Abstract
Primary Objective:
Impairments in attention following traumatic brain injury (TBI) can significantly impact recovery and rehabilitation effectiveness. This study investigated the multi-faceted construct of selective attention following TBI, highlighting the differences on visual nonsearch (focused attention) and search (divided attention) tasks.
Methods and Procedures:
Participants were 30 individuals with moderate to severe TBI who were tested acutely (i.e. following emergence from PTA) and 30 age and education matched controls. Participants were presented with visual displays that contained either two or eight items. In the focused attention, nonsearch condition, the location of the target (if present) was cued with a peripheral arrow prior to presentation of the visual displays. In the divided attention, search condition, no spatial cue was provided prior to presentation of the visual displays.
Main Outcomes and Results:
The results revealed intact focused, nonsearch, attention abilities in the acute phase of TBI recovery. In contrast, when no spatial cue was provided (divided attention condition), participants with TBI demonstrated slower visual search compared to the control group.
Conclusions:
The results of this study suggest that capitalizing on intact focused attention abilities by allocating attention during cognitively demanding tasks may help to reduce mental workload and improve rehabilitation effectiveness.
Keywords: Visual search, visual nonsearch, selective attention, closed head injury, deficits
Introduction
Many individuals who sustain a moderate to severe traumatic brain injury (TBI) complain of difficulties with attention [1,2]. Selective attention deficits, or the inability to filter out irrelevant information, have been documented as a particular problem following TBI. Such deficits can negatively impact rehabilitation effectiveness due to an individual’s increased distractibility, tendency to become overloaded when dealing with more than one thought at a time, and challenges with staying on task [3]. Given the complexity of selective attention, it has been examined within the context of several different visual search paradigms. However, because these paradigms appear to be measuring different components of selective attention, it is difficult to synthesize information about how selective attention is impacted following TBI.
For example, when searching the visual environment for a target, individuals rely heavily on selective attention to filter out irrelevant information. The ease with which visual search occurs, however, can be impacted by whether attention is first oriented to a specific direction or location. Consider searching for a friend in a room full of people. One needs to carefully scan the entire environment and ignore the other people in the room until the person is found. However, the searching process can be made easier and more efficient if someone first points out that the friend is located in a specific area of the room, allowing one to focus attention to that area first. These two scenarios demonstrate separate, but related selective attention components: visual search and visual nonsearch abilities (respectively). The former is often referred to as divided attention, which occurs when multiple inputs must be monitored to determine where to direct focus [4,5]. The latter is often referred to as focused attention, which occurs when one knows in advance which inputs are relevant to task performance and can ignore distractors [6]. However, because these constructs are not always well defined, researchers frequently intermix these terms [6,7,8]. In this study, we use visual search and visual nonsearch tasks to evaluate both divided and focused attention abilities in the acute phase of recovery from moderate to severe TBI.
Due to methodological differences, inconsistencies in defining attentional constructs, and study limitations, research on how selective attention is impacted following TBI has resulted in mixed study findings. Many studies use visual search tasks that emphasize inhibition of responses to examine selective attention, such as the Stroop colour-word task and go-no-go paradigms. Researchers have argued that individuals with TBI who take longer than controls to complete these tasks have impaired selective attention because they cannot efficiently filter out or inhibit irrelevant information (e.g. the written name of the colour on the Stroop colour-word task). Although several studies have found adults with TBI perform poorer on tasks of inhibition compared to control groups both acutely (< 2 months post-injury) and at long-term follow-up [3,9, 10,11], others have found no significant differences [12,13].
Researchers have also measured selective attention by examining completion times for paper and pencil visual search tasks. Research has shown that adults in the chronic phase of TBI demonstrated impaired selective attention abilities on the TEA map search and telephone search subtests [3,14]. Similarly, participants with TBI (2–69 months post-injury) demonstrated impaired performance on the Paced Cancellation Task and Ruff 2 and 7 compared to controls [3,15], suggesting deficits in selective attention both within the first year of recovery and extending into the chronic phase of TBI.
These inhibition and visual search tasks are, however, known to involve other processes such as interference control and processing speed, rather than selective attention exclusively [16]. Indeed, several studies have demonstrated that once processing speed is controlled for in studies using an inhibition task as a measure of selective attention, significant differences dissipate [13,17,18]. Thus, when making suppositions about how selective attention is impacted following TBI using the results from these studies, caution needs to be exercised.
Researchers have also used visual nonsearch tasks to examine selective attention. For example, Posner [19] developed the Covert Orienting of Attention Task (COAT). The COAT uses visual stimuli via an LED display unit in a dimmed room to present valid, neutral, or invalid cues to focus attention. A cost benefit analysis based off of valid, neutral, and invalid cue responses is conducted to assess various aspects of attention. Studies evaluating the COAT found that although participants with chronic TBI had slower reaction times (RTs), there were no group differences in focused attention [20,21,22]. Therefore, the slower RT in the group with TBI was argued to be a result of slowed processing speed, not impaired attention [20,21,22]. In one study, Cremona-Meteyard and colleagues [23,24] did find impaired focused attention deficits in individuals with chronic TBI using the COAT; however, Bait and colleagues [20] argued that this might reflect the study low sample size.
To assess selective attention more thoroughly, paradigms that measure both focused and divided components of attention have been developed. The Multiple Choice Reaction Time Tests (MCRT) is a computerized assessment in which participants are asked to press a button when the target shape appeared [25]. Two different versions of this test were created, the MCRT complex and MCRT redundant, to measure divided and focused attention, respectively. The MCRT redundant was simpler than the MCRT complex because the nontarget stimuli did not contain any of the states (e.g. colour, line orientation, shape) that the target items contained, whereas the MCRT complex nontarget stimuli did. Using this task, Stuss and colleagues [25] found deficits in both focused and divided attention in individuals with TBI from 2–144 months post-injury. In contrast, Schmitter-Edgecombe and Kibby [26] employed a task that included a search condition and a nonsearch condition and found that participants with chronic TBI (> 1 year post-injury) had intact performance on the simple nonsearch task when discriminability between targets and distractors was high, while deficits were found when the nonsearch task was made more difficult by reducing the discriminability between targets and distractors. Impairments were also found in both search conditions, but group differences were more pronounced when the discriminability between targets and distractors was more difficult [26].
Although the aforementioned studies have improved our understanding of selective attention, a number of study limitations remain to be addressed. The tasks used to assess focused and divided attention often measured other aspects of cognition, such as processing speed, which was not always taken into consideration when evaluating the results of many of the studies. Furthermore, the majority of studies examined visual search tasks (divided attention), rather than visual nonsearch tasks (focused attention) and no research has been conducted on visual nonsearch tasks in the earliest phase of recovery from TBI. Thus, the current literature does not provide sufficient evidence to draw conclusions about whether visual nonsearch abilities are impacted in the acute phase of TBI and how visual nonsearch abilities compare to visual search abilities in this early phase of recovery.
In this study, computerized visual search and visual nonsearch tasks were used to measure both focused and divided attention abilities in the acute phase of recovery (i.e. within the first two months of emergence from post-traumatic amnesia; PTA) from a moderate to severe TBI. Participants with TBI were tested while at an inpatient rehabilitation facility, and their performance compared to demographically matched control participants. The computerized tasks required participants to respond to relevant stimuli, while ignoring distractors. In the focused attention condition, participant’s attention was first oriented to the location of the target stimuli, while in the divided attention condition no orientation of attention was provided. The amount of distractors in each trial was either two or eight. Based on prior literature [3,15,14], we expected that the participants with TBI would be slower to search the visual displays in the visual search condition compared to controls. We were especially interested in whether participants with TBI would be able to make use of the location cues and effectively ignore distracting stimuli in the focused attention condition (i.e. visual nonsearch condition).
Method
Participants
Thirty participants with TBI (20 males, 10 females) and 30 matched control participants (18 males, 12 females) were tested. Participants with TBI for this study were recruited from a rehabilitation program in the Pacific Northwest and were a subset of participants who were included in a larger study on the recovery of visual search processes [27] and who had also received the focused visual attention task at baseline. In return for their participation, participants with TBI received feedback regarding their cognitive functioning. Participants were excluded from the study if they had a history of multiple head injuries, preexisting neurological, psychiatric, or developmental disorder, recent (i.e. past year) history of treatment for substance abuse, a visual field deficit that would disrupt viewing of a computer screen, poor visual acuity at a distance of 16 inches (i.e. 20/60 vision using both eyes), or severe motor deficits in both upper limbs that would preclude accurate measurement of RT.
Participants with TBI had sustained a moderate to severe TBI according to their Glasgow Coma Scale score (GCS) [28]. Moderate TBI was defined by a GCS score between 9 and 12 (n = 4) or a score of more than 12 that also had positive neuroimaging findings and/or neurosurgery (n=7) [29,30,31]. Severe TBI was defined by a GCS score of 8 or less (n=19) [29, 32]. GCS was obtained from medical records and represented the lowest GSC score documented either at the scene of the accident or in the emergency room. All of the participants exhibited a period of extended PTA (M = 18.57 days; SD = 12.01 days; range = 3–50 days). Emergence from PTA was measured either prospectively (n=21) by repeated administration of the Galveston Orientation and Amnesia Test (GOAT) [33] and an anterograde memory test at the rehabilitation facility, or retrospectively (n=9) when PTA had resolved prior to arrival at the rehabilitation facility, by carefully assessing recall of post-injury memories until the evaluator was persuaded that the participant displayed normal continuous memory [34,35]. Participants were tested in the acute phase of recovery (time since injury (TSI) M = 38.70 days; SD = 20.67 days; range = 12–89 days). All injuries were closed-head, non-penetrating. The majority of head injuries (n=23) resulted from a motor vehicle or motorcycle accident, three were a result of a fall, one was a pedestrian in a motor vehicle accident, one resulted from an assault, and two from a sports related injury. One-third of the participants with TBI (n = 10) had neuroimaging data (i.e., CT or MRI) which described the presence of diffuse axonal injury. Neuroimaging data from the remaining participants with TBI revealed primarily frontal lobe damage either alone (n = 2) or in conjunction with other areas of the brain (n = 15), with one participant sustaining primarily parietal lobe injury and two other participants primarily temporal lobe damage.
Comparisons between the TBI and control groups revealed the groups were well matched on the demographic variables of age, education level and gender, X2 (1, n=60) = 0.29 (see table 1). In addition, the TBI and control groups were well matched on an estimate of premorbid intelligence based on the Barona Index equation [36], which takes into account six demographic variables (i.e. age, sex, race, education, occupation and region). As seen in table 1, consistent with sustaining a recent moderate to severe brain injury, participants with TBI performed more poorly than controls on cognitive measures assessing general mental status (Telephone Interview of Cognitive Status [TICS, 37]), attention and speeded processing (Symbol Digit Modalities Test [SDMT, 38]), verbal learning and memory (Rey Auditory Verbal Learning Test [RAVLT, 39]), and executive functioning (Controlled Oral Word Association Test [COWAT, PRW, 40]; Letter-Number sequencing sub-test from the Wechsler Adult Intelligence Scale-Third Edition [WAIS-III, 41]; Trail Making Test, Part B [42]).
Table 1.
Demographic Data and Mean Neuropsychological Summary Data for Traumatic Brain Injury (TBI) and Control Groups.
| Group |
||||||
|---|---|---|---|---|---|---|
| TBI (n=30) | Control (n=30) | |||||
| Variable or Test | M | SD | M | SD | t-test | Cohen’s d |
| Demographics | ||||||
| Age | 30.43 | 13.51 | 29.87 | 12.84 | 0.17 | 0.04 |
| Education (years) | 12.57 | 1.89 | 13.30 | 2.49 | −1.28 | 0.33 |
| Gender (% male) | 67% | 60% | ||||
| eFSIQ | 103.27 | 8.36 | 104.47 | 9.10 | −0.52 | 0.14 |
| Global Cognitive Status | ||||||
| TICS total score | 33.64† | 4.16 | 38.42‡ | 3.81 | −4.29* | 1.20 |
| Attention/Processing Speed | ||||||
| SDMT Oral total | 46.97 | 10.27 | 68.00 | 10.96 | −7.67* | 1.98 |
| SDMT Written total | 39.33 | 11.90 | 56.17 | 7.52 | −6.55* | 1.69 |
| Verbal Memory | ||||||
| RAVLT List Learning | 45.53 | 8.89 | 56.03 | 7.63 | −4.91* | 1.27 |
| RAVLT Delayed Recall | 7.70 | 3.51 | 11.37 | 3.06 | −4.31* | 1.11 |
| Executive Skills | ||||||
| WAIS-III LN Sequencing | 9.50 | 2.67 | 11.63 | 2.85 | −2.99* | 0.77 |
| COWAT | 25.00 | 9.72 | 42.00 | 11.23 | −6.27* | 1.62 |
| Trails B | 104.97 | 48.21 | 56.83 | 22.68 | 4.95* | 1.28 |
Note. Unless otherwise indicated, mean scores are raw scores. TICS = Telephone Interview for Cognitive Status; SDMT = Symbol Digit Modalities Test; RAVLT = Rey Auditory Verbal Learning Test; LN Sequencing= Letter Number Sequencing subtest of the Weschler Adult Intelligence Scale- Third Edition; COWAT= Controlled Oral Word Association Test (PRW).
p < .01
n = 28
n = 24
Apparatus and Stimuli
The stimuli were displayed on an IBM-compatible personal computer programmed with SuperLab Pro Beta Version Experimental Lab Software [43]. Stimuli consisted of circles and circles with intersecting vertical lines. Each stimulus item subtended a visual angle of two degrees. The stimulus items were situated at the vertices of an imaginary octagon centred at fixation with radius of five degrees. A circular configuration was chosen as it allows all locations to have the same properties [44]. The stimuli were black against a white background. The number of stimuli present in one display (set size) was either two or eight.
Procedure
This experiment was completed as part of a larger test battery that included standardized neuropsychological tests and other experimental measures [45]. The neuropsychological measures were administered using standardized instructions across two days of testing. The focused and divided attention measures were both administered on the second day of testing. Both tasks consisted of 128 test trials preceded by 16 practice trials. Participants received a self-paced rest break half way through each task. Four different conditions were created by manipulating the number of items in the visual display (2 or 8) and the response required (target-present or target-absent). For both tasks, each trial was signaled by a computer-generated auditory warning tone (medium pitch) presented simultaneously with a 400 ms fixation cross displayed in the centre of the screen.
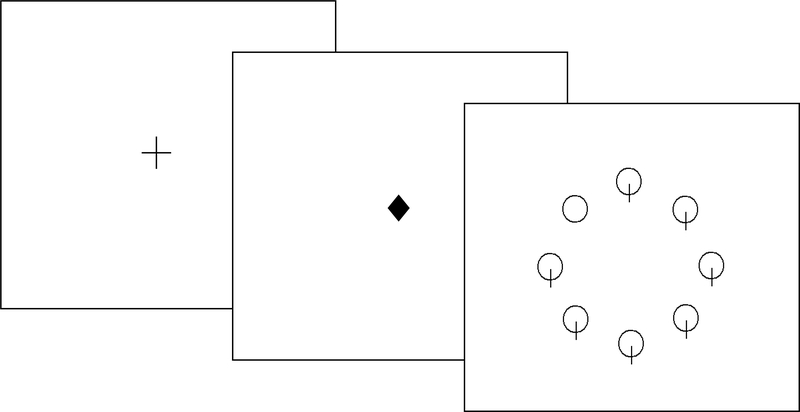
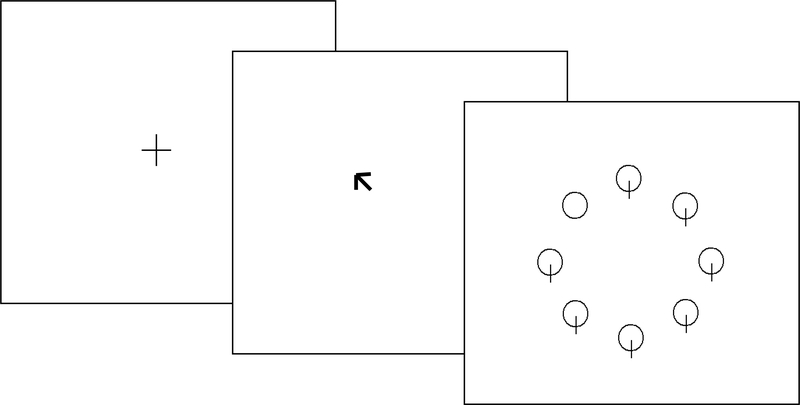
In the divided attention task, just prior to the presentation of the search display, the fixation cross changed to a central diamond for 200ms (see figure 1). Prior research has shown that a state of distributed attentional resources can be induced by presentation of an informationless, neutral cue (e.g. central diamond) prior to the visual display presentation [46]. However, in the focused attention task, a 200 ms arrow orienting the participant’s attention to the specific location that the target was going to appear preceded the display (see figure 2). Participants were told to focus on the location cued by the arrow and ignore all other information. The search display then appeared on the computer screen until the participant made a response. Participants were told to press the button labeled ‘yes’ if the target (i.e. circle without intersecting line) was present in the display and to press the button labeled ‘no’ if the target was absent from the display (see figure 1). Responses were made with the index and middle fingers of the participant’s dominant hand. To maximize RTs, participants kept their fingers resting on the response keys throughout the duration of the tasks. Half of the visual displays required target-present responses and the other half target-absent responses. Incorrect responses were indicated by a computer-generated feedback tone.
Figure 1.
Example of the divided attention stimuli with target-present.
Figure 2.
Example of the focused attention stimuli with target-present.
Analysis
SPSS statistical software was used for data analysis. RTs for error trials were removed from analysis. The data were trimmed by removing trials that were 2.5 standard deviations away from the individual means. For ease of interpretation, the raw data is presented; however, transformed RTs [i.e. log(RT)] were also analysed to examine the robustness of the interactions, normalize the data, and take effects of differences in processing speed into account [47, 48]. When the data did not yield identical findings, it is indicated in the results section.
Separate mixed-model ANOVAs with group (TBI, control) as the between subjects factor and display size (two, eight) and response type (target-present, target-absent) as the within-subjects factors were completed for RT and accuracy rates for the focused attention and divided attention tasks. Correlation analyses were performed for the TBI group to examine for relationships between target-present and target-absent search slope values and injury characteristics, demographic variables, and standardized neuropsychological tests (see table 1). The visual search slope values for the functions relating RT to the number of items in the visual display represented the increase in RT with additional distracters. Because of the large number of correlations conducted, a more conservative value of p < 0.01 was used to establish significance.
Focused Attention
Table 2 displays the mean RTs for correct responses as a function of group (TBI, control), display size (two, eight), and response type (target-present, target-absent). Analysis of the focused attention search RTs revealed that RTs were faster for the control group; F(1, 58) = 18.44, p < 0.01, ηp2= 0.24, for display size 2; F(1, 58) = 19.47, p < 0.01, ηp2= 0.25, and when the target was present; F(1, 58) = 46.67, p < 0.01, ηp2= 0.45. There was a significant interaction between display size and response type; F(1, 58) = 141.81, p < 0.01, ηp2= 0.17, which indicated that the difference between target-absent and target-present responses was greater for display size 8; t (58)= 3.07, p= 0.01, compared to display size 2; t (58)= 2.05, p= 0.04. There was also a significant interaction of response type by group; F(1, 58) = 5.72, p < 0.02, ηp2= 0.09, which reflected the finding that the participants with TBI had slower RTs for target-absent responses compared to target-present responses; t (58)= 2.62, p= 0.01, while the response type did not influence the RTs of the control participants; t (58)= 1.26, p= 0.22. No additional two-way interactions or three-way interactions reached significance, Fs<1. The log transformed data revealed a similar pattern of findings to the raw data, with the exception that the response type by group interaction only approached significance (p = 0.07). Of most importance, there was no significant group by display size interaction or three-way interaction for either analysis indicating that the TBI and control groups did not differ in their ability to focus attention to the target location. In addition, percent slowing between display sizes two and eight was minimal for both the TBI (8%) and control (5%) groups.
Table 2.
Mean Reaction Times, Standard Errors and Accuracy Rates as a Function of Group, Display Size, Response Type and Task (Log transformed RTs in parentheses)
| Focused Attention Visual Non-Search Task | ||||
| TBI (n = 30) | Control (n = 30) | |||
| Display Size | Display Size | |||
| Condition | 2 | 8 | 2 | 8 |
| Target-present | ||||
| M | 704 (6.53) | 727 (6.55) | 564 (6.32) | 570 (6.33) |
| SE | 25 (0.04) | 31 (0.04) | 25 (0.04) | 31 (0.04) |
| % Correct | 96.4 | 97.3 | 96.1 | 95.6 |
| Target-absent | ||||
| M | 781 (6.63) | 874 (6.73) | 593 (6.37) | 650 (6.45) |
| SE | 27 (0.04) | 42 (0.05) | 27 (0.04) | 42 (0.05) |
| % Correct | 96.6 | 97.3 | 97.5 | 96.6 |
| Divided Attention Visual Search Task | ||||
| TBI (n = 30) | Control (n = 30) | |||
| Display Size | Display Size | |||
| Condition | 2 | 8 | 2 | 8 |
| Target-present | ||||
| M | 810 (6.67) | 1089 (6.96) | 654 (6.47) | 850 (6.73) |
| SE | 29 (0.04) | 43 (0.04) | 29 (0.04) | 43 (0.04) |
| % Correct | 97.3 | 94.0 | 96.8 | 93.3 |
| Target-absent | ||||
| M | 901 (6.77) | 1767 (7.41) | 698 (6.53) | 1232 (7.06) |
| SE | 37 (0.04) | 102 (0.06) | 37 (0.04) | 102 (0.06) |
| % Correct | 98.2 | 97.5 | 97.0 | 97.1 |
A group by display size by response type ANOVA of accuracy rates revealed no significant main effects or interactions, Fs<3.2. The finding of no main effects of interactions suggests a similar level and pattern of response accuracy across groups (see table 2). Accuracy rates across conditions fell between 96.35%−97.29% for the TBI group and 95.63% - 97.50% for the control group.
Divided Attention
Analysis of the divided attention RTs revealed that RTs were faster for the control group; F(1, 58) = 16.55, p < 0.01, ηp2= 0.22, for display size 2; F(1, 58) = 185.30, p < 0.01, ηp2= 0.76, and when the target was present; F(1, 58) = 109.27, p < 0.01, ηp2= 0.65. There was a significant interaction between display size and response type; F(1, 58) = 120.47, p < 0.01, ηp2= 0.68, which indicated that the difference between target-absent and target-present responses was greater for display size 8; t (58)= 6.73, p= 0.01, compared to display size 2; t (58)= 2.03, p= 0.05. There was also a significant display size by group interaction; F(1, 58) = 9.07, p < 0.01, ηp2= 0.14, which reflected the finding that the addition of distractor stimuli increased the search rates of the RTs for participants with TBI more than the controls. In contrast to the nonsearch condition, percent slowing between display sizes two and eight in the search condition was large for the control group (54%), but even larger for the TBI group (67%). Furthermore, the response type by group interaction reached significance; F(1, 58) = 9.01, p < 0.01, ηp2= 0.13, as did the 3-way interaction between display size, response type, and group; F(1, 58) = 8.70, p < 0.01, ηp2= 0.13. This reflected the fact that the display size effect was larger in magnitude for the target-absent compared to the target-present trials and this pattern was more pronounced for the TBI group compared to controls (see table 2). The log transformed data revealed a similar pattern of findings to the raw data, except that the display size by group interaction (p = 0.09) and the 3-way interaction (p = 0.07) trended towards significance, but was not significant. However, this likely reflects a power issue because in a prior study with a larger sample size and this similar visual search task, we found a significant display size by group interaction and 3-way interaction with log transformed data [27].
Analysis of accuracy rates revealed significant main effects of display size; F(1, 58) = 17.431, p < 0.01, ηp2= 0.23, and response type; F(1, 58) = 23.16, p < 0.01, ηp2= 0.29. There was also a significant display size by response type interaction; F(1, 58) = 12.31, p < 0.01, ηp2= 0.18, which reflected a greater difference in response type accuracy with a display size of 8; t (58)= 3.60, p= 0.01, compared to a display size of 2; t (58)= 0.77, p= 0.45. Importantly, there were no main effects involving group suggesting a similar pattern of accuracy across groups. Accuracy rates across conditions fell between 94.0%−98.2% for the TBI group and 93.3% - 97.1% for the control group.
Correlations
For the TBI group, we conducted correlational analyses to determine whether the focused and divided visual search rates (defined by target-absent and target-present slope measures) exhibited a relationship with injury characteristics (i.e. GCS, PTA, TSI), demographic variables, and the standardized neuropsychological tasks administered in table 1. At a more conservative significance level of p < 0.01, no significant correlations emerged between the slope estimates and the standardized neuropsychological measures, rs between −0.29 and 0.17, with the exception of Trails B being positively correlated with the focused attention target-present slope; r=0.48, p < 0.01. There were also no significant correlations between the slope estimates and the demographic variables of age, education, gender and eFSIQ, rs between −0.44 and 0.29, or the injury characteristics of GCS, PTA, and TSI, rs between −0.31 and 0.42.
Discussion
Consistent with prior research on individuals with both acute and chronic TBI [e.g. 3,21,22, 25,26], we found impaired performance on the divided attention, search task in the acute recovery phase of TBI. This finding indicates that participants with TBI were more influenced by the display size manipulation when compared to healthy controls when participants had to search a visual display. Of interest to this study, the display size manipulation did not differentially impact performances between groups on the nonsearch task. This finding indicates that participants in the acute recovery phase of TBI did not demonstrate impaired focused attention, which suggests that this ability may remain relatively intact following TBI. Prior studies conducted with participants in the chronic (>1 year) phase of TBI have found similar results [e.g. 20,21,22,26].
The results of this study have several important implications to consider. First, the results highlight the importance of considering the different components of selective attention. As demonstrated by this study, as well as others [3,26], results differ depending on how selective attention is measured. The results of this study indicate that selective attention abilities are impacted differently based on whether or not attention is first allocated by the use of a spatial cue. Participants with TBI had difficulty ignoring distractors and/or searching the visual display when their attention was not first oriented by a spatial cue. In contrast, when attention was focused to the target location, participants with TBI were able to effectively ignore task-irrelevant information. It is thought that by first focusing attention, the individual’s limited-capacity attentional resources are allocated to the most task relevant information, which lessens processing demands [49,50]. Of note, target and distractors in this study were easily discriminable, which also likely lessened processing demands [26].
We found that participants with moderate to severe TBI demonstrated the greatest difficulty in selective attention when their attention was not first focused to a specific area. Several theoretical explanations can be drawn upon to explain the results of this study. It is possible that participants with TBI have more difficulty ignoring irrelevant task information in search situations when a spatial cue is not provided. Therefore, impaired performance on the divided attention task may reflect problems of inhibition because RT is slowed by an inability to inhibit the processing of distractor information. Alternatively, participants with TBI may have more difficulty with spatial localization and as a result their performance is impacted when they have to search for a target, rather than having their attentional resources first allocated to target information. Furthermore, the findings may provide additional evidence that an overall reduction in either the capacity or speed of attentional processes occurs following a moderate to severe TBI. For example, in the divided attention condition, attentional resources are being distributed throughout the visual field and both target and distractors are competing for attentional resources. This competition of attentional resources may result in an overall reduction in attentional capacity following TBI.
An additional finding from this study revealed that participants with TBI took longer to respond to target-absent trials compared to target-present trials in both the search and nonsearch conditions, while response type did not influence the control participants performance in the nonsearch condition. The results revealed that even when attention was allocated by a spatial cue, participants with TBI had difficulty recognizing when a target was not present. It appears that participants with TBI either experienced a problem with detecting the absence of a target or used a higher standard than controls for saying the target was not there [27]. Research on the aging population has also found similar difficulties with target-absent responses [51,52]; however, the underlying mechanism needs further investigation. This finding along with the finding that divided, but not focused, attention is impacted following TBI, adds evidence that there are specific aspects of the visual search process that are more impacted following TBI.
In regards to rehabilitation, selective attention deficits have been shown to limit intervention effectiveness due to increased distractibility, tendency to become overloaded, and challenges with staying on task [20]. Thus, the results of this study raise several implications for rehabilitation strategies. Based on the finding of intact performance in the focused attention condition, individuals with TBI can greatly benefit from spatial cues to allocate attentional resources before beginning a task. For example, locating a specific food item on a grocery shelf would be made easier if the approximate location of the item was first demonstrated. Also, it is important to note, that even when spatial cues are provided, RTs are generally slower in those with TBI and if the target item is not present (e.g. the specific food item is not located on any grocery shelf), it may take individuals with TBI longer to realize that the target is absent than to find it when it is present. Therefore, during rehabilitation, it is important to give individuals with TBI ample time to complete tasks that require visual search, especially if not all items to be searched for are present. Furthermore, investigating the different components of attention in the acute phase of TBI can provide a wealth of information that can guide rehabilitation strategies. This is especially true if clinicians can capitalize on intact attention abilities, such as focused attention, in order to reduce the cognitive demand of high mental workload tasks during rehabilitation. By first focusing attention, individuals with TBI can be fully engaged in the rehabilitation task at hand, increasing effectiveness.
It is important to note that our participants consisted of primarily Caucasian individuals and those with TBI suffered moderate to severe injuries that primarily resulted from a motor vehicle accident. Neuroimaging data also revealed a high proportion of participants with TBI sustained diffuse axonal injury and injury to the frontal lobes both alone and concomitant with other areas of the brain. Thus, the findings may not generalize to other ethnic populations or individuals with either mild TBIs or TBIs that have other underlying mechanisms (e.g. a blast injury) or injury locations. There are also components of our task, such as, stimulus onset asynchrony between cue and visual display [53] and saliency and distinctiveness of target compared to distractors [26] that may not generalize to other selective attention tasks. Similarly, further investigation of how this type of simple attention task is related to real world activities that utilize similar components of selective attention is needed.
In conclusion, differences in selective attention between individuals who suffered a moderate to severe TBI and control participants are dependent on whether visual search is required. In the acute phase of recovery, it appears that focused attention, or the ability to discriminate a target item from distractors when a spatial cue is provided and is relatively distinct, is intact. In contrast, divided attention, or the ability to discriminate target items from distractor items when no spatial cue is provided, is impaired in the acute phase of TBI. Findings from this study suggest that, during the rehabilitation process, individuals with TBI may require more time to complete visual search tasks, particularly when target items are absent. Furthermore, the visual search performance of participants with TBI will benefit from spatial cues that serve to orient attention to the target location.
Acknowledgements:
This study was supported by the National Institute of Neurological Disorders and Stroke under grant #R01 NS47690. We would like to thank Randi McDonald, Shital Pavawalla, Jonathan Anderson, Jennifer McWilliams, Michelle Nuegen, Matthew Wright, and Ellen Woo for their support in coordinating data collection. We would also like to thank the TBI participants and the members of the Head Injury Research Team for their help in collecting and scoring the data.
Footnotes
Declaration of Interest: The authors report no declarations of interest.
References
- 1.McKinlay WW, Brooks DN, Bond MR, Martinage DP, Marshall MM. The short term outcome of severe blunt head injury as reported by relatives of the injured persons. Journal of Neurology, Neurosurgery, and Psychiatry 1981;44:527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoccolotti P, Matano A, Deloche G, Cantagallo A, Passadori A, Leclercq M…Zimmermann P. Patterns of attentional impairment following closed head injury: A collaborative European study. Cortex 2000;36:93–107. [DOI] [PubMed] [Google Scholar]
- 3.Bate AJ, Mathias JL, Crawford JR. Performance on the Test of Everyday Attention and standard tests of attention following severe traumatic brain injury. The Clinical Neuropsychologist 2001;15(3):405–22. [DOI] [PubMed] [Google Scholar]
- 4.Davies DR, Jones DM, Taylor A. Selective- and sustained-attention tasks: Individual and group differences In: Parasuraman R, Davies DR, editors. Varieties of attention. New York: Academic Press; 1984. P. 395–447. [Google Scholar]
- 5.Plude DJ, Doussard-Roosevelt JA. Aging, selective attention, and feature integration. Psychology and Aging 1990;4:98–105. [DOI] [PubMed] [Google Scholar]
- 6.Plude DJ, Hoyer WJ. Attention and performance: Identifying and localizing age deficits In Charness N, editors. Aging and human performance. New York: John Wiley & Sons; 1985. p. 47–99. [Google Scholar]
- 7.Kahneman D. Attention and memory. Englewood Cliffs, NJ: Prentice Hall; 1973. [Google Scholar]
- 8.Shiffrin RM, Schneider W. Controlled and automatic information processing: II. Perceptual learning, automatic at- tending and a general theory. Psychological Review 1977;84:127–90. [Google Scholar]
- 9.Belmont A, Agar N, Azouvi P. Subjective fatigue, mental effort, and attention deficits after severe traumatic brain injury. Neurorehabilitation and Neural Repair 2009;23(9):939–44. [DOI] [PubMed] [Google Scholar]
- 10.Godefroy O, Rousseaux M. Divided and focused attention in patients with lesion of the prefrontal cortex. Brain and Cognition 1996;30:155–74. [DOI] [PubMed] [Google Scholar]
- 11.Spikman JM, Van Zomeren AH, Deelman BG. Deficits of attention after closed-head injury: slowness only? Journal of Clinical and Experimental Neuropsychology 1996;18:755–67. [DOI] [PubMed] [Google Scholar]
- 12.Ponsford J, Kinsella G. Attentional deficits following closed-head injury. Journal of Clinical and Experimental Neuropsychology 1992;14(5):822–38. [DOI] [PubMed] [Google Scholar]
- 13.Felmingham KL, Baguley IJ, Green AM. Effects of diffuse axonal injury on speed of information processing following severe traumatic brain injury. Neuropsychology 2004;18(3):564–71. [DOI] [PubMed] [Google Scholar]
- 14.Ziino C, Ponsford. Selective attention deficits and subjective fatigue following traumatic brain injury. Neuropsychology 2006;20(3):383–90. [DOI] [PubMed] [Google Scholar]
- 15.Michael GA, Masson M, Robert E, Bacon E, Desert JF, Rhein F…Colliot P. Disturbances of selective attention in traumatic brain injury and schizophrenia: What is common and what is different? Psychologie Francaise 2015;60:387–402. [Google Scholar]
- 16.Marchetta NDJ, Hurks PPM, De Sonneville LMJ, Krabbendam L, Jolles J. Sustained and focused attention deficits in adult ADHD. Journal of Attention Disorders 2008;11(6):664–76. [DOI] [PubMed] [Google Scholar]
- 17.Dymowski AR, Owens JA, Ponsford JL, Willmott C. Speed of processing and strategic control of attention after traumatic brain injury. Journal of Clinical and Experimental Neuropsychology 2015;37(10):1024–35. [DOI] [PubMed] [Google Scholar]
- 18.Rios M, Perianez JA, Munoz-Cespedes JM. Attentional control and slowness of information processing after severe traumatic brain injury. Brain Injury 2004;18(3):257–72. [DOI] [PubMed] [Google Scholar]
- 19.Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology 1980;41A:19–45. [DOI] [PubMed] [Google Scholar]
- 20.Bate AJ, Mathias JL, Crawford JR. The covert orienting of visual attention following severe traumatic brain injury. Journal of Clinical and Experimental Neuropsychology 2001;23(3):386–98. [DOI] [PubMed] [Google Scholar]
- 21.Pavese A, Heidrich A, Sohlberg MM, Laughlin KA, Posner MI. Pathologies of attentional networks following traumatic brain injury. Manuscript submitted for publication 2000. [Google Scholar]
- 22.Sandson J, Crosson B, Posner MI, Barco PP, Velozo CA, Brobeck TC. Attentional imbalances following head injury In: Williams JM, Long CJ, editors. Cognitive approaches to neuropsychology. New York: Plenum; 1988. [Google Scholar]
- 23.Cremona-Meteyard SL, Clark CR, Wright MJ, Geffen GM. Covert orientation of visual attention after closed head injury. Neuropsychologia 1992;30(2):123–32. [DOI] [PubMed] [Google Scholar]
- 24.Cremona-Meteyard SL, Geffen GM. Persistent visuospatial attention deficits following mild head injury in Australian rules football players. Neuropsychologia 1994;32(6):649–62. [DOI] [PubMed] [Google Scholar]
- 25.Stuss DT, Stethem LL, Hugenholtz H, Picton T, Pivik J, Richard MT. Reaction time after head injury: Fatigue, divided and focused attention, and consistency of performance. Journal of Neurology, Neurosurgery, and Psychiatry 1989;52:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitter-Edgecombe M, Kibby MK. Visual selective attention after severe closed head injury. Journal of the International Neuropsychological Society 1998;4:144–59. [DOI] [PubMed] [Google Scholar]
- 27.Schmitter-Edgecombe M, Robertson K. Recovery of Visual Search following Moderate to Severe Traumatic Brain Injury. Journal of Clinical and Experimental Neuropsychology 2015;37(2):162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2(7872):81–4. [DOI] [PubMed] [Google Scholar]
- 29.O’Neil ME, Calson K, Strozbach D, Brenner L, Freeman M, Quinones A…Kansagar D. Complications of mild traumatic brain injury in veterans and military personnel: A systematic review. Department of Veterans Affairs (US) 2013. [PubMed] [Google Scholar]
- 30.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery 1990;27:422–428. [DOI] [PubMed] [Google Scholar]
- 31.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcome after traumatic brain injury in children: Behavior and achievement. Neuropsychology 2002;16:15–27 [DOI] [PubMed] [Google Scholar]
- 32.Andriessen TMJC, Horn J, Frnaschman G, Van DN, Haitsma I, Jacobs B…Vos PE. Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: A prospective multicenter study. Journal of Neurotrauma 2011;28(10):2019–2031. [DOI] [PubMed] [Google Scholar]
- 33.Levin HS, O’Donnell VM, Grossman RG. The Galveston Orientation and Amnesia Test: A practical scale to assess cognition after head injury. Journal of Nervous and Mental Disease 1979;167(11):675–84. [DOI] [PubMed] [Google Scholar]
- 34.King NS, Crawford S, Wenden FJ, Moss N, Wade DT. Interventions and service need following mild and moderate head injury: The Oxford Head Injury Service. Clinical Rehabilitation 1997;11:13–27. [DOI] [PubMed] [Google Scholar]
- 35.McMillan TM, Jongen ELMM, Greenwood RJ. Assessment of post-traumatic amnesia after severe closed head injury: Retrospective or prospective? Journal of Neurology, Neurosurgery, & Psychiatry 1996;60(4):422–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barona A, Reynolds CR, Chastain, R. A demographically based index of premorbid intelligence for the WAIS-R. Journal of Consulting and Clinical Psychology 1984;52:885–7. [Google Scholar]
- 37.Brandt J, Folstein MF. Telephone Interview for Cognitive Status. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- 38.Smith A Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services; 1991. [Google Scholar]
- 39.Majdan A, Sziklas V, Jones-Gotman M. Performance of healthy subjects and patients with resection from the anterior temporal lobe on matched tests of verbal and visuoperceptual learning. Journal of Clinical and Experimental Neuropsychology 1996;18(3):416–30. [DOI] [PubMed] [Google Scholar]
- 40.Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. 3rd ed. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- 41.Wechsler D The WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 42.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills 1958;8:271–6. [Google Scholar]
- 43.Cedrus Corporation. SuperLab Pro 1.75 [Computer program] San Pedro, CA; 1999. [Google Scholar]
- 44.Yantis S, Johnson. Mechanisms of attentional priority. Journal of Exp Psychol Hum Percept Perform 1990;16(4):812–25. [DOI] [PubMed] [Google Scholar]
- 45.Schmitter-Edgecombe M, Rueda AD. Time estimation and episodic memory following traumatic brain injury. Journal of Clinical and Experimental Neuropsychology 2008;30:212–23. [DOI] [PubMed] [Google Scholar]
- 46.Eriksen CW, Yeh YY. Allocation of attention in the visual field. Journal of Experimental Psychology: Human Perception and Performance 1985;11(5):583–97. [DOI] [PubMed] [Google Scholar]
- 47.Van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genomics 2006;8(7):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faust ME, Balota DA, Spieler DH, Ferraro RF. Individual differences in information-processing rate and amount: Implication for group differences in response latency. Psychological Bulletin 1999;125(6):777–99. [DOI] [PubMed] [Google Scholar]
- 49., Johnston WA, Dark VJ. Selective attention In Rosenzweig MR, Porter LW, editors, Annual review of psychology. Palo Alto, CA: Annual Reviews; 1986, vol. 37, p. 43–75. [Google Scholar]
- 50.Wickens CD. Processing resources in attention In: Para- suraman R, Davies DR, editors. Varieties of attention. New York: Academic Press; 1984. p. 395–447. [Google Scholar]
- 51.Hommel B, Li KZ, Li SC. Visual search across the lifespan. Developmental Psychology 2004;40:545–58. [DOI] [PubMed] [Google Scholar]
- 52.Potter LM, Grealy MA, Elliott MA, Andres P. Aging and performance on an everyday-based visual search task. Acta Psychologica 2012;140:208–17. [DOI] [PubMed] [Google Scholar]
- 53.Colegate RL, Hoffman JE, Eriksen CW. Selective encoding from multielement visual displays. Perception and Psychophysics 1973;12:217–24. [Google Scholar]