Abstract
The first total synthesis of chaetoglobin A (1), which features a chiral axis between two identical highly oxygenated bicyclic cores, was successfully completed in 12 steps from 2,6-dimethoxytoluene. Vanadium-catalyzed oxidative phenol coupling, as a key step, enabled generation of the axial chirality.

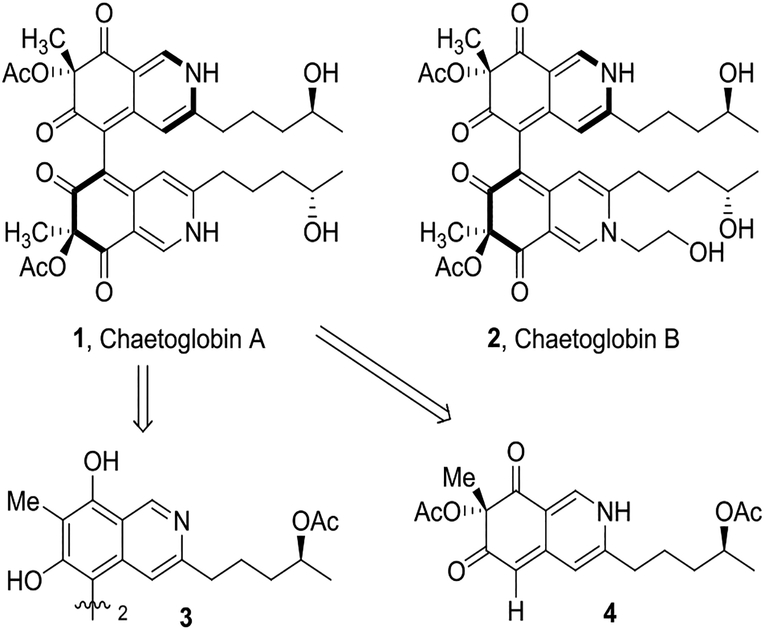
Chaetoglobin A is one of the azaphilone dimers, which contains two identical oxygenated bicyclic cores incorporating tertiary alcohol stereocenters that are connected through a chiral axis (Figure 1). It is isolated from the endophytic fungus chaetomium globosum, which lives in the stem of Imperata cylindrical. In 2008, Tan et al. reported its inhibitory ability on the propagation of human breast cancer and colon cancer cell lines.1 Previous studies on the biosynthesis of azaphilone alkaloids indicate that its core backbone is of polyketide origin.2 However, the nature of the dimerization and generation of attendant axial chiral stereochemistry has not been delineated. Specifically, it is unclear whether the stereochemistry of the chiral axis forms first and directs formation of the tertiary alcohol centers (via 3), if the tertiary alcohol centers form first and then direct formation of the chiral axis (via 4), or if these stereoelements are formed independently.
Figure 1.
Structure of chaetoglobin A (1) and B (2)
Due to their intriguing structures and biological activities, numerous attempts to synthesize the azaphilone alkaloids have been reported since the early 1970s.3 Approaches to introducing the bicyclic core proceed from either a keto-formyl precursor or alkynyl-formyl precursor that generate benzopyrylium salts or pyronoquinones as key intermediates. Often, acid-catalyzed cycloisomerization, followed by oxidation, was used to construct the 2H-isoquinoline-2,6-dione backbone. However, no synthetic efforts have been reported toward azaphilone dimers to date. Recently, we reported an efficient method to construct a chiral biaryl axis by means of vanadium-catalyzed enantioselective oxidative phenol coupling.4 The oxidative coupling permits the formation of chiral axes effectively. Here, we describe the total synthesis of chaetoglobin A utilizing this stereooselective oxidative phenol coupling to generate an axial chiral bisphenol dimer 7 as a key intermediate.
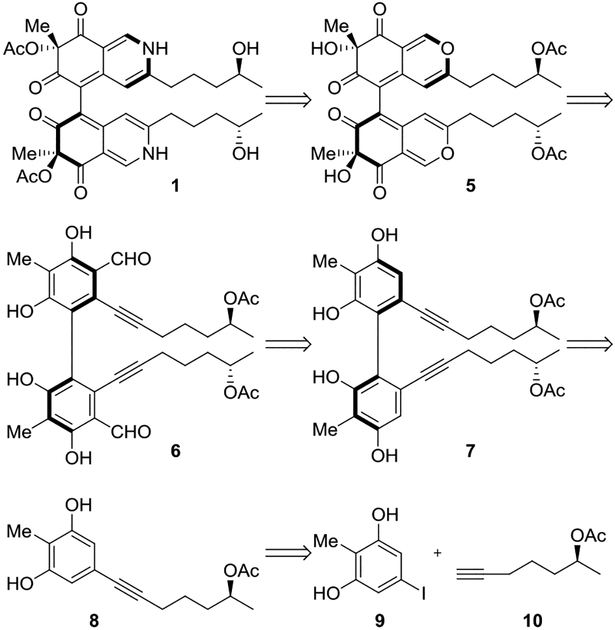
In our retrosynthetic analysis, the final isoquinoline moiety could be prepared by amination of 5 (Scheme 1). Lewis acid-catalyzed dearomatization followed by oxidation would allow for construction of the corresponding bicyclic core from formylated dimer 6. We hypothesize that the axial chirality of 6 could direct formation of the tertiary alcohol centers of 5. In doing so, we could determine the feasibility one of the possible biosynthetic pathways (i.e. via 3)( Figure 1). Vilsmeier-Haack formylation could install the necessary formyl groups on tetraphenol 7. We envisioned the atroposelective oxidative phenol coupling of 8 to afford pure atropoisomer 7. We anticipated that the acetoxy stereocenters of 8 would have little effect on stereochemical course of the phenol coupling with the chiral catalyst exerting control. Internal alkynyl monomer 8 would be prepared from the Sonogashira cross-coupling of bisphenol 9 and alkyne 10.
Scheme 1.
Retrosynthetic Analysis of Chaetoglobin A (1)
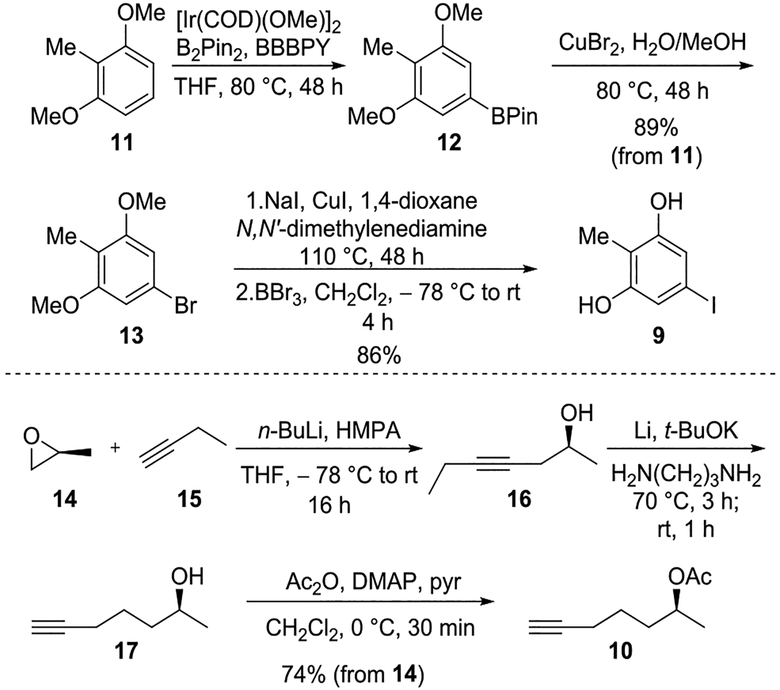
Synthetic efforts commenced with preparation of phenol 9 and alkyne 10 as shown in Scheme 2. Iridium-catalyzed borylation5 of commercially available 11, followed by halogenation allowed for the formation of bromide 13. Due to need for a more reactive electrophile for Sonogashira cross-coupling, halogen exchange was undertaken to generate iodophenol 9 after demethylation. Surprisingly, stepwise iodination via halogen exchange6 from bromide 13 proved to be more efficient than direct iodination7 from boronic ester 12. Following a literature report8 to prepare enantiomerically pure hydroxyalkyne 17, a nucleophilic ring opening with commercially available propylene oxide 14 and butyne 15 gave internal alkyne 16. Sequential alkyne isomerization was effected with a Li/KOt-Bu in 1,3-diaminopropane. Acetate protection of the free hydroxyl group provided the desired alkyne 10 in 74% yield over three steps.
Scheme 2.
Syntheses of Sonogashira Coupling Fragments 9 and 10
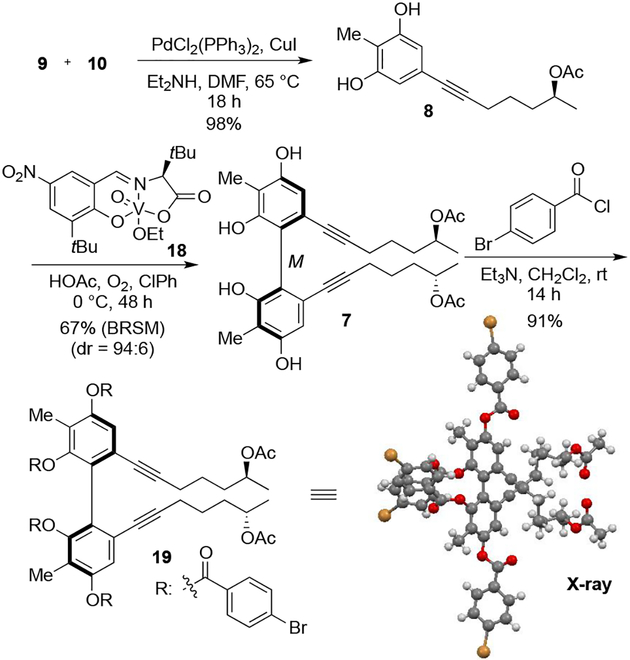
Optimization of Sonogashira coupling between iodide 9 and alkyne 10 led to the formation of oxidative phenol coupling precursor 8 in 98% yield (Scheme 3). Using our recently reported atroposelective oxidative coupling of phenols,4 vanadium catalyst 18 was applied to this transformation. Notably, complete catalyst control over the diastereoselectivity was observed with the opposite enantiomer of the catalyst providing the diastereomeric product with comparable dr. The additives, LiCl and HOAc, which are theorized to activate the vanadium catalyst result in significant improvements (Table 1). Treatment of 8 with LiCl as an additive gave high diastereoselectivity (Table 1, entry 2). Increased catalyst loading showed neither a significant improvement in yield, nor selectivity (Table 1, entry 3). Among alternative solvents, chlorobenzene seemed a promising candidate in terms of improving yield (Table 1, entry 2 vs 4 and entry 5 vs 6). Improved yield and comparable selectivity of dimer were realized with HOAc as the additive and chlorobenzene as solvent (Table 1, entry 6). Finally, further improved yield and diastereoselectivity was achieved under more concentrated reaction conditions (Table 1, entry 7). Use of chiral vanadyl catalyst 18 under the optimized conditions formed dimer 7 in 67% yield establishing the axial chiral element with >15:1 dr. The absolute stereochemistry of product 7 was confirmed by X-ray crystallographic analysis of the para-bromobenzoyl substituted derivative 19.9
Scheme 3.
Atroposelective Oxidative Phenol Coupling and Determination of Absolute Axial Stereochemistry
Table 1.
Diastereoselective Oxidative Coupling of 8
| entry | V-cat 18 | additivea | solventb | yield (%)c | dr |
|---|---|---|---|---|---|
| 1 | 20 mol % | – | chlorobenzened | 34 (44) | 79:21 |
| 2 | 20 mol % | LiCl | toluene | 52 (59) | 92:8 |
| 3 | 40 mol % | LiCl | toluene | 49 (52) | 93:7 |
| 4 | 20 mol % | LiCl | chlorobenzene | 54 (60) | 90:10 |
| 5 | 20 mol % | HOAc | toluene | 49 (55) | 87:13 |
| 6 | 20 mol % | HOAc | chlorobenzene | 49 (64) | 90:10 |
| 7 | 20 mol % | HOAc | chlorobenzened | 58 (67) | 94:6 |
20 mol %
0.3 M
Isolated yield based on recovery of substrate in parentheses
0.5 M
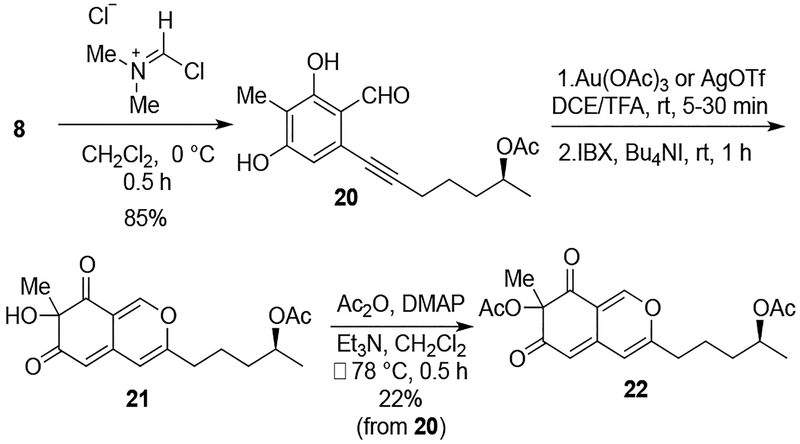
With the coupled atropoisomer in hand, our attention turned to the key oxidative dearomatization reaction, developed by Porco and coworkers, to generate the bicyclic core featuring a tertiary alcohol stereocenter.11 Initially, we tested this transformation using monomeric formylated phenol 20, which was easily prepared from monomer 8 in 85% yield (Scheme 4). Based on the literature, Au(OAc)3 was the optimal Lewis acid for this transformation. These conditions efficiently provided the desired oxygenated core 21 upon IBX oxidation as assessed by a monomer 22 after acetylation (72% yield over three steps).
Scheme 4.
Oxidative Dearomatization with Monomer 22
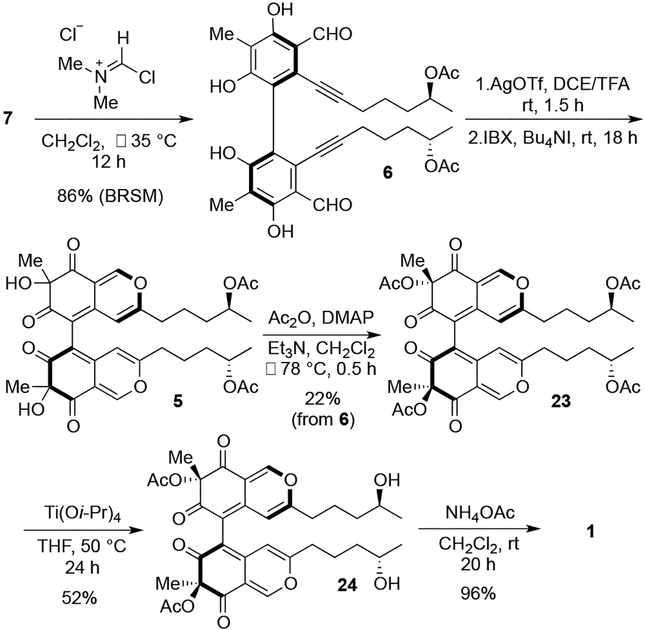
With dimer 7, several methods to formylate adjacent to the alkyne chain were surveyed.10 Ultimately, the Vilsmeier-Haack formylation with preformed (chloromethylene)dimethyliminium chloride at −35 °C provided bisformylated product 6 in 86% yield (Scheme 5) without acyl deprotection, which occurred in many of the other conditions screened due to acid byproducts formed during generation of the active acylating species.
Scheme 5.
Completion of Synthesis of Chaetoglobin A (1)
With dimer 6, however, Au(OAc)3-catalyzed dearomatization did not generate desired product 5, but caused decomposition. After screening several Lewis acids, we found that AgOTf12 gave a similar result in the model system from Scheme 4 (63% overall yield from 20). To our delight, cycloisomerization of 6 performed simlarly with AgOTf, and subsequent oxidation with a hypervalent iodine reagent, IBX, allowed access to bicyclic dimer 5 (Scheme 5). We had theorized that the chiral axis would influence the stereochemistry during formation of this tertiary alcohol center with the bulk of the 2H-isoquinoline-2,6-dione backbone blocking one stereoface. However, the reaction gave a 1:1:1 mixture of the three possible isomers (7S,7’S; 7S,7’R; 7R,7’R), indicating that the axial stereochemistry does not create a strong facial bias. Fortunately, sufficient amounts of each isomer could be obtained to proceed with the synthesis. The following section describes the results obtained with the faster eluting symmetric diastereomer, which could not be definitively assigned as 7S,7’S or 7R,7’R at this stage.
At this juncture, selective acylation of the tertiary alcohol centers was needed, which could be achieved either by deacylation of the secondary alcohols followed by selective acylation of the tertiary alcohols or by global acetylation followed by selection deacylation of the less hindered secondary alcohols. The former possibility takes advantage of the greater reactivity of the alcohols adjacent to the ketone.13 Upon removal of the acetyl groups from 5, the resultant product was found to be prone to decomposition. As such, the latter alternative became a focus. Acetylation of 5 afforded product 23 in 22% over three steps from 6, which represent six sets of chemical transformations due to the dimeric nature of the material (Scheme 4). Unexpectedly, selective deprotection of the secondary acetoxy group in the presence of the sterically congested tertiary acetoxy group was challenging. Attempted hydrolysis of 23 with acidic or basic conditions led decomposition that was faster than deprotection. We explored a wide range of hydrolysis conditions,14 including enzymatic methods,15 and finally established that 10 equiv of Ti(Oi-Pr)4 in CH2Cl2 at elevated temperature afforded free hydroxylated 24 in 52% yield (Scheme 4). Exposure of oxygenated bicycle 24 to excess NH4OAc furnished synthetic chaetoglobin A (1) in nearly quantitative yield.
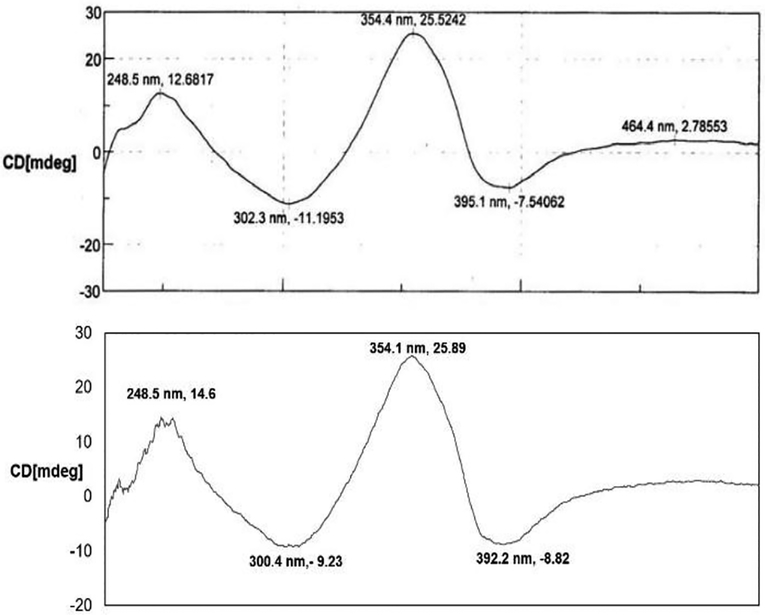
The spectroscopic data from synthetic 1 was in accord with those reported in the literature for chaetoglobin A.1 In particular, the 13C NMR gave 17 differentiable carbons supporting the symmetric structure. The chemical shifts of those signals closely matched those from the natural product securing evidence that the correct diastereomeric relationship between the tertiary alcohols and the stereoaxis had been generated. Importantly, circular dichroism data obtained from the synthetic material proved identical to that from the natural product (Figure 2), which indicates that both the same absolute and relative stereochemistries are established.
Figure 2.
Comparing circular dichroism of chaetoglobin A (1) (a) CD spectrum of natural product isolate. Adapted from reference 1 with permission of The Royal Society of Chemistry; (b) synthetic 1 CD spectrum.
In conclusion, we have accomplished the first total synthesis of chaetoglobin A in 4.3% overall yield, with 12 steps in the longest linear sequence. A vanadium-catalyzed atroposelective oxidative phenol coupling serves as a key step in the formation of stereoaxis of chiral azaphilone dimer 1. The stereochemical results from oxidative cyclization after formation of the stereoaxis raise interesting questions about the operating biosynthetic pathways.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to the NIH (GM-112684) and the NSF (CHE1464778, CHE1764298) for financial support of this research. Partial instrumentation support was provided by the NIH and NSF (1S10RR023444, 1S10RR022442, CHE 0840438, CHE-0848460, 1S10OD011980). Drs. Rakesh Kohli and Charles W. Ross III (UPenn) are acknowledged for obtaining HRMS data. We thank Prof. Virgil Percec and Benjamin Partridge (UPenn) for assistance with CD measurements. We are grateful to Dr. Patrick Carroll for assistance with crystallographic structures.
Footnotes
Supporting Information
Experimental procedures, compound characterization, crystallographic data, NMR spectra. The Supporting Information is available free of charge on the ACS Publications website.
REFERENCES
- (1).Ming Ge H; Yun Zhang W; Ding G; Saparpakorn P; Chun Song Y; Hannongbua S; Xiang Tan R Chaetoglobins A and B, Two Unusual Alkaloids from Endophytic Chaetomium Globosum Culture. Chem. Commun 2008, 5978–5980. [DOI] [PubMed] [Google Scholar]
- (2).(a) Colombo L; Gennari C; Ricca GS; Scolastico C; Aragozzini F Detection of One Symmetrical Precursor during the Biosynthesis of the Fungal Metabolite Austdiol Using [1,2–13C2]Acetate and [Me-13C]Methionine. J. Chem. Soc. Chem. Commun 1981, 575–576. (b) Takahashi M; Koyama K; Natori S Four New Azaphilones from Chaetomium Globosum Var. Flavo-Viridae. Chem. Pharm. Bull. (Tokyo) 1990, 38, 625,–628. (c) Seto H; Tanabe M Utilization of 13C-13C Coupling in Structural and Biosynthetic Studies. III. Ochrephilone - a New Fungal Metabolite. Tetrahedron Lett 1974, 15, 651,–654. (d) Duncan SJ; Williams DH; Ainsworth M; Martin S; Ford R; Wrigley SK On the Biosynthesis of an Inhibitor of the P53/MDM2 Interaction. Tetrahedron Lett 2002, 43, 1075,–1078. (e) Nakazawa T; Ishiuchi K; Sato M; Tsunematsu Y; Sugimoto S; Gotanda Y; Noguchi H; Hotta K; Watanabe K Targeted Disruption of Transcriptional Regulators in Chaetomium Globosum Activates Biosynthetic Pathways and Reveals Transcriptional Regulator-Like Behavior of Aureonitol. J. Am. Chem. Soc 2013, 135, 13446–13455. [Google Scholar]
- (3).(a) Chong R; King RR; Whalley WB The Synthesis of Sclerotiorin and of an Analogue of Rotiorin. J. Chem. Soc. Chem. Commun 1969, 1512–1513. (b) Chong R; Gray RW; King RR; Whalley WB The Synthesis of (±) Mitorubrin. J. Chem. Soc. Chem. Commun 1970, 0, 101a,- 101a, (c) Galbraith MN; Whalley WB The Chemistry of Fungi. Part LIX. The Synthesis of (±)-Ascochitine. J Chem Soc C 1971, 3557,–3559. (d) Chong R; Gray RW; King RR; Whalley WB The Chemistry of Fungi. Part LXII. The Synthesis of (+/−)-Mitorubrin, a Metabolite of Penicillium Rubrum. J Chem Soc C 1971, 3571,–3575. (e) Zhu J; Grigoriadis NP; Lee JP; Porco JA Synthesis of the Azaphilones Using Copper-Mediated Enantioselective Oxidative Dearomatization. J. Am. Chem. Soc 2005, 127, 9342,–9343. (f) Marsini MA; Gowin KM; Pettus TRR Total Synthesis of (±)-Mitorubrinic Acid. Org. Lett 2006, 8, 3481,–3483. (g) Zhu J; Porco JA Asymmetric Syntheses of (−)-Mitorubrin and Related Azaphilone Natural Products. Org. Lett 2006, 8, 5169,–5171 (h) Lee SY; Clark RC; Boger DL Total Synthesis, Stereochemical Reassignment, and Absolute Configuration of Chlorofusin. J. Am. Chem. Soc 2007, 129, 9860,–9861. (i) Qian W-J; Wei W-G; Zhang Y-X; Yao Z-J Total Synthesis, Assignment of Absolute Stereochemistry, and Structural Revision of Chlorofusin. J. Am. Chem. Soc 2007, 129, 6400,–6401. (j) Clark RC; Yeul Lee S; Boger DL Total Synthesis of Chlorofusin, Its Seven Chromophore Diastereomers, and Key Partial Structures. J. Am. Chem. Soc 2008, 130, 12355,–12369. (k) Clark RC; Lee SY; Searcey M; Boger DL The Isolation, Total Synthesis and Structure Elucidation of Chlorofusin, a Natural Product Inhibitor of the P53–MDM2 Protein–Protein Interaction. Nat. Prod. Rep 2009, 26, 465,–477. (l) Boulangé A; Peixoto PA; Franck X Diastereoselective IBX Oxidative Dearomatization of Phenols by Remote Induction: Towards the Epicocconone Core Framework. Chem. – Eur. J 2011, 17, 10241,–10245. (m) Germain AR; Bruggemeyer DM; Zhu J; Genet C; O’Brien P; Porco JA Synthesis of the Azaphilones (+)-Sclerotiorin and (+)-8-O-Methylsclerotiorinamine Utilizing (+)-Sparteine Surrogates in Copper-Mediated Oxidative Dearomatization. J. Org. Chem 2011, 76, 2577,–2584. (n) Somoza AD; Lee K-H; Chiang Y-M; Oakley BR; Wang CCC Reengineering an Azaphilone Biosynthesis Pathway in Aspergillus Nidulans To Create Lipoxygenase Inhibitors. Org. Lett 2012, 14, 972,–975. (o) Lin L; Mulholland N; Wu Q-Y; Beattie D; Huang S-W; Irwin D; Clough J; Gu Y-C; Yang G-F Synthesis and Antifungal Activity of Novel Sclerotiorin Analogues. J. Agric. Food Chem 2012, 60, 4480,–4491. (p) Achard M; Beeler AB; Porco JA Synthesis of Azaphilone-Based Chemical Libraries. ACS Comb. Sci 2012, 14, 236,–244. (q) Gao J-M; Yang S-X; Qin J-C Azaphilones: Chemistry and Biology. Chem. Rev 2013, 113, 4755,–4811. (r) Peixoto PA; Boulangé A; Ball M; Naudin B; Alle T; Cosette P; Karuso P; Franck X Design and Synthesis of Epicocconone Analogues with Improved Fluorescence Properties. J. Am. Chem. Soc 2014, 136, 15248,–15256. (s) Qiu H-B; Qian W-J; Yu S-M; Yao Z-J Stereodivergent Total Synthesis of Chlorofusin and Its All Seven Chromophore Diastereomers. Tetrahedron 2015, 71, 370–380. [Google Scholar]
- (4).Kang H; Lee YE; Reddy PVG; Dey S; Allen SE; Niederer KA; Sung P; Hewitt K; Torruellas C; Herling MR; Kozlowski MC Asymmetric Oxidative Coupling of Phenols and Hydroxycarbazoles. Org. Lett 2017, 19, 5505–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Murphy JM; Liao X; Hartwig JF Meta Halogenation of 1,3-Disubstituted Arenes via Iridium-Catalyzed Arene Borylation. J. Am. Chem. Soc 2007, 129, 15434–15435. [DOI] [PubMed] [Google Scholar]
- (6).Klapars A; Buchwald SL Copper-Catalyzed Halogen Exchange in Aryl Halides: An Aromatic Finkelstein Reaction. J. Am. Chem. Soc 2002, 124, 14844–14845. [DOI] [PubMed] [Google Scholar]
- (7).Partridge BM; Hartwig JF Sterically Controlled Iodination of Arenes via Iridium-Catalyzed C–H Borylation. Org. Lett 2013, 15, 140–143. [DOI] [PubMed] [Google Scholar]
- (8).Wu Y; Gao J Total Synthesis of (+)-Brefeldin A. Org. Lett 2008, 10, 1533–1536. [DOI] [PubMed] [Google Scholar]
- (9).Opposite chiral axis compound (P)-19 crystallographic data included in Supporting Information.
- (10).(a) Ramadas S; Krupadanam GLD Enantioselective Acylation of 2-Hydroxymethyl-2,3-Dihydrobenzofurans Catalysed by Lipase from Pseudomonas Cepacia (Amano PS) and Total Stereoselective Synthesis of (−)-(R)-MEM-Protected Arthrographol. Tetrahedron Asymmetry 2000, 11, 3375–3393. (b) Phan DHT; Kim B; Dong VM Phthalides by Rhodium-Catalyzed Ketone Hydroacylation. J. Am. Chem. Soc 2009, 131, 15608,–15609. (c) Ohsawa K; Yoshida M; Doi T A Direct and Mild Formylation Method for Substituted Benzenes Utilizing Dichloromethyl Methyl Ether–Silver Trifluoromethanesulfonate. J. Org. Chem 2013, 78, 3438–3444. [Google Scholar]
- (11).Zhu J; Germain AR; Porco JA Synthesis of Azaphilones and Related Molecules by Employing Cycloisomerization of O-Alkynylbenzaldehydes. Angew. Chem. Int. Ed 2004, 43, 1239–1243. [DOI] [PubMed] [Google Scholar]
- (12).Feng X; Wang H; Yang B; Fan R One-Pot Synthesis of Highly Substituted 4-Acetonylindoles via Sequential Dearomatization and Silver-Catalyzed Domino Reaction. Org. Lett 2014, 16, 3600–3603. [DOI] [PubMed] [Google Scholar]
- (13).Holton RA; Zhang Z; Clarke PA; Nadizadeh H; Procter DJ Selective Protection of the C(7) and C(10) Hydroxyl Groups in 10-Deacetyl Baccatin III. Tetrahedron Lett 1998, 39, 2883–2886. [Google Scholar]
- (14).(a) Plattner JJ; Gless RD; Rapoport H Synthesis of Some DE and CDE Ring Analogs of Camptothecin. J. Am. Chem. Soc 1972, 94, 8613–8615. (b) Mori K; Tominaga M; Takigawa T; Matsui M A Mild Transesterification Method. Synthesis 1973, 790,–791. (c) Askin D; Angst C; Danishefsky S An Approach to the Synthesis of Bactobolin and the Total Synthesis of N-Acetylactinobolamine: Some Remarkably Stable Hemiacetals. J. Org. Chem 1987, 52, 622,–635. (d) Nudelman A; Herzig J; Gottlieb HE; Keinan E; Sterling J Selective Deacetylation of Anomeric Sugar Acetates with Tin Alkoxides. Carbohydr. Res 1987, 162, 145,–152. (e) Baptistella LHB; Santos JFD; Ballabio KC; Marsaioli AJ 1,8-Diazabicyclo[5.4.0]Undec-7-Ene as a Mild Deprotective Agent for Acetyl Groups. Synthesis 1989, 21, 436,–439. (f) Roush WR; Lin X-F Studies on the Synthesis of Aureolic Acid Antibiotics: Highly Stereoselective Synthesis of Aryl 2-Deoxy-.Beta.-Glycosides via the Mitsunobu Reaction and Synthesis of the Olivomycin A-B Disaccharide. J. Am. Chem. Soc 1995, 117, 2236,–2250. (g) Yamamoto N; Nishikawa T; Isobe M Synthesis of Bicyclic Hydroxy Lactone Intermediates toward (−)-Tetrodotoxin. Synlett 1995, 505,–506. (h) Yanada R; Negoro N; Bessho K; Yanada K Metallic Samarium and Iodine in Alcohol. Deacylation and Dealkyloxycarbonylation of Protected Alcohols and Lactams. Synlett 1995, 1261,–1263. (i) Xu Y-C; Bizuneh A; Walker C A Reagent for Selective Deprotection of Alkyl Acetates. J. Org. Chem 1996, 61, 9086,–9089. (j) Kajiro H; Mitamura S; Mori A; Hiyama T Scandium Trifluoromethanesulfonate-Catalyzed Mild, Efficient, and Selective Cleavage of Acetates Bearing a Coordinative Group. Tetrahedron Lett 1999, 40, 1689,–1692. (k) Sharma GVM; Ilangovan A Ytterbium Triflate Mediated Selective Deprotection of Acetates. Synlett 1999, 1963,–1965. (l) Yoshimoto K; Kawabata H; Nakamichi N; Hayashi M Tris(2,4,6-Trimethoxyphenyl)Phosphine (TTMPP): A Novel Catalyst for Selective Deacetylation. Chem. Lett 2001, 30, 934,–935. (m) Ravindranathan Kartha KP; Mukhopadhyay B; Field RA Practical De-O-Acylation Reactions Promoted by Molecular Sieves. Carbohydr. Res 2004, 339, 729,–732. (n) Wang S-M; Ge W-Z; Liu H-M; Zou D-P; Yan X-B Syntheses of Acetylated Steroid Glycosides and Selective Cleavage of O-Acetyl Groups in Sugar Moiety. Steroids 2004, 69, 599,–604. (o) Ren B; Cai L; Zhang L-R; Yang Z-J; Zhang L-H Selective Deacetylation Using Iodine–methanol Reagent in Fully Acetylated Nucleosides. Tetrahedron Lett 2005, 46, 8083,–8086. (p) Yeom C-E; Lee SY; Kim YJ; Kim BM Mild and Chemoselective Deacetylation Method Using a Catalytic Amount of Acetyl Chloride in Methanol. Synlett 2005, 1527,–1530. (q) Ranu BC, Guchhait SK, Saha M, J. Indian Chem. Soc 1999, 76, 547. [Google Scholar]
- (15).(a) Sakaki J; Sakoda H; Sugita Y; Sato M; Kaneko C Lipase-Catalyzed Asymmetric Synthesis of 6-(3-Chloro-2-Hydroxypropyl)-1,3-Dioxin-4-Ones and Their Conversion to Chiral 5,6-Epoxyhexanoates. Tetrahedron Asymmetry 1991, 2, 343–346. (b) Lopez R; Montero E; Sanchez F; Canada J; Fernandez-Mayoralas A Regioselective Acetylations of Alkyl.Beta.-D-Xylopyranosides by Use of Lipase PS in Organic Solvents and Application to the Chemoenzymic Synthesis of Oligosaccharides. J. Org. Chem 1994, 59, 7027,–7032. (c) Houille O; Schmittberger T; Uguen D A Remarkably Simple Process for Monoprotecting Diols. Tetrahedron Lett 1996, 37, 625–628. [Google Scholar]
- (16).CCDC 1822188 ((P)-19), and 1822189 (19) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.