Abstract
Aims
Aircraft noise causes endothelial dysfunction, oxidative stress, and inflammation. Transportation noise increases the incidence of coronary artery disease, hypertension, and stroke. The underlying mechanisms are not well understood. Herein, we investigated effects of phagocyte-type NADPH oxidase (Nox2) knockout and different noise protocols (around-the-clock, sleep/awake phase noise) on vascular and cerebral complications in mice.
Methods and results
C57BL/6j and Nox2−/− (gp91phox−/−) mice were exposed to aircraft noise (maximum sound level of 85 dB(A), average sound pressure level of 72 dB(A)) around-the-clock or during sleep/awake phases for 1, 2, and 4 days. Adverse effects of around-the-clock noise on the vasculature and brain were mostly prevented by Nox2 deficiency. Around-the-clock aircraft noise of the mice caused the most pronounced vascular effects and dysregulation of Foxo3/circadian clock as revealed by next generation sequencing (NGS), suggesting impaired sleep quality in exposed mice. Accordingly, sleep but not awake phase noise caused increased blood pressure, endothelial dysfunction, increased markers of vascular/systemic oxidative stress, and inflammation. Noise also caused cerebral oxidative stress and inflammation, endothelial and neuronal nitric oxide synthase (e/nNOS) uncoupling, nNOS mRNA and protein down-regulation, and Nox2 activation. NGS revealed similarities in adverse gene regulation between around-the-clock and sleep phase noise. In patients with established coronary artery disease, night-time aircraft noise increased oxidative stress, and inflammation biomarkers in serum.
Conclusion
Aircraft noise increases vascular and cerebral oxidative stress via Nox2. Sleep deprivation and/or fragmentation caused by noise triggers vascular dysfunction. Thus, preventive measures that reduce night-time aircraft noise are warranted.
Keywords: Environmental stressor, Noise exposure, Endothelial dysfunction, NADPH oxidase-derived oxidative stress, eNOS uncoupling, Systemic inflammation, Cerebral redox balance, Sleep deprivation
Translational perspective
Noise leads to annoyance, a form of mental stress that is associated with pathophysiological changes such as a pro-atherothrombotic phenotype and increased incidence of cardiovascular events and disease. This study is the first to demonstrate (i) increased markers of oxidative stress and inflammation in noise-exposed patients with established coronary artery disease, (ii) an impact of sleep vs. awake phase aircraft noise exposure on the vasculature, (iii) the stimulatory effects of aircraft noise on cerebral superoxide production by uncoupled neuronal nitric oxide synthase (nNOS) as well as induction of a neuroinflammatory phenotype, and (iv) the protective effects of Nox2 deletion on aircraft noise-induced vascular dysfunction, cerebral superoxide production, and neuroinflammation. Thus, these results may explain at least in part why sleep phase rather than awake phase noise leads to cardiovascular diseases and may also provide an explanation why aircraft noise is linked with cognitive impairment including retardations of learning and memory capabilities.
Introduction
Transportation noise is becoming recognized as a cardiovascular risk factor (for review, see Refs1,2). Numerous studies indicate that continuous road, railway, and aircraft noise is associated with a significant increase in arterial hypertension, coronary artery disease, heart failure, and stroke (for review, see Refs3–5). Simulation of night-time aircraft noise (for one night) causes stress and endothelial dysfunction in healthy subjects6 and patients with established coronary artery disease,7 likely due to increased production of vascular reactive oxygen species (ROS).8 Importantly, night-time noise is more likely to cause cardiovascular disease than daytime noise,9–11 a phenomenon, that may be linked to activation of metabolic, endocrine,12 and immune pathways.13 Sleep restriction14 or fragmentation15 causes endothelial dysfunction and increases cerebral oxidative stress and increases cardiovascular mortality,16 likely due to increased NADPH oxidase (mainly Nox2) activation. Chronic aircraft noise also causes learning and memory impairment in children,17 all of which may be linked to an activation of the cerebral Nox2.18 In addition, Nox2 knockout mice were protected against endothelial dysfunction in numerous cardiovascular disease models including experimental hypertension.19
Thus, with these studies we sought to determine
the vascular and cerebral consequences of sleep (reflecting night-time noise) vs. awake phase aircraft noise,
the role of Nox2 in aircraft noise-induced vascular and cerebral oxidative stress by using a Nox2−/− (gp91phox−/−) knockout mouse,
the impact of aircraft noise on circadian clock gene regulation, and
as a translational approach, to investigate for the first time whether night-time aircraft noise increases oxidative stress biomarkers in humans.
Materials and methods
Noise exposure
All human data was collected in accordance with the declaration of Helsinki and Ethical approval was granted by the Landesärztekammer Rheinland-Pfalz (Mainz, Germany; permit number: 837.190.12 (8291-F)). Written consent was received from all included individuals. Human data are previously unpublished results of the FLuG-Risiko study.7 Serum of each individual was sampled after nights without or with aircraft noise exposure for 6 h. The aircraft noise consisted of 60 repetitive noise events, which had been recorded near Düsseldorf airport, interrupted by random silent periods, yielding a mean sound pressure level of 47 dB(A) (compared to 39 dB(A) in the silent nights). The detailed protocol and health status of included individuals was published previously.7
All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the U.S. National Institutes of Health and approval was granted by the Ethical Committee of the University Medical Center Mainz and the Landesuntersuchungsamt Rheinland-Pfalz (Koblenz, Germany; permit number: 23 177-07/G 15-1-094). After the indicated duration and protocol of noise exposure, animals were killed under isoflurane anaesthesia by transection of the diaphragm and removal of the heart and thoracic aorta. Glucose levels were assessed in whole blood using the ACCU-CHEK Sensor system from Roche Diagnostics GmbH (Mannheim, Germany).
For detailed description of all methods, see Supplementary material online.
Results
Effects of Nox2 (gp91phox) deficiency on vascular function and systemic oxidative stress caused by continuous noise exposure (24 h/day) for 1, 2, and 4 days
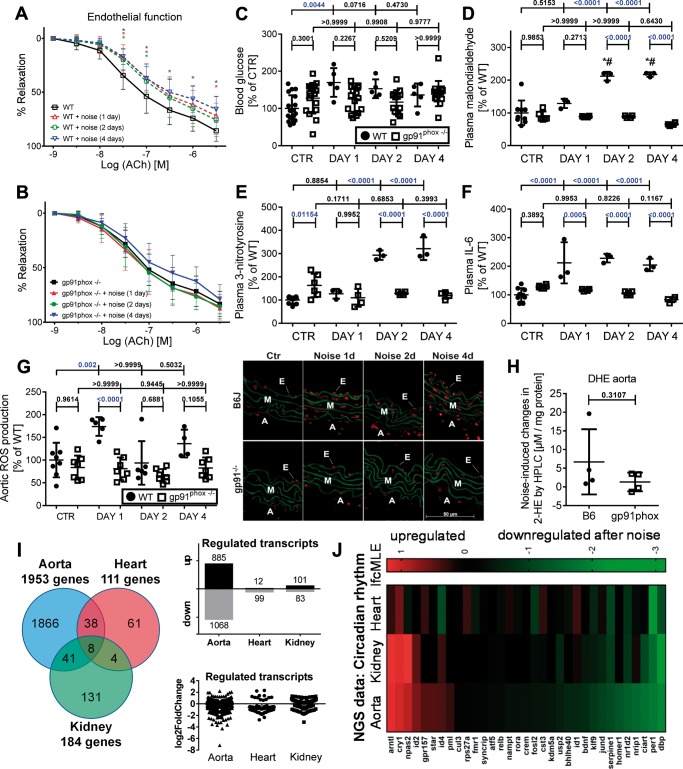
Animals were exposed to aircraft noise around-the-clock for 1, 2, and 4 days. This induced endothelial dysfunction in wild-type mice on all exposure days, whereas in Nox2−/− (gp91phox−/−) mice endothelial function was preserved (Figure 1A and B), while endothelium-independent relaxation in Nox2−/− mice was slightly impaired (see Supplementary material online, Figure S1). Blood glucose levels increased on Day 1 of noise exposure in wild type but not in Nox2−/− mice (Figure 1C). Plasma levels of malondialdehyde- and 3-nitrotyrosine-positive proteins, markers of systemic oxidative stress, and circulating levels of interleukin-6 (IL-6) were significantly increased (Figure 1D–F, for original blots see Supplementary material online, Figure S2). Oxidative burst in whole blood was augmented in noise-exposed wild type but not in Nox2−/− mice (see Supplementary material online, Figure S1). Aortic ROS formation was increased by around-the-clock noise exposure and normalized by Nox2 deficiency (Figure 1G and H). In contrast to our previous observations,8 the cGMP-dependent kinase-1 (cGK-I) activity (=P-VASP), protein levels of endothelin-1 (ET-1), and heme oxygenase-1 (HO-1) were not modified in aorta of aircraft noise-exposed Nox2 (gp91phox) deficient mice pointing to the crucial role of Nox2 in noise-triggered vascular damage (see Supplementary material online, Figure S3).
Figure 1.
Effects of continuous noise exposure (24 h/day) on blood glucose, vascular function, systemic oxidative stress, and gene regulation (next generation sequencing data) in wild-type and Nox2−/− (gp91phox−/−) mice. Around the clock noise exposure (24 h/day) impaired endothelial function significantly (A and B) and increased blood glucose (C) in wild-type mice. The endothelium-independent relaxation (NTG response) is shown in Supplementary material online, Figure S1. Levels of 3-nitrotyrosine- and malondialdehyde-positive proteins were significantly increased in mouse plasma in wild-type mice (D and E). Circulating IL-6 in mouse plasma was significantly elevated in wild-type mice (F). Representative dot blots are shown in Supplementary material online, Figure S2. Vascular ROS formation as envisaged by DHE staining was increased in noise-exposed wild-type mice (G). None of the parameters were changed in around-the-clock noise exposed Nox2−/− (gp91phox−/−) mice. Noise-induced aortic superoxide formation as measured by HPLC analysis was higher by trend in wild type than in Nox2−/− (gp91phox−/−) mice (H). Representative HPLC chromatograms are shown in Supplementary material online, Figure S24. The pie chart and box/scatter plots summarize the number of genes that are significantly and tissue-independently regulated (I). Changes of transcriptional profiles were determined by next generation sequencing in aorta, heart, and kidney of mice exposed to aircraft noise and unexposed controls. Clustering of genes in heat maps revealed tissue-conserved transcriptional changes in the circadian clock pathway (J). Most significantly up- or down-regulated genes for all tissues can be found in Supplementary material online, Tables S1–S5. Data are presented as mean ± SD from n = 14–15 (A and B) or 6 (C) animals/group, at least three samples/group (pooled from 2 to 3 mice/sample) (D–F), 4–6 WT and 7–8 gp91phox−/− (G), and 4 mice/group (H–J). (A and B) Statistical analysis was performed using two-way ANOVA comparing the values of the entire curves; *P < 0.05 vs. control without noise at the same ACh concentration (as indicated by colour code). (H) Unpaired t-test with Welch’s correction was used. For all other data: normality test passed and one-way ANOVA with Tukey’s correction was used. lfcMLE means Log2 fold change with the unshrunken maximum likelihood estimate (MLE).
Noise-induced changes in circadian clock genes assessed by next generation sequencing
Comparative analysis of the transcriptome of aortic tissue from around-the-clock aircraft noise-treated animals vs. controls showed numerous differentially expressed genes. Recently, we identified 224 transcripts that were either up- or down-regulated over the entire exposure period (applying a threshold of P < 0.05 and Log2 fold change ≥0.5).8 Several of these regulated genes are involved in essential cellular processes such as apoptosis, fibrosis, proliferation, antioxidant defence, and inflammation, with FOXO transcription factors representing a central signalling hub.8 Herein, we report that around-the-clock exposure to aircraft noise regulates almost 2000 genes in aorta (see pie chart as well as box/scatter plots in Figure 1I) and induces transcriptional changes in circadian rhythm (see heat map in Figure 1J), inflammation and oxidative stress response (see heat maps in Supplementary material online, Figure S4) in three different tissues (aorta, heart, and kidney) (see also Supplementary material online, Tables S1–S3). Most significant similarities in the transcriptional changes between the different tissues were found for circadian rhythm, which led us to assess the circadian clock system in more detail. Key changes in the aorta comprised impaired insulin signalling and down-regulation of Foxo3 expression, with significant impact on Npas2, Arntl, Clock, Bmal1, Cry1, Per1, Prlag1/2, Cul1, NR1D1/2, and Rora all genes of the circadian clock pathway, as identified by bioinformatic procedures (see Supplementary material online, Figure S5). At least the Bmal1 promoter has a direct binding site for FOXO3.
Since circadian clock dysregulation seemed to be centred around Foxo3 we tested the effect of the FOXO3 activator bepridil,20 a calcium antagonist, anti-anginal, and class IV anti-arrhythmic drug. Bepridil prevented noise-induced endothelial dysfunction, increased Foxo3 mRNA expression and prevented vascular/cerebral oxidative stress (see Supplementary material online, Figure S6).
Effects of Nox2 (gp91phox) deficiency on cerebral oxidative stress and inflammation caused by around-the-clock aircraft noise
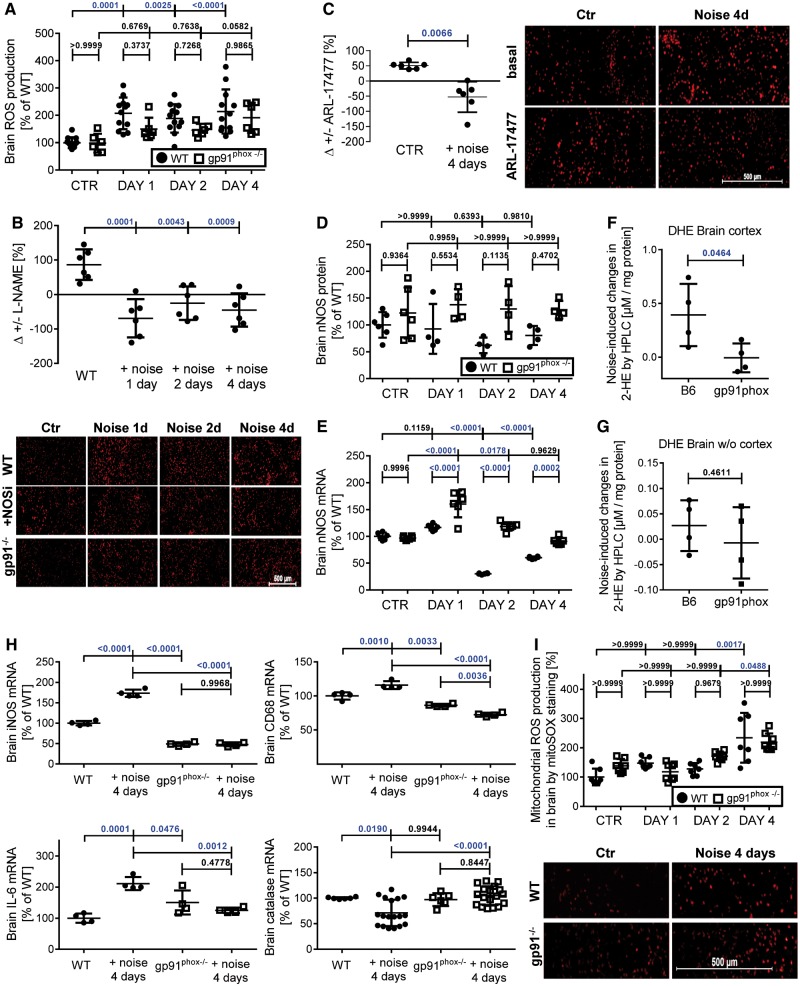
Around-the-clock aircraft noise increased ROS [dihydroethidium (DHE) cryo staining] in the frontal cortex of the brain, an effect that was severely blunted in Nox2−/− (gp91phox−/−) mice (Figure 2A). L-NG-nitroarginine methyl ester (L-NAME) increased ROS formation in the frontal cortex of the control group while decreasing it in noise-exposed animals, compatible with an uncoupled nitric oxide synthase (NOS) (Figure 2B), as described already for the vasculature.8 Using the highly specific inhibitor of nNOS (NOS1), ARL-17477, we identified uncoupled nNOS as a significant cerebral source of ROS formation (Figure 2C), while white noise had no adverse effects at all (see Supplementary material online, Figure S7).
Figure 2.
Effects of continuous noise (24 h/day) on cerebral oxidative stress and inflammation in wild type and Nox2−/− (gp91phox−/−) mice. Around the clock noise (24 h/day) increased oxidative stress in the cortex of wild-type mice significantly, whereas Nox2−/− (gp91phox−/−) mice were protected on the first two exposure days (A). Modulation of the ROS signal by L-NAME was used to identify ROS formation coming from uncoupled NOS (B). Representative minimized cerebral cryo-stainings of DHE fluorescence microtopography are shown below the quantification. Supplementary material online, Figures S24 and S25 show the full images and higher magnification as well as stainings of the entire brain. Modulation of the ROS signal by ARL-17477 was used to identify ROS formation coming from uncoupled nNOS (C). Cerebral nNOS expression was quantified at the protein and mRNA level in wild type and Nox2 deficient mice (D and E). Representative original western blots are shown in Supplementary material online, Figure S24. Noise-induced superoxide formation in frontal cortex as measured by HPLC analysis was significantly higher in WT than in Nox2−/− (gp91phox−/−) mice and whole brain tissue without frontal cortex showed the same trend (F and G). Representative HPLC chromatograms and full images of the representative DHE stainings are shown in Supplementary material online, Figure S24, respectively. Markers of inflammation (iNOS, CD68, IL-6) were increased and catalase was decreased at the mRNA level by noise, all of which was prevented by Nox2 deficiency (H). Cryo-sections of the frontal cortex were stained with mitoSOX red (1 µM) for detection of mitochondrial ROS formation (I). Representative full size staining images for all groups are shown in Supplementary material online, Figure S10. Data are presented as mean ± SD from n = 8 (A and B), at least 5 (C and D), 4 (F–H), and 7–8 (I) mice/group. (I) Normality test failed and non-parametric Kruskal–Wallis test with Dunn’s correction was used. (C) Paired and (F, G) unpaired t-test was used. For all other data: normality test passed and one-way ANOVA with Tukey’s correction was used.
Aircraft noise caused a down-regulation of nNOS in whole brain homogenate at the mRNA (significant) and the protein levels (by trend) (Figure 2D and E), which was prevented by genetic Nox2 deletion. nNOS phosphorylation at serine 847, which was reported to cause nNOS inactivation and to be associated with nNOS uncoupling,21,22 was increased in the frontal cortex upon exposure to noise (see Supplementary material online, Figure S8). Increased cerebral oxidative stress was observed in wild type but not Nox2−/− mice (Figure 2F and G). In the noise-exposed wild-type mice, cerebral oxidative stress was mainly derived from increased Nox2 activity secondary to activation by protein kinase C [increased phospho-MARCKS and p47phox phosphorylation at Ser328 (see Supplementary material online, Figure S9)] and due to nNOS uncoupling.
We also established increased mRNA expression of the inflammation markers inducible NOS, CD68, and IL-6 but decreased antioxidant defence gene catalase upon noise, all of which was prevented by Nox2 deletion (Figure 2H; 50 most significantly up- or down-regulated genes in brain are shown in Supplementary material online, Tables S4 and S5). On Day 4, Nox2 was replaced as the significant superoxide source in the brain by mitochondria (Figure 2I, Supplementary material online, Figure S10).
Effects of sleep/awake phase noise on selected genes
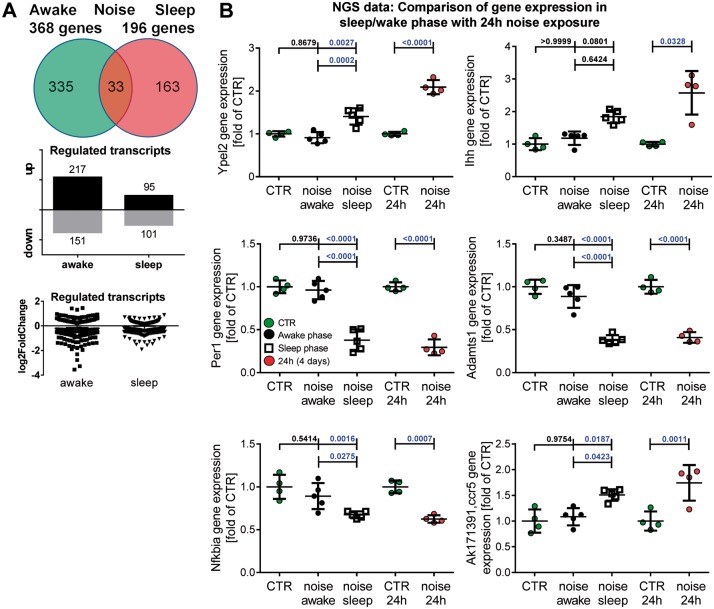
Recently, we identified eight potential risk marker genes that respond to continuous aircraft noise by next generation sequencing (NGS).8 Among the four most strongly up-regulated genes was Indian Hedgehog (Ihh), which is involved in cellular signalling, cartilage degeneration, and in TGFβ-driven processes.23,24 Aircraft noise during the sleep phase increased vascular Ihh gene expression to a greater extent than exposure during the awake phase in accordance with an increase in Ihh in aorta of mice exposed for 4 days to around-the-clock noise (see Supplementary material online, Figure S11A). The markers of endothelial cell activation and inflammation, Nos2 and Vcam1, were increased at the mRNA level in cardiac tissue, mostly of sleep phase or around-the-clock noise exposed mice (see Supplementary material online, Figure S11B and C), supporting the recently reported inflammatory phenotype in vessels of aircraft noise-exposed animals.8 We further confirmed the above-reported NGS data of previous and the present study by quantitative analysis of three selected genes of the postulated circadian clock pathway in Supplementary material online, Figure S5 using RT–PCR. Arntl was somewhat stronger up-regulated by exposure to noise during the sleep phase in accordance to 4 days exposure to around-the-clock aircraft noise (see Supplementary material online, Figure S11D). Foxo3 was down-regulated by exposure to noise during the sleep phase in accordance to 4 days exposure to around-the-clock aircraft noise (see Supplementary material online, Figure S11E). Parp1 was increased on almost all days in response to awake/sleep phase and around-the-clock aircraft noise exposure (see Supplementary material online, Figure S11F).
The changes in aircraft noise exposure-induced gene expression patterns were also supported by NGS data (see pie chart as well as box/scatter plots in Figure 3A), identifying key genes in cell cycle, transcription, inflammatory response, and circadian rhythm (Ypel2, Ihh, Per1, Adamts1, Nfkbia, Ccr5, Figure 3B and 27 more genes with a pie chart summary in Supplementary material online, Figures S12 and S13; 50 most significantly up- or down-regulated genes in Supplementary material online, Tables S6–S8).
Figure 3.
Effects of noise during the sleep or awake phase (12 h/day) on gene expression (NGS). The pie chart as well as box/scatter plots summarize the number of genes that are significantly and noise protocol-independently regulated in aortic tissue (A). NGS data for the three noise exposure protocols were used to identify genes that are similarly regulated in response to around-the-clock or sleep phase aircraft noise for 4 days (B). Most significantly up- or down-regulated genes (in aorta) for awake and sleep phase as well as around-the-clock noise can be found in Supplementary material online, Tables S1–S3 and S6–S9. Data are presented as mean ± SD from four samples in control group, five samples in sleep or awake group (each pooled from four mice) (B). Another 27 genes with high regulatory similarity between around-the-clock or sleep phase aircraft noise for 4 days and a summary of these comparisons can be found in Supplementary material online, Figures S12 and S13. (B, Ihh): Normality test failed and non-parametric Kruskal–Wallis test with Dunn’s correction was used. For all other data: normality test passed and one-way ANOVA with Tukey’s correction was used.
Effects of sleep/awake phase noise on haemodynamics, vascular function, and the •NO/cGMP signalling pathway
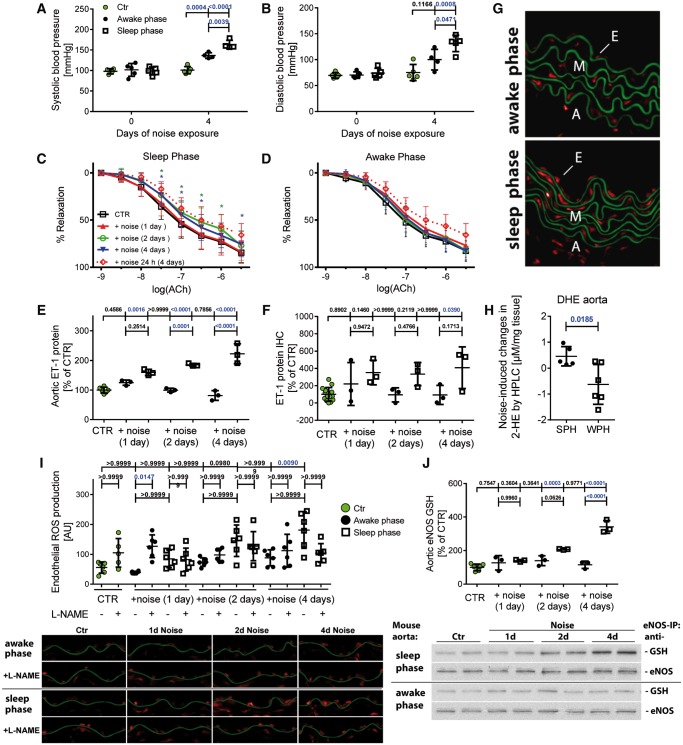
Four days of aircraft noise exposure during both the sleep and awake phases increased systolic and diastolic blood pressure significantly although noise exposure during the sleep phase had a more pronounced effect than awake phase exposures (Figure 4A and B; for time-dependence see Supplementary material online, Figure S14). Sleep phase but not awake phase noise caused endothelial dysfunction, impaired endothelium-independent relaxation by nitroglycerin (Figure 4C and D and Supplementary material online, Figure S15) and increased vascular expression of ET-1 (Figure 4E). Immunohistochemical analysis revealed an increase in ET-1 staining mainly within the endothelium (Figure 4F and see Supplementary material online, Figure S16). Likewise, an increase in serum leptin levels was observed (see Supplementary material online, Figure S17).
Figure 4.
Effects of noise during the sleep or awake phase (12 h/day) on blood pressure, vascular function, endothelin-1 levels, and vascular oxidative stress. Systolic and diastolic blood pressure was increased after sleep and awake phase noise. The increase of blood pressure was more pronounced after sleep phase noise (A and B). Time-dependent changes of blood pressure for 1, 2, 3, and 4 days of noise are shown in Supplementary material online, Figure S14. Relaxation by the endothelium-dependent vasodilator acetylcholine (ACh) was impaired by sleep phase but not awake phase noise (C and D). The dashed lines are reproduced data of Figure 1A above as reference values of mice exposed to around-the-clock aircraft noise for 4 days. The endothelium-independent relaxation (NTG response) is shown in Supplementary material online, Figure S15. Noise during the sleep phase but not the awake phase caused an increase in the expression ET-1 in aortic tissue (E). Immunohistochemical analysis revealed that noise during the sleep phase but not the awake phase enhanced vascular ET-1 levels on Day 1, mainly within the endothelium (F). Representative stained images and original blots are shown in Supplementary material online, Figure S16. In vascular tissue, sleep but not awake phase noise increased ROS production as revealed by DHE cryo staining (G, all staining images and quantification in Supplementary material online, Figure S19). Noise-induced aortic superoxide formation as measured by HPLC analysis was higher in the sleep than in the awake phase noise group (H). Representative HPLC chromatograms are shown in Supplementary material online, Figure S26. ROS production in the endothelial cell layer was increased in aortic segments of animals exposed to sleep phase noise but not awake phase noise and treatment with the eNOS inhibitor L-NAME exerted opposing effects on endothelial superoxide production in the sleep (rather down) vs. awake (rather up) phase noise groups on all days (I). Sleep phase but not awake phase noise lead to a substantial increase in S-glutathionylation of eNOS (J). Representative stained images and western blots are shown below the densitometric quantification. Data are presented as mean ± SD from n = 5 (A and B), 15–20 (C and D) mice per group, 6–8 samples (pooled from 2 to 3 mice per sample) (E), and 6–8 (F), 4–6 (H), and 6–10 (I, J) mice/group. Two-way ANOVA (C and D); *P < 0.05 vs. control without noise at the same ACh concentration (as indicated by color code). (A and I) Normality test failed and non-parametric Kruskal–Wallis test with Dunn’s correction was used. (H) Unpaired t-test was used. For all other data: normality test passed and one-way ANOVA with Tukey’s correction was used.
Sleep phase noise caused an up-regulation of eNOS expression over 1–4 days of noise and significant elevation of phosphorylation of eNOS at Ser1177 (see Supplementary material online, Figure S18A and B) and an increase in protein levels of GTP-cyclohydrolase-1 (GCH-1) and dihydrofolate reductase (DHFR), both being responsible for providing the eNOS cofactor tetrahydrobiopterin (BH4) (see Supplementary material online, Figure S18C and D). The •NO/cGMP/cGK-I signalling pathway was not impaired (data not shown) suggesting that the observed eNOS uncoupling in the present studies is not due to a deficit of BH4 synthesis or recycling, but rather related to eNOS glutathionylation and increased oxidative break-down of BH4.
Effects of sleep/awake noise on systemic and vascular oxidative stress and inflammation
In vascular tissue, noise exposure increased the global aortic ROS production being more pronounced in the sleep phase noise compared to the awake phase noise group (Figure 4G, H and Supplementary material online, Figure S19). DHE staining revealed that endothelial ROS production was increased by exposure to noise during the sleep phase and treatment with the eNOS inhibitor L-NAME had differential effects on endothelial superoxide production in sleep vs. awake phase noise groups, identifying eNOS as a significant superoxide source (Figure 4I). This was confirmed by the substantial increase in S-glutathionylation of eNOS in response to sleep phase noise (Figure 4J). Sleep but not awake phase noise increased mRNA of inflammation markers (iNOS, MCP-1, and CD68) in the aorta (see Supplementary material online, Figures S20 and S21). In the plasma, noise exposure during the sleep phase increased malondialdehyde-positive proteins, 3-nitrotyrosine-positive proteins, and interleukin-6 as established by dot blot measurements (see Supplementary material online, Figures S22 and S23).
Effects of sleep/awake aircraft noise on oxidative stress and inflammation in the brain
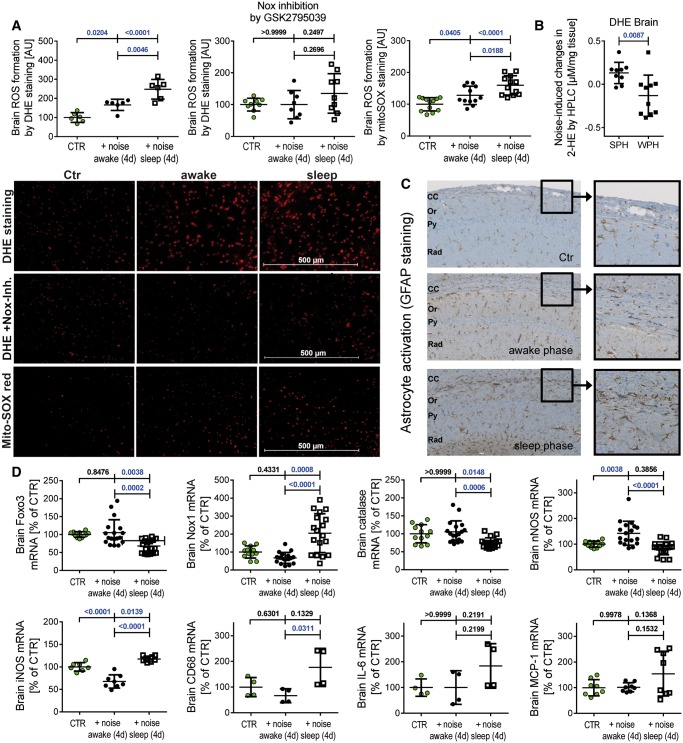
Aircraft noise during the sleep for 4 days increased cerebral cellular and mitochondrial ROS formation stronger than noise during the awake phase (Figure 5A and B). Cellular ROS formation was significantly blocked by the previously described Nox2 inhibitor GSK2795039.25 The inflammatory and pro-oxidative phenotype in sleep phase noise-exposed mice was also evident by increased glial fibrillary acidic protein (GFAP)-positive astrocytic processes and accumulation of astrocytes in the corpus callosum (CC) (Figure 5C).26,27 Microglia staining by ionized calcium binding adaptor molecule 1 (Iba1) showed homogenously distributed ramified microglia throughout the brain (not shown) but this does not necessarily mean that their function was not altered by noise since even microglia with apparently similar morphologies may exhibit diverse molecular and functional phenotypes.28 mRNA levels of protective genes (nNOS, Foxo3, and catalase) were down-regulated and mRNA expression of ROS generating Nox1 as well as markers of inflammation in the brain of mice upon noise were up-regulated during the sleep but not the awake phase (Figure 5D).
Figure 5.
Effects of sleep and awake phase noise (12 h/day for 4 days) on cerebral oxidative stress, inflammation, and gene regulation. Sleep phase noise caused a more pronounced increase in ROS formation in the frontal cortex than awake phase noise and this signal was blocked by Nox inhibition (DHE staining for cytoplasmic ROS and mitoSOX red for mitochondrial ROS, A). Representative stained images are shown together with the densitometric quantification. Noise-induced cerebral superoxide formation as measured by HPLC analysis was higher in the sleep than in the awake phase noise group (B). Representative HPLC chromatograms are shown in Supplementary material online, Figure S26. Astrocytes were activated in sleep phase noise exposed mice in the corpus callosum (C). Representative images for immunohistochemical staining for GFAP are shown at the level of the hippocampus sector CA1 [corpus callosum (CC), stratum oriens (Or), pyramidale (Py), and radiatum (Rad); 4 mice/group]. Markers of inflammation (iNOS, CD68 significant and IL-6, MCP-1 by trend) and ROS-producing Nox1 were increased (at least by trend), whereas the antioxidant/protective genes catalase, Foxo3, and nNOS were decreased at the mRNA level by sleep phase noise (D). Data are presented as mean ± SD from at least n = 6 (A), 9–10 (B), and at least 4 (D) mice/group. (D, Catalase): normality test failed and non-parametric Kruskal–Wallis test with Dunn’s correction was used. (B) Unpaired t-test was used. For all other data: normality test passed and one-way ANOVA with Tukey’s correction was used.
Evidence for noise-induced oxidative stress in human subjects
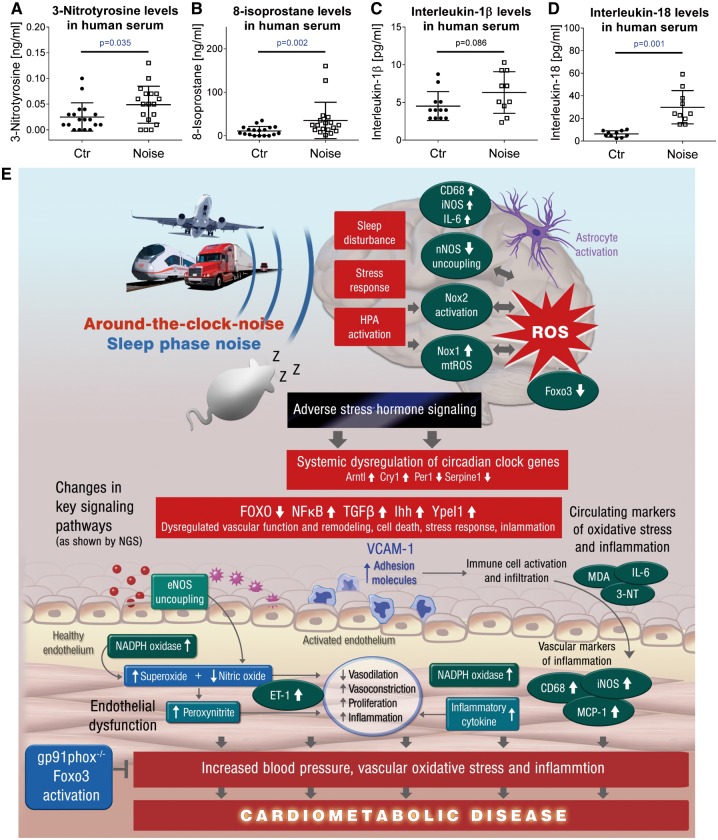
Noise exposure (simulated aircraft noise for 6 h, peak SPL 60 dB(A), mean SPL 47 dB(A)) caused a significant increase in 3-nitrotyrosine-positive proteins and 8-isoprostane concentrations in serum of human subjects (Figure 6A and B). Markers of inflammation such as IL-1β (by trend) and IL-18 were also found increased (Figure 6C and D).
Figure 6.
Oxidative stress markers in serum of noise-exposed human subjects. ELISA measurements revealed increased 3-nitrotyrosine (3NT)-positive proteins (A) and 8-isoprostane concentrations (B) in serum of human subjects. Multiplex measurements revealed increased markers of inflammation (IL-1β by trend, (C) IL-18 significant, (D)) in serum of human subjects. Data are presented as mean ± SD. Samples from n = 18 individuals (A and B) were compared before and after noise (aircraft noise for 6 h, mean SPL 47 dB(A)) using the Wilcoxon matched-pairs signed rank test. Cytokine measurements yielded results for n = 10–12 individuals using the unpaired t-test (without (IL-1β) and with Welch’s correction (IL-18)) (C and D). (E) Summarizing central scheme: around-the-clock and sleep phase noise triggers cerebral oxidative stress and a neuroinflammatory phenotype that translates the adverse effects of noise to the vascular and systemic level (e.g. by adverse stress hormone signalling and dysregulation of circadian clock inducing changes in key signalling pathways). Noise via neuronal pathways triggers vascular oxidative stress and inflammation with subsequent endothelial dysfunction, increases in blood pressure, all of which contributes the development and progression of cardiometabolic disease.
Discussion
With this study, we sought to determine the vascular and cerebral consequences of chronic aircraft noise when applied around-the-clock, during the awake phase and sleep phase (reflecting night time noise) for 1, 2, and 4 days. We could demonstrate that aircraft noise increased oxidative stress in the vasculature and the brain (mainly in the frontal cortex), caused cerebral nNOS down-regulation, all of which was almost completely prevented by Nox2 (gp91phox) deletion. Importantly, we established that noise during the sleep but not during the awake phase makes the difference. Mainly noise applied during the sleep phase comparable to night-time noise in humans caused endothelial dysfunction, increased oxidative stress, and inflammation within plasma, the heart and the vasculature and induced eNOS uncoupling.
In addition, we demonstrate for the first time that aircraft noise increases oxidative stress in the brain that is at least in part mediated by nNOS uncoupling and Nox2, all of which induces a neuroinflammatory phenotype as reflected by substantial astrocyte activation. Adverse gene regulation, as revealed by NGS in the aorta, kidney, heart, and brain, seems to be a key mechanism of noise-induced cardiovascular and cerebral damage. We here identified dysregulated circadian rhythm in different tissues, centered around Foxo3 down-regulation, which also adversely affects oxidative stress and inflammatory pathways. The link between adverse cerebral effects of noise and the subsequent cardiovascular damage is most likely based on detrimental stress hormone signalling as shown in animals8,29 and men.6
Aircraft noise and cardiovascular disease
An increasing portion of the population is exposed to aircraft, rail and road traffic noise and the evidence that transportation noise represents a significant cardiovascular risk factor has increased substantially. Noise leads to an increased incidence of cardiovascular diseases including coronary artery disease, stroke, and arterial hypertension30–34 and increases in the risk of diabetes mellitus type 2,35 and obesity30 development. Recent translational studies provided some mechanistic insight into the pathophysiology underlying aircraft noise-induced cardiovascular disease. Night-time of aircraft noise simulation stimulated the release of stress hormones such as adrenaline and caused endothelial dysfunction in healthy volunteers6 and patients with established coronary artery disease7 and as expected the deterioration of endothelial function was stronger in patients with established coronary artery disease as compared to healthy subjects.5 Importantly, endothelial dysfunction was markedly improved via acute administration of the antioxidant vitamin C pointing to increased production of ROS in the vasculature.6 With the present studies we can expand these observations by demonstrating for the first time that night-time aircraft noise of patients with established coronary artery disease leads to increased biomarkers of oxidative stress and inflammation (Figure 6).
Several studies indicate that in particular night-time aircraft noise consistently leads to the development of stress and arterial hypertension. For example, the HYENA trial established a significant exposure-response relationship for night-time but not daytime aircraft noise with stress hormone levels36 and the risk for arterial hypertension, heart disease, and stroke even after adjustment for major confounders.37–39 A similar phenomenon was recently demonstrated by more pronounced hypertensive effects of railway noise when exposure was during night-time.10 In addition, Eze et al.11 established that transportation noise may be more relevant than air pollution in the development of diabetes, potentially acting through noise-induced sleep disturbances. These observations are not surprising since the commonly used measurements of daytime noise have been suggested to be a source of exposure bias since during workdays most people spend their daytime hours out of the home and therefore night-time noise measurements might alleviate any exposure bias problem.
Studies comparing separately the adverse vascular effects of night-time vs. daytime aircraft noise are still missing. Around-the-clock aircraft noise has been demonstrated in a novel animal model to cause a marked increase in circulating neurohormonal stress hormones, to cause endothelial dysfunction, increased vascular inflammation, and oxidative stress in the plasma and within the vasculature occurred already within 1 day during a 4 days noise period.8 Enzymes being significantly involved in increased ROS production were the NADPH oxidase (Nox2 subunit, likely macrophages and neutrophils) and an uncoupled eNOS.8 Importantly, identical average SPL Leq(3) of white noise (72 dBA) were less damaging to the vasculature,8 suggesting that other parameters than noise energy such as dynamics or the frequency of the noise stimulus may be responsible for the vascular damage.
With the results of this studies, we provide for the first time the proof for a significant role of Nox2 for noise-triggered cerebral and vascular damage supporting the concept that Nox2-dependent oxidative stress represents an important constituent of noise-mediated cardiovascular disease progression. Nox2 deletion normalized noise-induced endothelial dysfunction, hyperglycemia, plasma malondialdehyde, 3-nitrotyrosine, IL-6 levels, and vascular superoxide production. Also around-the-clock noise-induced adversely altered protein (e.g. P-VASP, ET-1, HO-1) expression was normalized by Nox2 deletion.
Disturbance of sleep likely accounts for majority of aircraft noise-induced vascular damage
Importantly, with this studies, we were able to demonstrate that noise during the sleep (reflecting night-time noise) but not awake phase markedly adversely affects vascular function. Sleep phase but not awake phase noise caused endothelial dysfunction, increased vascular ET-1 expression and plasma leptin levels.
Sleep phase but not awake phase noise increased vascular ROS production and caused an uncoupling of the eNOS and increased plasma levels of markers for oxidative stress and inflammation such as malondialdehyde, 3-nitrotyrosine, and IL-6. Especially the increase in L-NAME-inhibitable endothelial ROS in vessels from animals with sleep phase noise was striking and goes parallel with observations made in human vascular tissue where this methodology was used to identify eNOS uncoupling in patients with an obstructive sleep apnoea syndrome and thus interrupted sleep.40 Of note, in that particular study the endothelial superoxide signal showed significant correlation with impaired •NO formation in the same samples, all of which was corrected by ex vivo treatment with eNOS cofactor BH4 and in vivo therapy by continuous positive airway pressure (CPAP).40
eNOS S-glutathionylation was reported as an important mechanism of eNOS uncoupling,41,42 which was here substantially increased in vessels from sleep but not awake noise exposed animals. We also observed an increase in eNOS phosphorylation at Ser1177 and expression of the BH4 synthesizing and recycling enzymes GCH-1 and DHFR, which may be interpreted as a compensatory response to other causes of eNOS uncoupling.
In our previous studies with healthy subjects and patients with coronary artery disease, exposure to 30 or 60 flight events per night, respectively was paralleled by a marked decrease in sleep quality.6,7 Interestingly, 2 h of sleep deprivation for eight days has been shown to cause endothelial dysfunction in healthy subjects, and the degree of deterioration of endothelial function was comparable to that observed in workers working 24-h shifts43 and in humans exposed to chronic sleep restriction.44
Using a mouse model, Carreras et al.15 demonstrated that endothelial dysfunction and arterial hypertension will develop upon exposure to 20 weeks of sleep deprivation. The authors observed a marked disruption of vascular elastic fibres and an increase in foam cells and macrophages within the vascular wall. In addition, sleep fragmentation reduced mRNA expression of the senescence markers TERT and cyclin A, the tumour suppressor p16INK4 and IL-6 levels.15 The same group also demonstrated that sleep fragmentation causes activation of the NADPH oxidase in the brain45 and increases oxidative stress,46 all of which was associated with insulin resistance. All of these features were also shared by mouse models of hypertension by angiotensin-II infusion47,48 and, as for the part on inflammation, oxidative stress, vascular dysfunction, and increases in blood pressure, also our mouse model of aircraft noise exposure.8
Thus, a likely explanation for the development of endothelial dysfunction caused by sleep phase noise exposure may be sleep deprivation and fragmentation, conditions also being linked with increased cardiovascular events and mortality.49,50
For more detailed discussion on the effects of night-time noise exposure on circadian clock pathways and vascular function, see Supplementary material online.
Aircraft noise causes oxidative stress in the brain
Chronic aircraft noise exposure has also been demonstrated to be associated with cognitive impairment of children17 and also mental disease in adults,51,52 all of which may be linked to increased oxidative stress in the brain located primarily in the frontal cortex.53
With this studies, we can demonstrate for the first time that aircraft noise markedly increases DHE staining and 2-hydroxyethidium formation (detected by HPLC) in the frontal cortex of the brain (but potentially also other brain regions). Importantly, inhibition of NOS by L-NAME increased the DHE signal indicating that ambient levels of NO scavenge baseline superoxide production in the brain, an observation, which goes parallel to observations made in the vasculature in the setting of arterial hypertension, diabetes mellitus and hypercholesterolemia.54–56 In the brain of aircraft noise exposed animals, however, inhibition of NOS by L-NAME reduced DHE signals compatible with NOS uncoupling. To further differentiate between eNOS and nNOS uncoupling we employed the highly specific nNOS inhibitor ARL-17477 and could demonstrate a marked reduction in the DHE signal compatible with nNOS uncoupling being a significant ROS source in the frontal cortex. This observation is further supported by noise-triggered phosphorylation of nNOS at serine 847, which causes nNOS inactivation and increased nNOS-dependent ROS formation (uncoupling).21,22 In addition, we established that Nox2 deletion ameliorated cerebral ROS production in response to aircraft noise, which could also affect the Ser847 phosphorylation and uncoupling of nNOS that is redox-regulated at the level of calcium/calmodulin-dependent protein kinase.22
The location of increased oxidative stress is important because the frontal cortex, is a critical regulator of autonomic and neuroendocrine stress responses57 and thus may contribute to the development of noise-induced cognitive impairment. The simultaneous decrease in nNOS expression in whole brain homogenate at the protein and mRNA level probably further contributes to the phenomenon by removing neuroprotective basal NO formation. Interestingly, sleep deprivation has also been shown to impair the cerebral redox balance and to cause increased cerebral oxidative stress and abnormal (manic-like) behaviour and memory impairment,58–60 conditions that are triggered by increased activity of the hypothalamic–pituitary–adrenal (HPA) axis, sympathetic activation and subsequent stress hormone production. In addition, Nox2 (gp91phox)-mediated increased oxidative stress was demonstrated to play an important role for learning and memory impairment.18 Thus, frequent awakening may cause sleep fragmentation and sleep restriction that may contribute to increased oxidative stress in the brain as well as to vascular damage as observed in this investigations.
For more detailed discussion on the effects of noise exposure on the brain see Supplementary material online.
Conclusions and clinical implications
Noise leads to annoyance, a form of mental stress that is associated with pathophysiological changes such as a pro-atherothrombotic phenotype,61,62 also found in response to tangible physical stress conditions such as pneumonia or myocardial infarction.63,64 This study is the first to demonstrate (i) increased markers of oxidative stress and inflammation in noise-exposed patients with established coronary artery disease, (ii) a significant impact of sleep vs. awake phase aircraft noise exposure on the vasculature, (iii) the stimulatory effects of aircraft noise on superoxide production in the brain by an uncoupled nNOS and Nox2 as well as induction of a neuro-inflammatory phenotype, and (iv) the protective effects of Nox2 deletion on aircraft noise-induced vascular dysfunction, cerebral superoxide production and neuro-inflammation. Our results clearly indicate that around-the-clock and sleep phase but not awake phase noise cause endothelial dysfunction, more inflammation and oxidative stress within plasma, the heart, the vasculature and the frontal cortex (and potentially other brain regions). It remains to be established which of these changes are easily reversible and which are more persistent, as already discussed previously.8 All these phenomena, including increased oxidative stress in the brain were strikingly reduced by the deletion of the Nox2 gene indicating a key role of Nox2 for aircraft noise-induced cerebrovascular damage.
Thus, the presented results may explain at least in part why sleep phase rather than awake phase noise leads to cardiovascular diseases and may also provide an explanation why aircraft noise is linked with cognitive impairment including retardations of learning and memory capabilities in children. Thus, preventive measures should be considered to reduce night-time aircraft noise.65
Supplementary Material
Acknowledgements
We are indebted to Angelica Karpi (University Medical Center Mainz, 55131 Mainz, Germany), Jörg Schreiner (University Medical Center Mainz, 55131 Mainz, Germany), Jessica Rudolph (University Medical Center Mainz, 55131 Mainz, Germany), Nicole Glas (University Medical Center Mainz, 55131 Mainz, Germany) and Bettina Mros (University Medical Center Mainz, 55131 Mainz, Germany) for expert technical assistance. We acknowledge the expert graphical assistance with preparation of the central figure by Margot Neuser. This work contains parts of the thesis of Katie Frenis (all data except ROS measurements and immunohistochemical stainings), Sanela Kalinovic and Ksenija Vujacic-Mirski (data on ROS measurements), Konstantina Filippou (immunohistochemical data). Katie Frenis, Sanela Kalinovic and Ksenija Vujacic-Mirski hold PhD stipends of the TransMed PhD Program at the University Medical Center Mainz.
Funding
This work was supported by a vascular biology research grant from the Boehringer Ingelheim Foundation for the collaborative research group ‘Novel and neglected cardiovascular risk factors: molecular mechanisms and therapeutic implications’ to study the effects of aircraft noise exposure on vascular function and oxidative stress (A.D., S.S., and T.M.). The specific studies on the effects of aircraft noise using different exposure protocols as well as the protective role of Nox2 deficiency were in part funded by the German Heart foundation (S.K.-S.). The pilot studies were supported by a vascular biology research grant from the Foundation Heart of Mainz (A.D., S.S., and T.M.). K.Fra. is recipient of a career development award of the Stavros Niarchos Foundation. T.M. is and P.S.W. PI of the DZHK (German Center for Cardiovascular Research), Partner Site Rhine-Main, Mainz, Germany.
Conflict of interest: none declared.
Footnotes
See page 3540 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy431)
References
- 1. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S.. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J 2017;38:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook J, Chen LC, Brook RD, Rajagopalan S.. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 2017;38:550–556. [DOI] [PubMed] [Google Scholar]
- 3. Munzel T, Gori T, Babisch W, Basner M.. Cardiovascular effects of environmental noise exposure. Eur Heart J 2014;35:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vienneau D, Schindler C, Perez L, Probst-Hensch N, Röösli M.. The relationship between transportation noise exposure and ischemic heart disease: a meta-analysis. Environ Res 2015;138:372–380. [DOI] [PubMed] [Google Scholar]
- 5. Munzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sorensen M.. Environmental noise and the cardiovascular system. J Am Coll Cardiol 2018;71:688–697. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt FP, Basner M, Kroger G, Weck S, Schnorbus B, Muttray A, Sariyar M, Binder H, Gori T, Warnholtz A, Munzel T.. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J 2013;34:3508–3314a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidt F, Kolle K, Kreuder K, Schnorbus B, Wild P, Hechtner M, Binder H, Gori T, Munzel T.. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin Res Cardiol 2015;104:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Münzel T, Daiber A, Steven S, Tran LP, Ullmann E, Kossmann S, Schmidt FP, Oelze M, Xia N, Li H, Pinto A, Wild P, Pies K, Schmidt ER, Rapp S, Kröller-Schön S.. Effects of noise on vascular function, oxidative stress, and inflammation: mechanistic insight from studies in mice. Eur Heart J 2017;38:2838–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jarup L, Babisch W, Houthuijs D, Pershagen G, Katsouyanni K, Cadum E, Dudley ML, Savigny P, Seiffert I, Swart W, Breugelmans O, Bluhm G, Selander J, Haralabidis A, Dimakopoulou K, Sourtzi P, Velonakis M, Vigna-Taglianti F.. Hypertension and exposure to noise near airports: the HYENA study. Environ Health Perspect 2008;116:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dratva J, Phuleria HC, Foraster M, Gaspoz J-M, Keidel D, Künzli N, Liu LJS, Pons M, Zemp E, Gerbase MW, Schindler C.. Transportation noise and blood pressure in a population-based sample of adults. Environ Health Perspect 2011;120:50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eze IC, Foraster M, Schaffner E, Vienneau D, Heritier H, Rudzik F, Thiesse L, Pieren R, Imboden M, von Eckardstein A, Schindler C, Brink M, Cajochen C, Wunderli JM, Roosli M, Probst-Hensch N.. Long-term exposure to transportation noise and air pollution in relation to incident diabetes in the SAPALDIA study. Int J Epidemiol 2017;46:1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spiegel K, Tasali E, Leproult R, Van Cauter E.. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 2009;5:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller MA, Cappuccio FP.. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol 2007;5:93–102. [DOI] [PubMed] [Google Scholar]
- 14. Calvin AD, Covassin N, Kremers WK, Adachi T, Macedo P, Albuquerque FN, Bukartyk J, Davison DE, Levine JA, Singh P, Wang S, Somers VK.. Experimental sleep restriction causes endothelial dysfunction in healthy humans. J Am Heart Assoc 2014;3:e001143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carreras A, Zhang SX, Peris E, Qiao Z, Gileles-Hillel A, Li RC, Wang Y, Gozal D.. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep 2014;37:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA.. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 17. Stansfeld SA, Berglund B, Clark C, Lopez-Barrio I, Fischer P, Ohrstrom E, Haines MM, Head J, Hygge S, van Kamp I, Berry BF;. RANCH study team. Aircraft and road traffic noise and children's cognition and health: a cross-national study. Lancet 2005;365:1942–1949. [DOI] [PubMed] [Google Scholar]
- 18. Kan H, Hu W, Wang Y, Wu W, Yin Y, Liang Y, Wang C, Huang D, Li W.. NADPH oxidase-derived production of reactive oxygen species is involved in learning and memory impairments in 16-month-old female rats. Mol Med Rep 2015;12:4546–4553. [DOI] [PubMed] [Google Scholar]
- 19. Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP.. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 2004;109:1795–1801. [DOI] [PubMed] [Google Scholar]
- 20. Park SH, Chung YM, Ma J, Yang Q, Berek JS, Hu MC.. Pharmacological activation of FOXO3 suppresses triple-negative breast cancer in vitro and in vivo. Oncotarget 2016;7:42110–42125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Komeima K, Hayashi Y, Naito Y, Watanabe Y.. Inhibition of neuronal nitric-oxide synthase by calcium/calmodulin-dependent protein kinase IIalpha through Ser847 phosphorylation in NG108-15 neuronal cells. J Biol Chem 2000;275:28139–28143. [DOI] [PubMed] [Google Scholar]
- 22. Kasamatsu S, Watanabe Y, Sawa T, Akaike T, Ihara H.. Redox signal regulation via nNOS phosphorylation at Ser847 in PC12 cells and rat cerebellar granule neurons. Biochem J 2014;459:251–263. [DOI] [PubMed] [Google Scholar]
- 23. Wang S, Yang K, Chen S, Wang J, Du G, Fan S, Wei L.. Indian hedgehog contributes to human cartilage endplate degeneration. Eur Spine J 2015;24:1720–1728. [DOI] [PubMed] [Google Scholar]
- 24. Handorf AM, Chamberlain CS, Li WJ.. Endogenously produced Indian Hedgehog regulates TGFbeta-driven chondrogenesis of human bone marrow stromal/stem cells. Stem Cells Dev 2015;24:995–1007. [DOI] [PubMed] [Google Scholar]
- 25. Hirano K, Chen WS, Chueng AL, Dunne AA, Seredenina T, Filippova A, Ramachandran S, Bridges A, Chaudry L, Pettman G, Allan C, Duncan S, Lee KC, Lim J, Ma MT, Ong AB, Ye NY, Nasir S, Mulyanidewi S, Aw CC, Oon PP, Liao S, Li D, Johns DG, Miller ND, Davies CH, Browne ER, Matsuoka Y, Chen DW, Jaquet V, Rutter AR.. Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid Redox Signal 2015;23:358–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frauenknecht K, Katzav A, Grimm C, Chapman J, Sommer CJ.. Neurological impairment in experimental antiphospholipid syndrome is associated with increased ligand binding to hippocampal and cortical serotonergic 5-HT1A receptors. Immunobiology 2013;218:517–526. [DOI] [PubMed] [Google Scholar]
- 27. Zhu C, Herrmann US, Falsig J, Abakumova I, Nuvolone M, Schwarz P, Frauenknecht K, Rushing EJ, Aguzzi A.. A neuroprotective role for microglia in prion diseases. J Exp Med 2016;213:1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wes PD, Sayed FA, Bard F, Gan L.. Targeting microglia for the treatment of Alzheimer's Disease. Glia 2016;64:1710–1732. [DOI] [PubMed] [Google Scholar]
- 29. Said MA, El-Gohary OA.. Effect of noise stress on cardiovascular system in adult male albino rat: implication of stress hormones, endothelial dysfunction and oxidative stress. Gen Physiol Biophys 2016;35:371–377. [DOI] [PubMed] [Google Scholar]
- 30. Sorensen M, Andersen ZJ, Nordsborg RB, Becker T, Tjonneland A, Overvad K, Raaschou-Nielsen O.. Long-term exposure to road traffic noise and incident diabetes: a cohort study. Environ Health Perspect 2013;121:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sorensen M, Andersen ZJ, Nordsborg RB, Jensen SS, Lillelund KG, Beelen R, Schmidt EB, Tjonneland A, Overvad K, Raaschou-Nielsen O.. Road traffic noise and incident myocardial infarction: a prospective cohort study. PLoS One 2012;7:e39283.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sorensen M, Hvidberg M, Andersen ZJ, Nordsborg RB, Lillelund KG, Jakobsen J, Tjonneland A, Overvad K, Raaschou-Nielsen O.. Road traffic noise and stroke: a prospective cohort study. Eur Heart J 2011;32:737–744. [DOI] [PubMed] [Google Scholar]
- 33. Sorensen M, Hvidberg M, Hoffmann B, Andersen ZJ, Nordsborg RB, Lillelund KG, Jakobsen J, Tjonneland A, Overvad K, Raaschou-Nielsen O.. Exposure to road traffic and railway noise and associations with blood pressure and self-reported hypertension: a cohort study. Environ Health 2011;10:92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang TY, Su TC, Lin SY, Jain RM, Chan CC.. Effects of occupational noise exposure on 24-hour ambulatory vascular properties in male workers. Environ Health Perspect 2007;115:1660–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dzhambov AM. Long-term noise exposure and the risk for type 2 diabetes: a meta-analysis. Noise Health 2015;17:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Selander J, Bluhm G, Theorell T, Pershagen G, Babisch W, Seiffert I, Houthuijs D, Breugelmans O, Vigna-Taglianti F, Antoniotti MC, Velonakis E, Davou E, Dudley ML, Jarup L, Consortium H.. Saliva cortisol and exposure to aircraft noise in six European countries. Environ Health Perspect 2009;117:1713–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haralabidis AS, Dimakopoulou K, Vigna-Taglianti F, Giampaolo M, Borgini A, Dudley ML, Pershagen G, Bluhm G, Houthuijs D, Babisch W, Velonakis M, Katsouyanni K, Jarup L, Consortium H.. Acute effects of night-time noise exposure on blood pressure in populations living near airports. Eur Heart J 2008;29:658–664. [DOI] [PubMed] [Google Scholar]
- 38. Jarup L, Babisch W, Houthuijs D, Pershagen G, Katsouyanni K, Cadum E, Dudley ML, Savigny P, Seiffert I, Swart W, Breugelmans O, Bluhm G, Selander J, Haralabidis A, Dimakopoulou K, Sourtzi P, Velonakis M, Vigna-Taglianti F.. team Hs. Hypertension and exposure to noise near airports: the HYENA study. Environ Health Perspect 2007;116:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Floud S, Blangiardo M, Clark C, de Hoogh K, Babisch W, Houthuijs D, Swart W, Pershagen G, Katsouyanni K, Velonakis M, Vigna-Taglianti F, Cadum E, Hansell AL.. Exposure to aircraft and road traffic noise and associations with heart disease and stroke in six European countries: a cross-sectional study. Environ Health 2013;12:89.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Varadharaj S, Porter K, Pleister A, Wannemacher J, Sow A, Jarjoura D, Zweier JL, Khayat RN.. Endothelial nitric oxide synthase uncoupling: a novel pathway in OSA induced vascular endothelial dysfunction. Respir Physiol Neurobiol 2015;207:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL.. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 2010;468:1115–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zweier JL, Chen CA, Druhan LJ.. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal 2011;14:1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amir O, Alroy S, Schliamser JE, Asmir I, Shiran A, Flugelman MY, Halon DA, Lewis BS.. Brachial artery endothelial function in residents and fellows working night shifts. Am J Cardiol 2004;93:947–949. [DOI] [PubMed] [Google Scholar]
- 44. Takase B, Akima T, Uehata A, Ohsuzu F, Kurita A.. Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clin Cardiol 2004;27:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, Gozal D.. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med 2011;184:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang SX, Khalyfa A, Wang Y, Carreras A, Hakim F, Neel BA, Brady MJ, Qiao Z, Hirotsu C, Gozal D.. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int J Obes (Lond) 2014; 38:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kroller-Schon S, Jansen T, Schuler A, Oelze M, Wenzel P, Hausding M, Kerahrodi JG, Beisele M, Lackner KJ, Daiber A, Munzel T, Schulz E.. Peroxisome proliferator-activated receptor gamma, coactivator 1alpha deletion induces angiotensin II-associated vascular dysfunction by increasing mitochondrial oxidative stress and vascular inflammation. Arterioscler Thromb Vasc Biol 2013;33:1928–1935. [DOI] [PubMed] [Google Scholar]
- 48. Kröller-Schön S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, Xia N, Hausding M, Mikhed Y, Zinßius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Münzel T, Daiber A.. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal 2014;20:247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chien KL, Chen PC, Hsu HC, Su TC, Sung FC, Chen MF, Lee YT.. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep 2010;33:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, Marmot MG.. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep 2007;30:1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beutel ME, Junger C, Klein EM, Wild P, Lackner K, Blettner M, Binder H, Michal M, Wiltink J, Brahler E, Munzel T.. Noise annoyance is associated with depression and anxiety in the general population—the contribution of aircraft noise. PLoS One 2016;11:e0155357.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stansfeld SA, Haines MM, Burr M, Berry B, Lercher P.. A Review of Environmental noise and mental health. Noise Health 2000;2:1–8. [PubMed] [Google Scholar]
- 53. Manikandan S, Padma MK, Srikumar R, Jeya Parthasarathy N, Muthuvel A, Sheela Devi R.. Effects of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial prefrontal cortex. Neurosci Lett 2006;399:17–22. [DOI] [PubMed] [Google Scholar]
- 54. Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T.. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 2001;88:E14–E22. [DOI] [PubMed] [Google Scholar]
- 55. Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T.. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 2002;90:E58–E65. [DOI] [PubMed] [Google Scholar]
- 56. Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Munzel T.. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric Oxide/cGMP signaling and endothelial dysfunction. Circ Res 2000;87:999–1005. [DOI] [PubMed] [Google Scholar]
- 57. McKlveen JM, Myers B, Herman JP.. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol 2015;27:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kanazawa LK, Vecchia DD, Wendler EM, Hocayen PA, Dos Reis Livero FA, Stipp MC, Barcaro IM, Acco A, Andreatini R.. Quercetin reduces manic-like behavior and brain oxidative stress induced by paradoxical sleep deprivation in mice. Free Radic Biol Med 2016;99:79–86. [DOI] [PubMed] [Google Scholar]
- 59. Alzoubi KH, Khabour OF, Albawaana AS, Alhashimi FH, Athamneh RY.. Tempol prevents chronic sleep-deprivation induced memory impairment. Brain Res Bull 2016;120:144–150. [DOI] [PubMed] [Google Scholar]
- 60. Schiavone S, Jaquet V, Trabace L, Krause KH.. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal 2013;18:1475–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zafar MU, Paz-Yepes M, Shimbo D, Vilahur G, Burg MM, Chaplin W, Fuster V, Davidson KW, Badimon JJ.. Anxiety is a better predictor of platelet reactivity in coronary artery disease patients than depression. Eur Heart J 2010;31:1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M.. Chronic variable stress activates hematopoietic stem cells. Nat Med 2014;20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Santos-Gallego CG, Badimon JJ.. The sum of two evils: pneumonia and myocardial infarction: is platelet activation the missing link? J Am Coll Cardiol 2014;64:1926–1928. [DOI] [PubMed] [Google Scholar]
- 64. Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M.. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.WHO and JRC report: burden of disease from environmental noise. http://www.euro.who.int/__data/assets/pdf_file/0008/136466/e94888.pdf (8 June 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.