Abstract
A large number of cardiovascular events are not prevented by current therapeutic regimens. In search for additional, innovative strategies, immune cells have been recognized as key players contributing to atherosclerotic plaque progression and destabilization. Particularly the role of innate immune cells is of major interest, following the recent paradigm shift that innate immunity, long considered to be incapable of learning, does exhibit immunological memory mediated via epigenetic reprogramming. Compelling evidence shows that atherosclerotic risk factors promote immune cell migration by pre-activation of circulating innate immune cells. Innate immune cell activation via metabolic and epigenetic reprogramming perpetuates a systemic low-grade inflammatory state in cardiovascular disease (CVD) that is also common in other chronic inflammatory disorders. This opens a new therapeutic area in which metabolic or epigenetic modulation of innate immune cells may result in decreased systemic chronic inflammation, alleviating CVD, and its co-morbidities.
Keywords: Immune cells, Epigenetic reprogramming, Trained immunity, Cardiovascular disease, Chronic inflammatory disease
Introduction
Cardiovascular diseases (CVD) are a major health challenge for modern societies. An estimated number of 17 million people die due to CVD each year, representing ≈30% of all deaths worldwide. The high burden of CVD is attributable to the increasing incidence of atherosclerosis, caused amongst others by worldwide adoption of the Western lifestyle.1 In parallel, the incidence of chronic inflammatory diseases (CID) such as rheumatoid arthritis (RA) is rising. Since CID are accompanied by a two- to three-fold higher CVD-risk,2 the increased CID prevalence further contributes to the overall CVD burden. Traditionally, risk factors for atherosclerosis are considered to be dyslipoproteinaemia, as well as smoking, hypertension, diabetes, and obesity. Subsequently, therapeutic measures have focused on lowering the most atherogenic cholesterol, predominantly low-density-lipoprotein cholesterol (LDL-C), which has successfully lowered CVD-risk by 25–35%.3 This success rate, however, also discloses a large residual risk not adequately addressed by current cholesterol-lowering treatment alone.
Atherosclerosis is a chronic inflammatory disease
Atherosclerosis, formerly considered a lipid storage disease, involves a chronic, low-grade inflammatory response of the arterial wall, initiated by lipid accumulation in the intimal layer.4 Low-density-lipoprotein-cholesterol or modified LDL-cholesterol can trigger pattern-recognition receptors, including toll-like receptors (TLRs).5 Subsequent activation and recruitment of innate immune cells contributes to plaque progression and eventually plaque destabilization.6 In more detail, it has been demonstrated that following accumulation of cholesterol in the arterial wall, the subendothelial lipids are modified leading to the formation of active signalling moieties. Particularly oxidized derivatives and cholesterol crystals7 trigger a variety of inflammatory pathways, immune cells and mediators8 that drive atherogenesis and co-morbidities of this disease. As the atherosclerotic lesion advances, the presence of immune cells in the lesion increases proportionally, creating a localized pro-inflammatory milieu within the subendothelial compartment.9 Cholesterol crystal uptake in macrophages for example promotes atherosclerosis and more importantly destabilization of the plaques via TLR-4 activation.5 Accumulation of cholesterol not only activates a pro-inflammatory response. Upon accumulation, the reverse cholesterol transporters ABCA1 and ABCG1 are upregulated via the LXR–RXR pathway.5 ABCA1 and ABCG1 subsequently promote the efflux of cholesterol from macrophages.10 Unfortunately, this negative feedback loop is not sufficient enough to prevent the activation of the immune system.
In addition to excessive amounts of lipids, atherosclerotic lesions thus harbour all classes of immune cells and moreover, serum levels of inflammatory mediators are linked to coronary heart disease.11 Recent advances in preclinical research have established a fundamental role for cellular inflammation throughout all stages of this disease from initiation through progression and, ultimately, the thrombotic complications following plaque rupture or erosion.6,12–14 A key role for innate immunity is illustrated by studies showing that monocytes and macrophages are abundantly present in atherosclerotic plaques,15 and moreover, inhibiting monocyte-entry into the plaque drastically attenuates atherogenesis16 as well as CVD-risk,9 whereas immune cell stimulation accelerates atherosclerosis.13
In parallel, systemic monocyte production and circulating monocyte number increase progressively with advancing disease.17,18 These innate immune cells help drive the formation of an extensive microvascular network penetrating the more advanced atherosclerotic lesions. These leaky, immature vessels provide an easy communication and access network for both cellular as well as humoral elements.19 The extravasated immune cells also have an intricate impact on plaque phenotype by contributing substantially to endothelial dysfunction, oxidative stress, and extracellular matrix degradation.9 From a therapeutic perspective, several randomized clinical trials are currently underway that will determine whether non-specific anti-inflammatory therapies with for instance interleukin-1-neutralizing antibodies or methotrexate can reduce the risk of cardiovascular events in patients with atherosclerosis.20–22
To date, little is known on the mechanism(s) maintaining the chronic inflammatory state in CV patients. Recent data have moved our focus from immune cells within the atherosclerotic plaque towards a systemic pro-inflammatory state, characterized by increased production and mobilization of innate immune cells by haematopoietic organs,7,13,23 lipoprotein-driven activation of innate immune cells already within the plasma compartment,24 and rapid, increased influx of immune cells into atherosclerotic plaques in patients,25 collectively driving the inflammatory state in the atherosclerotic plaque.
Whereas the contribution of immune cells to atherogenesis has been well established, it remains elusive why the strong inflammatory response in the arterial wall persists over time. In the adaptive arm of the immune system, dendritic cells in the plaque promote adaptive immune responses by presenting antigens, leading to enhanced T-cell function both in experimental and clinical atherosclerosis.26 The interplay between activated T-cells and resident macrophages perpetuates a local inflammatory cascade within the subendothelial compartment.4,27 In addition, increasing body of evidence directs towards a role for impaired resolution of inflammation.28–30
From a therapeutic standpoint, however, interventions targeting the adaptive immune system directly are hampered by a markedly increased incidence of infectious complications.31,32 A better understanding of the role of the innate immune system in the chronic inflammatory characteristics of atherosclerosis may overcome these limitations. However, boosting the adaptive immune system using vaccination strategies has therapeutic potential and is currently being studied for atherosclerosis treatment.26,33
Multi-level systemic immune cell activation contributes to atherogenesis and cardiovascular risk
The challenges in targeting inflammation in any chronic disease lie in three properties that are critical for evolutionary survival: redundancy, compensation, and necessity. Targeting one component may not be sufficient to attenuate a pro-inflammatory reaction. Inflammation is a carefully tuned process that has multiple feedback loops: thus, inhibition of a critical pathway may trigger a compensatory pro-inflammatory response. Finally and most importantly, the inflammatory response is critical for host defence, and even if the previous two challenges have been met, the risk–benefit ratio may still be unacceptable, exposing for example to an elevated risk of infections. Accordingly, broad immune pathway targeting is unlikely to confer therapeutic benefits. Therefore, developing anti-inflammatory therapies involves identifying suitable pathways in mice, pre-clinical testing of whether the pathway can be neutralized without causing collateral damage, translational studies identifying similarly relevant pathways in humans, and, finally, clinical trials. This challenging drug discovery path can be negotiated more efficiently with the help of immune system imaging, especially of immune cells, their subsets, and their migration, production, and function.
The rate of progression of atherosclerotic lesions is critically dependent on the number and characteristics of the circulating monocyte pool.34 In view of the short half-life of innate immune cells,14 attention has shifted towards a more prominent role of bone marrow mobilization and activation of immune cell progenitors13 and local proliferation.14 Recently, acute events, both myocardial infarction13 and stroke35 were found to elicit a spike in neutrophil and monocyte production in experimental models, which subsequently accumulated in the infarcted areas. In apoE−/− mice with advanced atherosclerosis, acute local events were also found to augment inflammation in atherosclerotic plaques at a distance, increasing plaque size, and inducing a more vulnerable lesion morphology with higher inflammatory cell content. An increased supply of innate immune cells was identified as the principal driver for this phenomenon.13 However, the residence time of innate immune cells in ischaemic tissue was estimated to be 19 h, implying the continued need for new immune cells to maintain the high cell number in atherosclerotic lesions.36 To support this demand, in mice after coronary ligation, increased sympathetic nervous activity released upstream precursors from bone marrow niches.13 Markedly, both the increased monocyte count and the pro-inflammatory changes in systemic plaques persisted for several months, the mechanism for which remains yet to be established.
Haematopoiesis in the bone marrow produces most of the circulating monocytes in human. Dysregulated haematopoiesis in the bone marrow can be caused by several stimuli, among which dyslipidaemia.37,38 Biased differentiation of the haematopoietic stem cells (HSCs) can, in the case of dyslipidaemia, increase monocyte production. The number of circulating innate immune cells was shown to be a strong predictor of future CV-risk in patients.39 Following an acute myocardial infarction (AMI), bone marrow activity as well as inflammatory signals in distant atherosclerotic lesions were found to be increased,23 implying a systemic cellular mobilization comparable to experimental data.13 In advanced atherosclerotic lesions, immune cell accumulation is also markedly increased, implying a role for systemic immune cell activity in maintaining the increased inflammatory state in the arterial wall.25 Furthermore, not only the monocyte number, but also the monocyte phenotype may be affecting atherogenesis. In vitro, several atherogenic risk factors were shown to induce a persistent activated state in monocytes,40 which can further contribute to arterial wall inflammation.
Somatic mutations in HSCs are commonly found in the general population, especially in increasing age categories; in the group of people above 70 years of age 10% have somatic mutations. Mutations in TET2, ASXL1, and DNMT3A are associated with not only increased risk of haematologic malignancies but also increased CVD risk.41 Patients with Clonal Haematopoiesis of Indeterminate Potential (CHIP), in comparison to patients without clones in peripheral blood have higher risk of dying. This increased risk could not only be explained by the higher incidence of haematopoietic malignancy and may be related to coronary heart disease and stroke. Clonal Haematopoiesis of Indeterminate Potential carriers have a 1.9 times greater chance of developing coronary heart disease, and a 4 times higher chance on myocardial infarction in comparison with non-CHIP carriers.42 Possibly these clones changes the differentiation of monocytes and macrophages and thereby aggravate atherosclerosis as shown in a murine model.42 In this murine model not only increased atherosclerosis was found in TET2 mutation but also increased levels of CXC chemokines, interleukin-8, and interleukin-1β.42,43
Trained innate immunity and epigenetic reprogramming: a memory for innate immune cells
In the classical immunological paradigm, activation of the innate immune cells (monocytes and macrophages) provides a rapid first line defence against infectious episodes, followed by a ‘memory’ response mediated by the adaptive immune system. Recently, Netea et al.44–46 challenged the classical dichotomy of innate vs. adaptive immunity by showing that brief exposure to microbial products induces a long-term pro-inflammatory phenotype in monocytes, which was found to be linked to metabolic and epigenetic reprogramming. After vaccination of healthy subjects with BCG, monocytes showed a profound pro-inflammatory phenotype, that persisted even 3 months after vaccination and could be observed even several months after the initial exposure.47,48 The concept that the innate immune system is incapable of mounting adaptive responses49 has already been contradicted by studies showing that organisms lacking a specific immune system are still capable of responding adaptively to infections.50 Epigenetic reprogramming, accompanied by markers of histone modifications such as methylation of histone 3 at lysine residue 4 (H3K4) or H3K27 acetylation, has been proposed as the molecular mechanism responsible for long-term memory of innate immunity and this process has been termed ‘trained immunity’.45,47,51
Epigenetic control denotes the regulation of gene transcription without altering the nucleotide sequence of the DNA by the modification of the chromatin structure. These modifications result in specific chromosomal regions becoming more or less accessible to transcription factors, leading to prolonged alterations in downstream gene-products. There are many different epigenetic modifications that are tightly regulated by a wide array of specific epigenetic writers and erasers that function in a lineage specific manner.52,53 Importantly, these modifications are reversible, making chromatin modifying enzymes a potentially interesting therapeutic target.54 The drug development area of inhibitors targeting histone deacetylases (HDACs) and DNA methyltransferases (DNMTs) in various disease states is expanding rapidly;55,56 though not yet in the cardiovascular arena. Interestingly, the epigenetic modulators suberoylanilide hydroxamic acid (SAHA, or Vorinostat) and valproic acid (HDAC inhibitors) have already been approved by the FDA for cancer treatment and epilepsy, respectively.57,58 In the past years, the potential for epigenetic intervention by targeting specific histone methyltransferases has increasingly received attention.59
In the cardiovascular domain, pro-atherogenic stimuli such as oxLDL and lipoprotein(a) [Lp(a)] have recently been shown to induce a prolonged, ‘primed’ or ‘trained’ state of the innate immune cells, already in the plasma compartment. In patients with elevated Lp(a), a major CV risk factor, monocytes showed a primed state by increased pro-inflammatory cytokine production upon ex vivo stimulation. In vitro priming of healthy monocytes with Lp(a) showed a training capacity: 6 days after first exposure to Lp(a), monocytes showed an increased pro-inflammatory phenotype. In vivo, this trained state coincided with activated monocytes which accumulated more rapidly into the arterial wall in vivo.60 In line, oxidized LDL-c also induced trained immunity via epigenetic reprogramming, eliciting an activated monocyte phenotype. Incubation of monocytes with oxLDL was associated with increased H3K4me3.40 The time course for the induction of these pro-inflammatory changes remains to be established. Because monocytes and macrophages have life spans in the order of hours to days, their upstream progenitors, including bone marrow haematopoietic progenitor cells (HSPCs) should also be investigated. Interestingly, recent findings by van der Valk et al. and Nahrendorf and colleagues61 did reveal a persistent inflammatory state in the arterial wall, as well as increased metabolic activity in the bone marrow more than 3 months after an AMI. These findings, supported by experimental data, imply that epigenetic changes in short-lived monocytes are likely to be maintained by similar changes in the haematopoietic precursor cells in the bone marrow (HSPC).61 Detailed in vivo studies on HSPCs are, however, absent to date.
How do these pro-atherogenic stimuli modify epigenetic markers? Accumulating evidence points to a pivotal role for rewiring of intracellular metabolism in innate immune cells 62 Various intermediate metabolites act as important cofactors for epigenetic enzymes, including NAD+, acetylCoA, SAM, FAD, and fumarate.63–65 Trained immunity by BCG or beta-glucan requires a shift from oxidative phosphorylation to aerobic glycolysis.66,67 In addition, accumulation of fumarate due to anaplerotic replenishment of the TCA cycle by glutamine projects onto epigenetic changes by inhibition of the lysine demethylase KDM5.68 The relevance of these metabolic changes for the development of atherosclerosis is illustrated by the up-regulation of glycolytic pathways in monocytes and macrophages isolated from patients with atherosclerosis.69,70 For a more detailed description of the role of immunometabolism in the (epigenetic) reprogramming of innate immune cells in the context of atherosclerosis, we refer to recent excellent reviews on this topic71 (Stienstra et al., in press).
In parallel, in CID, the increased inflammatory responsiveness of monocytes and macrophages due to trained immunity is likely to play a central role.72 The abundantly present danger associated molecular patterns (DAMPs) in CID and particularly in RA, including biglycan,73 S100 proteins,74 HMBG1,75 citrullinated proteins,76 heat shock proteins,77 and tenascin-C78 might induce a trained state of innate immune cells, leading to prolonged hyper responsiveness mediated at least partly via histone modifications. Interestingly, synovial fibroblasts from affected joints in RA also showed distinct global and promoter-specific changes in DNA methylation,79 collectively pointing towards a role for epigenetic modulation being involved in the persistent pro-inflammatory state in CID.
From this perspective, the capacity of innate immunity to mount adaptive responses both redefines the function of innate immunity and provides a potential therapeutic target in chronic inflammation, including atherosclerosis and RA. This also echoes the highly feared recurrent CV-events in the first months after an initial CV-event, attributed largely to persistent inflammatory activity.80 As active participants in arterial wall inflammation, ‘trained’ innate immune cells may represent promising therapeutic targets.81–83 Targeting innate immune cells is likely to also offer a wider therapeutic window compared to the adaptive immune system, since patients with innate immune deficiencies are much less prone to infectious complications compared to those with disturbances in the adaptive immune system.
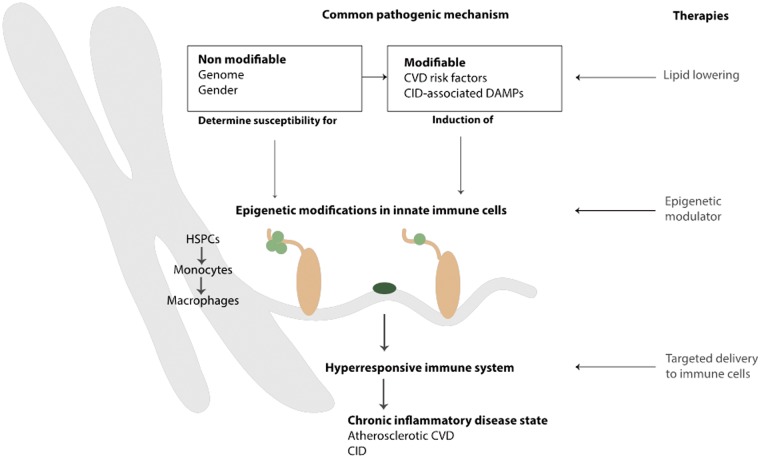
In the Horizon2020 project REPROGRAM,84 we propose that trained immunity is an important pathway promoting an activated state of innate immune cells in the context of atherosclerotic CVD. Hence, modulation of the trained immunity pathway may offer an attractive strategy to effectively attenuate the chronic inflammatory state in atherosclerosis as well as other CID (Figure 1).
Figure 1.
Innate immune cell activation as a common pathway perpetuating the upheld inflammatory state in atherosclerosis and other chronic inflammatory disease states. Both non-modifiable (i.e. genome and gender) and modifiable (i.e. danger associated molecular patterns and pathogen-associated molecular patterns) risk factors can lead to the induction of epigenetic modifications in innate immune cells. Changes can occur in either haematopoietic stem cells or monocytes, leading to differentiation into reprogrammed monocytes or macrophages. This results in a hyper-responsive immune system, contributing to a chronic inflammatory disease state such as atherosclerotic cardiovascular diseases or chronic inflammatory diseases. Current therapies aim to lower risk factors, for example by lipid lowering. Future therapies might include additional epigenetic modulators, preventing and reversing the modification of innate immune cells, ideally by specifically targeting immune cells.
Common immune responses in atherosclerosis and chronic inflammatory diseases
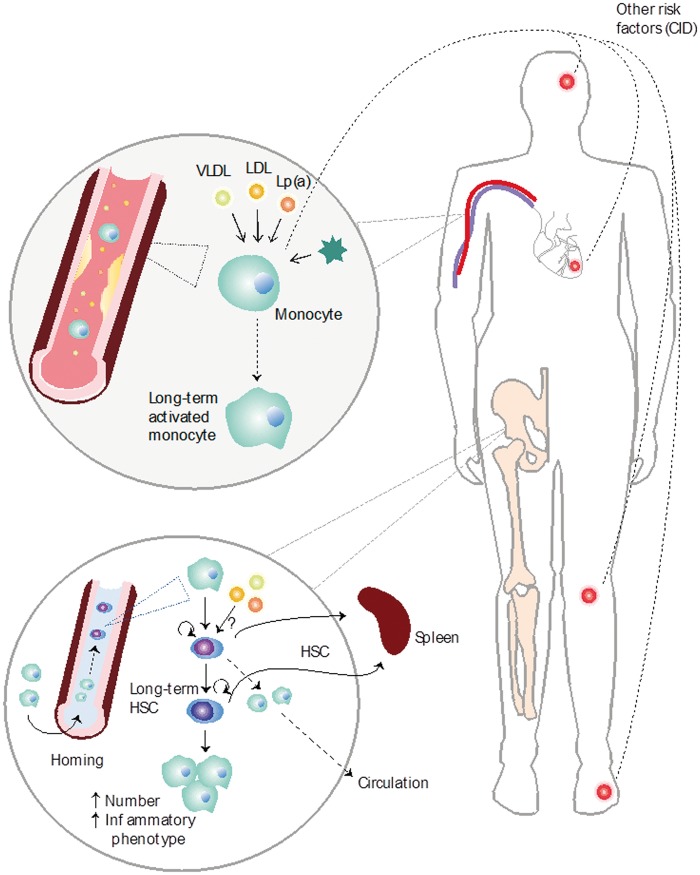
Many CIDs are associated with altered monocyte phenotype and function, which may alter the potential of these cells to influence atherogenesis.85 For example, in patients with well-controlled human immunodeficiency virus (HIV), arterial wall inflammation in the aorta is increased, associated with circulating markers of monocyte and macrophage activation.86 Furthermore, resident macrophages and TLR signalling play an important role in RA,87 atherosclerotic tissue,88 and acute coronary syndromes.89 MRP8/14, a physiological TLR4 ligand released by activated macrophages, is a prognostic biomarker in both RA90 and acute coronary syndromes.91 In mice, experimental osteoarthritis induced increased levels of Ly6C-high, as well as Ly6C-low monocytes, due to increased bone marrow activity.92 It has been recently proposed that therapeutically targeting interactions between TLRs and foam cell formation may reduce adverse cardiovascular outcomes in individuals with CID.93 These studies have contributed to uncover novel molecular mechanisms that modulate the inflammatory response in atherosclerotic lesions, and suggest that a parallel exists with chronic inflammatory diseases, such as RA (Figure 2). Particularly, a prominent role for endogenous danger signals, comprising both pathogen-associated molecular patterns (PAMPs) as well as DAMPs, in the immunological pathophysiology is emerging rapidly.94,95 In RA tissues and synovial fluids, multiple DAMPs are present, most of which have the capacity to act as TLR2 and/or TLR4 agonists.
Figure 2.
Monocyte reprogramming in atherosclerosis and chronic inflammatory diseases. Different chronic inflammatory disease states such as myocardial infarction, stroke, rheumatoid arthritis and gout as well as different atherosclerotic danger associated molecular patterns (DAMPs) such as low-density-lipoprotein, oxLDL, VLDL, and cholesterol crystals can trigger monocyte phenotype to change into a long-term activated monocyte. In the bone marrow, these pathogen-associated molecular patterns and danger associated molecular patterns alter the phenotype of the haematopoietic stem cells (HSCs) leading to the differentiation into a different monocyte phenotype and increased monocyte accumulation in the spleen and circulation. Furthermore, HSCs increasingly travel to the spleen leading to extramedullary haematopoiesis.
Several DAMPs have now been shown to contribute to the ‘memory’ response in monocytes, mediated by distinct histone modifications.96 The correlation between RA disease activity/duration and the markedly increased inflammatory activity in the arterial wall in RA patients97 lends further support to a common underlying pathophysiology, eventually translating into a two- to three-fold higher CV-risk in RA patients which is only partly attributable to known CV-risk factors.98 In support of immune cell hyperactivity as a causal factor of CVD in patients with RA, Bernelot Moens et al.96 recently observed that circulating monocytes in RA patients in remission requiring continued biological therapy are characterized by increased expression of activation/adhesion markers, which coincides with increased arterial wall inflammation in RA patients. A potential contribution of epigenetic modulation to the persistent inflammatory state in RA as well as its cardiovascular co-morbidity has been put forward,99 yet the evidence for this concept remains to be delivered.
Multimodal imaging in atherosclerosis and chronic inflammatory diseases
The optimal method to evaluate the presence or progression of atherosclerosis is to directly visualize the target organ for atherosclerosis: the arterial wall. In line with this, several imaging modalities have been used in patient studies to measure the dimension of the arterial wall, including ultrasound100 and magnetic resonance imaging (MRI).101 With the new insight that inflammation is a key component dictating both the progression of atherosclerotic lesions as well as the vulnerability of advanced plaques, attention has shifted towards novel imaging modalities able to quantify the functional aspects in the arterial wall/atherosclerotic lesions, including the inflammatory activity. Whereas previously, ultrasound imaging only gave information about intima media thickness, new contrast agents have emerged in contrast-enhanced ultrasonography (CEUS). Contrast-enhanced ultrasonography has shown in human studies to directly visualize the vasa vasorum in atherosclerotic carotid arteries and has the ability to show the adventitial wall and intra-plaque neovascularization. However widely available and easily accessible, robust quantitative assessment tools of the CEUS signal are lacking, which is a major limitation.102 Magnetic resonance imaging gives information about both vessel wall and lumen and can identify positive remodelling. In tracking arterial wall inflammation in MRI ultra-small super paramagnetic particles of iron oxide (USPIOs) are used, since these are phagocytosed by macrophages. The USPIO enhanced MRI signal does not only reflect the phagocytosis, but also local haemorrhage and calcification.101 SPECT imaging using 99mTc labelling of autologous leucocytes in humans can visualize leucocyte migration into the atherosclerotic plaque,25 but has lower spatial resolution in comparison to PET.103 Optical coherence tomography (OCT) is an intravascular method, using infra-red light. Optical coherence tomography has the capacity to detect macrophages, however motion artefacts limits the usability.7 Positron emission tomography with computed tomography is frequently used in clinical studies of atherosclerosis. A diversity of PET tracers have been investigated, however 18-fluordeoxyglucose (18F-FDG) PET/CT imaging is increasingly applied to serve as a measure of arterial wall inflammation.104 Although its primary target is to detect overall metabolic activity, 18F-FDG imaging in patients with atherosclerosis robustly correlates with macrophage density as measured by histology105 and correlates to plaque macrophage content and distribution.106 It is increasingly used to monitor inflammation in vascular beds as a function of therapy in patients, particularly in the aorta and carotid arteries.107–110
Clinical credence for PET-based imaging emerged after several retrospective111,112 and small-scale prospective studies showing that PET can identify active culprit lesions113,114 and predict the risk of a recurrent event. In addition, the first intervention studies have been performed using changes in 18F-FDG uptake PET to monitor therapeutic efficacy of novel anti-atherosclerotic strategies.109,115 More recently, several small 18F-FDG PET/computed tomography studies in patients with AMI reported increased PET signals in ischaemic myocardial regions in association with higher 18F-FDG uptake in remote non-culprit atherosclerotic lesions, as well as in haematopoietic organs,116,117 highlighting the systemic inflammation following acute ischaemic events, as previously described in pre-clinical models. A retrospective trial in patients with atherosclerosis reported that increased splenic 18F-FDG also predicted higher cardiovascular event rates.23 These trials exemplify the opportunities generated by whole-body imaging, including the ability to sample more than one organ system. In order to unravel systems-wide immune actions as well as connections between cardiovascular and haematopoietic organs, imaging studies allow integration of data from cardiovascular organs, non-culprit atherosclerotic lesions, spleen, and bone marrow, which can be combined with data derived from blood and bone-marrow analyses. In this scenario, whole-body multimodal imaging can translate preclinical findings, serve as companion imaging in clinical trials, and help guide individualized therapy.
Therapeutic implications of epigenetic reprogramming of innate immune cells
Chronic inflammatory diseases are the most common diseases of ageing and represent one of our major health threats. These include most forms of CVD, RA, type 2 diabetes, and virtually all neurodegenerative diseases. In these disease states, a non-autoimmune primary pathological process determines disease progression; for example, inflammation promotes the formation of oxidized phospholipids that may serve as DAMPs in atherosclerosis.94 As with autoimmune diseases, inhibition of inflammation could reduce the rate of disease progression to the point of substantial clinical benefit despite not altering the underlying pathogenic process. In contrast, in primary inflammatory or autoimmune diseases there is little evidence as yet for efficacy of this approach in humans.
The increased inflammatory responsiveness of monocytes and macrophages due to trained immunity is likely to play a central role in CIDs. From this perspective, the adaptive capacity of the innate immune system provides a potential therapeutic target in human diseases. It is thus essential to improve our understanding of the pathophysiology and cellular and molecular mechanisms common to chronic inflammation, starting with atherosclerosis. Moreover, it is essential to understand the cellular and molecular mechanisms that mediate trained immunity, in hopes of harnessing their therapeutic potential. An important finding in that respect is that trained immunity is characterized by a metabolic shift from oxidative phosphorylation to aerobic glycolysis, which closely interacts with the epigenetic reprogramming.65 This opens doors for new potential therapeutic possibilities. To prevent training, this mechanism can be targeted, for example using inhibitors of glycolysis,118 or inhibitors of micro RNAs that dictate the balance between glycolysis and OXPHOS.119
New knowledge about inflammatory signalling, particularly in the areas of endogenous homeostatic pathways and inflammation resolution also provides the promise for new therapeutic options that can adequately meet the therapeutic challenges in CID and atherosclerosis. The abundant presence of epigenetic alterations in both CID and atherosclerosis underlines the potential for clinical applications. In line with this, the potential of epigenetic alterations as molecular biomarkers are being explored for CVD risk evaluation, early detection, prognosis stratification, and treatment response prediction. On the other hand, unlike genetic mutations, epigenetic changes, including DNA methylation and histone modifications, are pharmacologically reversible, making them attractive targets in therapeutic strategies.
Novel insights that will develop by a systems biology approach of trained monocytes is highly likely to identify novel therapeutic targets to prevent or treat atherosclerosis.80 Several preclinical studies have already provided proof-of-concept data that drugs that modulate the activity of epigenetic writers or erasers, such as HDAC inhibitors, can modulate the development of atherosclerosis.120,121 Furthermore, BET-inhibitors such as RVX-208, JQ1, and I-Bet that inhibit the interaction of BET proteins with acetylated histone tails, showed a repression of pathways that contribute to CVD and inhibition of atherogenesis in mouse models.122–124 Drugs leading to the reverse reprogramming of macrophages are for example cyclodextrin. Via the LXR pathway, cyclodextrin supports atherosclerosis regression by transcriptional reprogramming leading to increased cholesterol efflux and anti-inflammatory effects,7 thereby reversing the initial pro-inflammatory response upon cholesterol crystal accumulation. Major advances have been made in the field of oncology with aberrant DNA methylation profiles and alterations in histone modification being linked to specific cancers and tumour progression, some of which are already used in the clinic.125 The discrepancy between major advances in the oncology field and scarcity of data in the cardiovascular and rheumatology field illustrates that an organized effort to address the potential of epigenetic modulation in atherosclerosis and CID is long overdue.
In conclusion, epigenetic reprogramming has been shown to play a role in perpetuation of the chronic inflammatory state in atherosclerosis by promoting activation of innate immune cells. Since innate immune cell activation serves as a mostly inappropriate amplification loop which is not critically involved in primary defence against infectious diseases, this novel pathway may offer a safe and novel method to lower this redundant inflammatory activity in absence of the danger of eliciting immune suppression. Therefore, further research is warranted to better understand cellular and molecular mechanisms mediating trained immunity, offering the opportunity of opening this area for future therapeutic agents in the cardiovascular area.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 667837.
Conflict of interest: none declared.
References
- 1. Alwan A, Armstrong T, Bettcher D.. Global status report on noncummunicable diseases. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 2. Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–874. [DOI] [PubMed] [Google Scholar]
- 3. Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ; American Heart Association Advocacy Coordinating Committee, Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Nursing, Council on the Kidney in Cardiovascular Disease, Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research . Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933–944. [DOI] [PubMed] [Google Scholar]
- 4. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 5. Tall AR, Yvan-Charvet L.. Cholesterol, inflammation and innate immunity. Nat Rev Immunol 2015;15:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swirski FK, Nahrendorf M.. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013;339:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, Ulas T, Skjelland M, Nardo DD, Labzin LI, Kerksiek A, Hempel C, Heneka MT, Hawxhurst V, Fitzgerald ML, Trebicka J, Björkhem I, Gustafsson J-Å, Westerterp M, Tall AR, Wright SD, Espevik T, Schultze JL, Nickenig G, Lütjohann D, Latz E.. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Transl Med 2016;8:333ra50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller YI, Choi S-H, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL.. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res 2011;108:235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Libby P, Hansson GK.. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res 2015;116:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N.. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab 2008;7:365–375. [DOI] [PubMed] [Google Scholar]
- 11. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005;352:20–28. [DOI] [PubMed] [Google Scholar]
- 12. Nahrendorf M, Swirski FK.. Innate immune cells in ischaemic heart disease: does myocardial infarction beget myocardial infarction? Eur Heart J 2016;37:868–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HWM, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M.. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo J-L, Gorbatov R, Sukhova GK, Gerhardt LMS, Smyth D, Zavitz CCJ, Shikatani EA, Parsons M, Rooijen N. V, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK.. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 2013;19:1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore KJ, Tabas I.. Macrophages in the pathogenesis of atherosclerosis. Cell 2011;145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boring L, Gosling J, Cleary M, Charo IF.. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998;394:894–897. [DOI] [PubMed] [Google Scholar]
- 17. Madjid M, Awan I, Willerson JT, Casscells SW.. Leukocyte count and coronary heart disease. J Am Coll Cardiol 2004;44:1945–1956. [DOI] [PubMed] [Google Scholar]
- 18. Olivares R, Ducimetière P, Claude JR.. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol 1993;137:49–53. [DOI] [PubMed] [Google Scholar]
- 19. Taqueti VR, Carli MFD, Jerosch-Herold M, Sukhova GK, Murthy VL, Folco EJ, Kwong RY, Ozaki CK, Belkin M, Nahrendorf M, Weissleder R, Libby P.. Increased microvascularization and vessel permeability associate with active inflammation in human atheromata. Circ Cardiovasc Imaging 2014;7:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ridker PM, Lüscher TF.. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J 2014;35:1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ridker PM, Thuren T, Zalewski A, Libby P.. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 2011;162:597–605. [DOI] [PubMed] [Google Scholar]
- 22. Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AAK, Glynn RJ, Ridker PM.. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 2013;166:199–207.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JHF, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, Fredrickson J, Rubin B, Hoffmann U, Truong QA, Min JK, Baruch A, Nasir K, Nahrendorf M, Tawakol A.. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans . JACC Cardiovasc Imaging 2015;8:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bernelot Moens SJ, Neele AE, Kroon J, Valk FM, van der Bossche J, Van den Hoeksema MA, Hoogeveen RM, Schnitzler JG, Baccara-Dinet MT, Manvelian G, Winther MPJ. D, Stroes ESG.. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J 2017;38:1584–1593. [DOI] [PubMed] [Google Scholar]
- 25. Valk FM. V D, Kroon J, Potters WV, Thurlings RM, Bennink RJ, Verberne HJ, Nederveen AJ, Nieuwdorp M, Mulder WJM, Fayad Z. A, Buul JD. V, Stroes ESG.. In vivo imaging of enhanced leukocyte accumulation in atherosclerotic lesions in humans. J Am Coll Cardiol 2014;64:1019–1029. [DOI] [PubMed] [Google Scholar]
- 26. Andersson J, Libby P, Hansson GK.. Adaptive immunity and atherosclerosis. Clin Immunol 2010;134:33–46. [DOI] [PubMed] [Google Scholar]
- 27. Hermansson A, Ketelhuth DFJ, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK.. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med 2010;207:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol 2010;10:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Libby P, Tabas I, Fredman G, Fisher EA.. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res 2014;114:1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fredman G, Tabas I.. Boosting inflammation resolution in atherosclerosis. Am J Pathol 2017;187:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sommerer C, Konstandin M, Dengler T, Schmidt J, Meuer S, Zeier M, Giese T.. Pharmacodynamic monitoring of cyclosporine a in renal allograft recipients shows a quantitative relationship between immunosuppression and the occurrence of recurrent infections and malignancies. Transplantation 2006;82:1280–1285. [DOI] [PubMed] [Google Scholar]
- 32. Singh N. Infectious complications in organ transplant recipients with the use of calcineurin-inhibitor agent-based immunosuppressive regimens. Curr Opin Infect Dis 2005;18:342–345. [DOI] [PubMed] [Google Scholar]
- 33. Lahoute C, Herbin O, Mallat Z, Tedgui A.. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol 2011;8:348–358. [DOI] [PubMed] [Google Scholar]
- 34. Woollard KJ, Geissmann F.. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010;7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Courties G, Herisson F, Sager HB, Heidt T, Ye Y, Wei Y, Sun Y, Severe N, Dutta P, Scharff J, Scadden DT, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M.. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ Res 2015;116:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M.. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 2012;209:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bernelot Moens SJ, Verweij SL, Schnitzler JG, Stiekema LCA, Bos M, Langsted A, Kuijk C, Bekkering S, Voermans C, Verberne HJ, Nordestgaard BG, Stroes ESG, Kroon J.. Remnant cholesterol elicits arterial wall inflammation and a multilevel cellular immune response in humans. Arterioscler Thromb Vasc Biol 2017;37:969–975. [DOI] [PubMed] [Google Scholar]
- 38. Rahman MS, Murphy AJ, Woollard KJ.. Effects of dyslipidaemia on monocyte production and function in cardiovascular disease. Nat Rev Cardiol 2017;14:387–400. [DOI] [PubMed] [Google Scholar]
- 39. Giugliano G, Brevetti G, Lanero S, Schiano V, Laurenzano E, Chiariello M.. Leukocyte count in peripheral arterial disease: a simple, reliable, inexpensive approach to cardiovascular risk prediction. Atherosclerosis 2010;210:288–293. [DOI] [PubMed] [Google Scholar]
- 40. Bekkering S, Quintin J, Joosten L. A B, Meer JWM. V D, Netea MG, Riksen NP.. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol 2014;34:1731–1738. [DOI] [PubMed] [Google Scholar]
- 41. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL.. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL.. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu C, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AAB, Cooper MA, Andrés V, Hirschi KK, Martin KA, Walsh K.. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quintin J, Cheng S-C, Meer JWM. V D, Netea MG.. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol 2014;29:1–7. [DOI] [PubMed] [Google Scholar]
- 45. Netea M, Quintin J, Meer J. V D.. Trained immunity: a memory for innate host defense. Cell Host Microbe 2011;9:355–361. [DOI] [PubMed] [Google Scholar]
- 46. Ifrim DC, Quintin J, Joosten LAB, Jacobs C, Jansen T, Jacobs L, Gow NAR, Williams DL, Meer JWM. V D, Netea MG.. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin Vaccine Immunol 2014;21:534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JW, van Crevel R, Netea MG.. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012;109:17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kleinnijenhuis J, Quintin J, Preijers F, Benn CS, Joosten LAB, Jacobs C, Loenhout JV, Xavier RJ, Aaby P, Meer JWMVD, Crevel RV, Netea MG.. Long-lasting effects of BCG vaccination on both heterologous th1/th17 responses and innate trained immunity. J Innate Immun 2014;6:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Medzhitov R, Janeway C.. Innate immune recognition: mechanisms and pathways. Immunol Rev 2000;173:89–97. [DOI] [PubMed] [Google Scholar]
- 50. Kurtz J. Specific memory within innate immune systems. Trends Immunol 2005;26:186–192. [DOI] [PubMed] [Google Scholar]
- 51. Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, ONeill LAJ, Xavier RJ.. Trained immunity: a program of innate immune memory in health and disease. Science 2016;352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heinz S, Romanoski CE, Benner C, Glass CK.. The selection and function of cell type-specific enhancers. Nat Rev Mol Cell Biol 2015;16:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hoeksema MA, Winther MPJ. D.. Epigenetic regulation of monocyte and macrophage function. Antioxid Redox Signal 2016;25:758–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Neele AE, van den Bossche J, Hoeksema MA, de Winther MP.. Epigenetic pathways in macrophages emerge as novel targets in atherosclerosis. Eur J Pharmacol 2015;763:79–89. [DOI] [PubMed] [Google Scholar]
- 55. Baylin SB, Jones PA.. A decade of exploring the cancer epigenome—biological and translational implications. Nat Rev Cancer 2011;11:726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 57. Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA.. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev 2008;34:206–222. [DOI] [PubMed] [Google Scholar]
- 58. Mann BS, Johnson JR, He K, Sridhara R, Abraham S, Booth BP, Verbois L, Morse DE, Jee JM, Pope S, Harapanhalli RS, Dagher R, Farrell A, Justice R, Pazdur R.. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res 2007;13:2318–2322. [DOI] [PubMed] [Google Scholar]
- 59. Wang Z, Patel DJ.. Small molecule epigenetic inhibitors targeted to histone lysine methyltransferases and demethylases. Q Rev Biophys 2013;46:349–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, Nieuwdorp M, Joosten LA, Netea MG, Koschinsky ML, Witztum JL, Tsimikas S, Riksen NP, Stroes ES.. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 2016;134:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Valk FM, van der, Kuijk C, Verweij SL, Stiekema LCA, Kaiser Y, Zeerleder S, Nahrendorf M, Voermans C, Stroes ESG.. Increased haematopoietic activity in patients with atherosclerosis. Eur Heart J 2017;38:425–432. [DOI] [PubMed] [Google Scholar]
- 62. Stienstra R, Netea-Maier RT, Riksen NP, Joosten LAB, Netea MG.. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metab 2017;26:142–156. [DOI] [PubMed] [Google Scholar]
- 63. Rodriguez H, Rafehi H, Bhave M, El-Osta A.. Metabolism and chromatin dynamics in health and disease. Mol Aspects Med 2017;54:1–15. [DOI] [PubMed] [Google Scholar]
- 64. Keating ST, El-Osta A.. Epigenetics and metabolism. Circ Res 2015;116:715–736. [DOI] [PubMed] [Google Scholar]
- 65. Montgomery DC, Sorum AW, Guasch L, Nicklaus MC, Meier JL.. Metabolic regulation of histone acetyltransferases by endogenous Acyl-CoA cofactors. Chem Biol 2015;22:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG.. mTOR- and HIF-1-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014;345:1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arts RJ, Carvalho A, Rocca CL, Palma C, Rodrigues F, Silvestre R, Kleinnijenhuis J, Lachmandas E, Gonçalves LG, Belinha A, Cunha C, Oosting M, Joosten LA, Matarese G, Crevel R. V, Netea MG.. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep 2016;17:2562–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Arts RJ, Novakovic B, ter Horst R, Carvalho A, Bekkering S, Lachmandas E, Rodrigues F, Silvestre R, Cheng S-C, Wang S-Y, Habibi E, Gonçalves LG, Mesquita I, Cunha C, van Laarhoven A, van de Veerdonk FL, Williams DL, van der Meer JW, Logie C, O’Neill LA, Dinarello CA, Riksen NP, van Crevel R, Clish C, Notebaart RA, Joosten LA, Stunnenberg HG, Xavier RJ, Netea MG.. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab 2016;24:807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, Goronzy JJ, Weyand CM.. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med 2016;213:337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, de Graaf J, Joosten LA, Netea MG, Gomes ME, Riksen NP.. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis 2016;254:228–236. [DOI] [PubMed] [Google Scholar]
- 71. Van den Bossche J, O’Neill LA, Menon D.. Macrophage immunometabolism: where are we (going)? Trends Immunol 2017;38:395–406. [DOI] [PubMed] [Google Scholar]
- 72. Bekkering S, Joosten LA, van der Meer JW, Netea MG, Riksen NP.. Trained innate immunity and atherosclerosis. Curr Opin Lipidol 2013;24:487–492. [DOI] [PubMed] [Google Scholar]
- 73. Barreto G, Soininen A, Ylinen P, Sandelin J, Konttinen YT, Nordström DC, Eklund KK.. Soluble biglycan: a potential mediator of cartilage degradation in osteoarthritis. Arthritis Res Ther 2015;17:379.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Senolt L, Grigorian M, Lukanidin E, Simmen B, Michel BA, Pavelka K, Gay RE, Gay S, Neidhart M.. S100A4 is expressed at site of invasion in rheumatoid arthritis synovium and modulates production of matrix metalloproteinases. Ann Rheum Dis 2006;65:1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kokkola R, Sundberg E, Ulfgren A-K, Palmblad K, Li J, Wang H, Ulloa L, Yang H, Yan X-J, Furie R, Chiorazzi N, Tracey KJ, Andersson U, Harris HE.. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum 2002;46:2598–2603. [DOI] [PubMed] [Google Scholar]
- 76. Chang X, Yamada R, Suzuki A, Kochi Y, Sawada T, Yamamoto K.. Citrullination of fibronectin in rheumatoid arthritis synovial tissue. Rheumatology 2005;44:1374–1382. [DOI] [PubMed] [Google Scholar]
- 77. Huang Q-Q, Koessler RE, Birkett R, Dorfleutner A, Perlman H, Haines GK, Stehlik C, Nicchitta CV, Pope RM.. Glycoprotein 96 perpetuates the persistent inflammation of rheumatoid arthritis. Arthritis Rheum 2012;64:3638–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hasegawa M, Nakoshi Y, Muraki M, Sudo A, Kinoshita N, Yoshida T, Uchida A.. Expression of large tenascin-C splice variants in synovial fluid of patients with rheumatoid arthritis. J Orthop Res 2007;25:563–568. [DOI] [PubMed] [Google Scholar]
- 79. Karouzakis E, Gay RE, Gay S, Neidhart M.. Increased recycling of polyamines is associated with global DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 2012;64:1809–1817. [DOI] [PubMed] [Google Scholar]
- 80. Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, Dolan E, Moroney J, Murphy S, O’Rourke K, O’Malley K, O’Donohoe M, McDonnell C, Noone I, Barry M, Crowe M, Kavanagh E, O’Connell M, Kelly PJ.. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol 2012;71:709–718. [DOI] [PubMed] [Google Scholar]
- 81. Bekkering S, Joosten LA, van der Meer JW, Netea MG, Riksen NP.. The epigenetic memory of monocytes and macrophages as a novel drug target in atherosclerosis. Clin Ther 2015;37:914–923. [DOI] [PubMed] [Google Scholar]
- 82. Christ A, Bekkering S, Latz E, Riksen NP.. Long-term activation of the innate immune system in atherosclerosis. Semin Immunol 2016;28:384–393. [DOI] [PubMed] [Google Scholar]
- 83. Tabas I, Glass CK.. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 2013;339:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.REPROGRAM horizon2020 research program. www.reprogram-horizon2020 (5 October 2017).
- 85. Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U.. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum 2012;64:671–677. [DOI] [PubMed] [Google Scholar]
- 86. Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK.. Arterial inflammation in patients with HIV. JAMA 2012;308:379.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huang Q, Ma Y, Adebayo A, Pope RM.. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum 2007;56:2192–2201. [DOI] [PubMed] [Google Scholar]
- 88. Edfeldt K, Swedenborg J, Hansson GK, Yan Z.. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 2002;105:1158–1161. [PubMed] [Google Scholar]
- 89. Wyss CA, Neidhart M, Altwegg L, Spanaus KS, Yonekawa K, Wischnewsky MB, Corti R, Kucher N, Roffi M, Eberli FR, Amann-Vesti B, Gay S, von Eckardstein A, Lüscher TF, Maier W.. Cellular actors, toll-like receptors, and local cytokine profile in acute coronary syndromes. Eur Heart J 2010;31:1457–1469. [DOI] [PubMed] [Google Scholar]
- 90. Choi IY, Gerlag DM, Holzinger D, Roth J, Tak PP.. From synovial tissue to peripheral blood: myeloid related protein 8/14 is a sensitive biomarker for effective treatment in early drug development in patients with rheumatoid arthritis. PLoS One 2014;9:e106253.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yonekawa K, Neidhart M, Altwegg LA, Wyss CA, Corti R, Vogl T, Grigorian M, Gay S, Lüscher TF, Maier W.. Myeloid related proteins activate toll-like receptor 4 in human acute coronary syndromes. Atherosclerosis 2011;218:486–492. [DOI] [PubMed] [Google Scholar]
- 92. Cremers N, Geven E, Blom A, Sloetjes A, DiCeglie I, van Dalen S, Bosch M, Kraan P, Lent P.. S100A8/A9 increases the mobilization of Ly6C high monocytes to the synovium during experimental osteoarthritis. Osteoarthr Cartil 2017;25:S47–S48. [Google Scholar]
- 93. Angelovich TA, Hearps AC, Jaworowski A.. Inflammation-induced foam cell formation in chronic inflammatory disease. Immunol Cell Biol 2015;93:683–693. [DOI] [PubMed] [Google Scholar]
- 94. Pisetsky DS. The origin and properties of extracellular DNA: from PAMP to DAMP. Clin Immunol 2012;144:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zimmer S, Grebe A, Latz E.. Danger signaling in atherosclerosis. Circ Res 2015;116:323–340. [DOI] [PubMed] [Google Scholar]
- 96. Bekkering S, Blok BA, Joosten LAB, Riksen NP, van Crevel R, Netea MG, In vitro experimental model of trained innate immunity in human primary monocytes Clin Vaccine Immunol 2016;23:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bernelot Moens SJ, van der Valk FM, Strang AC, Kroon J, Smits LP, Kneepkens EL, Verberne HJ, van Buul JD, Nurmohamed MT, Stroes ES.. Unexpected arterial wall and cellular inflammation in patients with rheumatoid arthritis in remission using biological therapy: a cross-sectional study. Arthritis Res Ther 2016;18:115.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA, The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis PLoS One 2015;10:e0117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Klein K, Kabala PA, Grabiec AM, Gay RE, Kolling C, Lin L-L, Gay S, Tak PP, Prinjha RK, Ospelt C, Reedquist KA.. The bromodomain protein inhibitor I-BET151 suppresses expression of inflammatory genes and matrix degrading enzymes in rheumatoid arthritis synovial fibroblasts. Ann Rheum Dis 2016;75:422–429. [DOI] [PubMed] [Google Scholar]
- 100. Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E; ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008;358:1431–1443. [DOI] [PubMed] [Google Scholar]
- 101. Bochem AE, van Wijk DF, Holleboom AG, Duivenvoorden R, Motazacker MM, Dallinga-Thie GM, de Groot E, Kastelein JJP, Nederveen AJ, Hovingh GK, Stroes ES.. ABCA1 mutation carriers with low high-density lipoprotein cholesterol are characterized by a larger atherosclerotic burden. Eur Heart J 2013;34:286–291. [DOI] [PubMed] [Google Scholar]
- 102. Magnoni M, Ammirati E, Camici PG.. Non-invasive molecular imaging of vulnerable atherosclerotic plaques. J Cardiol 2015;65:261–269. [DOI] [PubMed] [Google Scholar]
- 103. Sriranjan RS, Tarkin JM, Evans NR, Chowdhury MM, Rudd JHF.. Imaging unstable plaque. Q J Nucl Med Mol Imaging 2016;60:205–218. [PubMed] [Google Scholar]
- 104. Tarkin JM, Rudd JHF.. Techniques for noninvasive molecular imaging of atherosclerotic plaque. Nat Rev Cardiol 2015;12:79.. [DOI] [PubMed] [Google Scholar]
- 105. Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ.. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818–1824. [DOI] [PubMed] [Google Scholar]
- 106. Masteling MG, Zeebregts CJ, Tio RA, Breek J-C, Tietge UJ, de Boer JF, Glaudemans AW, Dierckx RA, Boersma HH, Slart RH.. High-resolution imaging of human atherosclerotic carotid plaques with micro 18F-FDG PET scanning exploring plaque vulnerability. J Nucl Cardiol 2011;18:1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lehrer-Graiwer J, Singh P, Abdelbaky A, Vucic E, Korsgren M, Baruch A, Fredrickson J, Bruggen N, Tang MT, Frendeus B, Rudd JHF, Hsieh F, Ballantyne CM, Ghoshhajra B, Rosenson RS, Koren M, Roth EM, Duprez DA, Fayad ZA, Tawakol AA.. FDG-PET imaging for oxidized LDL in stable atherosclerotic disease: a phase II study of safety, tolerability, and anti-inflammatory activity. JACC Cardiovasc Imaging 2015;8:493–494. [DOI] [PubMed] [Google Scholar]
- 108. Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif J-C, Rudd JHF, Farkouh ME, Tawakol A; dal-PLAQUE Investigators. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 2011;378:1547–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, Nunes IO, Beals CR, Shankar SS.. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation. J Am Coll Cardiol 2013;62:909–917. [DOI] [PubMed] [Google Scholar]
- 110. van Wijk DF, Sjouke B, Figueroa A, Emami H, van der Valk FM, MacNabb MH, Hemphill LC, Schulte DM, Koopman MG, Lobatto ME, Verberne HJ, Fayad ZA, Kastelein JJP, Mulder WJ, Hovingh GK, Tawakol A, Stroes ES.. Nonpharmacological lipoprotein apheresis reduces arterial inflammation in familial hypercholesterolemia. J Am Coll Cardiol 2014;64:1418–1426. [DOI] [PubMed] [Google Scholar]
- 111. Paulmier B, Duet M, Khayat R, Pierquetghazzar N, Laissy J, Maunoury C, Hugonnet F, Sauvaget E, Trinquart L, Faraggi M.. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol 2008;15:209–217. [DOI] [PubMed] [Google Scholar]
- 112. Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, Lawler MA, Grinspoon SK, Brady TJ, Nasir K, Hoffmann U, Tawakol A.. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging 2013;6:1250–1259. [DOI] [PubMed] [Google Scholar]
- 113. Davies JR, Rudd JHF, Fryer TD, Graves MJ, Clark JC, Kirkpatrick PJ, Gillard JH, Warburton EA, Weissberg PL.. Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke 2005;36:2642–2647. [DOI] [PubMed] [Google Scholar]
- 114. Rudd JHF, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnström P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL.. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708–2711. [DOI] [PubMed] [Google Scholar]
- 115. Tawakol A, Singh P, Rudd JHF, Soffer J, Cai G, Vucic E, Brannan SP, Tarka EA, Shaddinger BC, Sarov-Blat L, Matthews P, Subramanian S, Farkouh M, Fayad ZA.. Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging. J Am Coll Cardiol 2014;63:86–88. [DOI] [PubMed] [Google Scholar]
- 116. Assmus B, Iwasaki M, Schachinger V, Roexe T, Koyanagi M, Iekushi K, Xu Q, Tonn T, Seifried E, Liebner S, Kranert WT, Grunwald F, Dimmeler S, Zeiher AM.. Acute myocardial infarction activates progenitor cells and increases Wnt signalling in the bone marrow. Eur Heart J 2012;33:1911–1919. [DOI] [PubMed] [Google Scholar]
- 117. Wollenweber T, Roentgen P, Schafer A, Schatka I, Zwadlo C, Brunkhorst T, Berding G, Bauersachs J, Bengel FM.. Characterizing the inflammatory tissue response to acute myocardial infarction by clinical multimodality noninvasive imaging. Circ Cardiovasc Imaging 2014;7:811–818. [DOI] [PubMed] [Google Scholar]
- 118. Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquière B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, Bifari F, Wang X, Blanco R, Tembuyser B, Cornelissen I, Bouché A, Vinckier S, Diaz-Moralli S, Gerhardt H, Telang S, Cascante M, Chesney J, Dewerchin M, Carmeliet P.. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab 2014;19:37–48. [DOI] [PubMed] [Google Scholar]
- 119. Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, Rinehold K, Solingen C. V, Fullerton MD, Cecchini K, Rayner KJ, Steinberg GR, Zamore PD, Fisher EA, Loke P, Moore KJ.. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest 2015;125:4334–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cao Q, Rong S, Repa JJ, Clair RS, Parks JS, Mishra N.. Histone deacetylase 9 represses cholesterol efflux and alternatively activated macrophages in atherosclerosis development. Arterioscler Thromb Vasc Biol 2014;34:1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hoeksema MA, Gijbels MJ, Van den Bossche J, van der Velden S, Sijm A, Neele AE, Seijkens T, Stoger JL, Meiler S, Boshuizen MC, Dallinga-Thie GM, Levels JH, Boon L, Mullican SE, Spann NJ, Cleutjens JP, Glass CK, Lazar MA, de Vries CJ, Biessen EA, Daemen MJ, Lutgens E, Winther MP.. Targeting macrophage Histone deacetylase 3 stabilizes atherosclerotic lesions. EMBO Mol Med 2014;6:1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gilham D, Wasiak S, Tsujikawa LM, Halliday C, Norek K, Patel RG, Kulikowski E, Johansson J, Sweeney M, Wong NCW.. RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease. Atherosclerosis 2016;247:48–57. [DOI] [PubMed] [Google Scholar]
- 123. Jahagirdar R, Zhang H, Azhar S, Tobin J, Attwell S, Yu R, Wu J, McLure KG, Hansen HC, Wagner GS, Young PR, Srivastava RAK, Wong NCW, Johansson J.. A novel BET bromodomain inhibitor, RVX-208, shows reduction of atherosclerosis in hyperlipidemic ApoE deficient mice. Atherosclerosis 2014;236:91–100. [DOI] [PubMed] [Google Scholar]
- 124. Brown JD, Lin CY, Duan Q, Griffin G, Federation AJ, Paranal RM, Bair S, Newton G, Lichtman AH, Kung AL, Yang T, Wang H, Luscinskas FW, Croce KJ, Bradner JE, Plutzky J.. NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell 2014;56:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kelly TK, Carvalho DD, Jones PA.. Epigenetic modifications as therapeutic targets. Nat Biotechnol 2010;28:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]