Abstract
In both acute and chronic pain conditions, women tend to be more sensitive than men. This sex difference may be regulated by estrogens, such as estradiol, that are synthesized in the spinal cord and brainstem and act locally to influence pain processing. To identify a potential cellular source of local estrogen, here we examined the expression of aromatase, the enzyme that catalyzes the conversion of testosterone to estradiol. Our studies focused on primary afferent neurons and on their central targets in the spinal cord and medulla as well as in the nucleus of the solitary tract, the target of nodose ganglion-derived visceral afferents. Immunohistochemical staining in an aromatase reporter mouse revealed that many neurons in laminae I and V of the spinal cord dorsal horn and caudal spinal trigeminal nucleus and in the nucleus of the solitary tract express aromatase. The great majority of these cells also express inhibitory interneuron markers. We did not find sex differences in aromatase expression and neither the pattern nor the number of neurons changed in a sciatic nerve transection model of neuropathic pain or in the Complete Freund’s adjuvant model of inflammatory pain. A few aromatase neurons express Fos after cheek injection of capsaicin, formalin, or chloroquine. In total, given their location, these aromatase neurons are poised to engage nociceptive circuits, whether it is through local estrogen synthesis or inhibitory neurotransmitter release.
Keywords: aromatase, estrogens, pain, solitary nucleus, spinal cord dorsal horn, trigeminal caudal nucleus, RRID:MGI:4430066, RRID:MGI:5634564
1. INTRODUCTION
The great majority of clinical pain conditions predominate in women (Berkley, 1997; Mogil, 2012; Unruh, 1996). Women also exhibit lower pain thresholds and tolerances to a variety of noxious stimuli, which suggests that females are more sensitive to painful stimuli, even at baseline (Fillingim & Maixner, 1995; Mogil, 2012; Riley, Robinson, Wise, Myers, & Fillingim, 1998). As sex hormones are likely to facilitate these sex differences, particular attention has been paid to the contribution of estrogen in nociception (Aloisi & Bonifazi, 2006; Craft, Mogil, & Aloisi, 2004; Kuba & Quinones-Jenab, 2005). Unfortunately, because of the broad distribution of estrogen receptors in neural and non neural tissues as well as the wide range of effects linked to estrogen, determining unequivocally whether estrogen is selectively pro- or antinociceptive has proven difficult (Craft, 2007). For this reason, a more detailed analysis of the existence and function of estrogen and its receptors in discrete cell populations implicated in pain processing is essential (Amandusson & Blomqvist, 2013).
Although the gonads are the primary source of estrogen circulating in the blood, there is now considerable evidence that numerous other tissues, including skin, fat, and brain, produce estrogen that acts in or near those locations (McEwen & Alves, 1999; Simpson, 2003). In fact, several groups have proposed that there is estrogen synthesis in the spinal cord. For example, Evrard et al. (2000) detected aromatase, the enzyme that converts androgens to estrogens, in the spinal cord dorsal horn of Japanese quails and Zhang, LÜ, Zhao, and Zhang (2012) demonstrated electrically evoked estrogen release in rat spinal cord slices. Furthermore, in both quails and rats, the behavioral consequences of manipulating spinal estrogen levels by administering estrogens or aromatase inhibitors suggests that spinal cord-derived estrogen has pronociceptive effects (Evrard & Balthazart, 2004; Zhang, Xiao, Zhang, Zhao,&Zhang,2012).
The earlier work that characterized the distribution of aromatase used antibodies directed against the protein. Because of significant concerns with antibody specificity, here we took advantage of a transgenic mouse line in which an internal ribosome entry site coupled to a nuclear lacZ reporter has been knocked into the aromatase gene (Wu et al., 2009), allowing for very precise molecular mapping of the distribution of aromatase-expressing cells. We also characterized the cells based on co-expression of a host of molecular markers and examined the extent to which these cells are influenced in different mouse models of acute and chronic pain.
2. METHODS
2.1. Mouse lines
We used aromatase IRES-PLAP-IRES-nlacZ reporter mice (Wu et al., 2009; homozygous for reporter allele, RRID:MGI:4430066 and heterozygous for reporter allele, RRID:MGI:5634564). Animals were 8–12 weeks old at time of perfusion. All experiments were performed in accordance with the University of California, San Francisco’s Institutional Animal Care and Use Committee guidelines.
2.2. Immunohistochemistry
Animals were deeply anesthetized using 250–400 mg/kg 2,2,2-Tribro- moethanol (Avertin, Sigma-Aldrich, St. Louis, Missouri) and then transcardially perfused with 10 ml of phosphate-buffered saline (PBS) followed by 30 ml of 4% formaldehyde in PBS (37% formaldehyde diluted 1/10; ACROS Organics, Morris Plains, New Jersey). All perfusions and incubations used 1X PBS with the exception of those involving staining for sst2a, which required PBS containing 300 mM NaCl rather than the typical concentration of 137 mM NaCl. Dorsal root ganglia (DRGs), trigeminal ganglia (TGs), spinal cord, and brain were dissected out and post-fixed in 4% formaldehyde in PBS for 3–6 hr at room temperature. Tissue was cryo-protected in 30% sucrose for at least one night and then sectioned on a cryostat; spinal cord and medulla were cut at a thickness of 25 and 35 μm, respectively, and collected in PBS while DRG and TG were cut at 14 mm and directly mounted onto slides. For staining, tissue was blocked for 1 hr in 10% normal goat serum in PBS containing 0.3% Triton X-100 and then incubated overnight at room temperature in primary antibodies diluted in 1% normal goat serum in PBS containing 0.3% Triton X-100. Primary antibodies used are as indicated in Table 1. Following overnight incubation, tissue was washed three times with PBS and then incubated with secondary antibodies diluted 1:1,000 in PBS for a minimum of 2 hr at room temperature. Secondary antibodies were all Alexa Fluor 488, 546, 594, or 647-conjugated (Thermo Fisher Scientific, Waltham, Massachusetts) and raised in goat against the appropriate primary species. After several PBS washes, tissue was mounted onto slides (if necessary) and allowed to briefly dry before coverslipping with Fluoromount-G aqueous mounting medium (SouthernBiotech, Birmingham, Alabama).
Table 1.
Primary antibodies
| Antibody | Immunogen | Source | Concentration |
|---|---|---|---|
| beta Galactosidase (β-gal) | Purified full-length native protein from E. coli | Abcam, Cat# ab9361; RRID:AB_307210; chicken, polyclonal | 1:10,000 |
| NeuN | Purified mouse brain cell nuclei, clone A60 | Millipore, Cat# MAB377; RRID:AB_2298772; mouse, monoclonal | 1:5,000 |
| Fluorogold | Glutaraldehyde-conjugated Fluoro-Gold | Protos Biotech, Cat# NM-101 FluGgp; RRID: AB_2314409; guinea pig, polyclonal | 1:1,000 |
| TRPV1 | C-terminus 15aa (EDAEVFKDSMAPGEK) of mouse transient receptor potential vanilloid subtype 1 | D. Julius, University of California, San Francisco; guinea pig, polyclonal | 1:5,000 |
| Pax2 | Synthetic peptide within human paired box 2 protein, aa1–20 at N-terminus, clone EP3251 | Abcam, Cat# ab79389; RRID:AB_1603338; rabbit, monoclonal | 1:4,000 |
| sst2a | C-terminus 15aa (ETQRTLLNGDLQTSI) of mouse somatostatin receptor subtype 2A | Gramsch Laboratories, Cat# SS-870; RRID: AB_2491104; guinea pig, polyclonal | 1:10,000 |
| Lmx1b | Full-length LIM homeobox transcription factor 1 beta protein from mouse | T. Müller and C. Birchmeier, Max-Delbrück-Center for Molecular Medicine, Berlin, Germany; RRID: AB_2314752; guinea pig, polyclonal | 1:10,000 |
| ERα | 15aa (TYYIPPEAEGFPNTI) at C-terminus of rat estrogen receptor α | Millipore, Cat# 06–935; RRID:AB_310305; rabbit, polyclonal | 1:10,000 |
| Fos | Synthetic peptide within human c-Fos, aa4–17 (SGFNADYEASSSRC), Ab-5 | Calbiochem, Cat# PC38; RRID:AB_2106755; rabbit, polyclonal | 1:5,000 |
| ATF3 | C-terminus 19aa (PEDERNLFIQQIKEGTLQS) of human activating transcription factor 3 | Santa Cruz Biotechnology, Cat# sc-188; RRID: AB_2258513; rabbit, polyclonal | 1:2,000 |
| Iba1 | Synthetic peptide corresponding to C-terminus 14aa (PTGPPAKKAISELP) of ionized calcium-binding adapter molecule 1 | Wako, Cat# 019–19741; RRID:AB_839504; rabbit, polyclonal | 1:1,000 |
2.3. Antibody characterization
Chicken anti β-gal (Abcam, Cat# ab9361, RRID:AB_307210) does not produce staining in wild-type animals, that is, animals that do not express the aromatase reporter allele (unpublished observation).
Mouse anti NeuN (Millipore, Cat# MAB377, RRID:AB_2298772) recognizes neuronal nuclei and cytoplasm. Antibody specificity has been evaluated with immunohistochemistry and immunoblot analysis, showing that immunoreactivity is present in neurons and in nervous tissue but not in glia or in other organs (Mullen, Buck, & Smith, 1992).
Guinea pig anti Fluorogold (Protos Biotech, Cat# NM-101 FluGgp, RRID:AB_2314409) signal completely overlaps with Fluoro-Gold fluorescence observed with ultraviolet illumination (data not shown; Al- Khater & Todd, 2009). Specificity was also confirmed through a lack of immunoreactivity following preincubation with Fluoro-Gold (manufacturer’s information). Furthermore, there is no Fluorogold staining in animals that were not injected with Fluoro-Gold.
Rabbit anti Pax2 (Abcam, Cat# ab79389, RRID:AB_1603338) staining pattern in this study is in agreement with previous reports that characterize spinal cord dorsal horn Pax2-expressing cells as inhibitory interneurons (Kardon et al., 2014; Punnakkal, von Schoultz, Haenraets, Wildner, & Zeilhofer, 2014). In addition, for this particular antibody, Western blot from human fetal kidney tissue recognizes a band at the proper expected size of 45 kDa (manufacturer’s information). Furthermore, immunostaining in aromatase reporter mice that were crossed to a GAD67-GFP reporter line indicate that around 80% of the aromatase cells are GABAergic, which is similar to the percentage of aromatase cells that are Pax2-expressing (data not shown).
Guinea pig anti sst2a (Gramsch Laboratories, Cat# SS-870, RRID: AB_2491104) has been previously shown to label inhibitory neurons in the rodent spinal cord dorsal horn (Todd, Spike, & Polgár, 1998) and in a pattern that matches other reports of sst2a expression (Holloway, Feniuk, Kidd, & Humphrey, 1996; Schindler, Sellers, Humphrey, & Emson, 1997). Specificity of this antibody has been confirmed through dot-blot assays demonstrating detection of sst2a but not somatostatin receptors sst1, sst2b, or sst3; Western blots from rat brain tissue displaying a broad band of suitable size (80 kDa); and lack of immunoreac- tive cells following preadsorption with the immunizing peptide (Schulz et al., 1998). Antibody was kindly provided by Andrew J. Todd, University of Glasgow.
Guinea pig anti Lmx1b (gift of T. Muller and C. Birchmeier, Max- Delbruck-Center for Molecular Medicine; Berlin, Germany, RRID: AB_2314752) specifically marks excitatory dorsal horn interneurons of the dI5 and dlLB lineages (Yang, Cagle, & Honig, 2010). The staining pattern in our report is consistent with previously published articles (Del Barrio et al., 2013; Szabo et al., 2015).
Rabbit anti ERa (Millipore, Cat# 06–935, RRID:AB_310305) detects in Western blots a roughly 58 kDa band from MCF7 cell lysate (manufacturer’s information) and a 55 kDa band from cichlid whole brain extract (Munchrath & Hofmann, 2010). Preincubation with the antigen eliminates all bands (Friend, Resnick, Ang, & Shupnik, 1997). In addition, there was no detectable signal in spinal cord tissue immuno- stained from ERα conditional knockout mice (unpublished observation).
Rabbit anti c-Fos (Calbiochem, Cat# PC38, RRID:AB_2106755) labels c-Fos without cross-reactivity with other Fos-related antigens in the rat central nervous system (Hoffman, Smith, & Fitzsimmons, 1992; Rinaman, Verbalis, Stricker, & Hoffman, 1993). In tests by the manufacturer, the antibody was able to bind to c-Fos and v-Fos (55 and 62 kDa, respectively) but not Jun (39 kDa).
Guinea pig anti TRPV1 (gift of David Julius, University of California, San Francisco) marks a subset of sensory neurons in the DRG. Immunostaining this antibody matches previous reports and importantly, there is no antibody staining in tissue from TRPV1 knockout mice (Bráz & Basbaum, 2010) or in the spinal cord of mice in which TRPV1 central terminals have been ablated (Cavanaugh et al., 2009).
Rabbit anti ATF3 (Santa Cruz Biotechnology, Cat# sc-188, RRID: AB_2258513) is a well characterized antibody that labels injured DRG neurons (Bráz, Ackerman, & Basbaum, 2011; Starkey et al., 2009). Western blot from rat brain tissue produces an appropriate band approximately 21 kDa in size (Yamanaka, Kobayashi, Okubo, Fukuoka, & Noguchi, 2011). Our data show that ATF3 is only induced in neurons ipsilateral to the nerve injury, which is in agreement with previous studies (Bráz & Basbaum, 2010; Guan et al., 2016; Tsujino et al., 2000).
Rabbit anti Iba1 (Wako, Cat# 019–19741, RRID:AB_839504) has been used extensively as a microglia marker and the spinal cord staining pattern we observed is consistent with other reports (Guan et al., 2016; Pineau & Lacroix, 2007; Yamanaka et al., 2011). This antibody has been demonstrated to be specific to microglia as Western blotting displays a single band of correct expected size of 17 kDa only in microglia-containing tissue samples and immunohistochemistry detects no signal in astrocytes, oligodendrocytes, or neurons (manufacturer’s information; Imai, Ibata, Ito, Ohsawa, & Kohsaka, 1996; Ito etal., 1998; Yamanaka et al., 2011).
2.4. Retrograde tracing
Under intraperitoneal 80–100 mg/kg ketamine + 5–10 mg/kg xylazine anesthesia, mice were placed in stereotaxic apparatus (Kopf Instruments, Tujunga, California) and 0.5–1 μl of either Fluoro-Gold (Fluoro- chrome, Denver, Colorado) or red RetroBeads (Lumafluor, Durham, North Carolina) was injected into the left lateral parabrachial nucleus. Lateral parabrachial nucleus was located according to coordinates from Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates. Animals were perfused 3–9 days later and tissue was processed for immunohistochemistry.
2.5. Fos induction
Capsaicin (Sigma-Aldrich; 5 μg in 30 μl saline with 10% Tween-80 and 10% ethanol for cheek, 3 μg in 10 μl saline with 10% Tween-80 and 10% ethanol for hindpaw), 2% formalin (37% by weight formaldehyde, diluted 1/50 in saline; 50 μl for cheek, 10 μl for hindpaw), or chloro- quine (chloroquine diphosphate salt, Sigma-Aldrich; 200 μg in 50 μl saline for cheek, 40 μg in 20 μl saline for hindpaw) was injected into the left cheek (shaved the day before injection) or the plantar surface of the left hindpaw of mice that were lightly restrained with a towel. 90 min later, mice were perfused and tissue was processed for immu- nohistochemistry as above.
2.6. Chronic injury models
For infraorbital or sciatic nerve transection, mice were anesthetized in the same manner as they were for retrograde tracing experiments. The left cheek or left hind leg was shaved, a small incision was made in the whisker pad area or thigh, and then the appropriate nerve was exposed. Following the cutting of the nerve (and in the case of sciatic nerve transection, excision of 2 mm of nerve), cheek or leg was sutured and mice were allowed to recover from anesthesia. One week later, mice were perfused and tissue was processed for immunohistochemis- try. For Complete Freund’s adjuvant (CFA) injections, mice were lightly restrained with a towel and 20 μl of CFA (Sigma-Aldrich; 1:1 emulsion in saline) was injected into the left cheek or the plantar surface of the left hindpaw. Three days later, mice were perfused and tissue was processed for immunohistochemistry.
2.7. Confocal and epifluorescent imaging
All images except medulla images were taken on a LSM 700 confocal microscope (Zeiss, Oberkochen, Germany) equipped with 405, 488, 555, and 639 nm diode lasers, a main dichroic beam splitter URGB and a gradient secondary beam splitter for LSM 700 using a 10X EC Plan- Neofluar (10X/0.3) for sagittal spinal cord sections or a 20X Plan- Apochromat (20X/0.8) objective (Zeiss). Image acquisition was done with ZEN 2010 (Zeiss), and image dimensions were 1024 X 1024 pixels with an image depth of 12 bits. Two times averaging was applied during image acquisition. Laser power and gain were adjusted to avoid saturation of single pixels and kept constant for each experiment. Medulla images were taken on an Axioimager M2 (Zeiss) equipped with AF488, AF568, Cy5, and DAPI filter sets and an Axiocam 506 mono camera using a 20X Plan-Apochromat (20X/0.8) objective (Zeiss) in the “Tiling mode” of Zen2 Pro (Zeiss). Image acquisition was performed with fixed exposure times for each channel and with a 10% overlap of neighboring images. Stitching was done in Zen2 Pro based on the NeuN channel using the “stitching/fuse tiles” function. Adjustment of brightness/contrast, changing of artificial colors (LUT), and maximum projections of Z- stack images were done in Fiji/ImageJ (https://fiji.sc, RRID: SCR_002285). All images of the same experiment were processed in an identical manner. For images in Figures 3–7, and 8, the “Remove Outliers” filter in Fiji/ImageJ was applied to digitally remove artifacts and debris in areas outside of the tissue. This filter was set to sample the value of pixels in a 100-pixel radius and replace pixels that were more than 10 units brighter than the median with the median value.
FIGURE 3.
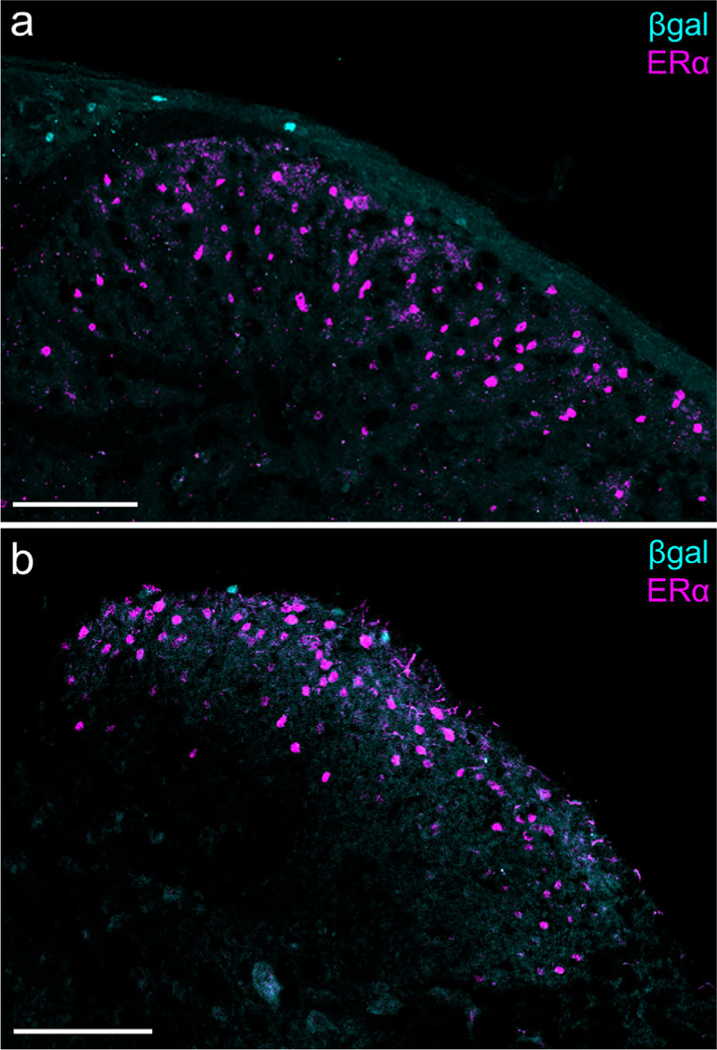
Aromatase and estrogen receptor α (ERα) expression in the medullary and spinal dorsal horns. (a) Caudal spinal trigeminal nucleus: β-gal+ cells are visible in lamina I, whereas ERα-expressing cells are distributed throughout laminae I and II. Scale bar: 100 μm. (b) Sacral spinal cord: There is also no overlap of β-gal and ERα in the spinal cord. Scale bar: 100 μm. [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 7.
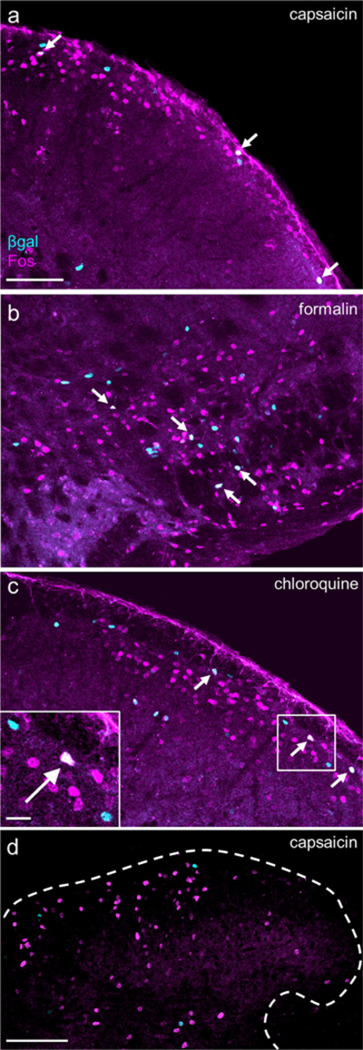
Algogens and a pruritogen induce Fos in a small number of aromatase-expressing neurons. (a, b, c) Cheek injections of a) capsaicin (5.0 μg/30 μl; 3 males, 3 females), (b) formalin (2% v/v in saline, 50 μl; n = 2 males, 2 females), and (c) chloroquine (200 μg in 50 μl; n = 2 males, 2 females) induced Fos expression in a subset of β-gal+ neurons in the cSTN (arrows). Scale bar (applies to a, b, and c): 100 μm; inset: 20 μm. (d) We did not observe Fos and β-gal overlap in lumbar spinal cord following hindpaw injection of capsaicin (3.0 μg/10 μl). Comparable results were obtained after hindpaw injection of formalin or chloroquine (data not shown). Dashed line outlines border of spinal gray matter. n = 2 males, 2 females. Scale bar: 100 μm. [Color figure can be viewed at wileyonlinelibrary.com]
2.8. Quantification of double-labeled cells
For counting cells in the spinal cord, the dorsal horn of 4–6 randomly selected spinal cord sections from each mouse was imaged using identical imaging parameters. Labeled cells in each channel were identified using the Isodata Threshold algorithm in Fiji/ImageJ, and counted with the “Particle Analyzer” function with a size range of 15–150 μm and a circularity of 0.5–1. An overlay of both masks was created to identify double-labeled cells in each section. For counting cells in the caudal spinal trigeminal nucleus (cSTN) and nucleus of the solitary tract (NST), the entirety of 3–4 randomly selected medulla sections from each mouse was imaged using identical imaging parameters. In the resulting stitched images, cSTN and NST were distinguished based on typical area morphology from Paxinos and Franklin’s mouse brain atlas. Labeled cells in each channel were manually counted in Fiji/ImageJ using the “Cell Counter” tool. Double-labeled cells were identified by color change; an overlap of cyan and magenta to produce white indicated that a cell was double-labeled. For both spinal cord and medulla, the percentage of double-labeled cells was calculated for each mouse as the total number of double-labeled cells divided by the total number of β-gal positive cells from the sections used for quantification. Final percentages are displayed as the averages of both male and female mice as there was no statistically significant difference in overlap between the sexes as determined by Student’s t test.
2.9. Statistical analysis
Unpaired (Student’s) t tests and two-way ANOVA with Tukey’s test to correct for multiple comparisons were run using GraphPad Prism (version 6.0e, https://www.graphpad.com/scientific-software/prism, RRID: SCR_002798). Tests are as indicated in figure legends.
3. RESULTS
3.1. Aromatase expression in spinal cord and medulla
Immunostaining for β-galactosidase (β-gal, the product encoded by lacZ) in the aromatase reporter mouse revealed an extensive distribution of β-gal immunoreactivity that overlapped remarkably with regions of the spinal cord and brainstem that process nociceptive information (Figures 1 and 2). In the medulla, chains of β-gal+ nuclei are visible in laminae I and V of caudal spinal trigeminal nucleus (cSTN; Figure 1a), an area that receives nociceptive input from primary sensory neurons of the trigeminal ganglion (TG; Price, Dubner, & Hu, 1976; Robertson & Arvidsson, 1985). We observed a comparable pattern, although with many fewer cells, in laminae I and V of the spinal cord dorsal horn (Figure 2a, b), which receives nociceptive input from primary sensory neurons of the dorsal root ganglion (DRG; Adrian, 1928; Foerster, 1933). Interestingly, we also found scattered aromatase-positive cells in the nucleus of the solitary tract (NST; Figure 1a), which receives input from nodose ganglion-derived visceral afferents (Contreras, Beckstead, & Norgren, 1982; Randich & Gebhart, 1992). Every β-gal+ cell co-labeled with the neuronal marker NeuN, indicating that these aromataseexpressing cells are neurons (Figures 1a and 2b, insets 1–2). In contrast, we never found β-gal-immunoreactive cells in the aromatase reporter mouse in either TG or DRG (Figures 1b and 2c), despite extensive estrogen receptor expression in these sensory ganglia (Papka, Sriniva- san, Miller, & Hayashi, 1997; Papka et al., 2001; Sohrabji, Miranda, & Toran-Allerand, 1994; Taleghany, Sarajari, DonCarlos, Gollapudi, & Oblinger, 1999). Similarly, although the aromatase-expressing cells are found in close proximity to estrogen receptor α (ERα)-expressing neurons, we found no evidence for overlap in the medulla or spinal cord (Figure 3a, b).
FIGURE 1.
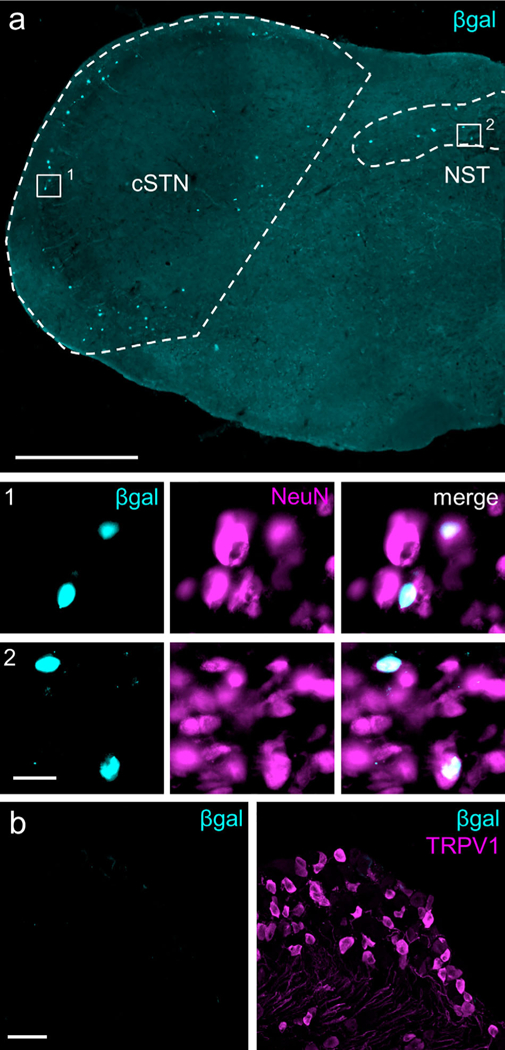
Aromatase expression in the medulla and trigeminal ganglia (TG). (a) Representative section from aromatase reporter mouse illustrates nuclear β-galactosidase (βgal) expression in laminae I and V of the caudal spinal trigeminal nucleus (cSTN, area demarcated by dashed lines) and in the nucleus of the solitary tract (NST, area demarcated by dashed line). Co-staining for the neuronal marker, NeuN, shows complete overlap with β-gal, examples of which can be seen in insets 1 and 2. Image is stitched from single 20X images. Scale bar: 500 μm; insets: 20 μm. (b) No β-gal signal was detected in the TG (left panel). For comparison, right panel illustrates TRPV1- immunoreactive neurons in the same section. Scale bar: 50 μm. [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
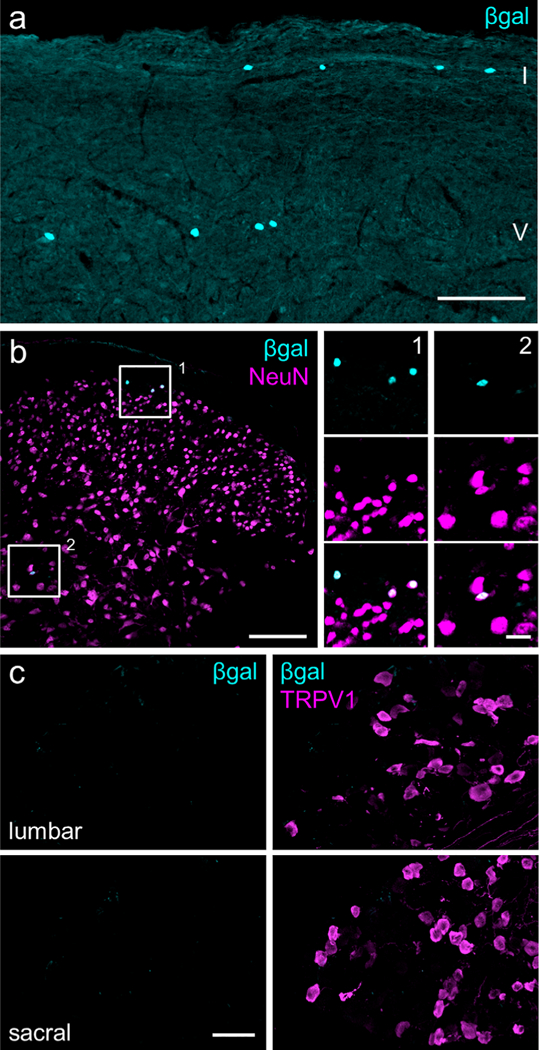
Aromatase expression in the spinal cord and dorsal root ganglia (DRG). (a) Sagittal section of the lumbar spinal cord demonstrates nuclear β-gal expression in laminae I and V of dorsal horn. Scale bar: 100 μm. (b) Coronal spinal cord section immuno- stained for NeuN. Insets 1 and 2 show co-localization of β-gal with NeuN. Scale bar: 100 μm; inset: 20 μm. (c) Lumbar and sacral DRG do not express β-gal. For comparison, right panels illustrate TRPV1- immunoreactive neurons in the same sections. Images represent maximum Z-projections of confocal images. Scale bar: 50 μm. [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Quantification of aromatase expression in males and females
As a sex difference in aromatase expression in the brain impacts sexually dimorphic behaviors (Unger et al., 2015; Wu et al., 2009), we next asked if there are different numbers of β-gal+ cells in the spinal cord and medulla of male and female mice, a difference that might underlie sexual dimorphisms in pain processing. cSTN and NST display no sex differences (Figure 4a; unpaired t test—cSTN, p = .84, male: 54.6 ± 5.9 and female: 56.1 ± 4.8 cells per section ± SEM; NST, p = .18, male: 10.5 ± 0.60 and female: 8.9 ± 0.85 cells per section ± SEM). Across the cervical, thoracic, lumbar, and sacral regions of the spinal cord, we found neither a significant difference between males and females nor a difference in numbers of cells at different segmental levels (Figure 4b; two-way ANOVA—sex effect: F(1,41) = 0.031, p = .86; region effect: F(3,41) = 0.17, p = .92; interaction: F(3,41) = 0.40, p = .75; overall male: 7.0 ± 0.26 cells per section ± SEM, overall female: 7.1 ± 0.38 cells per section ± SEM; see Figure 4 legend for breakdown by spinal region).
FIGURE 4.
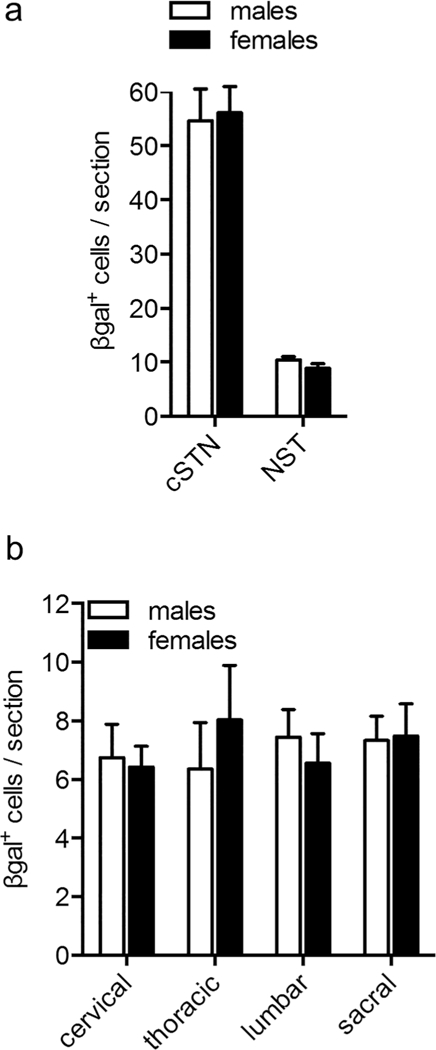
No sex differences in spinal or medullary aromatase expression. (a) Male and female mice have comparable numbers of β-gal+ cells in the cSTN and NST. Number of β-gai+ cells per section ± SEM—cSTN, males: 54.6 ± 5.9 and females: 56.1 ± 4.8; NST, males: 10.5 ± 0.60 and females: 8.9 ± 0.85. Unpaired t test— cSTN, p = .84; NST, p = .18. n = 5 males, 6 females (3–4 sections per animal). (b) Male and female mice also have comparable numbers of β-gal+ cells in cervical, thoracic, lumbar, and sacral spinal cord segments. Number of β-gal+ cells per section ± SEM— cervical, males: 6.7 ± 1.1 and females: 6.4 ± 0.71; thoracic, males: 6.4 ± 1.6 and females: 8.0 ± 1.9; lumbar, males: 7.4 ± 0.94 and females: 6.6 ± 1.1; sacral, males: 7.3 ± 0.83 and females: 7.5 ± 1.1. Two-way ANOVA—effect of sex: F(1,41) = 0.031, p = .86; effect of segment: F(3,41) = 0.17, p = .92; interaction: F(3,41) = 0.40, p = .75. n = 6 males, 5 females (4–6 sections counted per animal at each spinal region)
3.3. Overlap of aromatase and markers of projection neurons and interneurons
Laminae I and V contain major populations of nociceptive projection neurons (Bráz, Solorzano, Wang, & Basbaum, 2014; Klop, Mouton, & Holstege, 2005; Price, Greenspan, & Dubner, 2003; Todd, McGill, & Shehab, 2000), with the great majority (~85%) of neurons targeting the parabrachial nucleus of the rostral pons (Hylden, Anton, & Nahin, 1989; Spike, Puskár, Andrew, & Todd, 2003). Because of the striking concentration of the aromatase-expressing cells in laminae I and V, we injected retrograde tracers into one side of the lateral parabrachial nucleus of reporter mice and double-labeled tissue to identify aromatase-expressing projection neurons. Although we observed many projection neurons neighboring cells with β-gal+ nuclei, we never detected double-labeled cells (Figure 5a-c). Based on this finding, we conclude that aromatase-expressing neurons do not project, but rather are interneurons that predominate in laminae I and V.
FIGURE 5.
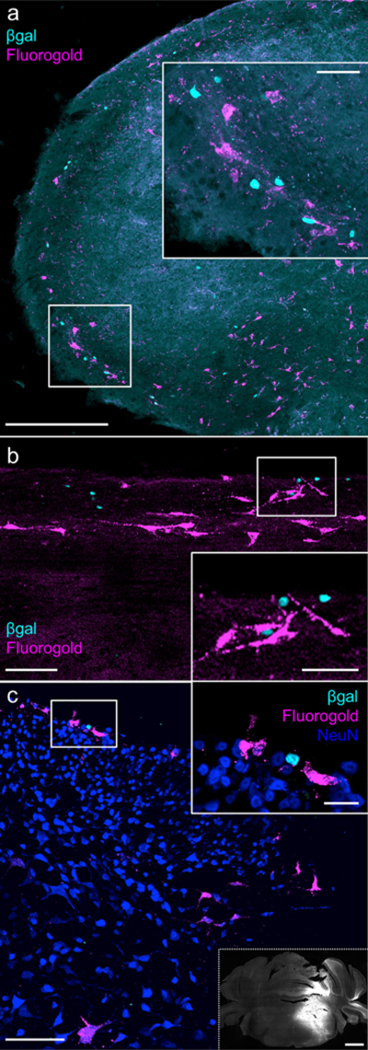
Aromatase-expressing neurons do not project to the parabrachial nucleus (a) Representative image of the cSTN 7 days after unilateral injection of a retrograde tracer (Fluoro-Gold) into the lateral parabrachial nucleus. Inset illustrates that β-gal and retrogradely labeled neurons in laminae I and V often appear in neighboring cells, but we never recorded overlap. n = 5 mice (2 males, 3 females). Scale bar: 300 μm; inset: 50 μm. (b, c) Representative images of sagittal (b) and coronal (c) sections of the lumbar spinal cord 8 days after injection of Fluoro-Gold into the lateral parabrachial nucleus. Insets: As for the medulla, we did not record β-gal+ retrogradely labeled neurons. In (c), NeuN immunoreactivity is included to demarcate the most superficial dorsal horn. Inset bordered by dashed line is a representative image of the Fluoro-Gold injection site, as shown by native, ultraviolet-excited Fluoro-Gold fluorescence. The injection site includes the area surrounding the brachium as well as the pontine reticular formation, capturing both spinoparabrachial and spinoreticular projection neuron targets. Scale bar: 100 μm; insets: (b) 50 μm, (c) 20 μm, dashed line) 1 mm. n = 6 mice (2 males and 2 females with Fluoro-Gold, 1 male and 1 female with RetroBeads). [Color figure can be viewed at wileyonlinelibrary.com]
Dorsal horn interneurons consist of both excitatory and inhibitory interneurons and within these general populations are neurochemically distinct subtypes. We first co-stained the aromatase cells with an antibody that recognizes Pax2, a selective marker of dorsal horn inhibitory interneurons (Del Barrio et al., 2013; Punnakkal et al., 2014). Figure 6a, b, e show that ~80% of the aromatase-expressing neurons in both the spinal cord and cSTN are Pax2-expressing and thus inhibitory. We next examined the subtypes of inhibitory interneurons, which Todd and colleagues (2010) defined by their differential expression of galanin, neuropeptide Y, neuronal nitric oxide synthase, and parvalbu- min. The somatostatin receptor subtype 2A (sst2A) marks about 50% of spinal inhibitory interneurons and encompasses the galanin, neuronal nitric oxide synthase, and some of the neuropeptide Y populations (Polgár, Durrieux, Hughes, & Todd, 2013; Polgár et al. 2013). Somewhat unexpectedly, we found no overlap of sst2A and β-gal in the spinal cord (Figure 6c; 0 sst+2A in 130 β-gal+ cells, n = 2 males, 2 females), which demonstrates that the aromatase-expressing cells belong to the sst2A-negative class of inhibitory interneurons. Finally, because a small number of aromatase-expressing cells were Pax2-negative, we also immunostained the cells for Lmx1b, a marker for spinal excitatory neurons (Del Barrio et al., 2013; Szabo et al., 2015). As expected we identified occasional Lmx1b-β-gal double-labeled cells (Figure 6d, e). Based on these findings, we conclude that the great majority of aromataseexpressing neurons are inhibitory and of the sst2a receptor-negative subtype.
FIGURE 6.
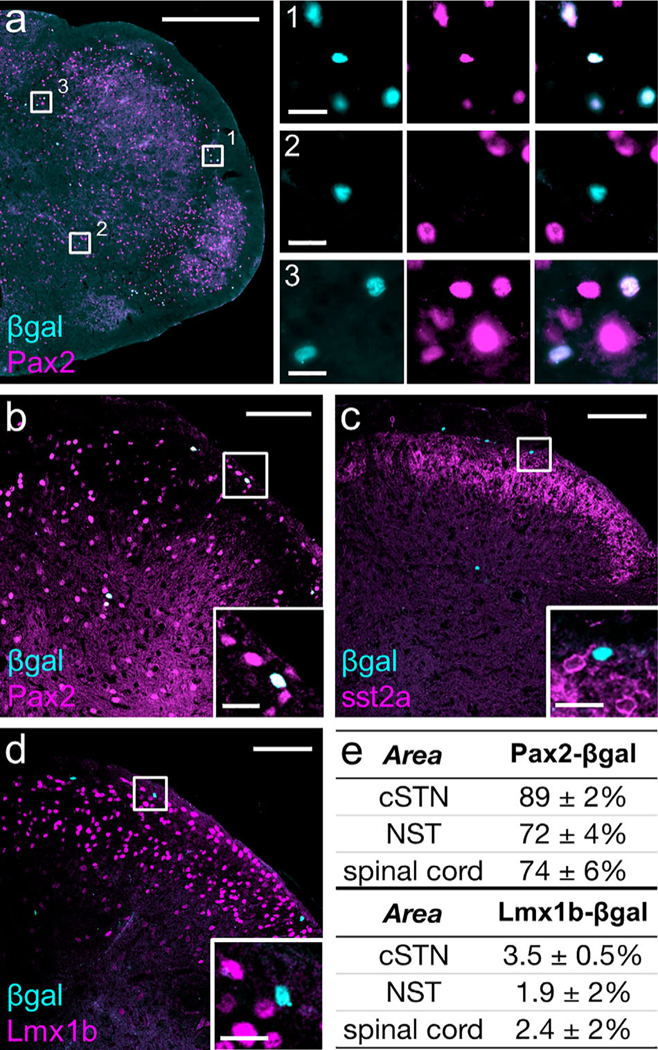
A small subpopulation of medullary and spinal inhibitory interneurons express aromatase. (a) Pax2, a marker of inhibitory interneurons, labels the majority of β-gai+ cells in the medulla. Insets 1 and 3 show examples of their co-localization in the cSTN and NST, respectively. Inset 2, in the cSTN, illustrates an example in which β-gal and Pax2 do not overlap. Scale bar: 500 μm; inset: 20 μm. (b) Pax2 also co-labels the majority of β-gal+ cells in the dorsal horn of the spinal cord. Scale bar: 100 mm; inset: 20 mm. (c) β-gal+ nuclei were never ringed by cytoplasmic sst2A receptor-immunoreactivity, indicating that aromatase neurons are in the sst2A- subpopulation of inhibitory interneurons. Scale bar: 100 μm; inset: 20 μm. (d) Co- staining for Lmxlb illustrates that very few β-gal+ cells are excitatory interneurons. Scale bar: 100 μm; inset: 20 μm. (e) Percentage of β-gal+ cells that co-label with Pax2 or Lmx1b. As an unpaired t test showed no difference between males and females for β-gal and Pax2 or Lmx1b overlap counts from male and female mice were pooled (Pax2: p = .48, .32, and .43 for cSTN, NST, and lumbar dorsal horn, respectively; Lmx1b: p = .72, .37, and .12 for cSTN, NST, and lumbar dorsal horn, respectively). n = 3 males, 3 females (3–4 sections per animal for the cSTN and NST, 4–6 sections per animal for lumbar dorsal horn). [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Algogen- and pruritogen-induced Fos expression
Various types of noxious stimulation induce expression of the immediate early gene Fos, prompting the production of the Fos protein in activated dorsal horn neurons (Hunt, Pini, & Evan, 1987; Menetrey, Gannon, Levine, & Basbaum, 1989). As different stimuli give rise to different patterns of Fos expression, co-localization with Fos could provide insights into whether the aromatase-expressing cells respond to specific pain modalities. Figure 7a-c illustrates examples of fb-gal1 cells in the medullary dorsal horn that overlap with Fos induced by the injection of the algogens capsaicin and formalin or the pruritogen chloroquine. Quantification showed that capsaicin induced Fos in 17.6% of cSTN aromatase neurons, formalin in 12.1% of these cells, and chloroquine in 13.9%. In contrast, the same agents injected into the hindpaw did not induce any overlap in the spinal cord dorsal horn (Figure 7d and data not shown).
3.5. Aromatase expression in models of chronic neuropathic or inflammatory pain
Two previous studies reported that nerve injury results in increased aromatase expression and estrogen synthesis in DRG neurons and in the spinal cord dorsal horn (Ghorbanpoor, Garcia-Segura, Haeri-Rohani, Khodagholi, & Jorjani, 2014; Schaeffer, Meyer, Patte-Mensah, Eckert, & Mensah-Nyagan, 2010). Here we sought to confirm those observations by assessing aromatase expression in the TG or DRG and cSTN or spinal cord after a complete unilateral transection of the infraorbital or sciatic nerves. We performed these studies in both male and female aromatase reporter mice and monitored β-gal expression 7 days post injury, a time point when rodents show behaviors indicative of spontaneous pain and significant upregulation of neurochemical markers of nerve injury (Basbaum, 1974; Bráz et al., 2011; Coderre, Grimes, & Melzack, 1986; Villar et al., 1989; Wall et al., 1979). As expected, we recorded activating transcription factor 3 (ATF3), a marker of injured sensory neurons (Braz & Basbaum, 2010; Tsujino et al., 2000), in large numbers of TG and L4/L5 DRG neurons ipsilateral to the transection (Figure 8a, c, upper right). However, we never detected β-gal signal in either the ipsilateral or contralateral DRG (Figure 8a, c, lower left and right). In a similar fashion, ionized calcium binding adaptor molecule 1 (Iba1)-expressing spinal cord microglia are readily apparent after nerve transection (Figure 8b, d, upper right; Ito et al., 1998; Romero- Sandoval, Chai, Nutile-McMenemy, & Deleo, 2008), but the β-gal expression pattern did not change (Figure 8b, d, lower left and right). Finally, in a separate set of experiments we evaluated aromatase expression under conditions of prolonged inflammation by injecting Complete Freund’s adjuvant (CFA) into the hindpaw. At 3 days postinjection when animals demonstrate profound thermal and mechanical hypersensitivity (Ma & Woolf, 1996; Malmberg, Gilbert, McCabe, & Basbaum, 2003), we again found no changes in the number of b-gal1 cells in the DRG or spinal cord dorsal horn (data not shown). Finally, in neither of these chronic pain models did we observe sex differences in aromatase expression in either DRG or spinal cord (data not shown). Taken together, our data indicate that neither peripheral nerve injury nor inflammation modifies aromatase expression in the spinal cord or DRG at the time points examined.
FIGURE 8.
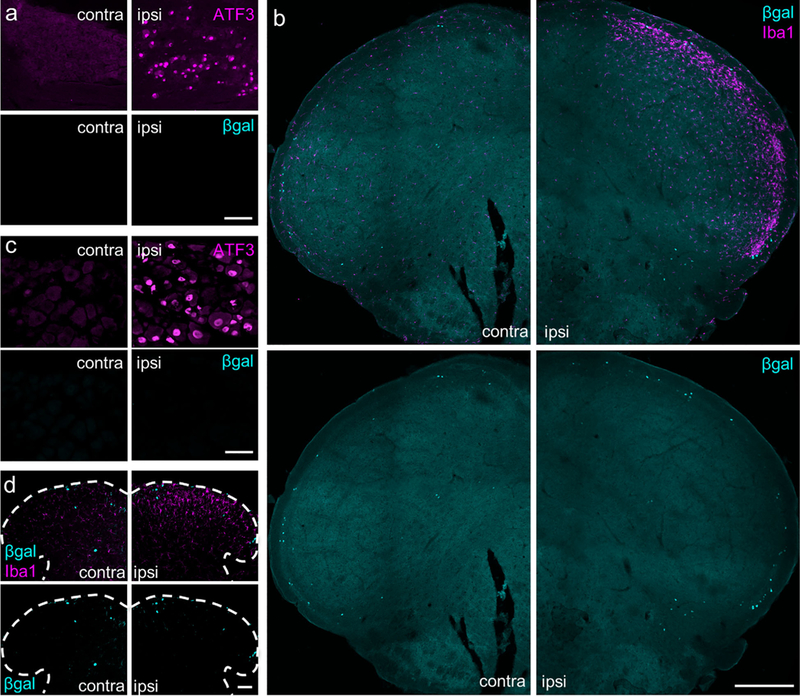
Peripheral nerve transection does not alter aromatase expression in primary sensory neurons or in the medullary or spinal dorsal horn. (a) Infraorbital nerve transection is a model of facial neuropathic pain. One week after infraorbital nerve transection, which models facial neuropathic pain, axotomized TG sensory neurons express ATF3 (contralateral vs. ipsilateral side, upper panels), but do not upregulate β-gal expression (lower panels). Comparable results were observed in male and female mice. n = 2 males, 2 females. Scale bar: 100 μm. (b) Nerve injury also provoked an upregulation of Iba1+ in microglia ipsilateral to the transection (upper panels), but did not alter the number of β-gal-expressing neurons in the cSTN (lower panels). Comparable results were observed in male and female mice. n = 2 males, 2 females. Scale bar: 300 μm. (c) One week post-injury in a partial sciatic nerve transection model of neuropathic pain, axotomized neurons of the L4/L5 DRG express ATF3 (upper panels), but there is no upregulation of fb-gal (lower panels). n = 5 males, 5 females. Scale bar: 50 μm. (d) Similarly, despite significant ipsilateral induction of the Iba 1 marker of microglial activation (upper panels), there was no apparent change in the numbers of aromatase expressing neurons (lower panels). n = 1 male, 3 females. Scale bar: 50 μm. [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In the present study, we identified and characterized a subpopulation of spinal and medullary neurons that express aromatase, a critical enzyme for estrogen synthesis. The aromatase-expressing cells are concentrated in laminae I and V of the spinal cord dorsal horn and in its trigeminal homologue, the caudal spinal trigeminal nucleus, as well as in the nucleus of the solitary tract. The spinal cord dorsal horn processes somatic sensory information, the caudal spinal trigeminal nucleus processes facial sensory information, and the nucleus of the solitary tract processes visceral sensory information. The great majority of aromatase-expressing cells is inhibitory interneurons, but appears not to express markers of the major inhibitory interneuron subpopulations. As some of these neurons express Fos in response to a variety of proalgesic or pruritic agents, it is likely that they are engaged in the setting of acute pain and itch. In contrast, their contribution in chronic pain models is unclear, given that we found no difference in the number or pattern of aromatase expressing neurons in several models of nerve or tissue injury. Finally, across the tissues and conditions examined, we found no difference in numbers of aromatase neurons in male and female mice.
4.1. Studies of aromatase expression and technical considerations
Aromatase is a member of the very large and structurally similar cytochrome P450 superfamily (Danielson, 2002). As a result, it can be difficult to generate antibodies that selectively recognize aromatase without crossreacting with any of the other 101 cytochrome P450 genes in the mouse (Nelson et al., 2004). In fact, this potential limitation of an immunocytochemical approach is what motivated several groups to develop reporter mouse lines (Wu et al., 2009; Stanić et al., 2014). In our experiments, we used the mouse generated by Wu and colleagues, which drives β-galactosidase expression under the control of aromatase promoters without disrupting normal aromatase function. Importantly, β-gal immunostaining in this reporter mouse successfully recapitulates the brain aromatase in situ hybridization pattern (Unger etal., 2015; Wu etal., 2009).
Our results using the aromatase reporter mouse are largely consistent with previous immunocytochemical reports of aromatase expression in the medullary and spinal cord dorsal horns in the Japanese quail, rat, and mouse (Horvath & Wikler, 1999; Evrard et al., 2000; O’Brien, Smeester, Michlitsch, Lee, & Beitz, 2015; Smeester, O’Brien, Michlitsch, Lee, & Beitz, 2016). On the other hand, although our finding that these cells are indeed neurons agrees with the conclusion of some groups (Horvath & Wikler, 1999; Evrard et al., 2000), it is at odds with another group that concluded that aromatase expression arises from astrocytes (O’Brien et al., 2015; Smeester et al., 2016). Furthermore, our finding of no expression of aromatase in sensory neurons contrasts significantly with the report of Schaeffer et al. (2010) that described aromatase immunoreactivity in DRG neurons using a mouse monoclonal antibody.
4.2. Neuronal estrogen and pain processing
Aromatase catalyzes the final steps that convert androgens, such as testosterone, to estrogens, such as estradiol. The presence of aromatase expression in spinal and medullary neurons thus indicates that these neurons are capable of synthesizing estrogens. As both the spinal and medullary dorsal horns express estrogen receptors (Amandusson, Hermanson, & Blomqvist, 1995; Bereiter, Cioffi, & Bereiter, 2005; Dun et al., 2009; Merchenthaler, Lane, Numan, and Dellovade, 2004; Shughrue, Lane, & Merchenthaler, 1997; Vanderhorst, Gustafsson, & Ulfhake, 2005), the aromatase cells in laminae I and V are ideally positioned to release estrogen in circuits that process nociceptive/pain messages. Given the broad distribution of estrogen receptor- expressing neurons in the superficial dorsal horn, the estrogen receptors may be located in the same cell (autocrine and intracrine signaling), in cells in the vicinity (paracrine signaling), or even in synaptic partners (synaptocrine signaling, see Remage-Healey, Saldanha, & Schlinger, 2011 for review). Our results showing that ERα is expressed by cells adjacent to but not in the aromatase neurons indicate that paracrine and synaptocrine signaling are more likely. Co-staining for other estrogen receptors as well as circuit tracing in the aromatase reporter mouse should provide valuable information about the targets of local estrogen.
Despite the abundance of superficial dorsal horn interneurons that express estrogen receptors, there is surprisingly little information as to the function of local estrogen synthesis in nociception. When applied to neurons, estrogen increases intrinsic excitability and excitatory transmission (Woolley, 2007), which suggests that local estrogen would be pronociceptive, promoting sensitization of circuits and subsequent heightened responses to painful stimuli. Indeed, for acute pain, estrogen synthesized in the spinal cord appears to be pronociceptive (Evrard & Balthazart, 2004; Zhang, Lü, et al., 2012; Zhang, Xiao, et al., 2012). Injection of estrogen into the paw, which would mimic the effects of locally synthesized estrogen acting on primary afferents, also generates hyperalgesia (Hucho, Dina, Kuhn, & Levine, 2006; Kuhn et al., 2008). In contrast, there is evidence that sensory neuron-derived estrogen is antinociceptive (Fusi et al., 2014). There is also a lack of agreement as to estrogen’s function in chronic pain models. It is of interest, for example, that some dorsal horn ERa-expressing neurons can synthesize enkephalin, an endogenous opioid peptide, and that its precursor, preproenkephalin, is acutely upregulated by estrogen (Amandusson, Hallbeck, Hallbeck, Hermanson, & Blomqvist, 1999; Amandusson et al., 1995). As such, estrogen synthesized by medullary and spinal aromatase-expressing cells could activate ERa-expressing neurons to engage a spinal endogenous analgesic system. On the other hand, although antinociceptive effects of primary afferent and spinal cord- derived estrogen were demonstrated in two neuropathic pain models (Ghorbanpoor et al., 2014; Schaeffer et al., 2010), a different group observed a pronociceptive effect in a bone cancer pain model (O’Brien et al., 2015; Smeester et al., 2016). Surprisingly, although aromatase expression was increased in each of these chronic pain models, using the reporter mouse we found no changes after nerve or tissue injury. Although these results are seemingly contradictory, the effects of estrogen are highly dependent on the anatomical location, the type of pain that is assessed, and a host of other factors, making direct comparison of studies difficult (Amandusson & Blomqvist, 2013; Craft, 2007).
The contribution of local estrogen to pain processing may also depend on the sex of the animal studied. In the rodent brain, aromatase neurons exhibit differential expression in males and females and contribute to sexually dimorphic behaviors, in part through the action of locally synthesized estrogen (Unger et al., 2015; Wu et al., 2009). Surprisingly, in spinal cord and medulla, comparing males and females, we found no statistical difference in numbers of aromatase-expressing cells. We appreciate, however, that numbers of cells may not be the critical contributor. For example, it is possible that these cells are more active in one sex compared to the other. Notably, males have a greater amount of circulating testosterone compared to females, which raises the possibility that the aromatase neurons in males are more likely to encounter testosterone that can be aromatized into estrogen. Consequently, although females have overall higher systemic levels of estrogen, males could experience greater local concentrations of estrogen that act more discretely on nearby nociceptive circuits. Studies that measure local estrogen in males and females, perhaps by microdialysis, would be useful to test this hypothesis.
4.3. Aromatase as an inhibitory cell marker in the spinal cord and medulla
In addition to being a source of estrogen, these cells likely release neurotransmitters that modify nociceptive circuits. Through our retrograde tracing and immunohistochemical co-labeling experiments, we found that the majority of the aromatase-expressing cells express g-aminobutyric acid (GABA; data not shown) and the Pax2 transcription factor, markers of inhibitory interneurons. On the other hand, we failed to define the subtype of inhibitory GABAergic interneuron, four of which have been demonstrated to date: neuronal nitric oxide synthase, galanin, neuropeptide Y, and parvalbumin (Laing, Todd, Heizmann, & Schmidt, 1994; Sardella, Polgar, Watanabe, & Todd, 2011; Sardella et al., 2011; Tiong, Polgar, van Kralingen, Watanabe, & Todd, 2011). Furthermore, although almost fifty percent of dorsal horn inhibitory interneurons express the sst2A subtype of somatostatin receptor (Polgar, Durrieux, et al., 2013), the aromatase expressing neurons did not. Defining the subtypes of inhibitory interneurons is of particular interest as these subtypes differentially modulate itch and pain (Bour- ane et al., 2015; Duan et al., 2014; Foster et al., 2015; Kardon et al., 2014; Petitjean et al., 2015; Ross et al., 2010). The sst-2A inhibitory interneurons, in contrast, are largely uncharacterized. The aromataseexpressing interneurons may, therefore, represent a unique subset of inhibitory interneurons, which could allow for specific molecular targeting of a nociceptive or pruritoceptive circuit. Future experiments involving ablation or silencing of aromatase neurons and subsequent pain and itch behavioral testing should indicate whether these cells engage modality-specific circuits.
Our finding that approximately 15% of aromatase neurons are activated by capsaicin, formalin, or chloroquine suggests that they indeed contribute to the processing of both pain and itch. Whether individual neurons receive convergent input from nociceptors and pru- ritoceptors, or whether they are part of hypothesized labeled lines that selectively transmit sensory information (Basbaum, Bautista, Scherrer, & Julius, 2009; Bráz et al., 2014), remains to be determined, preferably by electrophysiological analyses. Both capsaicin and formalin have previously been shown to stimulate Fos in inhibitory (GABAergic and/or glycinergic) neurons of the spinal cord dorsal horn (Hossaini, Duraku, Saraç, Jongen, & Holstege, 2010; Todd, Spike, Brodbelt, Price, & She- hab, 1994). Conceivably, engagement of these inhibitory interneurons by algogens and pruritogens underlies a feedforward regulation of output neurons, in a manner comparable to that proposed in Melzack & Wall’s Gate Control Theory (1965). Interestingly, Fos (or another marker of activation, phosphorylated extracellular signal-regulated kinase) is only induced in particular subsets of spinal dorsal horn inhibitory interneurons, namely the galanin, neuropeptide Y, and neuronal nitric oxide synthase populations (Polgár, Sardella, et al., 2013). Galanin and neuronal nitric oxide synthase are present in sst+2A cells, whereas neuropeptide Y is in a mix of both sst+2A and sst-2A cells. Because the aromatase-expressing neurons are sst-2A they may co-express neuropeptide Y. Unfortunately, we could not test this hypothesis as the aromatase reporter mouse produces a nuclear β-gal signal that we could not distinguish from the typical neuropeptide Y punctate staining pattern.
The Fos results also raise important questions about a possible dual estrogenic and inhibitory function of the aromatase-expressing neurons. On one hand, by converting androgens to estrogens via aromatase, these cells may be producing estrogen that increases the excitability of neighboring neurons (Woolley, 2007). On the other hand, because the majority of these cells also express inhibitory markers, they likely release GABA, which would reduce activity of neurons with which they communicate. These seemingly conflicting actions suggest that the aromatase-expressing neurons can concurrently regulate different cell populations by both inhibitory and facilitatory mechanisms. Pharmacological studies that block both estrogen synthesis and inhibitory neurotransmission by these cells could elucidate the functional consequences of a dorsal horn neuron releasing two opposing signaling molecules.
5. CONCLUSION
Several groups have suggested that estrogen is synthesized in pain processing areas and contributes to pain. In pursuit of the mechanisms that underlie pain-related local estrogen synthesis, this report represents the first attempt to define the molecular identity of the estrogen- producing cells. We demonstrate that aromatase-expressing cells in the spinal cord and medulla, including the neurons of the nucleus of the solitary tract, are anatomically positioned to receive somatic and visceral nociceptive inputs and have the potential to regulate multiple functions, not only through their capacity to release steroids but also through their neurochemical composition, as they form a distinct subpopulation of inhibitory interneurons. Determining whether the estrogenic and inhibitory components work independently or in concert with each other should generate valuable insights into how these cells exert their influence on nociception.
ACKNOWLEDGMENTS
The authors thank Drs. Nirao M. Shah and Elizabeth K. Unger at UCSF for providing the aromatase reporter mice. We are also grateful to Drs. David Julius at UCSF, Thomas Müller at the Max Delbrück Center for Molecular Medicine, and Andrew J. Todd at University of Glasgow for their generous donation of antibodies.
REFERENCES
- Adrian ED (1928). The basis of sensation: The action of the sense organs. London: Christophers. [Google Scholar]
- Al-Khater KM, & Todd AJ (2009). Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. The Journal of Comparative Neurology, 515, 629–646. doi: 10.1002/cne.22081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi AM, & Bonifazi M (2006). Sex hormones, central nervous system and pain. Hormones and Behavior, 50, 1–7. doi: 10.1016/j.yhbeh.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Amandusson Å, & Blomqvist A (2013). Estrogenic influences in pain processing. Frontiers in Neuroendocrinology, 34, 329–349. doi: 10.1016/j.yfrne.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Amandusson Å, Hallbeck M, Hallbeck A-L, Hermanson O, & Blomqv- ist A (1999). Estrogen-induced alterations of spinal cord enkephalin gene expression. Pain, 83, 243–248. doi: 10.1016/S0304-3959(99)00109-8 [DOI] [PubMed] [Google Scholar]
- Amandusson Å, Hermanson O, & Blomqvist A (1995). Estrogen receptor-like immunoreactivity in the medullary and spinal dorsal horn of the female rat. Neuroscience Letters, 196, 25–28. doi: 10.1016/0304-3940(95)11828-K [DOI] [PubMed] [Google Scholar]
- Amandusson Å, Hermanson O, & Blomqvist A (1996). Colocalization of oestrogen receptor lmmunoreactivity and preproenkephalin mRNA expression to neurons in the superficial laminae of the spinal and medullary dorsal horn of rats. European Journal of Neuroscience, 8, 2440–2445. doi: 10.1111/j.1460-9568.1996.tb01207.x [DOI] [PubMed] [Google Scholar]
- Basbaum AI (1974). Effects of central lesions on disorders produced by multiple dorsal rhizotomy in rats. Experimental Neurology, 42, 490–501. doi: 10.1016/0014-4886(74)90073-9 [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, & Julius D (2009). Cellular and molecular mechanisms of pain. Cell, 139, 267–284. doi: 10.1016/j.cell.2009.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter DA, Cioffi JL, & Bereiter DF (2005). Oestrogen receptor- immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Archives of Oral Biology, 50, 971–979. doi: 10.1016/j.archoralbio.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Berkley KJ (1997). Sex differences in pain. The Behavioral and Brain Sciences, 20, 513. doi: 10.1017/S0140525X97221485 [DOI] [PubMed] [Google Scholar]
- Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, ... Goulding M (2015). Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science (New York, N.Y.), 350, 550–554. doi: 10.1126/science.aac8653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Ackerman L, & Basbaum AI (2011). Sciatic nerve transection triggers release and intercellular transfer of a genetically expressed macromolecular tracer in dorsal root ganglia. The Journal of Comparative Neurology, 519, 2648–2657. doi: 10.1002/cne.22645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bráz JM, & Basbaum AI (2010). Differential ATF3 expression in dorsal root ganglion neurons reveals the profile of primary afferents engaged by diverse noxious chemical stimuli. Pain, 150, 290–301. doi: 10.1016/j.pain.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bráz JM, Solorzano C, Wang X, & Basbaum AI (2014). Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron, 82, 522–536. doi: 10.1016/j.neuron.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, & Anderson DJ (2009). Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proceedings of the National Academy of Sciences of the United States of America, 106, 9075–9080. doi: 10.1073/pnas.0901507106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre TJ, Grimes RW, & Melzack R (1986). Deafferentation and chronic pain in animals: An evaluation of evidence suggesting autot- omy is related to pain. Pain, 26, 61–84. doi: 10.1016/0304-3959(86)90174-0 [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Beckstead RM, & Norgren R (1982). The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: An autoradiographic study in the rat. Journal of the Autonomic Nervous System, 6, 303–322. doi: 10.1016/0165-1838(82)90003-0 [DOI] [PubMed] [Google Scholar]
- Craft RM (2007). Modulation of pain by estrogens. Pain, 132(Suppl 1), 3. doi: 10.1016/j.pain.2007.09.028 [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, & Aloisi AM (2004). Sex differences in pain and analgesia: The role of gonadal hormones. European Journal of Pain (London, England), 8, 397–411. doi: 10.1016/j.ejpain.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Danielson PB (2002). The cytochrome P450 superfamily: Biochemistry, evolution and drug metabolism in humans. Current Drug Metabolism, 3, 561–597. doi: 10.2174/1389200023337054 [DOI] [PubMed] [Google Scholar]
- Del Barrio MG, Bourane S, Grossmann K, Schüle R, Britsch S, O’Leary DDM, & Goulding M (2013). A transcription factor code defines nine sensory interneuron subtypes in the mechanosensory area of the spinal cord. PloS One, 8, e77928. doi: 10.1371/journal.pone.0077928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, ... Ma Q (2014). Identification of spinal circuits transmitting and gating mechanical pain. Cell, 159, 1417–1432. doi: 10.1016/j.cell.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun SL, Brailoiu GC, Gao X, Brailoiu E, Arterburn JB, Prossnitz ER, ... Dun NJ (2009). Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. Journal of Neuroscience Research, 87, 1610–1619. doi: 10.1002/jnr.21980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard HC, Baillien M, Foidart A, Absil P, Harada N, & Balthazart J (2000). Localization and controls of aromatase in the quail spinal cord. The Journal of Comparative Neurology, 423, 552–564. doi: [DOI] [PubMed] [Google Scholar]
- Evrard HC, & Balthazart J (2004). Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. The Journal of Neuroscience, 24, 7225–7229. doi: 10.1523/JNEUROSCI.1638-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, & Maixner W (1995). Gender differences in the responses to noxious stimuli. The Journal of Pain, 4, 209–221. doi: 10.1016/S1082-3174(11)80022-X [DOI] [Google Scholar]
- Foerster O (1933). The dermatomes in man. Brain, 56, 1–39. doi: 10.1093/brain/56.1.1 [DOI] [Google Scholar]
- Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, ... Zeilhofer HU (2015). Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron, 85, 1289–1304. doi: 10.1016/j.neuron.2015.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend KE, Resnick EM, Ang LW, & Shupnik MA (1997). Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Molecular and Cellular Endocrinology, 131, 147–155. doi: 10.1016/S0303-7207(97)00098-1 [DOI] [PubMed] [Google Scholar]
- Fusi C, Materazzi S, Benemei S, Coppi E, Trevisan G, Marone IM, ... Nassini R (2014). Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nature Communications, 5, 5736. doi: 10.1038/ncomms6736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbanpoor S, Garcia-Segura LM, Haeri-Rohani A, Khodagholi F, & Jorjani M (2014). Aromatase inhibition exacerbates pain and reactive gliosis in the dorsal horn of the spinal cord of female rats caused by spinothalamic tract injury. Endocrinology, 155, 4341–4355. doi: 10.1210/en.2014-1158 [DOI] [PubMed] [Google Scholar]
- Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, ... Basbaum AI (2016). Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nature Neuroscience, 19, 94–101. doi: 10.1038/nn.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, & Fitzsimmons MD (1992). Detecting steroidal effects on immediate early gene expression in the hypothalamus. Neuroprotocols, 1, 52–66. doi: 10.1016/1058-6741(92)90021-O [DOI] [Google Scholar]
- Holloway S, Feniuk W, Kidd EJ, & Humphrey PP (1996). A quantitative autoradiographical study on the distribution of somatostatin sst2 receptors in the rat central nervous system using [125I]-BIM-23027. Neuropharmacology, 35, 1109–1120. doi: 10.1016/S0028-3908(96)00082-2 [DOI] [PubMed] [Google Scholar]
- Horvath TL, & Wikler KC (1999). Aromatase in developing sensory systems of the rat brain. Journal of Neuroendocrinology, 11, 77–84. doi: 10.1046/j.1365-2826.1999.00285.x [DOI] [PubMed] [Google Scholar]
- Hossaini M, Duraku LS, Sara C, Jongen JLM, & Holstege JC(2010). Differential distribution of activated spinal neurons containing glycine and/or GABA and expressing c-fos in acute and chronic pain models. Pain, 151, 356–365. doi: 10.1016/j.pain.2010.07.023 [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina CA, Kuhn J, & Levine JD (2006). Estrogen controls PKCe-dependent mechanical hyperalgesia through direct action on nociceptive neurons. European Journal of Neuroscience, 24, 527–534. doi: 10.1111/j.1460-9568.2006.04913.x [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, & Evan G (1987). Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature, 328, 632–634. doi: 10.1038/328632a0 [DOI] [PubMed] [Google Scholar]
- Hylden JL, Anton F, & Nahin RL (1989). Spinal lamina I projection neurons in the rat: Collateral innervation of parabrachial area and thalamus. Neuroscience, 28, 27–37. doi: 10.1016/0306-4522(89)90229-7 [DOI] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, & Kohsaka S (1996). A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochemical and Biophysical Research Communications, 224, 855–862. doi: 10.1006/bbrc.1996.1112 [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, & Kohsaka S (1998). Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Research. Molecular Brain Research, 57, 1–9. doi: 10.1016/S0169-328X(98)00040-0 [DOI] [PubMed] [Google Scholar]
- Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, ... Ross SE (2014). Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron, 82, 573–586. doi: 10.1016/j.neuron.2014.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klop EM, Mouton LJ, & Holstege G (2005). Segmental and laminar organization of the spinothalamic neurons in cat: Evidence for at least five separate clusters. The Journal of Comparative Neurology, 493, 580–595. doi: 10.1002/cne.20777 [DOI] [PubMed] [Google Scholar]
- Kuba T, & Quinones-Jenab V (2005). The role of female gonadal hormones in behavioral sex differences in persistent and chronic pain: Clinical versus preclinical studies. Brain Research Bulletin, 66, 179–188. doi: 10.1016/j.brainresbull.2005.05.009 [DOI] [PubMed] [Google Scholar]
- Kuhn J, Dina OA, Goswami C, Suckow V, Levine JD, & Hucho T (2008). GPR30 estrogen receptor agonists induce mechanical hyper-algesia in the rat. European Journal of Neuroscience, 27, 1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x [DOI] [PubMed] [Google Scholar]
- Laing I, Todd AJ, Heizmann CW, & Schmidt HH (1994). Subpo-pulations of GABAergic neurons in laminae I-III of rat spinal dorsal horn defined by coexistence with classical transmitters, peptides, nitric oxide synthase or parvalbumin. Neuroscience, 61, 123–132. doi: 10.1016/0306-4522(94)90065-5 [DOI] [PubMed] [Google Scholar]
- Ma QP, & Woolf CJ (1996). Progressive tactile hypersensitivity: An inflammation-induced incremental increase in the excitability of the spinal cord. Pain, 67, 97–106. doi: 10.1016/0304-3959(96)03105-3 [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Gilbert H, McCabe RT, & Basbaum AI (2003). Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain, 101, 109–116. doi: 10.1016/S0304-3959(02)00303-2 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Alves SE (1999). Estrogen actions in the central nervous system. Endocrine Reviews, 20, 279–307. doi: 10.1210/edrv.20.3.0365 [DOI] [PubMed] [Google Scholar]
- Melzack R, & Wall PD (1965). Pain mechanisms: A new theory. Science (New York, N.Y.), 150, 971–979. doi: 10.1126/science.150.3699.971 [DOI] [PubMed] [Google Scholar]
- Menétrey D, Gannon A, Levine JD, & Basbaum AI (1989). Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. The Journal of Comparative Neurology, 285, 177–195. doi: 10.1002/cne.902850203 [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, & Dellovade TL (2004). Distribution of estrogen receptor a and p in the mouse central nervous system: In vivo autoradiographic and immunocytochemical analyses. The Journal of Comparative Neurology, 473, 270–291. doi: 10.1002/cne.20128 [DOI] [PubMed] [Google Scholar]
- Mogil JS (2012). Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nature Reviews. Neuroscience, 13, 859–866. doi: 10.1038/nrn3360 [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, & Smith AM (1992). NeuN, a neuronal specific nuclear protein in vertebrates. Development (Cambridge, England), 116, 201–211. [DOI] [PubMed] [Google Scholar]
- Munchrath LA, & Hofmann HA (2010). Distribution of sex steroid hormone receptors in the brain of an African cichlid fish, Astatotilapia burtoni. The Journal of Comparative Neurology, 518, 3302–3326. doi: 10.1002/cne.22401 [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SMG, Maltais LJ, Wain HM, & Nebert DW (2004). Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics, 14, 1–18. doi: 10.1097/00008571-200401000-00001 [DOI] [PubMed] [Google Scholar]
- O’Brien EE, Smeester BA, Michlitsch KS, Lee JH, & Beitz AJ (2015). Colocalization of aromatase in spinal cord astrocytes: Differences in expression and relationship to mechanical and thermal hyperalgesia in murine models of a painful and a non-painful bone tumor. Neuroscience, 301, 235–245. doi: 10.1016/j.neuroscience.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papka RE, Srinivasan B, Miller KE, & Hayashi S (1997). Localization of estrogen receptor protein and estrogen receptor messenger RNA in peripheral autonomic and sensory neurons. Neuroscience, 79, 1153–1163. doi: 10.1016/S0306-4522(97)00076-6 [DOI] [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, ... Shupnik M (2001). Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell and Tissue Research, 304, 193–214. doi: 10.1007/s004410100363 [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Franklin K (2001). Paxinos and Franklin’s the mouse brain in stereotaxic coordinates (2nd ed.). San Diego: Academic Press. [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, ... Sharif-Naeini R (2015). Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Reports, 13, 1246–1257. doi: 10.1016/j.celrep.2015.09.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau I, & Lacroix S (2007). Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. The Journal of Comparative Neurology, 500, 267–285. doi: 10.1002/cne.21149 [DOI] [PubMed] [Google Scholar]
- Polgár E, Durrieux C, Hughes DI, & Todd AJ (2013). A quantitative study of inhibitory interneurons in laminae I-III of the mouse spinal dorsal horn. PloS One, 8, e78309. doi: 10.1371/journal.pone.0078309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Sardella TCP, Tiong SYX, Locke S, Watanabe M, & Todd AJ (2013). Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn. Pain, 154, 2606–2615. doi: 10.1016/j.pain.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, Dubner R, & Hu HW (1976). Trigeminothalamic neurons in nucleus caudalis responsive to tactile, thermal, and nociceptive stimulation of monkey’s face. Journal of Neurophysiology, 39, 936–953. doi: 10.1016/0304-3959(77)90097-5 [DOI] [PubMed] [Google Scholar]
- Price DD, Greenspan JD, & Dubner R (2003). Neurons involved in the exteroceptive the exteroceptive function of pain. Pain, 106, 215–219. doi: 10.1016/j.pain.2003.10.016 [DOI] [PubMed] [Google Scholar]
- Punnakkal P, von Schoultz C, Haenraets K, Wildner H, & Zeilhofer HU (2014). Morphological, biophysical and synaptic properties of glutamatergic neurons of the mouse spinal dorsal horn. The Journal of Physiology, 592, 759–776. doi: 10.1113/jphysiol.2013.264937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, & Gebhart GF (1992). Vagal afferent modulation of nociception. Brain Research. Brain Research Reviews, 17, 77–99. doi: 10.1016/0165-0173(92)90009-B [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Saldanha CJ, & Schlinger BA (2011). Estradiol synthesis and action at the synapse: Evidence for “synaptocrine” signaling. Frontiers in Endocrinology, 2, 28. doi: 10.3389/fendo.2011.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, Robinson ME, Wise EA, Myers CD, & Fillingim RB (1998). Sex differences in the perception of noxious experimental stimuli: A meta-analysis. Pain, 74, 181–187. doi: 10.1016/S0304-3959(97)00199-1 [DOI] [PubMed] [Google Scholar]
- Rinaman L, Verbalis JG, Stricker EM, & Hoffman GE (1993). Distribution and neurochemical phenotypes of caudal medullary neurons activated to express cFos following peripheral administration of cho-lecystokinin. The Journal of Comparative Neurology, 338, 475–490. doi: 10.1002/cne.903380402 [DOI] [PubMed] [Google Scholar]
- Robertson B, & Arvidsson J (1985). Transganglionic transport of wheat germ agglutinin-HRP and choleragenoid-HRP in rat trigeminal primary sensory neurons. Brain Research, 348, 44–51. doi: 10.1016/0006-8993(85)90357-9 [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval A, Chai N, Nutile-McMenemy N, & Deleo JA (2008). A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Research, 1219, 116–126. doi: 10.1016/j.brainres.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, ... Greenberg ME (2010). Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron, 65, 886–898. doi: 10.1016/j.neuron.2010.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardella TCP, Polgár E, Garzillo F, Furuta T, Kaneko T, Watanabe M, & Todd AJ (2011). Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Molecular Pain, 7, 76.doi: 10.1186/1744-8069-7-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardella TCP, Polgár E, Watanabe M, & Todd AJ (2011). A quantitative study of neuronal nitric oxide synthase expression in laminae I-III of the rat spinal dorsal horn. Neuroscience, 192, 708–720. doi: 10.1016/j.neuroscience.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer V, Meyer L, Patte-Mensah C, Eckert A, & Mensah-Nyagan AG (2010). Sciatic nerve injury induces apoptosis of dorsal root ganglion satellite glial cells and selectively modifies neurosteroidogenesis in sensory neurons. Glia, 58, 169–180. doi: 10.1002/glia.20910 [DOI] [PubMed] [Google Scholar]
- Schindler M, Sellers LA, Humphrey PP, & Emson PC (1997). Immunohistochemical localization of the somatostatin SST2(A) receptor in the rat brain and spinal cord. Neuroscience, 76, 225–240. doi: 10.1016/S0306-4522(96)00388-0 [DOI] [PubMed] [Google Scholar]
- Schulz S, Schulz S, Schmitt J, Wiborny D, Schmidt H, Olbricht S, ... Höllt V (1998). Immunocytochemical detection of somatostatin receptors sst1, sst2A, sst2B, and sst3 in paraffin-embedded breast cancer tissue using subtype-specific antibodies. Clinical Cancer Research, 4, 2047–2052. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, & Merchenthaler I (1997). Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. The Journal of Comparative Neurology, 388, 507–525. doi: [DOI] [PubMed] [Google Scholar]
- Simpson ER (2003). Sources of estrogen and their importance. The Journal of Steroid Biochemistry and Molecular Biology, 86, 225–230. doi: 10.1016/S0960-0760(03)00360-1 [DOI] [PubMed] [Google Scholar]
- Smeester BA, O’Brien EE, Michlitsch KS, Lee JH, & Beitz AJ (2016) The relationship of bone-tumor-induced spinal cord astrocyte activation and aromatase expression to mechanical hyperalgesia and cold hypersensitivity in intact female and ovariectomized mice. Neuroscience, 324, 344–354. doi: 10.1016/j.neuroscience.2016.03.030 [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, & Toran-Allerand CD (1994). Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. The Journal of Neuroscience, 14, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike RC, Puskár Z, Andrew D, & Todd AJ (2003). A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. The European Journal of Neuroscience, 18, 2433–2448. doi: 10.1046/j.1460-9568.2003.02981.x [DOI] [PubMed] [Google Scholar]
- Stanić D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, & Boon WC (2014). Characterization of aromatase expression in the adult male and female mouse brain. I. coexistence with oestrogen receptors α and β, and androgen receptors. PloS One, 9, e90451. doi: 10.1371/journal.pone.0090451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey ML, Davies M, Yip PK, Carter LM, Wong DJN, McMahon SB, & Bradbury EJ (2009). Expression of the regeneration-associated protein SPRR1A in primary sensory neurons and spinal cord of the adult mouse following peripheral and central injury. The Journal of Comparative Neurology, 513, 51–68. doi: 10.1002/cne.21944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo NE, da Silva RV, Sotocinal SG, Zeilhofer HU, Mogil JS, & Kania A (2015). Hoxb8 intersection defines a role for Lmx1b in excitatory dorsal horn neuron development, spinofugal connectivity, and nociception. The Journal of Neuroscience, 35, 5233–5246. doi: 10.1523/JNEURUSCI.4690-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleghany N, Sarajari S, DonCarlos L. l., Gollapudi L, & Oblinger MM (1999). Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. Journal of Neuroscience Research, 57, 603–615. doi: [DOI] [PubMed] [Google Scholar]
- Tiong SYX, Polgár E, van Kralingen JC, Watanabe M, & Todd AJ (2011). Galanin-immunoreactivity identifies a distinct population of inhibitory interneurons in laminae I-III of the rat spinal cord. Molecular Pain, 7, 36. doi: 10.1186/1744-8069-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, & Shehab SA (2000). Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. The European Journal of Neuroscience, 12, 689–700. doi: 10.1046/j.1460-9568.2000.00950.x [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC, Brodbelt AR, Price RF, & Shehab SA (1994). Some inhibitory neurons in the spinal cord develop c-fos- immunoreactivity after noxious stimulation. Neuroscience, 63, 805–816. doi: 10.1016/0306-4522(94)90525-8 [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC, & Polgár E (1998). A quantitative study of neurons which express neurokinin-1 or somatostatin sst2a receptor in rat spinal dorsal horn. Neuroscience, 85, 459–473. doi: 10.1016/S0306-4522(97)00669-6 [DOI] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, ... Noguchi K (2000). Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Molecular and Cellular Neurosciences, 15, 170–182. doi: 10.1006/mcne.1999.0814 [DOI] [PubMed] [Google Scholar]
- Unger EK, Burke KJ, Yang CF, Bender KJ, Fuller PM, & Shah NM (2015). Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Reports, 10, 453–462. doi: 10.1016/j.celrep.2014.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh AM (1996). Gender variations in clinical pain experience. Pain, 65, 123–167. doi: 10.1016/0304-3959(95)00214-6 [DOI] [PubMed] [Google Scholar]
- Vanderhorst Veronique G J M, Gustafsson J, & Ulfhake B (2005). Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: Relationships to monoaminergic, cholinergic, and spinal projection systems. The Journal of Comparative Neurology, 488, 152–179. doi: 10.1002/cne.20569 [DOI] [PubMed] [Google Scholar]
- Villar MJ, Cortés R, Theodorsson E, Wiesenfeld-Hallin Z, Schalling M, Fahrenkrug J, ... Hökfelt T (1989). Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience, 33, 587–604. doi: 10.1016/0306-4522(89)90411-9 [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, & Tomkiewicz MM (1979). Autotomy following peripheral nerve lesions: Experimental anaesthesia dolorosa. Pain, 7, 103–111. doi: 10.1016/0304-3959(79)90002-2 [DOI] [PubMed] [Google Scholar]
- Woolley CS (2007). Acute effects of estrogen on neuronal physiology. Annual Review of Pharmacology and Toxicology, 47, 657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219 [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S,... Shah NM (2009). Estrogen masculinizes neural pathways and sex- specific behaviors. Cell, 139, 61–72. doi: 10.1016/j.cell.2009.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Kobayashi K, Okubo M, Fukuoka T, & Noguchi K (2011). Increase of close homolog of cell adhesion molecule L1 in primary afferent by nerve injury and the contribution to neuropathic pain. The Journal of Comparative Neurology, 519, 1597–1615. doi: 10.1002/cne.22588 [DOI] [PubMed] [Google Scholar]
- Yang M, Cagle MC, & Honig MG (2010). Identification of cerebel-lin2 in chick and its preferential expression by subsets of developing sensory neurons and their targets in the dorsal horn. The Journal of Comparative Neurology, 518, 2818–2840. doi: 10.1002/cne.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lü N, Zhao Z, & Zhang Y (2012). Involvement of estrogen in rapid pain modulation in the rat spinal cord. Neurochemical Research, 37, 2697–2705. doi: 10.1007/s11064-012-0859-1 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiao X, Zhang X, Zhao Z, & Zhang Y (2012). Estrogen facilitates spinal cord synaptic transmission via membrane-bound estrogen receptors: Implications for pain hypersensitivity. The Journal of Biological Chemistry, 287, 33268–33281. doi: 10.1074/jbc.M112.368142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran M, Kuhn JA, Bráz JM, Basbaum AI. Neuronal aromatase expression in pain processing regions of the medullary and spinal cord dorsal horn. J Comp Neurol. 2017;525:3414–3428. 10.1002/cne.24269 [DOI] [PMC free article] [PubMed] [Google Scholar]


