Abstract
Objective:
Systemic inflammation contributes to cardiovascular disease in patients with type 2 diabetes, and elevated white blood cell (WBC) counts are an established risk factor. Our goal is to describe changes in WBCs and inflammatory markers after glycemic reductions in diabetes.
Research Design and Methods:
This study enrolled 63 subjects with poorly controlled diabetes, defined as hemoglobin A1c (HbA1c) ≥8% [64 mmol/mol]. Circulating granulocytes and mononuclear cells were separated by histopaque double-density protocol. Inflammatory markers from these isolated WBCs were assessed at baseline and after 3 months of medical management.
Results:
After 3 months, significant glycemic reduction, defined as a decrease in HbA1c≥1.5%, occurred in 42 subjects. Fasting plasma glucose decreased by 47% (165.6 mg/dL), and HbA1c decreased from 10.2±1.8 to 6.8±0.9. Glycemic reductions were associated with a 9.4% decrease in total WBC counts, 10.96% decrease in neutrophils, and 21.74% decrease in monocytes. The mRNA levels of inflammatory markers from granulocytes and mononuclear cells decreased, including receptor for advanced glycation endproducts; S100 calcium binding proteins A8, A9, A12; krüppel-like factor 5; and IL-1. Also, circulating levels of IL-1β and C-reactive protein decreased. Insulin dose was a mediator between HbA1c and both total WBC and neutrophil counts, but not changes in WBC inflammatory markers. In contrast, the 17 subjects without significant glycemic reductions showed no significant differences in their WBC counts and proteins of inflammatory genes.
Conclusion:
Significant glycemic reduction in subjects with poorly controlled diabetes led to reduced circulating WBC counts and inflammatory gene expression.
Keywords: Glycemic Reduction, Inflammatory Biomarkers, Inflammation, Hyperglycemia
1. Introduction
Two-thirds of individuals with diabetes die from cardiovascular disease (CVD) or stroke [1]. Increased white blood cell (WBC) counts and their inflammatory phenotype in diabetes may play a pivotal role in micro- and macro-vascular disease [2-5]. For more than three decades, investigators have known that elevated WBC levels are an indicator of CVD risk [6]. Similarly, we reported that elevated WBCs from increased monocyte and neutrophil counts correlated with CVD events in patients with type 1 diabetes [7]. In mice, improvements in glycemic control reduced WBC numbers and repaired atherosclerotic arteries after cholesterol reduction [7].
Both macrophages and neutrophils are found in atherosclerotic plaques from mice and humans [8, 9]. Neutrophils, although the most abundant WBC, have received less attention in their contribution to atherosclerosis. Elevated circulating neutrophils predict CVD, independent of serum cholesterol levels [9]. While inflammatory cytokines and WBC counts are elevated in patients with diabetes [10-12], whether this is due solely to hyperglycemia, defective insulin actions, or other factors associated with diabetes remains unclear. To determine whether glycemic reductions lowered WBC levels and expression of inflammatory genes, we recruited a group of patients with poorly controlled diabetes and examined two WBC populations: granulocytes comprised of neutrophils, basophils, and eosinophils; and mononuclear cells, comprised of monocytes and lymphocytes. In addition, we assessed whether improved glucose levels reduced circulating levels of several inflammatory markers.
2. Subjects and methods
2.1. Subjects
This was a cohort study (n=63) that enrolled subjects with poorly controlled diabetes from Anhui Provincial Hospital in Hefei, China. The Anhui Provincial Hospital Ethics Committee approved the study protocol, and signed written informed consents were received from each participant. The inclusion criterion for entry into this study was diagnosis of poorly controlled diabetes, defined as fasting glucose >250 mg/dL and/or hemoglobin A1 c (HbA1c) ≥8% (64 mmol/mol). Individuals with a history of diabetic ketoacidosis, hyperosmolar hyperglycemic state, any active infections, pulmonary disease, severe acute cardiovascular or cerebrovascular disease, or renal impairment were excluded from the study. Subjects presented to the hospital with symptoms that included polydipsia, polyuria, or weight loss. All subjects had significantly elevated glucose levels and were admitted by the internal medicine team for glycemic control. Type 1 and type 2 diabetes diagnoses were confirmed by reviewing the medical records of subjects with chronic diabetes.
2.2. Study Protocol
2.2.1. Data collection
We obtained baseline information including sex, age, medications, HbA1c, and body mass index (BMI). The subjects underwent nutritional counseling as well as glycemic and medical management by their physician during hospitalization. Researchers were not involved in the medical management of subjects. All medications including anti-hyperglycemics and lipid lowering drugs, HbA1c, and BMI were recorded again at a 3-month follow-up visit.
2.2.2. Clinical specimens
Peripheral blood was collected from consenting donors into vacutainer tubes containing K2 EDTA and immediately placed on ice.
2.2.3. Blood chemistry and complete blood counts (CBCs)
We assessed BMI, HbA1c, CBC with differential, and lipid levels at baseline and 3 months. Baseline assessments took place during hospitalization. At each time point, histopaque separation was used to isolate granulocytes and mononuclear cells.
2.2.4. Histopaque double-density separation
Blood samples were diluted 1:1 with 0.9% saline solution and mixed well by gentle inversion. The diluted blood/saline solution was carefully layered in another conical tube containing equal volumes of histopaque 1.077 and histopaque 1.119. The tubes were centrifuged at 700 × g for 30 min at room temperature. After centrifugation, the opaque bands containing granulocytes and mononuclear cells were carefully aspirated and transferred to separate conical centrifuge tubes containing 8mL of ice-cold 0.9% saline. The tubes were centrifuged at 1500 rpm for 15 min at 4°C. The supernatant was removed and discarded, and the pellets were suspended in 800 μ of 0.2% and 1.6% ice-cold saline. Cells were centrifuged at 600 × g for 8 s. The supernatant was suctioned out, and cell pellets re-suspended in Trizol®. RNA was extracted from these cells for quantitative PCR (qPCR).
2.2.5. RNA isolation
RNA was extracted using the Thermo Fisher Scientific Trizol® Plus-RNA Purification Kit, and cDNA was synthesized according to the manufacturer’s protocols using the Superscript III First-Strand Synthesis System-Thermo Fisher Scientific. Then, qPCR analysis was performed using iQ™ SYBR® Green Supermix. Samples were analyzed in duplicates using Applied Biosystems 7500 Real-Time PCR System.Standard thermal cycling conditions (10 min at 95°C, 40 cycles for 15 s at 95°C, and 1 min at 60°C) were used for all genes.
2.2.6. Gene expression analysis
After qPCR was completed, 10 genes for each subject were analyzed at baseline and 3 months. These genes include: receptor for advanced glycation endproducts (AGER), S100A8, S100A9, S100A12, diaphanous related formin 1 (DIAPH1), glyoxalase I (GLO1), KLF5, IL-1, IL-4, and IL-6. (See Supplementary Table 1 for primers). Quantity values, calculated from cycle threshold (CT) values, were based on standard curves obtained for each gene in each sample using the Applied Biosystems 7500 Real-Time PCR System. RNA levels of each subject were normalized to their respective baseline and 3-month 18S levels. All genetic markers at baseline were normalized to a value of 100.
2.2.7. Circulating inflammatory markers
Plasma levels of IL-1β, IL-6, S100A8/A9, and C-reactive protein (CRP) were analyzed using the ELISA kits from R&D systems. All assays were performed in duplicate.
2.3. Statistical analyses
Statistical analyses were conducted using SPSS 23.0, with the alpha level set at p < 0.05. 18S genes were used as reference genes for normalization. Results were analyzed in duplicate. To compare mean changes in continuous variables from baseline to 3 months, and for non-normally distributed data, the Mann-Whitney U-test was used. Antiplatelet and statin usage, as well as new versus old diabetes were dichotomous variables. The Kruskal-Wallis test was used to compare the change in inflammatory markers in these groups. The pairwise correlation between individual gene expression and insulin and HbA1c levels were computed using Spearman’s rank correlation coefficient. Chi-square tests were used to compare the proportion of medication use between the diabetes groups. The McNemar’s test was used to compare the proportion of medication use before and after medical management for each subject. Regression analysis was performed to evaluate statistical mediation using the PROCESS macros for SPSS [13], including bootstrapping of the sampling distribution to derive estimates of indirect effects, standard error, and bias-corrected 95% confidence intervals (CI) [13, 14]. This path model examined the indirect effects of HbA1c on total WBC, neutrophil, monocyte, and lymphocytes counts, using insulin as a mediator. Clinical data are represented as mean ± standard deviation. A significance of 5% was used.
3. Results
3.1. Patient characteristics
The baseline characteristics of the 63 subjects are shown in Table 1. After 3 months, we categorized the subjects into two groups: 1) improved diabetes group (IDG) (n=42), defined as a reduction in HbA1c of ≥ 1.5% (16 mmol/mol), and 2) unimproved diabetes group (UDG) (n=21), defined as <1.5% HbA1c reduction. Of the 42 subjects in the IDG, 69% were men, 60% were newly diagnosed with diabetes, 36% had hypertension, 48% had a history of CVD, and 7% had a history of cerebral infarction. Additionally, 12% had type 1 diabetes and 88% had type 2 diabetes. The UDG had 21 subjects, of which 76% were men, 48% had hypertension, 57% had a history of CVD, and 9.5% had a history of cerebral infarction. In the UDG, 19% of patients had type 1 diabetes and 81% had type 2 diabetes. Nine patients were newly diagnosed with type 1 diabetes; 5 in the IDG and 4 in the UDG. In these patients, type 1 diabetes was confirmed by positive glutamic acid decarboxylase (GAD) antibodies, and C-peptide <0.26 nmol/L. Other medical conditions included nonalcoholic fatty liver disease, gastroesophageal reflux disease (GERD), articular gout, bronchial asthma, cholelithiasis, and benign prostate hyperplasia. The differences in the number of these medical conditions between the IDG and UDG were not statistically significant (Supplementary Table 2).
Table 1:
Characteristics of Subjects at Baseline and 3 Months
| Improved (n=42) | Unimproved (n=21) | |||
|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |
| Age (years) | 43.8±5.2 | 41.3±4.6 | ||
| Type 1 diabetes n (%) | 5 (12) | 4 (19) | ||
| Type 2 diabetes n (%) | 37 (88) | 17 (81) | ||
| Diabetes duration (years) | 3 [0-11] | 3 [0-8] | ||
| Men n (%) | 29 (69) | 16 (76) | ||
| New cases of diabetes n (%) |
25(60) | 15 (71) | ||
| Average days hospitalized | 11±2.2 | 14±2.0 | ||
| BMI (kg/m2) | 26.8±2.3 | 25.5±2.1* | 27.3±2.6 | 26.9±2.5 |
| Fasting glucose (mg/dL) | 312.6±41.2 | 147.1±19.3* | 215.6±20.2 | 209.5±16.8 |
| HbA1c (%) | 10.2±1.7 (88 mmol/mol) |
6.8±0.9* (51 mmol/mol) |
9.1±0.9 (76 mmol/mol) |
8.8±0.8 (73 mmol/mol) |
| History of hypertension n (%) |
15 (35.7) | 10 (47.6) | ||
| History of cerebral infarction n (%) |
3 (7.1) | 2 (9.5) | ||
| History of CVD n (%) | 20 (48) | 12 (57) | ||
| Medications | ||||
| Antiplatelet n (%) | 14 (33.3) | 35 (83.3)* | 6 (29) | 10 (47.6)% |
| Calcium channel blockers n (%) |
8 (19) | 11 (26.2) | 4 (19) | 6 (28.6) |
| Angiotensin II receptor blocker n (%) |
3 (7.1) | 3 (7.1) | 1 (4.8) | 3 (14.3) |
| Angiotensin converting enzyme inhibitor n (%) |
1 (2.4) | 1 (2.4) | 0 (0) | 0 (0) |
| Nitrates n (%) | 2 (7.4) | 2 (7.4) | 1 (4.8) | 1 (4.8) |
| Subjects on Diabetes medications |
17 (40) | 42 (100)* | 6 (29) | 21 (100)* |
| Only oral antihyperglycemics n (%) |
4 (9.5) | 8 (19) | 2 (9.5) | 7 (33.3) |
| Number of subjects on insulin only n (%) |
6 (14.3) | 20 (47.6)* | 2 (9.5) | 6 (28.6)* |
| Combination therapy (insulin+oral antihyperglycemics) n (%) |
7 (16) | 14 (33.3) | 2 (9.5) | 9 (42.9) |
| Mean total insulin dosage-per-person (units/day) |
10.8±3.7 | 38.8±18.1* | 13.5±3.4 | 14.2±6.0* |
| Number of subjects on statins n (%) |
13 (31) | 36 (86)* | 6 (29) | 16 (76.2)* |
| Total cholesterol (mg/dL) |
280.5±68.92 | 270.6±60.5* | 290.8±48 | 285±42* |
| LDL (mg/dL) | 167.2±41.7 | 161.2±36.2* | 177.0±30.24 | 174.9±29.1 |
| HDL (mg/dL) | 53.7±13.5 | 55.2±13.5 | 50.5±12.1 | 51.28±9.0 |
| Triglycerides (mg/dL) | 115±62.8 | 107±55.6* | 130.9±60 | 123.5±54.4 |
Subjects were divided into 2 groups: 1) improved group had significant glycemic reductions after 3 months of medical management, and 2) unimproved group did not have significant glycemic reductions after 3 months. Data are reported as mean ± SD, mean [range].
p<0.05
After 3 months, we observed significant reductions in mean fasting plasma glucose by 47% (165.6 ± 35.3 mg/dl_), HbA1c by 3.4%±1.4% (37.2 mmol/mol), and BMI by 4.8%± 2.3% in the IDG (Table 1). Unlike the IDG, the UDG did not experience significant reductions in mean fasting plasma glucose, HbA1c, or BMI.
3.2. Medications
At 3 months, there was an increase in oral antihyperglycemic usage, with 67% of all subjects taking such medications (Supplementary Table 3). The oral antihyperglycemics include metformin, acarbose, gliclazide, and repaglinide. The total number of subjects on insulin, as well as insulin dose, increased at 3 months in both groups (Table 1). About 40% of subjects in the IDG were either on insulin, oral antihyperglycemics, or a combination of both at baseline, whereas only 29% of UDG subjects were on these medications. The remaining subjects were not taking any form of diabetes medication. At the end of the study, there was a significant increase in the number of subjects on diabetes medications. All of the patients in both groups were taking diabetes medications at 3 months. There were more subjects who were only on insulin in the IDG than in the UDG, but there was no significant difference in oral antihyperglycemics or combination therapy usage in both groups. (Table 1).
In China, patients that are diagnosed with diabetes are commonly given oral antiplatelet medications. In this study, the antiplatelets that were administered were aspirin and clopidogrel, and their usage increased in both groups. Also, the number of subjects on statins significantly increased from 31% to 86% in the IDG, and from 29% to 76% in the UDG (Table 1). Comparing subjects who were taking antiplatelet and statin therapy to those who were not, there were no differences in the genetic expression of inflammatory markers (Supplementary Table 3).
Other medications that were used in both groups were antihypertensives that included calcium channel blockers, angiotensin II receptor blockers, angiotensin converting enzyme inhibitors, and nitrates (Table 1). There was no significant change in usage of these medications at 3 months.
3.3. Circulating lipid levels
Subjects had elevated levels of circulating total cholesterol and low-density lipoprotein (LDL) at baseline (Table 1). Total cholesterol significantly decreased by 9.9±21 mg/dL in the IDG and 5.8±11 mg/dL in the UDG. LDL levels were significantly decreased by 6.0±15 mg/dL in the IDG. High-density lipoprotein (HDL) and triglyceride levels did not significantly change in both groups. Diet and other lifestyle alterations were not documented in detail, but most likely aided the reduction of HbA1c and lipid levels.
3.4. Relationship of HbA1c with WBCs
We evaluated how glycemic reductions altered total WBC, monocyte, and neutrophil counts after 3 months of diabetes management. In the IDG, there were significant decreases (p<0.001) of 9.4% in total WBCs, 10.96% in neutrophils, and 21.7% in monocytes. No significant reductions were found in lymphocyte levels (Table 2). In contrast, the UDG had no significant changes in WBC counts (Table 2). Additionally, neither group showed significant changes in platelet, hemoglobin, and hematocrit levels (not shown). WBC counts of subjects with type 1 diabetes were compared to those of subjects with type 2 diabetes. At 3 months, there were no significant differences in total WBC counts, neutrophils, monocytes, and lymphocytes between these two groups. However, there was a greater reduction in neutrophil counts in subjects with type 2 diabetes (Supplementary Figure 1).
Table 2:
WBC Counts Decreased in Subjects with Improved Glucose Control
| WBCs (×109/L) |
Neutrophils (×109/L) |
Monocytes (×109/L) |
Lymphocytes (×109/L) |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | |
| Improved | 8.40 ±1.7 |
7.61* ±1.32 |
5.75 ±1.4 |
5.12* ±1.1 |
0.69 ±0.21 |
0.54* ±0.13 |
1.74 ±0.3 |
1.76 ±0.43 |
| Unimproved | 7.94 ±1.3 |
7.90 ±1.15 |
5.46 ±1.1 |
5.52 ±1.0 |
0.62 ±0.17 |
0.61 ±0.16 |
1.63 ±0.4 |
1.58 ±0.36 |
Circulating total WBC, neutrophil, and monocyte levels decreased in the improved group after 3 months of medical management (*p<0.05), but there were no significant changes in lymphocytes. The unimproved group did not have any significant changes.
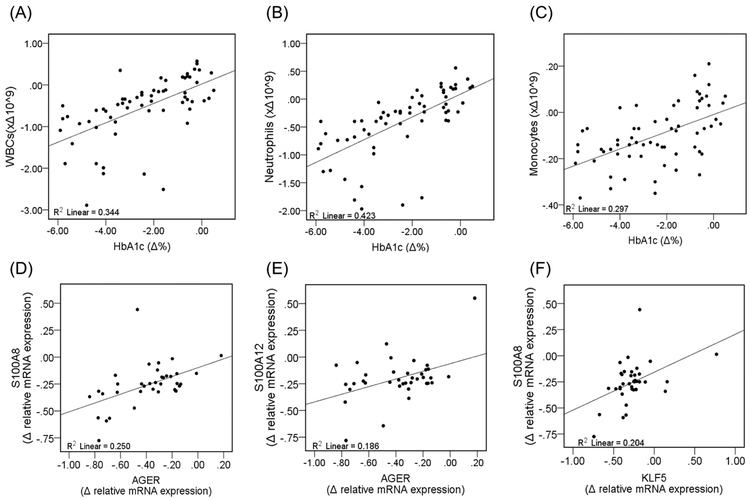
We also determined correlations between changes in HbA1c and WBCs (Figure 1) Changes in HbA1c correlated with changes in total WBCs (ρ: 0.607; p <0.001) and neutrophils (ρ: 0.602; p <0.001), but not with changes in monocytes and lymphocytes (p>0.05) (lymphocytes not shown).
Figure 1: Correlation of Changes in WBCs and Inflammatory Markers in the Improved Diabetes Group.
WBCs were assessed using a Coulter counter to determine numbers of circulating cells. Histopaque double-density separation was also used to separate WBCs into granulocytes and mononuclear cells. Then, mRNA levels were assessed from granulocytes and mononuclear cells using qPCR. (A) There is a significant linear and positive correlation between relative changes in HbA1c and WBCs (F =8.8, p<0.01). Changes in HbA1c significantly correlated with (B) neutrophil counts (F =10.8, p<0.01), but not (C) monocytes (F=2.26, p=0.142) and lymphocytes (F=0.111, p<0.740) (data for lymphocytes are not shown). There was a significant linear and positive correlation between relative changes in AGER and (D) S100A8 (F=11.7, p<0.01) and (E) S100A12 (F=8.0, p<0.01). Relative changes in the gene expression of (F) KLF5 positively and significantly correlated with relative changes in S100A8 (F=6.3, p<0.05).
At baseline, there was no correlation between insulin dose and total WBC, neutrophil, monocyte, or lymphocyte counts in both the IDG and UDG. However, at 3 months there was a positive correlation between insulin dose and HbA1c (ρ: 0.466, p=0.002), as well as total WBC (ρ:0.693, p<0.001), neutrophil (ρ:0.668, p<0.001) and monocyte (ρ: 0.423, p=0.005) counts in the IDG, but not in the UDG. There was no correlation between insulin dose and lymphocyte counts in both groups. Regression analysis was performed to determine if the insulin dose was a mediator in the relationship between HbA1c and WBC counts at 3 months. Insulin was a full mediator (F=22.2, p=0.007) between HbA1c and only WBCs and neutrophils in the IDG. With bootstrap estimation, there was a significant indirect effect of HbA1c on WBC (effect size=0.4.44, 95%CI= 0.1265, 0.7697) and neutrophil counts (effect size=(0.3509, 95%CI=0.1178, 0.7053) with insulin dose as a mediator (Supplementary Figure 2 and Supplementary Table 4 and 5). Insulin was not a mediator between HbA1c and monocytes. Thus, at 3 months, the positive association between HbA1c levels and total WBC and neutrophils counts are controlled by insulin dose.
3.5. Gene expression of cytokines, AGER, and AGER ligands decreased with glycemic reduction
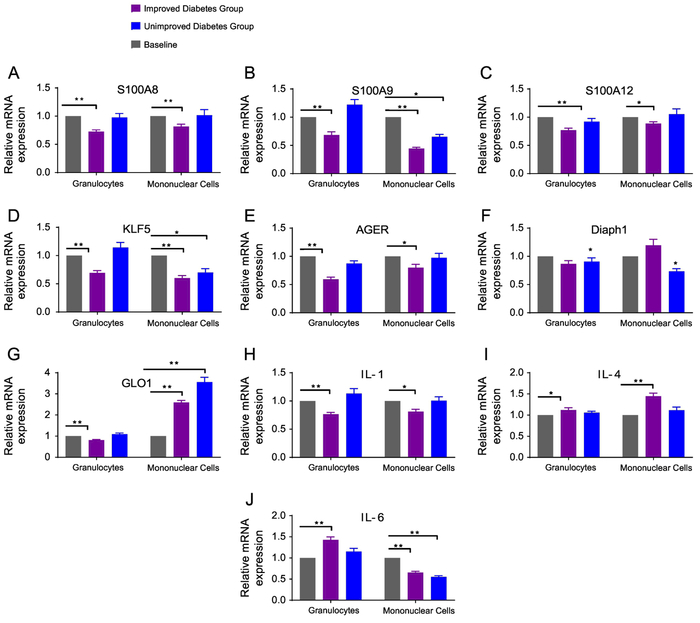
IL-1 expression was reduced in granulocytes and mononuclear cells, IL-4 increased in granulocytes and mononuclear cells, and IL-6 increased in granulocytes in the IDG (Figure 2). Granulocyte and mononuclear cell mRNA levels of AGER and its ligands—S100A8, S100A9, and S100A12—decreased significantly in the IDG (p<0.001) (Figure 2). In comparison, the UDG showed no reductions in mRNA levels of S100A8 or S100A12, while S100A9 decreased only in mononuclear cells (Figure 2). We also found a significant decrease (p<0.001) in KLF5 expression in both WBC populations after glycemic reductions. Thus, improved glucose control was associated with reductions in mRNA levels of AGER, KLF5, and S100 proteins. Moreover, the changes in AGER mRNA levels correlated with S100A8 and A12; KLF5 correlated with S100A8 (Figure 1).
Figure 2: Relative Gene Expression of Inflammatory Markers from Granulocytes and Mononuclear Cells after 3 months of Diabetes Treatment.
Granulocytes and mononuclear cells were isolated from subjects with type 2 diabetes. Inflammatory markers from these WBCs were measured at baseline and 3 months in the improved and unimproved diabetes groups. Inflammatory markers include (A) S100A8 (B) S100A9 (C) S100A12 (D) KLF5 (E) AGER (F) Diaph1 (G) GLO1 (H) IL-1 (I) IL-4, and (J) IL-6. Data are represented as mean and SEM. All inflammatory markers are compared to a relative baseline expression of 1. * p<0.05, ** p<0.001
The cytoplasmic domain of AGER binds to the formin homology 1 (FH1) domain and DIAPH1, causing AGER ligand-stimulated signal transduction [15]. Also, the effect of hyperglycemia-induced expression of AGER and its ligands is mediated by methylglyoxal, a major substrate of GLO1 [16]. In this study, DIAPH1 expression was significantly reduced in granulocytes and mononuclear cells of subjects in the UDG (Figure 2). Furthermore, GLO1 mRNA levels decreased at 3 months in granulocytes, but increased significantly in mononuclear cells in the IDG. The reason for this inverse relationship in GLO1 expression between the two WBC populations is unclear. Similar to the IDG, the UDG had reductions in S100A9, KLF5, and IL-6, as well as an increase in GLO1 in mononuclear cells. We determined if insulin dose was associated with changes in inflammatory markers in granulocytes and mononuclear cells. With the exception of KLF5, insulin dose at baseline and 3 months was not correlated with changes in inflammatory markers (data not shown).
When granulocyte inflammatory markers from type 1 and type 2 diabetes were compared, changes in S100A12 (p<0.5) and KLF5 (p <0.05) were significantly different. Subjects with type 1 diabetes had greater reductions in these inflammatory markers (Supplementary Figure 3). However, changes in inflammatory markers from mononuclear cells in these diabetes groups were not different (data not shown). Differences in gender did not correlate with changes in WBC counts and genetic expression of inflammatory markers.
3.6. Reduction in serum levels of IL-6, S100A8/A9, and CRP after glycemic reduction
Serum was obtained at baseline and after 3 months, and proteins levels were measured in a single assay. In the IDG, there were significant reductions in IL-1β and CRP (Table 3). These changes were not seen in UDG. We then compared the change in protein levels from baseline to 3 months from subjects with type 1 and type 2 diabetes; there was no difference between these two groups.
Table 3:
Inflammatory Protein Levels at Baseline and 3 months
| IL-1β (pg/ml) | IL-6 (pg/ml) | S100A8/A9 (ng/ml) | CRP (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | |
| Improved | 1.41 ± 0.57 |
1.25* ± 0.62 |
1.36 ±0.40 |
1.17* ±0.58 |
3.80 ±1.27 |
3.35 ±1.24 |
20.1 ±7.44 |
17.8* ±5.32 |
| Unimproved | 1.42 ±0.68 |
1.47 ±0.49 |
1.25 ±0.54 |
1.37 ±0.42 |
3.3 ±1.2 |
3.04 ±1.45 |
22.83 ±7.03 |
20.2 ±8.6 |
Circulating IL-6, S100A8/A9, and CRP—but not IL-1β— protein levels decreased in the improved group after 3 months of medical management (*p<0.05). The unimproved group did not have any significant changes.
4. Discussion
Aside from hypertension and hyperlipidemia, the other major risk factor for CVD is increased inflammation. Multiple studies in animal models support a role for inflammation as a driver of atherosclerosis [17]. In animal models, reducing levels of circulating monocytes decreased atherosclerosis [17].Thus, it is not surprising that indices of inflammation, such as CRP, track with disease [18]. Similarly, WBC levels are increased in subjects with CVD [6]; however, the variability of this measurement over time and between people makes it a difficult clinical tool.
We sought to determine whether reduction of hyperglycemia would alter WBC counts and their expression of inflammatory genes, as was found in mice [7]. We used a cohort study design to analyze WBCs of patients with poorly controlled diabetes at baseline and after 3 months of medical management; thus, each patient was their own control for the effects of the intervention. We demonstrated that glycemic reduction was associated with a reduction in WBC counts and inflammatory markers. Insulin, through its glucose lowering effects, may also play a role in this relationship. Improved glycemic levels reduced mRNA levels of several inflammatory cytokines, such as IL-1 and IL-6. Anti-inflammatory factor IL-4 increased with glycemic reduction. AGER and S100 proteins from WBCs, which are implicated in CVD, as well as circulating IL-1β and CRP decreased after glycemic reduction [19]. Our data support previous animal studies that found associations between glycemic reduction and WBC reductions [7].
Not all WBCs decreased with improved glycemic control. Reductions of HbA1c ≥1.5% (16 mmol/mol) at 3 months were associated with significant decreases in total WBC, neutrophil, and monocyte counts. However, there were no significant changes in WBC counts when the 3-month HbA1c reduction was <1.5% (16 mmol/mol). Lymphocyte numbers, platelet counts, and hematocrit were not altered.
In many studies, including the Multiple Risk Factor Intervention Trial (MRFIT) Study, WBC counts were a marker for CVD risk [6]. For each interval increase of 1000/cu mm in WBC count, the risk for CVD death increased by 14% [6]. The United Kingdom Prospective Diabetes Study (UKPDS) describes a linear relationship between HbA1c and CVD. Nevertheless, if and how chronic hyperglycemia drives changes in WBCs counts and inflammatory markers as well as its impact on CVD risk is not well understood [20]. Our data suggest that hyperglycemia affects CVD risk in part by affecting the circulating levels and inflammatory status of neutrophils and monocytes.
How might hyperglycemia lead to these changes in WBCs? Nagareddy et al. proposed a mechanism for leukocytosis in diabetic mice [7]. S100A8/A9, through its interaction with AGER, drives the production of neutrophils and monocytes during hyperglycemia. In their mouse study, glycemic reduction was associated with better atherosclerosis regression—the reduction of arterial macrophages with decreased cholesterol—along with decreased WBC counts, S100A8/A9 levels, and monocyte AGER expression [7]. Similarly, we demonstrated a significant decrease in AGER gene expression from granulocytes and mononuclear cells after glycemic reduction, which corresponded to reduced expression of S100A8, S100A9, S100A12, and circulating protein levels of S100A8/A9.
Studies have associated S100A8, S100A9, and S100A12 with CVD pathogenesis [19, 21-23]. S100A8/A9 recruits and activates neutrophils and promotes monocyte recruitment into the arterial wall [21]. Also, S100A8/A9 interacts with cluster of differentiation (CD) 36, a protein expressed on platelets and monocytes/-macrophages that leads to platelet activation [24] and is thought to induce inflammation in macrophages [25]. In humans with diabetes, elevated S100A8/A9 levels were associated with leukocytosis and CVD [7]. What stimulates the production of S100A8/A9 is unknown, but one study suggested that the transcription factor KLF5 promotes S100A8/A9 expression in the kidney [26]. KLF5 is a master transcription factor important for regulation of hematopoietic stem cells as well as growth and differentiation of myeloid-derived cells [27, 28]. Its role in CVD is not delineated. We demonstrate a reduction in KLF5 in granulocytes and mononuclear cells.
Furthermore, elevated circulating levels of IL-1 and IL-6 have been found in patients with CVD. The expression of IL-1 α and β correlate with progression of atherosclerotic plaques [29, 30], and increased levels of IL-1 and IL-6 predicted adverse outcomes in patients with unstable angina [31, 32]. In addition, in vitro studies show that glucose stimulates IL-1β [33]. In humans, IL-1 antagonism decreased CRP [34] and lowered the rate of recurrent cardiovascular events [35]. Our study connects these observations by demonstrating that improved glucose control decreases the gene expression of IL-1 in granulocytes and in mononuclear cells. This is significant since atherosclerotic plaques have higher expression of inflammatory receptors and proteins, and increased infiltration of inflammatory cells in type 2 diabetes [36]. Elevation in HbA1c is associated with coronary artery disease in type 2 diabetes [20], and increased expression of inflammatory markers at baseline in our study may reflect an underlying atherosclerotic process [37]. Although data suggests that both metformin [38] and sulfonylureas [39] can affect inflammasome activation, and consequently IL-1β production, in our study, only a small number of subjects were taking these medications, and the use of these drugs did not differ between the IDG and UDG subjects.
We also found that IL-6 increased in granulocytes and decreased in mononuclear cells in the IDG. IL-6 has both anti-inflammatory and inflammatory roles [40]. One example of an anti-inflammatory action was improved insulin secretion by IL-6 treated human pancreatic cells [41]. In other studies, IL-6 gene expression was increased in WBCs from patients with diabetes [42]. Perhaps, IL-6 has alternate functions in different WBCs during hyperglycemia.
Fewer inflammatory markers changed in the UDG; mRNA levels of S100A9, KLF5, and IL-6 decreased in mononuclear cells, while GLO1 increased. These changes may be attributed to the small glycemic reduction in this group. Also, there was a greater reduction in the IDG granulocyte S100A12 and KLF5 from subjects with type 1 diabetes than with type 2 diabetes, but no differences in the changes in other inflammatory markers. The sample size of subjects with type 1 diabetes was small, and a larger study would be beneficial to reveal any differences between these diabetes groups.
Could the increased use of insulin, rather than reduced glycemia, cause a decrease in inflammatory markers? Effects of insulin and insulin signaling on inflammation are inconsistent. Insulin treatment did not alter atherosclerosis in cholesterol-fed rabbits [43] and, in some studies, atherosclerosis-prone mice [44]. Recently, Park et al. found that insulin treatment decreased atherosclerosis and inflammatory markers in high fat diet (HFD)-fed apolipoprotein E knockout (ApoE−/−) mice [45]. However, insulin treatment also improved the lipid profile. Studies in mice with tissue-specific deletions show that complete loss of insulin receptors in endothelial cells increases atherosclerosis [46]. The effects of loss of insulin signaling in monocytes/macrophages are mixed; macrophage knockout of the insulin receptor increased necrotic core formation within lesions [47], but led to no overall increase in lesions [48].
Mouse studies in which HFD and leptin deficiency were used to create obesity and insulin resistance showed that lower blood glucose did not alter circulating levels of monocytes and neutrophils, as it had done in an insulin-deficient hyperglycemic model [7, 49]. We suspect that both the initial glucose level and the magnitude of its reduction were not sufficient in the obese mice. In contrast, our subjects had marked hyperglycemia at baseline, with an initial average HbA1c of 10.2% that was reduced to 6.7%, a 3.4% reduction. The subjects in the IDG had more insulin usage than the UDG, which may have contributed to better glycemic control in the IDG. There was also an indirect effect of HbA1c on total WBCs and neutrophils through insulin dose, but there was no association between insulin dose and change in genetic expression of inflammatory markers. We cannot, however, exclude the possibility that some of the beneficial effects seen in the IDG subjects were not due to greater weight loss, diet, or changes in circulating lipid levels.
5. Conclusion
In summary, we show that glycemic reduction decreased gene expression of cytokines, AGER, S100 proteins, and KLF5. These genes may be potential drug targets to reduce inflammation and secondary complications of diabetes. In addition, the reduction in circulating WBCs suggests a reduction in CVD risk with marked improvement in glycemic control. It is likely that such changes in inflammatory status are but one of many beneficial effects of improved medical care that occurs along with reduced blood pressure and circulating LDL levels. We note that the small sample size is a limitation of this study. For that reason, larger studies that analyze glycemic reductions in both type 1 and type 2 diabetes should be performed in the future.
Supplementary Material
Acknowledgments
Funding: This work was supported by Anhui Provincial Hospital (XF and S-LH) and the National Institutes of Health [DK095684, P01 HL092969] (IJG). In addition, assistance with the statistical analysis was obtained from the New York University School of Medicine Clinical Translational Science Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no relevant conflicts of interest to disclose.
References
- [1].Duncan BB, Schmidt MI, Pankow JS, et al. , Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study, Diabetes, 2003;52:1799–1805. [DOI] [PubMed] [Google Scholar]
- [2].Kolseth IB, Reine TM, Parker K, et al. , Increased levels of inflammatory mediators and proinflammatory monocytes in patients with type I diabetes mellitus and nephropathy, J. Diabetes Complications, 2017;31:245–252. [DOI] [PubMed] [Google Scholar]
- [3].Tong PC, Lee K-F, So, W-Y, et al. , White Blood Cell Count Is Associated With Macro- and Microvascular Complications in Chinese Patients With Type 2 Diabetes, Diabetes Care, 2004;27:216–222. [DOI] [PubMed] [Google Scholar]
- [4].Devaraj S, Cheung AT, Jialal I, et al. , Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications, Diabetes, 2007;56:2790–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Devaraj S, Glaser N, Griffen S, et al. , Increased Monocytic Activity and Biomarkers of Inflammation in Patients With Type 1 Diabetes, Diabetes, 2006;55:774–779. [DOI] [PubMed] [Google Scholar]
- [6].Grimm RH Jr., Neaton JD and Ludwig W, Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality, JAMA, 1985;254:1932–1937. [PubMed] [Google Scholar]
- [7].Nagareddy PR, Murphy AJ, Stirzaker RA, et al. , Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis, Cell Metab, 2013;17:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen Y-C, Huang AL, Kyaw TS, et al. , Atherosclerotic Plaque Rupture Identifying the Straw That Breaks the Camel’s Back, Arterioscler. Thromb. Vasc. Biol, 2016;36:e63–e72. [DOI] [PubMed] [Google Scholar]
- [9].Soehnlein O, Multiple roles for neutrophils in atherosclerosis, Circ. Res, 2012;110:875–888. [DOI] [PubMed] [Google Scholar]
- [10].Vinagre I, Sanchez-Quesada JL, Sanchez-Hernandez J, et al. , Inflammatory biomarkers in type 2 diabetic patients: effect of glycemic control and impact of LDL subfraction phenotype, Cardiovasc. Diabetol, 2014; 13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heier M, Margeirsdottir HD, Brunborg C, et al. , Inflammation in childhood type 1 diabetes; influence of glycemic control, Atherosclerosis, 2015;238:33–37. [DOI] [PubMed] [Google Scholar]
- [12].Vozarova B, Weyer C, Lindsay RS, et al. , High White Blood Cell Count Is Associated With a Worsening of Insulin Sensitivity and Predicts the Development of Type 2 Diabetes , Diabetes, 2002;51:455–461. [DOI] [PubMed] [Google Scholar]
- [13].Hayes A, Introduction to Mediation, Moderation and Conditional Process Analysis: A Regression Based Approach, New York, NY, Guilford Press, 2013. [Google Scholar]
- [14].Preacher KJ and Hayes AF, SPSS and SAS procedures for estimating indirect effects in simple mediation models, Behav. Res. Methods Instrum. Comput, 2004;36:717–731. [DOI] [PubMed] [Google Scholar]
- [15].Manigrasso MB, Pan J, Rai V, et al. , Small Molecule Inhibition of Ligand-Stimulated RAGE-DIAPH1 Signal Transduction, Sci. Rep, 2016;6:22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yao D and Brownlee M, Hyperglycemia-Induced Reactive Oxygen Species Increase Expression of the Receptor for Advanced Glycation End Products (RAGE) and RAGE Ligands, Diabetes, 2010;59:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Libby P, History of Discovery: Inflammation in Atherosclerosis, Arterioscler. Thromb. Vasc. Biol, 2012;32:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Singh SK, Suresh MV, Voleti B, et al. , The connection between C-reactive protein and atherosclerosis, Ann. Med, 2008;40:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Oesterle A and Hofmann Bowman MA, S100A12 and the S100/Calgranulins - Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets, Arterioscler. Thromb. Vasc. Biol, 2015;35:2496–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Turner RC, Millns H, Neil HA, et al. , Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23), BMJ, 1998;316:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schiopu A and Cotoi OS, S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease, Mediators Inflamm, 2013;2013:828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ehlermann P, Eggers K, Bierhaus A, et al. , Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products, Cardiovasc. Diabetol, 2006;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kosaki A, Hasegawa T, Kimura T, et al. , Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes, J. Clin. Endocrinol. Metab, 2004;89:5423–5428. [DOI] [PubMed] [Google Scholar]
- [24].Wang Y, Fang C, Gao H, et al. , Platelet-derived S100 family member myeloid-related protein-14 regulates thrombosis, J. Clin. Invest, 2014;124:2160–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moore KJ, Sheedy FJ and Fisher EA, Macrophages in atherosclerosis: a dynamic balance, Nat. Rev. Immunol, 2013;13:709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fujiu K, Manabe I and Nagai R, Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice, J. Clin. Invest, 2011;121:3425–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang P, Iwasaki-Arai J, Iwasaki H, et al. , Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP alpha, Immunity, 2004;21:853–863. [DOI] [PubMed] [Google Scholar]
- [28].Shahrin NH, Diakiw S, Dent LA, et al. , Conditional knockout mice demonstrate function of Klf5 as a myeloid transcription factor, Blood, 2016;128:55–59. [DOI] [PubMed] [Google Scholar]
- [29].Bacchiega BC, Bacchiega AB, Usnayo MJG, et al. , Interleukin 6 Inhibition and Coronary Artery Disease in a High-Risk Population: A Prospective Community-Based Clinical Study, J. Am. Heart Assoc, 2017;6:e005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Van Tassell BW, Toldo S, Mezzaroma E, et al. , Targeting interleukin-1 in heart disease, Circulation, 2013;128:1910–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fanola CL, Morrow DA, Cannon CP, et al. , Interleukin-6 and the Risk of Adverse Outcomes in Patients After an Acute Coronary Syndrome: Observations From the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib—Thrombolysis in Myocardial Infarction 52) Trial, J. Am. Heart Assoc, 2017;6:e005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Van Tassell BW, Toldo S, Mezzaroma E, et al. , Targeting Interleukin-1 in Heart Disease, Circulation, 2013;128:1910–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Maedler K, Sergeev P, Ris F, et al. , Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets, J. Clin. Invest, 2002;110:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Larsen CM, Faulenbach M, Vaag A, et al. , Interleukin-1-receptor antagonist in type 2 diabetes mellitus, N. Engl. J. Med, 2007;356:1517–1526. [DOI] [PubMed] [Google Scholar]
- [35].Ridker PM, Everett BM, Thuren T, et al. , Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease, N. Engl. J. Med, 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- [36].Virmani R, Burke AP and Kolodgie F, Morphological characteristics of coronary atherosclerosis in diabetes mellitus, Can. J. Cardiol, 2006;22:81B–84B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ray A, Huisman MV, Tamsma JT, et al. , The role of inflammation on atherosclerosis, intermediate and clinical cardiovascular endpoints in type 2 diabetes mellitus, Eur. J. Intern. Med, 2009;20:253–260. [DOI] [PubMed] [Google Scholar]
- [38].Kelly B, Tannahill GM, Murphy MP, et al. , Metformin Inhibits the Production of Reactive Oxygen Species from NADH:Ubiquinone Oxidoreductase to Limit Induction of Interleukin-1beta (IL-1beta) and Boosts Interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-activated Macrophages, J. Biol. Chem, 2015;290:20348–20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhou R, Tardivel A, Thorens B, et al. , Thioredoxin-interacting protein links oxidative stress to inflammasome activation, Nat. Immunol, 2010;11:136–140. [DOI] [PubMed] [Google Scholar]
- [40].Scheller J, Chalaris A, Schmidt-Arras D, et al. , The pro- and anti-inflammatory properties of the cytokine interleukin-6, Biochim. Biophys. Acta, 2011;1813:878–888. [DOI] [PubMed] [Google Scholar]
- [41].Ellingsgaard H, Hauselmann I, Schuler B, et al. , Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells, Nat. Med, 2011;17:1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Devaraj S, Venugopal SK, Singh U, et al. , Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta}, Diabetes, 2005;54:85–91. [DOI] [PubMed] [Google Scholar]
- [43].Nordestgaard BG, Agerholm-Larsen B and Stender S, Effect of exogenous hyperinsulinaemia on atherogenesis in cholesterol-fed rabbits, Diabetologia, 1997;40:512–520. [DOI] [PubMed] [Google Scholar]
- [44].Rask-Madsen C, Buonomo E, Li Q, et al. , Hyperinsulinemia does not change atherosclerosis development in apolipoprotein E null mice, Arterioscler. Thromb. Vasc. Biol, 2012;32:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Park K, Li Q, Evcimen ND, et al. , Exogenous Insulin Infusion Can Decrease Atherosclerosis in Diabetic Rodents by Improving Lipids, Inflammation, and Endothelial Function, Arterioscler. Thromb. Vasc. Biol, 2017;38:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rask-Madsen C, Li Q, Freund B, et al. , Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice, Cell Metab, 2010;11:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Han S, Liang CP, DeVries-Seimon T, et al. , Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions, Cell Metab, 2006;3:257–266. [DOI] [PubMed] [Google Scholar]
- [48].Bornfeldt KE and Tabas I, Insulin resistance, hyperglycemia, and atherosclerosis, Cell Metab, 2011;14:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nagareddy PR, Kraakman M, Masters SL, et al. , Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity, Cell Metab, 2014;19:821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.