflect differences in culture conditions, the length of HFD treatment, or the precise location of the dissected tissue used to prepare the neurospheres. Indeed, the precise origin of the stem-like cells used in these assays is unclear, although in both studies they are interpreted as residing in the mediobasal hypothalamic parenchyma. However, it has been established for some time that cells from the hypothalamic ventricular zone proliferate robustly in response to EGF and FGF treatment, and consequently have much greater potential to generate neurospheres than do parenchymal cells [12, 32, 35, 54]. It remains possible that stem-like cells from the parenchyma and from the ventricular zone may show differential responses to HFD treatment.
Several studies have reported that short-term treatment with HFD induces cells of the hypothalamic ventricular zone to proliferate and give rise to neurons. One recent report observed increased proliferation in mice within 3 days of HFD treatment as measured by BrdU incorporation, which within two weeks led to an increase in both glial cells and anorexigenic POMC-positive neurons [55]. Administration of AraC by i.c.v. both blocked cell proliferation and led to an increase in body weight in HFD-fed mice. This may represent a mechanism to partially counteract the effects of increased caloric intake in HFD-fed mice by reducing food intake, a response that is normally seen in mice after one week of HFD [56].
Our group has likewise shown that HFD treatment stimulates neurogenesis in the ME. This effect was seen in early postweaning mice of both sexes, but occurred only in females in pubescent animals [22, 57]. Focal X-irradiation of the ME of P45 females (but not males) fed HFD blocked both cell proliferation and led to a long-term increase in activity levels and reduction in weight gain, although food intake was unaffected. Interestingly, this effect of HFD in stimulating neurogenesis, as measured by BrdU delivered by i.p. injection, was confined to the ME, with one month of HFD feeding reducing neurogenesis in the ArcN [57], as observed in other studies [17, 19].
This work highlights the importance of analyzing both sexes when studying this highly sexually dimorphic brain region [58]. Other studies have also identified sex-specific differences in regulation of HFD-induced hypothalamic neurogenesis [59, 60]. In this study, proliferating cells in ovariectomized female mice fed either HFD or normal chow were labeled by BrdU delivered for 9 days by i.c.v. infusion, followed by a 34-day chase. HFD increased the number of BrdU-positive neurons in the ArcN and VMH, while estradiol (E2) treatment blocked this increase in proliferation. Interestingly, HFD treatment selectively increased the number of estrogen receptor alpha-positive neurons in the ArcN and VMH [59]. These results differ from other studies performed on male mice using i.c.v. delivery of BrdU, where HFD was reported to inhibit neurogenesis in the ArcN [17, 19], as well as studies performed in intact female mice that used i.p. injections to deliver BrdU [57]. In the latter case, the high levels of psychological stress that associated with intraperitoneal injection, as well as the fact that animals had not been ovarectomized, may have affected these results [61].
Although these studies have all suggested that dietary signals – such as HFD itself, along with diet-regulated hormonal signals such as leptin – may regulate hypothalamic neurogenesis, there is still no obvious link between the phenotypes observed and the specific subtypes of neurons generated. The fraction of newly generated neurons that express markers of neuronal subtypes that control feeding and body weight is quite small, and is not always correlated with the observed behavioral phenotypes. In one study that reported HFD-induced inhibition of neurogenesis [19], 10% of all newborn neurons were POMC-positive, while 4% were NPY-positive. In a second study, 6% of all newborn cells were POMC-positive in mice fed either normal chow or HFD [55]. These and other studies have also shown that no more than 25% of newborn neurons are leptin-responsive, as measured by leptin-induced phospho-STAT3 staining [15, 18, 22, 62]. Further studies, possibly using a combination of lineage mapping and highly sensitive techniques such as scRNA-Seq, will be needed to more fully characterize the identity of these newborn neurons and provide clues as to how they might influence feeding, metabolism and body weight.
Reproduction and sex-specific behaviors
The studies discussed above identified significant sex-dependent differences in dietary regulation of hypothalamic neurogenesis. Other work has observed that both gonadal sex hormones and photoperiodic changes in reproduction may themselves have a major influence on hypothalamic neurogenesis. Gonadal steroids have long been known to play a central role in controlling the development of multiple different subtypes of hypothalamic neurons [63], and in particular play a major role in promoting survival of neurons the sexually dimorphic nucleus (SDN) of the preoptic area (POA) in males [64, 65]. The first study to report a role of gonadal steroids in postnatal hypothalamic neurogenesis observed sexually dimorphic patterns of neurogenesis during puberty (P30-32 in rats) in sexually dimorphic regions in the anterior hypothalamus. Specifically, neurogenesis was observed in the anterioventral paraventricular (AVPV) nucleus in females and the SDN in males [66]. Gonadectomy at P20 blocked all sex-specific differences in neurogenesis. In this study, no attempt was made to identify the cells of origin of these newborn neurons, which are found considerably anterior to areas of active neurogenesis described in other studies. A more recent study directly addressed the functional significance of sex-specific neurogenesis in female rats [67]. This study showed that i.c.v. canulation of AraC blocked both pubertal neurogenesis and attenuated the preovulatory surge in luteinizing hormone (LH) release.
A second study, which has been previously mentioned in the context of HFD-dependent regulation of adult neurogenesis, reported that while E2 treatment blocked neurogenesis in HFD-treated ovarectomized female mice, E2 stimulated neurogenesis in mice fed normal chow [59, 60]. E2 treatment downregulated hypothalamic Fgf10 expression in HFD-treated mice, suggesting that proliferation of Fgf10-sensitive ventricular progenitors might be reduced. Interestingly, the number of estrogen receptor alpha-positive neurons increased following HFD treatment, regardless of hormonal status. These studies are interesting in light of epidemiological evidence demonstrating that both post-menopausal women [68] and aged female rodents [69] are both vulnerable to diet-induced obesity, which can in many cases be ameliorated by E2 treatment, and suggest that defective hormone-dependent regulation of diet-regulated neurogenesis could mediate these effects.
Other studies have focused on the role of adult hypothalamic neurogenesis in mediating seasonal-dependent changes in reproduction. A large fraction of animals that live in temperate and polar climates exhibit photoperiod-dependent changes in mating and fertility [70], although they have been attenuated or eliminated in most laboratory mice through a process of artificial selection [71]. Changes in photoperiod have also been linked to seasonal changes in levels of adult neurogenesis. This was first reported in songbirds, where seasonal variation in both cell proliferation and incorporation newborn neurons into the brain song control system were observed [72–75]. The Syrian hamster, which shows strong reproductive photoperiodism and breeds only during long days, was reported to show increased rates of neurogenesis during short days in SVZ and SGZ. Increased numbers of BrdU-positive cells were also observed in the hypothalamus, but these were not immunostained for neuronal markers [76]. This increase in proliferation occurs opposite to the reproductive period, and may related to other physiological processes that show seasonal variation, such as body weight and metabolism [77].
More direct evidence for a role of adult hypothalamic neurogenesis in control of photoperiodic changes in reproduction comes from studies of sheep, a long-lived mammal that is more sensitive to seasonal photoperiodic changes than rodents [78]. Ewes injected with BrdU in December showed increased cell proliferation in thalamus and hypothalamus relative to treatment in July [79]. After a 24-hour chase, 8% of BrdU-positive cells were still actively proliferating, as measured by Ki67 immunoreactivity, and 33% were colocalized with the stem/progenitor cell marker Sox2. After 4 weeks’ chase, 17% of the BrdU-positive cells were positive for the mature neuronal marker NeuN, and about 70% of them expressed either GFAP or S100b, markers of astrocytes. This increased rate of neurogenesis and gliogenesis during the period of sexual activity was unaffected by estrogen levels, as similar changes were observed both in untreated ovariectomized animals and in ovariectomized animals rescued by estradiol treatment [79].
The same group performed comparative studies in mouse, sheep, and human hypothalamus to characterize the localization of Doublecortin (DCX)-expressing migrating neuronal precursor cells, and in each species found numerous DCX-positive cells in the ArcN and ME [80]. Interestingly, they observed that the DCX-positive cells located close to the 3rd ventricle are small and rounded without processes, much like immature neuronal precursors. These cells were morphologically distinct from larger and fusiform bipolar DCX-positive cells that were located in the parenchymal regions of the ArcN and VMH, which also co-labeled with the neuronal marker HuC/D. These more mature precursor-like cells also expressed Cdk5 and p35, kinases that directly phosphorylate DCX and regulate its activity. Most importantly, the number of DCX-positive cells in the ArcN and VMH was controlled by seasonal photoperiod and was substantially higher during winter days when animals were sexually active [81]. The presence of cells that resemble immature neuronal precursors adjacent to the ventricular region of the mediobasal hypothalamus is consistent with a tanycytic origin for these cells. Further support for this comes from recent studies that have demonstrates a substantial increase in the thickness of the ventricular zone of the mediobasal hypothalamus during short day periods in winter, with tanycyte basal processes closely associated with actively proliferating cells [82]. It differs, however, from the results reported in the first of this series of studies, which observed BrdU-positive cells broadly distributed throughout the hypothalamic parenchyma [79].
Temperature homeostasis
The hypothalamus is the central regulator of core body temperature, maintaining it around a tightly controlled set point by controlling activity levels, sweating, and adaptive thermogenesis through burning of brown fat, to name just some of the mechanisms used [83–85]. Core homeostatic regulatory circuitry for control of body temperature resides in the POA, although other hypothalamic regions such as the PVN and DMH play essential roles controlling behaviors that mediate heating or cooling. Adult neurogenesis has been specifically implicated in the induction of heat acclimation in response to chronic exposure to moderate-high heat. This process results in lower core body temperatures, and slower increases in body temperature in response to heat exposure than naive individuals [86]. In the first of a series of studies on this topic, substantially increased numbers of BrdU-positive cells were observed in the rat hypothalamus at 6 days of moderate heat exposure, with lower but still elevated numbers observed following longer exposure times [87]. Most BrdU-positive cells were initially observed in the ventricular zone adjacent to the 3rd ventricle, and were later found mostly in the parenchyma, consistent with origin from tanycytes. The number of NeuN/BrdU-double positive cells in hypothalamus was increased seven-fold after 33 days of heat exposure relative to controls. Labeled cells were distributed throughout the hypothalamic parenchyma, including POA, AH, DMH and VMH. Later studies demonstrated that BrdU-positive POA neurons generated in response to heat exposure expressed GABAergic and glutamatergic markers, and induced c-fos in response to heat stress [88]. These same studies also showed that inhibition of cell proliferation via i.c.v. delivery of AraC disrupted heat acclimation. These intriguing studies suggest that long-term heat acclimation may result in stable and long-lasting changes in thermoregulation as a result of changes to hypothalamic circuitry as a result of increased neurogenesis following heat stress. These studies have thus far only been conducted in rats, and extending them to mice would allow more extensive characterization of the cell of origin of these neurons, along with the molecular mechanisms controlling heat-induced neurogenesis. In the meantime, they are one of the more compelling potential examples of direct modification of homeostatic regulatory circuitry in the hypothalamus in response to long-term environmental changes.
Injury-induced neurogenesis
Adult neurogenesis can be induced in cold-blooded vertebrates [89], and also possibly in mammalian cortex and striatum [90], to regenerate individual neuronal subtypes lost as a result of injury. Several studies have investigated whether this can also occur in the hypothalamus. While a recent study did not observe clear evidence for adult neurogenesis in response to excitotoxic injury in ArcN and ME [35], an older study from the same group reported an induction of adult neurogenesis in mice that were genetically modified so that AgRP-expressing neurons underwent slow degeneration by cell-specific selective deletion of the transcription factor Tfam [18]. An increase in proliferating cells was observed in the ArcN of mutant mice, and i.c.v. canulation of AraC blocked increased food intake in these mutant mice but, as previously reported, did not affect wildtype controls [15]. This study observed that actively proliferating Ki67- and PCNA-positive cells expressed AgRP and POMC, which are expressed exclusively in postmitotic neural precursors during normal embryonic development [91, 92]. It is unclear to what extent this represents selective regeneration of AgRP-positive neurons, since roughly equal numbers of POMC- and AgRP-positive cells were observed to be labeled with PCNA and Ki67 [18]. Likewise, in the absence of BrdU labeling or genetic lineage analysis to label the progeny of these progenitors, it is not possible to quantify the extent to which adult neurogenesis is actually occurring in these animals.
Exercise
Voluntary exercise has been shown to promote neurogenesis in the SGZ of the hippocampus [93], which in turn has been linked to enhanced cognitive function, particularly during aging [93–95]. Exercise-induced adult neurogenesis has also been linked to recovery of function following stroke, and reduced incidence of future stroke, in stroke-prone spontaneously hypertensive rats (SHRSP) [96]. More recently, exercise-induced hypothalamic neurogenesis has been directly investigated in SHRSP rats [97]. Long-term voluntary exercise leads to beneficial effects in SHRSP rats, reducing systolic blood pressure and increasing food intake and body weight, leading to a substantially enhanced survival rate as compared to sedentary animals [97]. Exercise substantially increased both total levels of cell proliferation (as measured by BrdU incorporation), as well as adult neurogenesis, in the ArcN and ME in both wildtype and SHRSP rats, relative to sedentary controls. Furthermore, even in the sedentary state, SHRSP rats showed increased levels of cell proliferation and neurogenesis relative to wildtype controls. Elevated levels of neurogenesis were also seen after experimentally induced stroke in SHRSP rats. Substantial levels of BrdU labeling were observed in both α2 and β-tanycytes, implying that these are the source of the newborn neurons seen here. Interestingly, exercise led to increased expression of both FGF-2 and EGF in the ventricular zone, suggesting a possible mechanism by which tanycyte proliferation might be stimulated, although this was observed only following stroke. While these effects are substantial and exciting, further work is needed to definitively determine whether tanycytes are indeed the source of these new neurons, as well as to determine whether they play a functional role in exercise-induced increases in food intake and body weight.
Aging and control of postnatal hypothalamic neurogenesis
Adult neurogenesis declines with advancing age in all brain regions studied to date [94, 98], and the hypothalamus does not appear to be an exception to this rule. Several of the previously mentioned studies that explored hypothalamic neurogenesis in different age groups using identical BrdU labeling methods also observed an age-related decline in the number of newborn cells. Our group has previously shown a dramatic reduction in the number of newborn neurons in the ME after P28 under baseline conditions, with a more modest reduction in animals fed HFD [22]. A similar age-related reduction in BrdU incorporation was observed in another study comparing post-weaning (P28-32) with adult (P60-70) mice that were chased for identical periods. Intriguingly, the number of both BrdU-positive tanycytes and neurons derived from Fgf10-Cre expressing cells showed a similar age-related reduction [26]. This substantial reduction neurogenesis after puberty could be regulated by gonadal hormones and/or related to control of sex-specific behaviors, as discussed above [66, 67]. Other reports directly studying the effect of aging in hypothalamic neurogenesis revealed an additional reduction later in life, demonstrated an age-related reduction in the number of neurogenic α-tanycytes after 6 months of age, along with a substantial reduction in neurogenesis in elderly (16 month-old) animals [33]. This data suggests that the progressive age-related depletion of neurogenic tanycytes may drive the reduction in age-related hypothalamic neurogenesis.
It has been known for some time that age-related changes in morphology and gene expression occur in rat tanycytes [99, 100]. These studies observed a reduction in the number of tanycytic process immunolabelled with GFAP, leading to speculation that tanycytes may be lost during aging. This hypothesis was supported by a recent study of postmortem human tissue, which showed a dramatic reduction in Vimentin-immunoreactive tanycytic processes in the elderly post-mortem female hypothalamus compared to the juveniles or adults [101], although the causes of these differences are still unclear. One potential factor controlling age-related decreases in hypothalamic neurogenesis in females are declines in the levels of circulating estrogen. Ovariectomized female mice shows increased proliferation as measured by BrdU incorporation when implanted with capsules containing estradiol in comparison to the vehicle infusion [59, 60]. However, the systemic age-related decline in the production of new neurons occurs in both sexes, as do age-related changes in tanycyte morphology and gene expression [99, 100], implying that other mechanisms also control this process. Related to this, a study of male mice has reported that suppression of IGF-1 signaling by conditional knockout of IGF-1R in Nestin-positive adult neural stem cells, as well as tanycytes, attenuated the age-derived reduction of neurogenesis in both hippocampus and hypothalamus, and also led to functional improvements in both brain regions [33, 102]. Although this result seemingly conflicts with an earlier finding that observed short-term treatment with IGF-1 stimulated tanycyte proliferation [30], this may imply that elevated levels of IGF signaling early in life exhausts a limited supply of neurogenic tanycytes, leading to reduced neurogenesis later in life. Neuroinflammation has also been proposed as a causative factor for impaired neurogenesis in old animals [103, 104]. Neurospheres grown from the hypothalamus from obese mice, in which neuroinflammation has been activated, showed less proliferative and neural differentiation potential [19], although it has not been directly tested whether this is also related to age-related reductions in hypothalamic neurogenesis.
Potential functional consequences of age-related decreases in hypothalamic neurogenesis have been observed with respect to temperature homeostasis. The authors showed that the heat-acclimated aged (22–25 months) rats exhibited less heat tolerance in conjunction with no increase in the proliferative cell number [105]. Even the mature adult (10–11 months) had a similar phenotype. Only heat-acclimated young (5 weeks) rats displayed strong heat tolerance and significantly enhanced cell proliferation followed by neuronal differentiation including DCX and NeuN-expressing neurons, consistent with other reports [87, 88].
Parallels between hypothalamic tanycytes and retinal Müller glia
Insight into the mechanism by which neurogenic competence is regulated in tanycytes may come from retinal Müller glia, a subtype of radial glia that closely resemble tanycytes in morphology, development and function, and have been more extensively investigated as potential sources of adult neurogenesis [106–109]. The hypothalamus and retina both derive from the ventral anterior neural tube, and both retain radial glia into adulthood. Table 4 summarizes relevant key parallels between tanycytes and Müller glia.
Table 4.
Comparison of retinal Müller glia and hypothalamic tanycytes.
| Müller glia | Tanycytes | ||
|---|---|---|---|
| Shared features | Morphology | Radial glia (bipolar) | Radial glia (unipolar) |
| Developmental origin | Ventral anterior neural tube | ||
| Status before injury | Quiescent/non-proliferative | ||
| Barrier function | Blood retina barrier | Blood-CSF barrier/ Blood-hypothalamus barrier | |
| Stem cell-like characters | Self-renewal, multipotency (retinal neurons) | Self-renewal, multipotency (neuron, astrocyte, oligodendrocyte) | |
| Progenitor markers expressed | Rax, Lhx2, Notch1, Sox2 | Rax, Lhx2, Notch, Sox2, GFAP, Nestin | |
| Signaling pathways involved in development | Notch, Wnt | Notch, Wnt | |
| Regenerative capacity | Dedifferentiation | Essential for regeneration | Unknown |
| Injury models studied | Chemical treatments (NMDA, MNU, MSG, ouabain), light damage, needle poke, genetic models of retinal degeneration | MSG | |
| Extrinsic factors that induce neurogenesis | TNFα, IL6 and leptin, FGF-2 and Insulin, IGF-1, EGF, HB-EGF [142] | IGF-1, bFGF, BDNF, EGF | |
| Genes upregulated following injury | GFAP, Ascl1, Lin28, cell cycle regulators | GFAP | |
| Reprogramming factors | Ascl1, Lin28, let-7[118], Sox2 [143] | Unknown | |
| Neurogenesis-promoting signaling pathways | Wnt, Notch (later stages), Shh, FGF, Insulin, EGF, BMP, Jak/Stat | IGF, FGF | |
| Inhibitory signaling pathways | TGFb, [144, 145]Notch | Unknown | |
Müller glia, which are the only glial cell type that derive from retinal progenitors, span the full radial axis of the retina, and are essential for maintenance of retinal lamination and photoreceptor survival [110]. Müller glia perform many essential physiological functions conducted by astrocytes in other brain regions, such as uptake of synaptic glutamate, control of extracellular potassium concentration, and lactate production [110]. Furthermore, mammalian Müller glia have long been known to undergo a set of characteristic morphological and transcriptional changes in response to injury that are shared with astrocytes. These include hypertrophy and increased stiffness, which partially results from induction of the intermediate filament protein GFAP, and expression of a diverse set of secreted neuroprotective factors, including LIF and BDNF [111–113]. In cases where this question has been directly studied, tanycytes have been shown to share these functions and features with Müller glia.
Müller glia closely resemble tanycytes in their gene expression profile and retain expression of many genes that are selectively expressed in retinal and/or hypothalamic progenitors. Both cell types are specified by overlapping sets of transcription factors, including the retinal and hypothalamic progenitor-expressed homeodomain factors Rax and Lhx2 [23, 24, 113, 114]. Both cell types also retain relatively high levels of Notch signaling into adulthood [22, 24, 115, 116]. These intrinsic and extrinsic signals likely interact to maintain the radial morphology and progenitor-like gene expression patterns of both cell types into adulthood, at a time when radial glial cells are no longer found in the great majority of CNS regions.
Despite closely resembling neural progenitors in their gene expression profile, however, both tanycytes and Müller glia show little or no detectable proliferation under baseline, uninjured conditions, but show varying ability to undergo neurogenesis in response to injury or stressors. In zebrafish, for instance, Müller glia function as quiescent, injury-inducible neural stem cells throughout life [108, 117]. Loss of any specific subtype of retinal neuron will rapidly induce transcription of genes that promote neurogenic competence such as ascl1a and lin28a, and lead Müller glia to reenter the cell cycle within 36 hours following injury [118, 119]. Treatment with a wide variety of extrinsic factors – including TNF, IGF, FGF, Wnt and BMP family members – can readily induce neurogenic competence, although Notch signaling must be transiently downregulated to initiate this process [120, 121]. Zebrafish Müller glia that have undergone dedifferentiation in this manner go on to produce all major subtypes of retinal neuron, with the missing cell type(s) undergoing terminal differentiation and functional integration, while all other neuronal subtypes undergo apoptotic cell death [122].
In contrast, the Müller glia of early post-hatch chickens retain a modest level of neurogenic competence. They readily proliferate and activate expression of proneural bHLH factors following excitotoxic injury or treatment with IGF and FGF, but only give rise to a limited number and subtypes of retinal neurons [123–126]. Moreover, chick Müller glia lose neurogenic competence by one month post-hatch [124, 127]. Müller glia of mice possess extremely limited injury-induced neurogenic competence in early postnatal life, and this too is lost upon eye opening at the end of the second postnatal week [128, 129]. Recent studies have shown that a limited level of neurogenic competence can be induced in adult mouse Müller glia by overexpression of Ascl1 and treatment with histone deacetylase inhibitors [130–132], and that these reprogrammed Müller glia can give rise to a small number of retinal interneurons which undergo functional integration.
These findings show that while the neurogenic competence of Müller glia is much higher in cold-blooded vertebrates and at younger ages, it is also inducible even in adult mammals to a limited extent. Given their many similarities with Müller glia, it is reasonable to propose that this may also hold for tanycytes. The neurogenic competence of hypothalamic tanycytes in non-mammalian species has not been directly investigated, but may well be substantially higher than in mice.
What genes might control neurogenic competence in Müller glia and tanycytes, and why are these so much lower in mammals? Recent studies of Müller glia in non-mammalian species have reported a mutually exclusive relationship between genes associated with non-proliferative reactive gliosis and neurogenic competence. In zebrafish, for instance, injury initially induces expression characteristic markers of reactive gliosis such as GFAP, which are suppressed once cells begin to re-enter into mitosis [133]. Pharmacological inhibition of mitosis blocks both induction of neurogenic competence and leads to persistent expression of GFAP. Likewise in chick, there appears to be a mutually exclusive relationship between glia that upregulate GFAP and those that upregulate Ascl1 following injury [127].
These results suggest that there exists a mutually inhibitory relationship between the transcriptional regulatory networks that induce neurogenic competence and non-proliferative reactive gliosis, and suggest that injury triggers the former program in zebrafish Müller glia, but the latter in mammals. This hypothesis remains to be directly tested. Although a considerable amount is known about the gene regulatory networks that control neurogenic competence, much less is known about those that control reactive gliosis. Identifying and inhibiting key negative regulators of neurogenic competence in radial glia, in combination with induced expression of positive regulators of neurogenesis, may ultimately prove the best way to induce production of substantial numbers of functional neurons from both Müller glia and tanycytes.
Conclusion
The past two decades have seen a steady accumulation of evidence suggesting that neurogenesis can occur in the adult mammalian hypothalamus (Tables 1–3). The first studies to hint at this possibility arose from in vitro analysis of neurosphere formation [12], or used slow i.c.v. infusion of BrdU to label neurons in the hypothalamic parenchyma [13]. Later studies used viral or genetic cell lineage analysis to identify both tanycytes [14, 22, 26, 32] and Sox2-expressing parenchymal cells [19, 36] as the source of these newly generated neurons. Many reports have identified changes in diet, behavior or hormone levels as regulating hypothalamic neurogenesis, and a more limited number of studies has observed that either global [15, 18] or focal [19, 22] inhibition of cell proliferation can modulate behavioral states that are associated with elevated levels of neurogenesis.
Given the immense potential functional importance of adult neurogenesis in the plasticity of hypothalamic circuitry, it may seem puzzling as to why this topic has not attracted the same level of attention as studies of adult neurogenesis in the SVZ and SGZ. There are several reasons for this. First is the fact that even the highest reported levels of hypothalamic neurogenesis are very low – as much as several orders of magnitude lower than in age-matched SVZ or SGZ. This is a formidable technical obstacle that substantially slows all research on this topic and makes it particularly difficult to obtain morphological and functional evidence that newly-generated cells actually integrate into hypothalamic circuitry, which is the gold standard in the field to convincingly demonstrate the functional importance of adult neurogenesis.
A second related fact is that much of the evidence put forward in support of adult hypothalamic neurogenesis has been incomplete, and therefore open to various interpretations. For instance, the ability of hypothalamic cells to form neurospheres in culture does not demonstrate that they actually do so in vivo. Likewise, although all cells going through S-phase will show BrdU incorporation, BrdU incorporation alone not necessarily indicate that cells have undergone mitosis, and can instead reflect damage-induced abortive DNA replication or extensive DNA repair [43]. Some more recent studies using essentially identical conditions for BrdU delivery have failed to replicate these findings altogether [35]. Differences in the length of the chase interval following BrdU treatment may lead to dilution to the point of undetectibility in some cases, if hypothalamic neural progenitors undergo many rounds of division during this period (see Table 1).
Techniques used to analyze cell lineage have their own set of limitations. The viral vectors that have been used in several studies to investigate the cell of origin of adult-born neurons [14, 19] do not selectively infect dividing cells, and thus are not really suitable for cell lineage analysis. While transgenic lines expressing inducible Cre recombinase are powerful and effective tools for performing lineage analysis, no studies have combined BrdU labeling with Cre-based lineage analysis to directly show that adult-generated hypothalamic neurons actually derive from proliferating progenitor cells [26, 32]. Furthermore, none of the studies that report behavior effects following disruption of hypothalamic neurogenesis, using either irradiation or chemotherapy drugs, selectively disrupted the birth of new neurons – instead, they disrupted all cell division, and in some cases did so throughout the entire brain (Table 3). The time lag between the inhibition of cell proliferation and observation of physiological effects is either somewhat shorter [22] or much shorter [15] than those observed in SVZ and SGZ, raising questions about whether these effects are indeed mediated by disruption of neurogenesis. Lastly, no group has yet produced definitive evidence that adult-born neurons form synaptic connection and functionally integrate into hypothalamic circuitry. Without such evidence, many researchers who might otherwise be drawn to work on this topic may consider that the case for adult hypothalamic neurogenesis not proven beyond a reasonable doubt.
A third reason is a general lack of coordination among investigators in the field, which thus far has not allowed much of a body of knowledge to accumulate about how adult neurogenesis is regulated in any specific context. In contrast to studies of adult neurogenesis in the SVZ and SGZ, studies of postnatal hypothalamic neurogenesis have used a very broad range of model organisms, experimental protocols to detect neurogenesis, and environmental and behavioral conditions. The seemingly conflicting reports that HFD either inhibits [17, 19] or activates [22, 55, 60] neurogenesis likely come down to differences in age, sex, duration of HFD administration, and/or mode of BrdU administration, but currently there is not enough independent replication of any of these findings to identify which of these variables are most relevant.
Knowing which physiological or environmental conditions consistently give rise to the highest levels of neurogenesis would greatly accelerate mechanistic studies of how this process is regulated. In the mouse, however, only a relatively small fraction of all potentially relevant conditions have been studied, and the observed rates of neurogenesis remain stubbornly low. Likewise, some of highest levels of adult hypothalamic neurogenesis have been observed in organisms – such as the Ile de France ewe or the SHRSP rat [97, 134], – that are not widely used, and which lack transgenic tools for identifying both cell lineage and functional manipulation of candidate genes that regulate neurogenesis.
To overcome these obstacles, several steps need to be taken. First, it is essential that the behavioral and environmental factors that produce the highest levels of neurogenesis in adult mice be identified. Moreover, in light of the short half-life of adult-generated neurons in the SVZ and SGZ [135], it is imperative to identify conditions that promote the survival of the newly generated hypothalamic neurons. Identifying the optimal conditions in which to investigate adult hypothalamic neurogenesis will allow a vast array of transgenic lines and other molecular reagents to be brought to bear on mechanistic analysis of this topic, and will likely draw many other researchers into the field. In particular, a number of genetic tools to either selectively kill or promote the survival of newborn neurons have been generated to study adult neurogenesis in the SVZ and SGZ, and may be useful in the study of hypothalamic neurogenesis [136, 137]. In parallel, CRISPR-based genetic reagents and new viral vectors should be developed for species in which high levels of adult hypothalamic neurogenesis have already been identified. This should make it possible to unambiguously demonstrate whether new neurons are indeed being born, are fully differentiating, and if they are integrating appropriately into adult hypothalamic circuitry.
Other technical advances, and insights from studies of other cell types, are likely to further accelerate our understanding of adult hypothalamic neurogenesis. Emerging techniques such as scRNA-Seq analysis will allow molecular phenotyping and classification of adult-born neurons, and also identify genes that control the generation and differentiation of these newly generated neurons. Recently, several scRNA-Seq studies have made first attempts to classify the major cell types of the adult mouse hypothalamus [28, 29, 138]. Several of these have identified unexpected molecular diversity among tanycyte subtypes [28, 29]. A modified form of scRNA-Seq that enriches for very rare proliferating cells known as Div-Seq has been used to identify very rare populations of neural progenitors in adult mouse spinal cord [139]. Similar approaches may help identify genes that control neurogenic and proliferative competence in tanycytes and/or other candidate hypothalamic progenitor cell types. Finally, analysis of postmortem samples may help molecular markers for candidate neural progenitor populations in humans [140], making it possible to successfully conduct scRNA-Seq analysis to determine whether neurogenesis occurs in adult human hypothalamus [141].
Studies of adult neurogenesis in other forebrain regions are also likely to help inform studies of hypothalamic neurogenesis. One cell type of particular relevance is retinal Müller glia – which closely resemble tanycytes in developmental origin, function and gene expression profile – and may help guide the design of studies of tanycyte-derived neurogenesis and identify genes that control neurogenic competence in tanycytes.
Highlights.
Over the past two decades, evidence has accumulated from multiple species that neurogenesis can occur in the adult mammalian hypothalamus.
Levels of adult hypothalamic neurogenesis are very low relative to the SVZ and SGZ, is age-dependent, and is regulated by a range of environmental, behavioral and hormonal conditions.
The source of these newborn neurons is still controversial. Tanycytes are the most likely candidate, although multiple different progenitor cell types may exist.
Standardization of experimental protocols and model systems is likely to substantially accelerate future progress in the field.
Studies of the control of neurogenic competence in retinal Müller glia, which share many features with hypothalamic tanycytes, may help identify both general principles and specific genes that regulate adult hypothalamic neurogenesis.
Acknowledgments
We thank W. Yap for comments on the manuscript. This work was supported by a grant from NIH (R01DK108230) to S.B.
Abbreviations used
- 3V
third ventricle
- AH
anterior hypothalamus
- ArcN
arcuate nucleus
- AVPV
anterioventral periventricular nucleus
- CSF
cerebrospinal fluid
- DIO
diet-induced obesity
- DMH
dorsomedial hypothalamus
- EM
electron microscopy
- GDX
Gonadectomy
- HFD
high fat diet
- KO
knockout
- LH
lateral hypothalamus
- ME
median eminence
- n.s.
not significant
- OVX
ovariectomy
- PH
posterior hypothalamus
- POA
preoptic area
- PVN
paraventricular nucleus
- SCN
suprachiasmatic nucleus
- SDN
sexually dimorphic nucleus
- VMH
ventromedial hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colucci-D'Amato L, Bonavita V, di Porzio U. The end of the central dogma of neurobiology: stem cells and neurogenesis in adult CNS. Neurol Sci. 2006;27(4):266–70. doi: 10.1007/s10072-006-0682-z. [DOI] [PubMed] [Google Scholar]
- 2.Horstman J. The Scientific American Brave New Brain: How Neuroscience, Brain-Machine Interfaces, Neuroimaging, Psychopharmacology, Epigenetics, the Internet, and Our Own Minds are Stimulating and Enhancing the Future of Mental Power. John Wiley & Sons; 2010. p. 208. [Google Scholar]
- 3.Lim DA, Alvarez-Buylla A. The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb Perspect Biol. 2016;8(5) doi: 10.1101/cshperspect.a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song J, et al. Modification of hippocampal circuitry by adult neurogenesis. Dev Neurobiol. 2012;72(7):1032–43. doi: 10.1002/dneu.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christian KM, Song H, Ming GL. Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci. 2014;37:243–62. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valley MT, et al. Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Front Neurosci. 2009;3:51. doi: 10.3389/neuro.22.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun S, et al. Stress-Induced Anxiety- and Depressive-Like Phenotype Associated with Transient Reduction in Neurogenesis in Adult Nestin-CreERT2/Diphtheria Toxin Fragment A Transgenic Mice. PLoS One. 2016;11(1):e0147256. doi: 10.1371/journal.pone.0147256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakamoto M, et al. Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc Natl Acad Sci U S A. 2011;108(20):8479–84. doi: 10.1073/pnas.1018782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Y, Zhang W, Yang P. Current states of endogenous stem cells in adult spinal cord. J Neurosci Res. 2015;93(3):391–8. doi: 10.1002/jnr.23480. [DOI] [PubMed] [Google Scholar]
- 10.Bjornsson CS, et al. It takes a village: constructing the neurogenic niche. Dev Cell. 2015;32(4):435–46. doi: 10.1016/j.devcel.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swaab DF. Handbook of Clinical Neurology. 1. Vol. 80. Elsevier; 2004. Human Hypothalamus: Basic and Clinical Aspects. [Google Scholar]
- 12.Weiss S, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16(23):7599–609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pencea V, et al. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21(17):6706–17. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, et al. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192(2):251–64. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310(5748):679–83. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 16.Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505(2):209–20. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- 17.McNay DE, et al. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest. 2012;122(1):142–52. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce AA, Xu AW. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 2010;30(2):723–30. doi: 10.1523/JNEUROSCI.2479-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14(10):999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez EM, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 21.Bolborea M, Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36(2):91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DA, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15(5):700–2. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miranda-Angulo AL, et al. Rax regulates hypothalamic tanycyte differentiation and barrier function in mice. J Comp Neurol. 2014;522(4):876–99. doi: 10.1002/cne.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvatierra J, et al. The LIM Homeodomain Factor Lhx2 Is Required for Hypothalamic Tanycyte Specification and Differentiation. J Neurosci. 2014;34(50):16809–20. doi: 10.1523/JNEUROSCI.1711-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pak T, et al. Rax-CreERT2 knock-in mice: a tool for selective and conditional gene deletion in progenitor cells and radial glia of the retina and hypothalamus. PLoS One. 2014;9(4):e90381. doi: 10.1371/journal.pone.0090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haan N, et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013;33(14):6170–80. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma MS, et al. Multipotent stem cell factor UGS148 is a marker for tanycytes in the adult hypothalamus. Mol Cell Neurosci. 2015;65:21–30. doi: 10.1016/j.mcn.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Campbell JN, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484–496. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen R, et al. Single-Cell RNA-Seq Reveals Hypothalamic Cell Diversity. Cell Rep. 2017;18(13):3227–3241. doi: 10.1016/j.celrep.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Martin M, et al. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci. 2010;31(9):1533–48. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- 31.Cifuentes M, et al. A comparative analysis of intraperitoneal versus intracerebroventricular administration of bromodeoxyuridine for the study of cell proliferation in the adult rat brain. J Neurosci Methods. 2011;201(2):307–14. doi: 10.1016/j.jneumeth.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Robins SC, et al. alpha-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun. 2013;4:2049. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- 33.Chaker Z, et al. Hypothalamic neurogenesis persists in the aging brain and is controlled by energy-sensing IGF-I pathway. Neurobiol Aging. 2016;41:64–72. doi: 10.1016/j.neurobiolaging.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Mirzadeh Z, et al. Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat Commun. 2017;8:13759. doi: 10.1038/ncomms13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yulyaningsih E, et al. Acute Lesioning and Rapid Repair of Hypothalamic Neurons outside the Blood-Brain Barrier. Cell Rep. 2017;19(11):2257–2271. doi: 10.1016/j.celrep.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robins SC, et al. Evidence for NG2-glia derived, adult-born functional neurons in the hypothalamus. PLoS One. 2013;8(10):e78236. doi: 10.1371/journal.pone.0078236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Migaud M, et al. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci. 2010;32(12):2042–52. doi: 10.1111/j.1460-9568.2010.07521.x. [DOI] [PubMed] [Google Scholar]
- 38.Jourdon A, et al. Prss56, a novel marker of adult neurogenesis in the mouse brain. Brain Struct Funct. 2016;221(9):4411–4427. doi: 10.1007/s00429-015-1171-z. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida S, Kato T, Kato Y. Regulatory System for Stem/Progenitor Cell Niches in the Adult Rodent Pituitary. Int J Mol Sci. 2016;17(1) doi: 10.3390/ijms17010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405(6789):951–5. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 41.Arvidsson A, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 42.Nakatomi H, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–41. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 43.Breunig JJ, et al. Everything that glitters isn't gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 2007;1(6):612–27. doi: 10.1016/j.stem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Dimou L, et al. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28(41):10434–42. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang SH, et al. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68(4):668–81. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008;4(1):19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- 47.Zhu X, et al. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138(4):745–53. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishiyama A, Suzuki R, Zhu X. NG2 cells (polydendrocytes) in brain physiology and repair. Front Neurosci. 2014;8:133. doi: 10.3389/fnins.2014.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8(9):e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sousa-Ferreira L, de Almeida LP, Cavadas C. Role of hypothalamic neurogenesis in feeding regulation. Trends Endocrinol Metab. 2014;25(2):80–8. doi: 10.1016/j.tem.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol Behav. 2001;74(4–5):703–8. doi: 10.1016/s0031-9384(01)00613-8. [DOI] [PubMed] [Google Scholar]
- 52.Xu B, Xie X. Neurotrophic factor control of satiety and body weight. Nat Rev Neurosci. 2016;17(5):282–92. doi: 10.1038/nrn.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipovich L, et al. Activity-dependent Human Brain Coding/Non-coding Gene Regulatory Networks. Genetics. 2012 doi: 10.1534/genetics.112.145128. 2012/09/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu AW, et al. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3(12):e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gouaze A, et al. Cerebral cell renewal in adult mice controls the onset of obesity. PLoS One. 2013;8(8):e72029. doi: 10.1371/journal.pone.0072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams LM, et al. The development of diet-induced obesity and glucose intolerance in C57BL/6 mice on a high-fat diet consists of distinct phases. PLoS One. 2014;9(8):e106159. doi: 10.1371/journal.pone.0106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee DA, et al. Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice. Front Neurosci. 2014;8:157. doi: 10.3389/fnins.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7(10):1034–9. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 59.Bless EP, et al. Adult Neurogenesis in the Female Mouse Hypothalamus: Estradiol and High-Fat Diet Alter the Generation of Newborn Neurons Expressing Estrogen Receptor alpha. eNeuro. 2016;3(4) doi: 10.1523/ENEURO.0027-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bless EP, et al. Oestradiol and diet modulate energy homeostasis and hypothalamic neurogenesis in the adult female mouse. J Neuroendocrinol. 2014;26(11):805–16. doi: 10.1111/jne.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meijer MK, et al. Effect of restraint and injection methods on heart rate and body temperature in mice. Lab Anim. 2006;40(4):382–91. doi: 10.1258/002367706778476370. [DOI] [PubMed] [Google Scholar]
- 62.Fritsche LG, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45(4):433–9. doi: 10.1038/ng.2578. 439e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lenz KM, McCarthy MM. Organized for sex - steroid hormones and the developing hypothalamus. Eur J Neurosci. 2010;32(12):2096–104. doi: 10.1111/j.1460-9568.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murakami S, Arai Y. Neuronal death in the developing sexually dimorphic periventricular nucleus of the preoptic area in the female rat: effect of neonatal androgen treatment. Neurosci Lett. 1989;102(2–3):185–90. doi: 10.1016/0304-3940(89)90076-1. [DOI] [PubMed] [Google Scholar]
- 65.Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138(3):929–38. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Ahmed EI, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11(9):995–7. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohr MA, DonCarlos LL, Sisk CL. Inhibiting Production of New Brain Cells during Puberty or Adulthood Blunts the Hormonally Induced Surge of Luteinizing Hormone in Female Rats. eNeuro. 2017;4(5) doi: 10.1523/ENEURO.0133-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lizcano F, Guzman G. Estrogen Deficiency and the Origin of Obesity during Menopause. Biomed Res Int. 2014;2014:757461. doi: 10.1155/2014/757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giles ED, Jackman MR, MacLean PS. Modeling Diet-Induced Obesity with Obesity-Prone Rats: Implications for Studies in Females. Front Nutr. 2016;3:50. doi: 10.3389/fnut.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hazlerigg D. The evolutionary physiology of photoperiodism in vertebrates. Prog Brain Res. 2012;199:413–22. doi: 10.1016/B978-0-444-59427-3.00023-X. [DOI] [PubMed] [Google Scholar]
- 71.Peirson SN, et al. Light and the laboratory mouse. J Neurosci Methods. 2017 doi: 10.1016/j.jneumeth.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5(1):101–9. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- 73.Kirn JR, et al. Fate of new neurons in adult canary high vocal center during the first 30 days after their formation. J Comp Neurol. 1999;411(3):487–94. [PubMed] [Google Scholar]
- 74.Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214(4527):1368–70. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 75.Nottebohm F, et al. The life span of new neurons in a song control nucleus of the adult canary brain depends on time of year when these cells are born. Proc Natl Acad Sci U S A. 1994;91(17):7849–53. doi: 10.1073/pnas.91.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang L, DeVries GJ, Bittman EL. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J Neurobiol. 1998;36(3):410–20. doi: 10.1002/(sici)1097-4695(19980905)36:3<410::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 77.Bartness TJ, Wade GN. Photoperiodic control of seasonal body weight cycles in hamsters. Neurosci Biobehav Rev. 1985;9(4):599–612. doi: 10.1016/0149-7634(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 78.Migaud M, Butrille L, Batailler M. Seasonal regulation of structural plasticity and neurogenesis in the adult mammalian brain: focus on the sheep hypothalamus. Front Neuroendocrinol. 2015;37:146–57. doi: 10.1016/j.yfrne.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Migaud M, et al. Seasonal changes in cell proliferation in the adult sheep brain and pars tuberalis. J Biol Rhythms. 2011;26(6):486–96. doi: 10.1177/0748730411420062. [DOI] [PubMed] [Google Scholar]
- 80.Batailler M, et al. DCX-expressing cells in the vicinity of the hypothalamic neurogenic niche: a comparative study between mouse, sheep, and human tissues. J Comp Neurol. 2014;522(8):1966–85. doi: 10.1002/cne.23514. [DOI] [PubMed] [Google Scholar]
- 81.Batailler M, et al. Sensitivity to the photoperiod and potential migratory features of neuroblasts in the adult sheep hypothalamus. Brain Struct Funct. 2016;221(6):3301–14. doi: 10.1007/s00429-015-1101-0. [DOI] [PubMed] [Google Scholar]
- 82.Butruille L, et al. Seasonal reorganization of hypothalamic neurogenic niche in adult sheep. Brain Struct Funct. 2017 doi: 10.1007/s00429-017-1478-z. [DOI] [PubMed] [Google Scholar]
- 83.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1207–28. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- 84.Ramsay DS, Woods SC. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol Rev. 2014;121(2):225–47. doi: 10.1037/a0035942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrison SF. Central control of body temperature. F1000Res. 2016:5. doi: 10.12688/f1000research.7958.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horowitz M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp Biochem Physiol A Mol Integr Physiol. 2002;131(3):475–83. doi: 10.1016/s1095-6433(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 87.Matsuzaki K, et al. Proliferation of neuronal progenitor cells and neuronal differentiation in the hypothalamus are enhanced in heat-acclimated rats. Pflugers Arch. 2009;458(4):661–73. doi: 10.1007/s00424-009-0654-2. [DOI] [PubMed] [Google Scholar]
- 88.Matsuzaki K, et al. Neural progenitor cell proliferation in the hypothalamus is involved in acquired heat tolerance in long-term heat-acclimated rats. PLoS One. 2017;12(6):e0178787. doi: 10.1371/journal.pone.0178787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghosh S, Hui SP. Regeneration of Zebrafish CNS: Adult Neurogenesis. Neural Plast. 2016;2016:5815439. doi: 10.1155/2016/5815439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37(2):267–74. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med. 2010;16(4):403–5. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shimogori T, et al. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13(6):767–75. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 94.Lazarov O, et al. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33(12):569–79. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marlatt MW, et al. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol. 2012;72(6):943–52. doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noguchi T, et al. Effects of voluntary exercise and L-arginine on thrombogenesis and microcirculation in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1999;26(4):330–5. doi: 10.1046/j.1440-1681.1999.03037.x. [DOI] [PubMed] [Google Scholar]
- 97.Niwa A, et al. Voluntary exercise induces neurogenesis in the hypothalamus and ependymal lining of the third ventricle. Brain Struct Funct. 2016;221(3):1653–66. doi: 10.1007/s00429-015-0995-x. [DOI] [PubMed] [Google Scholar]
- 98.Klempin F, Kempermann G. Adult hippocampal neurogenesis and aging. Eur Arch Psychiatry Clin Neurosci. 2007;257(5):271–80. doi: 10.1007/s00406-007-0731-5. [DOI] [PubMed] [Google Scholar]
- 99.Zoli M, et al. Age-related alterations in tanycytes of the mediobasal hypothalamus of the male rat. Neurobiol Aging. 1995;16(1):77–83. doi: 10.1016/0197-4580(95)80010-o. [DOI] [PubMed] [Google Scholar]
- 100.Brawer JR, Walsh RJ. Response of tanycytes to aging in the median eminence of the rat. Am J Anat. 1982;163(3):247–56. doi: 10.1002/aja.1001630305. [DOI] [PubMed] [Google Scholar]
- 101.Koopman ACM, Taziaux M, Bakker J. Age-related changes in the morphology of tanycytes in the human female infundibular nucleus/median eminence. J Neuroendocrinol. 2017;29(5) doi: 10.1111/jne.12467. [DOI] [PubMed] [Google Scholar]
- 102.Chaker Z, et al. Suppression of IGF-I signals in neural stem cells enhances neurogenesis and olfactory function during aging. Aging Cell. 2015;14(5):847–56. doi: 10.1111/acel.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–4. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith LK, et al. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21(8):932–7. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsuzaki K, et al. Aging attenuates acquired heat tolerance and hypothalamic neurogenesis in rats. J Comp Neurol. 2015;523(8):1190–201. doi: 10.1002/cne.23732. [DOI] [PubMed] [Google Scholar]
- 106.Goldman D. Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15(7):431–42. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lenkowski JR, Raymond PA. Muller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014;40:94–123. doi: 10.1016/j.preteyeres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wan J, Goldman D. Retina regeneration in zebrafish. Curr Opin Genet Dev. 2016;40:41–47. doi: 10.1016/j.gde.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chohan A, et al. Muller stem cell dependent retinal regeneration. Clin Chim Acta. 2017;464:160–164. doi: 10.1016/j.cca.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 110.Bringmann A, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 111.Iandiev I, et al. Atypical gliosis in Muller cells of the slowly degenerating rds mutant mouse retina. Exp Eye Res. 2006;82(3):449–57. doi: 10.1016/j.exer.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 112.Chang ML, et al. Reactive changes of retinal astrocytes and Muller glial cells in kainate-induced neuroexcitotoxicity. J Anat. 2007;210(1):54–65. doi: 10.1111/j.1469-7580.2006.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Melo J, et al. Injury-independent induction of reactive gliosis in retina by loss of function of the LIM homeodomain transcription factor Lhx2. Proc Natl Acad Sci U S A. 2012;109(12):4657–62. doi: 10.1073/pnas.1107488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Furukawa T, et al. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26(2):383–94. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 115.Roesch K, et al. The transcriptome of retinal Muller glial cells. J Comp Neurol. 2008;509(2):225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nelson BR, et al. Genome-wide analysis of Muller glial differentiation reveals a requirement for Notch signaling in postmitotic cells to maintain the glial fate. PLoS One. 2011;6(8):e22817. doi: 10.1371/journal.pone.0022817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bernardos RL, et al. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27(26):7028–40. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12(11):1101–7. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Powell C, Elsaeidi F, Goldman D. Injury-dependent Muller glia and ganglion cell reprogramming during tissue regeneration requires Apobec2a and Apobec2b. J Neurosci. 2012;32(3):1096–109. doi: 10.1523/JNEUROSCI.5603-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Conner C, et al. Repressing notch signaling and expressing TNFalpha are sufficient to mimic retinal regeneration by inducing Muller glial proliferation to generate committed progenitor cells. J Neurosci. 2014;34(43):14403–19. doi: 10.1523/JNEUROSCI.0498-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wan J, et al. Retinal injury, growth factors, and cytokines converge on beta-catenin and pStat3 signaling to stimulate retina regeneration. Cell Rep. 2014;9(1):285–97. doi: 10.1016/j.celrep.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Powell C, et al. Zebrafish Muller glia-derived progenitors are multipotent, exhibit proliferative biases and regenerate excess neurons. Sci Rep. 2016;6:24851. doi: 10.1038/srep24851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fischer AJ, et al. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002;22(21):9387–98. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fischer AJ, Bongini R. Turning Muller glia into neural progenitors in the retina. Mol Neurobiol. 2010;42(3):199–209. doi: 10.1007/s12035-010-8152-2. [DOI] [PubMed] [Google Scholar]
- 125.Gallina D, Todd L, Fischer AJ. A comparative analysis of Muller glia-mediated regeneration in the vertebrate retina. Exp Eye Res. 2014;123:121–30. doi: 10.1016/j.exer.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fischer AJ, et al. Different aspects of gliosis in retinal Muller glia can be induced by CNTF, insulin, and FGF2 in the absence of damage. Mol Vis. 2004;10:973–86. [PubMed] [Google Scholar]
- 127.Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4(3):247–52. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 128.Karl MO, et al. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008;105(49):19508–13. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Loffler K, et al. Age-dependent Muller glia neurogenic competence in the mouse retina. Glia. 2015;63(10):1809–24. doi: 10.1002/glia.22846. [DOI] [PubMed] [Google Scholar]
- 130.Pollak J, et al. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development. 2013;140(12):2619–31. doi: 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ueki Y, et al. Transgenic expression of the proneural transcription factor Ascl1 in Muller glia stimulates retinal regeneration in young mice. Proc Natl Acad Sci U S A. 2015;112(44):13717–22. doi: 10.1073/pnas.1510595112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jorstad NL, et al. Stimulation of functional neuronal regeneration from Muller glia in adult mice. Nature. 2017;548(7665):103–107. doi: 10.1038/nature23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thomas JL, et al. Reactive gliosis in the adult zebrafish retina. Exp Eye Res. 2016;143:98–109. doi: 10.1016/j.exer.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 134.Migaud M, Butrille L, Batailler M. Seasonal regulation of structural plasticity and neurogenesis in the adult mammalian brain: Focus on the sheep hypothalamus. Front Neuroendocrinol. 2014 doi: 10.1016/j.yfrne.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 135.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 136.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–70. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 138.Romanov RA, et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci. 2017;20(2):176–188. doi: 10.1038/nn.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Habib N, et al. Div-Seq: Single-nucleus RNA-Seq reveals dynamics of rare adult newborn neurons. Science. 2016;353(6302):925–8. doi: 10.1126/science.aad7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pellegrino G, et al. A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and grey mouse lemur (Microcebus murinus) J Comp Neurol. 2017 doi: 10.1002/cne.24376. [DOI] [PubMed] [Google Scholar]
- 141.Darmanis S, et al. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci U S A. 2015;112(23):7285–90. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22(2):334–47. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gorsuch RA, et al. Sox2 regulates Muller glia reprogramming and proliferation in the regenerating zebrafish retina via Lin28 and Ascl1a. Exp Eye Res. 2017;161:174–192. doi: 10.1016/j.exer.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Close JL, Gumuscu B, Reh TA. Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling. Development. 2005;132(13):3015–26. doi: 10.1242/dev.01882. [DOI] [PubMed] [Google Scholar]
- 145.Todd L, et al. BMP- and TGFbeta-signaling regulate the formation of Muller glia-derived progenitor cells in the avian retina. Glia. 2017;65(10):1640–1655. doi: 10.1002/glia.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]