Abstract
In elementary neural circuits, changes in excitability can have a strong impact in the expression of a given behavior. One example is provided by B51, a neuron with decision-making properties in the feeding neural circuit of the mollusk Aplysia. The excitability of B51 is bidirectionally modulated by external and internal stimuli in a manner that is consistent with the corresponding induced changes in feeding behavior. For example, in operant reward learning, which up-regulates feeding, B51 excitability is increased via a cAMP-dependent mechanism. Conversely, following training protocols with aversive stimuli, which down-regulate feeding, B51 excitability is decreased. In this study, we tested the hypothesis that B51 decreased excitability may be mediated by another cyclic nucleotide, cGMP. Our results revealed that iontophoretic injection of cGMP was capable of inducing both short-term (45 min) and long-term (24 h) reduction of B51 excitability. We next investigated which biochemical trigger could increase cGMP cytosolic levels. The neurotransmitter nitric oxide was found to decrease B51 excitability through the activation of the soluble guanylyl cyclase. These findings indicate that a cGMP-dependent pathway modulates B51 excitability in a manner opposite of cAMP, indicating that distinct cyclic-nucleotide pathways bidirectionally regulate the excitability of a decision-making neuron.
Keywords: Aplysia, Excitability, Neuronal plasticity, Second messengers
1. Introduction
In recent years, the role of plasticity of neuronal excitability has become increasingly apparent in diverse physiological and pathological areas, including: brain-metabolism axis [1], fragile X syndrome [2], depressive [3,4], and other neurological disorders [5], addiction [6], and learning and memory [7,8]. In circumstances of elementary computation within simple neural circuits, changes in excitability may be highly effective in influencing the expression of a given behavior [7–9]. One example is provided by neurons with decision-making features in which modifications in excitability are correlated with changes in behavior, such as neuron B51 in the mollusk Aplysia califomica [10,11].
In Aplysia, the goal-oriented behavior of feeding is controlled by a well-characterized neural circuit, which generates the rhythmic output activity underlying the cycles of protraction, closure and retraction of the radula that constitute bites [12,13]. Within the feeding neural circuit, B51 is a neuron with in vitro decision-making features that contributes to the elicitation of ingestive buccal motor programs (BMPs), which are in vitro neurophysiological representations of bites/swallows [13–16]. B51 exhibits intrinsically-generated all-or-nothing bursts of action potentials, known as plateau potentials [14,17], which participate in the selection of ingestive BMPs [10,15]. B51 is capable of switching in an all-or-nothing fashion from an inactive sub-threshold state to an active plateau potential during the occurrence of ingestive BMPs [11,15,18]. Notably, artificial suprathreshold activation of B51 biases the feeding neural circuit to generate ingestive BMPs, whereas hyperpolarization of B51 prevents their occurrence [15].
Various external and internal stimuli modulate the expression of feeding [11] and, subsequently, induce modifications of B51 excitability that are consistent with the observed changes in feeding [11]. For example, an increase in B51 excitability has been associated with the increased number of ingestive BMPs in vitro and bites in vivo produced by operant reward learning [15,17,19]. Conversely, a decrease of B51 excitability has been observed following nonassociative training with aversive stimuli, which induces sensitization of defensive reflexes and reduces feeding [20]. A decrease of B51 excitability has been reported also following a food-satiation paradigm, which suppresses feeding for several days [18]. These findings highlight an interesting example of bidirectional modulation of excitability in a decision-making neuron, which can influence the outcome of a behaviorally-relevant neural circuit in opposite directions.
Previous work has identified cAMP as the second messenger underlying the increase of B51 excitability induced by operant reward learning [19,21]. In particular, iontophoretic injections of cAMP, mimicking short-term and long-term conditioning protocols, induced increases of B51 excitability lasting a few minutes and 24 h, respectively [19,21]. The goal of this study was to determine which second messenger would induce a decrease of B51 excitability. We focused on another cyclic nucleotide, cGMP, which is known to modulate neuronal excitability in numerous systems, including Aplysia [22,23]. In addition, cGMP signaling has been implicated in a form of learning that results in decreased feeding behavior in Aplysia [24,25], thus positing it as an interesting candidate to modulate B51 activity in a way that is consistent with feeding suppression.
2. Materials and methods
2.1. Animals
Adult specimen of Aplysia califomica were obtained from South Coast Bio-Marine (San Pedro, CA), and were individually housed in two aquaria (Aquatic Enterprises Inc., WA) of continuously circulating 15 °C aquarium seawater (Instant Ocean) on a 12-h light/dark cycle. Animals were fed one strip of dried seaweed (3 ×19 cm; 0.5 g; Emerald Cove® Organic Pacific Nori; Great Eastern Sun, Asheville, NC) three times a week [26]. Animals were food deprived for 2–3 days before the beginning of an experiment [27]. Prior to each experiment, the general health and motivational state of an animal were tested by presenting it with a food stimulus. An animal was included in the study only if it generated bites in response to the food stimulus [27].
2.2. Dissection and ganglion preparation
Aplysia were anesthetized by injecting a volume of isotonic MgCl2 (equivalent to 50% of the weight of the animal) through the foot [27,28]. The buccal ganglion was removed and pinned rostral-side up on the Sylgard-coated bottom of a Plexiglas recording chamber containing artificial seawater with a high concentration of divalent cations (high-divalent ASW), composed of 210 mM NaCl, 10 mM KCl, 145 mM MgCl2, 20 mM MgSO4, 33 mM CaCl2, and 10 mM HEPES, with the pH adjusted to 7.5 using NaOH [28]. Ganglia were subsequently desheathed to access the soma of neuron B51 [15,20].
After desheathing, the high-divalent ASW was exchanged for normal ASW (composed of 450 mM NaCl, 10 mM KCl, 30 mM MgCl2, 20 mM MgSO4, 10 mM CaCl2, and 10 mM HEPES, with pH adjusted to 7.5 using NaOH; Mozzachiodi et al. [28]) and the preparation was rested for 30 min. The recording chamber was maintained at 15° C throughout the electrophysiological recordings and experimental treatments with a feedback-controlled cooling device (Model BTC-100/BTC-S, Bioscience Tools, CA).
2.3. B51 identification and measurement of membrane properties
The soma of neuron B51 was identified based on its relative size and position (Fig. 1A1) and the occurrence of its characteristic intrinsically-generated plateau potentials [14,15,17]. Although standard two-electrode current-clamp technique was used for intracellular recording and stimulation in all experiments [15,19], different electrode configurations were employed. In the first two experiments, in which iontophoretic injection of cGMP was used, B51 was initially impaled with one electrode containing 3-M potassium acetate and one iontophoresis electrode loaded with either cGMP or vehicle (Figs. 1A1, Fig. 2A1) [19,21]. In all the remaining experiments, B51 was impaled with two electrodes containing 3-M potassium acetate [15].
Fig. 1.

Time course of the effects of single injection of cGMP on B51 excitability. (A) Placements of recording and iontophoretic electrodes in B51 during pre-test (A1) and post-tests (A2), and timeline of B51 measurements prior to and after the single-injection (A3). (B) Sample traces of B51 burst threshold from cGMP-injected (B1) and vehicle-injected cells (B2). (C) Summary data illustrate that the single cGMP injection significantly increased B51 burst threshold for up to 45 min after treatment. In this and in the following figure, statistical significance is indicated by an asterisk.
Fig. 2.

A four-cGMP injection protocol induced a decrease of B51 excitability 24 h after treatment. (A) Placement of recording and iontophoretic electrodes in B51 during pre-test (A1) and 24-h post-test (A2), and timeline of B51 measurements prior to and 24 h after the four-injections (A3). (B) Sample traces of B51 burst threshold from cGMP-injected (B1) and vehicle-injected cells (B2). (C) Summary data illustrate that four cGMP injections significantly increased B51 burst threshold 24 h after treatment.
Five min after impalement, a pre-test was conducted to measure resting membrane potential (Vm), input resistance (Rm) and excitability. Cells were included in the study only if they displayed a resting membrane potential of at least −45 mV [20]. After recording the Vm, B51 was clamped at −60 mV. In experiments using an iontophoresis electrode, a subthreshold impulse of depolarizing current (5-s duration, 2-nA intensity) was used to measure Rm, because injection of negative current would lead to cGMP iontophoresis into B51 [23]. In all other experiments, Rm was measured using a 5-s, 5-nA hyperpolarizing current impulse [15].
Excitability was assessed by measuring the threshold to elicit a plateau potential, defined as the minimum amount of depolarizing current necessary to elicit a burst of activity that outlasted the duration of a 5-s pulse of depolarizing current [15]. The burst threshold was measured by injecting 5-s depolarizing current pulses of incremental intensity at 10-s intervals, beginning at 5 nA, until the cell fired a plateau potential [15]. In cases in which B51 elicited a plateau potential at 5 nA, the neuron’s response to 2 nA, 3 nA and 4 nA was also assessed to ensure that the burst threshold was not overestimated. Cells that did not exhibit a plateau potential by 25 nA were excluded from the study [27]. Post-tests were conducted identically to the pre-tests at different time points after injection/treatment protocols [19,27]. An increase of burst threshold would correspond to a decrease of excitability, whereas a decrease of burst threshold would correspond to an increase of excitability.
2.4. Protocols for cGMP injection experiments
The first two experiments involved the iontophoretic injection of cGMP into the cytosol of B51. The iontophoresis electrode was loaded with either cGMP (30 mM in 1% NaHCO3) or vehicle (1% NaHCO3) [23]. We developed two iontophoretic injection protocols, single injection and four injections, to mimic in-vitro learning paradigms that use electrical nerve stimulations to induce short-term (15 min) and long-term (24 h) decrease of B51 excitability, respectively [27]. In both protocols, each injection consisted of 30 s of 20-nA hyperpolarizing current delivered through the iontophoresis electrode. Similar protocols, which previously delivered iontophoretic injection of cAMP, successfully increased B51 excitability [19,21]. Preparations were randomly assigned to receive either cGMP or vehicle injection.
In the single-injection experiment, one potassium acetate electrode and one iontophoresis electrode were used for the entire duration of the experiment (Fig. 1A1, A2). During the pre-test, Vm, Rm and excitability were measured. Ten min after the end of the pre-test a single injection of either cGMP or vehicle was delivered. In a pilot experiment, a post-test was performed 5 min after the injection. In the subsequent experiment, post-tests were conducted 15 min, 30 min, 45 min, 1 h, after injection (Fig. 1A3).
In the four-injection experiment, one potassium acetate electrode and one iontophoresis electrode were used for the pre-test and treatment with cGMP/vehicle (Fig. 2A1). A pre-test was conducted as illustrated above for the single injection (Fig. 2A1). Ten min after the end of the pre-test, four consecutive injections of either cGMP or vehicle were delivered, spaced 30 min apart (Fig. 2A3). Ten min after the end of the injection protocol, electrodes were gently removed from B51 and ASW was exchanged with a modified L-15 culture medium. This medium consisted of L-15 medium (Sigma) supplemented with (in mM): NaCl 293.1, KC1 4.6, MgCl2 26, MgSO4 18.9, CaCl2 10.9, HEPES 30 and 0.10 g/1 of streptomycin (Sigma) and 0.12 g/l of penicillin-G (Sigma), with the pH adjusted to 7.5 with NaOH [19,27]. The recording chamber was then covered and stored in an incubator at 15 °C for 23.5 h. On the next day, ganglia were rinsed in normal ASW and allowed to rest for 30 min [19,27]. Post-test measurements of membrane properties were conducted 24 h after the end of treatment using two potassium acetate electrodes (Fig. 2A2, A3).
2.5. Protocols for pharmacological inhibition/activation of the nitric oxide – cGMP signaling pathway
In the remaining experiments, we employed a series of chemicals to manipulate the nitric oxide (NO) – cGMP signaling pathway. We used 1H-(1,2,4) oxidazole (4,3-a) quinoxalin-l-one (ODQ) to inhibit the soluble guanylyl cyclase (sGC) [29]. ODQ was delivered via bolus application (final chamber concentration: 25 μM in 0.1% DMSO [30]. Control preparations were treated with bolus application of DMSO (final chamber concentration: 0.l%). B51 membrane properties were measured prior to (pre-test) and 15 min after ODQ/DMSO application (post-test, Fig. 3A).
Fig. 3.
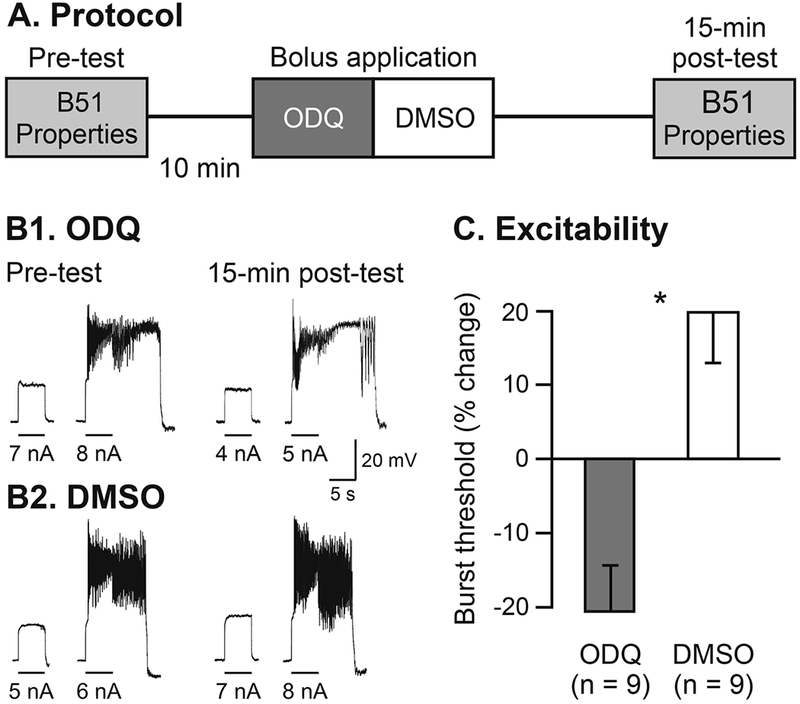
Inhibition of sGC activity increased B51 excitability. (A) Protocol of B51 measurements prior to and 15 min after ODQ/DMSO treatment. (B) Sample traces of B51 burst threshold from ODQ-treated (B1) and DMSO-treated cells (B2). (C) Summary data illustrate that ODQ significantly decreased B51 burst threshold 15 min after treatment.
We used the NO donor S-nitroso-N-acetyl-penicillamine (SNAP) to trigger release of NO in the buccal ganglia [31]. SNAP was delivered via a bolus application (final chamber concentration: 100 μM in 0.1% DMSO) [25]. Control preparations were treated with bolus application of DMSO (final chamber concentration: 0.1%). B51 membrane properties were measured prior to (pre-test) and after SNAP/DMSO application (post-test, Fig. 4A). To ensure that we captured the effects of released NO, post-tests were conducted 3 min after SNAP application.
Fig. 4.

B51 excitability is modulated by NO signaling. (A) Protocol of B51 measurements prior to and 3 min after treatments with either SNAP/DMSO or L-NAME/ASW. (B) Sample traces of B51 burst threshold from SNAP-treated (B1) and DMSO-treated cells (B2). (C) Summary data illustrate that SNAP significantly increased B51 burst threshold 3 min after treatment. (D) Sample traces of B51 burst threshold from L-NAME-treated (D1) and ASW-treated cells (D2). (E) Summary data illustrate that L-NAME significantly decreased B51 burst threshold 3 min after treatment.
We used NG-Nitro-L-arginine-methyl ester hydrochloride (L-NAME) to inhibit the NO synthase [29]. L-NAME was delivered via a bolus application (final chamber concentration: 0.37 mM in ASW) [31]. Control preparations were treated with bolus application of ASW. B51 membrane properties were measured prior to (pre-test) and 3 min after L-NAME/ASW application (post-test, Fig. 4A).
In the final experiment, ganglia were initially incubated in either ODQ (final chamber concentration: 25 μM in 0.1% DMSO) or DMSO (final chamber concentration: 0.1% DMSO) for 30 min before pre-test measurements were taken (Fig. 5A). SNAP was then delivered via bolus application and post-test measurements were taken 3 min after treatment (Fig. 5A).
Fig. 5.

The NO-dependent decrease of B51 excitability is mediate by the sGC. (A) Protocol of incubation with ODQ/DMSO and subsequent measurements of B51 properties prior to and 3 min after SNAP treatment. (B) Sample traces of B51 burst threshold from SNAP-treated cells incubated with ODQ (B1) and SNAP-treated cells incubated with DMSO (B2). (C) Summary data illustrate that ODQ incubation prevented the SNAP-induced increase of B51 threshold.
2.6. Data analysis
For each membrane property of each neuron recorded, the percent change was calculated as [(post-pre/pre) × 100] to assess modifications due to treatments [15,19,28]. We were not able to collect every measurement from B51 at each of the time points, due to the inability to maintain recordings until the end of the experiment.
For the experiment in which the effects of the single cGMP/vehicle injection on B51 membrane properties were analyzed at multiple time points (Fig. 1A3), data were normally distributed with equal variance. Therefore, we used a repeated-measure one-way ANOVA to compare changes in each membrane property between cGMP and vehicle groups at different time points [32]. The Student-Newman-Keuls pairwise post-hoc comparisons (q) was then used to isolate the sources of significance. In all the remaining experiments, data were not normally distributed and, therefore, the comparisons between treated and control groups were conducted using the Mann-Whitney U test [33]. Data were reported as mean ± SEM and were analyzed using the statistical package of SigmaPlot 11.0 (Jandel Scientific, San Rafael, CA). Statistical significance was set at p < 0.05.
3. Results
3.1. Single cGMP injection induces short-term decrease of B51 excitability
We initially investigated whether cGMP injection altered B51 membrane properties. To ensure that we captured cGMP-dependent cellular changes, we first measured B51 membrane properties 5 min after a single injection of cGMP/vehicle. B51 excitability was significantly decreased in cGMP-injected cells (change in burst threshold: 24.57 ± 11.54% of pre-test; n = 8), compared to the vehicle-injected cells (change in burst threshold: 7.65 ± 3.05% of pre-test; n = 10; p < 0.05; U = 16.00). The cGMP-dependent decrease of B51 excitability was not accompanied by significant changes in either Vm (change in Vm, cGMP: 7.22 ± 1.84% of pre-test; n = 8; vehicle: −4.70 ± 1.17% of pre-test; n = 10; p = 0.31; U = 28.00), or Rm (change in Rm, cGMP: −4.10 ± 8.22% of pre-test; n = 7; vehicle: 4.65 ± 3.78% of pre-test; n = 9; p = 0.17; U = 18.00).
In the next experiment, we analyzed the time course of the decreased excitability induced by a single injection of cGMP (Fig. 1A3). Analysis of B51 excitability revealed an overall statistical significance among the different time points (F7,103 = 5.75; p < 0.05). Post-hoc analysis highlights that B51 excitability was significantly decreased in cGMP-injected cells (Fig. 1B1, C), compared to the vehicle-injected cells (Fig. 1B2, C) at the following time points: 15 min (p < 0.05; q = 4.30), 30 min (p < 0.05; q = 4.20) and 45-min (p < 0.05; q = 4.82). B51 excitability was no longer significantly different between cGMP-injected cells and vehicle-injected cells at 1 h after the injection (p ≥ 0.05; q = 1.89). In contrast to excitability, neither Vm (F7,102 = 0.71; p = 0.67) nor Rm (F7,85 = 0.90; p = 0.51) were significantly altered by cGMP injection at any of the time points examined.
3.2. Four cGMP injections induce long-term decrease of B51 excitability
We next analyzed the long-term (24 h) effects of cGMP injection on B51 membrane properties. Four cGMP injections induced a significant decrease of B51 excitability 24 h after the treatment (Fig. 2B1, C), compared to vehicle-injected cells (p < 0.05; U = 16.00; Fig. 2B2, C). Conversely, significant differences were not observed between cGMP-injected and vehicle-injected cells for either Vm (p = 0.15; U = 27.00) or Rm (p = 0.82; U = 37.00) 24 h after treatment.
3.3. Inhibition of sGC activity increases B51 excitability
To confirm the role of cGMP signaling in the modulation of B51 excitability, we investigated the effects of blocking the sGC enzyme with ODQ [29,30]. Application of ODQ induced a significant increase of B51 excitability 15 min after treatment (Fig. 3B1, 3C), compared to application of DMSO (p < 0.05; U = 6.00; Fig. 3B2, C). ODQ did not alter either Vm (p = 0.51; U = 32.00) or Rm (p = 0.08; U = 14.00) 15 min after application.
3.4. B51 excitability is modulated by NO signaling
In the next series of experiments, we began to characterize which neurotransmitter could lead to a cytosolic increase of cGMP, and to the subsequent decrease of B51 excitability. Because in numerous model systems this role is played by NO [34], we analyzed its contribution in modulating B51 excitability by: 1) artificially increasing NO levels in the ganglia with SNAP (Fig. 4A) [31] and 2) inhibiting the activity of the NO synthase with L-NAME (Fig. 4A) [29]. Application of SNAP significantly decreased B51 excitability 3 min after treatment (Fig. 4B1, C), compared to application of DMSO (p < 0.05; U = 6.00; Fig. 4B2, C), without altering either Vm (p = 0.27; U = 10.00) or Rm (p = 1.00; U = 17.00). Conversely, application of L-NAME significantly increased B51 excitability 3 min after treatment (Fig. 4D1, E), compared to application of ASW (p < 0.05; U = 13.00; Fig. 4D2, E), without altering either Vm (p = 0.86; U = 52.00) or Rm (p = 0.31; U = 40.00).
3.5. The NO-dependent decrease of B51 excitability is mediate by the sGC
In the final experiment, we investigated whether the NO-induced decrease of B51 excitability occurred through the activation of the sGC. In preparations incubated with ODQ, SNAP application failed to decrease B51 excitability (Fig. 5B1, C), as occurred in preparations in which SNAP was applied following incubation with DMSO (Fig. 5B2, C; p < 0.05; U = 10.00). No significant changes were found in either Vm (p = 0.40; U = 20.00) or Rm (p = 0.69; U = 24.00).
4. Discussion
In this study, we analyzed the contribution of the second messenger cGMP to the decreased excitability of B51, a decision-making neuron in the Aplysia feeding neural circuit. Our results indicate that cGMP decreases B51 excitability through an increase of the neuron’s plateau potential threshold. In particular, a single cGMP injection leads to a rapid decrease of B51 excitability, which lasts for up to 45 min (Fig. 1C). Four consecutive cGMP injections induced a more persistent decrease of B51 excitability that lasted for at least 24 h (Fig. 2C). The use of ODQ revealed that B51 excitability is physiologically modulated by the activity of the sGC (Fig. 3C). The inhibition of sGC increased B51 excitability, suggesting that a basal tonic activity of the cyclase controls the neuron’s excitability. Together with the iontophoretic injection results, these findings indicate that a cGMP signal regulates B51 excitability.
We next investigated which neurotransmitter might elevate the cytosolic levels of cGMP and, subsequently, decrease B51 excitability. The cGMP signaling cascade is activated by the neurotransmitter NO in numerous systems, including: hippocampal neurons [35], olfactory neurons [36], dorsal-root ganglia neurons [37] as well as Aplysia sensory neurons [23]. Furthermore, NO signalling has been shown to regulate Aplysia feeding [38]. In particular, in vivo manipulations of the NO pathway modulate the feeding response, with the NO synthase inhibitor L-NAME increasing feeding and the NO donor SNAP reducing feeding [31]. The in vitro effects of SNAP and L-NAME on B51 paralleled those in vivo: SNAP decreased excitability, whereas L-NAME increased excitability (Figs. 4C, E). The up and down regulation exerted by L-NAME and SNAP on B51 excitability indicates that the basal levels of NO tonically control the neuron’s activity. The final experiment of this study demonstrated that the SNAP-induced decrease of B51 excitability was blocked by ODQ (Fig. 5C), thus indicating that NO modulates B51 excitability through the activation of the sGC. Overall, these results are consistent with the previously reported regulation of feeding behavior by the NO-cGMP pathway [31,38], and suggest that NO modulates feeding, at least in part, by regulating B51 excitability.
Neither the short-term nor the long-term cGMP-induced decrease of B51 excitability were accompanied by modifications of Vm or Rm. This evidence indicates that the plasticity is intrinsic to B51 [17,19,21,33]. The lack of changes in the neuron’s resting membrane properties also suggests that cGMP may modulate voltage-gated conductances responsible for the genesis of the plateau potential in B51. Recent findings have implicated voltage-gated Na+ channels as the intrinsic mechanism contributing to the decrease of B51 excitability induced by sensitization training [39]. Therefore, it is plausible that a cGMP-mediated signalling cascade may reduce B51 excitability by acting on the properties of voltage-gated Na+ channels, a modulatory mechanism that has been previously reported in the olfactory receptor cells of the newt Cynops pyrrhogaster [36].
Combined with previous findings, our results provide an interesting model of bidirectional modulation in which B51 excitability is regulated by different cyclic-nucleotide pathways. In particular, a cAMP-mediated pathway leads to an increase of B51 excitability [19,21]. This pathway is activated in operant reward learning, which up-regulates feeding and increases B51 excitability [15,17,21]. The cAMP-mediated pathway is triggered in B51 by the convergence of Ca2+ entry during the plateau potential and dopamine released onto B51 during contingent reinforcement [11,21]. Our current results reveal that B51 excitability is modulated, in an opposite manner, by cGMP, which can be elevated in the cytosol through the NO-dependent activation of the sGC.
Previous evidence of bidirectional modulation of excitability has been provided in both vertebrates and invertebrates. In rodents, opposite changes in excitability have been reported in pyramidal neurons of the baso-lateral amygdala following different forms of learning, such as positive and negative olfactory conditioning [40]. In the leech Hirudo medicinalis, the excitability of the S interneurons, which participate in the defensive whole-body shortening, is increased following sensitization training and is decreased following habituation training [41]. These examples illustrate that bidirectional changes in behaviorally-relevant neurons contribute to the encoding of stimuli with opposite values, such as appetitive vs. aversive, or aversive vs. neutral. In the case of B51, the present findings, combined with previous observations, delineate a scenario in which opposite changes in B51 excitability are sustained by distinct cyclic nucleotides mediated cascades.
Examples of both convergence and divergence of second-messenger cascades modulating neuronal excitability can be found in the Aplysia nervous system. For instance, in the sensory neurons that mediate defensive withdrawal reflexes [42], cyclic-nucleotide signals show converging effects in which different triggers, such as noxious stimuli via cAMP [42], and injury via cGMP [23], both increase neuronal excitability. Similarly, distinct neuromodulators, such as serotonin via cAMP, and NO via cGMP, lead to an increase in the excitability of the modulatory metacerebral cells [43]. Now, this study, together with previous research [19,21], provides evidence of divergence of excitability modulation by second-messenger cascades within B51, a neuron with decision-making characteristics. Due to the decision-making nature of B51 [10,18], and the great flexibility exhibited by feeding to changes in external and internal state [11,38], the bidirectional modulation of its excitability by distinct second-messenger cascades may represent a key mechanism to regulate the output of the feeding neural circuit in opposite directions.
Future investigations will examine which external triggers physiologically activate the NO-cGMP pathway in B51. Previous studies revealed that the same NO-cGMP pathway that modulates feeding and B51 excitability is also activated during a form of learning in which Aplysia learns that food is not edible and downregulates the expression of bites accordingly [24,25]. The involvement of the NO-cGMP pathway might extend to other forms of learning in which feeding is reduced. For example, sensitization training suppresses feeding and decreases B51 excitability [20,26]. That activation of the NO-cGMP pathway induces changes in B51 excitability that are analogous to those induced by in vivo and in vitro sensitization training [20,27], suggesting that this signalling cascade might also contribute to the behavioral and cellular modifications induced by sensitizing stimuli. Finally, we recently reported that, in Aplysia, food satiation suppresses feeding for several days and decreases B51 excitability [18]. It is plausible to hypothesize that the NO-cGMP pathway might also play a role in this other process by which feeding and B51 excitability are down regulated by the animal’s internal state.
Acknowledgments
This work was supported by NIH-NIGMS grant SC3GM111188 to R.M. The funding source had no involvements in preparation of the article, collection, analysis and interpretation of data, writing of the report, and in the decision to submit the article for publication.
Footnotes
Conflict of Interest
The authors have no actual or potential conflicts of interest.
References
- [1].Katsu-Jiménez Y, Alves RMP, Giménez-Cassina A, Food for thought: impact of metabolism on neuronal excitability, Exp. Cell. Res. 360 (2017) 41–46. [DOI] [PubMed] [Google Scholar]
- [2].Contractor A, Klyachko VA, Portera-Cailliau C, Altered neuronal and circuit excitability in fragile X syndrome, Neuron 87 (2015) 699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim JI, Cho HY, Han JH, Kaang BK, Which neurons will be the engram–activated neurons and/or more excitable neurons? Exp. Neurobiol. 25 (2016) 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ku SM, Han MH, HCN channel targets for novel antidepressant treatment, Neurotherapeutics 14 (2017) 698–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Beck H, Yaari Y, Plasticity of intrinsic neuronal properties in CNS disorders, Nat. Rev. Neurosci. 9 (2008) 357–369. [DOI] [PubMed] [Google Scholar]
- [6].Kourrich S, Calu DJ, Bonci A, Intrinsic plasticity: an emerging player in addiction, Nat. Rev. Neurosci. 16 (2015) 173–184. [DOI] [PubMed] [Google Scholar]
- [7].Mozzachiodi R, Byrne JH, More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory, Trends Neurosci. 33 (2010) 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Titley HK, Brunel N, Hansel C, Toward a neurocentric View of learning, Neuron 95 (2017) 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Crow T, Pavlovian conditioning of Hermissenda: current cellular, molecular, and circuit perspectives, Learn. Mem. 11 (2004) 229–238. [DOI] [PubMed] [Google Scholar]
- [10].Nargeot R, Simmers J, Functional organization and adaptability of a decision-making network in Aplysia, Front. Neurosci. 6 (2012) 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mozzachiodi R, Baxter DA, Byrne JH, Comparison of operant and classical conditioning of feeding behavior in Aplysia, in: Menzel R, Benjamin P (Eds.), Invertebrate Learning and Memory, Academic Press, London, U.K, 2013, pp. 183–193. [Google Scholar]
- [12].Kupfermann I, Feeding behavior in Aplysia: a simple system for the study of motivation, Behav. Biol. 10 (1974) 1–26. [DOI] [PubMed] [Google Scholar]
- [13].Cropper EC, Evans CG, Hurwitz I, Jing J, Proekt A, Romero A, Rosen SC, Feeding neural networks in the mollusc Aplysia, Neurosignals 13 (2004) 70–86. [DOI] [PubMed] [Google Scholar]
- [14].Plummer MR, Kirk MD, Premotor neurons B51 and B52 in the buccal ganglia of Aplysia californica: synaptic connections, effects on ongoing motor rhythms, and peptide modulation, J. Neurophysiol. 63 (1990) 539–558. [DOI] [PubMed] [Google Scholar]
- [15].Nargeot R, Baxter DA, Byrne JH, In vitro analog of operant conditioning in Aplysia. I. Contingent reinforcement modifies the functional dynamics of an identified neuron, J. Neurosci. 19 (1999) 2247–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sasaki K, Cropper EC, Weiss KR, Jing J, Functional differentiation of a population of electrically coupled heterogeneous elements in a microcircuit, J. Neurosci. 33 (2013) 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brembs B, Lorenzetti FD, Reyes F, Baxter DA, Byrne JH, Operant reward learning in Aplysia: neuronal correlates and mechanisms, Science 296 (2002) 1706–1709. [DOI] [PubMed] [Google Scholar]
- [18].Dickinson K, Wainwright ML, Mozzachiodi R, Change in excitability of a putative decision-making neuron in Aplysia serves as a mechanism in the decision not feed following food satiation, Behav. Brain Res. 281 (2015) 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mozzachiodi R, Lorenzetti FD, Baxter DA, Byrne JH, Changes in neuronal excitability serve as a mechanism of long-term memory for operant conditioning, Nat. Neurosci. 11 (2008) 1146–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shields-Johnson M, Hernandez JS, Torno C, Adams KM, Wainwright M, Mozzachiodi R, Effects of aversive stimuli beyond defensive neural circuits: reduced excitability in an identified neuron critical for feeding in Aplysia, Learn. Mem 20 (2013) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lorenzetti FD, Baxter DA, Byrne JH, Molecular mechanisms underlying a cellular analog of operant reward learning, Neuron 59 (2008) 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Threlfell S, West AR, Modulation of striatal neuron activity by cyclic nucleotide signaling and phosphodiesterase inhibition, Basal Ganglia 3 (2013) 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lewin MR, Walters ET, Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons, Nat. Neurosci. 2 (1999) 18–23. [DOI] [PubMed] [Google Scholar]
- [24].Susswein AJ, Schwartz M, A learned change of response to inedible food in Aplysia, Behav. Neural Biol. 39 (1983) 1–6. [DOI] [PubMed] [Google Scholar]
- [25].Katzoff A, Ben-Gedalya T, Hurwitz I, Miller N, Susswein YZ, Susswein AJ, Nitric oxide signals that Aplysia have attempted to eat, a necessary component of memory formation after learning that food is inedible, J. Neurophysiol. 96 (2006) 1247–1257. [DOI] [PubMed] [Google Scholar]
- [26].Acheampong A, Kelly K, Shields-Johnson M, Hajovsky J, Wainwright M, Mozzachiodi R, Rapid and persistent suppression of feeding behavior induced by sensitization training in Aplysia, Learn. Mem 19 (2012) 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Weisz HA, Wainwright ML, Mozzachiodi R, A novel in vitro analog expressing learning-induced cellular correlates in distinct neural circuits, Learn. Mem. 24 (2017) 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mozzachiodi R, Lechner HA, Baxter DA, Byrne JH, In vitro analog of classical conditioning of feeding behavior in Aplysia, Learn. Mem 10 (2003) 478–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kemenes I, Kemenes G, Andrew RJ, Benjamin PR, O’Shea M, Critical timewindow for NO-cGMP-dependent long-term memory formation after one-trial appetitive conditioning, J. Neurosci. 22 (2002) 1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koh HY, Jacklet JW, Nitric oxide stimulates cGMP production and mimics synaptic responses in metacerebral neurons of Aplysia, J. Neurosci. 19 (1999) 3818–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miller N, Saada R, Markovich S, Hurwitz I, Susswein AJ, L-arginine via nitric oxide is an inhibitory feedback modulator of Aplysia feeding, J. Neurophysiol. 105 (2011) 1642–1650. [DOI] [PubMed] [Google Scholar]
- [32].Michel M, Green CL, Eskin A, Lyons LC, PKG-mediated MAPK signaling is necessary for long-term operant memory in Aplysia, Learn. Mem 18 (2011) 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lorenzetti FD, Mozzachiodi R, Baxter DA, Byrne JH, Classical and operant conditioning differentially modify the intrinsic properties of an identified neuron, Nat. Neurosci. 9 (2006) 17–19. [DOI] [PubMed] [Google Scholar]
- [34].Jacklet JW, Nitric oxide signaling in invertebrates, Invert. Neurosci 3 (1997) 1–14. [DOI] [PubMed] [Google Scholar]
- [35].Doerner D, Alger BE, Cyclic GMP depresses hippocampal Ca2+ current through a mechanism independent of cGMP-dependent protein kinase, Neuron 1 (1988) 693–699. [DOI] [PubMed] [Google Scholar]
- [36].Kawai F, Miyachi EI, Modulation by cGMP of the voltage-gated currents in newt olfactory receptor cells, Neurosci. Res. 39 (2001) 327–337. [DOI] [PubMed] [Google Scholar]
- [37].Yoshimura N, Seki S, de Groat WC, Nitric oxide modulates Ca(2+) channels in dorsal root ganglion neurons innervating rat urinary bladder, J. Neurophysiol. 86 (2001) 304–311. [DOI] [PubMed] [Google Scholar]
- [38].Susswein AJ, Chiel HJ, Nitric oxide as a regulator of behavior: New ideas from Aplysia feeding, Prog. Neurobiol. 97 (2012) 304–317. [DOI] [PubMed] [Google Scholar]
- [39].Hernandez JS, Wainwright ML, Mozzachiodi R, Long-term sensitization training in Aplysia decreases the excitability of a decision-making neuron through a sodiumdependent mechanism, Learn. Mem 24 (2017) 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Motanis H, Mouna M, Barkai E, Learning-induced bidirectional plasticity of intrinsic neuronal excitability reflects the valence of the outcome, Cereb. Cortex 24 (2014) 1075–1087. [DOI] [PubMed] [Google Scholar]
- [41].Burrell BD, Sahley CL, Muller KJ, Non-associative learning and serotonin induce similar bi-directional changes in excitability of a neuron critical for learning in the medicinal leech, J. Neurosci 21 (2001) 1401–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Byrne JH, Hawkins RD, Nonassociative learning in invertebrates, Cold Spring Harb. Perspect. Biol 7 (2015) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jacklet JW, Grizzaffi J, Tieman DG, Serotonin, nitric oxide and histamine enhance the excitability of neuron MCC by diverse mechanisms, Acta Biol. Hung. 55 (2004) 201–210. [DOI] [PubMed] [Google Scholar]


